Abstract
Lay Abstract
Autism Spectrum Disorders (ASD) are predominantly characterized by impairments in social communication, as well as by restricted and repetitive behaviors. Among these are atypical responses to sensory stimuli, which are commonly observed in ASD. While some children with ASD are easily overwhelmed by sensory stimuli, others may seem unaware of their environment. Vision and audition are two of the main sensory modalities involved in social interactions and language. To examine how basic perceptual processes that may form the foundation for these cognitive abilities are affected in ASD, 16 children and adolescents with ASD and 16 matched typically developing (TD) participants were tested using functional magnetic resonance imaging. Participants were presented with auditory (high or low pitch) and visual stimuli (dot located high or low in a display), and were asked to indicate for each stimulus whether it was “high” or “low”. During the auditory condition, the TD group showed decreased neural activity in visual processing regions. By contrast, the ASD group showed increased neural activity in these regions. This unusual activity in visual regions was associated with autism symptomatology. Overall, these findings suggest that simple nonverbal perceptual discrimination may be impaired for auditory (but not visual) stimuli in ASD, and that individuals with ASD atypically recruit visual brain regions during processing of simple auditory stimuli.
Scientific Abstract
Autism Spectrum Disorders (ASD) are pervasive developmental disorders characterized by impairments in language development and social interaction, along with restricted and stereotyped behaviors. These behaviors often include atypical responses to sensory stimuli; some children with ASD are easily overwhelmed by sensory stimuli, while others may seem unaware of their environment. Vision and audition are two sensory modalities important for social interactions and language, and are differentially affected in ASD. In the present study, 16 children and adolescents with ASD and 16 typically developing (TD) participants matched for age, gender, nonverbal IQ, and handedness were tested using a mixed event-related/blocked functional magnetic resonance imaging (fMRI) paradigm to examine basic perceptual processes that may form the foundation for later-developing cognitive abilities. Auditory (high or low pitch) and visual conditions (dot located high or low in the display) were presented, and participants indicated whether the stimuli were “high” or “low”. Results for the auditory condition showed downregulated activity of the visual cortex in the TD group, but upregulation in the ASD group. This atypical activity in visual cortex was associated with autism symptomatology. These findings suggest atypical crossmodal (auditory-visual) modulation linked to sociocommunicative deficits in ASD, in agreement with the general hypothesis of low-level sensorimotor impairments affecting core symptomatology.
Keywords: Autism spectrum disorders, Development, Visual, Auditory, fMRI
1 Introduction
Autism Spectrum Disorder (ASD) is a pervasive neurodevelopmental disorder primarily characterized by two core domains of atypical behavior: impaired social communication, and stereotyped, restricted, and repetitive behaviors (American Psychiatric Association, 2013). Although once considered rare, recent findings indicate a rising prevalence of ASD with estimates of 1 in 45 children (or 2.24%) born in the U.S. (Zablotsky et al., 2015). A variety of features are commonly seen in ASD; many of these consist of atypical responses to sensory stimuli (Kern et al., 2006).
Empirical, clinical, and anecdotal reports suggest that the incidence of sensory and perceptual issues among individuals with ASD ranges from 30% to 100% (Dawson & Watling, 2000). Some researchers have argued that atypical responses to sensory stimuli can differentiate ASD from other developmental disorders (Gillberg & Coleman, 2000). Indeed, sensory symptoms are often the first issues that parents notice in a child later diagnosed with ASD (Baker et al., 2008). Kern and colleagues (2007) administered the Sensory Profile (Dunn, 1999) to 104 individuals with ASD ranging in age from 3 to 56 years and correlated those measures with diagnostic scores on the Childhood Autism Rating Scale (Schopler et al., 1994). Results indicated a positive correlation between sensory disturbances and symptom severity in children with ASD.
Although sensory and perceptual symptoms are common in ASD, the manifestation of these symptoms is heterogeneous. In some instances, differences in perceptual processing result in superior performance on certain tasks. For example, some individuals with ASD demonstrate enhanced pitch discrimination (Bonnel et al., 2003), particularly those with a history of delayed speech onset (Jones et al., 2009; Bonnel et al., 2010), and superior detection of novel auditory targets (Gomot et al., 2008). Furthermore, it has been suggested that visuospatial abilities in particular are not only spared, but also are a relative strength for many individuals with ASD despite evidence of altered oculomotor function (Brenner et al., 2006; Simmons et al., 2009). Individuals with ASD often outperform their typically developing (TD) peers on visuospatial tasks such as visual search or embedded figures tasks, with superior performance being most pronounced in more difficult conditions (Plaisted et al., 1998; O’Riordan et al., 2001). This enhanced perceptual functioning, however, may result in focus directed at specific sensory details to the detriment of global information such as social context (Mottron & Burack, 2001; Mottron et al., 2006; Keehn et al., 2008).
Given the high prevalence of perceptual issues in ASD and the importance of sensory input during early development, disturbances in the auditory and visual systems may have cascading effects that contribute to impairments in higher-order cognitive and sociocommunicative abilities. Several studies have shown that individuals with ASD activate visual cortices in response to linguistic or auditory stimuli more so than their TD peers (Kemner et al., 1995; Kana et al., 2006; Gaffrey et al., 2007; Samson et al., 2012). Kana and colleagues (2006) employed a sentence comprehension task using visually presented text with low-imagery and high-imagery content. Their findings indicated that young adults with ASD, but not TD control participants, recruited areas associated with visual processing and imagery even during the low-imagery condition. Another study demonstrated extrastriate activation during a semantic decision task, suggesting that individuals with ASD engage visual processes to assist with language comprehension (Gaffrey et al., 2007). This was supported by the finding of atypically increased functional connectivity between visual cortices and left inferior frontal gyrus in ASD (Shen et al., 2012). Yet, the involvement of visual areas in children with ASD has also been shown to occur with more basic auditory stimuli such as during an auditory oddball task consisting of phonemes as the standard and deviant stimuli, and a complex non-verbal sound as the novel stimulus (Kemner et al., 1995). This over-activation of visual processing areas in ASD may be indicative of a strong reliance on vision even in the context of sensory stimulation from other modalities, such as audition.
In TD adults, activity in visual cortex is downregulated in the context of auditory stimulation. Laurienti and colleagues (2002) used nonverbal visual and auditory stimuli presented individually and concurrently to examine activity in sensory specific cortices. They showed that auditory stimuli produced a reliable deactivation in visual cortices, particularly in the lingual gyri and cuneus. Similarly, visual stimuli decreased activity in superior and middle temporal gyri, although the modulation of auditory cortices by visual stimuli was not as robust. Evidence of atypical recruitment of visual cortex in ASD described above raises the question whether the typical crossmodal downregulation observed by Laurienti and colleagues (2002) may be affected in ASD.
To assess the neural mechanisms underlying auditory and visual processing in children and adolescents with ASD, and specifically to examine the effects of auditory processing on visual cortical activity in nonlinguistic contexts, the current study implemented functional magnetic resonance imaging during a perceptual discrimination task. Based on adult findings described above, we predicted (i) that the TD group would show a downregulation of sensory cortices during the presentation of stimuli from a different modality. We further predicted (ii) that the ASD group would show greater activity in visual cortex in response to auditory stimuli as compared to the TD group.
2 Methods
2.1 Participants
A total of 58 children and adolescents between the ages of 8 and 18 years were recruited. Participants with ASD (n = 29) were recruited through an ongoing collaboration with a local clinical expert (Dr. Alan J. Lincoln, Alliant International University). A diagnosis of Autism Spectrum Disorder was confirmed using the Autism Diagnostic Interview Revised (ADI-R; Lord et al., 1994) and the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 1989) for participants in the ASD group (Table 1). These assessments were administered and scored by Dr. Lincoln, a research-reliable assessor and certified trainer of these instruments. All participants completed the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 2000). Typically developing (TD) children and adolescents (n = 29) were recruited from an existing participant pool available to the Brain Development Imaging Laboratory, as well as through advertisements in the community.
Table 1.
Participant demographics per group.
| ASD (n=16) | TD (n=16) | ||||
|---|---|---|---|---|---|
|
| |||||
| Mean | SD | Mean | SD | ||
| Age in years | 12.62 | 2.61 | 14.16 | 2.67 | |
| WASI | Full Scale IQ | 116.00 | 16.35 | 113.44 | 11.12 |
| Verbal IQ | 112.88 | 17.59 | 110.13 | 10.42 | |
| Nonverbal IQ | 115.44 | 13.99 | 112.63 | 10.73 | |
| Motion | RMSD | 0.18 | 0.10 | 0.13 | 0.11 |
| ADOS | Social Interaction | 8.44 | 2.73 | - | - |
| Communication | 3.06 | 1.61 | - | - | |
| Combined | 11.50 | 3.79 | - | - | |
| Repetitive/Restricted | 1.87 | 1.54 | - | - | |
| ADI-R† | Social Interaction | 17.00 | 4.58 | - | - |
| Communication | 13.45 | 7.57 | - | - | |
| Repetitive Behavior | 5.45 | 1.69 | - | - | |
reported data consist of a subset of participants (n=11) with available data
Participants were screened for MRI contraindications (e.g., ferrous objects in body, claustrophobia) and to ensure that there was no personal or family history of neurological, psychiatric, or developmental disorders in the TD group or co-morbid ASD-related medical conditions (e.g., Fragile-X syndrome, tuberous sclerosis) or other neurological conditions (e.g., epilepsy, Tourette syndrome) in the ASD group. Imaging data were collected from all 29 children and adolescents with ASD and 29 TD participants matched in age, gender, IQ (verbal, nonverbal and full scale), and handedness (Table 1). Eight participants were excluded due to equipment malfunction and an additional 18 participants were excluded due to excessive motion (>20% of time-points lost in one condition due to movement greater than 2.0mm). Independent sample t-tests confirmed that the final fMRI groups (ASD: n=16, 2 female; TD: n=16, none female) were not significantly different in age (t(30) = 1.65, p = .11), full scale IQ (t(30) = .52 p = .61), verbal IQ (t(30) = .54, p = .60), nonverbal IQ (t(30) = .74, p = .47), or root-mean-square displacement (RMSD) across the entire time series to determine motion (t(30) = 1.54, p = .13; see also Table 1). Thus, the final sample size primarily included high-functioning children with autism who were able to stay still in the scanner well enough and long enough to provide sufficient neuroimaging data. The study protocol was approved by the Institutional Review Boards at the University of California, San Diego and San Diego State University. Written informed consent and assent was acquired from participants and their caregivers prior to beginning study procedures.
2.2 Scored assessments
The Autism Diagnostic Observation Schedule (ADOS; Lord et al., 1989)—a semi-structured, standardized assessment used to evaluate behaviors that are indicative of symptoms described by the DSM-IV criteria for ASD—was administered to the ASD participants. Three subsections (Communication, Social Interaction, and Stereotyped Behaviors and Restricted Interests) and a composite subsection (Communication and Social Interaction Combined) were scored, with higher scores indicating greater autism symptomatology (Table 1).
The Adolescent/Adult Sensory Profile (AASP; Brown & Dunn, 2002) was administered to evaluate sensory symptoms in all participants with the exception of 3 ASD participants who failed to complete the assessment. This measure consisted of 60 items scored on a Likert scale with response options ranging from almost never (1) to almost always (5). Behaviors were divided into four quadrants based on sensory threshold (high or low) and the pattern of response to sensory stimuli (passive or active): 1) low registration (high-threshold with passive response); 2) sensation seeking (high-threshold with active response); 3) sensory sensitivity (low-threshold with passive response); 4) and sensation avoiding (low-threshold with active response). The AASP was explained to the participants who completed the assessment either independently or with a trained experimenter if help with comprehension was needed.
2.3 Stimuli and procedure
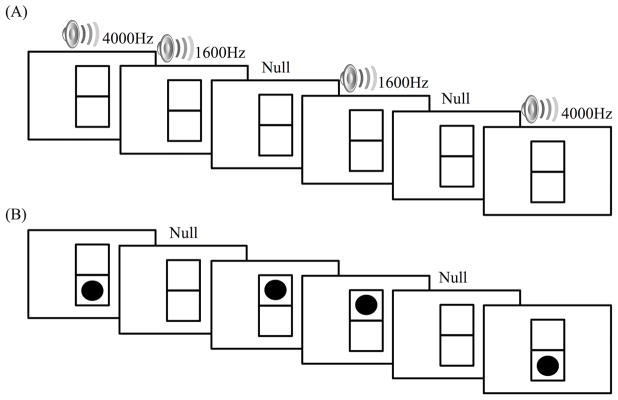
The experimental task consisted of a single run in a mixed event-related/blocked design with unisensory auditory and visual conditions (Figure 1). Each condition included 36 experimental trials (2000ms per trial) and 26 jittered null trials (2000ms per trial) that were presented using PsyScope X (http://psy.ck.sissa.it/). Each participant was pseudo-randomly assigned a block order of auditory then visual, or visual then auditory trials; block orders were counterbalanced within each group. A vertical rectangle with a horizontal bisection was present for the entire duration of the task (including null trials). The auditory stimuli consisted of two tones (high: 4000Hz, low: 1600Hz) with a 2000ms duration. Selection of frequencies served pragmatic purposes (being easily distinguished by participants on the background of gradient noise during fMRI). The visual stimulus was a black dot that appeared either in the top or bottom half of the rectangle for 2000ms. Participants were instructed to distinguish between “high” and “low” stimuli, and to respond as quickly and correctly as possible via a button box; they indicated their responses with the right index finger (“high”) or right middle finger (“low”). Response times and accuracy measures were collected throughout the experiment. Participants viewed the visual stimuli on a screen through a mirror placed on top of an 8 channel head coil located inside the bore of the scanner, and listened to the auditory stimuli with noise-cancelling headphones.
Figure 1.
Task paradigm depicting a blocked subset of auditory (A) and visual (B) trials. Each condition consisted of 36 experimental trials and 26 jittered null trials; each individual trial lasted 2000ms.
The task was practiced on a laptop (Pentium III 1.7GHz/512MB PC) during a mock scan session to acclimate the participants to the MR environment prior to the actual experiment, which was conducted during a separate MRI session. Feedback was not provided during the practice task; accuracy was used to assess instruction comprehension. Both ASD and TD groups were at least 90% accurate for each condition, with a minimum individual accuracy of 81% in the ASD group and 86% in the TD group for the auditory condition and a minimum individual accuracy of 85% in the ASD group and 92% in the TD group for the visual condition.
2.4 MRI Data Acquisition
Functional Magnetic Resonance Imaging (fMRI) data were collected on a General Electric 3 Tesla HD Signa Excite system located at the UCSD Center for Functional MRI. High-resolution anatomical images were acquired using a standard spoiled gradient recalled (SPGR) T1-weighted sequence. During the fMRI run, 217 whole-brain volumes were acquired in 32 interleaved slices using a single-shot echo-planar image (EPI) pulse sequence (echo time [TE] = 30 ms; repetition time [TR] = 2000 ms; flip angle = 90°; 64×64 matrix; slice thickness = 3.2 mm; in-plane resolution = 3.4 mm2).
2.5 Data analysis procedures
Data were processed and analyzed with conventional blood-oxygen-level-dependent signal (BOLD) analysis procedures using Analysis of Functional NeuroImages (AFNI; http://afni.nimh.nih.gov/afni/; Cox, 1996). Data underwent a standard preprocessing pipeline of motion correction, slice timing correction, and spatial smoothing via a Gaussian kernel of 6×6×6mm (full-width half-maximum). Time points with motion exceeding 2.0mm were censored prior to analysis. Functional and structural images were coregistered and normalized to the Talairach atlas (Talairach & Tournoux, 1988) using the AFNI TT N27 template. Datasets with greater than 20% data loss within any task condition due to motion were excluded from analysis. The hemodynamic response was estimated using a general linear model with stimulus-specific regressors. Additionally, six rigid-body motion parameters (3 rotations, 3 translations) were used as orthogonal regressors. Global signal regression, a method often employed to reduce noise in a functional data set, was not performed to avoid spurious deactivation effects (Fox et al., 2009; Murphy et al., 2009; Weissenbacher et al., 2009; Jones et al., 2010).
Contrasts were thresholded with a minimum significance level of p < .05, and corrected for multiple comparisons with an alpha-level of .05 using a cluster threshold of at least 40 contiguous voxels as determined by AFNI’s cluster simulation algorithm (Forman et al., 1995). This algorithm implements Monte Carlo simulation to estimate the cluster-size threshold required to produce an alpha-level of .05 based on specific voxel-wise p-values. Individual statistical parametric maps were combined for pair-wise t-tests to explore group differences. Further analyses were performed for the auditory condition in anatomically defined visual cortex (Brodmann areas 17–19). Contrasts in this region were thresholded at a significance level of p < .05, and cluster corrected with an alpha-level of .05.
Statistical analyses were conducted on the sensory profile data, behavioral reaction times and accuracy, and neural activation data. Additionally, several correlations with auditory activity were calculated. In brief, analyses of variance (ANOVAs) were conducted to test group differences for the four AASP quadrants, and for reaction time and accuracy; four sets of contrasts were performed within and between groups; and correlations were calculated between the four sensory profile quadrants and neural activity (in visual cortex, and in right and left lingual gyri) for each group, and between two subdomains of the ADOS (Communication; Stereotyped Behaviors and Restricted Interests) and neural activity for the ASD group to examine the relation between symptomatology and neural activity.
3 Results
3.1 Sensory profile results
Complete sensory profiles were collected from a total of 14 ASD participants and 25 TD participants. A one-way analysis of variance (ANOVA) was conducted to test differences between the groups for the four AASP quadrant scores. Means and standard deviations for each quadrant are displayed in Table 2.
Table 2.
Adolescent/Adult Sensory Profile quadrant scores.
| All | ASD (n = 14) | TD (n = 25) | ||
|---|---|---|---|---|
|
| ||||
| Mean | SD | Mean | SD | |
| Low Registration | 36.79 | 7.3 | 31.52 | 7.9* |
| Sensation Seeking | 42.43 | 7.6 | 47.52 | 5.5 * |
| Sensory Sensitivity | 37.57 | 8.0 | 32.28 | 6.8 * |
| Sensory Avoidance | 38.71 | 8.1 | 32.92 | 8.7 * |
|
| ||||
| Useable fMRI | ASD (n = 13) | TD (n = 16) | ||
|
| ||||
| Mean | SD | Mean | SD | |
|
| ||||
| Low Registration | 31.54 | 11.4 | 30.81 | 6.8 |
| Sensation Seeking | 38.85 | 7.2 | 47.38 | 5.9 * |
| Sensory Sensitivity | 36.77 | 8.1 | 31.81 | 6.9 |
| Sensory Avoidance | 36.69 | 8.0 | 32.56 | 8.6 |
denotes significant between-group difference (p < .05)
For all participants who had completed the sensory profile, the ASD group reported significantly more sensory behaviors than the TD group for the low registration quadrant (F(1,37) = 4.23, p < .05), the sensory sensitivity quadrant (F(1,37) = 4.77, p < .05), and the sensation avoiding quadrant (F(1,37) = 4.19, p < .05). The TD group reported significantly more behaviors than the ASD group for the sensation seeking quadrant (F(1,37) = 5.87, p < .05). For participants who had both a completed sensory profile and usable fMRI data (ASD: n = 13; TD: n = 16), the TD group reported significantly more behaviors than the ASD group in the sensation seeking quadrant (F(1, 27) = 12.32, p = .002).
3.2 Behavioral results
A natural log transformation was performed on reaction times (RT) to normalize the distribution for statistical analysis. Separate 2×2 repeated measures ANOVAs were conducted on mean RT and on accuracy (% correct) with condition (auditory, visual) as the within-subjects factor, and group (ASD, TD) as the between-subjects factor. Means and standard deviations of RT and accuracy data are presented in Table 3.
Table 3.
Mean response time (natural log) and accuracy (% correct).
| ASD (n = 16) | TD (n = 16) | |||
|---|---|---|---|---|
|
| ||||
| Mean | SD | Mean | SD | |
| Response Time | ||||
| Auditory Trials | 6.40 | 0.27 | 6.30 | 0.16 |
| Visual Trials | 6.43 | 0.26 | 6.27 | 0.15 |
| Accuracy | ||||
| Auditory Trials | 83.7% | 12.0% | 92.0% | 7.6% * |
| Visual Trials | 96.4% | 3.9% | 95.2% | 3.5% |
denotes significant between-group difference (p < .05)
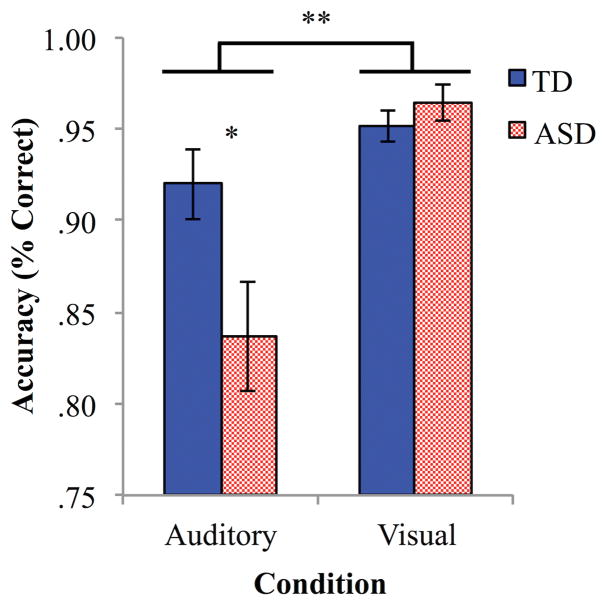
Analyses of RT showed neither a main effect of condition or group, nor any interaction effects. Analyses of accuracy, however, showed a significant main effect of condition (F(1,30) = 20.89, p < .001, MSe = .10), with overall greater accuracy for visual than auditory discrimination (t(31) = 4.15, p < .001; Figure 2). Additionally, there was a significant condition by group interaction effect (F(1,30) = 7.58, p < .01, MSe = .04). Post-hoc t-tests revealed that this effect was driven by weaker accuracy in the auditory condition for the ASD group as compared to the TD group (t(30) = 2.29, p = .03), as well as weaker auditory than visual accuracy within the ASD group (t(15) = 4.10, p = .001).
Figure 2.
Accuracy (% correct) for auditory and visual conditions in ASD and TD groups. Error bars represent SEM; ** denotes p < .001, * p < .05.
3.3 Functional MRI activation results
Table 4 provides activation results from analyses of the whole-brain and in the visual cortex.
Table 4.
Peak activation clusters in each group (p < .05, corrected).
| TD group | ASD group | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Peak location | Tal. coordinates | Volume | Peak location | Tal. coordinates | Volume | |||||||||
| Additional regions [% volume] | x | y | z | (μl) | voxels | t(15) | Additional regions [% volume] | x | y | z | (μl) | voxels | t(15) | |
| Auditory > Null | R Superior Temporal Gyrus | 44 | −2 | −4 | 24975 | 925 | 6.05 | L Superior Temporal Gyrus | −38 | −28 | 6 | 83835 | 3105 | 8.49 |
| R Middle Temporal Gyrus [9] | L Postcentral Gyrus [9] | |||||||||||||
| R Insula Lobe [9] | L Precentral Gyrus [6] | |||||||||||||
| L Superior Temporal Gyrus | −58 | −38 | 20 | 24273 | 899 | 6.57 | L Inferior Parietal Lobule [5] | |||||||
| L Insula Lobe [5] | L Insula Lobe [5] | |||||||||||||
| L Postcentral Gyrus | −50 | −22 | 48 | 9261 | 343 | 6.23 | R Insula | 46 | −4 | 6 | 50301 | 1863 | 8.19 | |
| L Precentral Gyrus [30] | R Superior Temporal Gyrus [22] | |||||||||||||
| L Inferior Parietal Lobule [6] | L SMA | −4 | −14 | 50 | 18009 | 667 | 6.03 | |||||||
| R Cerebellum (VI) | 8 | −56 | −18 | 7830 | 290 | 4.27 | R SMA [32] | |||||||
| L Cerebellum (IV-V) [14] | R Middle Cingulate Cortex [15] | |||||||||||||
| Cerebellar Vermis (6) [12] | L Middle Cingulate Cortex [10] | |||||||||||||
| Cerebellar Vermis (4/5) [12] | R Superior Frontal Gyrus [7] | |||||||||||||
| R Cerebellum (IV-V) [7] | ||||||||||||||
|
| ||||||||||||||
| Visual > Null | L Postcentral Gyrus | −52 | −20 | 44 | 17739 | 657 | 4.68 | L Thalamus | −2 | −32 | 8 | 24894 | 922 | 5.60 |
| L Precentral Gyrus [30] | R Middle Temporal Gyrus [10] | |||||||||||||
| L Inferior Parietal Gyrus [9] | R Inferior Temporal Gyrus [10] | |||||||||||||
| R Thalamus | 2 | −4 | 14 | 13662 | 506 | 4.60 | R Cerebellum [8] | |||||||
| L Thalamus [15] | Cerebellar Vermis (4/5) [7] | |||||||||||||
| L Putamen [10] | R Fusiform Gyrus [5] | |||||||||||||
| L Rolandic Operculum [6] | L Inferior Parietal Lobule | −38 | −34 | 38 | 17226 | 638 | 4.99 | |||||||
| R SMA | 8 | −4 | 54 | 9315 | 345 | 5.27 | L Postcentral Gyrus [32] | |||||||
| L SMA [48] | L Precentral Gyrus [24] | |||||||||||||
| L Middle Cingulate Cortex [13] | L SMA | −10 | −10 | 62 | 6318 | 234 | 4.98 | |||||||
| R Middle Cingulate Cortex [6] | R SMA [34] | |||||||||||||
| R Middle Cingulate Cortex [9] | ||||||||||||||
| L Middle Cingulate Cortex [6] | ||||||||||||||
| R Thalamus | 26 | −28 | 6 | 5886 | 218 | 4.61 | ||||||||
| R Caudate Nucleus [7] | ||||||||||||||
|
| ||||||||||||||
| Auditory > Null (Visual cortex) | L Lingual Gyrus | −26 | −80 | −12 | 3132 | 116 | −4.09 | R Lingual Gyrus | 8 | −62 | −6 | 13797 | 511 | 4.36 |
| L Inferior Occipital Gyrus [41] | R Calcarine Sulcus [21] | |||||||||||||
| L Middle Occipital Gyrus [27] | L Calcarine Sulcus [19] | |||||||||||||
| L Lingual Gyrus [19] | ||||||||||||||
| L Cuneus [5] | ||||||||||||||
| R Cuneus [5] | ||||||||||||||
|
| ||||||||||||||
| Visual > Null (Visual cortex) | L Middle Temporal Gyrus | −46 | −67 | 11 | 915 | 41 | 3.15 | R Middle Temporal Gyrus | 47 | −61 | 5 | 1242 | 60 | 3.23 |
| L Middle Occipital Gyrus [44] | R Superior Temporal Gyrus [5] | |||||||||||||
3.3.1 Auditory vs. Null
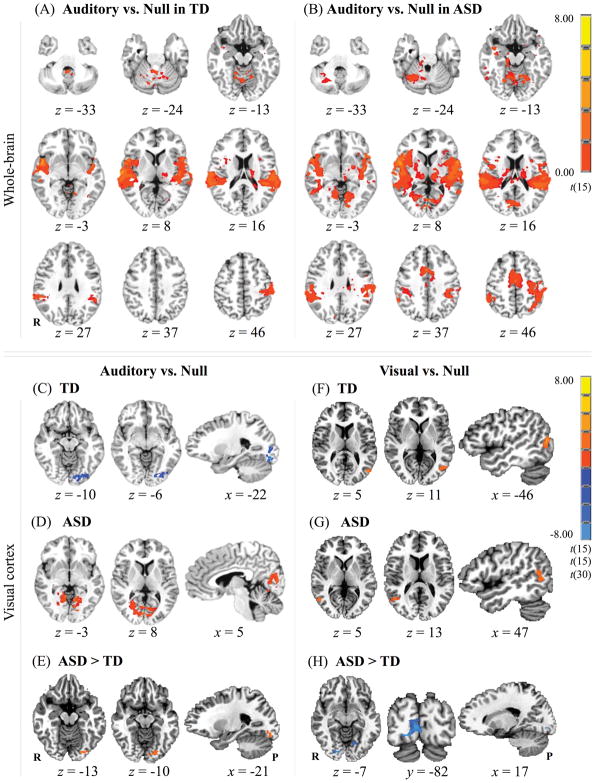
Both groups showed expected activation in auditory processing regions, as well as in additional cortical areas (Figure 3A–B). The TD group showed peak activation in superior temporal gyrus bilaterally; in the ASD group, peak activity in this region was located in the left hemisphere, and extended to the right (Table 4). In the TD group, additional areas of activation were found in the left postcentral gyrus extending into the left precentral gyrus, and in the right cerebellum. The ASD group activated areas in the left supplementary motor area (SMA), and in the right insula that extended into the right superior temporal gyrus. A t-test for between-group differences showed no statistically significant clusters.
Figure 3.
Statistical parametric maps. (A–B) Whole-brain clusters of activity for the auditory vs. null contrast in: (A) TD; and (B) ASD. (C–E) Activation clusters within the visual cortex for the auditory vs. null contrast in: (C) TD; (D) ASD; and (E) ASD > TD. (F–H) Clusters of activity within the visual cortex for the visual vs. null contrast in: (C) TD; (D) ASD; and (E) ASD > TD. Data are and presented in radiological convention (R = right, P = posterior).
3.3.2 Visual vs. Null
Neither group showed activation in expected visual processing areas that survived cluster correction. Instead, both groups showed significant bilateral activity in the thalamus, as well as in the somatosensory cortex and SMA that extended into the cingulate cortex (Table 4). A post-hoc t-test comparing the ASD to the TD group revealed no significant clusters for this contrast.
3.3.3 Auditory vs. Null, limited to visual cortex ROI
In order to test hypothesis (ii), we also performed analyses limited to visual cortex. We found differing effects between the two groups for the auditory condition. The TD group showed deactivation in the left lingual and inferior/middle occipital gyri (Figure 3C). Conversely, the ASD group showed a bilateral cluster of activation in the visual cortex with peak intensity in the right lingual gyrus (Figure 3D). This activity extended to the left lingual gyrus and cuneus bilaterally. A contrast of ASD vs. TD revealed a significant effect (ASD > TD) for activity in the left lingual gyrus (Figure 3E).
Post-hoc independent-samples t-tests were performed on mean z-scores calculated for the anatomically-defined visual cortex overall, as well as on mean beta weights in the group activation-derived right and left lingual gyri (Table 5). Results indicated a significant group difference in the left lingual gyrus (t(30) = −3.417, p = .002); ASD participants showed increased activity in this region as compared to TD participants.
Table 5.
Mean z-scores of neural activity in the visual cortex during the auditory and visual conditions, and mean beta weights in the lingual gyri during the auditory condition.
| Condition in visual cortex (BA17-19) (z-scores) | ASD (n=16) | TD (n=16) | ||
|---|---|---|---|---|
|
| ||||
| Mean | SD | Mean | SD | |
| Auditory | 0.05 | 0.60 | −0.23 | 0.82 |
| Visual | 0.07 | 0.39 | 0.26 | 0.67 |
| Region during auditory condition (beta weights) | Mean | SD | Mean | SD |
|
| ||||
| Right Lingual Gyrus | 4.76 | 4.76 | 2.08 | 6.14 |
| Left Lingual Gyrus | 0.95 | 8.75 | −9.33 | 8.26 * |
denotes significant between-group difference (p < .05)
3.3.4 Visual vs. Null, limited to visual cortex ROI
Since the visual vs. null whole-brain contrast (Visual > Null) did not activate expected visual processing areas, further analyses were performed in the visual cortex. A small cluster of activation was found in posterior middle temporal gyrus, in the left hemisphere for the TD group (Figure 3F), and in the right for the ASD group (Figure 3G). The contrast of ASD vs. TD within the visual cortex revealed weaker activation in the ASD as compared to the TD group in right lingual gyrus extending into left lingual gyrus and calcarine sulci (Figure 3H). A post-hoc independent-samples t-test performed on the mean z-scores calculated from activity in the visual cortex overall (Table 5), however, revealed no significant group differences.
3.4 Correlations with auditory activity
Correlations were calculated for each group between the scores for each of the four quadrants of the AASP and the mean z-scores of activity during the auditory condition in the visual cortex, as well as the mean beta weights in the right and left lingual gyri. Neither group showed significant correlations between their sensory profile scores and auditory activity in these regions. Further correlations were calculated for each group between the auditory subscores comprising the four quadrants and the mean z-scores and beta weights of neural activity in visual regions during the auditory condition; no correlations were significant.
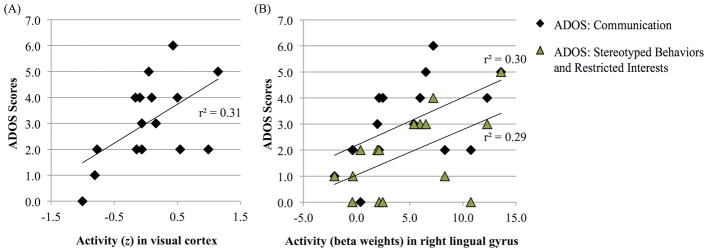
Correlations were also calculated between the ADOS scores from the ASD group and the mean z-scores in the visual cortex, and the mean beta weights in the right and left lingual gyri (Figure 4). Results showed a significant positive correlation between auditory activity in visual cortex overall and the communication domain of the ADOS (r2(14) = .31, p = .03; Figure 4A). Activity in the right lingual gyrus was also positively correlated with ADOS Communication subscores (r2(14) = .30, p = .03), and the repetitive/restricted behaviors domain (r2(14) = .29, p = .03; Figure 4B). No significant correlations were found between activity in the left lingual gyrus and the ADOS scores.
Figure 4.
Correlations between ADOS scores and neural activity (z-scores) in (A) visual cortex; correlations between ADOS scores and neural activity (beta weights) in (B) right lingual gyrus. Trend lines depict significant r2-values (p < .05).
Additional post-hoc correlations were performed to examine the relationship between behavioral and neural findings. Correlations between auditory accuracy scores and neural activity (mean z-scores) in the visual cortex, as well as neural activity (beta weights) in the right and left lingual gyri, were calculated for each participant in the ASD group. This analysis, however, showed no significant correlations between behavioral accuracy and neural activity in visual regions during the auditory condition (auditory accuracy and visual cortex: r2(14) = .03, p = .51; auditory accuracy and right lingual gyrus: r2(14) = .001, p = .91; auditory accuracy and left lingual gyrus: r2(14) = .12, p = .19).
4 Discussion
The prevalence of sensory and perceptual abnormalities is relatively high in autism (Dawson & Watling, 2000). Atypical sensory responses are also some of the earliest signs seen in children who are later diagnosed with ASD (Zwaigenbaum et al., 2005; Rogers, 2009;). In the current study, functional MRI was implemented during a simple perceptual discrimination task to investigate the neural mechanisms underlying auditory and visual processing in children and adolescents with ASD as compared to their TD peers. Primary findings for the auditory condition indicated downregulation of visual cortex in the TD group, but upregulation in the ASD group. Atypical activity in visual cortex was associated with symptomatology in the ASD group. Moreover, behavioral measures showed markedly lower accuracy for auditory discrimination in ASD compared to TD participants. These findings suggest that ASD individuals may recruit brain resources typically allocated to vision during the processing of stimuli from other sensory modalities such as audition.
4.1 Impaired downregulation of visual cortex in ASD
Analyses examining functional activity in visual cortex (BA17-19) during the auditory condition showed significant deactivation in the left lingual gyrus for the TD group. Deactivations are often not reported in fMRI studies as activation is defined relative to a control condition. It is important to note, however, that the deactivation reported here could not simply be attributed to a relative decrease in activity between stimulus conditions, as there was a reduction in the BOLD signal when compared to a task-free null condition. Our results for TD children and adolescents are in agreement with previous findings of downregulation of visual cortex during presentation of auditory stimuli in TD adults (Laurienti et al., 2002; Eckert et al., 2008). By contrast, our ASD group showed significant activation in visual cortex for the auditory condition, specifically in left cuneus and lingual gyrus bilaterally. This may relate to the finding of an atypical P3 event-related potential over occipital cortex in children with ASD by Kemner and colleagues (1995), interpreted as suggesting “availability” of visual cortex in the processing of simple auditory stimuli. Less specifically, our finding also bears some analogy to the observation of adults with ASD failing to deactivate ‘task-negative’ (or default mode) areas that are typically suppressed, or downregulated, during cognitive tasks (Kennedy et al., 2006). This previous finding may be analogous to ours because both reflect impaired downregulation or failed deactivation in ASD of cortical areas that are deactivated for a given condition or task in TD individuals.
Individuals with ASD often allocate unusually strong resources to visual cortices during a wide range of cognitive and perceptual tasks (Samson et al., 2012). The results of the current study suggest that this over-allocation of resources to visual cortex in ASD also applies to non-visual auditory tasks, indicating an absence or even paradoxical reversal of crossmodal downregulation. Activation of visual cortex during an auditory task may reflect a failure to disengage visual processing and shift attention to other sensory modalities. Such impairment is an early marker of ASD (Zwaigenbaum et al., 2005; Elsabbagh et al., 2009; Elsabbagh et al., 2013) and may be reflected in atypical over-activity of visual cortex in the context of auditory stimuli observed in our cohort of children and adolescents with ASD. Our findings therefore add to previous evidence of enhanced resource allocation to visual regions for a variety of perceptual and cognitive tasks in ASD (Samson et al., 2012), suggesting that this atypical visual participation also applies to low-level perceptual processing in another modality (e.g., audition). Furthermore, our finding of impaired downregulation of visual cortex appears consistent with the hypothesis of enhanced crossmodal processing and an over-representation of synesthesia in ASD (Mottron et al., 2013; Neufeld et al., 2013), which have been related to hyperplasticity and may be supported by converging evidence of ASD risk genes affecting synaptic function (Baudouin, 2014; Sahin & Sur, 2015) and plasticity (Ebert & Greenberg, 2013; see review in Mottron et al., 2014).
In a visual task, increased activation of early visual cortices might suggest greater sensory-driven, or bottom-up, processing of information (Corbetta & Shulman, 2002), and could therefore be expected to contribute to superior performance. However, behavioral results for the visual condition showed no differences in RT and equally high accuracy in TD and ASD participants. While this indicates that participants were engaged throughout the task, it also suggests ceiling effects due to the very simple nature of the visual discrimination required. Neural activation in visual regions for this condition was also weak with small clusters of activity detectable only in analyses limited to visual cortex. Notably, activity in lingual gyrus was decreased in ASD compared to TD participants in these ROI analyses. The overall modest visual activity in both groups could be attributed to continuous visual stimulation (the bisected vertical rectangle; see Fig. 1), which remained onscreen throughout the experiment.
With respect to the auditory task, atypically increased activity in visual cortex could specifically relate to this feature of the task paradigm, which had been selected for primarily pragmatic reasons (i.e., providing a visual reference grid for the “high” vs. “low” discrimination). The bisected rectangle was presented continuously (i.e., also during null and auditory trials), analogous to the common procedure of presenting a fixation cross throughout an fMRI experiment. Such a constant visual stimulus is similar, for example, to the edge of a projection screen, which may be located in the visual field throughout an experiment, and which would likely require negligible processing demands in the TD brain. Atypically high activity levels in visual cortex may suggest that this was not true for ASD participants, but that on the contrary, during the auditory condition the task-irrelevant bisected rectangle may have remained an actively processed stimulus. However, while this might account for the failure to downregulate visual cortex in ASD, it cannot explain increased activity in comparison to the null condition during which the rectangle was also visible. The observed positive activation effect therefore likely reflects abnormal auditory processing mechanisms in ASD, rather than the specifics of the task paradigm employed here. Abnormal auditory processing in ASD may also encompass compensatory mechanisms (e.g., the implementation of a visual strategy) during the more difficult auditory task (see section 4.2), which would result in upregulation, and by complement a failure in downregulation, of visual cortex.
4.2 Diminished auditory accuracy in ASD
ASD participants were significantly less accurate than TD participants in the auditory condition, whereas they performed near ceiling in the visual condition. These behavioral findings, as well as the neural patterns of activity, suggest that the auditory system may be overall more affected in individuals with ASD than the visual system. Indeed, visual cortex and visual abilities are considered to be intact and in some respects even enhanced in ASD (Dakin & Frith, 2005; Brenner et al., 2006; Samson et al., 2012). For example, individuals with ASD often outperform their TD peers in difficult visuospatial tasks (O’Riordan et al., 2001; Plaisted et al., 1998; Simmons et al., 2009).
Yet, the “sparing” of vision may also have deleterious implications, particularly in the auditory domain. For instance, people with ASD often have difficulties integrating auditory and visual information (Smith & Bennetto, 2007). In the current study, however, decreased auditory accuracy did not correlate with increased neural activity in visual cortical areas. It is conceivable that visual cortical over-activity during the auditory condition may reflect a combination of auditory processing anomalies (interfering with behavioral abilities) and compensatory mechanisms (aiding behavioral abilities), in which case robust correlations with behavioral performance could not be expected. Alternatively, the marked decrease in auditory accuracy may be due to attentional mechanisms with impaired top-down regulation in ASD. However, since reaction times were not significantly longer in the ASD group, attentional differences are likely not the sole factor.
An additional consideration concerns the specific setting of the fMRI studies in which standard imaging protocols were used. Although noise-cancelling headphones were provided to minimize the scanner noise, this noise may nevertheless have added to task difficulty in the auditory condition, and may have affected ASD more so than TD participants. Previous behavioral reports have suggested that infants and children with ASD show difficulties with auditory filtering tasks (Rogers et al., 2003; Wiggins et al., 2009). Scanner noise may have thus made high vs. low tone discrimination particularly demanding for ASD participants in this context, resulting in diminished auditory accuracy.
4.3 Correlations with autism symptomatology
Activity during the auditory condition in several visual cortical clusters and in visual cortex as a whole was positively correlated with ADOS scores. These results indicate that reduced downregulation of visual cortex in the ASD cohort was related to greater deficits in communication and increased presence of repetitive and restricted behaviors, both of which are core domains of ASD symptomatology. These correlations may appear surprising given that imaging measures were derived from sensory brain regions for a simple perceptual discrimination task. They are, however, consistent with evidence linking abnormal sensory symptoms with sociocommunicative symptom severity (Watson et al., 2011) as well as stereotyped and restricted behaviors (Kern et al., 2007; Gabriels et al., 2008; Wiggins et al., 2009; Hilton et al., 2010) in young children with ASD.
Activity in visual cortex in response to the auditory condition was not significantly correlated with summary quadrant scores or auditory subscores of the sensory profile in either group. For the entire sample of participants with complete sensory profiles, there were more sensory symptoms for the low registration, sensory sensitivity, and sensory avoidance quadrants, but fewer symptoms in the sensation seeking quadrant in the ASD group than in the TD group. These results are consistent with previous findings in infants and children that have indicated both hyposensitivity and hypersensitivity to auditory stimuli in ASD (Dahlgren & Gillberg 1989; Kern et al. 2007; Tomchek & Dunn, 2007). In the subsample with useable fMRI data, means for sensory symptoms were still higher in the ASD than in the TD group for sensory sensitivity and sensory avoidance quadrants, but these differences did not reach significance, possibly due to reduced statistical power. Consistent with earlier studies, however, the TD group reported significantly more behaviors than the ASD group in the sensation seeking quadrant, which includes social components and is not strictly reflective of basic perceptual processing (Dunn et al., 2002; Crane et al., 2009; Jones et al., 2009; for a discussion, see Stewart et al. (2015)). Overall, group differences in sensory profiles were not as robust as those previously reported (Crane et al., 2009). This may be due to small sample size coupled with strong variability seen in the ASD group. Additionally, given the wide age range of participants in this study, maturational effects may have impacted these findings, as sensory symptoms in ASD have been shown to diminish with age (Kern et al., 2007).
4.3 Limitations
Several limitations of the study have been acknowledged and addressed in the preceding subsections. Additional limitations consist of (a) the small sample sizes, and (b) the level of functioning in the ASD group. First, due to the constraints of the sample size, further subdivision of the ASD group was not feasible. It was therefore not possible to test whether auditory activation differed between ASD individuals with and without delay in speech onset, as recently suggested by Samson and colleagues (2015). Second, high-functioning children with autism were primarily included in the current study as dictated by the demands of the testing environment. As such, the findings are limited to those with ASD who are able to lie still inside the scanner bore for prolonged periods of time.
4.4 Conclusions
In the current study, functional MRI, behavioral measures, assessments, and sensory questionnaires were used to examine the mechanisms underlying perceptual processing of auditory and visual information in children and adolescents with ASD. Imaging results indicated divergent patterns of activity in visual cortex in response to auditory stimuli: the TD group showed deactivation, whereas the ASD group showed increased activity, and this increased activity was correlated with greater symptomatology. Our findings suggest that impaired crossmodal downregulation (of visual cortex during auditory processing) may be linked to ASD symptomatology.
Acknowledgments
Grant sponsor:National Institutes of Health; Grant numbers: R01 DC006155, R01 MH081023, and R01 MH101173.
This research was supported by the National Institutes of Health, grants R01 DC006155, R01 MH081023, and R01 MH101173. Special thanks are extended to the participants and families involved in this study. The authors have no conflict of interest to declare.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, D.C: American Psychiatric Association; 2013. [Google Scholar]
- Baker AEZ, Lane A, Angley MT, Young RL. The relationship between sensory processing patterns and behavioural responsiveness in autistic disorder: A pilot study. Journal of Autism & Developmental Disorders. 2008;38(5):867–875. doi: 10.1007/s10803-007-0459-0. [DOI] [PubMed] [Google Scholar]
- Baudouin SJ. Heterogeneity and convergence: The synaptic pathophysiology of autism. European Journal of Neuroscience. 2014;39(7):1107–1113. doi: 10.1111/ejn.12498. [DOI] [PubMed] [Google Scholar]
- Bonnel A, McAdams S, Smith B, Berthiaume C, Bertone A, Ciocca V, et al. Enhanced pure-tone pitch discrimination among persons with autism but not Asperger syndrome. Neuropsychologia. 2010;48(9):2465–2475. doi: 10.1016/j.neuropsychologia.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Bonnel A, Mottron L, Peretz I, Trudel M, Gallun E, Bonnel AM. Enhanced pitch sensitivity in individuals with autism: A signal detection analysis. Journal of Cognitive Neuroscience. 2003;15(2):226–235. doi: 10.1162/089892903321208169. [DOI] [PubMed] [Google Scholar]
- Brenner LA, Turner KC, Müller RA. Eye movement and visual search: Are there elementary abnormalities in autism? Journal of Autism and Developmental Disorders. 2006;37(7):1289–1309. doi: 10.1007/s10803-006-0277-9. [DOI] [PubMed] [Google Scholar]
- Brown C, Dunn W. Adolescent/adult sensory profile manual. San Antonio, TX: The Psychological Corporation; 2002. [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, An International Journal. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crane L, Goddard L, Pring L. Sensory processing in adults with autism spectrum disorders. Autism: The International Journal of Research & Practice. 2009;13(3):215–228. doi: 10.1177/1362361309103794. [DOI] [PubMed] [Google Scholar]
- Dahlgren SO, Gillberg C. Symptoms in the first two years of life. A preliminary population study of infantile autism. European Archives of Psychiatry and Neurological Sciences. 1989;238(3):169–174. doi: 10.1007/BF00451006. [DOI] [PubMed] [Google Scholar]
- Dakin S, Frith U. Vagaries of visual perception in autism. Neuron. 2005;48:497–507. doi: 10.1016/j.neuron.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Dawson G, Watling R. Interventions to facilitate auditory, visual, and motor integration in autism: A review of the evidence. Journal of Autism & Developmental Disorders. 2000;30(5):415. doi: 10.1023/a:1005547422749. [DOI] [PubMed] [Google Scholar]
- Dunn W. Sensory profile. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Dunn W, Myles BS, Orr S. Sensory processing issues associated with Asperger syndrome: A preliminary investigation. The American Journal of Occupational Therapy: Official Publication of the American Occupational Therapy Association. 2002;56(1):97–102. doi: 10.5014/ajot.56.1.97. [DOI] [PubMed] [Google Scholar]
- Ebert DH, Greenberg ME. Activity-dependent neuronal signalling and autism spectrum disorder. Nature. 2013;493(7432):327–337. doi: 10.1038/nature11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Kamdar NV, Chang CE, Beckmann CF, Greicius MD, Menon V. A cross-modal system linking primary auditory and visual cortices: Evidence from intrinsic fMRI connectivity analysis. Human Brain Mapping. 2008;29(7):848–857. doi: 10.1002/hbm.20560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Fernandes J, Jane Webb S, Dawson G, Charman T, Johnson MH, et al. Disengagement of visual attention in infancy is associated with emerging autism in toddlerhood. Biological Psychiatry. 2013;74(3):189–194. doi: 10.1016/j.biopsych.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Volein A, Holmboe K, Tucker L, Csibra G, Baron-Cohen S. Visual orienting in the early broader autism phenotype: Disengagement and facilitation. Journal of Child Psychology and Psychiatry. 2009;50:637–642. doi: 10.1111/j.1469-7610.2008.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. Journal of Neurophysiology. 2009;101(6):3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriels RL, Agnew JA, Miller LJ, Gralla J, Pan Z, Goldson E, et al. Is there a relationship between restricted, repetitive, stereotyped behaviors and interests and abnormal sensory response in children with autism spectrum disorders? Research in Autism Spectrum Disorders. 2008;2:660–670. [Google Scholar]
- Gaffrey MS, Kleinhans NM, Haist F, Akshoomoff N, Campbell A, Courchesne E, et al. A typical participation of visual cortex during word processing in autism: An fMRI study of semantic decision. Neuropsychologia. 2007;45(8):1672–1684. doi: 10.1016/j.neuropsychologia.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillberg C, Coleman M. The biology of the autistic syndromes. New York, NY: Mac Keith Press; 2000. [Google Scholar]
- Gomot M, Belmonte MK, Bullmore ET, Bernard FA, Baron-Cohen S. Brain hyper-reactivity to auditory novel targets in children with high-functioning autism. Brain. 2008;131(9):2479–2488. doi: 10.1093/brain/awn172. [DOI] [PubMed] [Google Scholar]
- Hilton CL, Harper JD, Kueker RH, Lang AR, Abbacchi AM, Todorov A, et al. Sensory responsiveness as a predictor of social severity in children with high functioning Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2010;40(8):937–945. doi: 10.1007/s10803-010-0944-8. [DOI] [PubMed] [Google Scholar]
- Jones TB, Bandettini PA, Kenworthy L, Case LK, Milleville SC, Martin A, et al. Sources of group differences in functional connectivity: An investigation applied to autism spectrum disorder. NeuroImage. 2010;49(1):401–414. doi: 10.1016/j.neuroimage.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CRG, HappÈ F, Baird G, Simonoff E, Marsden AJS, Tregay J, et al. Auditory discrimination and auditory sensory behaviours in autism spectrum disorders. Neuropsychologia. 2009;47(13):2850–2858. doi: 10.1016/j.neuropsychologia.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: Thinking in pictures with decreased functional connectivity. Brain: A Journal of Neurology. 2006;129:2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Brenner L, Palmer E, Lincoln AJ, Müller RA. Functional brain organization for visual search in ASD. Journal of the International Neuropsychological Society. 2008;14(6):990–1003. doi: 10.1017/S1355617708081356. [DOI] [PubMed] [Google Scholar]
- Kemner C, Verbaten MN, Cuperus JM, Camfferman G. Auditory event-related brain potentials in autistic children and three different control groups. Biological Psychiatry. 1995;38(3):150–165. doi: 10.1016/0006-3223(94)00247-Z. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: Resting functional abnormalities in autism. Proceedings of the National Academy of Sciences. 2006;103(21):8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern JK, Trivedi MH, Garver CR, Grannemann BD, Andrews AA, Savla JS, et al. The pattern of sensory processing abnormalities in autism. Autism: The International Journal of Research & Practice. 2006;10(5):480–494. doi: 10.1177/1362361306066564. [DOI] [PubMed] [Google Scholar]
- Kern JK, Trivedi MH, Grannemann BD, Garver CR, Johnson DG, Andrews AA, et al. Sensory correlations in autism. Autism. 2007;11(2):123–134. doi: 10.1177/1362361307075702. [DOI] [PubMed] [Google Scholar]
- Laurienti PJ, Burdette JH, Wallace MT, Yen YF, Field AS, Stein BE. Deactivation of sensory-specific cortex by cross-modal stimuli. Journal of Cognitive Neuroscience. 2002;14(3):420–429. doi: 10.1162/089892902317361930. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, et al. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. Journal of Autism and Developmental Disorders. 1989;19(2):185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- Mottron L, Belleville S, Rouleau GA, Collignon O. Linking neocortical, cognitive, and genetic variability in autism with alterations of brain plasticity: The Trigger-Threshold-Target model. Neuroscience and Biobehavioral Reviews. 2014;47:735–752. doi: 10.1016/j.neubiorev.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Mottron L, Bouvet L, Bonnel A, Samson F, Burack JA, Dawson M, et al. Veridical mapping in the development of exceptional autistic abilities. Neuroscience and Biobehavioral Reviews. 2013;37(2):209–228. doi: 10.1016/j.neubiorev.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Mottron L, Burack JA. Enhanced perceptual functioning in the development of autism. In: Burack JA, Charman T, Yirmiya N, Zelazo PR, editors. The development of autism: Perspectives from theory and research. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers; 2001. pp. 131–148. [Google Scholar]
- Mottron L, Dawson M, Soulières I, Hubert B, Burack J. Enhanced perceptual functioning in autism: An update, and eight principles of autistic perception. Journal of Autism and Developmental Disorders. 2006;36(1):27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? NeuroImage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld J, Roy M, Zapf A, Sinke C, Emrich HM, Prox-Vagedes V, et al. Is synesthesia more common in patients with Asperger syndrome? Frontiers in Human Neuroscience. 2013;7:847. doi: 10.3389/fnhum.2013.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Riordan MA, Plaisted KC, Driver J, Baron-Cohen S. Superior visual search in autism. Journal of Experimental Psychology: Human Perception and Performance. 2001;27(3):719–730. doi: 10.1037//0096-1523.27.3.719. [DOI] [PubMed] [Google Scholar]
- Plaisted K, O’Riordan M, Baron-Cohen S. Enhanced visual search for a conjunctive target in autism: A research note. Journal of Child Psychology & Psychiatry & Allied Disciplines. 1998;39(5):777. [PubMed] [Google Scholar]
- Rogers SJ. What are infant siblings teaching us about autism in infancy? Autism Research. 2009;2(3):125–137. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn S, Wehner E. Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. Journal of Autism and Developmental Disorders. 2003;33(6):631–642. doi: 10.1023/b:jadd.0000006000.38991.a7. [DOI] [PubMed] [Google Scholar]
- Sahin M, Sur M. Genes, circuits, and precision therapies for autism and related neurodevelopmental disorders. Science. 2015;350(6263) doi: 10.1126/science.aab3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson F, Mottron L, Soulières I, Zeffiro TA. Enhanced visual functioning in autism: An ALE meta-analysis. Human Brain Mapping. 2012;33:1553–1581. doi: 10.1002/hbm.21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson F, Zeffiro TA, Doyon J, Benali H, Mottron L. Speech acquisition predicts regions of enhanced cortical response to auditory stimulation in autism spectrum individuals. Journal of Psychiatric Research. 2015;68:285–292. doi: 10.1016/j.jpsychires.2015.05.011. [DOI] [PubMed] [Google Scholar]
- Schopler E, Reichler R, Renner B. The childhood autism rating scale. Los Angeles, CA: Western Psychological Services; 1994. [Google Scholar]
- Shen MD, Shih P, Öttl B, Keehn B, Leyden KM, Gaffrey MS, et al. Atypical lexicosemantic function of extrastriate cortex in autism spectrum disorder: Evidence from functional and effective connectivity. NeuroImage. 2012;62:1780–1791. doi: 10.1016/j.neuroimage.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DR, Robertson AE, McKay LS, Toal E, McAleer P, Pollick FE. Vision in autism spectrum disorders. Vision Research. 2009;49(22):2705–2739. doi: 10.1016/j.visres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Smith EG, Bennetto L. Audiovisual speech integration and lipreading in autism. Journal of Child Psychology & Psychiatry. 2007;48(8):813–821. doi: 10.1111/j.1469-7610.2007.01766.x. [DOI] [PubMed] [Google Scholar]
- Stewart CR, Sanchez SS, Grenesko EL, Brown CM, Chen CP, Keehn B, et al. Sensory symptoms and processing of nonverbal auditory and visual stimuli in children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders. 2015 doi: 10.1007/s10803-015-2367-z. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system – an approach to cerebral imaging. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- Tomchek SD, Dunn W. Sensory processing in children with and without autism: A comparative study using the short sensory profile. The American Journal of Occupational Therapy: Official Publication of the American Occupational Therapy Association. 2007;61(2):190–200. doi: 10.5014/ajot.61.2.190. [DOI] [PubMed] [Google Scholar]
- Watson LR, Patten E, Baranek GT, Poe M, Boyd BA, Freuler A, et al. Differential associations between sensory response patterns and language, social, and communication measures in children with autism or other developmental disabilities. Journal of Speech, Language, and Hearing Research. 2011;54(6):1562–1576. doi: 10.1044/1092-4388(2011/10-0029). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler’s abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: A quantitative comparison of preprocessing strategies. NeuroImage. 2009;47(4):1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Wiggins LD, Robins DL, Bakeman R, Adamson LB. Brief report: Sensory abnormalities as distinguishing symptoms of autism spectrum disorders in young children. Journal of Autism & Developmental Disorders. 2009;39(7):1087–1091. doi: 10.1007/s10803-009-0711-x. [DOI] [PubMed] [Google Scholar]
- Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral D, Van de Water J. Autoantibodies in autism spectrum disorders (ASD) Annals of The New York Academy of Sciences. 2007;1107:79–91. doi: 10.1196/annals.1381.009. [DOI] [PubMed] [Google Scholar]
- Zablotsky B, Black LI, Maenner MJ, Schieve LA, Blumberg SJ. Estimated prevalence of autism and other developmental disabilities following questionnaire changes in the 2014 national health interview survey. National Health Statistics Reports. 2015;87:1–20. [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23(2–3):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]