Abstract
The reinforcing efficacy of vaporized methamphetamine HCl (0.3 mg/kg) was determined in baboons with minimal previous drug exposure. A group of 8 adult male baboons was tested prior to a group of 7 adult female baboons. Baboons were initially trained to suck on a brass stem activating a pressure-sensitive relay (i.e., puff), to receive one M&M® candy. Five of the 8 males and 6 of the 7 females learned to activate the relay. 0.05 ml of 95% ethyl alcohol containing 0.3 mg/kg methamphetamine was vaporized and delivered to the mouth of the baboon after he/she completed 2 puffs; a single candy was given after an additional 5 puffs to ensure that baboons continued puffing after the aerosol entered their mouths. Puffing was recorded but not reinforced by candy or drug for 2 min after each aerosol delivery for males and 1 minute for females. Males could earn 10 and females could earn 20 aerosol deliveries. Males made between 225 and 650 puffs each session. Females made between 200 and 400 puffs each session. When only candy and placebo aerosol were delivered the number of puffs decreased in all 6 females but increased in all 5 males. When candy was delivered without aerosol, puffing decreased in 4 of 5 males, but this manipulation was not tested in females. Methamphetamine aerosol delivery maintained lower rates of puffing behavior in females than males, but procedural differences weaken interpretation of this sex comparison. Although training non-human primates to inhale drug vapors is time consuming, if successful, their long lifespan could provide years of valuable data justifying further work with non-human primates using models of vaporized drug self-administration.
Keywords: Methamphetamine, Baboon, Sex Differences, Self-administration, Aerosol Inhalation, Vapor Inhalation, Smoking
1. Introduction
Humans commonly inhale drugs by smoking drugs in cigarette forms (e.g., marijuana, tobacco) or by directly inhaling aerosols (e.g., heroin, THC, nicotine), yet compared to intravenous or oral routes of drug delivery, preclinical self-administration studies with non-humans using inhaled drugs are relatively few. Liquid aerosol is formed by heating a drug until it produces a vapor that upon rapid cooling condenses to form an aerosol (small liquid particles suspended in air; Wood, 1990). Aerosol production is possible for drugs that vaporize before they are pyrolized. Early studies examined the effects of the delivery of drug by combusting it in combination with plant material (see review by Wood, 1990). For example, Cole and colleagues trained 2 chimpanzees and 1 orangutan to inhale tobacco cigarettes containing methamphetamine (Pieper & Cole; 1973) or THC (Cole et al., 1971) by reinforcing the great apes with M&M® candy for successively longer puffs on a stem. Reinforcing long puffs with candy was effective in training great apes to “smoke” up to 8 tobacco cigarettes laced with methamphetamine or THC. This procedure was later used to train rhesus monkeys to puff on lettuce leaf cigarettes containing cocaine base (Siegel et al., 1976). Ando and Yanagita (1981) attempted to train 14 monkeys to self-administer, via puffing, tobacco cigarette smoke without any non-drug reinforcement and only 2 of the 14 animals acquired tobacco self-administration.
Studies looking at drug aerosols using rodents have relied most often on ventilated chambers such that drug aerosol can be delivered in a controlled manner and the animals will necessarily take drug as they breath (Wong, 2007). Such procedures have been commonly used to study the behavior or toxicological effects of inhaled chemical exposure (e.g., Phalen, 1997) and alcohol (e.g., O’Dell et al., 2004; Gilpin et al., 2008). Several early studies modified this approach to work with non-human primates. For example, in order to examine the behavioral toxicology of marijuana smoke, a large group of rhesus monkeys was exposed, via a face mask, to the smoke of marijuana cigarettes (e.g., Schulze et al., 1989; Slikker et al., 1991; Pryor & Rebert, 1989). In another early study (Katz et al. 1991), squirrel monkeys, who had been trained to discriminate intravenous cocaine from placebo, responded as if they had been given intravenous cocaine after exposure to vaporized cocaine base in a ventilated chamber. These studies clearly demonstrated that inhalation of drug vapors and other components of some plant products produces significant behavioral effects.
While nicotine and cannabis are commonly smoked as plant material, other abused drugs are administered in a more direct form such as inhalants. Yanagita et al. (1970) demonstrated the reinforcing efficacy of chloroform, ether and lacquer thinner when delivered via indwelling intranasal cannulas in rhesus monkeys. Twenty years later, Carroll made significant contributions to the study of self-administered “smoked” drugs using a device similar to that used by Hatsukami et al. (1990) for delivering precise amounts of vaporized cocaine base to human cocaine smokers. Rhesus monkeys were trained to activate a pressure-sensitive relay in order to receive a single dose of cocaine base vaporized by rapid heating of a metal coil (Carroll et al., 1990), similar to the technology currently used in e-cigarettes (Harrell et al., 2014). Responding for cocaine was greater than for lidocaine, cocaine produced the expected physiological changes in heart rate (Carroll et al., 1990), response rates were increased, as observed with intravenous self-administration, when the monkeys were food deprived (Comer et al., 1995), and response rates were decreased by buprenorphine (Rodefer et al., 1997). Vaporized heroin was also self-administered under these procedures (Mattox & Carroll, 1996). Using this technology, 10 years later Newman & Carroll (2006) reported that rhesus monkeys who had been trained to self-administer cocaine base would self-administer methamphetamine: methamphetamine was less efficacious than cocaine and the dose-response function was relatively flat.
In a similar time-frame Lichtman, Martin and Boni developed devices that provided for aerosol delivery to mice either by generating the aerosol directly on a heating coil or pulling aerosol from lit marijuana cigarettes through a system connecting to a nose piece for mice (e.g., Lichtman et al., 1996, 2000, 2001). Using this system Meng et al. (1999) reported that methamphetamine aerosol and intravenous methamphetamine had similar pharmacokinetic profiles. More recently a similar system was used to deliver synthetic cannabinoids (“Spice”) to mice (Weibelhaus et al., 2012). While these systems deliver behaviorally active drug directly to a mouse’s nose, the required restraint of the mouse limits its utility for self-administration studies. The current vaping technology that provides for rapid, precise and well controlled volatilization of drugs of abuse has led to a resurgence of work looking at the effects of inhaled drugs in laboratory rodents. Studies have demonstrated the utility of commercially-available vaporizers for the administration of nicotine (Lefever et al., 2017a), THC (Lefever et al., 2017b; Manwell et al., 2014a, 2014b), methamphetamine (Juarez-Portilla et al., 2017; Marusich et al., 2016; Nguyen et al., 2016b) and synthetic cannabinoids (Nguyen et al., 2016a) to rodents. Of note, Nguyen et al. (2016a) reported a decrease in intracranial self-stimulation threshold with inhaled methamphetamine and synthetic cannabinoids, which suggest that the aerosol would function as a positive reinforcer. Clearly, e-cigarette technology has been instrumental in conducting research with inhaled drugs and has opened up a wide range of opportunities for future research.
We have used a procedure for administering drug aerosols to rhesus monkeys that is based upon the way human cocaine users smoke cocaine. Monkeys activated a pressure-sensitive relay by puffing on a brass stem that was attached to a heated glass tube that contained stainless steel mesh. Upon completion of the response requirement drug dissolved in 95% ethanol was dropped onto the screen and vaporized such that continued puffing on the stem delivered drug aerosol. Four of six rhesus monkeys acquired heroin self-administration, developed a place-preference for the experimental space associated with heroin self-administration (Foltin & Evans, 2001) and chose heroin over a preferred fluid reinforcer during choice trials (Evans et al., 2003). The first purpose of the current study was to determine if this procedure could be used with another non-human primate species with a larger brain, the baboon, to engender methamphetamine self-administration. Baboons were chosen because of their utility in PET imaging studies (VandeBerg et al., 2009). If successful, the study would lay the foundation for future work with baboons that would allow PET imaging of receptor binding and neurotransmitter release as a function of aerosol inhalation. Of the studies involving non-human primates and inhaled drug self-administration cited in this paper, 120 males and 1 female (the sex of 9 additional animals was not specified) participated. Of the 15 studies involving rodents and inhaled drug self-administration cited in this paper only 1 study tested females. Clearly, there is a significant paucity of data on aerosol administration in females. Therefore, the second purpose of the current study was to compare methamphetamine aerosol self-administration between male and female baboons.
2. Method
2.1. Animals
One group of 8 adult male baboons (Papio cynocephalus anubis), initially weighing 19.8 to 26.5 (Mean = 23.6) kg completed the study and then a group of 7 adult female baboons, initially weighing 8.4 to 15.5 (Mean = 11.7) kg completed the study. All baboons had experienced acute (<10) injections of intramuscular (i.m.) amphetamine and i.m. dexfenfluramine, while the males also had experienced acute (< 10) injections of i.m. heroin and i.m. naloxone. All baboons also had previous experience responding for food pellets or M&M® candy under a daily schedule similar to that described below. Baboons were individually housed in custom-designed non-human primate cages (1.4 × 1.2 × 1.5 m high) at The New York State Psychiatric Institute. The room was illuminated with fluorescent lighting from 7:00 AM to 7:00 PM daily. In addition to food and candy earned during experimental sessions, two chewable vitamins, two pieces of fresh fruit, and a dog biscuit were also given daily. Water was available ad libitum from a spout located at the back of each cage. All aspects of animal maintenance and experimental procedures complied with the U.S. National Institutes of Health Guide for Care and Use of Laboratory Animals, and were approved by the New York State Psychiatric Institute Animal Care and Use Committee.
2.2. Apparatus
Two response panels were located on the front wall of the cage. Six session lights (CM 1820, 24 V; Chicago Miniature, Buffalo Grove, Ill., USA) with white lenses were evenly spaced around the outside edges of each panel. From the baboon’s perspective the right panel was used for food delivery, while the left panel was used for aerosol delivery. At approximately waist height for a sitting baboon the food panel had one Lindsley lever response manipulanda (BRS-LVE, Beltsville, Md., USA) mounted at the baboon’s left and one mounted at the baboon’s right. There were two stimulus lights mounted above each lever. A pellet dispenser (BRS-LVE model PDC-005) was also mounted on the outside of response panel with a tube that ran to a pellet catch cup that the baboons could reach into to pick up the food pellets. At approximately waist height for a sitting baboon the aerosol panel had one Lindsley lever, with a single light over it, mounted at the baboon’s left. Two stimulus lights were mounted over a brass pipe mouthpiece that was at approximately mouth height for a sitting baboon at the baboon’s right, i.e., lever to the left, stem to the right. A pressure-activated relay (Micro Pneumatic Logic, Fort Lauderdale, Fla., USA) signaled the computer whenever a monkey sucked on the pipe. A heated stem (Boni et al., 1991), similar to that used by humans when smoking cocaine (Foltin et al., 1990), was mounted on the outside of the aerosol panel. A glass tube (10 mm) fitted with a screen for holding drug was set inside another glass tube (12 mm) mounted on the outside of the panel. The external pipe was wrapped by a heating coil (Cole-Parmer Co., Vernon Hills, Ill., USA), encased in fiberglass insulation (Cole-Parmer) with temperature controlled with a heat controller (#515, George Ulanet Co., Newark, N.J., USA). The heat source was maintained at a temperature of 210–230°C. A brass stopper was fitted to the top of the stem arrangement in order to provide a sealed system increasing the sensitivity of the pressure-sensitive switch. The inside glass stem and screens were changed daily. A Cole-Palmer peristaltic pump (flow rate of 10 ml/min) and a fluid reservoir connected to the aerosol stem were also mounted on the outside of the panels.
2.3. Methamphetamine Sessions (Responding on Aerosol Panel)
A single 90-min meal session occurred at 7:00 AM followed at 9:00 AM by a 60-min session during which baboons could respond for food or vaporized drug. Methamphetamine self-administration sessions occurred on weekdays beginning each day at 9:00 AM. The beginning of each session was signaled by illumination of the session lights on the aerosol panel and the light above the aerosol stem. Puffing on the pipe 2 times resulted in the delivery of 0.05 ml of the drug solution. After aerosol delivery the lights above the aerosol stem remained illuminated for 10 sec to encourage baboons to continue puffing on the stem. Baboons received 1 M&M® candy for making an additional 5 puffs. Baboons always completed the additional puffs after aerosol delivery. The next aerosol dose was available after 2-min of additional puffing for males and 1-min for females. Male baboons could earn up to 10 methamphetamine deliveries and 10 M&Ms and female baboons could earn up to 20 methamphetamine deliveries and 20 M&Ms. The number of doses was increased for the females in hopes that responding would increase from the low levels observed with 10 drug deliveries. Once methamphetamine self-administration stabilized methamphetamine sessions occurred on Monday, Wednesday and Friday and food pellet self-administration sessions occurred on Tuesday, Thursday and Saturday. Then beginning at 11:00 AM baboons had 4 consecutive opportunities for a pellet meal through the day. Food was not available overnight when baboons rarely eat. The 8 males completed the study prior to the 7 females.
2.4. Morning Food Pellet Sessions (Responding on Right Lever on Food Panel)
The 9:00 AM food session was similar to the methamphetamine session except that food pellets were delivered instead of aerosol. The beginning of the session was signaled by illumination of the session lights on the food panel and right light above the right lever. Pulling the right lever 10 times resulted in the delivery of 10 food pellets [one-g food pellets containing 3.4 kcal (13.22 kJ); Bio-Serv, Inc., Frenchtown, NJ] accompanied by the lights above the right lever flashing 10 times. Food delivery was followed by a 7-min timeout during which responding had no consequences. Baboons could earn up to 100 food pellets.
2.5. Pellet Meals (Responding on Left Lever on Food Panel)
Pellet meals were available once at 7:00 AM each morning (“breakfast”) and 4 times beginning at 11:00 AM. Pellet meal availability was signaled by the illumination of the session lights on the food panel and the right light over the left lever. If a baboon wanted to eat a meal of pellets it had to pull the left lever. The first pull on the left lever started a 30-min timer and during that interval each time the left lever was pulled 10 times both lights above the left lever flashed 10 times. The same pattern of flashing lights was paired with food pellet delivery such that responding during the initial 30 min was reinforced by cues paired with food, i.e., conditioned reinforcement. After the 30 min were up, the left light above the left lever was illuminated signaling the availability of food pellets. Each time the left lever was pulled 10 times baboons received 1 food pellet and the lights above the left lever flashed 10 times. There was a 10-s interval after each pellet delivery when responding had no consequences. Baboons could then respond for food pellets until the total 90-min session ended. All baboons responded for a food pellet meal at least once each day.
2.6. Procedure
Training to work for drug aerosol occurred at 9:00 AM on weekdays. Training consisted of: 1) training baboons to drink and then actively suck a sweet fluid (Cherry Koolaid®) from a syringe; 2) training baboons to drink and then actively suck a sweet fluid from the brass stem that was used for aerosol delivery; 3) training baboons to suck hard enough on the brass stem to activate a pressure-sensitive relay for fluid delivery; 4) reinforcing puffing, i.e., activation of the pressure-sensitive relay, behavior with 1 candy M&M® rather than fluid; 5) reinforcing puffing when puffing provided air heated to the melting point of methamphetamine; 6) reinforcing puffing when puffing resulted in delivery of vaporized alcohol (0.05 ml); 7) reinforcing puffing when puffing resulted in delivery of vaporized methamphetamine (0.3 mg/kg); and 8) reducing the number puffs that were reinforced with candy until only 1 candy was given after the baboon received methamphetamine and that occurred after an additional 5 puffs on the stem. An investigator or technician provided fluid and candy reinforcement by hand.
For male baboons, once responding was stable, they had access to vaporized methamphetamine during the morning on Mondays, Wednesdays and Fridays for 4 months. Food pellets were available during the morning on Tuesdays, Thursdays and Saturdays for 4 months (there were no sessions on Sunday mornings when the cages were sanitized weekly). Baboons received occasional acute i.m. doses of amphetamine, dexfenfluramine, heroin and naloxone (~18 total doses) on the mornings when food was available to test the effects of these drugs on food intake (a second drug was never given on days baboons had access to aerosol). The effects of removing methamphetamine from the aerosol but still providing candy was then tested for 9 days followed candy alone (no aerosol) for 3 days and a return to methamphetamine aerosol plus candy for 5 days.
For the female baboons, initial training went more quickly, but rates of puffing were low, so the minimal interval between drug doses was decreased to 1 min (from 2 min) and the number of does available was increased to 20 from 10. This was done in hopes that having more drug available would increase rates of puffing. For female baboons, once responding was stable, they had access to vaporized methamphetamine during the morning on Mondays, Wednesdays and Fridays for 3 months. Food pellets were available during the morning on Tuesdays, Thursdays and Saturdays for 3 months (there were no sessions on Sunday mornings when the cages were sanitized weekly). Baboons received occasional acute i.m. doses of amphetamine and dexfenfluramine (~9 total doses) on the mornings when food was available to test the effects of these drugs on food intake (a second drug was never given on days baboons had access to aerosol). The effects of removing methamphetamine from the aerosol but still providing candy were then tested for 10 days.
Total activations of the pressure-sensitive switch, i.e., puffs, including the 2 required prior to aerosol delivery, the 5 puffs after aerosol delivery to earn a candy and those that followed aerosol delivery, was used as the primary dependent measure because it included puffing behavior that occurred after aerosol delivery as well as those required to earn the delivery.
2.7. Drug
Methamphetamine HCl (0.3 mg/kg; Sigma Chemical Corp., St. Louis, MO, USA) was dissolved in 95% ethanol. The volume for a single dose was 0.05 ml. One concentration was used for the males based on the mean weight of the 5 males and one concentration was used for the females based on the mean weight of the 6 females. Baboons were weighed every 4–6 weeks and the concentrations adjusted to account for mean change in body weight across the study.
3. Results
The initial training of the male baboons required over 5 months. While all 8 baboons would touch the brass stem in order to receive a candy only 5 of the male baboons learned to suck, i.e., puff, on the stem hard enough to activate the pressure-sensitive relay. Baboons puffing behavior was initially reinforced by handing them one candy per puff. The number of candies given was then decreased to a single candy following each aerosol delivery after 5 additional puffs to encourage drug inhalation. Once responding was stable in the male baboons they were receiving 8 to 10 aerosol deliveries per day and the number of candies was gradually decreased with the goal that baboons would puff solely for aerosol delivery. With the exception of 1 baboon, the puffing behavior decreased unless an occasional M&M (5–10 per session) was given; baboons would touch the pipe, but not inhale. Also 1 baboon would periodically stop working entirely and had to be reinforced with frequent M&Ms to encourage him to puff again. It also became apparent that humans had become a discriminative stimulus for smoking such that puffing nearly ceased when a human was not in the room. To insure consistent behavior within the 5 baboons who would puff, a single candy was then given after an additional 5 puffs following each aerosol delivery.
The reinforcing efficacy of methamphetamine aerosol was assessed following 4 months of aerosol sessions 3 days a week by presenting only the alcohol placebo aerosol following completion of the minimal 2 puffs but continuing to provide candy reinforcement.
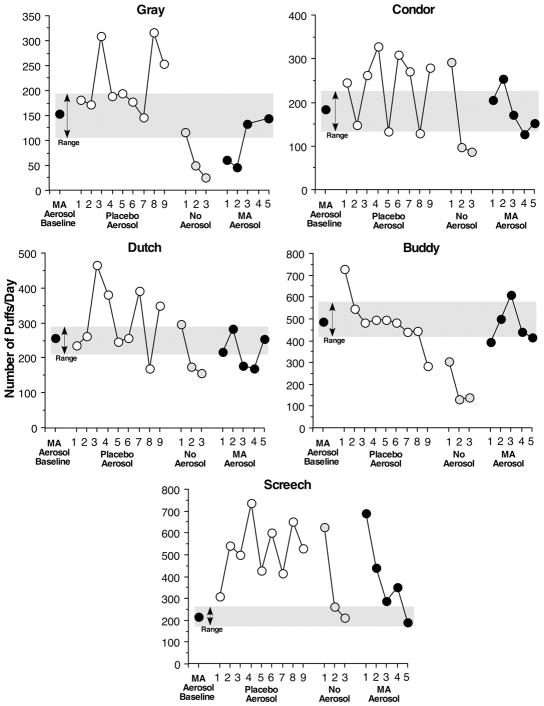
The mean number of puffs varied widely amongst male baboons with 4 of the 5 baboons puffing 150 – 275 times per day and one baboon (Buddy) puffing nearly 500 times each day (Figure 1). A minimum of 70 puffs were required to earn all 10 drug vapors and 10 candies. All of the baboons exceeded this minimum by 90 to 430 puffs. On the first day that 0.05 ml of alcohol alone was delivered the baboon who puffed the most (Buddy) increased his puffing by about 200 puffs and then over days the number of puffs decreased such that puffing was below the baseline range by the 9th day of alcohol: a pattern consistent with extinction. The remaining 4 baboons displayed a more variable saw-tooth pattern with the number of puffs increasing for one or two days and then decreasing for a day or two, with responding generally exceeding the range seen when methamphetamine was delivered. On the extreme end, Screech puffed about 600 times per day when alcohol (total of 1 ml during a session) was available compared to 225 puffs when methamphetamine was available. When aerosol was not delivered (just warm air), but a single candy was delivered, the number of puffs decreased substantially for all 5 baboons, with 4 of the 5 baboons decreasing the number of puffs below the range observed when methamphetamine was available. Responding promptly returned to baseline levels when methamphetamine was again delivered.
Figure 1.
Mean baseline number and range of total daily puffs made by 5 male baboons when methamphetamine (MA) aerosol was available, when placebo aerosol was available for 9 days, when no aerosol was available for 3 days and when methamphetamine aerosol was again available for 5 days. Male baboons could earn up to 10 aerosol deliveries. One candy was delivered after a baboon puffed five additional times following each aerosol delivery. One candy was always delivered when aerosol or warm air was delivered.
When grouped together, the 5 male baboons puffed on methamphetamine aerosol 260 ± 66 times (mean ± SEM) and puffing significantly increased the first 3 days that only placebo was delivered to 359 ± 76 times (P <0.04). While puffing, on average remained elevated throughout the remaining 6 days of alcohol delivery the increase was not statistically significant. The last day when warm air and candy only were delivered the male baboons puffed only 124 ± 35 times (P <0.08). On the days that the male baboons inhaled methamphetamine aerosol in the morning they consumed 273 ± 43 food pellets in the afternoon. Food pellet consumption significantly increased when the male baboons inhaled placebo to 347 ± 51 pellets (P < 0.05).
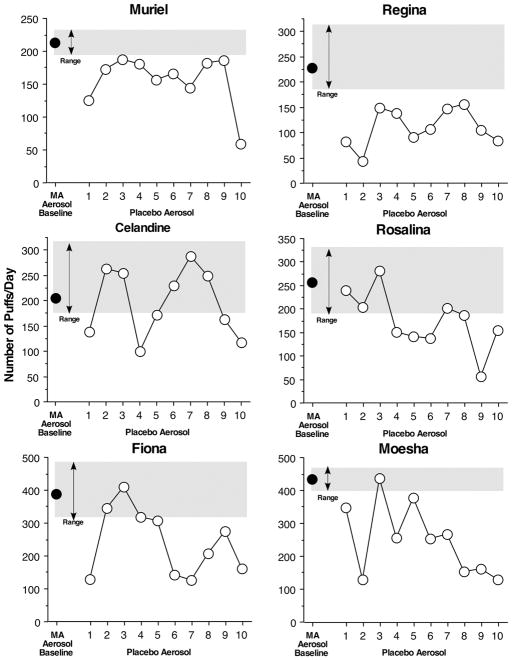
Initial training of the female baboons required less time, most likely related to the experience of the investigators rather than potential sex differences. Six of the 7 female baboons acquired stable methamphetamine aerosol self-administration in about 3 months. The female baboons then had 3 months of responding for methamphetamine 3 days a week. At this point, 4 of female baboons puffed between 200 to 260 times per day, while 2 of the female baboons puffed about 400 times per day (Figure 2). A minimum of 140 puffs were required to earn all 20 drug vapors and 20 candies. Alcohol was substituted for methamphetamine for 10 days. Changes in puffing were rapidly observed when only candy and placebo were delivered. Two female baboons consistently puffed less throughout the 10 days when placebo was available. As with the males, 4 females displayed a sawtooth pattern of puffing when alcohol was available. For three of these baboons (Rosalina, Fiona and Moesha) puffing for placebo was consistently lower than for methamphetamine aerosol by the end of the 10 days of alcohol. Puffing by Celandine remained within the range observed for methamphetamine on about one half of the alcohol days. Thus, the numbers of puffs reinforced with alcohol and candy during the second 5 days of alcohol were below the range of puffs observed for methamphetamine in 5 of the 6 female baboons.
Figure 2.
Mean baseline number and range of total daily puffs made by 6 female baboons when methamphetamine (MA) aerosol was available and when placebo aerosol was available for 10 days. Female baboons could earn up to 20 aerosol deliveries. One candy was delivered after a baboon puffed five additional times following each aerosol delivery. One candy was always delivered when aerosol was delivered.
When grouped together, the 6 female baboons puffed on methamphetamine aerosol 290 ± 4 times and puffing significantly decreased the first 5 days that only placebo was delivered to 211 ± 36 times (P <0.004) and significantly decreased the last 5 days that only placebo was delivered to 161 ± 17 times (P <0.01). On the days that the female baboons inhaled methamphetamine aerosol in the morning they consumed 277 ± 2 food pellets in the afternoon. Food pellet consumption slightly decreased when the female baboons inhaled placebo to 263 ± 26 pellets.
4. Discussion
After extensive training and with continued reinforcement with 1 piece of candy for puffing after aerosol delivery, 5 of 8 male and 6 of 7 female baboons acquired puffing behavior on a brass stem approximating human smoked drug taking. All attempts to maintain puffing while eliminating candy delivery in the males failed: only 1 male baboon puffed consistently without candy delivery. For this reason, the females were never tested without candy delivery. After 3–4 months of methamphetamine aerosol administration there was evidence for methamphetamine self-administration in 4 of the 5 male baboons and 5 of the 6 female baboons that learned to puff on the stem. In females, the number of puffs for aerosol containing methamphetamine was greater than the number of puffs for the placebo aerosol under conditions where puffing was also reinforced by candy delivery, i.e., methamphetamine + candy > alcohol + candy. In males, the number of puffs for aerosol containing methamphetamine was greater than the number of puffs for warm air under conditions where puffing was also reinforced by candy delivery, i.e., methamphetamine + candy > candy.
In our previous studies with male rhesus monkeys self-administering heroin aerosol, after sufficient training no reinforcement of puffing behavior other than the delivery of heroin was required (Evans et al., 2003; Foltin & Evans, 2001). Compared to heroin methamphetamine aerosol has minimal reinforcing efficacy in non-human primates. A reasonable amount of aerosol develops from 0.05 ml drug solution and it could be easily seen in the baboons’ mouths. Also, some baboons were observed waving the aerosol away from their faces. We expect that baboons, because they have excellent senses of smell and taste had difficulty adapting to the aerosol.
An interesting pattern was observed in 4 of the 5 males when placebo (0.05 ml alcohol) was substituted for methamphetamine. These males developed a sawtooth pattern with responding going quite high, often doubling, relative to responding for methamphetamine and then going back down and up again. As a group, substitution of placebo aerosol for methamphetamine aerosol initially significantly increased puffing behavior in males. This may represent an extinction burst of responding. In the male baboons removing aerosol such that only warm air was in the stem, but continuing to reinforce puffing with candy decreased responding below the methamphetamine range in 4 of 5 male baboons. Responding returned to previous levels when methamphetamine aerosol was again delivered. This pattern suggests that placebo aerosol may have acquired conditioned reinforcing effects. Four of the six females also displayed a saw-tooth pattern when alcohol was substituted for methamphetamine, but as a group, substitution of placebo for methamphetamine aerosol significantly decreased puffing behavior in females.
Methamphetamine aerosol delivery was associated with greater rates of responding in a previous study conducted in 5 male rhesus monkeys (Newman & Carroll, 2006). In that study, monkeys completed a ratio requirement on a response lever prior to activating a pressure-sensitive relay 5 times in order to earn methamphetamine aerosol delivery. In addition to the species difference, the monkeys in the Mattox & Carroll (2006) study had extensive previous experience (129–213 months) inhaling cocaine and heroin aerosols that may have facilitated methamphetamine aerosol self-administration.
Although there are fewer female than male drug users, and much fewer research studies with female non-human primates, it is well known that females “telescope” more quickly into troubled drug use than males (e.g., Greenfield et al., 2007; Holdcraft & Iacono, 2004), and numerous preclinical studies of substance use confirm a range of sex differences (see review by Fattore et al., 2008). For example, with respect to methamphetamine in rodents 1) female mice showed greater locomotor activity after methamphetamine aerosol inhalation than male mice (Marusich et al., 2016); 2) female rats showed greater locomotor activity and stereotypy following intravenous methamphetamine (Milesi-Halle et al., 2007); 3) more females than males acquired methamphetamine self-administration and at a faster rate (Roth & Carroll, 2004); 4) females responded for more methamphetamine than males under both fixed ratio and progressive ratio schedules of reinforcement (Roth & Carroll, 2004); 5) females’ methamphetamine intake escalated faster than males’ intake and females self-administered more methamphetamine than males when given access to methamphetamine during 6-hr sessions (Reichel et al., 2012); 6) during reinstatement tests females reinstated responding for methamphetamine following a lower “priming” dose than males (Reichel et al., 2012); and 7) during reinstatement tests females reinstated responding for methamphetamine following drug-paired cues more than males (Reichel et al., 2012).
Some sex differences were also observed in the current study. Food consumption was significantly decreased by methamphetamine aerosol inhalation compared to placebo inhalation in male baboons, but not in female baboons. Substitution of placebo for methamphetamine significantly increased puffing behavior in male baboons, and significantly decreased puffing behavior in female baboons. Also, more of the females than the males acquired methamphetamine aerosol self-administration, but this difference is most likely due to the fact that more females than males learned to activate the pressure-sensitive relay, i.e., a possible sex difference unrelated to drug action. With the exception of one male who puffed as much as 600 times each session, the range of puffs observed during sessions was similar between the males and the females. Females, however, were puffing for up to 20 doses, while males only could earn 10 doses in the same time frame. Rates of puffing per dose in males were nearly twice that observed in females. The decision to increase the number of aerosol deliveries for females was based on the low rate of puffing observed when only 10 doses were available. Clearly, it is not possible to make direct comparisons between males and females because the researchers were more experienced in training puffing when the females began the study and the number of drug deliveries varied. While the data do not support the rodent data showing that females are more likely to self-administer methamphetamine, the differences in food intake and puffing behavior between methamphetamine and placebo aerosol conditions between the sexes demonstrates a significant effect of sex in moderating the response to stimulant inhalation. A direct comparison of males and females trained and tested using identical procedures is needed. Regardless these female baboons may be the only female non-human primates used in aerosol research since a female chimpanzee in 1973 (Peiper & Cole, 1973).
While new technologies are increasing the ease with which aerosol for inhalation can be generated, conducting inhalation studies is still technically challenging. These challenges are even more difficult when attempting to assess self-administration of aerosols and drawing conclusions about abuse effects as a function of route. When aerosol is administered to an animal in an aerosol chamber it can be absorbed through the skin, the nasal mucosa, as well as the lungs making it impossible to accurately identify how much drug was absorbed via each route. Even the technology that limits aerosol exposure to the nose does not guarantee that the drug is absorbed in the lungs as it still must pass nasal mucosa. Thus, drug is absorbed via the nasal mucosa and the lungs. Although procedures with non-human primates that involve puffing on a stem for aerosol delivery most closely model human drug taking it is likely that drug is absorbed both in the lungs and buccally in the mouth. Thus, with all techniques there is variability in the actual route of delivery that is difficult to quantify. Caution needs to be used when drawing conclusions about the route of absorption in inhalation studies.
There are several limitations to the current study. Although the initial sample sizes were 7 females and 8 males, the final sample size of 6 females and 5 males who learned the puffing behavior was relatively small. For both sexes, the majority of the baboons responded in a similar way to the manipulations and statistical significance was obtained with the small sample size. More significantly, there were no biological samples taken to measure how much inhaled drug was absorbed. Thus, we cannot validate that the behavioral differences observed in puffing behavior between methamphetamine and placebo were due to methamphetamine intake.
In summary, puffing reinforced by methamphetamine exceeded puffing reinforced by the placebo aerosol when puffing was occasionally reinforced by candy or when puffing was reinforced by candy alone. In contrast to another study with vaporized methamphetamine (Newman & Carroll, 2006) and a study done with nearly identical procedures with vaporized heroin (Foltin & Evans, 2001), puffing behavior had to be reinforced with candy as well as drug aerosol. In spite of this difficulty baboons could be trained to inhale drug aerosol demonstrating that future work involving aerosol administration to baboons paired with PET imaging would be possible. Because there were differences in procedural details, and males and females were not run concurrently, it is not possible to draw strong conclusions about sex differences. Differences that did exist suggest that sex affects the response to methamphetamine aerosol. Given the popularity of inhaled drugs of abuse and the minimal amount of data in male and female non-human primates with this dosing methodology additional research is clearly warranted.
Highlights.
Male and female baboons could be trained to inhale aerosolized methamphetamine
Females had lower rates of puffing behavior than males
Acknowledgments
This research was supported by DA-004130 from The National Institute on Drug Abuse, and approved by the New York State Psychiatric Institute Animal Care and use Committee. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The veterinary assistance of Drs. Moshe Shalev, Amy Cassano and Mr. Girma Asfaw, and the technical assistance of Angel Ramirez, Jean Willi and Tanya Lalwani is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ando K, Yanagita T. Cigarette smoking in rhesus monkeys. Psychopharmacology. 1981;72:117–127. doi: 10.1007/BF00431644. [DOI] [PubMed] [Google Scholar]
- Boni JP, Barr WH, Martin BR. Cocaine inhalation in the rat: pharmacokinetics and cardiovascular response. Journal of Pharmacology & Experimental Therapeutics. 1991;257(1):307–315. [PubMed] [Google Scholar]
- Carroll ME, Krattiger KL, Gieske D, Sadoff DA. Cocaine-base smoking in rhesus monkeys: reinforcing and physiological effects. Psychopharmacology. 1990;102:443–450. doi: 10.1007/BF02247123. [DOI] [PubMed] [Google Scholar]
- Cole JM, Pieper WA, Rumbaugh DM. Effects of Δ9-tetrahydrocannabinol on spaced responding in great apes. Communications in Behavioral Biology. 1971;6:285–293. [Google Scholar]
- Comer SD, Turner DM, Carroll ME. Effects of food deprivation on cocaine base smoking in rhesus monkeys. Psychopharmacology. 1995;119:127–132. doi: 10.1007/BF02246152. [DOI] [PubMed] [Google Scholar]
- Evans SM, Nasser J, Comer SD, Foltin RW. Smoked heroin in rhesus monkeys: effects of heroin extinction and fluid availability on measures of heroin seeking. Pharmacology Biochemistry and Behavior. 2003;74(3):723–737. doi: 10.1016/s0091-3057(02)01070-5. [DOI] [PubMed] [Google Scholar]
- Fattore L, Altea S, Fratta W. Sex differences in drug addiction: a review of animal and human studies. Women’s Health. 2008;4:51–65. doi: 10.2217/17455057.4.1.51. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Nestadt G, Stromberger H, Cornell EE, Pearlson GD. Demonstration of naturalistic methods for cocaine smoking by human volunteers. Drug & Alcohol Dependence. 1990;26(2):145–154. doi: 10.1016/0376-8716(90)90121-t. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Evans SM. Location preference related to smoked heroin self-administration by rhesus monkeys. Psychopharmacology. 2001;155:419–425. doi: 10.1007/s002130100721. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Current Protocols in Neuroscience. 2008;44:1–9. doi: 10.1002/0471142301.ns0929s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Trucco EM, McHugh RK, Lincoln M, Gallop RJ. The Women’s Recovery Group Study: A Stage I trial of women-focused group therapy for substance use disorders versus mixed-gender group drug counseling. Drug and Alcohol Dependence. 2007;90(1):39–47. doi: 10.1016/j.drugalcdep.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell PT, Marquinez NS, Correa JB, Meltzer LR, Unrod M, Sutton SK, Simmons VN, Brandon TH. Expectancies for cigarettes, e-cigarettes, and nicotine replacement therapies among e-cigarette users. Nicotine & Tobacco Research. 2014;17:193–200. doi: 10.1093/ntr/ntu149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami D, Keenan R, Carroll ME, Colon E, Geiske D, Wilson B, Huber M. A method for delivery of precise doses of smoked cocaine-base to humans. Pharmacology Biochemistry & Behavior. 1990;36:1–7. doi: 10.1016/0091-3057(90)90116-y. [DOI] [PubMed] [Google Scholar]
- Holdcraft LC, Iacono WG. Cross-generational effects on gender differences in psychoactive drug abuse and dependence. Drug and Alcohol Dependence. 2004;74(2):147–158. doi: 10.1016/j.drugalcdep.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Juarez-Portilla C, Kim RD, Robotham M, Tariq M, Pitter M, LeSauter J, Silver R. Voluntary inhalation of methamphetamine: a novel strategy for studying intake non-invasively. Psychopharmacology. 2017;234:739–747. doi: 10.1007/s00213-016-4510-8. [DOI] [PubMed] [Google Scholar]
- Katz JL, Witkin JM. Behavioral effects of cocaine alone and in combination with selective dopamine antagonists in the squirrel monkey. Psychopharmacology. 1991;103:33–40. doi: 10.1007/BF02244070. [DOI] [PubMed] [Google Scholar]
- Lefever TW, Lee YO, Kovach AL, Silinski MA, Marusich JA, Thomas BF, Wiley JL. Delivery of nicotine aerosol to mice via a modified electronic cigarette device. Drug & Alcohol Dependence. 2017;172:80–87. doi: 10.1016/j.drugalcdep.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefever TW, Marusich JA, Thomas BF, et al. Vaping synthetic cannabinoids: a novel preclinical model of e-cigarette use in mice. Substance Abuse: Research & Treatment. 2017;11:1178–2218. doi: 10.1177/1178221817701739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Meng Y, Martin BR. Inhalation exposure to volatilized opioids produces antinociception in mice. Journal of Pharmacology & Experimental Therapeutics. 1996;279(1):69–76. [PubMed] [Google Scholar]
- Lichtman AH, Peart J, Poklis JL, Bridgen DT, Razdan RK, Wilson DM, Poklis A, Meng Y, Byron PR, Martin BR. Pharmacological evaluation of aerosolized cannabinoids in mice. European Journal of Pharmacology. 2000;399(2–3):141–149. doi: 10.1016/s0014-2999(00)00321-6. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Varvel S, Hamm R, Martin B. Differential effects of Δ9 -THC on spatial reference and working memory in mice. Psychopharmacology. 2001;157(2):142. doi: 10.1007/s002130100780. [DOI] [PubMed] [Google Scholar]
- Manwell LA, Ford B, Matthews BA, Heipel H, Mallet PE. A vapourized Δ9-tetrahydrocannabinol (Δ9-THC) delivery system part II: Comparison of behavioural effects of pulmonary versus parenteral cannabinoid exposure in rodents. Journal of Pharmacological & Toxicological Methods. 2014;70(1):112–119. doi: 10.1016/j.vascn.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Manwell LA, Charchoglyan A, Brewer D, Matthews BA, Heipel H, Mallet PE. A vapourized Δ9-tetrahydrocannabinol (Δ9-THC) delivery system part I: Development and validation of a pulmonary cannabinoid route of exposure for experimental pharmacology studies in rodents. Journal of Pharmacological & Toxicological Methods. 2014;70(1):120–127. doi: 10.1016/j.vascn.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Mattox AJ, Carroll ME. Smoked heroin self-administration in rhesus monkeys. Psychopharmacology. 1996;125:195–201. doi: 10.1007/BF02247328. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Lefever TW, Blough BE, Thomas BF, Wiley JL. Pharmacological effects of methamphetamine and alpha-PVP vapor and injection. Neurotoxicology. 2016;55:83–91. doi: 10.1016/j.neuro.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Dukat M, Bridgen DT, Martin BR, Lichtman AH. Pharmacological effects of methamphetamine and other stimulants via inhalation exposure. Drug & Alcohol Dependence. 1999;53:111–120. doi: 10.1016/s0376-8716(98)00120-3. [DOI] [PubMed] [Google Scholar]
- Milesi-Hallé A, McMillan DE, Laurenzana EM, Byrnes-Blake KA, Owens SM. Sex differences in (+)-amphetamine and (+)-methamphetamine-induced behavioral response in male and female Sprague–Dawley rats. Pharmacology Biochemistry & Behavior. 2007;86:140–149. doi: 10.1016/j.pbb.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JL, Carroll ME. Reinforcing effects of smoked methamphetamine in rhesus monkeys. Psychopharmacology. 2006;188:193–200. doi: 10.1007/s00213-006-0479-z. [DOI] [PubMed] [Google Scholar]
- Nguyen JD, Aarde SM, Cole M, Vandewater SA, Grant Y, Taffe MA. Locomotor stimulant and rewarding effects of inhaling methamphetamine, MDPV, and mephedrone via electronic cigarette-type technology. Neuropsychopharmacology. 2016;41:2759–2771. doi: 10.1038/npp.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Aarde SM, Vandewater SA, Grant Y, Stouffer DG, Parsons LH, Cole M, Taffe MA. Inhaled delivery of Δ9-tetrahydrocannabinol (THC) to rats by e-cigarette vapor technology. Neuropharmacology. 2016;109:112–120. doi: 10.1016/j.neuropharm.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcoholism: Clinical & Experimental Research. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Pieper WA, Cole JM. Operant control of smoking in great apes. Behavior Research Methods & Instrumentation. 1973;5:4–6. [Google Scholar]
- Phalen R. Methods in Inhalation Toxicology. CRC Press; Boca Raton, FL: 1997. [Google Scholar]
- Pryor GT, Rebert CS. A method for exposing primates to marihuana smoke that simulates the method used by human marihuana smokers. Pharmacology Biochemistry & Behavior. 1989;34(3):521–525. doi: 10.1016/0091-3057(89)90552-2. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Chan CH, Ghee SM, et al. Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology. 2012;223:371–380. doi: 10.1007/s00213-012-2727-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodefer JS, Mattox AJ, Thompson SS, Carroll ME. Effects of buprenorphine and an alternative nondrug reinforcer, alone and in combination on smoked cocaine self-administration in monkeys. Drug & Alcohol Dependence. 1997;45(1–2):21–29. doi: 10.1016/s0376-8716(97)01341-0. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology. 2004;172:443. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- Schulze GE, McMillan DE, Bailey JR, Scallet AC, Ali SF, Slikker W, Paule MG. Acute effects of marijuana smoke on complex operant behavior in rhesus monkeys. Life Sciences. 1989;45:465–475. doi: 10.1016/0024-3205(89)90096-9. [DOI] [PubMed] [Google Scholar]
- Siegel RK, Johnson CA, Brewster JM, Jarvik ME. Cocaine self-administration in monkeys by chewing and smoking. Pharmacology Biochemistry & Behavior. 1976;4:461–467. doi: 10.1016/0091-3057(76)90064-2. [DOI] [PubMed] [Google Scholar]
- Slikker W, Fligiel SE, Beals TF, Tashkin DP, Paule MG, Scallet AC, Bailey JR. Marijuana exposure and pulmonary alterations in primates. Pharmacology Biochemistry & Behavior. 1991;40:637–642. doi: 10.1016/0091-3057(91)90375-c. [DOI] [PubMed] [Google Scholar]
- VandeBerg JL, Williams-Blangero S, Tardif SD. The Baboon in Biomedical Research. Springer Science + Business Media; New York, NY: 2009. [Google Scholar]
- Wiebelhaus JM, Poklis JL, Poklis A, Vann RE, Lichtman AH, Wise LE. Inhalation exposure to smoke from synthetic “marijuana” produces potent cannabimimetic effects in mice. Drug & Alcohol Dependence. 2012;126(3):316–323. doi: 10.1016/j.drugalcdep.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BA. Inhalation exposure systems: design, methods and operation. Toxicologic Pathology. 2007;35:3–14. doi: 10.1080/01926230601060017. [DOI] [PubMed] [Google Scholar]
- Wood RW. Animal models of drug self-administration by smoking, in Research Findings on Smoking of Abused Substances. In: Chiang CN, Hawks RL, editors. NIDA Research Monograph 99. U.S. Government Printing Office; Washington, D.C: 1990. pp. 159–171. [PubMed] [Google Scholar]
- Yanagita T, Takahashi S, Ishida K, Funamoto H. Voluntary inhalation of volatile anesthetics and organic solvents by monkeys. Japanese Journal of Clinical Pharmacology & Therapeutics. 1970;1:13–16. [Google Scholar]