The history of how to diagnosis cerebral amyloid angiopathy (CAA) tells the story of the disease itself. CAA is defined by histopathology—deposition of β-amyloid in the cerebrovasculature—and through the 1980s the disorder was only diagnosed in patients with available brain tissue from hematoma evacuation, biopsy, or most commonly post-mortem exam.1 Introduction of the imaging-based Boston Criteria for diagnosis of CAA in the 1990s2, 3 allowed a diagnosis of “probable CAA” in living patients with no available brain tissue and substantially moved the field from the pathologist’s realm to the clinician’s. The Boston Criteria for CAA have become the basis for clinical decision-making as well as a rapidly growing body of literature4 investigating the disease’s clinical manifestations, phenotypic spectrum, progression, and potential for disease-modifying therapy.
The history of CAA diagnostic criteria also illustrates broader issues for other major central nervous system diseases. If the brain were as accessible to direct tissue examination during life as the blood or even the liver, diagnosis and staging of brain disorders such as cerebral small-vessel or neurodegenerative disease would be relatively straightforward and the state of clinical trials would presumably be more advanced. Given the relative inaccessibility of brain tissue, however, diagnostic approaches have needed to rely on indirect but nonetheless powerful methods such as MRI. The current article will review the evolution and application of the Boston Criteria, how the criteria have contributed to the search for CAA biomarkers, and future directions in this still evolving field.
Development and Validation of the Boston Criteria for CAA
The Boston Criteria for diagnosing CAA arose from discussions between one of the authors (SMG), Drs. Carlos Kase, Daniel Kanter, and the late Michael Pessin. The criteria were first published in 1995 in the Methods section of an analysis of CAA and the apolipoprotein E ε4 allele2 and in 1996 as a table in a clinical-pathologic case report.3 Using the category terminology applied to other brain disorders such as Alzheimer’s disease,5, 6 they defined definite CAA based on full autopsy, probable or possible CAA based on brain imaging plus clinical exclusions, and an additional category of probable CAA with supporting pathology based on clinical scenarios of having limited brain tissue from biopsy or hematoma evacuation (Table 1). Definite CAA requires high neuropathological severity (including features of advanced vasculopathy such as amyloid replacement and splitting of the blood vessel wall)7, 8 in order to avoid diagnosing the condition when the pathology is only mild and incidental. Lesser histopathological severity is required for probable CAA with supporting pathology to reflect the smaller amount of sampled tissue and consequent lower likelihood of identifying the most advanced foci of disease.9
Table 1.
The Modified Boston Criteria for Cerebral Amyloid Angiopathy
|
1. Definite CAA Full post-mortem examination demonstrating:
|
|
2. Probable CAA with supporting pathology Clinical data and pathologic tissue (evacuated hematoma or cortical biopsy) demonstrating:
|
|
3. Probable CAA Clinical data and MRI or CT demonstrating:
|
|
4. Possible CAA Clinical data and MRI or CT demonstrating:
|
Other causes of hemorrhage (differential diagnosis of lobar haemorrhages): antecedent head trauma, hemorrhagic transformation of an ischemic stroke, arteriovenous malformation, haemorrhagic tumour, warfarin therapy with international normalisation ratio > 3, vasculitis
The key diagnostic category for clinical practice and research is probable CAA, the highest level of diagnostic certainty currently achievable without obtaining brain tissue. As first formulated (“original Boston Criteria”), probable CAA entailed neuroimaging demonstration of multiple (i.e. two or more) hemorrhages restricted to lobar brain regions,2, 3 defined as cerebral cortex, the corticosubcortical (grey-white) junction, and subcortical white matter. A modification to count blood products in cortical sulci (cortical superficial siderosis, cSS) as one additional hemorrhagic lesion (“modified Boston Criteria”), was proposed and validated in 2010.10 The most recent version of the criteria are thus known as the modified Boston Criteria (Table 1). The requirement for multiple strictly lobar hemorrhages is based on the lobar predilection of CAA pathology and recurrent intracerebral hemorrhages (ICHs),1 an anatomic distribution that is in near-perfect contrast to the deep hemispheric and brainstem locations favored by ICHs due to hypertensive arteriopathy.11 Since CAA typically spares these deep territories, the presence of any hemorrhagic lesions in basal ganglia, thalamus, or pons preclude the probable CAA diagnosis. Cerebellar hemorrhages can result from either CAA or hypertensive arteriopathy and are therefore not counted by the Boston Criteria, either in favor or against a probable CAA diagnosis.
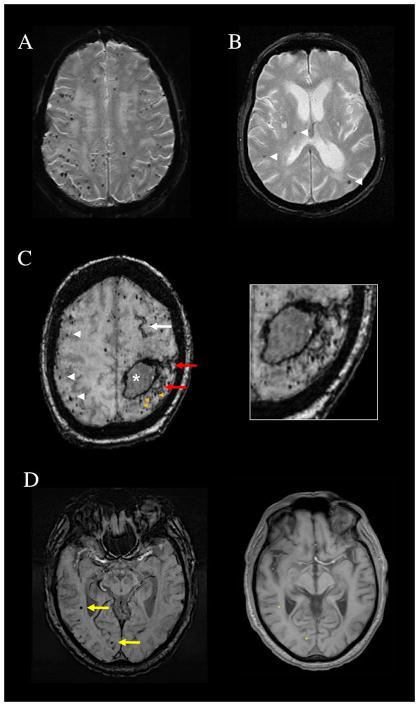
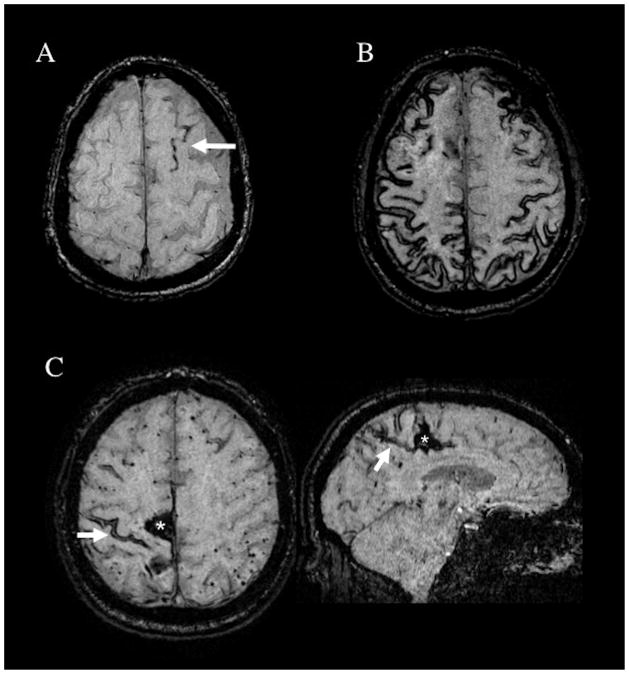
Several methodologic issues arise in applying the Boston Criteria in practice. One is that all types of hemorrhagic lesions—ICHs, cerebral microbleeds (CMBs),12 and (since publication of the modified Boston Criteria)10 acute convexity subarachnoid bleeds or cSS13—count towards the multiple lobar hemorrhages required for probable CAA, or alternatively preclude probable CAA if in deep territories. The rationale for counting all types of hemorrhagic lesions is that while different hemorrhage sizes may arise by distinct pathogenic mechanisms,14 each presumably represents a distinct event of vessel leakage and therefore provides independent evidence for the underlying small vessel condition. The increasingly widespread use of blood-sensitive T2*-weighted MRI methods has greatly influenced detection of CMBs (Fig. 1) and cSS (Fig. 2) and thus the diagnosis of CAA as discussed below. Conversely hemorrhagic lesions that may be part of a larger hemorrhage, such as smaller extensions in proximity to a larger hemorrhage or foci of cSS near or directly connected to ICHs that have ruptured into the subarachnoid space, are considered as part of a single bleeding event and thus not counted as separate hemorrhages (see Fig. 1C and 2C). A second practical issue is that a hemorrhagic lesion in the centrum semiovale can appear a long distance from the outer surface of the brain and still be quite close to the corticosubcortical junction (Fig. 1D) because of the undulating curves of the cortical gyri. Hemorrhages are considered deep hemispheric only when clearly involving the basal ganglia, thalamus, or internal capsule.
Figure 1.
Patterns of cerebral microbleeds (CMB). (A) Multiple strictly lobar CMB on T2*-weighted MRI of a 69-year-old woman who presented with a spontaneous lobar intracerebral hemorrhage. Brain autopsy showed advanced CAA. (B) Mixed CMB (arrowheads) affecting the right thalamus, a deep hemispheric territory, as well as lobar brain regions and therefore not fulfilling Boston criteria for probable CAA. (C) Subacute left frontoparietal lobar hemorrhage (asterisk), numerous strictly lobar CMB (white arrowheads), and a left frontal focus of cortical superficial siderosis (white arrow) on susceptibility-weighted imaging (SWI) MRI of a 78-year-old woman. The image additionally shows foci of CMB (orange arrowheads) and cortical superficial siderosis (red arrows, also seen in magnified image) that are immediately adjacent to the lobar hemorrhage and therefore not counted as separate lesions in determining the number of lobar hemorrhagic foci. (D) SWI from a 71-year-old man with memory loss and CAA on brain biopsy. The yellow arrows point to CMB in the right temporal and right occipital lobes that might appear distant from the brain surface, but would be counted as lobar microbleeds. The aligned T1-weighted slice shows that their positions (asterisks on the right panel) are within or very close to the cortical ribbon.
Figure 2.
Patterns of cortical superficial siderosis (cSS) in CAA patients. (A) Susceptibility-weighted imaging (SWI) MRI showing a single sulcus with cSS (arrow), classified as focal cSS. (B) SWI showing cSS affecting multiple cortical sulci, classified as disseminated (>3 affected sulci). (C) SWI images from a 68-year-old man with a right parasagittal CAA-related spontaneous lobar intracerebral hemorrhage (asterisk). The arrow points to an area of cSS close to the hematoma on the axial slice. The corresponding sagittal slice (right panel) shows that this cSS focus connects to the lobar hemorrhage and thus would not be counted as an independent hemorrhagic lesion. There are multiple lobar cerebral microbleeds in the left hemisphere.
MRI-histopathological studies to date10, 15–17 have provided validating evidence for the Boston Criteria probable CAA diagnosis (Table 2), with sensitivities that appear to depend in part on the clinical presentation of the patients examined. Among three hospital-based studies of patients presenting primarily with ICH who underwent T2*-weighted MRI,10, 15, 16 probable CAA by original Boston Criteria showed sensitivities ranging from 57.9% to 76.9% and specificities of 87.5% to 100%. One head-to-head comparison of original to modified criteria10 suggested that incorporation of cSS presence improved sensitivity without lowering specificity (Table 2). A fourth study17 analyzed a hospital-based cohort of non-ICH individuals with other clinical presentations such as cognitive impairment or transient focal neurological episodes, and found lower sensitivity of 42.4% and similar specificity of 90.9%. A regression model of these data showed that increasing lobar CMB counts predicted greater likelihood of CAA pathology. The same study17 also analyzed a community-based cohort of individuals with T2*-weighted MRI and subsequent autopsy, finding the probable CAA diagnosis to have low sensitivity of only 4.5% for pathological CAA with specificity of 88.0%.
Table 2.
Summary of validation studies of Boston Criteria probable CAA
| Setting | CAA pathology + subjects (ICH+/ICH-) | CAA pathology -subjects (ICH+/ICH-) | Sensitivity | Specificity |
|---|---|---|---|---|
| MRI-neuropathology studies | ||||
| Hospital-based15 | 11 (11/0) | 4 (4/0) | 72.7% | 100% |
| Hospital-based10 | 38 (27/11) | 22 (22/0) | 57.9% 71.1%* |
95.5% 95.5%* |
| Hospital-based16 | 14 (9/5) | 10 (10/0) | 76.9% | 87.5% |
| Hospital-based Population-based17 |
33 (0/33) 22 (0/22) |
22 (0/22) 25 (0/25) |
42.4% 4.5% |
90.9% 88.0% |
| MRI-genetic studies | ||||
| CAA mutation+ subjects | ||||
| Hospital-based, Dutch-type CAA18 | 15 ICH+ 12 ICH- |
NA | 100% 16.7% |
NA |
Data only from individuals with both T2*-weighted MRI and histopathologic or genetic diagnosis.
Using modified Boston criteria. All other values use original Boston criteria.
The Boston Criteria have also been validated by MRI-genetic correlation in individuals carrying CAA-specific, fully penetrant amyloid precursor protein (APP) mutations (Table 2). Among carriers of the Dutch-type APP mutation, 15 of 15 with symptomatic hemorrhagic stroke had multiple strictly lobar hemorrhagic lesions meeting original probable CAA criteria (outside of the age≥55 requirement).18 Only 2 of 12 mutation carriers without symptomatic ICH met this definition, suggesting high sensitivity for symptomatic Dutch-type hereditary disease but low sensitivity for the pre-symptomatic phase. Specificity could not be estimated in this study, as mutation-negative individuals were not scanned. Another report identified five individuals carrying other CAA-associated APP mutations (Iowa-, Italian-, and Flemish-types) whose hemorrhagic lesions also met modified probable CAA criteria.19
The above validation studies have notable limitations: small sample sizes, restriction primarily to a single site and to white participants, and varying T2*-weighted MRI methods (discussed below). These concerns notwithstanding, the studies suggest that the modified probable CAA criteria have 1) reasonably high specificity for pathologic CAA in all settings, and 2) high sensitivity among patients presenting with symptomatic hemorrhages, possibly lower sensitivity for non-ICH presentations, and quite low sensitivity in the general population. The trend for sensitivity to increase with greater severity or later stage of CAA presumably reflects the long-recognized observation that CAA pathology needs to be substantially advanced before it is severe enough to trigger hemorrhages.7, 8 The regression analysis showing increasing specificity with higher CMB counts17 points to the additional possibility that likelihood of CAA follows a graded relationship with hemorrhage number rather than a sharp cutoff at ≥2 hemorrhages.
Variation in MRI hemorrhage detection adds a further layer of complexity. CMB detection in particular12 is strongly influenced by a range of factors in MRI acquisition and processing, including magnetic field strength, echo time, scan resolution, incorporation of phase information (used in susceptibility-weighted imaging20), or weighted averaging across multiple echo times (used in susceptibility weighted angiography21). Systematic comparisons of concurrently obtained MRIs indicate that these parameters substantially affect the number of CMBs counted by raters,22, 23 which means any study of CAA-associated CMB will be influenced by the precise MRI method used. Recent ex vivo MRI analysis of postmortem brains24 suggests that the theoretical goal of close to 100% CMB detection might ultimately be achievable, but only with very high resolution (on the order of 200 μm isotropic voxels). Additional lesions detected on even further reductions in voxel size (to 75 μm isotropic) mostly represented CMB mimics such as small vessel occlusions or microaneurysms rather than bona fide microbleeds.
The possible CAA Boston Criteria category refers to individuals with exactly one hemorrhagic lesion,15 or (per modified Criteria) cSS only without ICH or CMB.10 Little MRI-pathological correlation has been performed in possible CAA. Of the 8 individuals in the initial validation study15 with only an isolated lobar ICH on T2*-weighted MRI, 3 had CAA pathology, supporting the interpretation that possible CAA carries less diagnostic certainty than probable CAA. The impact of MRI parameters on which individuals are diagnosed with probable vs possible CAA has not been systematically analyzed, but based on the above is likely considerable. Another commonly encountered pattern is “mixed” hemorrhagic lesions located in both lobar and deep territories (Fig. 1B). A recent analysis of 75 mixed-ICH patients25 found 66 (88%) to be hypertensive and have other markers of hypertensive small vessel disease such as higher serum creatinine and abundant enlarged perivascular spaces in the basal ganglia relative to patients with probable CAA. These findings suggest contribution from hypertensive arteriopathy, but do not preclude overlapping CAA in at least a subset. Because of the high level of diagnostic uncertainty, patients in this mixed category pose substantial challenges to clinicians (see Future Directions below).
Role of the Boston Criteria in the Search for Biomarkers
The ability to diagnose CAA during life with good specificity is a prerequisite for identifying other biomarkers of the disease’s presence, severity, and future behavior. The probable CAA diagnosis—derived from the number and distribution of hemorrhagic lesions—has indeed been the basis for identifying a range of nonhemorrhagic biomarkers of CAA. Basing a biomarker on probable CAA rather than requiring histopathologic confirmation runs the risk that individuals wrongly diagnosed will yield spurious findings, either false-positives or more likely false-negatives due to misclassification of the “exposure” variable of CAA. The experience in CAA research, however, suggests this approach is preferable to the alternative of restricting studies solely to pathologically verified CAA. This latter approach carries its own major limitations in both sample size and generalizability, as brain tissue (whether from hematoma evacuation, biopsy, or autopsy) becomes available not at random but rather in select subgroups with particularly severe or atypical clinical courses.
Among the long (and likely still growing) list of CAA biomarkers made possible by the Boston Criteria are: 1) white matter T2 hyperintensities (WMH),26 with tendency for posterior predominance27 or a multiple subcortical spot pattern;28 2) altered diffusion-tensor imaging parameters such as global mean diffusion29 or diffusion-tensor-derived global efficiency;30 3) vascular reactivity to functional stimulation;31, 32 4) cortical thickness;33 5) punctate diffusion-weighted imaging hyperintensities suggestive of acute microinfarcts;34, 35 6) enlarged perivascular spaces in the centrum semiovale (CSO-EPVS);36, 37 7) positron-emission tomography (PET) labeling with the amyloid ligands Pittsburgh Compound B and Florbetapir;38–42 and 8) reduced cerebrospinal fluid concentrations of β-amyloid.43–45 These multimodal biomarkers serve as both important windows into the pathogenesis of CAA and candidate outcome markers for clinical trials.46
The dependence of the Boston Criteria CAA diagnosis on hemorrhagic lesions limits analysis of any biomarkers that appear prior to or entirely without CAA-related bleeding. The major approach to circumventing this limitation has been to study pre-hemorrhage carriers of penetrant CAA-related APP mutations. Analysis of such carriers of the Dutch-type mutation suggest that reduced functional reactivity, microinfarcts, and WMH may precede CAA-related CMBs or ICH.47, 48
One further application of the Boston Criteria has been as a starting point for formulating diagnostic criteria for the autoimmune syndrome of CAA-related inflammation (CAA-ri).49 Being able to diagnose CAA-ri by clinical and imaging features alone is clinically important, as it allows patients to begin immunosuppressive treatment without the morbidity of brain biopsy. Proposed criteria for probable CAA-ri50 require hemorrhagic lesions consistent with probable CAA as well as additional clinical and imaging features: presentation with headache, decreased consciousness, behavioral changes, focal signs or seizure, and MRI evidence of WMH lesions that are asymmetric and extend to the immediately subcortical white matter. In a validation study,50 probable CAA-ri criteria were met by 14 of 17 individuals with pathologically confirmed CAA-ri vs 1 of 37 with pathologically confirmed non-inflammatory CAA, yielding a sensitivity of 82% and specificity of 97%. The high specificity is particularly relevant from a clinical standpoint, as it suggests brain biopsy can be safely avoided in patients meeting probable CAA-ri criteria. The subcortical WMH lesions in CAA-ri have similar appearance to edema-type amyloid-related imaging abnormalities (ARIA-E) observed in association with anti-amyloid immunotherapy trials,51 suggesting possible common mechanisms.
Future Directions in CAA Diagnosis
The history of the Boston Criteria highlights some broader issues in devising criteria for diseases where definitive tissue diagnosis is often not feasible. One is the inherent tradeoff between sensitivity and specificity, whereby highly sensitive criteria run the risk of false-positive diagnoses and highly specific criteria yield more false-negatives. There is no single correct balance, and indeed different applications may require different priorities. Diagnostic specificity may be the overriding consideration for determining eligibility for research trials, for example, whereas sensitivity may be key to assessing clinical risk for antithrombotic treatment. A second tension is how to balance ease of use versus complexity and comprehensiveness. The probable CAA definition is reasonably straightforward to apply with a single cutoff set at two or more strictly lobar hemorrhagic lesions, but more complex criteria incorporating other lesion categories and biomarkers might improve accuracy and be more useful for research.
A few aspects of probable CAA seem like promising opportunities for improvement. One is incorporating cSS severity as well as presence. Disseminated cSS, defined as involving more than three sulci (Fig. 2B), is associated with clinical markers of disease severity such as recurrent ICH52, 53 and post-ICH dementia54 as well as other imaging biomarkers such as abundant CSO-EPVS,55 suggesting that extent of cSS carries useful diagnostic information. Another area for potential improvement is diagnosing CAA in individuals with hemorrhagic lesions in mixed lobar and deep territories (Fig. 1B), particularly when the lobar CMB greatly outnumber the deep. A recent analysis, for example, defined a CMB ratio of lobar to deep CMB for individuals in this mixed category and found higher ratios to correlate with increasing Pittsburgh Compound B-PET signal,56 suggesting likely CAA. Finally, one can imagine improvements to the Boston Criteria via incorporation of non-hemorrhagic imaging biomarkers. Counting severe CSO-EPVS as an additional lesion, for example, enhanced the Boston Criteria’s sensitivity without worsening specificity in a small series.16 Any incorporation of additional markers will need to account for the possibility noted above that their sensitivity/specificity may vary with CAA presentation (ICH, non-ICH symptoms, or asymptomatic).
As a next step towards updating and improving the diagnosis of CAA, a multicenter effort to update and externally validate the Boston Criteria has recently been undertaken by the International CAA Association. This project will analyze all available clinical and neuroimaging data from individuals age ≥50 with any of the potential CAA-related clinical presentations, MRI imaging, and histopathologic diagnoses. The goal is to produce and validate a data-driven “version 2.0” of the Boston Criteria that will meet the needs of clinicians and investigators and help maintain the rapid pace of progress towards better treatment of CAA.
Acknowledgments
Sources of Funding
Dr. Greenberg is supported by grants from the National Institutes of Health (R01 AG26484, R01 NS070834, R01 NS096730, U24 NS100591).
Dr Charidimou is supported from the Bodossaki Foundation (post-doctoral fellowship).
Footnotes
Disclosures
None
References
- 1.Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke. 1987;18:311–324. doi: 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg SM, Rebeck GW, Vonsattel JP, Gomez-Isla T, Hyman BT. Apolipoprotein e epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol. 1995;38:254–259. doi: 10.1002/ana.410380219. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg SM, Edgar MA. Case records of the massachusetts general hospital, case 22–1996. N Engl J Med. 1996;335:189–196. doi: 10.1056/NEJM199607183350308. [DOI] [PubMed] [Google Scholar]
- 4.Charidimou A, Fox Z, Werring DJ, Song M. Mapping the landscape of cerebral amyloid angiopathy research: An informetric analysis perspective. J Neurol Neurosurg Psychiatry. 2016;87:252–259. doi: 10.1136/jnnp-2015-310690. [DOI] [PubMed] [Google Scholar]
- 5.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of alzheimer’s disease: Report of the nincds-adrda work group under the auspices of department of health and human services task force on alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 6.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to alzheimer’s disease: Recommendations from the national institute on aging-alzheimer’s association workgroups on diagnostic guidelines for alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP. Cerebral amyloid angiopathy without and with cerebral hemorrhages: A comparative histological study. Ann Neurol. 1991;30:637–649. doi: 10.1002/ana.410300503. [DOI] [PubMed] [Google Scholar]
- 8.Mandybur TI. Cerebral amyloid angiopathy: The vascular pathology and complications. Journal of neuropathology and experimental neurology. 1986;45:79–90. [PubMed] [Google Scholar]
- 9.Greenberg SM, Vonsattel JP. Diagnosis of cerebral amyloid angiopathy. Sensitivity and specificity of cortical biopsy. Stroke. 1997;28:1418–1422. doi: 10.1161/01.str.28.7.1418. [DOI] [PubMed] [Google Scholar]
- 10.Linn J, Halpin A, Demaerel P, Ruhland J, Giese AD, Dichgans M, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010;74:1346–1350. doi: 10.1212/WNL.0b013e3181dad605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher CM. Pathological observations in hypertensive cerebral hemorrhage. J Neuropathol Exp Neurol. 1971;30:536–550. doi: 10.1097/00005072-197107000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: A guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charidimou A, Linn J, Vernooij MW, Opherk C, Akoudad S, Baron JC, et al. Cortical superficial siderosis: Detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain. 2015;138:2126–2139. doi: 10.1093/brain/awv162. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg SM, Nandigam RN, Delgado P, Betensky RA, Rosand J, Viswanathan A, et al. Microbleeds versus macrobleeds: Evidence for distinct entities. Stroke. 2009;40:2382–2386. doi: 10.1161/STROKEAHA.109.548974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: Validation of the boston criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 16.Charidimou A, Jaunmuktane Z, Baron JC, Burnell M, Varlet P, Peeters A, et al. White matter perivascular spaces: An mri marker in pathology-proven cerebral amyloid angiopathy? Neurology. 2014;82:57–62. doi: 10.1212/01.wnl.0000438225.02729.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez-Ramirez S, Romero JR, Shoamanesh A, McKee AC, Van Etten E, Pontes-Neto O, et al. Diagnostic value of lobar microbleeds in individuals without intracerebral hemorrhage. Alzheimers Dement. 2015;11:1480–1488. doi: 10.1016/j.jalz.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Rooden S, van der Grond J, van den Boom R, Haan J, Linn J, Greenberg SM, et al. Descriptive analysis of the boston criteria applied to a dutch-type cerebral amyloid angiopathy population. Stroke. 2009;40:3022–3027. doi: 10.1161/STROKEAHA.109.554378. [DOI] [PubMed] [Google Scholar]
- 19.Sellal F, Wallon D, Martinez-Almoyna L, Marelli C, Dhar A, Oesterle H, et al. App mutations in cerebral amyloid angiopathy with or without cortical calcifications: Report of three families and a literature review. J Alzheimers Dis. 2017;56:37–46. doi: 10.3233/JAD-160709. [DOI] [PubMed] [Google Scholar]
- 20.Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (swi) Magn Reson Med. 2004;52:612–618. doi: 10.1002/mrm.20198. [DOI] [PubMed] [Google Scholar]
- 21.Boeckh-Behrens T, Lutz J, Lummel N, Burke M, Wesemann T, Schopf V, et al. Susceptibility-weighted angiography (swan) of cerebral veins and arteries compared to tof-mra. Eur J Radiol. 2012;81:1238–1245. doi: 10.1016/j.ejrad.2011.02.057. [DOI] [PubMed] [Google Scholar]
- 22.Vernooij MW, Ikram MA, Wielopolski PA, Krestin GP, Breteler MM, van der Lugt A. Cerebral microbleeds: Accelerated 3d t2*-weighted gre mr imaging versus conventional 2d t2*-weighted gre mr imaging for detection. Radiology. 2008;248:272–277. doi: 10.1148/radiol.2481071158. [DOI] [PubMed] [Google Scholar]
- 23.Nandigam RN, Viswanathan A, Delgado P, Skehan ME, Smith EE, Rosand J, et al. Mr imaging detection of cerebral microbleeds: Effect of susceptibility-weighted imaging, section thickness, and field strength. AJNR Am J Neuroradiol. 2009;30:338–343. doi: 10.3174/ajnr.A1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Veluw SJ, Charidimou A, van der Kouwe AJ, Lauer A, Reijmer YD, Costantino I, et al. Microbleed and microinfarct detection in amyloid angiopathy: A high-resolution mri-histopathology study. Brain. 2016;139:3151–3162. doi: 10.1093/brain/aww229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasi M, Charidimou A, Boulouis G, Auriel E, Ayres A, Schwab KM, et al. Mixed location (deep and lobar) intracererbral hemorrhage and microbleeds: Underlying microangiopathy and risk of recurrence. Neurology. 2017 doi: 10.1212/WNL.0000000000004797. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurol ME, Irizarry MC, Smith EE, Raju S, Diaz-Arrastia R, Bottiglieri T, et al. Plasma beta-amyloid and white matter lesions in ad, mci, and cerebral amyloid angiopathy. Neurology. 2006;66:23–29. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- 27.Thanprasertsuk S, Martinez-Ramirez S, Pontes-Neto OM, Ni J, Ayres A, Reed A, et al. Posterior white matter disease distribution as a predictor of amyloid angiopathy. Neurology. 2014;83:794–800. doi: 10.1212/WNL.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charidimou A, Boulouis G, Haley K, Auriel E, van Etten ES, Fotiadis P, et al. White matter hyperintensity patterns in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology. 2016;86:505–511. doi: 10.1212/WNL.0000000000002362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viswanathan A, Patel P, Rahman R, Nandigam RN, Kinnecom C, Bracoud L, et al. Tissue microstructural changes are independently associated with cognitive impairment in cerebral amyloid angiopathy. Stroke. 2008;39:1988–1992. doi: 10.1161/STROKEAHA.107.509091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reijmer YD, Fotiadis P, Martinez-Ramirez S, Salat DH, Schultz A, Shoamanesh A, et al. Structural network alterations and neurological dysfunction in cerebral amyloid angiopathy. Brain. 2015;138:179–188. doi: 10.1093/brain/awu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dumas A, Dierksen GA, Gurol ME, Halpin A, Martinez-Ramirez S, Schwab K, et al. Functional magnetic resonance imaging detection of vascular reactivity in cerebral amyloid angiopathy. Ann Neurol. 2012;72:76–81. doi: 10.1002/ana.23566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peca S, McCreary CR, Donaldson E, Kumarpillai G, Shobha N, Sanchez K, et al. Neurovascular decoupling is associated with severity of cerebral amyloid angiopathy. Neurology. 2013;81:1659–1665. doi: 10.1212/01.wnl.0000435291.49598.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fotiadis P, van Rooden S, van der Grond J, Schultz A, Martinez-Ramirez S, Auriel E, et al. Cortical atrophy in patients with cerebral amyloid angiopathy: A case-control study. Lancet Neurol. 2016;15:811–819. doi: 10.1016/S1474-4422(16)30030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimberly WT, Gilson A, Rost NS, Rosand J, Viswanathan A, Smith EE, et al. Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology. 2009;72:1230–1235. doi: 10.1212/01.wnl.0000345666.83318.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gregoire SM, Charidimou A, Gadapa N, Dolan E, Antoun N, Peeters A, et al. Acute ischaemic brain lesions in intracerebral haemorrhage: Multicentre cross-sectional magnetic resonance imaging study. Brain: a journal of neurology. 2011;134:2376–2386. doi: 10.1093/brain/awr172. [DOI] [PubMed] [Google Scholar]
- 36.Charidimou A, Meegahage R, Fox Z, Peeters A, Vandermeeren Y, Laloux P, et al. Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: A multicentre mri cohort study. Journal of neurology, neurosurgery, and psychiatry. 2013;84:624–629. doi: 10.1136/jnnp-2012-304434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez-Ramirez S, Pontes-Neto OM, Dumas AP, Auriel E, Halpin A, Quimby M, et al. Topography of dilated perivascular spaces in subjects from a memory clinic cohort. Neurology. 2013;80:1551–1556. doi: 10.1212/WNL.0b013e31828f1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson KA, Gregas M, Becker JA, Kinnecom C, Salat DH, Moran EK, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62:229–234. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- 39.Ly JV, Donnan GA, Villemagne VL, Zavala JA, Ma H, O’Keefe G, et al. 11c-pib binding is increased in patients with cerebral amyloid angiopathy-related hemorrhage. Neurology. 2010;74:487–493. doi: 10.1212/WNL.0b013e3181cef7e3. [DOI] [PubMed] [Google Scholar]
- 40.Baron JC, Farid K, Dolan E, Turc G, Marrapu ST, O’Brien E, et al. Diagnostic utility of amyloid pet in cerebral amyloid angiopathy-related symptomatic intracerebral hemorrhage. J Cerebr Blood F Met. 2014;34:753–758. doi: 10.1038/jcbfm.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gurol ME, Becker JA, Fotiadis P, Riley G, Schwab K, Johnson KA, et al. Florbetapir-pet to diagnose cerebral amyloid angiopathy: A prospective study. Neurology. 2016;87:2043–2049. doi: 10.1212/WNL.0000000000003197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charidimou A, Farid K, Baron JC. Amyloid-pet in sporadic cerebral amyloid angiopathy: A diagnostic accuracy meta-analysis. Neurology. 2017;89:1490–1498. doi: 10.1212/WNL.0000000000004539. [DOI] [PubMed] [Google Scholar]
- 43.Verbeek MM, Kremer BP, Rikkert MO, Van Domburg PH, Skehan ME, Greenberg SM. Cerebrospinal fluid amyloid beta(40) is decreased in cerebral amyloid angiopathy. Ann Neurol. 2009;66:245–249. doi: 10.1002/ana.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renard D, Wacongne A, Ayrignac X, Charif M, Fourcade G, Azakri S, et al. Cerebrospinal fluid alzheimer’s disease biomarkers in cerebral amyloid angiopathy-related inflammation. J Alzheimers Dis. 2016;50:759–764. doi: 10.3233/JAD-150621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinez-Lizana E, Carmona-Iragui M, Alcolea D, Gomez-Choco M, Vilaplana E, Sanchez-Saudinos MB, et al. Cerebral amyloid angiopathy-related atraumatic convexal subarachnoid hemorrhage: An aria before the tsunami. J Cereb Blood Flow Metab. 2015;35:710–717. doi: 10.1038/jcbfm.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenberg SM, Al-Shahi Salman R, Biessels GJ, van Buchem M, Cordonnier C, Lee JM, et al. Outcome markers for clinical trials in cerebral amyloid angiopathy. Lancet Neurol. 2014;13:419–428. doi: 10.1016/S1474-4422(14)70003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Rooden S, Goos JD, van Opstal AM, Versluis MJ, Webb AG, Blauw GJ, et al. Increased number of microinfarcts in alzheimer disease at 7-t mr imaging. Radiology. 2014;270:205–211. doi: 10.1148/radiol.13130743. [DOI] [PubMed] [Google Scholar]
- 48.van Opstal AM, van Rooden S, van Harten T, Ghariq E, Labadie G, Fotiadis P, et al. Cerebrovascular function in presymptomatic and symptomatic individuals with hereditary cerebral amyloid angiopathy: A case-control study. Lancet Neurol. 2017;16:115–122. doi: 10.1016/S1474-4422(16)30346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eng JA, Frosch MP, Choi K, Rebeck GW, Greenberg SM. Clinical manifestations of cerebral amyloid angiopathy-related inflammation. Ann Neurol. 2004;55:250–256. doi: 10.1002/ana.10810. [DOI] [PubMed] [Google Scholar]
- 50.Auriel E, Charidimou A, Gurol ME, Ni J, Van Etten ES, Martinez-Ramirez S, et al. Validation of clinicoradiological criteria for the diagnosis of cerebral amyloid angiopathy-related inflammation. JAMA Neurol. 2016;73:197–202. doi: 10.1001/jamaneurol.2015.4078. [DOI] [PubMed] [Google Scholar]
- 51.Sperling RA, Jack CR, Jr, Black SE, Frosch MP, Greenberg SM, Hyman BT, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: Recommendations from the alzheimer’s association research roundtable workgroup. Alzheimers Dement. 2011;7:367–385. doi: 10.1016/j.jalz.2011.05.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boulouis G, Charidimou A, Pasi M, Roongpiboonsopit D, Xiong L, Auriel E, et al. Hemorrhage recurrence risk factors in cerebral amyloid angiopathy: Comparative analysis of the overall small vessel disease severity score versus individual neuroimaging markers. J Neurol Sci. 2017;380:64–67. doi: 10.1016/j.jns.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Charidimou A, Peeters AP, Jager R, Fox Z, Vandermeeren Y, Laloux P, et al. Cortical superficial siderosis and intracerebral hemorrhage risk in cerebral amyloid angiopathy. Neurology. 2013;81:1666–1673. doi: 10.1212/01.wnl.0000435298.80023.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moulin S, Labreuche J, Bombois S, Rossi C, Boulouis G, Henon H, et al. Dementia risk after spontaneous intracerebral haemorrhage: A prospective cohort study. Lancet Neurol. 2016;15:820–829. doi: 10.1016/S1474-4422(16)00130-7. [DOI] [PubMed] [Google Scholar]
- 55.Charidimou A, Jager RH, Peeters A, Vandermeeren Y, Laloux P, Baron JC, et al. White matter perivascular spaces are related to cortical superficial siderosis in cerebral amyloid angiopathy. Stroke. 2014;45:2930–2935. doi: 10.1161/STROKEAHA.114.005568. [DOI] [PubMed] [Google Scholar]
- 56.Tsai HH, Tsai LK, Chen YF, Tang SC, Lee BC, Yen RF, et al. Correlation of cerebral microbleed distribution to amyloid burden in patients with primary intracerebral hemorrhage. Sci Rep. 2017;7:44715. doi: 10.1038/srep44715. [DOI] [PMC free article] [PubMed] [Google Scholar]