Abstract
Background and Objectives
The majority of individuals in substance use disorder (SUD) treatment also smoke cigarettes; yet, the availability of smoking cessation services in SUD treatment remains limited. In this study, we developed and piloted a brief intervention for smokers in SUD treatment intended to motivate engagement in tobacco quitline treatment (TIME-TQ).
Methods
First, we interviewed 19 smokers in SUD treatment to inform the development of TIME-TQ (Phase 1). Second, we delivered a prototype TIME-TQ to 16 smokers in the same SUD treatment program and followed them for 3 months post-discharge (Phase 2).
Results
Feedback from Phase 1 participants was used to refine response choices and video segments included in the prototype TIME-TQ. Phase 2 participants rated TIME-TQ high on relevance, interest, respectfulness, and helpfulness. Additionally, they reported significant increases in readiness to quit and perceived importance of quitting after receiving TIME-TQ. Eight of the 16 accepted a quitline referral, and 8 of 13 reached for follow-up (4 referral acceptors, 4 decliners) reported efforts to quit or reduce smoking during the follow-up period. However, only 3 received quitline counseling and none achieved a sustained period of abstinence.
Discussion and Conclusions
Our results suggest that TIME-TQ activated these patients to quit smoking, but our referral method (standard fax referral) was unsuccessful in helping participants fully engage in quitline treatment or achieving a period of abstinence.
Scientific Significance
We are now conducting an RCT to evaluate TIME-TQ with a revised referral procedure intended to increase treatment engagement and, ultimately, abstinence rates.
Keywords: smoking cessation, tobacco cessation, substance use treatment, quitline
INTRODUCTION
The prevalence of cigarette smoking among individuals receiving treatment for non-nicotine substance use disorder (SUD) remains 70% or higher1, even as the smoking rate in the general population has declined to 16.8%2. Ultimately, smokers in SUD treatment are more likely to die from tobacco use than from their other substance use3. However, less than half of SUD treatment facilities offer pharmacotherapy or counseling for smoking cessation, and less than 15% provide both4. Therefore, the integration of effective smoking cessation interventions into SUD treatment programs is a public health priority5.
Barriers to Integrating Smoking Cessation Services in SUD Treatment
Belief that Quitting Smoking Will Jeopardize Sobriety
The majority of smokers in SUD treatment want to quit smoking eventually6. However, concern that quitting will jeopardize their sobriety7 may account for the finding that many are reluctant to quit immediately8 and may prefer to quit after achieving a period of abstinence from alcohol and drugs. In fact, the overwhelming majority of existing research supports a simultaneous treatment approach, suggesting that participating in smoking cessation treatment during SUD treatment does not increase the risk of alcohol or drug relapse and, on the contrary, may actually promote alcohol and drug abstinence9.
Lack of Staff Time and Resources
To date, randomized controlled trials (RCTs) for smoking cessation in SUD treatment have generally tested intensive, face-to-face interventions that require large amounts of time, counselor training, and resources to implement and would be difficult to disseminate to community-based SUD treatment9–11. Furthermore, quit rates have been low9, and they have only infrequently provided follow-up smoking intervention upon discharge from SUD treatment.
In the current study, we developed (Phase 1) and conducted a pilot test (Phase 2) of a brief, tablet computer-based intervention intended to motivate smokers in SUD treatment to use a no-cost, readily available, efficacious tobacco cessation treatment option in the community – free tobacco quitlines available in every U.S. state (1-800-QUIT-NOW)12,13. Our intervention, “Tablet Intervention to Motivate Engagement with Tobacco Quitlines” (TIME-TQ), was designed following known brief intervention best practices13, while also focusing on practicality to address barriers to integration of smoking cessation into SUD treatment.
The TIME-TQ Intervention
Motivational Interviewing
The TIME-TQ relies heavily on motivational interviewing (MI) principles14. Substantial evidence exists for the efficacy of MI in improving substance use outcomes15. However, with regard to tobacco use specifically, although MI is associated with increased quit attempts16 and self-efficacy17, meta-analyses indicate that as a standalone intervention MI has only a small effect on abstinence rates18,19. MI has also been evaluated as an approach for motivating engagement in formal substance use treatments. In this context, MI has demonstrated considerable promise20–22. Few previous studies have used MI to facilitate engagement in tobacco cessation interventions specifically, but results are promising23. Given that TIME-TQ was intended to facilitate engagement in tobacco quitline treatment, and is not a standalone tobacco cessation intervention in itself, we therefore selected MI to inform the content of TIME-TQ.
Use of a Tablet-Computer Based Intervention
Relative to face-to-face interventions, computer-based interventions afford distinct advantages24, including: (a) ready disseminability and appeal, even in populations with low literacy and limited computer experience; (b) flexibility, while preserving replicability and standardization; (c) privacy25; and (d) cost-effectiveness26,27.
Tobacco Quitlines
Tobacco quitlines are a major vehicle through which smoking cessation counseling services are delivered in the U.S. Quitline counseling has impressive odds ratios of 1.41 to 1.6 in meta-analyses12,13, which compare favorably to face-to-face counseling13. For example, a large study conducted at the California Smokers’ Helpline28 found that rates of continuous abstinence in the treatment group (20.7% at 1 month, 15.9% at 3 months, 11.7% at 6 months, 7.5% at 12 months) were double the rates in the control group (9.6%, 6.7%, 5.2%, 4.1%). Although to our knowledge no previous studies have evaluated the efficacy of quitlines specifically for SUD-treated smokers, over 60% of quitlines provide outreach to smokers with other SUDs29. In a recent study of 125,261 callers to the CA quitline, 12% said they had problems with alcohol or drug use30.
TIME-TQ Intervention
Software
The prototype TIME-TQ was created using the Computer Intervention Authoring Software (CIAS) platform, developed by Steven Ondersma, Ph.D. We deployed TIME-TQ on touch-screen tablet computers running Windows operating system that we believed would be optimal for delivering the intervention in our clinic setting. However, CIAS is compatible with all devices running Windows. CIAS features an animated narrator capable of over 50 specific motions and gestures (e.g., smile, point, etc.) that has received high feasibility and acceptability ratings31. Relevant graphics rotate on each screen, and video segments increase visual and auditory appeal. The animated narrator serves as a “guide” throughout the program and engages participants by addressing them by name, introducing each topic, asking pertinent questions, reflecting back selected responses, sharing information in a motivational style, and using occasional humor. CIAS interventions may be built in a modular fashion, whereby intervention components can be tailored to participant responses by strategically branching to specific components. CIAS interventions have produced positive outcomes in low-income post-partum women who used illicit drugs prior to pregnancy24,32 and in pregnant smokers31.
Prototype TIME-TQ
The prototype TIME-TQ consisted of a 30-minute intervention that aimed to motivate acceptance of a tobacco quitline referral. It wove together two evidence-based brief intervention approaches33: the 5 A’s (Ask, Advise, Assess, Assist, Arrange Follow-Up), a model for brief smoking intervention in health care settings, served as the basic framework for addressing tobacco use13, while the counseling style of MI34 engaged participants to explore their smoking in a safe and respectful manner, and was used more specifically to tailor responses. Participants were asked questions that followed the 5 A’s and MI (e.g., pros and cons of smoking), to which they could select multiple response choices. The narrator provided MI-consistent feedback that differed based on the participant’s readiness to quit (high vs. low) as indicated by their response(s). Gain-framed messages (i.e., focused on positive benefits of quitting rather than risks of smoking) served to reinforce confidence and desire for those who were ready to quit, and to motivate those who were not ready. The narrator also presented two graphs showing improved sobriety among smokers with SUDs who participated in tobacco cessation treatment or quit smoking. Additional messages depicted the cycle of nicotine dependence, illustrating that rather than relieving stress, smoking actually induces a state of ongoing nicotine withdrawal that is only relieved by smoking another cigarette.
Video clips appeared at two distinct points. The first video, depicting an actress portraying an addictions medicine physician, was shown to all participants and aimed to provide clear advice, while recognizing the participant may not be ready to quit. The second clip, which aimed to assist and was tailored to readiness to quit (high vs. low), included another message from the physician and also empowering testimonials from actors of varying gender and ethnicity who portrayed patients in SUD treatment who quit smoking with the help of the quitline. At the end of TIME-TQ, participants indicated by clicking “yes” or “no” whether they would like a referral to the quitline.
The Current Study
TIME-TQ has now been finalized and is being evaluated in an RCT. This manuscript describes the two-phase development process. In Phase 1, we conducted interviews and a brief survey with 19 patients in SUD treatment to learn about their smoking and preferences for intervention. This information was used to refine a prototype of TIME-TQ that we had already outlined. In Phase 2, we administered the prototype TIME-TQ to 16 patients in SUD treatment who had not participated in Phase 1 and revised TIME-TQ based on their feedback. Assessments were conducted before and after TIME-TQ, and at 1 and 3 months post-discharge from SUD treatment. We hypothesized that TIME-TQ would motivate and prepare smokers in SUD treatment to engage in the more active, skills-based quitline treatment. Primary outcomes included readiness to quit, proportion of participants who accepted the quitline referral and engaged in quitline counseling, and engagement in quit attempts. This study was approved by the Butler Hospital Institutional Review Board.
PHASE 1
METHOD
Participants
Participants (n = 19) were recruited during an admission to the alcohol and drug day treatment program (ADP) at Butler Hospital in Providence, RI. ADP provides treatment including counseling and medication management from 9:00 am-3:30 pm on weekdays, with a typical length of stay of 5 consecutive days and a typical daily census of about 15 patients. Patients are typically discharged from ADP with a referral to a less intensive level of care to continue their SUD treatment. All participants were between 18–70 years of age, smoked at least 10 cigarettes per day, had not used pharmacotherapy for smoking cessation or other tobacco products within the last 7 days, and had reliable access to a telephone.
Procedure
Recruitment
A research assistant screened the medical records of all patients admitted to ADP. Patients who appeared to meet study criteria were approached in person on day 2 or later of their ADP stay, given a brief explanation of the study, screened to confirm eligibility, and scheduled for an hour-long interview appointment if eligible and interested.
Interview
After providing written informed consent, patients completed a brief questionnaire assessing demographics, smoking history, nicotine dependence and readiness to quit (1–10 scale), and then completed a structured interview about their smoking and preferences for intervention. Interview topics were drawn from the content we planned to include in the prototype TIME-TQ, which had already been outlined and partially programmed in CIAS prior to conducting Phase 1. These topics included details about their smoking, pros and cons of smoking and quitting for themselves and for other smokers in SUD treatment, importance of quitting (i.e., 1–10 scale) and why they didn’t select a lower number (common MI-based technique), relationship of smoking to personal values (given a list of typical values such as relationships, health, etc. to generate discussion), barriers to quitting for themselves and other smokers in SUD treatment, awareness of and receptivity to using a tobacco quitline, telephone access, perceived impact of quitting on sobriety, and general preferences and suggestions for smoking intervention in SUD treatment. During the discussion of perceived impact of quitting on sobriety, the interviewer shared research findings and graphs indicating that quitting does not jeopardize, and may increase the likelihood of maintaining sobriety. At the end of the interview, participants were asked again whether they would accept a referral to the quitline if offered, and again rated their readiness to quit. During the discussion of suggestions for intervention, participants were also informed about the planned video components of the pending intervention, and asked specifically about preferred sources of information about smoking cessation while in treatment (e.g., from a physician or a patient in substance use treatment who had used the quitline).
RESULTS
Demographic and smoking characteristics are shown in Table 1. Regarding pros of smoking, an overwhelming majority endorsed negative reinforcement (e.g., relief of negative emotions, general mood management) and positive reinforecement (e.g.,. enjoyment and socialization with other smokers). Aspects of smoking that were cons or concerned participants were health and aesthetic concerns, expense, disapproval from others, second-hand smoke exposure of loved ones, and not wanting children or grandchildren to start smoking.
Table 1.
Demographic and Smoking Characteristics
| Phase 1 (N = 19) | Phase 2 (N = 16) | |
|---|---|---|
| Age | 32.6 (12.5) | 35.8 (8.5) |
| Gender | 63% Male | 50% Male |
| Cigarettes per day | 18.6 (13.8) | 16.3 (7.8) |
| FTND | 4.7 (2.4) | 5.6 (2.4) |
| Ready to quit within 30 days | 32% | 50%* |
| Readiness to quit (1–10) | 5.5 (1.3) | 3.5 (2.4)* |
Note. Means and standard deviations unless specified.
Significance difference between quitline acceptors and decliners, p < .05.
Participants indicated that quitting smoking was important (on a 1 to 10 scale, M = 7.50, SD = 2.32), and 58% reported that smoking did not fit in with their personal values. When asked about the perceived benefits of quitting, the most common responses pertained to improved physical health, including ability to be more physically active, not smelling like smoke, and saving money. Anticipated barriers to quitting included withdrawal symptoms, being around other people smoking, and being triggered to smoke in stressful situations, after meals, and while drinking coffee. Most participants (14/19, 74%) were not familiar with the local quitline. After being provided with a brief description of the quitline, 14/19 (74%) indicated that they would consider using the quitline if they decided they wanted to quit smoking and 8/19 (42%) said they would be interested in being connected with the quitline upon discharge from ADP. Participants were roughly equally divided as to whether they believed that quitting smoking would jeopardize or promote sobriety from alcohol and drugs. After being presented with graphs showing that quitting smoking does not jeopardize sobriety, the proportion of participants interested in being connected to the quitline upon discharge from ADP increased from 42% to 53% (10/19). Participants reported preferring to receive information about the quitline from both patients and a physician; they preferred a female doctor or had no gender preference, and a doctor wearing a white lab coat or had no clothing preference.
This feedback was used primarily to develop the response choice lists for the questions in TIME-TQ. Also, in the video segments we included an actress portraying an addictions medicine physician wearing a white lab coat who shares information about the effectiveness and availability of the local quitline, and actors of both genders and diverse ethnicities portraying patients describing their experiences with using the quitline.
PHASE 2
METHOD
Participants
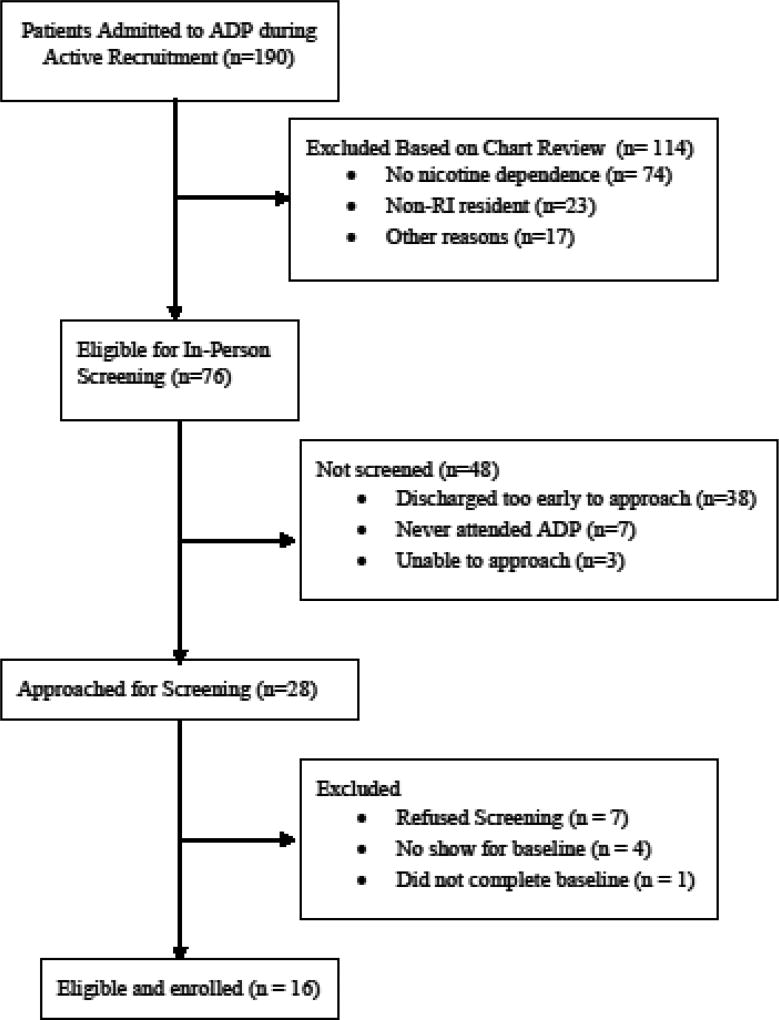
Phase 2 participants (n = 16) were also recruited during admission to the Butler Hospital ADP program with the same procedures and inclusion and exclusion criteria as in Phase 1. Phase 2 enrollment statistics are shown in Figure 1.
Figure 1.
Procedure
Phase 2 participants attended two, one-hour-long appointments during their ADP stay, on consecutive days whenever possible. During the first appointment, participants provided informed consent and completed a baseline assessment. At the second appointment, participants viewed the TIME-TQ program. A study clinician completed a fax referral form for participants who accepted a referral to the quitline at the end of TIME-TQ. The referral was then faxed to the quitline immediately. Any health care provider may use the quitline’s fax referral form to refer patients. The quitline makes up to 3 attempts to call all referred individuals to initiate the quitline’s program, with the first call attempt occurring within 3 business days of receiving the individual’s referral. Therefore, it would be unlikely for study participants to initiate quitline counseling before ADP discharge given the 5-day average length of stay. However, most would still be receiving SUD treatment at a lower level of care and therefore we consider TIME-TQ to be consistent with a simultaneous approach to treatment of tobacco dependence and other SUD(s). All participants, regardless of whether they accepted a referral, received printed materials describing how to access smoking cessation resources. After completing the fax referral form (if applicable) and receiving the resources, the clinician interviewed the participant to obtain feedback on TIME-TQ. Participants returned for follow-up appointments at 1 and 3-months post-discharge from ADP.
Phase 2 participants were recruited in two cohorts. The initial prototype TIME-TQ developed after Phase 1 was revised after the first cohort of 10 participants in Phase 2 provided feedback. This revised TIME-TQ was then administered to a second cohort of 6 additional participants who then provided additional feedback.
Measures
Smoking History
At baseline, participants described their smoking history including current smoking pattern, nicotine dependence (The Fagerström Test for Nicotine Dependence, FTND)35, and quit attempt history.
Feasibility and Acceptability of the TIME-TQ
Participants rated the acceptability of TIME-TQ on 5-point scales (1 = not at all to 5 = very much), including how relevant, interesting, respectful, and helpful it was to them, and how much they liked using the tablet computer.
State Motivation and Readiness to Quit
Participants reported on their readiness to quit smoking within the next 30 days on a 1–10 scale during the baseline assessment and at two points during TIME-TQ (embedded in the intervention). Additionally, just prior to and immediately following TIME-TQ, state motivation was assessed using 100-point visual analogue scale (VAS) items31, including likelihood of quitting smoking within 30 days, importance of and confidence in quitting, and concern about quitting smoking jeopardizing sobriety.
Smoking Outcomes
At follow-ups, participants reported on attempts to quit or cut down on smoking since the previous assessment. Participants also reported their tobacco use (cigarettes and other tobacco products) for each day since the previous assessment (i.e., since their baseline at 1 month and since their 1-month follow-up at 3 months) using the Timeline Followback calendar method36.
RESULTS
Participant Characteristics
Demographics and baseline smoking characteristics are shown in Table 1. Half of participants (4/10 from the first cohort and 4/6 from the second) accepted the quitline referral. At baseline, participants who went on to accept the quitline referral were more likely to endorse readiness to quit within 30 days (75% vs. 25%, (χ2) = 4.00, p =.046) and also reported higher readiness to quit on a 1–10 scale (M = 4.75, SD = 2.71 vs. M = 2.25, SD = 1.39, t (14) = −2.32, p = .04). Retention was 13/16 at 1 month and 9/16 at 3 months.
Revisions to TIME-TQ After Cohort 1
While Phase 1 participant feedback helped to generate the content and specific response choices to include in the prototype TIME-TQ, Phase 2 pilot feedback helped to guide the refinement of content and message delivery style. Overall, Phase 2 participants provided positive feedback. Two minor modifications were made after the first cohort and included in the revised TIME-TQ administered to the second cohort. First, we streamlined the spoken content and removed some “humor” elements that were disliked. Second, we added the response choice “Premature aging (yellow teeth, nails, wrinkles)” to an item that asked about “cons” of smoking
An additional modification for cohort 2 was made to the smoking cessation resources provided, based on clinical intuition. All cohort 1 participants, regardless of whether they accepted the quitline referral, received a quitline brochure, a self-help guide published by the National Cancer Institute (Clearing the Air), and a list of local smoking cessation programs. However, by the end of cohort 1 we became concerned that the additional resources might diminish or detract from engagement in the quitline services. Therefore, in cohort 2 we provided only the quitline brochure to those who accepted a quitline referral. We continued providing the full complement of resources to participants who declined the referral.
Debriefing interviews with this second cohort indicated that no additional pilot testing was needed and we were ready to begin the RCT. We have grouped together both cohorts for other results described below.
Feasibility and Acceptability of TIME-TQ
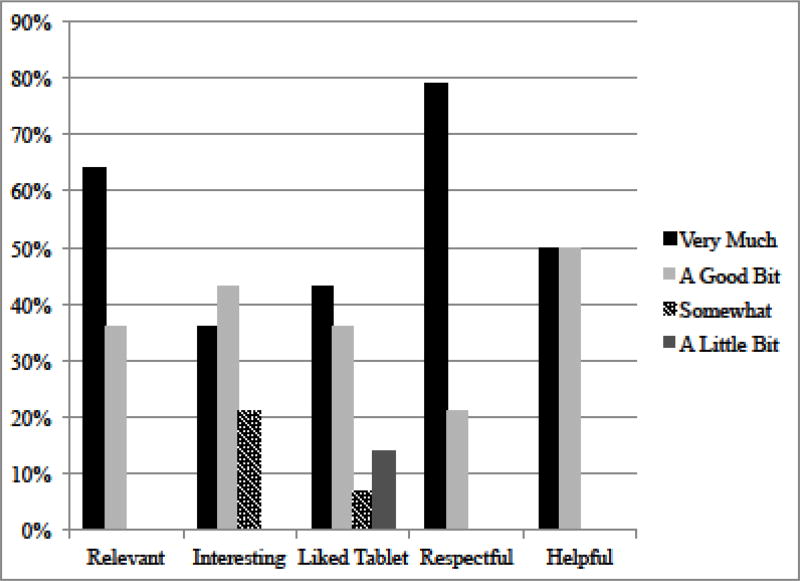
TIME-TQ was rated highly by participants, with most rating it as a 5 (“very much”) or a 4 (“a good bit”) on relevance, interest, how much they liked the tablet, respectfulness, and helpfulness (see Figure 2).
Figure 2.
Smoking Outcomes
State Motivation and Readiness to Quit
Paired samples t-tests (pre- vs. post-TIME-TQ) were conducted for quitline referral acceptors and decliners separately. Results indicated that both acceptors and decliners significantly increased their overall readiness to quit and perceived importance of quitting from pre- to post-TIME-TQ (ps < .05 for both of these pre-post comparisons). However, only acceptors reported increased likelihood of quitting within 30 days following TIME-TQ (p = .01). See Table 2.
Table 2.
Changes in State Motivation and Readiness to Quit (Phase 2)
| Accepted Quitline (n = 8) | Declined Quitline (n = 8) | |||
|---|---|---|---|---|
|
| ||||
| Before TIME-TQ |
After TIME-TQ |
Before TIME-TQ |
After TIME-TQ |
|
| Readiness to quit (1–10) | 4.7 (2.7) | 6.5 (2.5)* | 2.3 (1.4) | 3.9 (1.6)* |
| Smoking is a problem | 84.4 (15.3) | 87.0 (13.5) | 73.8 (21.8) | 68.1 (19.3) |
| Likelihood of quitting within 30 Days | 44.5 (24.3) | 68.8 (24.5)* | 30.5 (26.0) | 34.6 (29.3) |
| Confidence in quitting | 53.2 (28.9) | 65.0 (27.4) | 33.8 (25.4) | 36.5 (29.0) |
| Importance of quitting | 86.9 (16.4) | 95.5 (11.2)* | 58.4 (22.2) | 75.9 (27.8)* |
| Degree to which quitting would jeopardize sobriety | 52.0 (33.2) | 51.0 (38.2) | 36.6 (29.0) | 55.0 (21.8) |
Note.
= significant increase from before to after TIME-TQ, p < .05
Use of Quitline Services
Three (n = 3) of the 8 acceptors enrolled in quitline counseling and received at least 1 session; 3 could not be reached by the quitline, and 2 were reached but did not receive counseling (1 declined, 1 did not complete the enrollment process).
Use of Other Smoking Cessation Treatments
Eight of 13 participants who completed a follow-up assessment reported engaging in at least one effort to quit or cut down on smoking between the baseline and their last follow-up, including 4 of 6 quitline referral acceptors and 4 of 7 decliners. Methods used included cold turkey (3), nicotine patch (2), nicotine gum (1), nicotine lozenge (2), electronic cigarettes (1), print materials (1), individual counseling (2), and nicotine inhaler (1).
Abstinence and Reduction
No participants reported 7-day point prevalence abstinence at any follow-up. However, at the 1-month follow-up, 2 participants (both acceptors) reported reductions in smoking of at least 50% from their baseline level; at 3-months one had maintained this reduction and the other had reduced by another 50% (reporting <2 cigs/day). Also, at the 3–month follow-up, 2 additional participants (both decliners) had reduced their smoking by at least 50% from baseline.
DISCUSSION
In this research, we developed a brief tablet computer-based intervention to motivate engagement with tobacco quitlines (TIME-TQ) among smokers attending a day treatment program for non-nicotine substance use disorder (SUD) (Phase 1) and conducted a preliminary pilot test of TIME-TQ that established its feasibility and acceptability (Phase 2). Phase 2 participants found TIME-TQ relevant, interesting, respectful, and helpful. Furthermore, their overall readiness to quit and their perceived importance of quitting increased significantly after receiving TIME-TQ, and half (n = 8) accepted a referral to the quitline after completing TIME-TQ. At follow-ups, a majority of Phase 2 participants reported that they had engaged in efforts to reduce or quit smoking since receiving TIME-TQ. Despite the high rate of quitline referral acceptance (50%), only 3 of the 8 acceptors actually enrolled in the quitline’s program and received at least one counseling session, few reported smoking reductions, and none reported 7-day abstinence.
For the RCT, we implemented several procedural changes intended to increase the likelihood that referral acceptors will enroll in and receive quitline counseling, and will achieve a period of abstinence. First, we changed the referral procedure from the standard fax mechanism to a “warm transfer” in which the study clinician calls the quitline immediately after the participant chooses to accept the referral. The clinician informs the quitline that there is a patient seeking counseling, and hands the phone to the participant to complete the initial intake and schedule the first counseling session. Second, we decided to offer medically eligible participants who schedule their first quitline counseling session two weeks of nicotine patches at no cost. We originally intended for participants to receive free patches from the local quitline, which was offering them at the time we proposed the study. However, during follow-up appointments, we learned that the free patches promotion had been discontinued.
Results of this study challenge a prevailing assumption that smokers receiving SUD treatment are neither interested in nor ready to quit smoking. Our findings suggest that when these smokers are provided with evidence-based information to reduce primary barriers to quitting and offered the opportunity to engage in smoking cessation treatment with a quitline, a large proportion will become activated to quit smoking. More specifically, they will report high motivation to quit and receptivity to use of the quitline, and will engage in efforts to reduce and quit smoking. At the same time, our results suggest that TIME-TQ combined with a fax referral to the quitline was not a powerful enough intervention to help these patients fully engage in the quitline treatment or achieve a period of abstinence. We are hopeful that the referral procedure changes we implemented in the RCT will result in increased quitline enrollment and abstinence rates.
Limitations of this pilot study include: (1) there was no control condition; therefore, it is unknown how many participants would have accepted the quitline referral in the absence of TIME-TQ and (2),although we tried to recruit all smokers in the ADP program, it is possible that participants were more motivated to quit than those who refused.
Conclusions
In conclusion, we established the feasibility and acceptability of a brief tablet computer-based intervention to motivate patients in SUD treatment to accept a referral to a tobacco quitline. Ongoing research is evaluating this intervention with a revised referral procedure intended to increase treatment engagement and, ultimately, abstinence rates.
Acknowledgments
Funding: This work was supported by the National Institute on Drug Abuse, grant R34DA034312 to Richard A. Brown, Ph.D.
The authors thank the staff of the Addictions Research Group and the Alcohol and Drug Day Treatment Program at Butler Hospital who assisted with this research.
DECLARATION OF INTEREST
Dr. Ondersma is part owner of Interva, Inc., the company that markets the intervention authoring tool used to develop the TIME-TQ used in this study. Interva had no role in study design, data analysis, or write-up of results.
References
- 1.Guydish J, Passalacqua E, Tajima B, Chan M, Chun J, Bostrom A. Smoking prevalence in addiction treatment: a review. Nicotine Tob Res. 2011;13(6):401–411. doi: 10.1093/ntr/ntr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. Current Cigarette Smoking Among Adults - United States, 2005–2014. Morb Mortal Wkly Rep. 2015;64(44):1233–1240. doi: 10.15585/mmwr.mm6444a2. [DOI] [PubMed] [Google Scholar]
- 3.Hurt RD, Offord KP, Croghan IT, et al. Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. J Am Med Assoc. 1996;275(14):1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- 4.Knudsen HK, Studts JL, Boyd S, Roman PM. Structural and cultural barriers to the adoption of smoking cessation services in addiction treatment organizations. J Addict Dis. 2010;29(3):294–305. doi: 10.1080/10550887.2010.489446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Substance Abuse and Mental Health Services Administration. The NSDUH Report: Nicotine Dependence among Persons Who Received Substance Use Treatment. 2011 [Google Scholar]
- 6.Moore D, Langlois M, Gerber BM, Gaddis R, Hallam JS, Arnold R. Intention to quit tobacco use among clients in substance use disorder treatment settings. Subst Use Misuse. 2007;42(5):871–879. doi: 10.1080/10826080701202528. [DOI] [PubMed] [Google Scholar]
- 7.Ziedonis D, Guydish J, Williams J, Steinberg MB, Foulds J. Barriers and solutions to addressing tobacco dependence in addiction treatment programs. Alcohol Res Health. 2006;29(3):228–235. [PMC free article] [PubMed] [Google Scholar]
- 8.Ellingstad TP, Sobell LC, Sobell MB, Cleland PA, Agrawal S. Alcohol abusers who want to quit smoking: implications for clinical treatment. Drug Alcohol Depend. 1999;54(3):259–265. doi: 10.1016/s0376-8716(98)00180-x. [DOI] [PubMed] [Google Scholar]
- 9.Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. J Consult Clin Psychol. 2004;72(6):1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- 10.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thurgood SL, McNeill A, Clark-Carter D, Brose LS. A Systematic Review of Smoking Cessation Interventions for Adults in Substance Abuse Treatment or Recovery. Nicotine Tob Res. 2016;18(5):993–1001. doi: 10.1093/ntr/ntv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stead LF, Perera R, Lancaster T. A systematic review of interventions for smokers who contact quitlines. Tob Control. 2007;16(Suppl 1):i3–8. doi: 10.1136/tc.2006.019737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiore MC, Jaen CR, Baker TB, et al. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. Treating Tobacco Use and Dependence: 2008 Update. [Google Scholar]
- 14.Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. 2. New York: The Guilford Press; 2002. [Google Scholar]
- 15.Lundahl BW, Kunz C, Brownell C, Tollefson D, Burke BL. A meta-analysis of Motivational Interviewing: twenty-five years of empirical studies. Res Social Work Practice. 2010;20(2):137–160. [Google Scholar]
- 16.Borrelli B, Novak S, Hecht J, Emmons K, Papandonatos G, Abrams D. Home health care nurses as a new channel for smoking cessation treatment: outcomes from project CARES (Community-nurse Assisted Research and Education on Smoking) Prev Med. 2005;41(5–6):815–821. doi: 10.1016/j.ypmed.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Brown RA, Ramsey SE, Strong DR, et al. Effects of motivational interviewing on smoking cessation in adolescents with psychiatric disorders. Tob Control. 2003;12(Suppl 4):IV3–IV10. doi: 10.1136/tc.12.suppl_4.iv3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hettema JE, Hendricks PS. Motivational interviewing for smoking cessation: a meta-analytic review. J Consult Clin Psychol. 2010;78(6):868–884. doi: 10.1037/a0021498. [DOI] [PubMed] [Google Scholar]
- 19.Heckman CJ, Egleston BL, Hofmann MT. Efficacy of motivational interviewing for smoking cessation: a systematic review and meta-analysis. Tob Control. 2010;19(5):410–416. doi: 10.1136/tc.2009.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carroll KM, Libby B, Sheehan J, Hyland N. Motivational interviewing to enhance treatment initiation in substance abusers: an effectiveness study. Am J Addict. 2001;10(4):335–339. doi: 10.1080/aja.10.4.335.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connors GJ, Walitzer KS, Dermen KH. Preparing clients for alcoholism treatment: effects on treatment participation and outcomes. J Consult Clin Psychol. 2002;70(5):1161–1169. doi: 10.1037//0022-006x.70.5.1161. [DOI] [PubMed] [Google Scholar]
- 22.Martino S, Carroll KM, O'Malley SS, Rounsaville BJ. Motivational interviewing with psychiatrically ill substance abusing patients. Am J Addict. 2000;9(1):88–91. doi: 10.1080/10550490050172263. [DOI] [PubMed] [Google Scholar]
- 23.Steinberg ML, Ziedonis DM, Krejci JA, Brandon TH. Motivational interviewing with personalized feedback: a brief intervention for motivating smokers with schizophrenia to seek treatment for tobacco dependence. J Consult Clin Psychol. 2004;72(4):723–728. doi: 10.1037/0022-006X.72.4.723. [DOI] [PubMed] [Google Scholar]
- 24.Ondersma SJ, Chase SK, Svikis DS, Schuster CR. Computer-based brief motivational intervention for perinatal drug use. J Subst Abuse Treat. 2005;28(4):305–312. doi: 10.1016/j.jsat.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman MG, Szkodny LE, Llera SJ, Przeworski A. A review of technology-assisted self-help and minimal contact therapies for drug and alcohol abuse and smoking addiction: is human contact necessary for therapeutic efficacy? Clin Psychol Rev. 2011;31(1):178–186. doi: 10.1016/j.cpr.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Olmstead TA, Ostrow CD, Carroll KM. Cost-effectiveness of computer assisted training in cognitive-behavioral threapy as an adjunct to standard care for addiction. Drug Alcohol Depend. 2010;110:200–207. doi: 10.1016/j.drugalcdep.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCrone P, Knapp M, Proudfoot J, et al. Cost-effectiveness of computerised cognitive-behavioural therapy for anxiety and depression in primary care: randomised controlled trial. Br J Psychiatry. 2004;185:55–62. doi: 10.1192/bjp.185.1.55. [DOI] [PubMed] [Google Scholar]
- 28.Zhu SH, Anderson CM, Tedeschi GJ, et al. Evidence of real-world effectiveness of a telephone quitline for smokers. N Engl J Med. 2002;347(14):1087–1093. doi: 10.1056/NEJMsa020660. [DOI] [PubMed] [Google Scholar]
- 29.North American Quitline Consortium. Results from the 2015 NAQC Annual Survey of Quitlines. 2015 http://naquitline.org/?page=2015Survey.
- 30.Tedeschi GJ, Cummins SE, Anderson CM, Anthenelli RM, Zhuang YL, Zhu SH. Smokers with Self-Reported Mental Health Conditions: A Case for Screening in the Context of Tobacco Cessation Services. PLoS One. 2016;11(7):e0159127. doi: 10.1371/journal.pone.0159127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ondersma SJ, Svikis DS, Lam PK, Connors-Burge VS, Ledgerwood DM, Hopper JA. A randomized trial of computer-delivered brief intervention and low-intensity contingency management for smoking during pregnancy. Nicotine Tob Res. 2012;14(3):351–360. doi: 10.1093/ntr/ntr221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ondersma SJ, Svikis DS, Schuster CR. Computer-based brief intervention a randomized trial with postpartum women. Am J Prev Med. 2007;32(3):231–238. doi: 10.1016/j.amepre.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiore MC, Baker TB. Treating smokers in the health care setting. N Engl J Med. 2011;365:1222–1231. doi: 10.1056/NEJMcp1101512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai DT, Cahill K, Qin Y, Tang JL. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev. 2010;(1):CD006936. doi: 10.1002/14651858.CD006936.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 36.Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998;12(2):101–112. [Google Scholar]