Abstract
Background
The apnea-hypopnea index (AHI) and the mean apnea-hypopnea duration (MAD) are used to measure the severity of the symptoms of obstructive sleep apnea (OSA). The aim of this study was to compare the use of the MAD with the AHI as indicators of clinical and demographic parameters, blood oxygenation, and sleep parameters in patients diagnosed with OSA by polysomnography (PSG).
Material/Methods
A retrospective study included 511 patients with OSA diagnosed by PSG and who had the AHI and the MAD measured according to the guidelines from the American Academy of Sleep Medicine (AASM). The patients were divided into two groups: patients with a short MAD and with a long MAD, according to median duration, and using the inter-quartile range (IQR), as the data were not normally distributed. Clinical and demographic parameters were recorded. Pulse oximetry was used to measure blood oxygen saturation during sleep, sleep structure was recorded, and the Epworth Sleepiness Scale (ESS) questionnaire was used to measure daytime sleepiness.
Results
In all 511 patients with OSA, the MAD was significantly, but weakly, correlated with the AHI (r=0.17, P<0.01), but showed no significant associations with patient age (r=0.08, P=0.06), body weight (r=0.014, P=0.75), and height (r=0.06, P=0.16). Patients with a long MAD or severe OSA (n=260) had significantly worse blood oxygen levels and sleep parameters.
Conclusions
For patients with severe OSA, this study showed that the MAD was a useful indicator of blood oxygenation and sleep parameters.
MeSH Keywords: Anoxia; Sleep Apnea, Obstructive; Sleep Stages
Background
Obstructive sleep apnea (OSA) is a condition that is reported to affect between 2–4% of adults in the United States and China [1,2]. OSA is associated with a reduced quality-of-life, daytime hypersomnolence, increased frequency of accidents, cardiovascular complications, and reduced life expectancy mortality [3,4].
Polysomnography (PSG) is the ‘gold standard’ for the diagnosis of OSA, and the apnea-hypopnea index (AHI) has been used as the main parameter to stratify the severity of the disease. The AHI is derived from sleep apnea and hypopnea events detected within one hour during sleep. The duration of apnea in patients with OSA can vary widely, with periods of apnea or hypopnea lasting from between ten seconds to more than one minute. In some patients, although the AHI can be high, the duration of apnea or hypopnea may be short, and the AHI level may not reflect the degree of symptoms in patients with OSA, which include lethargy and mental sluggishness during the day. The main limitation of the use of the AHI is that it might not reflect the severity of the disturbance of breathing events during sleep in patients with OSA. Therefore, additional parameters are necessary that more accurately reflect demographic parameters, blood oxygenation, and sleep parameters in patients with OSA.
The mean apnea-hypopnea duration (MAD) is a parameter that incorporates the severity of breathing events during sleep. Although the use of the MAD has been previously studied in the pathophysiology of OSA, the relationship between the clinical utility of the MAD compared with the AHI, and with other sleep apnea risk factors and patient demographics remain unclear [5,6].
Therefore, the aim of this study was to compare the use of the MAD with the AHI as indicators of clinical and demographic parameters, blood oxygenation, and sleep parameters in patients diagnosed with OSA by polysomnography (PSG).
Material and Methods
Patients and study design
A retrospective clinical study included patients who were >18 years-of-age and who had a diagnosis of obstructive sleep apnea (OSA) by polysomnography (PSG) between 2012 to 2017 in the Sleep Center, Beijing Anzhen Hospital. The diagnosis of OSA by PSG was supported with the use of he apnea-hypopnea index (AHI) of ≥5 events per hour), as recommended by the diagnostic criteria of the American Academy of Sleep Medicine (AASM) [7]. Demographic and clinical information was extracted from the patient medical records. The Ethics Committee of the Anzhen Hospital approved the study protocol, and informed consent was obtained from all subjects who participated in the study.
Polysomnography (PSG) and sleep evaluation
The procedure of PSG in the sleep laboratory included continuous electroencephalographic (EEG) polygraphic recording using EEG leads, the use of right and left electro-oculographic leads, and chin electromyography for sleep staging. Electrocardiography (ECG monitoring during sleep, airflow measurement at the nose and mouth, and chest and abdominal respiratory movements were measured during sleep. Arterial oxygen saturation (SaO2) was measured with pulse oximetry. Episodes and duration of snoring were recorded, and body positioning was recorded during sleep.
All sleep studies were interpreted according to the manual of the AASM for the Scoring of Sleep and Associated Events, by certified sleep physicians in China [7]. Sleep-stage scoring was done during 30-second intervals, by trained technicians, according to standard criteria. Apnea was identified when the airflow amplitude in the nasal cannula was <10% of baseline and when no flow occurred on the oral airflow sensor (thermistor). Hypopnea was identified when the amplitude of the airflow was reduced by 30% from the baseline, and the event was followed by 4% O2 desaturation. The AHI was defined as the total number of apnea events and hypopnea events per hour of EEG-monitored sleep. The Epworth Sleepiness Scale (ESS) questionnaire was used to evaluate daytime sleepiness.
Statistical analysis
Data were expressed as the mean ± standard deviation (SD) or the median (interquartile range). The correlations between the MAD and patient demographic parameters were analyzed, and the patients were divided into two groups, a short MAD group and a long MAD group, according to the median value of the MAD. Blood oxygen parameters, sleep structure, and the ESS score were compared between the two MAD groups using an independent t-test or the Wilcoxon rank sum test. The effect of the MAD was further explored by adjusting for the AHI. Correlations were tested using Pearson’s correlation test. Data were analyzed using SPSS version 20.0 software (SPSS Inc., Chicago, IL, USA). A P-value of P≤0.05 was considered as statistically significant.
Results
Baseline demographics
This retrospective study included 511 patients with obstructive sleep apnea (OSA) diagnosed by polysomnography (PSG). The patient ages ranged from 18–75 years and included 421 men and 90 women. Patient demographic and clinical parameters are summarized in Table 1.
Table 1.
Baseline characteristics of the study population (mean ±SD).
| Characteristics (n=511) | Values |
|---|---|
| Male | 421 |
| Female | 90 |
| Age, years | 49.3±10.9 |
| BMI, kg/m2 | 27.5±6.31 |
| Neck circumference, cm | 43.0±4.41 |
| Waist circumference, cm | 98.6±6.52 |
| Hip circumference, cm | 96.7±7.12 |
| Waist/hip ratio | 1.03±0.15 |
| Systolic BP, mm Hg | 128.1±12.07 |
| Diastolic BP, mm Hg | 79.2±13.71 |
| ESS score | 10.3±4.97 |
BMI – body mass index; BP – blood pressure; ESS – Epworth Sleepiness Scale.
Characteristics of the mean apnea-hypopnea duration (MAD)
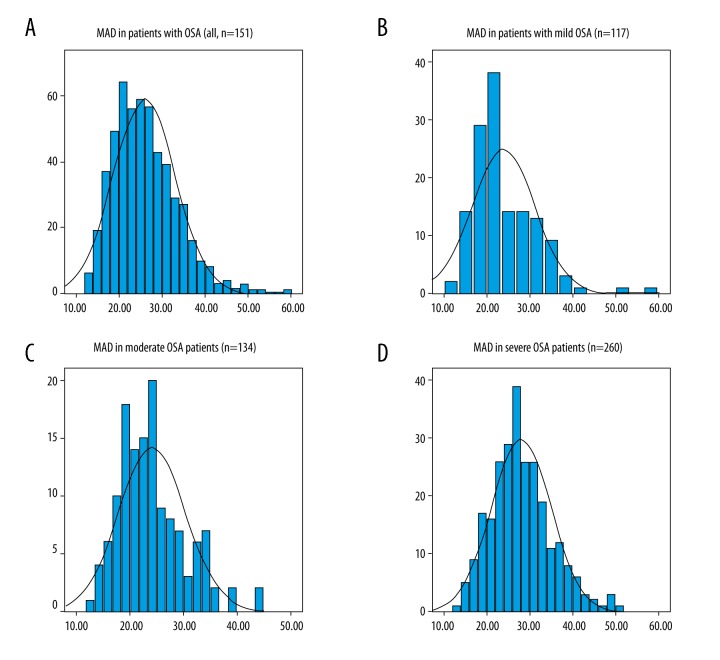
The mean apnea-hypopnea duration (MAD) results showed a non-normal distribution. The overall MAD for all patients in the study (n=511) was 25 seconds (interquartile range, 20.4–30.4 seconds) (Figure 1A). The MAD in patients with mild OSA (n=117) was 21.5 seconds (interquartile range, 18.2–28.7 seconds) (Figure 1B). The MAD in patients with moderate OSA (n=134) was 24.0±6.3 seconds (Figure 1C). The MAD in patients with severe OSA (n=260) was 27.9±7.0 seconds (Figure 1D). The MAD was significantly, but mildly, correlated with the AHI (r=0.17, P<0.01). The MAD results showed no significant associations with patient age (r=0.08, P=0.06), body weight (r=0.014, P=0.75), and height (r=0.06, P=0.16).
Figure 1.
Characteristics of the mean apnea-hypopnea duration (MAD) in patients with obstructive sleep apnea (OSA). (A) The mean apnea-hypopnea duration (MAD) in all patients included in the study who had a diagnosis of obstructive sleep apnea (OSA) (n=511). (B) The MAD in patients with mild OSA (n=117). (C) The MAD in patients with moderate OSA (n=134). (D) The MAD in patients with severe OSA (n=260).
Patients with OSA in the short MAD group and the long MAD group and blood oxygen and sleep parameters
Patients with OSA were divided into two groups, with a short MAD of <25 seconds, and with a long MAD of >25 seconds. The groups were defined by the finding that the median value for the MAD in the study population was 25 seconds. The differences in the two patients groups in the parameters of blood oxygen levels, sleep structure, and the Epworth Sleepiness Scale (ESS) were compared.
There were no significant differences between the two MAD groups in patient age, weight, and height. However, the blood oxygen parameters (average oxygen saturation, lowest oxygen saturation, and mean oxygen desaturation) of the long MAD group were significantly reduced when compared with those in the short MAD group. Also, in the long MAD group, the sleep latency was significantly shorter. Duration and proportion of Stage N3 or non-rapid eye movement (NREM) 3 sleep were significantly decreased. The duration and proportion of Stage N1 sleep, the arousal index, and oxygen desaturation index (ODI) were significantly increased in the long MAD group (all, P<0.05) (Table 2).
Table 2.
The differences between short MAD group and long MAD group.
| Parameters | Long MAD (n=256) | Short MAD (n=255) | P value |
|---|---|---|---|
| AHI, no/h | 43.1±24.8 | 31.7±24.7 | <0.01* |
| MAD, sec | 31.7±5.5 | 20.5±3.0 | <0.01* |
| AI, no/h | 36.5±23.7 | 22.4±17.0 | <0.01* |
| ODI, no/h | 41.4±26.5 | 30.7±23.6 | <0.01* |
| ESS | 11.6±5.2 | 9.9±4.9 | <0.01* |
| Sleep efficiency, % | 81.6±10.9 | 78.8±11.3 | <0.01* |
| sleep latency, min | 15.9±17.8 | 23.1±25.6 | <0.01* |
| N1 duration, min | 55.7±49.6 | 48.7±47.7 | 0.10 |
| N1, % of TST | 14.5±13.1 | 12.6±10.7 | 0.07 |
| N2 duration, min | 234.0±65.0 | 214.2±57.0 | <0.01* |
| N2, % of TST | 58.4±12.6 | 56.3±15.6 | 0.09 |
| N3 duration, min | 39.4±34.5 | 57.1±64.0 | <0.01* |
| N3, % of TST | 9.8±8.5 | 14.3±9.0 | <0.01* |
| R duration, min | 69.1±28.5 | 68.5±29.7 | 0.82 |
| R, % of TST | 17.1±6.3 | 17.7±7.4 | 0.32 |
| AOS, % | 89.1±5.9 | 92.1±3.8 | <0.01* |
| LOS, % | 72.3±12.3 | 78.9±9.0 | <0.01* |
| MOD, % | 9.7±4.9 | 6.9±2.7 | <0.01* |
AHI – apnea-hypopnea index; AI – arousal index; AOS – average oxygen saturation; ESS – Epworth Sleepiness Scale; LOS – lowest oxygen saturation; MAD – mean apnea/hypopnea duration; MOD – mean oxygen desaturation; ODI – oxygen desaturation index; TST – total sleep time.
Means significant difference between short MAD group and long MAD group, P<0.05.
Patients with OSA in the short MAD group and the long MAD group and the AHI
The AHI results were different in the patients with OSA and a short MAD of <25 seconds, and with a long MAD of >25 seconds. Therefore, the impact of AHI was excluded in the following analysis to determine whether the MAD was related to blood oxygen and sleep parameters. After adjusting for AHI, the MAD correlated with ESS, sleep latency, Stage N1 sleep duration, Stage N1 sleep component (%), Stage N3 sleep duration, Stage N3 sleep component (%), the arousal index, the average oxygen saturation (AOS), the lowest oxygen saturation (LOS), and the mean oxygen desaturation (MOD) (Table 3).
Table 3.
The relationship between MAD and other PSG parameters.
| Parameters | R | P value | Parameters | R | P value |
|---|---|---|---|---|---|
| AI | 0.252 | <0.01* | N2, % of TST | −0.003 | 0.952 |
| ODI | 0.064 | 0.151 | N3 duration | −0.173 | <0.01* |
| ESS | 0.09 | 0.04* | N3, % of TST | −0.225 | <0.01* |
| Sleep efficiency | 0.036 | 0.42 | R duration | 0.023 | 0.612 |
| sleep latency | −0.105 | 0.02* | R, % of TST | 0.01 | 0.821 |
| R latency | −0.062 | 0.17 | AOS | −0.19 | <0.01* |
| N1 duration | 0.131 | <0.01* | LOS | −0.242 | <0.01* |
| N1, % of TST | 0.144 | <0.01* | MOD | 0.273 | <0.01* |
| N2 duration | 0.016 | 0.713 |
AI – arousal index; AOS – average oxygen saturation; ESS – Epworth Sleepiness Scale; LOS – lowest oxygen saturation; MOD – mean oxygen desaturation; ODI – oxygen desaturation index, TST – total sleep time. AHI adjusted and * means P<0.05.
In the overall study population, patients in the mild to moderate OSA group (n=251) and patients in the severe OSA group (n=260) did have significant associations with sleep pattern. In the mild to moderate OSA group, the MAD was only significantly correlated with the duration of Stage N3 or non-rapid eye movement (NREM) 3 sleep (r=−0.14, P=0.02) and with the proportion (%) of Stage N3 sleep (r=−0.16, P=0.01). Table 4 shows the associations between the MAD and the ESS, the duration of Stage N1 sleep, the proportion (%) of Stage N1 sleep, the duration of Stage N3 sleep, the proportion (%) of Stage N3 sleep, the arousal index (AI), oxygen desaturation index (ODI), AOS, LOS, MOD, in the group of patients with severe OSA (n=260), with increased R-values when compared with the whole study group (n=511).
Table 4.
The relationship between MAD and other PSG parameters in Mild-moderate patients group and Severe OSA patients group.
| MAD | ||||
|---|---|---|---|---|
| Mild-moderate patients (n=251) | Severe OSA patients (n=260) | |||
| R | P value | R | P value | |
| AI | 0.10 | 0.10 | 0.40 | <0.01* |
| ODI | −0.10 | 0.10 | 0.16 | 0.01* |
| ESS | 0.07 | 0.28 | 0.17 | <0.01* |
| N1 duration | 0.01 | 0.90 | 0.21 | <0.01* |
| N1, % of TST | 0.03 | 0.63 | 0.19 | <0.01* |
| N2 duration | 0.06 | 0.35 | 0.002 | 0.97 |
| N2, % of TST | 0.06 | 0.38 | 0.05 | 0.40 |
| N3 duration | −0.14 | 0.02* | −0.31 | <0.01* |
| N3, % of TST | −0.16 | 0.01* | −0.37 | <0.01* |
| R duration | 0.03 | 0.68 | 0.004 | 0.504 |
| R, % of TST | 0.04 | 0.55 | 0.005 | 0.94 |
| AOS | −0.09 | 0.88 | −0.36 | <0.01* |
| LOS | −0.09 | 0.17 | −0.34 | <0.01* |
| MOD | 0.08 | 0.24 | 0.43 | <0.01* |
AI – arousal index; AOS – average oxygen saturation; ESS – Epworth Sleepiness Scale; LOS – lowest oxygen saturation; MOD – mean oxygen desaturation; ODI – oxygen desaturation index; TST – total sleep time. AHI adjusted and * means P<0.05.
Discussion
The aim of this study was to compare the use of the mean apnea-hypopnea duration (MAD) with the apnea-hypopnea index (AHI) as indicators of clinical and demographic parameters, blood oxygenation, and sleep parameters in patients diagnosed with obstructive sleep apnea (OSA) by polysomnography (PSG). The findings of this study showed that for patients with severe OSA, the MAD was an indicator of levels of blood oxygenation and sleep parameters that may be a useful addition to the clinical evaluation of patients with OSA.
In OSA patients, the collapse of upper airway leads to intermittent hypoxia, changes in respiratory arousal, and negative intrathoracic pressure, which result in other pathophysiological changes. The findings of the present study supported that the MAD was a parameter that captured some important aspects of the pathophysiology of OSA. The distribution of the MAD changed with the severity of OSA, which might have been due to the required length of apnea and hypoapnea used in the current definitions from the American Academy of Sleep Medicine (AASM) [7]. In this study, the MAD was significantly, but mildly, correlated with AHI and had no correlation with patient demographic data, which are factors that can affect blood oxygen and sleep structure. The findings of this study support that the AHI reflected the frequency of respiratory events, while the MAD represented the severity of respiratory events, and that these two indices are relatively independent, but may be complementary in the evaluation of patients with OSA.
The findings of the present study indicated that a longer MAD was associated with lower oxygen saturation in patients with severe OSA. Intermittent hypoxia is a major pathophysiological change caused by OSA, resulting in an increase in sympathetic nerve activity, systemic inflammation, metabolic dysregulation, endothelial cell injury, which may also aggravate cardiovascular disease including hypertension, arrhythmia, and arteriosclerosis. In a previously published study by our research group, patients with OSA and with a longer MAD had more severe hypertension when compared with patients with OSA and a shorter MAD [8]. Also, in this previously published study, when patients with OSA and hypertension were divided into three groups according to the severity of blood pressure, there was no significant difference in the AHI between the groups [8].
In the present study, the results showed that with the increase in the MAD in patients with OSA, the respiratory arousal index (AI) deteriorated more than the oxygen desaturation index (ODI). The explanation for this finding might have been that the oxygen reserve was reduced when the AHI score was high, and a short period of apnea or hypopnea might cause a significant oxygen desaturation, but this may not trigger respiratory arousal. In this study, patients with a longer MAD had a shorter Stage N3 or non-rapid eye movement (NREM) 3 sleep and a longer Stage N1 of sleep. When the MAD increased, sleep apnea appeared to be more likely to cause respiratory arousal, which might interrupt sleep stability, resulting in sleep fragmentation. The outcome might then be that the transition of Stage N2 (the longest stage of sleep) to Stage N3 is a vulnerable period that is interrupted in patients with OSA, and the overall sleep pattern becomes lighter. Therefore, the MAD might significantly interfere with sleep structure, especially Stage N3, the stage of deep sleep, even for patients with mild OSA. Because adequate Stage N3 sleep is required to prevent daytime sleepiness and to maintain normal daily function, the length of the MAD was also shown to be associated with an increased Epworth Sleepiness Scale (ESS) score, which with a shorter sleep latency, is also a manifestation of excessive sleepiness caused by Stage N3 sleep deprivation [9]. The secretion of hormones, including human growth hormone and the melanin-concentrating hormone (MCH) also occurs in Stage N3 sleep and may affect growth and development [10,11]. Stage N3 sleep also plays an important role in cognition and memory, and reduction of Stage N3 sleep in OSA may be an important factor for impairment of cognitive function [12,13]. Because of the possibility that an increase in the MAD can lead to Stage N3 sleep deprivation that results in cognitive impairment, further studies are warranted to explore the impact of the MAD on cognitive function.
In this study, there was no difference in rapid eye movement (REM) sleep time between the OSA patients in the long MAD group and the short MAD group. During REM sleep, reduction of the pharyngeal muscle activity substantially increases the propensity for upper airway collapse. The duration of apnea and hypopnea in the REM stage of sleep is different with that in non-REM (NREM) sleep. Also, REM sleep time is relatively short (about 15% of total sleep time), so the during the whole night, the MAD is usually different from the duration of apnea and hypopnea in the REM stage. The OSA patient with a short MAD might also have a long duration of apnea and hypopnea in the REM stage, and vice versa. Therefore, if OSA patients are divided into two groups according to the duration of the MAD, as in this study, the REM sleep times between these two groups may be similar.
Previously published studies have shown that following surgical treatment for OSA, the AHI remained unchanged in some patients, but subjective symptoms were improved, possibly due to improvement in the MAD, and an improvement in the CT90 (the cumulative time to reach a SaO2 <90%) was a better indicator than the AHI, indicating that the benefit of surgery include reduction in both the frequency and degree of apnea [14,15]. Therefore, the AHI alone may not be a sufficient method to evaluate the effect of surgery for OSA, especially in those procedures that are unlikely to have an impact on AHI (such as nasal septoplasty). Postoperative evaluation of patients with OSA with AHI alone may underestimate the beneficial physiological effects.
There were some limitations of this study. This study was retrospective, was performed in a single center, and included a relatively small study population. To evaluate the effects of using the MAD, there is still a need for further prospective, large-scale, controlled studies to examine the relationships between the MAD and other objective parameters, especially cardiovascular complications associated with OSA. Also, the value of the use of the MAD in evaluating the clinical effects of upper airway structural abnormalities should also be studied in future.
Conclusions
The findings of this study showed that, for patients with severe obstructive sleep apnea (OSA) diagnosed by polysomnography (PSG), the mean apnea-hypopnea duration (MAD) was a useful indicator of blood oxygenation and sleep parameters, which could better demonstrate the severity of apnea independent of the apnea-hypopnea index (AHI), patient age, height, and body weight. The use of the MAD, in combination with the AHI, in patients with OSA might provide more information in evaluating the detrimental effects of OSA.
Acknowledgments
The authors would like to thank all the staff for their help in extracting the clinical and demographic patient data from the Beijing Anzhen Hospital Sleep Center.
Footnotes
Source of support: This study was supported by the Beijing Municipal Administration of Hospitals Ascent Plan (Code: DFL20150602) and by the National Natural Science Foundation of China (Project: 81470567)
Conflict of interest
None.
References
- 1.Young T, Palta M, Dempsey J, et al. The occurrence of sleep disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–35. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Zheng T, Zhang L, Tian GY, et al. [An epidemiological survey of snoring disease and OSAHS among 374 truck drivers in Guangzhou, China]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2013;31(6):422–24. [In Chinese] [PubMed] [Google Scholar]
- 3.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, et al. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: A cohort study. Ann Intern Med. 2012;156(2):115–22. doi: 10.7326/0003-4819-156-2-201201170-00006. [DOI] [PubMed] [Google Scholar]
- 4.Terán-Santos J, Jiménez-Gómez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999;340(11):847–51. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 5.Mediano O, Barceló A, de la Peña M, et al. Daytime sleepiness and polysomnographic variables in sleep apnoea patients. Eur Resp J. 2007;30(1):110–13. doi: 10.1183/09031936.00009506. [DOI] [PubMed] [Google Scholar]
- 6.Koo BB, Mansour A. Correlates of obstructive apnea duration. Lung. 2014;192:185–90. doi: 10.1007/s00408-013-9510-4. [DOI] [PubMed] [Google Scholar]
- 7.Thornton AT, Singh P, Ruehland WR, et al. AASM criteria for scoring respiratory events: Interaction between apnea sensor and hypopnea definition. Sleep. 2012;35:425–32. doi: 10.5665/sleep.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu H, Zhan X, Zhao M, Wei Y. Mean apnea-hypopnea duration (but not apnea-hypopnea index) is associated with worse hypertension in patients with obstructive sleep apnea. Medicine (Baltimore) 2016;95(48):e5493. doi: 10.1097/MD.0000000000005493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dijk DJ, Groeger JA, Stanley N, et al. Age-related reduction in daytime sleep propensity and nocturnal slow wave sleep. Sleep. 2010;33(2):211–23. doi: 10.1093/sleep/33.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284(7):861–68. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 11.Monti JM, Torterolo P, Lagos P. Melanin-concentrating hormone control of sleep-wake behavior. Sleep Med Rev. 2013;17(4):293–98. doi: 10.1016/j.smrv.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Hoedlmoser K, Heib DP, Roell J, et al. Slow sleep spindle activity, declarative memory, and general cognitive abilities in children. Sleep. 2014;37(9):1501–12. doi: 10.5665/sleep.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo JC, Bennion KA, Chee MW. Sleep restriction can attenuate prioritization benefits on declarative memory consolidation. J Sleep Res. 2016;25(6):664–72. doi: 10.1111/jsr.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Paula Soares CF, Cavichio L, Cahali MB. Lateral pharyngoplasty reduces nocturnal blood pressure in patients with obstructive sleep apnea. Laryngoscope. 2014;124(1):311–16. doi: 10.1002/lary.24312. [DOI] [PubMed] [Google Scholar]
- 15.Zhan X, Li L, Wang N, et al. Can upper airway surgery for OSA protect against cardiovascular sequelae via effects on coagulation? Acta Otolaryngol. 2016;136(3):293–97. doi: 10.3109/00016489.2015.1112031. [DOI] [PubMed] [Google Scholar]