Abstract
Introduction
Aerobic exercise may improve smoking abstinence via reductions in craving and negative affect and increases in positive moods. Acute changes in craving and affect before and after structured exercise sessions have not been examined during the weeks prior to and following quit attempts nor has smoking status been examined in relation to these effects. Given that regular cigarette smoking can be perceived as affect enhancing and craving reducing, it is not known whether exercise could contribute additional affective benefit beyond these effects.
Method
Participants (N = 57; 68.4% women) were low-active daily smokers randomized to cessation treatments plus either group-based aerobic exercise (AE) or a health-education control (HEC). Mood, anxiety, and craving were assessed before and after each intervention session for each of the 12 weeks. Carbon monoxide (CO) breath samples ≤ 5ppm indicated smoking abstinence.
Results
During the prequit sessions, significantly greater decreases in anxiety following AE sessions relative to HEC sessions were observed. Changes in mood and craving were similar after AE and HEC sessions prior to quitting. Postquit attempt, significant reductions in craving and anxiety were observed after AE sessions but not following HEC. During the postquit period, positive mood increased following AE sessions relative to HEC only among individuals who were abstinence on that day.
Conclusions
AE may be effective in acutely reducing anxiety prior to a quit attempt and both anxiety and craving following the quit attempt regardless of abstinence status. The mood-enhancing effects of AE may occur only in the context of smoking abstinence.
Implications
The current findings underscore the importance of examining the acute effects of aerobic exercise prior to and after a cessation attempt and as a function of smoking status. Given the equivocal results from previous studies on the efficacy of exercise for smoking cessation, increasing our understanding of how aerobic exercise produces its reinforcing benefits for smokers attempting to quit could potentially inform the refinement (e.g., timing/sequencing) of exercise interventions within smoking cessation programs.
Introduction
Smoking remains the leading cause of preventable morbidity and mortality in the United States, accounting for approximately 448000 deaths annually.1 While the majority (68.8%) of smokers report motivation to quit,2 roughly 40 million adults in the United States still smoke (16.8% of the population3). Indeed, despite the wide range of available efficacious smoking cessation interventions (e.g., self-help manuals, brief interventions, intensive clinical interventions, and pharmacotherapy), 70–85% of smokers who attend treatment programs relapse within 1 year.4 Research focused on identifying and testing the efficacy/effectiveness of adjunctive treatment approaches to combat known risk factors precipitating lapse after attempts at smoking cessation continues to be critically important.
Over the last two decades, structured aerobic exercise (AE) has been incorporated as a natural aid to smoking cessation.5 Existing support for exercise interventions for smoking cessation has been equivocal. Methodological limitations (e.g., small sample sizes and poor adherence to exercise programs) and variations in implementation of exercise into smoking cessation programs (timing, duration, and intensity5) have prevented definitive conclusions about program efficacy. Optimism for eventual success stems from a body of work that demonstrates the acute effects of aerobic exercise on processes relevant to smoking cessation. For example, exercise leads to reductions in smoking craving and nicotine withdrawal symptom severity and increases in positive affect and decreases in negative affect.6–9 These changes are important, given increases in negative affect,10–13 decreases in positive affect,14,15 and more severe craving and withdrawal symptoms are consistently associated with poorer cessation outcomes.16–21 As such, through its acute effect on craving and affect, aerobic exercise may play an important role in smokers’ quit attempts.
A series of systematic reviews convincingly indicate that aerobic exercise has a significant acute and larger effects on craving and affect in smokers.6,8,9,22 Impressively, both aerobic and anaerobic exercise at varied intensities (e.g., light-to-moderate and vigorous) and for brief (e.g., 5–10 minutes) and longer (e.g., 30–40 minutes) bouts of activity all result in acute decreases in urges to smoke.5 Several laboratory-based studies among nontreatment-seeking smokers9,22–24 and smokers motivated for smoking cessation25,26 suggest that following exercise, smokers report acute reductions in smoking desire, withdrawal symptoms, cue-induced craving, and delayed initiation of ad libitum smoking. Importantly, while these simulate quit attempts in continuing smokers using periods of smoking abstinence prior to exercise, the acute effects of exercise on craving and affect among smokers making a smoking cessation attempt is less understood.
To date, few studies have examined the acute effects of exercise on mood, anxiety, and craving among smokers within the context of a cessation attempt. Generally, data indicate that smokers enrolled in moderate- or vigorous-intensity weekly exercise interventions (relative to health education control) experience greater decreases in negative affect and cigarette craving.27,28 These effects appear to be somewhat attenuated with lower intensity activity (e.g., walking).29 Additionally, a bout of moderate-intensity exercise appears to produce acute reductions in craving across three different exercise sessions during the nicotine patch step-down (i.e., 21, 14, and 7 mg30) and during use of the 2 mg nicotine lozenge.31 Collectively, these studies provide some preliminary evidence that, within the context of a quit attempt, AE generally has an acute effect on reduced craving and increased affect, above and beyond the effect of nicotine (at varying doses). One limitation of existing studies is that they have not considered the acute effect of AE on affect (mood and anxiety) and craving in the context of preparation to quit, immediately following smoking cessation or in the context of lapse events. Regular smokers report experiencing an affective benefit and reduction in craving with cigarettes smoked. Therefore, it is not known the extent to which bouts of exercise can further enhance affect and reduce craving in the context of regular smoking or lapses.
We aimed to explore the acute effects of an AE treatment on affect (mood and anxiety) and craving during the 4 weeks prior to a smoking cessation attempt and during the 8 weeks following the cessation attempt. Explication of these associations would increase specificity in our understanding of how AE produces its acute (reinforcing) benefits for smokers attempting to quit, which would potentially inform the refinement (timing/sequencing) of exercise interventions within smoking cessation programs.5
Method
Participants
Data analyzed were part of a randomized controlled smoking cessation trial of standard phone-based cognitive behavioral therapy (CBT) for smoking cessation and nicotine replacement therapy, with one of two adjunctive treatments: (1) 12-session, group AE intervention or (b) 12-session, group health education control (HEC) intervention.32 Participants (N = 61; 65.5% female) were community-recruited, treatment-seeking, adult daily smokers. Inclusion criteria included smoking 10 or more cigarettes per day and had not participated regularly in AE for at least 20 minutes per day, 3 days per week for the past 6 months. Exclusion criteria included current Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Axis I criteria for alcohol or drug abuse or dependence, bipolar disorder, eating disorder, psychotic disorder, current suicidality or homicidality, physical disabilities or medical problems contraindicated to AE (i.e., denied medical clearance by primary care physician), current pregnancy or intention to become pregnant during the following 3 months treatment period, and current use of pharmacotherapy for smoking cessation (e.g., nicotine replacement therapy). Of the randomized participants, four did not start the intervention; thus, data from 57 cases were included in the analyses. Complete demographic data are presented in Abrantes et al.32
Procedure
After participants received medical clearance to participate in an exercise program, stratified randomization based on body mass index (BMI), level of tobacco dependence, gender, and age was used to assign participants to either AE or HEC; treatment conditions were equated for contact time. All participants began the study with a set Quit Date, which was on week 5 of the study. Due to concerns about simultaneous onset of multiple behavior change among smokers negatively impacting outcomes,33 a month of exercise was initiated prior to cessation. In addition to the 12-week group intervention (either AE or HEC), all participants received 8 sessions of standard cognitive–behavioral smoking cessation treatment34 delivered by telephone (weekly, starting at week 1), and an 8-week supply of transdermal nicotine patches beginning on Quit Day (4 weeks of 21 mg patches, followed by 2 weeks of 21 mg, and ending with 2 weeks of 7 mg patches). Each intervention and the smoking cessation protocol are detailed in Abrantes et al. and briefly described below.
AE Intervention
Participants in the AE condition first received 20-mininute group CBT which focused on behavioral techniques to increase physical activity. After the group CBT sessions, participants attended AE sessions at the research fitness facility. The intensity and duration of the exercise were guided by the study exercise physiologist who monitored heart rate and adjusted equipment settings to ensure moderate-intensity aerobic exercise (55–69% of age-predicted maximal heart rate). In addition, gradual increases in weekly exercise were collaboratively determined by the exercise physiologist and the individual participant, based on their fitness level and progress each week. Exercise sessions began at 15 minutes/session and gradually increased to 40 minutes/session by week 12; only a subset of participants (51.7%) reached 40 minutes of exercise. Average duration of exercise sessions was 26.7(SD = 4.5) minutes prior to quitting (weeks 1–4) and 33.6 (SD = 7.4) minutes postquitting (weeks 5–12).
Types of exercise equipment available to participants included treadmills, recumbent bicycles, and elliptical machines. The exercise physiologist encouraged participants to try all equipment. The vast majority of participants did some part of their exercise session on a treadmill and the recumbent bicycle. The elliptical was less preferred, with 25% of participants electing not to utilize this piece of equipment at all. Participants were also prescribed home-based exercise of at least 2–4 additional times a week in their own environment, with a 12-week goal of achieving 150 min/week. They completed self-monitoring logs of these activities. As reported in the primary outcome paper35 participants increased minutes of moderate-to-vigorous physical activity (MVPA) per week from an average of 77.3 (SD = 112.5) minutes at baseline to 134 (SD = 94) minutes by the end of the intervention. Participants were instructed to remove the nicotine patch 1 hour prior to engaging in exercise sessions and reapply it after the exercise sessions were completed.
HEC Intervention
Participants in the HEC condition attended weekly 45–60-minute health education sessions on topics such as oral health, heart disease, cancer, sleep hygiene, and second-hand smoke, as they related to the effects of smoking. Health information was conveyed through lectures, handouts, in-group exercises, and Internet resources. Physical activity was not discussed. Participants were not encouraged to make any changes in their behavior, although it was possible that participants in the HEC condition would decide to begin exercising on their own, though few did. As reported in the primary outcome paper (Abrantes et al.35), participants went from an average of 60.3 (SD = 99.5) MVPA minutes at baseline to 70.1 (SD = 105.2) MVPA minutes by the end of the intervention.
Measures
Smoking Status at Weekly Session
Beginning on week 5 of the intervention (i.e., Quit Day), expired carbon monoxide (CO) breath samples were measured for all participants for each of the remaining weekly sessions to verify self-reported smoking status. Expired CO values > 5 ppm were used to denote current smoking, and CO ≤ 5 ppm was indicative of abstinence on the day of the session.
Nicotine Dependence
The Fagerstrom Test for Nicotine Dependence (FTND36) was used as a continuous measure of tobacco dependence.
BMI
The BMI was calculated by dividing body weight in kilograms by height in meters squared, consistent with American College of Sports Medicine guidelines. A Detecto medical scale was used to weight and a standiometer was used to measure height.
Acute Symptoms Self-Rating Scale
The National Institute of Mental Health self-rating scale has been used to capture acute changes in mood, anxiety, and craving as a function of AE in previous studies.37,38 At every AE and HEC intervention visit, participants were asked to rate, on a 10-point Likert-type scale, mood (0 = Feel worst ever to 10 = Feel best ever), anxiety (0 = No anxiety to 10 = Extreme anxiety), and craving to smoke (0 = No urge to 10 = Extreme urge) just prior to and again immediately following either the AE or the HEC session.
Data Analytic Strategy
Separate multilevel models were built in which postsession craving, mood, or anxiety was regressed on the treatment condition (AE or HEC) and smoking status for the prequit (4 weekly sessions before quit date) and postquit period (8 weekly sessions following quit date) with R Statistical Software 3.3 using the “lme” function in the “nlme” package. In order to examine changes in mood, anxiety, and craving before and after each weekly intervention session, presession levels of mood, anxiety, or craving were entered in the corresponding model. Time variables for the prequit and postquit periods were centered within each period. First, in order to test overall treatment effects on acute changes in mood, anxiety, and craving over the 4-week prequit and 8-week postquit treatment periods (in separate models), treatment condition (AE vs. HEC: a level-2 predictor) was included in the models, controlling for gender (coded 0 = male, 1 = female) and time (in weeks from quit date). For the postquit models, abstinence status (0 = smoking, 1 = abstinent on the day of the session) was also included as a covariate. Level-1 regression coefficients (e.g., smoking status and time) were allowed to vary across individuals if doing so improved model fit. In addition, the effects of smoking status on treatment differences over the postquit 8-week treatment period were tested by including a treatment by smoking status cross-level interaction in the models, controlling for presession levels of the corresponding variable, gender, and time (i.e., weeks in the treatment).
Results
Participants (N = 57, 68.4% female, Mage = 47.95, SD = 9.18) were primarily white (88%), employed (63%), and approximately one-third (31%) completed college. Participants had moderate levels of tobacco dependence per the FTND (M = 5.85, SD = 1.86), and average BMI was in the overweight range (M = 28.85, SD = 5.86). Of the 12-weekly AE or HEC group sessions, participants completed an average of 9.3 sessions (SD = 2.86). An average of 6.9 (SD = 1.68) of 8 telephone-based CBT smoking cessation sessions were completed. There were no differences in demographic characteristics or attendance rates across treatment conditions (ps > .05). In addition, there were no changes in cigarettes/day during the intervention weeks before quit day in either the AE or HEC condition.
Treatment and Presession Craving and Affect
Average mood, anxiety, and craving before weekly sessions did not differ across treatments either during the prequit attempt or postquit attempt period (ps > .05). Changes in average rating of mood, anxiety, and craving before weekly sessions from pre- to postquit attempt, prequit slope (changes over 4 weeks pre-quit), or postquit slope (changes over 8 weeks post-quit) did not vary across treatments (ps > .05). During the post-quit period, there was a significant treatment by smoking status interaction effect (p = .0004), such that among those who recently smoked, the HEC condition reported significantly better presession mood (p = .03), compared to the AE condition while no such differences across conditions were observed among those were abstinent on the day of the session (p > 0.05).
Precessation Treatment Period (4 Weeks Before Quit Date)
See Table 1 for summarized results of multilevel models, and Figures 1, 2, and 3 for visualization of mean mood, anxiety, and craving by treatment condition, over the course of treatment.
Table 1.
Effects of Treatment on Acute Changes in Craving, Mood, and Anxiety from Before to After Session During the 4-week Precessation Treatment Perioda
| Coefficient | Standard error | T-ratio | df | p value | |
|---|---|---|---|---|---|
| Craving (after session) | |||||
| Exercise condition | −0.461 | 0.321 | −1.438 | 54 | .156 |
| Female | −0.154 | 0.312 | −0.349 | 54 | .260 |
| Craving before session | 0.716 | 0.052 | 13.64 | 133 | .000* |
| Time (weeks in prequit) | −0.321 | 0.092 | −3.482 | 133 | .007* |
| Mood (after session) | |||||
| Exercise condition | 0.243 | 0.262 | 0.927 | 54 | .358 |
| Female | 0.243 | 0.284 | 0.857 | 54 | .395 |
| Mood before session | 0.621 | 0.055 | 11.36 | 139 | .000* |
| Time (weeks in prequit) | 0.008 | 0.071 | 0.118 | 139 | .906 |
| Anxiety (after session) | |||||
| Exercise condition | −0.850 | 0.260 | −3.269 | 54 | .002* |
| Female | −0.569 | 0.279 | −2.041 | 54 | .046 |
| Anxiety before session | 0.371 | 0.055 | 6.811 | 140 | .000* |
| Time (weeks in prequit) | −0.170 | 0.092 | −1.843 | 140 | .664 |
* p < .05.
aLevel-1 variables (mood, anxiety, and craving, time); level-2 variables (condition and gender).
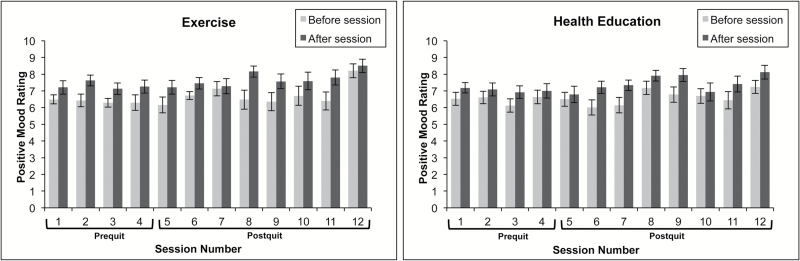
Figure 1.
Changes in positive mood before and after intervention sessions by prequit and postquit status.
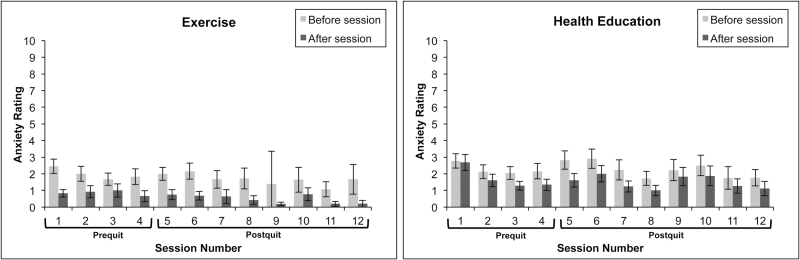
Figure 2.
Changes in anxiety before and after intervention sessions by prequit and postquit status.
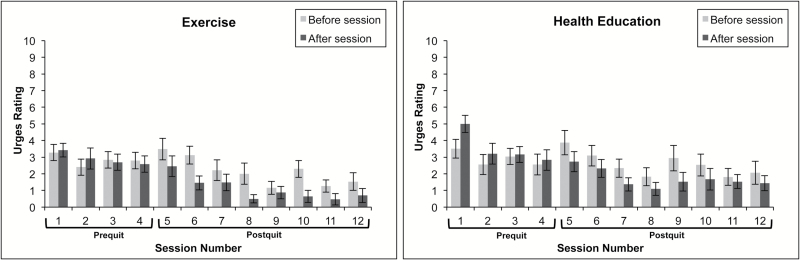
Figure 3.
Changes in smoking craving before and after intervention sessions by prequit and postquit status.
Mood
During the prequit period, there were no treatment differences in acute pre- to postsession changes in mood, controlling for gender and time. No Treatment × Time interaction effect was found.
Anxiety
A significant treatment effect was found (p = .0019) such that greater pre- to postsession decreases in anxiety was reported among the AE group, compared to the HEC group.
Craving
In general, over the course of 4-week intervention prior to the quit date, no differences in acute changes in craving (from before to after weekly sessions) across treatment conditions were observed, controlling for gender and time.
Postcessation Treatment Period (8 Weeks After Quit Date)
See Table 2 for the multilevel models for the postquit period.
Table 2.
Effects of Treatment and Smoking Status on Acute Changes in Mood, Anxiety, and Craving from Before to After Session During the 8-week Postcessation Treatment Period
| Coefficient | Standard error | T-ratio | df | p value | |
|---|---|---|---|---|---|
| Mood (after session) | |||||
| Exercise condition | 0.300 | 0.204 | 1.475 | 49 | .147 |
| Female | 0.024 | 0.227 | 0.106 | 49 | .916 |
| Mood before session | 0.587 | 0.042 | 14.06 | 228 | .000* |
| Smoking Abstinence | 0.217 | 0.20 | 1.088 | 228 | .278 |
| Time (weeks in pre-quit) | 0.055 | 0.199 | 1.623 | 228 | .106 |
| Abstinence × Exercise conditiona | 1.580 | 0.373 | 4.240 | 227 | .000* |
| Anxiety (after session) | |||||
| Exercise condition | −0.634 | 0.284 | −2.238 | 49 | .030* |
| Female | −0.079 | 0.312 | −0.253 | 49 | .801 |
| Anxiety before session | 0.350 | 0.034 | 10.21 | 229 | .000* |
| Smoking Abstinence | −0.182 | 0.185 | −0.984 | 229 | .326 |
| Time (weeks in prequit) | 0.011 | 0.026 | 0.436 | 229 | .664 |
| Abstinence × Exercise conditiona | 0.012 | 0.371 | 0.032 | 228 | .974 |
| Craving (after session) | |||||
| Exercise condition | −0.631 | 0.273 | −2.307 | 49 | .025* |
| Female | −0.343 | 0.301 | −1.139 | 49 | .260 |
| Craving before session | 0.452 | 0.032 | 14.54 | 218 | .000* |
| Smoking Abstinence | −0.704 | 0.327 | −2.154 | 218 | .032* |
| Time (weeks in prequit) | −0.053 | 0.031 | −1.738 | 218 | .084 |
| Abstinence × Exercise conditiona | −0.847 | 0.647 | −1.308 | 217 | .192 |
*p < .05.
aInteraction terms (treatment condition x abstinence status) were evaluated in subsequent models, controlling for gender, time and smoking status (not shown); level-1 variables (craving, mood, anxiety, time, and smoking status); level-2 variables (condition and gender). Smoking Abstinence (CO ≤ 5).
Mood
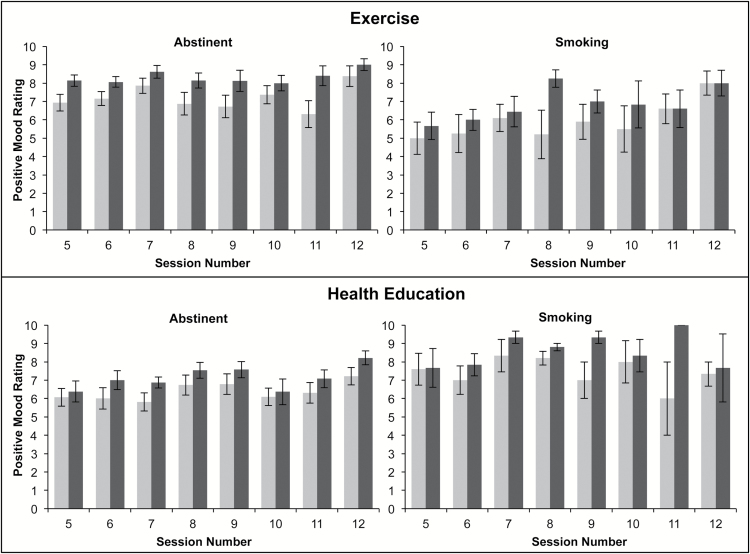
Over the course of 8 weeks postquit, the differences in pre- to postsession changes in mood across treatment conditions did not reach statistical significance, adjusting for gender, time, and abstinence status. Abstinence status did not predict pre- to postsession changes in mood (see Table 2). However, a significant Treatment × Abstinence status interactions indicated that the AE group reported significantly improved mood, compared to the HEC group, only among those who were abstinent on the day of the session (CO ≤ 5; Table 2 and Figure 4).
Figure 4.
Changes in positive mood before and after intervention sessions during the postquit period by smoking status (Abstinent = carbon monoxide [CO] ≤ 5; Smoking=CO > 5).
Anxiety
A significantly greater pre- to postsession reduction in anxiety was observed in the AE group, compared to the HEC group, over the course of 8 weeks postquit, adjusting for gender and smoking status. Abstinence status did not predict pre- to postsession changes in anxiety (Table 2). In addition, abstinence status did not moderate the effects of treatment on anxiety.
Craving
A significant treatment effect was found over the 8-week postquit period (p = .025) such that AE group reported a significantly greater reduction in craving after the session, compared to the HEC group, controlling for gender, time, and abstinence status. Abstinence status also predicted pre- to postsession changes in craving such that a greater reduction in craving was observed when CO indicated that participants did not smoke on the day of the session, compared to those who recently smoked. However, no significant Treatment × Abstinence status interaction was found, indicating that the effects of abstinence status on craving did not differ across treatment (Table 2).
Discussion
The current study examined the acute effects of an AE treatment on mood, anxiety, and craving, relative to HEC treatment, during the 4 weeks prior to a smoking cessation attempt and during the 8 weeks following the cessation attempt. In the weeks prior to the cessation attempt, smokers who received AE reported significant decreases in pre–post session anxiety, relative to smokers who received HEC. This effect was specific to anxiety and not seen for mood or craving.
There is consistent evidence that anxiety or fear of arousal sensations is a barrier to smoking cessation (e.g.,39,40), commonly reported as an antecedent to smoking lapse (e.g.,41) and that reduction in anxiety vulnerability prior to a smoking cessation attempt is associated with better cessation outcomes (e.g.,42). Thus, “prescribing” AE sessions prior to the established quit day may facilitate acute periods of anxiety reduction, which could help decrease fear to and avoidance of arousal sensations43 and in turn could promote smoking cessation.44 Indeed, it has been recommended that smoking cessation programs start prior to the established quit date4; however approximately half of published exercise-based intervention trial do not prescribe exercise prior to the quit date (see5 for review). The current data further underscore the potential anxiolytic benefits of exercise prior to a cessation attempt.
During the postcessation period, smokers who received AE, relative to HEC, reported significantly greater acute reductions in pre–post session anxiety. Moreover, significant reductions in pre–post session craving were observed for smokers who received AE relative to HEC. This set of findings is broadly consistent with results from Bock and colleagues27 who found that vigorous-intensity exercise, relative to a control treatment, produced significant acute reductions in negative affect, nicotine withdrawal symptoms, and craving following a cessation attempt. Importantly, these observed effects did not vary based on smoking status. That is, AE after the cessation attempt appears to produce its acute effects on anxiety- and craving reduction, regardless of whether individuals have recently smoked. Though, we make this interpretation with caution given the unequal patch use between conditions before and after exercise sessions. The AE sessions could potentially serve as an acute negative reinforcer (of anxiety and craving) even in the context of smoking lapse, which theoretically could motivate the reinitiation of a smoking quit attempt or reinforce continued engagement in AE.
The acute effects of AE on increased mood were not observed during the 4 weeks prior to quit day. Interestingly, during the postquit period, smokers who completed AE sessions (relative to HEC) on days they were abstinent reported significantly greater acute improvements in mood. Taken together, the data suggest that AE may only produce its acute enhancing effects on negative mood states during abstinence. Indeed, the majority of existing (laboratory-based) studies on the acute-enhancing effects of AE on mood have been conducted among smokers who are nicotine deprived (1–15 hours of abstinence8). The current data offer unique evidence that a bout of AE may enhance the abstinence-induced effects on negative mood. There are many possible reasons for the observed effects. It is possible that recent smoking prior to AE produces mood-enhancing effects via its pharmacological effects and that any additional acute benefit of acute AE was not incremental to the nicotinic effects. Alternatively, those who recently smoked may have activated various cognitive processes (e.g., distraction and expectancies of mood activation from smoking), which temporally enhanced mood. In contrast, however, smokers who were smoking may have had negative cognitions related to the recent lapse. Finally, we cannot rule out the possibility that the observed effects are due to measurement error. For example, more comprehensive measurement of mood (as used in 27,28) may have resulted in different outcomes, thus the current findings warrant replication.
There are several limitations to the current study. First, data were from a pilot study of AE versus HEC for smoking cessation32; thus, the small sample size may have affected power to detect significant effects. Second, the sample was relatively homogeneous in terms of racial background, and participants were generally highly educated; thus, findings may not necessarily be generalizable to more ethnically/racially diverse or lower educated smokers. Third, participants in the AE condition were instructed to remove the patch 1 hour prior to exercise, thus providing preexercise affect and cravings ratings without the patch, while participants in the HE condition remained with the patch on when providing those ratings. Nicotine replacement may bolster the acute effects of AE on craving (e.g.,30,31); thus, it would be important to understand how nicotine replacement therapies, relative to no use, during the cessation attempts, influences the acute effects of AE on affect and craving. Therefore, the results of this study allow us to generate some potential mechanisms by which the acute effects of exercise may influence smoking outcomes. However, these need to be tested with larger, well-controlled randomized trials, including more heterogeneous samples of smokers (e.g., with varying degrees of concomitant psychopathology or physical activity levels).
The current findings underscore the importance of examining the acute effects of AE prior to and after a cessation attempt and as a function of smoking status. Future work is needed to further explicate the extent to which acute changes in craving and affect via AE results in change in actual smoking behavior, in the context of a smoking cessation attempt. The timing of AE may have important implications for precessation benefits and the maintenance of a cessation attempt.
Funding
This work was supported by a grant funded by the National Institute on Drug Abuse (K23 DA019950) awarded to Dr. Abrantes. Dr. Farris is supported by a training grant (T32-HL076134) funded by the National Heart, Lung, and Blood Institute.
Declaration of Interests
The authors declare that there is no conflict of interest.
References
- 1. National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking—50 years of progress: a report of the surgeon general. Atlanta, GA: Centers for Disease Control and Prevention (US); 2014. 1–36. doi:NBK179276. [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Quitting smoking among adults - United States, 2001–2010. Morb Mortal Wkly Rep. 2011;60(44):1513–1519. doi:10.1016/j.ypdi.2012.01.021. [PubMed] [Google Scholar]
- 3. Jamal A, Homa DM, O’Connor E et al. . Current cigarette smoking among adults – United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2015;64(44):1233–1240. http://www.ncbi.nlm.nih.gov/pubmed/26562061. [DOI] [PubMed] [Google Scholar]
- 4. Fiore MC, Jaén CR, Baker TB et al. . A clinical practice guideline for treating tobacco use and dependence: 2008 update. Am J Prev Med. 2008;35(May):158–176. doi:10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ussher MH, Taylor AH, Faulkner GEJ. Exercise interventions for smoking cessation. Cochrane Database Syst Rev. 2014;8(8):CD002295 http://www.ncbi.nlm.nih.gov/pubmed/25170798. [DOI] [PubMed] [Google Scholar]
- 6. Haasova M, Warren FC, Ussher M et al. . The acute effects of physical activity on cigarette cravings: systematic review and meta-analysis with individual participant data. Addiction. 2013;108(1):26–37. doi:10.1111/j.1360-0443.2012.04034.x. [DOI] [PubMed] [Google Scholar]
- 7. Haasova M, Warren FC, Ussher M et al. . The acute effects of physical activity on cigarette cravings: exploration of potential moderators, mediators and physical activity attributes using individual participant data (IPD) meta-analyses. Psychopharmacology (Berl). 2014;231(7):1267–1275. [DOI] [PubMed] [Google Scholar]
- 8. Roberts V, Maddison R, Simpson C, Bullen C, Prapavessis H. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect, and smoking behaviour: systematic review update and meta-analysis. Psychopharmacology (Berl). 2012;222(1):1–15. [DOI] [PubMed] [Google Scholar]
- 9. Taylor A, Katomeri M. Walking reduces cue-elicited cigarette cravings and withdrawal symptoms, and delays ad libitum smoking. Nicotine Tob Res. 2007;9(11):1183–1190. [DOI] [PubMed] [Google Scholar]
- 10. Burgess ES, Brown RA, Kahler CW et al. . Patterns of change in depressive symptoms during smoking cessation: who’s at risk for relapse?J Consult Clin Psychol. 2002;70(2):356–361. http://www.ncbi.nlm.nih.gov/pubmed/11952193%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1808225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Piasecki TM, Niaura R, Shadel WG et al. . Smoking withdrawal dynamics in unaided quitters. J Abnorm Psychol. 2000;109(1):74–86. http://www.ncbi.nlm.nih.gov/pubmed/10740938. [DOI] [PubMed] [Google Scholar]
- 12. Kang E, Lee J. A longitudinal study on the causal association between smoking and depression. J Prev Med Public Health. 2010;43(3):193–204. [DOI] [PubMed] [Google Scholar]
- 13. Boden JM, Fergusson DM, Horwood LJ. Cigarette smoking and depression: tests of causal linkages using a longitudinal birth cohort. Br J Psychiatry. 2010;196(6):440–446. [DOI] [PubMed] [Google Scholar]
- 14. Cook JW, Spring B, McChargue DE et al. . Influence of fluoxetine on positive and negative affect in a clinic-based smoking cessation trial. Psychopharmacology (Berl). 2004;173(1-2):153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lerman C, Roth D, Kaufmann V et al. . Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug Alcohol Depend. 2002;67(2):219–223. [DOI] [PubMed] [Google Scholar]
- 16. Xian H, Scherrer JF, Madden PA et al. . The heritability of failed smoking cessation and nicotine withdrawal in twins who smoked and attempted to quit. Nicotine Tob Res. 2003;5(2):245–254. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12745498. [PubMed] [Google Scholar]
- 17. Killen JD, Fortmann SP. Craving is associated with smoking relapse: findings from three prospective studies. Exp Clin Psychopharmacol. 1997;5(2):137–142. doi:10.1037/1064-1297.5.2.137 [DOI] [PubMed] [Google Scholar]
- 18. Allen SS, Bade T, Hatsukami D, Center B. Craving, withdrawal, and smoking urges on days immediately prior to smoking relapse. Nicotine Tob Res. 2008;10(1):35–45. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18188743%5Cnhttp://ntr.oxfordjournals.org/content/10/1/35.full.pdf. [DOI] [PubMed] [Google Scholar]
- 19. Kahler CW, Brown RA, Ramsey SE et al. . Negative mood, depressive symptoms, and major depression after smoking cessation treatment in smokers with a history of major depressive disorder. J Abnorm Psychol. 2002;111(4):670–675. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med4&NEWS=N&AN=12428781. [DOI] [PubMed] [Google Scholar]
- 20. Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation. Who will quit with and without the nicotine patch. JAMA. 1994;271(8):589–594. [DOI] [PubMed] [Google Scholar]
- 21. Bränström R, Penilla C, Pérez-Stable EJ, Muñoz RF. Positive affect and mood management in successful smoking cessation. Am J Health Behav. 2010;34(5):553–562. [PubMed] [Google Scholar]
- 22. Ussher M, Nunziata P, Cropley M, West R. Effect of a short bout of exercise on tobacco withdrawal symptoms and desire to smoke. Psychopharmacology (Berl). 2001;158(1):66–72. [DOI] [PubMed] [Google Scholar]
- 23. Daniel J, Cropley M, Ussher M, West R. Acute effects of a short bout of moderate versus light intensity exercise versus inactivity on tobacco withdrawal symptoms in sedentary smokers. Psychopharmacology (Berl). 2004;174(3):320–326. http://www.ncbi.nlm.nih.gov/pubmed/14997270. [DOI] [PubMed] [Google Scholar]
- 24. Elibero A, Janse Van Rensburg K, Drobes DJ. Acute effects of aerobic exercise and Hatha yoga on craving to smoke. Nicotine Tob Res. 2011;13(11):1140–1148. doi:10.1093/ntr/ntr163 [DOI] [PubMed] [Google Scholar]
- 25. Kurti AN, Dallery J. Effects of exercise on craving and cigarette smoking in the human laboratory. Addict Behav. 2014;39(6):1131–1137. [DOI] [PubMed] [Google Scholar]
- 26. Kurti AN, Dallery J. A laboratory-based evaluation of exercise plus contingency management for reducing cigarette smoking. Drug Alcohol Depend. 2014;144:201–209. [DOI] [PubMed] [Google Scholar]
- 27. Bock BC, Marcus BH, King TK, Borrelli B, Roberts MR. Exercise effects on withdrawal and mood among women attempting smoking cessation. Addict Behav. 1999;24(3):399–410. [DOI] [PubMed] [Google Scholar]
- 28. Williams DM, Dunsiger S, Whiteley JA, Ussher MH, Ciccolo JT, Jennings EG. Acute effects of moderate intensity aerobic exercise on affective withdrawal symptoms and cravings among women smokers. Addict Behav. 2011;36(8):894–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arbour-Nicitopoulos KP, Faulkner GE, Hsin A, Selby P. A pilot study examining the acute effects of exercise on cigarette cravings and affect among individuals with serious mental illness. Ment Health Phys Act. 2011;4(2):89–94. [Google Scholar]
- 30. Harper T, Fitzgeorge L, Tritter A, Prapavessis H. Acute Exercise Effects on Craving and Withdrawal Symptoms among Women Attempting to Quit Smoking Using Nicotine Replacement Therapy. J Smok Cessat. 2012:1–8.22936953 [Google Scholar]
- 31. Tritter A, Fitzgeorge L, Prapavessis H. The effect of acute exercise on cigarette cravings while using a nicotine lozenge. Psychopharmacology (Berl). 2015;232(14):2531–2539. [DOI] [PubMed] [Google Scholar]
- 32. Abrantes AM, Bloom EL, Strong DR et al. . A preliminary randomized controlled trial of a behavioral exercise intervention for smoking cessation. 2014;(2005):1–10. doi:10.1093/ntr/ntu036. [DOI] [PMC free article] [PubMed]
- 33. King TK, Marcus BH, Pinto BM, Emmons KM, Abrams DB. Cognitive-behavioral mediators of changing multiple behaviors: smoking and a sedentary lifestyle. Prev Med. 1996;25(6):684–691. doi:10.1006/pmed.1996.0107 [DOI] [PubMed] [Google Scholar]
- 34. Brown RA. Intensive behavioral treatment. In: Abrams DB, Niaura RS, Brown RA, Emmons KM, Goldstein MG, Monti PM, eds. The Tobacco Dependence Treatment Handbook: A Guide to Best Practices. New York: Guildford Press; 2003:118–177. [Google Scholar]
- 35. Abrantes AM, Bloom EL, Strong DR et al. . A preliminary randomized controlled trial of a behavioral exercise intervention for smoking cessation. Nicotine Tob Res. 2014;16(8):1094–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br J Addict. 1991;86(9):1119–1127. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1932883. [DOI] [PubMed] [Google Scholar]
- 37. Abrantes AM, Strong DR, Cohn A et al. . Acute changes in obsessions and compulsions following moderate-intensity aerobic exercise among patients with obsessive-compulsive disorder. J Anxiety Disord. 2009;23(7):923–927. doi:DOI 10.1016/j.janxdis.2009.06.008 [DOI] [PubMed] [Google Scholar]
- 38. Brown RA, Prince MA, Minami H, Abrantes AM. An exploratory analysis of changes in mood, anxiety and craving from pre- to post-single sessions of exercise, over 12 weeks, among patients with alcohol dependence. Ment Health Phys Act. 2016;11:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and Mental Illness. Jama. 2000;284(20):2606 http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 40. Piper ME, Smith SS, Schlam TR et al. . Psychiatric disorders in smokers seeking treatment for tobacco dependence: relations with tobacco dependence and cessation. J Consult Clin Psychol. 2010;78(1):13–23. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2813467&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shiffman S, Balabanis MH, Gwaltney CJ et al. . Prediction of lapse from associations between smoking and situational antecedents assessed by ecological momentary assessment. Drug Alcohol Depend. 2007;91(2-3):159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Assayag Y, Bernstein A, Zvolensky MJ, Steeves D, Stewart SS. Nature and role of change in anxiety sensitivity during NRT-aided cognitive-behavioral smoking cessation treatment. Cogn Behav Ther. 2012;41(1):51–62. [DOI] [PubMed] [Google Scholar]
- 43. Smits JAJ, Tart CD, Rosenfield D, Zvolensky MJ. The interplay between physical activity and anxiety sensitivity in fearful responding to carbon dioxide challenge. Psychosom Med. 2011;73(6):498–503. http://www.ncbi.nlm.nih.gov/pubmed/21700713%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC3131468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smits JAJ, Zvolensky MJ, Davis ML et al. . The efficacy of vigorous-intensity exercise as an aid to smoking cessation in adults with high anxiety sensitivity. Psychosom Med. 2015:1 http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006842-900000000-99022. [DOI] [PMC free article] [PubMed]