High tumor expression levels of glutamate-cysteine ligase catalytic subunit may be a potential predictor of treatment failure in lung adenocarcinoma patients.
Keywords: glutamate-cysteine ligase catalytic subunit, CD44, lung adenocarcinoma, drug resistance, prognosis
Abstract
Background
Cisplatin is a key drug for treating lung adenocarcinoma, and its sensitivity to cisplatin is directly related to prognosis. We aimed to reveal the roles of genes related to glutathione synthesis (glutamate-cysteine ligase catalytic subunit, GCLC) and cystine uptake (cystine/glutamate transporter, xCT and CD44v8-10) in cisplatin resistance and prognosis in lung adenocarcinoma.
Methods
We established cell lines stably expressing GCLC, xCT, standard isoform of CD44, and CD44v8-10, and investigated their sensitivities to cisplatin. We also measured mRNA expression levels of these genes in the tumor tissues from 92 lung adenocarcinoma patients. Patients were divided into high-expression (upper quartile, N = 23) and low-expression groups (remaining patients, N = 69). Recurrence-free survival, overall survival (N = 92), and post-recurrence survival (N = 22) were selected as endpoints.
Results
Compared with the control green fluorescent protein-expressing cell line (inhibitory concentration 50:6.9 μM), all the stable cell lines were more resistant to cisplatin (12.9 μM, P = 0.025; 13.9 μM, P = 0.028; 26.7 μM, P = 0.001; 17.7 μM, P = 0.008, respectively). In contrast, there was no significant difference in recurrence-free or overall survival between the high- and low-expression groups for any of the genes. However, high expression of GCLC was a risk factor for poorer post-recurrence survival (hazard ratio, 6.26; 95% confidence interval, 1.37–28.7; P = 0.018).
Conclusion
High expression levels of genes related to glutathione synthesis and cystine uptake promote cisplatin resistance in lung adenocarcinoma cell lines. High expression of GCLC in tumor tissue may be a potential predictor of treatment failure.
Introduction
Lung cancer is a leading cause of cancer-related death worldwide (1), and adenocarcinoma is the most common histological subtype of lung cancer in Japan (2). Platinum doublet therapy has generally been used as conventional chemotherapy for lung cancer patients (3). However, cancer cells often acquire gradual resistance to these anti-cancer agents, including the key lung cancer chemotherapy agent cisplatin, by decreasing their intracellular concentrations (4).
Glutathione conjugates with various electrophilic toxic substances such as cisplatin, thus enhancing their efflux and detoxifying them (5, 6). Glutathione can also scavenge free radicals, thus aiding the survival of cancer cells exposed to environmental stresses such as reactive oxygen species (7). Glutamate cysteine ligase (GCL) is the first and rate-limiting enzyme in the biosynthesis of glutathione from cysteine, glutamate and glycine. GCL is composed of a large catalytic subunit (GCLC) and a small modified subunit (5, 8). GCLC was recently reported to be involved in tamoxifen resistance in breast cancer (9) and cisplatin resistance in head and neck squamous cell carcinoma (10). Cysteine is mainly supplied from outside of the cell as a form of cystine via a cystine-glutamate transporter, xc(-), which was previously reported to be related to cancer chemoresistance (11, 12).
Cancer stem cells have been reported to play an important role in the efficacy of cancer therapies (13–15). In particular, one of the splicing variant of CD44, CD44v8-10, which is a known cancer stem cell marker, interacts with and stabilizes the xCT subunit of the xc(-), and thereby promotes glutathione synthesis (16). CD44v8-10 has been reported to serve as a marker of poor prognosis in a type of ovarian cancer (17) and in early gastric cancer (18). Inhibition of xCT in CD44v8-10-positive cells is therefore attracting attention as a potential new therapeutic target for improving sensitivity to chemotherapy (19–21).
The impacts of the expression levels of genes related to redox regulation on lung cancer prognosis have not been well studied. In this study, we investigated the prognostic values of tumor expression levels of GCLC, xCT and CD44v8-10 in lung adenocarcinoma.
Patients, materials and methods
Cell cultures
Lenti-X 293t cells (Takara Bio USA, Inc., CA, USA) were cultured under 5% CO2 at 37°C in Dulbecco’s modified Eagle’s medium (Wako Pure Chemical Industries, Ltd, Osaka, Japan) supplemented with 10% fetal bovine serum. The human lung cancer cell line NIH-H358 (ATCC) and its transgenic cell lines created as below were cultured under 5% CO2 at 37°C in RPMI-1640 (Wako Pure Chemical Industries, Ltd) supplemented with 10% fetal bovine serum.
Preparation of lentiviral vectors for exogenous overexpression
The lung adenocarcinoma cell line H358 showed relatively low expression levels of GCLC, xCT and CD44. We therefore created new stable cell lines by infecting them with lentiviral expression vectors for each gene, using the cDNA expression plasmid (CSII-CMV-MCS-IRES2-Bsd) and packaging plasmids (pCMV-VSVG-RSV-Rev and pCAG-HIVgp) (kindly provided by RIKEN BioResource Center) (22). The complete coding DNA sites of human GCLC (NCBI Reference Sequence: NM_001498.3), xCT (NM_014331.3), CD44s (NM_001001391.1), and CD44v8-10 (NM_001001390.1) were targeted. These cDNAs were cloned individually into the multiple cloning sites of the cDNA expression plasmid. Green fluorescent protein (GFP) was also cloned as a control. The resulting cell lines were named as H358-GCLC, H358-xCT, H358-CD44s, H358-CD44v and H358-GFP, respectively. Each cDNA expression plasmid and packaging plasmids were cotransfected into Lenti-X 293t cells using the lipofection agent HilyMax (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) according to the manufacturer’s protocol. The following day, the medium was removed and replaced with new complete culture medium with 10 μM forskolin. Each aliquot of Lenti-X 293t cells was incubated for 48 h at 37°C, and the vector-containing medium was then collected and filtered through a 0.45-μm Millipore filter.
Lentiviral infection
NIH-H358 cells were cultured to 25% confluence, the medium was then removed, and equivalent amounts of vector-containing medium and RPMI-1640 were added. Cells stably expressing each gene were obtained by selection with 10 μg/ml blasticidin S hydrochloride (Wako Pure Chemical Industries, Ltd) for 1week.
Cisplatin treatment and cell viability assay
Each cell line (1 × 104 cells in 100 μl of medium) was seeded in a well of a 96-well plate, and each seeding was performed in triplicate. The medium was changed the following day to medium containing with various concentrations of cisplatin (0–50 μM) for 48 h. Cell viability was then assessed using a Cell Counting Kit-8 (Dojindo Molecular Technologies, Inc.) according to the manufacturer’s protocol. The absorbance at 450 nm was measured using a multilabel counter ARVO MX (Perkin Elmer, Inc., MA, USA). The inhibitory concentration (IC)50 was calculated from the rate of cell survival after normalization by the probit transformation.
Patients’ selection
Among patients with pathological Stage IB–IIIA lung adenocarcinoma who underwent curative resection at the University of Tokyo Hospital (Tokyo, Japan) between March 2007 and June 2013, 92 patients had tumors large enough to allow the collection of adequate tumor specimens. The following clinical and pathological data were collected retrospectively: age, sex, smoking status, tumor size, pathological TNM classification, pathological stage, lymph node invasion and vascular invasion. Recurrence-free survival (RFS) was defined as the period from the date of lung resection until the date of radiologic evidence of disease recurrence. Overall survival (OS) was defined as the period from the date of lung resection until the date of death or last recall. Post-recurrence survival was defined as the period from the date of recurrence until the date of death or last recall. This study was approved by the Institutional Review Board at the University of Tokyo Hospital, and written informed consent was obtained from each patient.
Measurements of glutathione synthesis-related gene expression
Total RNA was extracted from cultured cells or patient tumor specimens and reverse transcribed into cDNA, as described previously (23). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using the following primers: glyceraldehyde 3-phosphate dehydrogenase (GAPDH), forward 5′-CAC CAC CAA CTG CTT AGC AC-3′ and reverse 5′-TGG CAG GTT TTC TAG ACG G-3′; GCLC, forward 5′-ACG GAG GAA CAA TGT CCG AG-3′ and reverse 5′-TAC TGA AGC GAG GGT GCT TG-3′; xCT, forward 5’-CAG GAG AAA GTG CAG CTG AA-3′ and reverse 5′-CTC CAA TGA TGG TGC CAA TG-3′; all CD44, forward 5′-TCG CTA CAG CAT CTC TCG GA-3′ and reverse 5′-TGC TGC ACA GAT GGA GTT GG-3′; and CD44v8-10, forward 5′-GAC AGA ATC CCT GCT ACC AAT A-3′ and reverse 5′-ATG TGT CTT GGT CTC CTG ATA A-3′. The validated primers targeting GAPDH were designed using Primer Analysis Software (OLIGO; Molecular Biology Insights, Inc., CO, USA). The other primers were optimally designed by Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/, last accessed 30 January 2018). The primer set for all CD44 was designed to amplify all splicing variant of CD44 (from exons 2 to 3), whereas that for CD44v8-10 was designed to be specific for CD44v8-10 (from variant exons 8 to 10). qRT-PCR analysis was performed using a 7500 Fast Real-Time PCR System (Applied Biosystems, CA, USA) with Thunderbird SYBR qPCR Mix (TOYOBO CO., LTD, Osaka, Japan). The amplification protocol comprised an initial incubation at 95°C for 1 min and 35 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 45 s, followed by dissociation-curve analysis to confirm specificity. Relative expression of each gene was calculated after normalization to GAPDH using the -ΔΔCt method.
Statistical analysis
Cell line data were represented as the mean ± standard error based on at least three independent experiments, each conducted in triplicate. IC50 values of cell lines were compared using Student’s t-test.
RFS, OS and post-recurrence survival were analyzed by the Kaplan–Meier method, and differences in survival were analyzed by the log-rank test. Differences were considered significant when the P value was <0.05. Statistical analysis was performed using SPSS version 22 (SPSS, Inc., Chicago, IL, USA).
Results
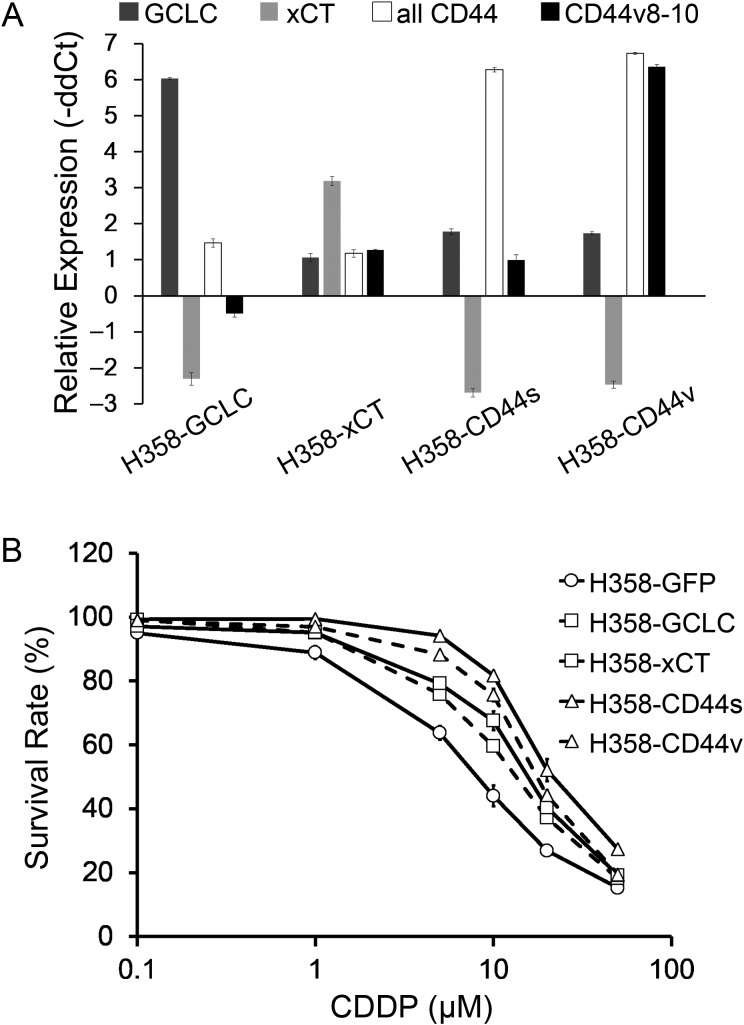
The newly created stable cell lines, H358-GCLC, H358-xCT, H358-CD44s and H358-CD44v, showed increased expression levels of the transfected genes compared with H358-GFP (Fig. 1A). Survival curves of the cell lines exposed to various concentrations of cisplatin are shown in Fig. 1B. The IC50 value of each cell line was significantly increased compared with that for H358-GFP (Table 1).
Figure 1.
mRNA expression levels of GCLC, xCT, all CD44 and CD44v8-10, and survival rate of cisplatin-exposed stable H358 cell lines. (A) mRNA expression levels in the stable cell lines relative to H358-GFP analyzed by qRT-PCR with GAPDH expression as an internal calibrator. Whiskers indicate the standard error from triplicate experiments. (B) Survival rates of stable cell lines exposed to cisplatin at various concentrations. Whiskers show standard error calculated from the results of more than three independent experiments.
Table 1.
The IC50 values of cisplatin in the five stable cell lines
| IC50 (μM) | P value (vs. H358-GFP) | |
|---|---|---|
| H358-GFP | 6.9 ± 0.7 | |
| H358-GCLC | 12.9 ± 1.6 | 0.025 |
| H358-xCT | 13.9 ± 1.1 | 0.028 |
| H358-CD44s | 26.7 ± 0.7 | 0.001 |
| H358-CD44v | 17.7 ± 0.4 | 0.008 |
Data presented as mean ± standard error, taken from at least three independent experiments.
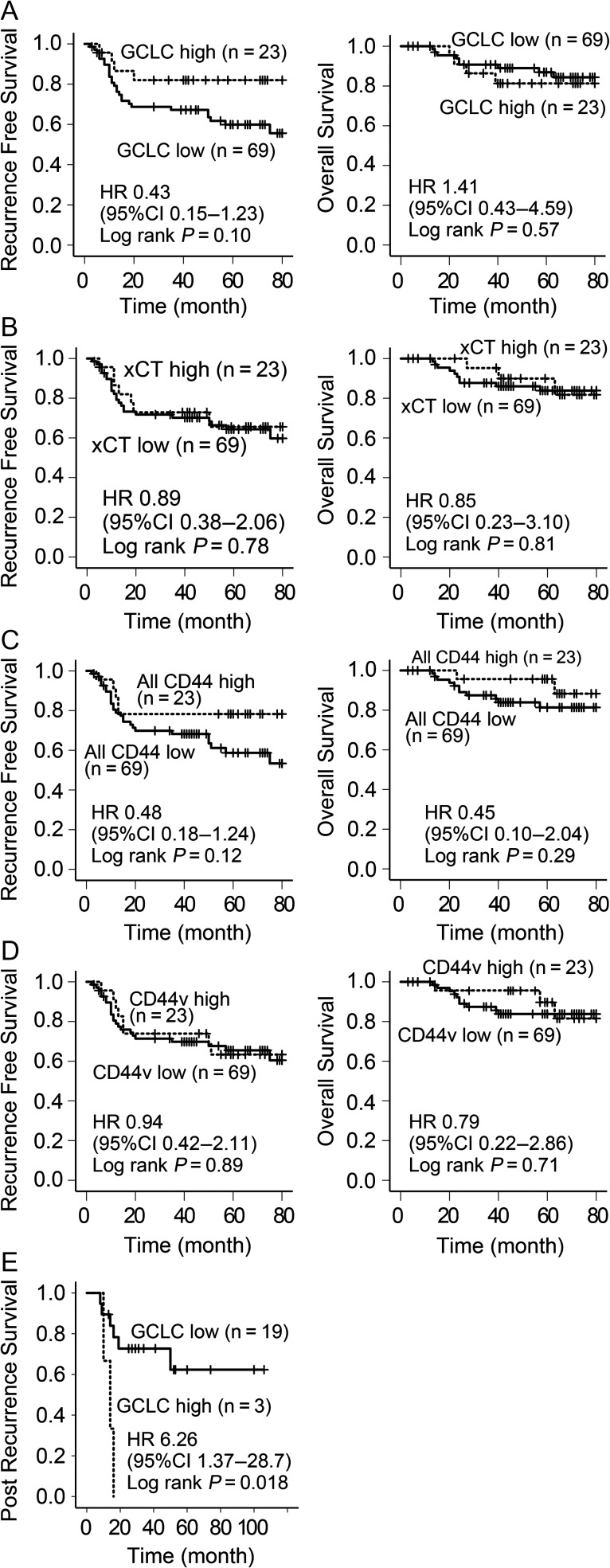
The clinical characteristics of the 92 patients with lung adenocarcinoma are shown in Table 2. The gene expression levels of GCLC, xCT, all CD44 and CD44v8-10 in tumor tissue were investigated by qRT-PCR and compared with normal lung tissues (Fig. S1). The expression levels of these genes in tumors were relatively low, with higher expression levels of GCLC, xCT, all CD44 and CD44v8-10 compared with normal tissues only found in 30, 47, 8 and 28 patients, respectively. To allow us to perform statistical analysis with the same numbers of cases for all genes, we set the top 25% (23 patients) as the high-expression group and the remaining patients (N = 69) as the low-expression group, such that the expression levels were not lower than in normal lung, except for all CD44. RFS and OS curves are shown in Fig. 2A–D. There were no significant differences in survival between the high- and low-expression groups for any of the genes. High expression of GCLC tended to be associated with a better prognosis in terms of RFS (hazard ratio (HR) 0.43, 95% confidence interval (CI) 0.15–1.23, log rank P = 0.10), and a poorer OS (HR 1.41, 95% CI 0.43–4.59, log rank P = 0.57), but the differences were not significant. Twenty-two patients received radical treatment for postoperative recurrence (including stereotactic radiation therapy for metastasis, chemotherapy with cisplatin/carboplatin and/or paclitaxel, docetaxel, pemetrexed or epidermal growth factor receptor tyrosine kinase inhibitors). Among these, high GCLC expression was associated with a significantly poorer prognosis in terms of post-recurrence survival (HR 6.26, 95%CI 1.37–28.7, log rank P = 0.006) (Fig. 2E), but expression levels of the other genes were not (Fig. S2).
Table 2.
Clinical and pathological features of lung adenocarcinoma patients
| Variable | N = 92 (%) |
|---|---|
| Age (years) | |
| ≤65 | 30 (32.6) |
| >65 | 62 (67.4) |
| Sex | |
| Male | 54 (58.7) |
| Female | 38 (41.3) |
| Smoking status | |
| Never | 36 (39.1) |
| Current/former | 54 (58.7) |
| pT | |
| 1 | 8 (8.7) |
| 2 | 71 (77.2) |
| 3 | 12 (13.0) |
| 4 | 1 (1.1) |
| Mean tumor size (mm) ± SD | 37.4 ± 16.8 |
| pN | |
| 0 | 64 (69.6) |
| 1 | 11 (12.0) |
| 2 | 17 (18.5) |
| pStage | |
| IB | 51 (55.4) |
| II | 21 (22.8) |
| III | 20 (21.7) |
| Vascular invasion | |
| Positive | 39 (42.4) |
| Lymphatic invasion | |
| Positive | 60 (65.2) |
SD, standard deviation.
Figure 2.
Survival curves of surgically treated lung adenocarcinoma patients. (A) RFS and OS according to GCLC mRNA expression level; (B) according to xCT; (C) according to all CD44; (D) according to CD44v8-10 (A–D: N = 92) and (E) post-recurrence survival compared with GCLC expression level (N = 22).
Discussion
The results of the present study showed that lung cancer cells acquired resistance to cisplatin under stable expression of GCLC, xCT, CD44s and CD44v8-10. Interestingly, xCT expression levels tended to decrease when other genes were overexpressed. The transcription factor Nrf2 has previously been reported to regulate the expression of antioxidant proteins, including GCLC and xCT (24), and Nrf2 is activated by oxidative stress (25). We speculated that the intracellular oxidative stress status may be changed by other genes, followed by inactivation of Nrf2. In addition, both standard and variant forms of CD44 contributed to cisplatin resistance. Previous studies using gastric and colon cancer cell lines showed that CD44v8-10 increased cystine uptake by stabilizing xCT on the cell membrane (16). Another study using ovarian and breast cancer cell lines, however, reported that CD44s increased P-glycoprotein-mediated multi-drug resistance (26). Further studies are needed to determine the mechanism responsible for promoting cisplatin resistance in lung cancer.
According to the present analysis, mRNA expression levels of none of the tested genes were significant prognostic factors. However, high expression of GCLC significantly shortened post-recurrence survival in patients after surgery, suggesting that it could be a potential marker for predicting treatment failure. These results appear to contradict the results obtained from the experimental cell lines. One possible reason for this apparent discrepancy is that the mRNA expression levels may not reflect the biological availability of the xCT and CD44 gene products, because they are expressed and function on the cell surface and are stabilized by each other. Higher levels of transmembrane CD44v8-10 protein expression were previously shown to correlate with good prognosis, but conversely, detection of the proteolytically cleaved and soluble extracellular domain of CD44v8-10 in patient ascites samples was correlated with a poorer prognosis (17). Furthermore, our cohort was limited to surgically treated cases, and the recurrence rate and mortality were therefore relatively low, and further studies in more cases may be needed to detect a significant difference in survival rate. In particular, only 22 of 27 recurrent patients had received curative chemotherapy or radiotherapy, and only one received cisplatin for recurrence. Carboplatin was used as a cisplatin analog in five cases, and 17 patients used platinum agents, including for postoperative adjuvant therapy. Third, GCLC, xCT and CD44 are also expressed in normal lung tissue. Compared with the total mRNA expression levels in background lung tissues from three patients who underwent lung resection for benign lung nodules, most of our tumor cohort showed low expression levels of these genes (Fig. S1). It is also possible that the RNA was contaminated by RNA from cells other than tumor cells.
High mRNA expression of GCLC in cancer tissue is a potential predictor of cisplatin resistance in lung adenocarcinoma patients.
Supplementary Material
Supplementary data
Supplementary data are available at Japanese Journal of Clinical Oncology online.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Funding
This work was supported by a Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Young Scientists (B) (15K20917 to K.W. and 15K18440 to Y.A.), and a JSPS Grant-in-Aid for Scientific Research (A) (16H02653 to T.N.).
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2. Sawabata N. Prognosis of lung cancer patients in Japan according to data from the Japanese Joint Committee of Lung Cancer Registry. Respir Investig 2014;52:317–21. [DOI] [PubMed] [Google Scholar]
- 3. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–57. [DOI] [PubMed] [Google Scholar]
- 4. Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 2007;7:573–84. [DOI] [PubMed] [Google Scholar]
- 5. Meister A, Anderson ME. Glutathione. Annu Rev Biochem 1983;52:711–60. [DOI] [PubMed] [Google Scholar]
- 6. Masella R, Di Benedetto R, Vari R, Filesi C, Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem 2005;16:577–86. [DOI] [PubMed] [Google Scholar]
- 7. Chiang AC, Massague J. Molecular origins of cancer molecular basis of metastasis. N Engl J Med 2008;359:2814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen Y, Shertzer HG, Schneider SN, Nebert DW, Dalton TP. Glutamate cysteine ligase catalysis: dependence on ATP and modifier subunit for regulation of tissue glutathione levels. J Biol Chem 2005;280:33766–74. [DOI] [PubMed] [Google Scholar]
- 9. Fiorillo M, Sotgia F, Sisci D, Cappello AR, Lisanti MP. Mitochondrial ‘power’ drives tamoxifen resistance: NQO1 and GCLC are new therapeutic targets in breast cancer. Oncotarget 2017;8:20309–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu CW, Hua KT, Li KC, et al. Histone methyltransferase G9a drives chemotherapy resistance by regulating the glutamate-cysteine ligase catalytic subunit in head and neck squamous cell carcinoma. Mol Cancer Ther 2017;16:1421–34. [DOI] [PubMed] [Google Scholar]
- 11. Huang Y, Dai Z, Barbacioru C, Sadee W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance. Cancer Res 2005;65:7446–54. [DOI] [PubMed] [Google Scholar]
- 12. Lo M, Wang YZ, Gout PW. The x(c)- cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J Cell Physiol 2008;215:593–602. [DOI] [PubMed] [Google Scholar]
- 13. Li XX, Lewis MT, Huang J, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 2008;100:672–9. [DOI] [PubMed] [Google Scholar]
- 14. Takaishi S, Okumura T, Tu SP, et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 2009;27:1006–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang S, Balch C, Chan MW, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res 2008;68:4311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ishimoto T, Nagano O, Yae T, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011;19:387–400. [DOI] [PubMed] [Google Scholar]
- 17. Sosulski A, Horn H, Zhang L, et al. CD44 splice variant v8-10 as a marker of serous ovarian cancer prognosis. PLoS One 2016;11:e0156595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Go SI, Ko GH, Lee WS, et al. CD44 variant 9 serves as a poor prognostic marker in early gastric cancer, but not in advanced gastric cancer. Cancer Res Treat 2016;48:142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoshikawa M, Tsuchihashi K, Ishimoto T, et al. xCT inhibition depletes CD44v-expressing tumor cells that are resistant to EGFR-targeted therapy in head and neck squamous cell carcinoma. Cancer Res 2013;73:1855–66. [DOI] [PubMed] [Google Scholar]
- 20. Shitara K, Doi T, Nagano O, et al. Phase 1 study of sulfasalazine and cisplatin for patients with CD44v-positive gastric cancer refractory to cisplatin (EPOC1407). Gastric Cancer 2017;20:1004–9. [DOI] [PubMed] [Google Scholar]
- 21. Shitara K, Doi T, Nagano O, et al. Dose-escalation study for the targeting of CD44v+ cancer stem cells by sulfasalazine in patients with advanced gastric cancer (EPOC1205). Gastric Cancer 2017;20:341–9. [DOI] [PubMed] [Google Scholar]
- 22. Miyoshi H. Gene delivery to hematopoietic stem cells using lentiviral vectors. Methods Mol Biol 2004;246:429–38. [DOI] [PubMed] [Google Scholar]
- 23. Sakatani T, Maemura K, Hiyama N, et al. High expression of IRE1 in lung adenocarcinoma is associated with a lower rate of recurrence. Jpn J Clin Oncol 2017;47:543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci 2009;34:176–88. [DOI] [PubMed] [Google Scholar]
- 25. Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 2007;47:89–116. [DOI] [PubMed] [Google Scholar]
- 26. Ravindranath AK, Kaur S, Wernyj RP, et al. CD44 promotes multi-drug resistance by protecting P-glycoprotein from FBXO21-mediated ubiquitination. Oncotarget 2015;6:26308–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.