Abstract
Within the root meristem of flowering plants is a group of mitotically inactive cells designated the quiescent center (QC). Recent work links the quiescent state to high levels of the growth regulator auxin that accumulates in the QC via polar transport. This in turn results in elevated levels of the enzyme ascorbic acid oxidase (AAO), resulting in a reduction of ascorbic acid (AA) within the QC and mitotic quiescence. We present evidence for additional interactions between auxin, AAO, and AA, and report that, in vitro, AAO oxidatively decarboxylates auxin, suggesting a mechanism for regulating auxin levels within the QC. We also report that oxidative decarboxylation occurs at the root tip and that an intact root cap must be present for this metabolic event to occur. Finally, we consider how interaction between auxin and AAO may influence root development by regulating the formation of the QC.
It has long been known that auxin is transported from the shoot to the root in a polar manner (Scott and Wilkins, 1968; Morris et al., 1969). However, little attention has been given to the fate of the auxin accumulating in the root tip. Since root cells are significantly more sensitive to auxin concentrations than are cells of the shoot (Thimann, 1937), it seems unlikely that high concentrations of auxin could be tolerated in a growing root tip.
Our previous work has provided evidence for polar transport of indole-3-acetic acid (IAA) and its accumulation in maize (Zea mays) root tips (Kerk and Feldman, 1994, 1995). We showed that this resulted in an increased level of ascorbic acid oxidase (AAO) mRNA, protein, and enzyme activity, as well as the localized depletion of ascorbic acid (AA) within the quiescent center (QC), a group of mitotically inactive cells within the root meristem. Since AA is necessary for the G1 to S transition in the cell cycle in root tips (Liso et al., 1988), and regulation of AA is thought to be dependent on AAO, we proposed that its depletion in root tips may be responsible for the formation and maintenance of the QC (Kerk and Feldman, 1995).
In the course of these experiments we discovered additional interactions between auxin and AAO. Using the radish (Raphanus sativus) root auxin bioassay system (Blakely et al., 1982), we found that increased levels of AAO in root segments in sterile culture decreased the response of radish roots to auxin. Moreover, if auxin is first reacted in vitro with AAO and then bioassayed, root cultures fail to exhibit a normal response.
Finally, we report on a possible mechanism underlying these auxin responses. We report here that AAO oxidatively decarboxylates auxin in vitro, suggesting a mechanism for regulating auxin levels within the QC and other root tissues. Oxidative decarboxylation occurs at the root tip, and an intact root cap must be present for this metabolic event to occur. These observations provide a robust model for the organization and functioning of the maize root tip, in which auxin transported from the shoot both regulates and is regulated by the level of AAO, which then regulates entry into the S phase by controlling levels of AA.
MATERIALS AND METHODS
Plant Growth Conditions
Maize (Zea mays var Merit, Asgrow Seed Co., Kalamazoo, MI) caryopses were imbibed and germinated in the dark at 25°C for 2 d. Bioassays were carried out on 3-d-old radish (Raphanus sativus Scarlet Globe, Asgrow Seed Co., Kalamazoo, MI) roots. Radish seeds were surface-sterilized and germinated aseptically in the dark at 25°C for 3 d. The terminal 2- to 5-mm tips were removed and 1-cm segments were placed into liquid culture. Medium was complete Murashige and Skoog (MS) medium (Murashige and Skoog, 1962) with added CuSO4, auxin, or auxin previously reacted with AAO, as reported in “Results.” AAO activity was assayed as described in Kerk and Feldman (1995).
In Vitro Auxin Metabolism
In a total volume of 0.5 mL, 10−7 to 10−8 m IAA (Sigma-Aldrich, St. Louis) ± 5-3H-IAA and/or 1-14C-IAA were incubated with 50 μm p-coumaric acid, 100 μm Mn2+, and 25 units of AAO (Biozyme Labs, San Diego) in a 7 mm Na-phosphate buffer, pH 5.3, at 27°C in the dark on a shaker for 1 to 4 h. For assessing decarboxylation activity, aliquots were periodically removed from the incubation mixture and counted on a scintillation counter set (model 60001C, Beckman Instruments, Fullerton, CA) to separately window 3H and 14C. To determine the degree to which this reaction is mediated by a copper-containing oxidase (such as AAO), we also performed the incubation in the presence of 1 to 5 mm bathocuproinedisulfonic acid (Sigma-Aldrich), a compound with high specificity for inhibiting copper-containing enzymes (Li et al., 1996), and in 1 mm diethyldithiocarbamate (DDC) (Sigma-Aldrich), another copper-chelating agent and inhibitor of AAO (Wang and Fuast, 1992).
For HPLC analysis, the incubation mixture was acidified to pH 3.0, extracted three times against ethyl acetate, dried, solubilized in a small amount of methanol, and subjected to HPLC analysis on a HPLC (Kratos, Chestnut Ridge, NY) using a reversed phase small pore silica C18 10-μm column (4.6 mm × 25 cm; Vydac, Hesperia, CA) and a 40-min linear gradient of 5% to 30% (v/v) acetonitrile in 0.08% (v/v) trifluoroacetic acid with a flow rate of 0.7 mL/min; fractions were collected every 30 s. Elution profiles were obtained by monitoring at 254 nm, and fractions representing products of IAA catabolism were collected, characterized, and counted as described above. For each run typically 90% of the injected radioactivity was recovered. After identifying the radioactive elution profiles of the IAA metabolites, we scaled up the reaction 10× and repeated the previously described incubation but instead used non-radioactive auxin. The products were then separated by HPLC and the two fractions corresponding to the radiolabeled IAA metabolites collected and subjected to diffuse-reflectance Fourier transform infrared (IR) spectroscopy. In preparation for IR analysis, each HPLC fraction was dried down under a stream of N2 and the residue was dissolved in 20 μL of CH2Cl2. The solution was applied to powdered IR-grade potassium bromide in a 3-mm microsampling cup and the IR spectrum (32 scans) obtained after evaporation of the CH2Cl2. The same HPLC fractions were also examined using UV spectroscopic analysis.
In Vivo Auxin Metabolism
For monitoring in vivo auxin metabolism the terminal 2 cm of roots from aseptically grown maize seedlings were excised from 48-h-old seedlings and placed tip down into microfuge tubes containing 0.5 mL of one-half-strength MS medium, pH 6.8, supplemented with 1% Suc and 0.9% agar. For some experiments roots were decapped (Kerk and Feldman, 1995) prior to excising the terminal 2 cm, which, as before, were placed tip down into the microfuge tubes. On the basal cut surface (the surface protruding from the tube) was placed a 1% agar block (approximately 1 mm2) containing 1 × 10−9 m non-radioactive IAA, plus 10−8 m 5-3H-IAA (specific activity, 16.7 Ci/mm; Amersham) and/or 1-14C-IAA (specific activity, 9.4 mCi/mm; Sigma). The roots with the attached agar blocks were returned to a moistened chamber and incubated for 12 h in the dark. For most experiments 40 roots were used.
Following incubation the root was divided into three sections: the distal terminal millimeter, the subtending 1 cm (root minus terminal millimeter), and the 1- to 2-cm section contacting the agar block. The terminal millimeter and subtending 1-cm sections of roots were harvested separately, pooled, homogenized, extracted in 80% (v/v) methanol (McDougall and Hillman, 1978), and subjected to HPLC analysis as described above. Fractions were collected every 30 s. To determine whether any decarboxylation occurred, 14C- and 3H-IAA in a known ratio were together incubated with root tissues. At the end of the 12-h incubation period, the ratios of 14C- and 3H-IAA were compared for each fraction using a scintillation counter set to separately window 3H and 14C, and the counts were normalized for any change in the ratio (indicating a loss of 14C). 14C-3H ratios were calculated after setting the background to 50 dpm.
RESULTS
Bioassay Results
Response of Cultured Roots to Exogenous IAA
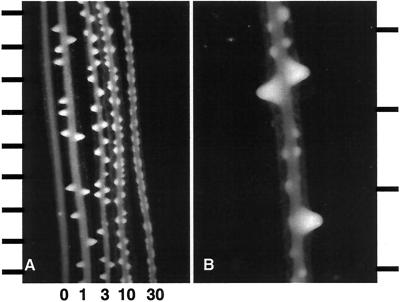
To establish a baseline for experiments involving the interaction between IAA and AAO, we examined the effect of supplying IAA in vitro to radish root segments. Roots exposed to a range of concentrations of IAA initiated increasing numbers of lateral roots, but the outgrowth of these roots was progressively inhibited (Fig. 1A). This result confirms the work of previous investigators (Blakely et al., 1982). Furthermore, we found that when roots that had produced laterals in response to a particular concentration of exogenous auxin were subsequently exposed to a higher concentration of IAA, numerous supernumerary lateral roots spaced between existing ones were formed (Fig. 1B).
Figure 1.
Radish root segments cultured with IAA. A, Root segments cultured for 48 h in MS medium containing from left to right, 0, 1, 3, 10, and 30 μm IAA. Scale bar increments indicate 1 mm. B, Root segment cultured for 24 h in MS plus 3 μm IAA and then shifted to MS plus 30 μm IAA for 48 h. Scale bar increments indicate 0.5 mm.
Response of Cultured Roots to Increased AAO
Esaka et al. (1992) have demonstrated a marked increase in ascorbate oxidase protein in pumpkin cells by adding copper to the culture medium. We used this method to cause a 1.7-fold increase in AAO in seedling radish roots grown in culture supplemented with 10 μm CuSO4. We also measured the response of these roots to auxin by determining the lateral root frequency, which increases with auxin concentration (Blakely et al., 1982; Kerk, 1990) (Fig. 1A).
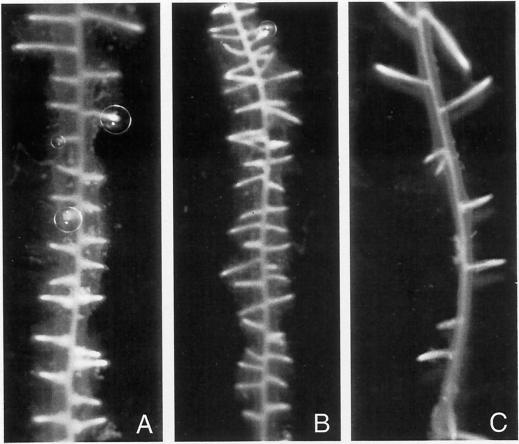
When auxin was added to the culture medium simultaneously with copper, there was no diminution in the number of lateral roots (Fig. 2, A and B). However, when roots were preincubated in a copper-containing culture medium for 24 h before auxin was added, there was a significant decrease in the number of lateral roots produced, yet these roots were healthy and showed no toxic effect of the copper (Fig. 2C). This suggests that levels of AAO in root tissues affect the auxin response.
Figure 2.
Radish root segments cultured in MS medium with 3 μm IAA only for 72 h (A); with 3 μm IAA and 10 μm CuSO4 added simultaneously for 72 h (B); roots incubated first for 24 h with 10 μm CuSO4 and then 3 μm IAA was added for a further 48 h (C).
In Vitro Reactions of IAA and AAO as Visualized by the Bioassay
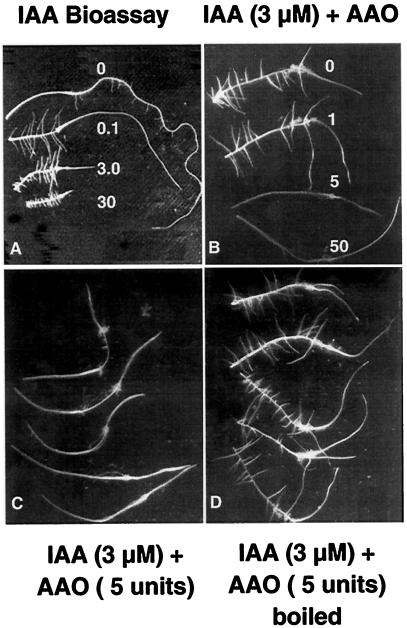
Experiments were carried out to determine if AAO could directly react with auxin as a substrate. In vitro reactions were set up containing auxin and AAO in buffers and at concentrations comparable to those found at physiological conditions. The reaction mixtures were filter-sterilized and used as the auxin source for bioassays. Reaction mixtures with increasing units of AAO resulted in a marked decrease in the number of lateral roots and failed to inhibit tip growth, as would be expected in a standard auxin bioassay in roots (Fig. 3, A–C). When the AAO was denatured by boiling before being reacted with IAA in the buffer, lateral root formation was similar to that in roots treated with IAA alone (Fig. 3D).
Figure 3.
Radish root segments cultured in MS and IAA, or IAA prereacted with AAO and then filter-sterilized and added to cultures. A, Effect of increasing concentrations of IAA. As the concentration is increased, tip growth becomes inhibited and lateral root frequency increases. B, Effects of IAA prereacted with increasing amounts of AAO. As AAO units are increased, inhibition of tip growth and lateral root frequency are decreased. C, Representative repetition of the effect on roots in culture with IAA pretreated with 5 units of AAO. The original tip region of the root before elongation growth in culture is marked by the swollen cortical region located proximally about one-third the distance to the present tip. D, Radish root segments cultured with 3 μm IAA reacted with 5 units of AAO previously boiled to denature the protein.
Tips of Lateral Root Primordia Lower the Effective Auxin Levels in Roots in Culture
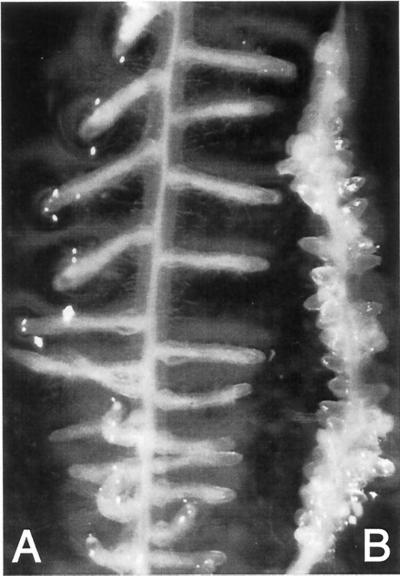
Roots were cultured in 3 μm IAA. The tips of the lateral roots that developed in response to IAA treatment were excised and the roots were returned to the same culture medium. The roots with decapitated tips formed a large number of supernumerary laterals spaced between the stumps of the pre-existing laterals. In control roots in which needle punctures were made in the cortical region but tips were left intact (to simulate the wounding effect of decapitation), no supernumerary laterals were formed (Fig. 4).
Figure 4.
Effect of removing lateral root tips on new lateral root initiation. A, Root cultured for 5 d in MS plus 3 μm IAA. B, Root grown as in A, but after 5 d lateral root tips were clipped off, leaving stumps, and placed back in 3 μm IAA medium. New laterals were initiated at high frequency on the parent root.
Biochemical Results
The bioassay experiments described in the preceding sections have provided evidence that AAO and auxin interact to decrease the effective auxin concentration in roots. To investigate this reaction, its products, and the likely reaction mechanism, we examined HPLC profiles of reactions carried out with AAO and radioactively labeled auxin.
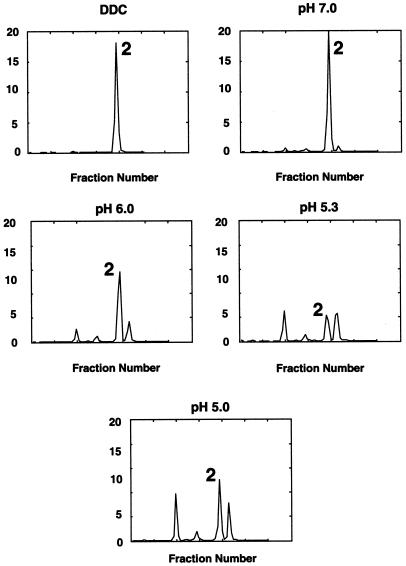
We identified two major metabolic products of the reaction that had a pH optimum of 5.3 (Fig. 5). The reaction was completely inhibited when DDC was added to the reaction mix (Fig. 5). DDC is an inhibitor of AAO that chelates copper away from this blue copper protein, permitting the subunits to dissociate (Wang and Fuast, 1992). The addition of ascorbic acid, dehydroascorbate, and ascorbate free radical to reaction mixes with or without AAO had no effect on auxin metabolism (data not shown).
Figure 5.
HPLC profiles of the reaction products of AAO and 3H-IAA. The pH optimum was pH 5.3 and the inclusion of DDC, an inhibitor of AAO, completely blocked the reaction. Peak 2, IAA.
HPLC Elution Profiles of in Vitro 14C-IAA and/or 3H-IAA Catabolism
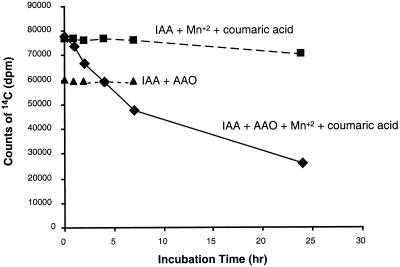
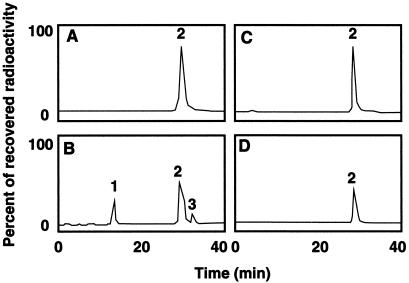
IAA is oxidatively decarboxylated in vitro by AAO, forming as one of the primary products oxindole-3-methanol (Figs. 6 and 7). Identification of this compound was based on its IR spectrum (diffuse reflectance on powdered KBr) (3,397 cm−1 [OH], 3,207 cm−1 [NH], 1,704 cm−1, and 1,621 cm−1 [C = O]) and on its UV spectrum (in methanol, λmax = 250 nm with a shoulder at 280 nm) (Hinman and Lang, 1965; Kobayashi et al., 1984). Additional support for this identification was obtained from the non-biological conversion of oxindole-3-methanol to a compound identified as 3-methyleneoxindole, based on its twin UV absorption peaks at λmax 248 and 252 nm (Hinman and Lang, 1965). This in vitro activity is dependent on the addition of Mn2+ and p-coumaric acid (Fig. 6). The addition of bathocuproinedisulfonic acid, a compound with high specificity for inhibiting copper-containing enzymes, resulted in a decrease in the decarboxylation of IAA, suggesting that the decarboxylating activity was due to a copper-containing enzyme such as AAO (Li et al., 1996) (Table I).
Figure 6.
AAO facilitates IAA decarboxylation. Oxidative decarboxylation of 1-14C-IAA, measured as a loss of 14CO2. Data reflect the amount of radioactivity in a 75-μL aliquot after various periods of incubation with and without AAO and with and without co-factors (Mn2+ and p-coumaric acid).
Figure 7.
HPLC elution profiles of in vitro 14C-IAA and/or 3H-IAA catabolism. A, 5-3H-IAA plus Mn2+ plus p-coumaric acid. B, 5-3H-IAA plus Mn2+ plus p-coumaric acid plus AAO. C, 1-14C-IAA plus Mn2+ plus p-coumaric acid. D, 1-14C-IAA plus AAO plus Mn2+ plus p-coumaric acid. Peak 1, Oxindole-3-methanol, the primary in vitro catabolic product; peak 2, IAA; peak 3, 3-methyleneoxindole, a non-biological breakdown product of peak 1.
Table I.
Effects of an inhibitor of copper-containing oxidases on IAA decarboxylation
| Incubation Time | +BA | −BA |
|---|---|---|
| h | dpm | |
| 0 | 69,203 | 67,909 |
| 2 | 67,528 | 61,068 |
| 5 | 61,739 | 48,920 |
Effect of bathocuproinedisulfonic acid (BA), an inhibitor of copper-containing enzymes, on the oxidative decarboxylation of 1-14C-IAA in the presence of AAO, Mn+2, and p-coumaric acid. Data reflect the amount of radioactivity in a 75 μL-aliquot after various periods of incubation with and without BA.
HPLC Elution Profiles of in Vivo 14C-IAA and/or 3H-IAA Catabolism
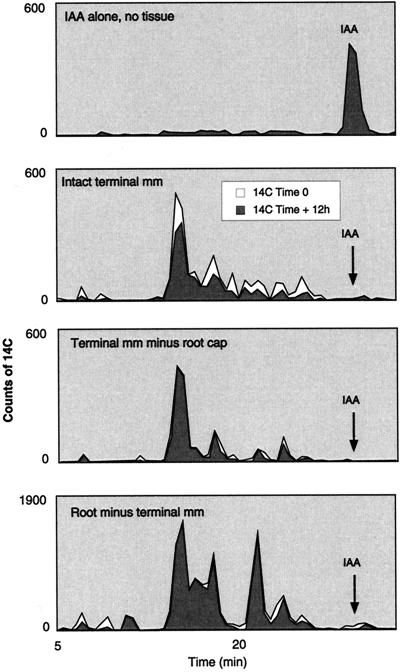
At least 30% of polarly transported auxin is oxidatively decarboxylated in vivo by intact root tips (terminal millimeter) (Fig. 8). Oxidative decarboxylation in the root occurs almost exclusively at the tip (Fig. 8). Excision of the cap prevents decarboxylation (Fig. 8).
Figure 8.
HPLC elution profiles of 1-14C-IAA metabolites in various maize root tissues. Black portions for each curve represent the actual recoverable 14C counts in each fraction after a 12-h incubation. The white portions under the curve represent the 14C counts that would have been recovered had there not been any decarboxylation. To determine which fractions show decarboxylation, 1-14C- and 5-3H-IAA in a known ratio were together incubated with root tissues. At the end of the 12-h incubation period the ratios of 14C- and 3H-IAA were compared for each fraction and the curve normalized for any change in the ratio (indicating a loss of 14C) (white portion of the curve). The only significant changes in the ratio are in IAA metabolites in intact terminal millimeter root tissues. For all other treatments the ratios are more or less unchanged and the white and black curves overlap. Note that all root tissues completely catabolize the radiolabeled IAA.
DISCUSSION
The major findings reported here suggest that: (a) elevated levels of AAO in roots are correlated with a decreased auxin response, as visualized by bioassays in radish root culture; and (b) the decreased auxin response may be due to the fact that AAO oxidatively decarboxylates auxin. This reaction renders the reacted auxin ineffective in stimulating an auxin response in root cultures. Support for the likely occurrence of this reaction was demonstrated by in vitro experiments and in vivo through bioassays for auxin response and by recovering metabolites of radiolabeled auxin generated through oxidative decarboxylation in intact maize root tips. We suggest that AAO, previously reported to be highly expressed in the root meristem, may function in vivo in auxin catabolism. This was demonstrated visually in the bioassays.
In the experiment shown in Figure 4, the tips, or putative auxin-catabolizing regions were removed from laterals on a cultured parent root and the result was a dramatic production of supernumerary lateral root primordia on the parent root. If roots with an existing, stable pattern of lateral roots are shifted to medium with higher auxin concentration, as shown in Figure 1B, this same effect is demonstrated. Our interpretation of this tip removal experiment is that we have removed the main sites of auxin metabolism in root tissue and, once removed, the parent root is now in an environment of high auxin and reacts by organizing new lateral root meristems, thus generating new root tips to replace these sites of auxin catabolism.
Until recently, it was believed that there were two major mechanisms for auxin turnover in plants: (a) a peroxidase- and/or oxidase-mediated oxidative decarboxylation, resulting in a loss of the number 1 carbon (the carboxyl group) from the indole side chain; and (b) a non-decarboxylative pathway (Normanly, 1997; Östin et al., 1998). While the non-decarboxylative pathway is today accepted as the likely process mediating free auxin levels, the significance of the decarboxylation pathway has recently been challenged (Normanly, 1997; Östin et al., 1998). Because the products of in vitro decarboxylation could not be shown to occur in vivo (Reinecke and Bandurski, 1983; Ernstsen et al., 1987; Bandurski et al., 1995), oxidative decarboxylation is now considered an unimportant, perhaps even an artifactual, route for auxin turnover. The reason for this conclusion is evident when one considers the plant materials (primarily shoot tissues) recently used to study auxin catabolism (Normanly, 1997; Östin et al., 1998). Root tissues have rarely been employed, and, when occasionally used, either the terminal 1 to 4 mm was excised prior to initiating IAA turnover experiments or the entire plant was extracted, which could lead to a masking of any decarboxylation occurring in the small amounts of tissue comprising the root tips (Nonhebel et al., 1983; Sztein et al., 1995). Additionally, when decarboxylation products have been detected in extracts, these products have often been viewed as resulting from experimentally induced (artifactual) exposure of auxin to peroxidases (Bandurski et al., 1995; Normanly, 1997; Östin et al., 1998). However, our results support a specific (non-artifactual) capacity of root tips to decarboxylate IAA, as suggested by several previous investigators (Morris et al., 1969; Bourbouloux and Bonnemain, 1974; Pernet and Pilet, 1979).
Here we have shown that at least 30% of polarly transported auxin is oxidatively decarboxylated in vivo by intact root tips (terminal millimeter) (Fig. 8). Oxidative decarboxylation in the root occurs almost exclusively at the tip (Fig. 8). Excision of the cap prevents decarboxylation (Fig. 8). We also show that IAA is oxidatively decarboxylated in vitro by AAO, forming as one of the primary products oxindole-3-methanol (Fig. 7).
Although we have not yet identified the in vivo-generated, auxin-decarboxylated products, it is clear that decarboxylation is a significant route for free auxin turnover in root tips (as much as 30% of the auxin is oxidatively decarboxylated) (Fig. 8). Moreover, our results do not support the contention that these decarboxylated products arise from an artifactual mixing of IAA and a peroxidase/oxidase, because if this were so, decarboxylation of IAA in both intact and decapped root tips would have been seen, and instead decarboxylation only occurred in intact tips (Fig. 8).
Furthermore, recent data with transgenic plants show that significant alterations in endogenous peroxidases (increases or decreases) have no effect on endogenous auxin levels (Lagrimini et al., 1992). This was shown for either a 10-fold increase or a 90% decrease in peroxidase levels (Lagrimini et al., 1992). These data thus argue against the likelihood that the observed in vivo IAA decarboxylation was due to peroxidase activity.
Previously we advanced a model in which polarly transported auxin accumulated at the root apex, resulting in an enhancement in AAO activity, a localized depletion of AA, and as a consequence, the formation of the QC (Kerk and Feldman, 1995). Here we add an additional step to that model by presenting evidence that AAO can also metabolize auxin. We suggest that in the intact root tip, AAO functions as an auxin oxidase regulating endogenous auxin levels at the root tip. Our previous study localized high levels of AAO specifically in the QC and cap of intact root tips (Kerk and Feldman, 1995).
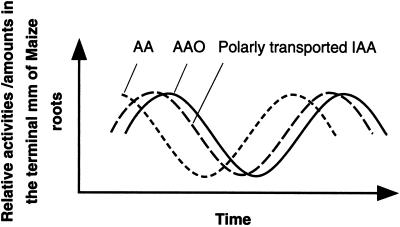
One way of integrating the proposed auxin/AAO interactions in regard to the maintenance of the QC is to suggest that a classical feedback loop involving AAO and auxin exists: high auxin enhances AAO activity, which leads to a decline in auxin, promoting a decline in AAO, allowing auxin levels to again increase (Fig. 9). Layered over this hypothesized feedback loop we suggest a parallel regulation in the levels of AA (Fig. 9). While it is widely known that AAO is present in most if not all higher plants, its regulation and biological function are not clearly defined (Arrigoni, 1994; Córdoba and González-Reyes, 1994; Smirnoff, 1996). That ascorbate is a substrate in vivo for AAO is highly probable (Avigliano and Finazzi-Agro, 1997). Recent data reported by Kato and Esaka (1996, 1999) showed correlations between levels of AAO mRNA and ascorbate metabolism in synchronous, non-synchronous, and elongating cultured tobacco cells. Kato and Esaka (1999) proposed that AAO expression and metabolic reaction are under control of the cell cycle and may be involved in the process of cell elongation.
Figure 9.
Hypothetical scheme of possible interactions between polarly transported auxin (IAA), AA, and AAO.
Our earlier data showing low levels of AA in regions with high AAO support this suggestion of cell cycle regulation of AAO, and as suggested earlier, could result in the formation of the QC. Therefore, the maintenance and functioning of the QC may be a consequence not only of the accumulation of auxin (Kerk and Feldman, 1995), but may also be a result of the regulation by auxin of an auxin-catabolizing enzyme. Whether AAO could regulate endogenous auxin levels within the QC has not yet been proven. However, its in vitro ability to oxidize auxin, its high concentration within the QC, and the property of auxin to regulate AAO mRNA and protein levels and activity (Esaka et al., 1992; Kerk and Feldman, 1995) argue in favor of in vivo catabolic interactions between AAO and auxin.
Many interrelated factors need to be considered when proposing a complex interaction between a hormone, an abundant metabolic enzyme, and the cell cycle. Kato and Esaka (1999) suggested a role for AAO-mediated metabolism in the apoplast during the process of cell elongation. Our findings showing a pH optimum of 5.3 for the oxidative decarboxylation of auxin are consistent with pH measurements in cell walls. In this regard, and considering the acid-growth hypothesis (Goodwin and Mercer, 1983), it is interesting to speculate on the possible metabolism of auxin in the walls by AAO. One could speculate that the subcellular pH and other forms of compartmentalization play important roles in favoring different phases of the feedback loop at different times of the cell cycle, in different regions of the cell, and even in different regions of the root.
Thus, while oxidative decarboxylation may be a minor pathway for regulating auxin levels in the whole plant, the occurrence of this pathway within the root may have consequences for root development. Determining whether this pathway operates in roots should provide an understanding of the establishment, maintenance, and function of the QC, and compartmentalization of the cap and other classic zones of the root such as the zone of elongation.
ACKNOWLEDGMENTS
We thank Ian Sussex for discussions and review of the manuscript, Steve Ruzin for his gracious and skilled assistance in figure preparation, Larry Cool of the University of California Forest Products Laboratory for the IR analysis, Sarah Reisinger for assistance with AAO assays, Richard Malkin for biochemical advice, and Robert Bandurski for sharing his enthusiasm and thoughts on auxin metabolism.
Footnotes
This work was supported by the National Science Foundation (grant no. IBN–9404842).
LITERATURE CITED
- Arrigoni O. Ascorbate system in plant development. J Bioenerg Biomembr. 1994;26:407–419. doi: 10.1007/BF00762782. [DOI] [PubMed] [Google Scholar]
- Avigliano L, Finazzi-Agro A. In: Multi-Copper Oxidases. Messerschmidt A, editor. Singapore: World Scientific; 1997. [Google Scholar]
- Bandurski RS, Cohen JD, Slovin JP, Reinecke DM. Auxin biosynthesis and metabolism. In: Davies P, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 39–65. [Google Scholar]
- Blakely LM, Durham M, Evans TA, Blakely RM. Experimental studies on lateral root formation in radish seedling roots: I. General methods, developmental stages, and spontaneous formation of laterals. Bot Gaz. 1982;143:341–352. [Google Scholar]
- Bourbouloux A, Bonnemain J-L. Transport, distribution et metabolisme de l'auxine dans la racine de Vicia faba L. apres application de [14C]AIA ou de [3H] AIA sur le bourgeon. Planta. 1974;119:169–182. doi: 10.1007/BF00429042. [DOI] [PubMed] [Google Scholar]
- Córdoba F, González-Reyes JA. Ascorbate and plant cell growth. J Bioenerg Biomembr. 1994;26:399–405. doi: 10.1007/BF00762781. [DOI] [PubMed] [Google Scholar]
- Ernstsen A, Sandberg G, Lundstrom K. Identification of oxindole-3-acetic acid in seeds of Pinus sylvestris. Planta. 1987;172:47–52. doi: 10.1007/BF00403027. [DOI] [PubMed] [Google Scholar]
- Esaka M, Fujisawa K, Goto M, Kisu Y. Regulation of ascorbate oxidase expression in pumpkin by auxin and copper. Plant Physiol. 1992;88:656–660. doi: 10.1104/pp.100.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin TW, Mercer EI. Introduction to Plant Biochemistry. Elmsford, NY: Pergamon Press; 1983. [Google Scholar]
- Hinman RL, Lang J. Peroxidase-catalyzed oxidation of indole-3-acetic acid. Biochemistry. 1965;4:144–158. doi: 10.1021/bi00877a023. [DOI] [PubMed] [Google Scholar]
- Kato N, Esaka M. cDNA cloning and gene expression of ascorbate oxidase in tobacco. Plant Mol Biol. 1996;30:833–837. doi: 10.1007/BF00019015. [DOI] [PubMed] [Google Scholar]
- Kato N, Esaka M. Changes in ascorbate oxidase gene expression and ascorbate levels in cell division and cell elongation in tobacco cells. Physiol Plant. 1999;105:321–329. [Google Scholar]
- Kerk N, Feldman L. The quiescent center in the roots of maize: initiation, maintenance and role in organization of the root apical meristem. Protoplasma. 1994;183:100–106. [Google Scholar]
- Kerk NM. Gene expression during meristem initiation and organization. PhD thesis. New Haven, CT: Yale University; 1990. [Google Scholar]
- Kerk NM, Feldman LJ. A biochemical model for the initiation and maintenance of the quiescent center: implications for organization of root meristems. Development. 1995;121:2825–2833. [Google Scholar]
- Kobayashi S, Sugioka K, Nakano M, Tero-Kubota S. Analysis of the stable end products and intermediates of oxidative decarboxylation of indole-3-acetic acid by horseradish peroxidase. Biochemistry. 1984;23:4589–4594. [Google Scholar]
- Lagrimini ML, Vaughn J, Finer J, Klotz K, Rubaihayo P. Expression of a chimeric tobacco peroxidase gene in transgenic tomato plants. Plant Cell. 1992;2:7–18. [Google Scholar]
- Li Y, Kuppusamy P, Zweier JL, Trush MA. Role of Cu-Zn-superoxide dismutase in xenobiotic activation: II. Biological effects resulting from the Cu-Zn superoxide dismutase-accelerated oxidation of the benzene metabolite 1,4-hydroquinone. Mol Pharmacol. 1996;49:412–421. [PubMed] [Google Scholar]
- Liso BR, Innocenti AM, Bitonti MB, Arrigoni O. Ascorbic acid-induced progression of quiescent centre cells from G1 to S phase. New Phytol. 1988;110:469–471. [Google Scholar]
- McDougall J, Hillman JR. Analysis of indole-3-acetic acid using GC-MS techniques. In: Hillman JR, editor. Isolation of Plant Growth Substances. Cambridge, UK: Cambridge University Press; 1978. pp. 1–25. [Google Scholar]
- Morris DA, Briant RE, Thomson PG. The transport and metabolism of 14C-indole acetic acid in intact pea seedlings. Planta. 1969;89:178–197. doi: 10.1007/BF00386984. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Nonhebel HM, Crozier A, Hillman JR. Analysis of [14C]indole-3-acetic acid metabolites from primary roots of Zea maysseedlings using reverse-phase high-performance liquid chromatography. Physiol Plant. 1983;57:129–134. [Google Scholar]
- Normanly J. Auxin metabolism. Physiol Plant. 1997;100:431–442. [Google Scholar]
- Östin A, Kowalyczk M, Bhalerao RP, Sandberg G. Metabolism of indole-3-acetic acid in Arabidopsis. Plant Physiol. 1998;118:285–296. doi: 10.1104/pp.118.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet J-J, Pilet P-E. Importance of the tip on the (5-3H)-indol-3yl-acetic acid transport in maize roots. Z Pflanzenphysiol. 1979;94:273–279. [Google Scholar]
- Reinecke DM, Bandurski RS. Oxindole-3-acetic acid, an indole-3-acetic acid catabolite in Zea mays. Plant Physiol. 1983;86:868–872. doi: 10.1104/pp.71.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott TK, Wilkins MB. Auxin transport in roots II: polar flux of IAA in Zearoots. Planta. 1968;83:323–334. doi: 10.1007/BF00387614. [DOI] [PubMed] [Google Scholar]
- Smirnoff N. The function and metabolism of ascorbic acid in plants. Ann Bot. 1996;78:661–669. [Google Scholar]
- Sztein AE, Cohen JD, Slovin JP, Cooke TJ. Auxin metabolism in representative land plants. Am J Bot. 1995;82:1514–1521. [Google Scholar]
- Thimann KV. On the nature of inhibitions caused by auxin. Am J Bot. 1937;24:407–412. [Google Scholar]
- Wang SY, Fuast M. Ascorbic acid oxidase activity in apple buds: relation to thidiazuron-induced lateral budbreak. Hortscience. 1992;27:1102–1105. [Google Scholar]