Abstract
Almost one third of the three million people in China suffering severe deafness are children, and 50% of these cases are believed to have genetic components to their etiology. Newborn hearing genetic screening can complement Universal Neonatal Hearing Screening for the diagnosis of congenital hearing loss as well as identifying children at risk for late-onset and progressive hearing impairment. The aim of this joint academic and Ministry of Health project was to prototype a cost effective newborn genetic screen in a community health setting on a city-wide level, and to ascertain the prevalence of variation at loci that have been associated with non-syndromic hearing loss. With the participation of 143 local hospitals in the city of Wuhan, China we screened 142,417 neonates born between May 2014 and Dec. 2015. The variants GJB2 c.235delC, SLC26A4 c.919-2A>G, and mitochondrial variants m.1555A>G and m.1494C>T were assayed using real time PCR. Newborns found to carry a variant were re-assayed by sequencing in duplicate. Within a subset of 707 newborns we assayed using real-time PCR and ARMS-PCR to compare cost, sensitivity and operating procedure. The most frequent hearing loss associated allele detected in this population was the 235delC variant in GJB2 gene. In total, 4289 (3.01%) newborns were found to carry at least one allele of either GJB2 c.235delC, SLC26A4 c.919-2A>G or two assayed MT-RNR1 variants. There was complete accordance between the real-time PCR and the ARMS PCR, though the real-time PCR had a much lower failure rate. Real-time PCR had a lower cost and operating time than ARMS PCR. Ongoing collaboration with the participating hospitals will determine the specificity and sensitivity of the association of the variants with hearing loss at birth and arising in early childhood, allowing an estimation of the benefits of newborn hearing genetic screening in a large-scale community setting.
Introduction
Hearing loss is one of the most common human disorders, and genetic causes contribute to more than half of congenital hearing loss cases[1, 2]. Although 30,000 neonates with hearing loss are identified each year in China via universal neonatal hearing screening, the number of people with hearing defects who are not registered as such by the government is far greater[3]. In addition, it has been shown that the prevalence of permanent non-syndromic hearing loss (NSHL) increases about 50 percent during childhood, and doubles during adolescence. This is due to delayed detection of congenital hearing loss, late-onset of hearing loss, and aminoglycoside-induced hearing loss[4, 5]. Early detection of hearing loss in newborns is very important, hearing-impaired neonates show improved outcome when their hearing loss is recognized before 6 months after birth[6–10]. Newborn deafness genetic screening can be a complementary tool to traditional physical hearing tests[11]. More than 20 cities in China have carried out newborn deafness genetic screening projects, but few large studies have been performed and the frequency of common hearing loss mutations across the Chinese population has not been well estimated[1, 2].
Deafness is characterized by its etiological heterogeneity. It is estimated that about 50% of cases of childhood hearing loss are associated with genetic factors. Loci in more than 70 genes have been found to be associated with NSHL[1, 2]. Additionally, genetic variants with known associations with congenital hearing loss have been identified[12, 13].Variations in GJB2, SLC26A4 and MT-RNR1 are the most common variants associated with NSHL in China[1]. Mutations in GJB2, encoding gap junction beta 2 protein (connexin 26), are the most common variants linked to non-syndromic hearing impairment worldwide[14–16]. However, the variants and their prevalence vary significantly in different ethnic populations[1, 17]. GJB2 c.35delG and GJB2 c.167delT are found to be the most frequent mutations in Caucasian and Ashkenazi Jewish groups, while GJB2 c.235delC is the most frequently seen mutation in East Asian populations[1]. GJB2 biallelic variants have been found in approximately 25% of infants diagnosed with hearing loss[17, 18]. Infants who failed newborn hearing screens were 11.8 times more likely to have carry GJB2 variants than infants who passed the hearing screen [17, 19]. Variations in SLC26A4 are the second most common genetic cause of sensorineural hearing loss[20], and are responsible for Pendred syndrome, an autosomal recessive disorder marked by enlarged vestibular aqueducts and concomitant sensorineural hearing loss. Mutations in this gene are associated with 3% of newborn incidences of NSHL, but the frequency increases significantly in later years[21], and appears to be associated with enlargement of the vestibular aqueduct[22]. SLC26A4 encodes a transmembrane exchanger of negative ions, and is expressed in the inner ear. The most common mutation of SLC26A4 found in the Chinese population is c.919-2A>G, its carrier frequency can be as high as 12.5%[1, 23]. Aminoglycoside antibiotics have been associated with high rates of nephrotoxicity and ototoxicity in some people, especially among carriers of variants in the mitochondrial 12S gene[24]. The mitochondrial variant m.1555A>G, though infrequent in a general population of NSHL cases[21], is the most common allele associated with aminoglycoside-induced deafness and NSHL in several ethnic groups, and m.1494C>T was the second most prevalent mutant[1, 25].
Early-detection is of vital importance in addressing the needs of newborns with hearing loss. The costs of identifying newborns with hearing loss via universal screening are relatively low[26–28], and can be economically beneficial even in developing countries[29]. Additionally, identification of mutations in mitochondrial genes associated with aminoglycoside-induced deafness can prevent the inappropriate use of these drugs. Studies have shown that newborn hearing concurrent gene screening was an effective complement to standard hearing assessments for the improved care of infants[30] and can identify children whose hearing loss occurs later in childhood[31]. It remains unanswered what is the most efficient and reliable means of identifying hearing loss related genetic variants in a public health effort. Next-generation sequencing (NGS) can be an effective tool for identifying known and novel variants related with hearing loss[32], but the cost and complexity of analysis may limit its applications to community level health care delivery, especially in developing regions. Chip based assays may be more cost effective, but it is difficult to add novel genetic loci related to hearing loss, the number of which are continually growing[30, 32]. PCR is highly cost effective for a small number of variants and samples, readily adoptable in a variety of health care delivery sites, and assays specifically targeted towards variants associated with NSHL have been approved for use in clinical diagnoses[33]. However standard PCR can have difficulties in scaling to large numbers of samples, though several different variants of the technique have been used in the screening of NSHL associated variants[34].
In the present study we performed one of the largest scale genetic screenings in a joint academic and Ministry of Health project, assaying 142,417 neonates born between May 2014 and December 2015 in Wuhan, China. 98.7% of newborns at the contributing 143 local hospitals participated in this screening during the time of the project. Deafness-related variants in three genes (GJB2, SLC26A4 and MT-RNR1 (mitochondrially encoded 12S RNA)) were assayed using real time PCR. The aim was to develop a low-cost, fast, accurate and high-throughput platform for large scale genetic screening applicable to a community health care setting and to compare to other methodologies. This also allows a comparison of the frequency of the chosen variants in Wuhan to other regions and to better assess the variants best suited for this assay.
Materials and methods
Recruitment of the subjects
The population enrolled in our study consisted of 142,417 newborns in Wuhan, which is located in the central of China. From May 2014 to Dec. 2015, 142,417 newborns were recruited from 143 hospitals, including both maternity and general hospitals. The participation rate was 98.7%. The screening protocol was approved by the Health and Family Planning Commission of Wuhan Municipality and all the participating hospitals. Three to four heel blood spots (diameter≥12 mm, 30–40 μL) were collected within 72 hours after birth according to standard protocol with FTA cards. The protocol was as follows: the heel of the foot was cleaned with an alcohol wipe, punctured at the edge of the plantar surface using an automated lancet, the first drop of blood was discarded, and a single blood spot was collected per card, and the cards were air dried four hours. The sampling card also contained the participants’ information (sample’s unique ID, newborn’s or mother’s name, sex and ethnicity, birth date, hospital, and blood collection date). All newborns’ information were gathered into a newborn deafness genetic database, and unused blood spots were stored as a biobank. Samples with inadequate number or size of blood spots, samples that were stored improperly, or samples with incomplete information were disqualified from analysis. Written informed consent was obtained from all the neonates’ parents or guardians who participated in the project. The study protocol was reviewed and approved by the internal review board of Huazhong University (#[S189]).
Selection of variants
A review of published meta-analyses was used to nominate candidate variants. Criteria for inclusion were repeated strong associations with risk of NSHL, potential actionability upon diagnosis, and prevalence in East Asian populations. Variants in GJB2, SLC26A4, MTRNR1 were among the most commonly related to non-syndromic hearing loss[35–38]. The c.235delC mutation of GJB2 gene, the c.919-2A>G mutation of SLC26A4 gene and the MTRNR1 m.1555A>G and m.1494 C>T were selected for further study.
DNA isolation and real-time PCR analysis
Genetics variants with known associations with hearing loss were assessed. The variants GJB2 c.235delC, SLC26A4 c.919-2A>G, and mitochondrial variants in the MT-RNR1 12S gene m.1555A>G and m.1494C>T were assayed using three independent fluorescent PCR kits. All variants were detected using a real time fluorescent PCR method with a commercial kit (Yingsheng, Jinan, China). The detection kit is comprised of genomic DNA extraction reagents and the fluorescent PCR reagents, including positive and negative control, and amplification reaction mixtures. All reactions for each variant were performed in a single well. Amplification was performed with ViiA™ 7 system on 384-well plates (ThermoFisher Scientific, Singapore). The genotypes of each allele were determined via amplification curves and the Ct value. Newborns found to carry a variant were confirmed by sequencing in duplicate. Heteroplasmy and homoplasmy of the mitochondrial variants were identified by the presence or absence of unique amplification curves.
The Taqman-MGB genotyping platform
The specific Taqman-MGB probe sets for (GJB2 c.235delC, SLC26A4 c.919-2A>G, MT-RNR1 m.1555A>G and m.1494C>T) with wild and mutant type were designed using Primer 3.0 and validated by Sanger sequencing. Each loci required a pair of primers common to both wild type (P1) and the mutant sequences (P2), as well as two different MGB (Minor Groove Binder) probes for each assay. The probe for the normal sequence was labeled with the FAM fluorophore, and the probe for the mutant sequence was labeled with the VIC fluorophore. A pair of regular PCR primers (F1, F2) for each mutation is also designed at the same time.
Each PCR reaction was carried out in a total volume of 5 μl. The appropriate concentration of a mixture of probes and primers (mixture: primer F1, F2 and Taqman-MGB probes: P1, P2) was added at a volume of 0.25μl, along with 2.5 μl 2X PCR Buffer (GeneCore, China), 1μl genomic DNA template and 1.25μl ddH2O (Sangon Biotech, China) (Table 1).
Table 1. Primers and Taqman-MGB probes for the four mutations.
| Genes | Mutations | SNP | Primers or Probes names | Primers or Probes sequences(5'-3') |
|---|---|---|---|---|
| SLC26A4 | c.919-2A>G | rs111033313 | SLC26A4-Forward | AAAGTTCAGCATTATTTGGTTGACAA |
| SLC26A4-Reverse | TTCCAGGTTGGCTCCATATGA | |||
| SLC26A4-P1 | FAM-CATCTTTTGTTTTATTTCAGACG-MGB | |||
| SLC26A4-P2 | VIC-TCTTTTGTTTTATTTCGGACGA-MGB | |||
| GJB2 | c.235delC | rs80338943 | GJB2-235-Forward | TGGCGTGGACACGAAGATC |
| GJB2-235-Reverse | CTACTTCCCCATCTCCCACATC | |||
| GJB2-235-P1 | FAM-CTGCAGGGCCCATA-MGB | |||
| GJB2-235-P2 | VIC-CTGCAGGCCCATAG-MGB | |||
| MTRNR1 | m. 1555A>G | rs267606617 | mit-1555-Forward | TGCACTTTCCAGTACACTTACCATGT |
| mit-1555-Reverse | GCCCGTCACCCTCCTCA | |||
| mit-1555-P1 | FAM-ACGACTTGTCTCCTCTA-MGB | |||
| mit-1555-P2 | VIC-ACGACTTGCCTCCT-MGB | |||
| MTRNR1 | m.1494C>T | rs267606619 | mit-1494-Forward | GCCCTGAAGCGCGTACAC |
| mit-1494-Reverse | CCATGTTACGACTTGTCTCCTCTATATAA | |||
| mit-1494-P1 | FAM-CGCCCGTCACCCT-MGB | |||
| mit-1494-P2 | VIC-CCGTCACTCTCCT-MGB |
PCR amplification was commenced with an initial denaturation step at 94 °C for 10 min, followed of the cycling conditions and annealing temperatures indicated in Table 1. The final extension step was at 72 °C for 5 min. 707 randomly chosen samples were also assayed using a Tetra-primer ARMS PCR kit (BioSino Bio, Beijing, China) in order to validate and compare identification of the four mutations. Gel electrophoresis was used to distinguish the ARMS PCR products. Heteroplasmy and homoplasmy of the mitochondrial variants could not be distinguished in this manner, and sequencing was performed to distinguish these products (data not shown). Anonymized genotype results are found in the supplementary S1 Table.
Sequencing validation
Additionally, all variants cases detected by these two PCR assays were validated via Sanger sequencing using sequencing primers designed with Primer 3.0 (Table 2). Three pairs of primers were designed and the targeted sequence was amplified. All the PCR products were purified on QIAquick Gel Extraction Kit (Qiagen, Valencia, CA) and subjected to direct sequencing by BigDye Terminator Cycle Sequencing kit (version v.3.1) and ABI genetic analyzer 3730. If the sequencing was discrepant from real time PCR result, the genotypes result from the sequencing would be reported.
Table 2. Sequencing primers for four NSHL associated loci.
| Genes | Mutations | Primer names | Primer sequence(5'-3') | Bases(bp) | Purification |
|---|---|---|---|---|---|
| GJB2 | c.235delC | GJB2-246-F1 | AGAGTTGGTGTTTGCTCAGG | 20 | PAGE |
| GJB2-1011-R1 | TTCAGTGACATTCAGCAGGA | 20 | PAGE | ||
| SLC26A4 | c.919-2A>G | SLC26A4-253-F1 | GATTTCACTGCTGGATTGCT | 20 | PAGE |
| SLC26A4-756-R1 | GCATATACGGGCTGCTTTTA | 20 | PAGE | ||
| MTRNR1 | m.1555A>G/m.1494C>T | MTRNR1-192-F1 | TAATCGATAAACCCCGATCA | 20 | PAGE |
| MTRNR1-761-R1 | TATCTATTGCGCCAGGTTTC | 20 | PAGE |
Statistical analysis
The inter-city differences in frequency of overall and single site carriers were compared using the two-tailed Chi-square test. A P-value less than 0.05 was considered statistically significant.
Results
The workflow of newborns deafness gene screening
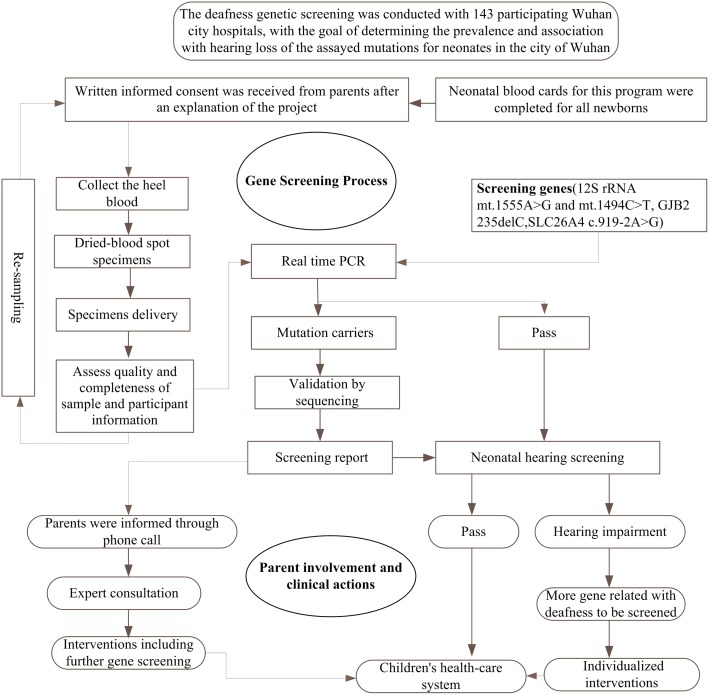
A total of 142,417 neonates were enrolled into the newborn deafness genetic screening study, and a high throughput genetic screening standard procedure was needed to assay their genotypes. Via discussion with the Health and Family Planning Commission and a collaborating otolaryngologist, a workflow was proposed and tested on a pilot project of 500 participants. As the initial results were favorable, the project was expanded to the total size reported in this study.
The protocol was comprised of four stages, including blood specimen collection, genetic screening, result interpretation, and follow-up intervention (Fig 1). All participants provided written informed consent before heel blood samples were collected. Three dried blood spots were collected and delivered to a single genetic screening center. Qualified samples were put into the genetic testing flow. Doctors and genetic counselors were responsible for interpreting the screened results and for suggesting possible intervention measures for mutation carriers. Those who carried alleles associated with increased risk of NSHL were registered with their local women and children health-care system for follow-up studies.
Fig 1. Flow chart of neonates’ deafness gene screening.
Results of the genetic screening
Genetic screening data of four deafness-associated loci in three genes is shown in Table 3. In total, 4289 newborns were found to carry at least one pathogenic variant. In the nuclear genome, 23 cases were homozygous for the risk allele, and 4030 were heterozygous. Among the cases carrying MT-RNR1 mutations, 218 were homoplasmic for the risk allele, and 22 were heteroplasmic. The total mutation frequency was 3.01% in this study. GJB2 c.235delC was the most prevalent variant (1.89%), contributing about 62.8% of the total carrier frequency. SLC26A4 c.919-2A>G presented a carrier frequency of 0.98%. The most frequent variant of the MT-RNR1 gene was m.1555A>G, with a carrier frequency of 0.154%, while m.1494C>T was the rarest allele, and had a frequency of 0.015%.
Table 3. Spectrum of four deafness associated alleles in 142,417 neonates.
| Gene | Cases counts | Carrier frequency* (%) |
|---|---|---|
|
GJB2 c.235delC heterozygous |
2677 | 1.890 |
| c.235delC homozygous | 16 | |
|
SLC26A4 c.919-2A>G heterozygous |
1386 | 0.978 |
| c.919-2A>G homozygous | 7 | |
|
MT-RNR1 m.1555A>G heteroplasmic |
22 | 0.154 |
| m.1555A>G homoplasmic | 197 | |
| m.1494C>T heteroplasmic | 0 | 0.015 |
| m.1494C>T homoplasmic | 21 | |
| Total | 4289 | 3.012 |
* Carrier frequency is calculated as the frequency of cases either heterozygous or homozygous for the minor allele.
37 neonates carried multiple screened variants. GJB2 c.235delC was the variant most frequently found in combination with other variants (Table 4): 33 neonates were GJB2 c.235delC heterozygous and SLC26A4 c.919-2A>G heterozygous, 3 neonates were GJB2 c.235delC heterozygous and m.1494C>T mutation, and one neonate was SLC26A4 c.919-2A>G heterozygous and m.1555A>G.
Table 4. Frequency of compound-mutations among 142,417 neonates.
| Mode of compound mutations | Cases counts | Frequency (%) |
|---|---|---|
| c.235delC heterozygous vs c.919-2A>G heterozygous |
33 | 0.023 |
| c.235delC heterozygous vs m.1494C>T homoplasmic |
3 | 0.002 |
| c.919-2A>G heterozygous vs m.1555A>G homoplasmic |
1 | 0.0007 |
Sequencing validation
A total of 4289 neonates were found to be carriers of at least one of the four variants via real time PCR. The targeted regions of these variants were amplified using appropriate specific primers and sequenced in duplicate via Sanger sequencing. Approximately 53 samples were found to not carry a mutation when sequenced, giving a concordance of 98.7% (1-53/4289).
Comparison of deafness genetic screening in different cities of China
Studies in more than 20 Chinese cities have assayed common loci associated with NSHL. We compared the results of this study with those of several studies, collected in cities or provinces in China from 2007 to 2015 (Table 5). The total mutation carrier frequency of four variants varied from 1.54% to 5.21%. The frequency identified in our study was 3.01%, slightly lower than the average mutation rate (3.24%). The overall carrier rates reported in 16 areas of China were significantly different via a chi-square test (p<2.2e-16), though large differences were primarily found in cities with relatively small studies.
Table 5. Comparison of carrier frequency of mutations associated with hearing loss in 21 cities in China.
| Cities | Case counts | Screening period | GJB2 c.235delC | SLC26A4 c.9192A>G | 12S rRNA m.1494C>T | 12S rRNA m.1555A>G | Total | references | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Homozygote | Heterozygote | Total | Homozygote | Heterozygote | Total | Homoplasmic | Heteroplasmic | Total | Homoplasmic | Heteroplasmic | Total | Total positive case | Frequency of Carrier(%) | ||||
| Wuhan | 142417 | May 2014-Dec 2015 | 16 | 2677 | 2693 | 7 | 1386 | 1393 | 21 | 0 | 21 | 197 | 22 | 219 | 4326 | 3.01 | |
| Hefei | 2363 | Sept 2014-Dec 2014 | 0 | 53 | 53 | 0 | 40 | 40 | 0 | 0 | 0 | 5 | 0 | 5 | 98 | 4.15 | [39] |
| Kaifeng | 9038 | Jan 2013-Mar 2014 | 3 | 159 | 162 | 3 | 79 | 82 | 1 | 0 | 1 | 3 | 17 | 20 | 265 | 2.93 | [40] |
| Luoyang | 2788 | Nov 2007-Aug 2008 | 1 | 40 | 41 | 0 | 34 | 34 | 0 | 0 | 0 | - | - | 6 | 81 | 2.91 | [41] |
| Liaocheng | 11046 | Dec 2013-Dec 2014 | 3 | 229 | 232 | 3 | 162 | 165 | 0 | 1 | 1 | 32 | 2 | 34 | 432 | 3.91 | [42] |
| Jinan | 646 | Nov 2010-Oct 2011 | - | 13 | 13 | - | 8 | 8 | - | - | 2 | - | - | 2 | 25 | 3.87 | [43] |
| Beijing | 89924 | Oct 2012-Sept 2014 | 9 | 1693 | 1702 | 7 | 1197 | 1204 | 11 | 1 | 12 | 135 | 45 | 180 | 3098 | 3.45 | [44] |
| Changzhi | 19113 | Jun 2013-Dec 2013 | 2 | 341 | 343 | 0 | 370 | 370 | - | - | 4 | - | - | 65 | 782 | 4.04 | [45] |
| Tianjin | 58397 | Dec 2011-Dec 2012 | 8 | 1135 | 1143 | 7 | 902 | 909 | 8 | 0 | 8 | 84 | 17 | 101 | 2161 | 3.7 | [30] |
| Shijiazhuang | 10948 | Jan 2014-Aug 2015 | 1 | 182 | 183 | 1 | 155 | 156 | 0 | 0 | 0 | 17 | 3 | 20 | 358 | 3.27 | [46] |
| Foshan | 10238 | May 2012-Dec 2013 | 4 | 165 | 169 | 3 | 82 | 85 | 1 | 0 | 1 | 18 | 4 | 22 | 277 | 2.71 | [47] |
| Shaoguan | 863 | 2014 | 0 | 12 | 12 | 0 | 24 | 24 | 0 | 2 | 2 | 5 | 7 | 12 | 48 | 5.56 | [48] |
| Suzhou | 5800 | Oct 2011-Feb 2012 | - | - | 109 | - | - | 94 | - | - | 1 | - | - | 8 | 212 | 3.66 | [49] |
| Wuxi | 2553 | Jan 2013-Dec 2013 | 0 | 67 | 67 | 1 | 21 | 22 | 0 | 0 | 0 | 3 | 1 | 4 | 93 | 3.64 | [50] |
| Nantong | 765 | Jan 2014-Apr 2014 | - | - | 25 | - | - | 12 | 1 | - | 1 | 0 | 0 | 0 | 38 | 4.97 | [51] |
| Yangzhou | 965 | Apr 2013-Aug 2014 | 2 | 30 | 32 | 0 | 16 | 16 | 0 | 0 | 0 | - | - | 1 | 49 | 5.08 | [52] |
| Shaoxing | 5121 | Jun 2013-Dec 2013 | 2 | 111 | 113 | 0 | 60 | 60 | 0 | 0 | 0 | 6 | 0 | 6 | 179 | 3.5 | [53, 54] |
| Nanning | 10224 | Jan 2014-Jun 2015 | 3 | 131 | 134 | 2 | 46 | 48 | 0 | 0 | 0 | 20 | 4 | 24 | 206 | 2.01 | [55, 56] |
| Chengdu | 17000 | Aug 2012-Jun 2013 | 2 | 254 | 256 | 0 | 130 | 130 | 4 | 0 | 0 | 34 | 8 | 42 | 431 | 2.54 | [57] |
| Gansu | 10043 | Dec 2009-Apr 2010 | 2 | 117 | 119 | 0 | 93 | 93 | - | - | 3 | - | - | 16 | 230 | 2.29 | [58] |
| Xinjiang | 1038 | - | 0 | 4 | 4 | - | 9 | 9 | - | - | - | 3 | 0 | 3 | 16 | 1.54 | [59] |
| Total | 411290 | - | 59 | 7362 | 7555 | 35 | 4776 | 4917 | 49 | 4 | 59 | 565 | 127 | 790 | 13320 | 3.24 | |
Method comparison of qPCR with ARMS-PCR
We used real-time PCR with Taqman technology and ARMS-PCR to assay the variant sites simultaneously in 707 neonates. The results for GJB2 c.235delC, m.1555A>G, m.1494C>T in these 707 newborns showed complete accordance. With SLC26A4 c.919-2A>G, 378 samples were consistent between methods, the remaining 314 newborns had no results at this site with the ARMS-PCR (Table 6). Three rounds of PCR were attempted if assays failed to produce detectable product (except for SLC26A4 c.919-2A>G, which demonstrated such a high failure rate with ARMS-PCR that efforts to evaluate it with this method were discontinued after the first round). Real-time PCR had a substantially lower failure rate than ARMS-PCR (Tables 7 and 8).
Table 6. Genotype results comparison of the PCR methods.
| ARMS-PCR | |||||||||||||
| GJB2 | SLC26A4 | MT-RNR1 | Total | ||||||||||
| c.235delC Heterzygous | c.235delC Homozyous | Wild type | c.919-2A>G Heterzygous | c.919-2A>G Homozyous | Wild type | m.1555 A>G Wild type | m.1555 A>G Mutation type | m.1494 C>T Wild type | m.1494 C>T Mutation type | ||||
| Taqman PCR | GJB2 | c.235delC Heterzygous | 8 | 0 | 0 | 707 | |||||||
| c.235delC Homozyous | 0 | 0 | 0 | ||||||||||
| Wild type | 0 | 0 | 699 | ||||||||||
| SLC26A4 | c.919-2A>G Heterzygous | 4 | 0 | 0 | 378 | ||||||||
| c.919-2A>G Homozyous | 0 | 0 | 0 | ||||||||||
| Wild type | 0 | 0 | 374 | ||||||||||
| MT-RNR1 | m.1555 A>G Wild type | 705 | 0 | 707 | |||||||||
| m.1555A>G Mutation type | 0 | 2 | |||||||||||
| m.1494 C>T Wild type | 707 | 0 | 707 | ||||||||||
| m.1494 C>T Mutation type | 0 | 0 | |||||||||||
| Total | 707 | 378 | 707 | 707 | |||||||||
Table 7. Failure rate of ARMS-PCR method.
| SLC26A4 | GJB2 | m.1555 A>G | m.1494 C>T | |
|---|---|---|---|---|
| Number assayed | 692 | 707 | 707 | 707 |
| Not- detected (first round) | 314 | 38 | 16 | 18 |
| Not-detected (second round) | NA | 16 | 3 | 8 |
| Not-detected (third round) | NA | 1 | 2 | 0 |
Table 8. Failure rate of real-time PCR.
| SLC26A4 | GJB2 | m.1555 A>G | m.1494 C>T | |
|---|---|---|---|---|
| Number assayed | 707 | 707 | 707 | 707 |
| Not- detected (first round) | 5 | 3 | 1 | 0 |
| Not-detected (second round) | 0 | 0 | 0 | 0 |
The time required for real time PCR was significantly lower than for ARMS-PCR, primarily due to differences in the requirements for DNA preparation and visualization of the amplification product. Processing each batch of ARMS-PCR entailed in total six hours, vs two hours for real time PCR. In addition, the reagents costs were 25% higher for ARMS-PCR.
Discussion
Hearing loss is a relatively common disorder, featured by high heterogeneity and phenotypic diversity. Early identification of infants with NSHL can avoid social and language difficulties that can occur in infants with undiagnosed hearing loss[8, 60, 61]. Universal programs to screen newborns for hearing defects are greatly aiding the identification of children with hearing loss, however estimates of the test failure rates range from 2 to 4 percent, and the tests can have poor specificity[4]. Additionally, late onset hearing loss and deafness induced by ototoxicity cannot be identified by these programs. One study of physical screening of newborns found that almost all carriers of 12S rRNA mutations passed the physical test[62]. Besides aiding in improved sensitivity for the detection of newborns with severe hearing loss, genetic testing could support the use of physical testing for newborns with only moderate hearing loss by detecting newborns with greater risk of hearing loss, and thus help avoid the trade-off of improved sensitivity for decreased specificity. Genetic screening, as a compliment to physical hearing assessments, could be an important aid in managing this disorder in children.
More than 70 genes have been identified to harbor variations linked to non-syndromic hearing loss. Previous studies have identified GJB2 as in important contributor to hereditary NSHL, and mutations in GJB2 can be detected in nearly 50% of patients with autosomal recessive hearing loss[63]. The c.235delC mutation rate was 18.16% in 1680 cases of Chinese NSHL patients from 13 provinces. Hearing loss related to single-site mutations is relatively infrequent and is unlikely to account for the heterogeneity of a disorder like hearing loss[64]. In this study, 2693 neonates were found to carry 235delC mutations (1.9% of all cases), and we found that c.235delC alleles are the most frequent compound mutations, accounting for 0.025% (36 cases) of all the neonates. About 80% of large vestibular aqueduct syndrome patients have been found to harbor SLC26A4 c.919-2A>G mutations[65]. In this study we found that 0.98% of neonates were c.919-2A>G carriers. SLC26A4 c.919-2A>G compound heterozygosity in hearing-impaired patients is common[66], this study found one case to carry that variant and the mitochondrial A1555G variant.
The m.1555A>G and m.1494C>T are the main pathogenic mutations of 12S rRNA gene. These rare variants have been shown to have a direct relationship with aminoglycoside induced hearing loss[67]. There were no heteroplasmic cases and 21 neonates were homoplasmic for m.1494C>T variant, while 10.0% of m.1555A>G variants occurred as heteroplasmic mutations (22/219). Mitochondrial heteroplasmy can occur at various rates in different populations[68]. Heteroplasmy of m.1555A>G has been observed as an uncommon phenomenon in both European and Asian populations [69–72]. Other studies have found that individuals with a higher proportion of m.1555A>G variants were more likely to exhibit hearing loss[69, 70].
Several methods for the detection of genetic variants related to hearing loss have been employed in the clinic. ARMS-PCR offers consistent and accurate results with inexpensive equipment. However, the running time for every target is about three hours. Additionally, different loci require different reaction conditions with this methodology. MALDI-TOF mass spectrometry high-throughput genotyping [73] and chip based assays can be effective[74], but can entail high costs. Therefore, additional technologies that allow efficient, flexible, and high throughput genotyping with low cost barriers to entry would likely facilitate the adoption of genetic screening, particularly in developing regions. We found that real-time PCR based on Taqman technology, which can be performed in a conventional real-time PCR machine, can be an effective method for genetic screening in a community health care environment. This method, using fluorescently labeled allele-specific probes, allows rapid and reliable detection of DNA mutations, including single nucleotide polymorphisms, insertions, and deletions. 384 samples can be assayed on one machine in only 1.5 hours, and two hours are required to prepare samples, thus the procedure is suitable for large-scale screenings. Additionally, we found that lower amounts of reagents are required for real-time PCR compared to conventional PCR. Finally, the failure rate of the real time PCR was lower than that of conventional PCR as well. All real-time PCR assays which failed in the first attempt succeeded in the second attempt, while ARMS-PCR failed several times after even three attempts.
The next stage of this study is a comparison between the variants identified via PCR and the results of physical hearing screening. The neonates carrying the positive variants will be tested again by otoacoustic emission testing or automated auditory brainstem response to identify associations with late onset hearing loss. In addition, it is hoped that the identification of alleles which are contraindicative for the use of aminoglycoside antibiotics will prevent their use. Further collaboration with the participating hospitals will determine the specificity and sensitivity of the association of the studied variants with hearing loss at birth and arising in early childhood, allowing an estimation of costs and benefits of delivering newborn hearing genetic screening in a large-scale community setting.
Supporting information
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Bitnerglindzicz M. Hereditary deafness and phenotyping in humans. British Medical Bulletin. 2002;63(1):73. [DOI] [PubMed] [Google Scholar]
- 2.Angeli S, Lin X, Liu XZ. Genetics of hearing and deafness. Anatomical record. 2012;295(11):1812–29. doi: 10.1002/ar.22579 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han B, Qian LI, Zong L, Lan L, Zhao YL, Wang DY, et al. An Clinical Research on Newborn Hearing Concurrent Genetic Screening in 106,513 Neonates. Chinese Journal of Otology. 2013;11(3):380–3. [Google Scholar]
- 4.Morton CC, Nance WE. Newborn hearing screening—a silent revolution. New England Journal of Medicine. 2006;354(20):2151 doi: 10.1056/NEJMra050700 [DOI] [PubMed] [Google Scholar]
- 5.Fortnum HM, Summerfield AQ, Marshall DH, Davis AC, Bamford JM. Prevalence of permanent childhood hearing impairment in the United Kingdom and implications for universal neonatal hearing screening: questionnaire based ascertainment study. Bmj. 2001;323(7312):536–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshinaga-Itano C. Early intervention after universal neonatal hearing screening: Impact on outcomes. Ment Retard Dev Disabil Res Rev. 2003;9(4):252–66. doi: 10.1002/mrdd.10088 [DOI] [PubMed] [Google Scholar]
- 7.Yoshinagaitano C, Apuzzo ML. Identification of hearing loss after age 18 months is not early enough. American Annals of the Deaf. 1998;143(5):380–7. [DOI] [PubMed] [Google Scholar]
- 8.Yoshinagaitano C, Apuzzo ML. The development of deaf and hard of hearing children identified early through the high-risk registry. Am Ann Deaf. 1998;143(5):416–24. [DOI] [PubMed] [Google Scholar]
- 9.Korres S, Peraki EE, Tsiakou M, Karakitsou M, Apostolopoulos N, Economides J, et al. Outcomes and Efficacy of Newborn Hearing Screening: Strengths and Weaknesses (Success or Failure?) Laryngoscope. 2010;118(7):1253–6. [DOI] [PubMed] [Google Scholar]
- 10.Cp VDP, Uilenburg NN, Kauffman-de Boer MA, Oudesluys-Murphy AM, Verkerk PH. Newborn hearing screening in youth health care in the Netherlands: National results of implementation and follow-up. International Journal of Audiology. 2012;51(8):584–90. doi: 10.3109/14992027.2012.684402 [DOI] [PubMed] [Google Scholar]
- 11.Xin F, Yuan Y, Deng X, Han M, Wang G, Zhao J, et al. Genetic mutations in nonsyndromic deafness patients of Chinese minority and han ethnicities in Yunnan, China. Journal of Translational Medicine. 2013;11(1):312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tranebjaerg L. Genetic causes of hearing loss—status and perspectives. Ugeskrift for Laeger. 2000;162(21):3044–51. [PubMed] [Google Scholar]
- 13.Yang T, Wei X, Chai Y, Li L, Wu H. Genetic etiology study of the non-syndromic deafness in Chinese Hans by targeted next-generation sequencing. Orphanet Journal of Rare Diseases. 2013;8(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsukada K, Nishio SY, Hattori M, Usami S. Ethnic-specific spectrum of GJB2 and SLC26A4 mutations: their origin and a literature review. Annals of Otology Rhinology & Laryngology. 2015;124 Suppl 1(1 suppl):61S. [DOI] [PubMed] [Google Scholar]
- 15.Pandya A, Arnos KS, Xia XJ, Welch KO, Blanton SH, Friedman TB, et al. Frequency and distribution of GJB2 (connexin 26) and GJB6 (connexin 30) mutations in a large North American repository of deaf probands. Genetics in Medicine Official Journal of the American College of Medical Genetics. 2003;5(4):295 doi: 10.1097/01.GIM.0000078026.01140.68 [DOI] [PubMed] [Google Scholar]
- 16.Propst EJ, Stockley TL, Gordon KA, Harrison RV, Papsin BC. Ethnicity and mutations in GJB2 (connexin 26) and GJB6 (connexin 30) in a multi-cultural Canadian paediatric Cochlear Implant Program. International Journal of Pediatric Otorhinolaryngology. 2006;70(3):435–44. doi: 10.1016/j.ijporl.2005.07.013 [DOI] [PubMed] [Google Scholar]
- 17.Schimmenti LA, Martinez A, Telatar M, Lai CH, Shapiro N, Fox M, et al. Infant hearing loss and connexin testing in a diverse population. Genetics in medicine: official journal of the American College of Medical Genetics. 2008;10(7):517–24. doi: 10.1097GIM.0b013e31817708fa . [DOI] [PubMed] [Google Scholar]
- 18.Putcha GV, Bejjani BA, Bleoo S, Booker JK, Carey JC, Carson N, et al. A multicenter study of the frequency and distribution of GJB2 and GJB6 mutations in a large North American cohort. Genetics in medicine: official journal of the American College of Medical Genetics. 2007;9(7):413–26. doi: 10.1097GIM.0b013e3180a03276 . [DOI] [PubMed] [Google Scholar]
- 19.Schimmenti LA, Warman B, Schleiss MR, Daly KA, Ross JA, McCann M, et al. Evaluation of newborn screening bloodspot-based genetic testing as second tier screen for bedside newborn hearing screening. Genetics in medicine: official journal of the American College of Medical Genetics. 2011;13(12):1006–10. doi: 10.1097/GIM.0b013e318226fc2e . [DOI] [PubMed] [Google Scholar]
- 20.Yuan Y, You Y, Huang D, Cui J, Wang Y, Wang Q, et al. Comprehensive molecular etiology analysis of nonsyndromic hearing impairment from typical areas in China. Journal of Translational Medicine. 2009;7(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morton CC, Nance WE. Newborn hearing screening—a silent revolution. The New England journal of medicine. 2006;354(20):2151–64. doi: 10.1056/NEJMra050700 . [DOI] [PubMed] [Google Scholar]
- 22.Pryor SP, Madeo AC, Reynolds JC, Sarlis NJ, Arnos KS, Nance WE, et al. SLC26A4/PDS genotype-phenotype correlation in hearing loss with enlargement of the vestibular aqueduct (EVA): evidence that Pendred syndrome and non-syndromic EVA are distinct clinical and genetic entities. J Med Genet. 2005;42(2):159–65. doi: 10.1136/jmg.2004.024208 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan Y, Guo W, Tang J, Zhang G, Wang G, Han M, et al. Molecular epidemiology and functional assessment of novel allelic variants of SLC26A4 in non-syndromic hearing loss patients with enlarged vestibular aqueduct in China. PLoS One. 2012;7(11):e49984 doi: 10.1371/journal.pone.0049984 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bindu LH, Reddy PP. Genetics of aminoglycoside-induced and prelingual non-syndromic mitochondrial hearing impairment: a review. International Journal of Audiology. 2008;47(11):702–7. doi: 10.1080/14992020802215862 [DOI] [PubMed] [Google Scholar]
- 25.Lu J, Li Z, Zhu Y, Yang A, Li R, Zheng J, et al. Mitochondrial 12S rRNA variants in 1642 Han Chinese pediatric subjects with aminoglycoside-induced and nonsyndromic hearing loss. Mitochondrion. 2010;10(4):380–90. doi: 10.1016/j.mito.2010.01.007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uus K, Bamford J, Taylor R. An analysis of the costs of implementing the National Newborn Hearing Screening Programme in England. Journal of medical screening. 2006;13(1):14–9. doi: 10.1258/096914106776179764 . [DOI] [PubMed] [Google Scholar]
- 27.Hessel F, Grill E, Schnell-Inderst P, Siebert U, Kunze S, Nickisch A, et al. Economic evaluation of newborn hearing screening: modelling costs and outcomes. German medical science: GMS e-journal. 2003;1:Doc09 . [PMC free article] [PubMed] [Google Scholar]
- 28.Kezirian EJ, White KR, Yueh B, Sullivan SD. Cost and cost-effectiveness of universal screening for hearing loss in newborns. Otolaryngology—head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2001;124(4):359–67. doi: 10.1067/mhn.2001.113945 . [DOI] [PubMed] [Google Scholar]
- 29.Huang LH, Zhang L, Tobe RY, Qi FH, Sun L, Teng Y, et al. Cost-effectiveness analysis of neonatal hearing screening program in China: should universal screening be prioritized? BMC health services research. 2012;12:97 doi: 10.1186/1472-6963-12-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Wang P, Han B, Ding Y, Pan L, Zou J, et al. Newborn hearing concurrent genetic screening for hearing impairment—A clinical practice in 58,397 neonates in Tianjin, China. International Journal of Pediatric Otorhinolaryngology. 2013;77(12):1929 doi: 10.1016/j.ijporl.2013.08.038 [DOI] [PubMed] [Google Scholar]
- 31.Wu CC, Tsai CH, Hung CC, Lin YH, Lin YH, Huang FL, et al. Newborn genetic screening for hearing impairment: a population-based longitudinal study. Genetics in medicine: official journal of the American College of Medical Genetics. 2017;19(1):6–12. doi: 10.1038/gim.2016.66 . [DOI] [PubMed] [Google Scholar]
- 32.Kim SY, Kim AR, Han KH, Min YK, Jeon EH, Koo JW, et al. Residual Hearing in DFNB1 Deafness and Its Clinical Implication in a Korean Population. Plos One. 2015;10(6):e0125416 doi: 10.1371/journal.pone.0125416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han B, Liang Z, Li Q, Zhang Z, Wang D, Lan L, et al. Newborn genetic screening for high risk deafness-associated mutations with a new Tetra-primer ARMS PCR kit. International Journal of Pediatric Otorhinolaryngology. 2013;77(9):1440 doi: 10.1016/j.ijporl.2013.05.040 [DOI] [PubMed] [Google Scholar]
- 34.Hilgert N, Smith RJ, Van CG. Forty-six genes causing nonsyndromic hearing impairment: which ones should be analyzed in DNA diagnostics? Mutation Research/reviews in Mutation Research. 2009;681(3):189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao J, Lu Y, Wei Q, Xin C, Xing G. A systematic review and meta-analysis of 235delC mutation of GJB2 gene. Journal of Translational Medicine. 2012;10(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du W, Guo Y, Wang C, Wang Y, Liu X. A systematic review and meta-analysis of common mutations of SLC26A4 gene in Asian populations. International Journal of Pediatric Otorhinolaryngology. 2013;77(10):1670–6. doi: 10.1016/j.ijporl.2013.07.023 [DOI] [PubMed] [Google Scholar]
- 37.Lu YJ, Yao J, Wei QJ, Xing GQ, Cao X. Diagnostic Value of SLC26A4 Mutation Status in Hereditary Hearing Loss With EVA: A PRISMA-Compliant Meta-Analysis. Medicine. 2015;94(50):e2248 doi: 10.1097/MD.0000000000002248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jing W, Zongjie H, Denggang F, Na H, Bin Z, Aifen Z, et al. Mitochondrial mutations associated with aminoglycoside ototoxicity and hearing loss susceptibility identified by meta-analysis. Journal of Medical Genetics. 2015;52(2):95 doi: 10.1136/jmedgenet-2014-102753 [DOI] [PubMed] [Google Scholar]
- 39.Junxiang Tang, Sun Y, Wang C, Wang Y, Tong K, Zhu S. Analysis of 4 common mutations sites of genetic deafness genes in 2363 newborns. Shandong Medical Journal. 2015;(32):76–8. [Google Scholar]
- 40.Chu J. Combined hearing and deafness gene mutation screening in Kaifeng area. Maternal and Child Health Care of China. 2016;31(3):525–6. [Google Scholar]
- 41.Hu S, Li J, Zhang P, Lan L, Zheng J, Li L, et al. A Clinical Study of 2788 Newborns Screened for Hearing and Gene. Journal of Audiology & Speech Pathology. 2010;18(3):222–4. [Google Scholar]
- 42.Sun X, Xi Z, Zhang J, Liu B, Xing X, Huang X, et al. Combined hearing and deafness gene mutation screening of 11,046 Chinese newborns. Zhonghua yi xue yi chuan xue za zhi = Zhonghua yixue yichuanxue zazhi = Chinese journal of medical genetics. 2015;32(6):766 doi: 10.3760/cma.j.issn.1003-9406.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 43.Li SX, Chen DL, Zhao SB, Guo LL, Feng HQ, Zhang XF, et al. Cordblood-Based High-Throughput Screening for Deafness Gene of 646 Newborns in Jinan Area of China. Clinical & Experimental Otorhinolaryngology. 2015;8(3):211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han P, Shao Y. Analysis on the management situation of deafness-related gene screening results in Haidian district of Beijing from 2012 to 2014. Chinese Journal of Women and Children Health. 2015;(3):62–5. [Google Scholar]
- 45.Xiaoze L, Weiping M, Zhipeng H, Zerong Y, Wei W. ChangZhi District 19113 Cases Of Neonatal Deafness Gene Detection. Chinese Journal of Otology. 2015;(4):654–7. [Google Scholar]
- 46.Feng J, Li S, Li T, Xiao H, Mei Z. Synchronous detection on screening of newborn hearing and deafness gene from Shijiazhuang. Journal of Hebei Medical University. 2015;36(11):1271–5. [Google Scholar]
- 47.Li Z, Liang S, Yu F, He T, Liu Y, He Q. An Analysis of Newborn Hearing Concurrent Genetic Screening Results. Journal of Audiology & Speech Pathology. 2014. [Google Scholar]
- 48.Lan P, Chen Y, Liu Y. Analysis of 20 mutation sites in 4 genes in neonates in shaoguan city. Lin chuang er bi yan hou tou jing wai ke za zhi = Journal of clinical otorhinolaryngology, head, and neck surgery. 2016;30(2):150 [PubMed] [Google Scholar]
- 49.Chen Y, Cao Y, Li HB, Mao J, Liu MJ, Liu YH, et al. SNaPshot reveals high mutation and carrier frequencies of 15 common hearing loss mutants in a Chinese newborn cohort. Clinical genetics. 2015;87(5):467–72. doi: 10.1111/cge.12452 [DOI] [PubMed] [Google Scholar]
- 50.Yongjun P, Xinye J, Weihong L, Zhongxiu S, Xiaoyan W. Application Experience of newborn hearing screening combined with deafness predisposing gene screening. Chinese Journal of Birth Health and Heredity. 2015;(12):66–7. [Google Scholar]
- 51.Gao H, Zhu Q, Zhou J. Clinical Application of 765 Neonatal Deaf Genes in Nantong City. Journal of Nantong University (Medical Sciences). 2014;(6):577–8. [Google Scholar]
- 52.Wang Y, Guan B, Ye S, Chang L, Yu A. A Study of Universal Newborn Hearing Screening Combined with Deafness Predisposing Gene Screening in 965 Newborns. Journal of Audiology and Speech Pathology. 2015;23(3):248–51. [Google Scholar]
- 53.Yu H, Liu D, Yang J, Wu Z, Sun D, Ma W. Analysis of common mutations of deafness-related genes in 2725 newborns. Zhonghua yi xue yi chuan xue za zhi = Zhonghua yixue yichuanxue zazhi = Chinese journal of medical genetics. 2015;32(3):335–8. doi: 10.3760/cma.j.issn.1003-9406.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 54.Yu H, Yang J, Liu D, Wu Z. The results analysis for newborns’ hearing screening combined with testing deafness predisposing genes. Chinese Journal of Birth Health and Heredity. 2014;(10):34–5. [Google Scholar]
- 55.Du J, Xu J, Huang P, Fu H. Screening results of deafness gene mutations in 4 679 newborns in Nanning. Shandong Medical Journal. 2015;(2015 年 43):17–9. [Google Scholar]
- 56.Caijuan L, Chao L, Wang L, Jinwu Y, Guoxing G, Ying H, et al. Analysis on detection results of deafness genes among 5545 neonates. Maternal and Child Health Care of China. 2015;30(34):6007–9. [Google Scholar]
- 57.Lyu K, Xiong Y, Yu H, Zou L, Ran L, Liu D, et al. Screening of common deafness gene mutations in 17 000 Chinese newborns from Chengdu based on microarray analysis. Chinese journal of medical genetics. 2014;31(5):547–52. doi: 10.3760/cma.j.issn.1003-9406.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 58.Zhang Z, Ding W, Liu X, Xu B, Du W, Nan S, et al. Auditory screening concurrent deafness predisposing genes screening in 10,043 neonates in Gansu province, China. International journal of pediatric otorhinolaryngology. 2012;76(7):984–8. doi: 10.1016/j.ijporl.2012.03.016 [DOI] [PubMed] [Google Scholar]
- 59.Zhang J, Yu M, Wu G, Zai N, Ding W, Xia Z, et al., editors. Newborns’ hearing combined with the sensitive deafness genes screening of Uighur from Xingjiang. Chinese Medical Association National Otolaryngology—Head and Neck Surgery Conference; 2013.
- 60.Sarafraz M, Hekmatshoar M, Zaheri S. Determination of Hearing Loss Prevalence in Preschool Children of Ahwaz. Iranian Journal of Otorhinolaryngology. 2011;2364(64). [Google Scholar]
- 61.Lü J, Huang Z, Yang T, Li Y, Mei L, Xiang M, et al. Screening for delayed-onset hearing loss in preschool children who previously passed the newborn hearing screening. International Journal of Pediatric Otorhinolaryngology. 2011;75(8):1045 doi: 10.1016/j.ijporl.2011.05.022 [DOI] [PubMed] [Google Scholar]
- 62.Xiangjun LI. Analysis of the Results of Combined Screening of Newborn Hearing and Deafness Related Genes. Journal of Medical Theory & Practice. 2017. [Google Scholar]
- 63.Zheng J, Ying Z, Cai Z, Sun D, He Z, Gao Y, et al. GJB2 Mutation Spectrum and Genotype-Phenotype Correlation in 1067 Han Chinese Subjects with Non-Syndromic Hearing Loss. Plos One. 2015;10(6):e0128691 doi: 10.1371/journal.pone.0128691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hernándezjuárez AA, Lugotrampe JJ, Camposacevedo LD, Lugotrampe A, Treviñogonzález JL, Delacruzávila I, et al. GJB2 and GJB6 mutations are an infrequent cause of autosomal-recessive nonsyndromic hearing loss in residents of Mexico. International Journal of Pediatric Otorhinolaryngology. 2014;78(12):2107–12. doi: 10.1016/j.ijporl.2014.09.016 [DOI] [PubMed] [Google Scholar]
- 65.Dai P, Li Q, Huang D, Yuan Y, Kang D, Miller DT, et al. SLC26A4 c.919-2A>G varies among Chinese ethnic groups as a cause of hearing loss. Genetics in Medicine Official Journal of the American College of Medical Genetics. 2008;10(8):586 doi: 10.1097GIM.0b013e31817d2ef1 [DOI] [PubMed] [Google Scholar]
- 66.Wang QJ, Zhao YL, Rao SQ, Guo YF, Yuan H, Zong L, et al. A distinct spectrum of SLC26A4 mutations in patients with enlarged vestibular aqueduct in China. Clinical Genetics. 2007;72(3):245 doi: 10.1111/j.1399-0004.2007.00862.x [DOI] [PubMed] [Google Scholar]
- 67.Zhao H, Li R, Wang Q, Yan Q, Deng JH, Han D, et al. Maternally Inherited Aminoglycoside-Induced and Nonsyndromic Deafness Is Associated with the Novel C1494T Mutation in the Mitochondrial 12S rRNA Gene in a Large Chinese Family. American Journal of Human Genetics. 2005;74(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ye K, Lu J, Ma F, Keinan A, Gu Z. Extensive pathogenicity of mitochondrial heteroplasmy in healthy human individuals. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(29):10654–9. doi: 10.1073/pnas.1403521111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elschahawi M, López dMA, Sarrazin AM, Shanske AL, Basirico M, Shanske S, et al. Two large Spanish pedigrees with nonsyndromic sensorineural deafness and the mtDNA mutation at nt 1555 in the 12s rRNA gene: evidence of heteroplasmy. Neurology. 1997;48(2):453–6. [DOI] [PubMed] [Google Scholar]
- 70.del Castillo FJ, Rodríguez-Ballesteros M, Martín Y, Arellano B, Gallo-Terán J, Morales-Angulo C, et al. Heteroplasmy for the 1555A>G mutation in the mitochondrial 12S rRNA gene in six Spanish families with non-syndromic hearing loss. Journal of Medical Genetics. 2003;40(8):632 doi: 10.1136/jmg.40.8.632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan B, Wang Z, Dai W, Li Q, Chen G, Ning C, et al. A six-generation Chinese family in haplogroup B4C1C exhibits high penetrance of 1555A > G-induced hearing Loss. BMC Medical Genetics. 2010;11(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qi-Shui OU, Cheng ZJ, Yang B, Jiang L, Chen J. Analysis of the ratio of mitchondrial DNA with A1555G mutant to wild type in deaf patients of Fujian province in China by a new method and its relationship with the severity of hearing loss. Chinese Medical Journal. 2011;124(20):3347–52. [PubMed] [Google Scholar]
- 73.Svidnicki MCCC, Silvacosta SM, Ramos PZ, Santos NZPD, Martins FTA, Castilho AM, et al. Screening of genetic alterations related to non-syndromic hearing loss using MassARRAY iPLEX® technology. Bmc Medical Genetics. 2015;16(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi SY, Kim YE, Ahn DB, Kim TH, Choi JH, Lee HR, et al. Construction of a DNA chip for screening of genetic hearing loss. Clinical & Experimental Otorhinolaryngology. 2009;2(1):44–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.