Abstract
Background
The ankle-brachial index (ABI) and pulse wave velocity (PWV) are indices of atherosclerosis and arterial stiffness. The Japan-made measuring devices of those indices have spread widely because of their convenience and the significance of the parameters. However, studies that comprehensively discuss the various pitfalls in using these indices are not available.
Methods
This study presents several representative pitfalls in using the ABI and brachial-ankle PWV (baPWV) by showing the result sheets of the device, “the Vascular Profiler”. Furthermore, some considerations when utilizing these indices in the future are also discussed.
Results
Several diseases such as arteriosclerosis obliterans (ASO), arterial calcification in the lower limb, arterial stenosis in the right upper-limb, aortic valve diseases, arterial stenosis in the upper-limb of the contralateral side of the hemodialysis access, are the representative pitfalls when evaluating ABI and baPWV. Moreover, a measurement error is found to actually exist. Furthermore, same phenomena are considered most likely to occur when using other similar indices and devices.
Conclusion
The ABI and baPWV are the useful and significant biomarkers. Nevertheless, caution is sometimes necessary when interpreting them. Moreover, rigorous patient exclusion criteria should be considered when using those indices in the severely conditioned patient population. And the results of this study can be applied to enhance the literacy using other indices, such as the cardio-ankle vascular index and other similar devices.
Keywords: ankle-brachial index, pulse wave velocity, peripheral arterial disease, aortic valve disease, hemodialysis
Introduction
The total number of atherosclerotic diseases has increased worldwide, and its prevention has become a global issue. The quantitative assessment of atherosclerosis and vascular function is important. Surrogate markers, such as the ankle-brachial index (ABI), pulse wave velocity (PWV), carotid intima media thickness, and flow-mediated dilatation, are used in clinical settings worldwide. Devices that measure four-limb blood pressure (BP) and PWVs were first manufactured in Japan, and recently, these devices have become popular in other Asian countries. They are now used mainly for research purposes in Western countries as well. Some of these devices are the Vascular Profiler (BP-203RPE, VP-1000, or VP-2000 series; Omron Healthcare Co., Kyoto, Japan) and VaSera (VS-1000 or VS-1500 series; Fukuda Denshi, Tokyo, Japan). Both devices are used to measure ABI, brachial-ankle pulse wave velocity (baPWV), cardio-ankle vascular index (CAVI), and other similar indices. It has been more than a decade since both devices were produced. In fact, the year 2019 marks the 20th year since the Vascular Profiler was first sold in Japan. At present, a lot of articles written in English have been published. Approximately 1800 and 550 articles have used the indices measured by the Vascular Profiler and VaSera, respectively, and were published globally. Statistical investigations have shown numerous results and clinical significance. On the other hand, some studies performed statistical analysis without excluding data with poor reliability. If we continue to use these indices without understanding their pitfalls, discrepancies from the guidelines1–3 and previous reports may be observed in some cases. As a result, the confidence in using the indices and the devices themselves may be affected. Nevertheless, studies that accurately and comprehensively discuss this topic have not been published. Therefore, in this study, several cases were presented along with the data from the result sheets of the Vascular Profiler, which must be cautiously interpreted. In addition, the results of this study can be applied when interpreting other indices, such as CAVI and other similar devices. The result sheets of Vascular Profiler were fully anonymized and provided by a few attending physicians who had the same awareness of the issues. Obtainment of the informed consents were exempted by the physicians because of the educational purpose and the retrospective data.
Case presentation
Normal participants
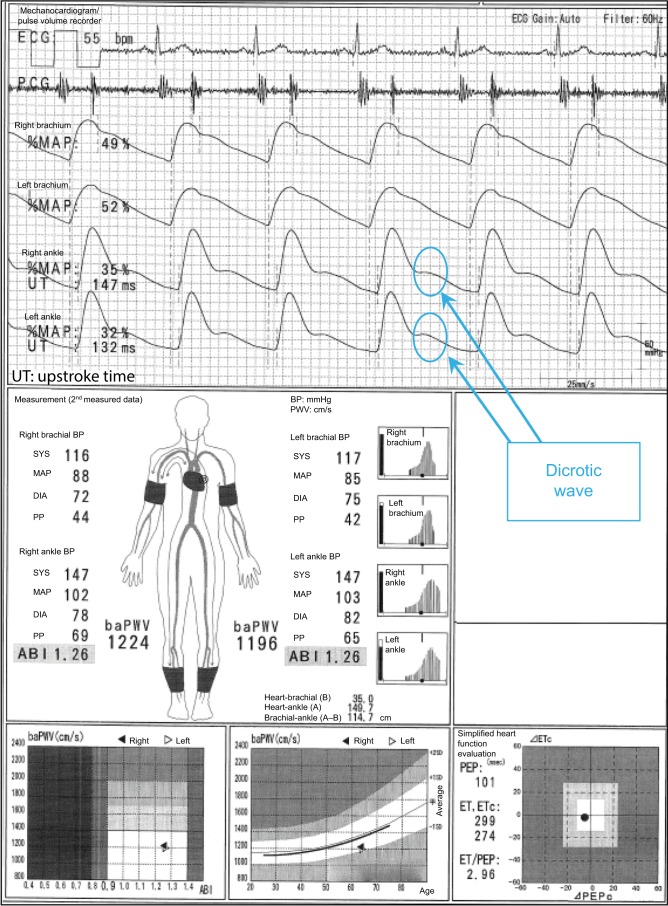
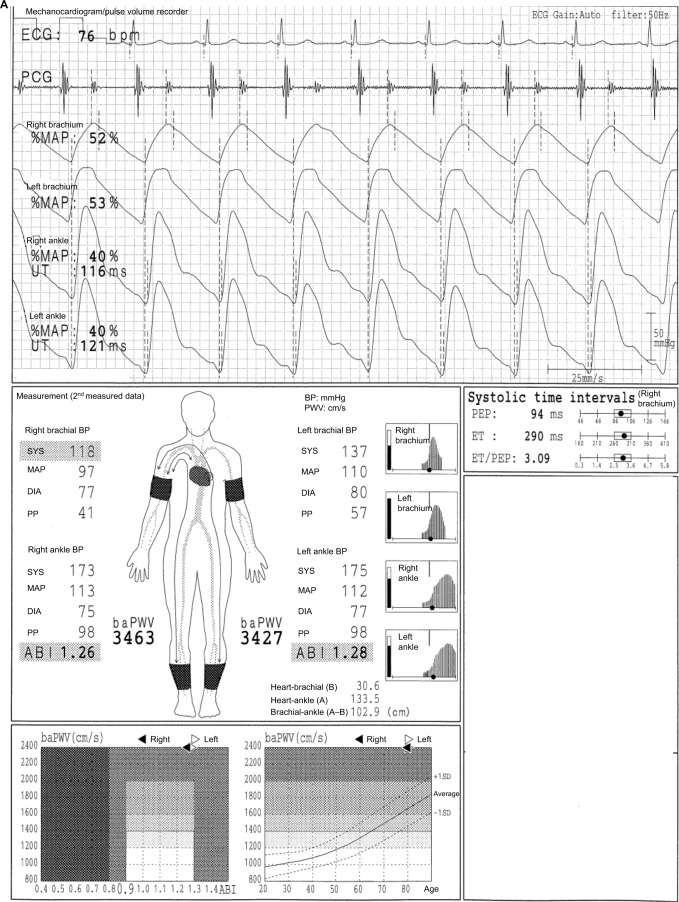
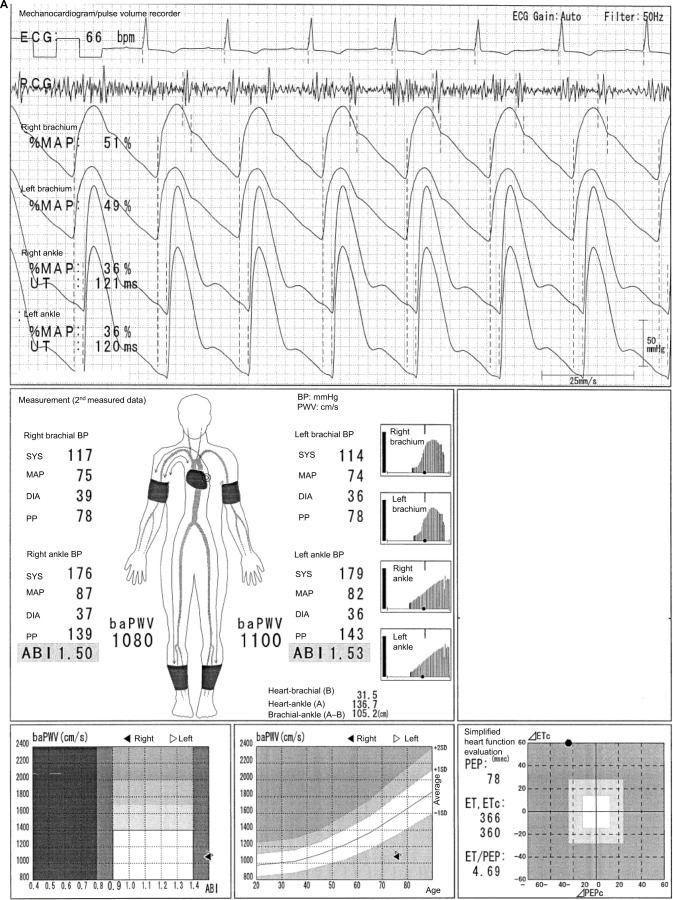
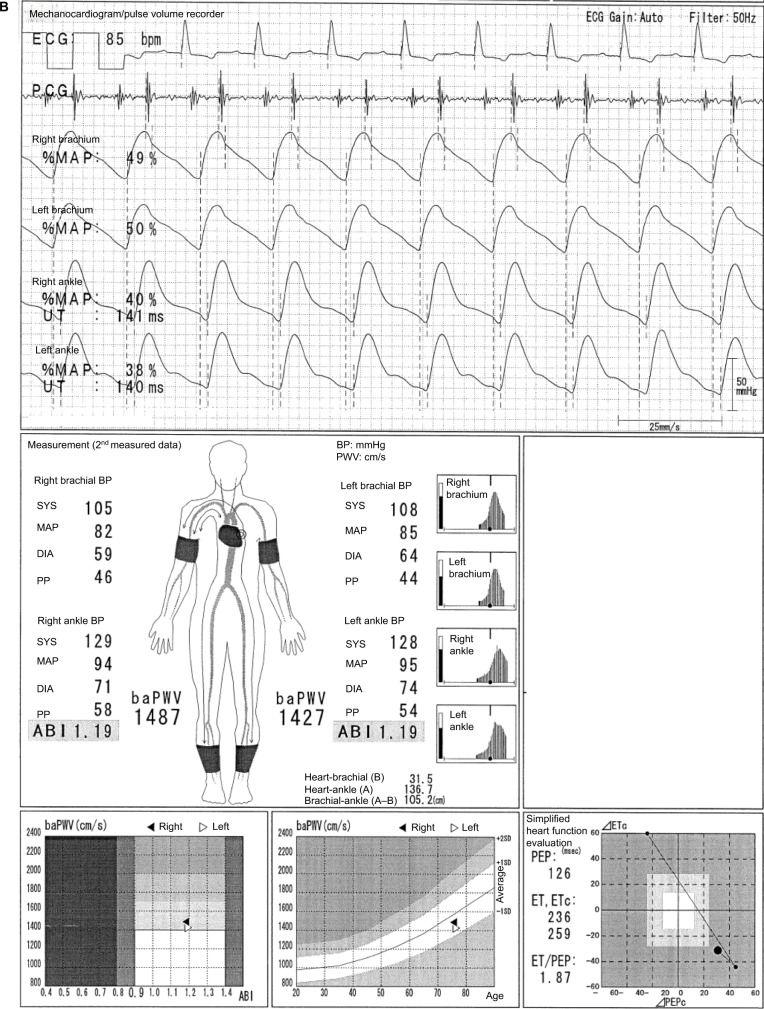
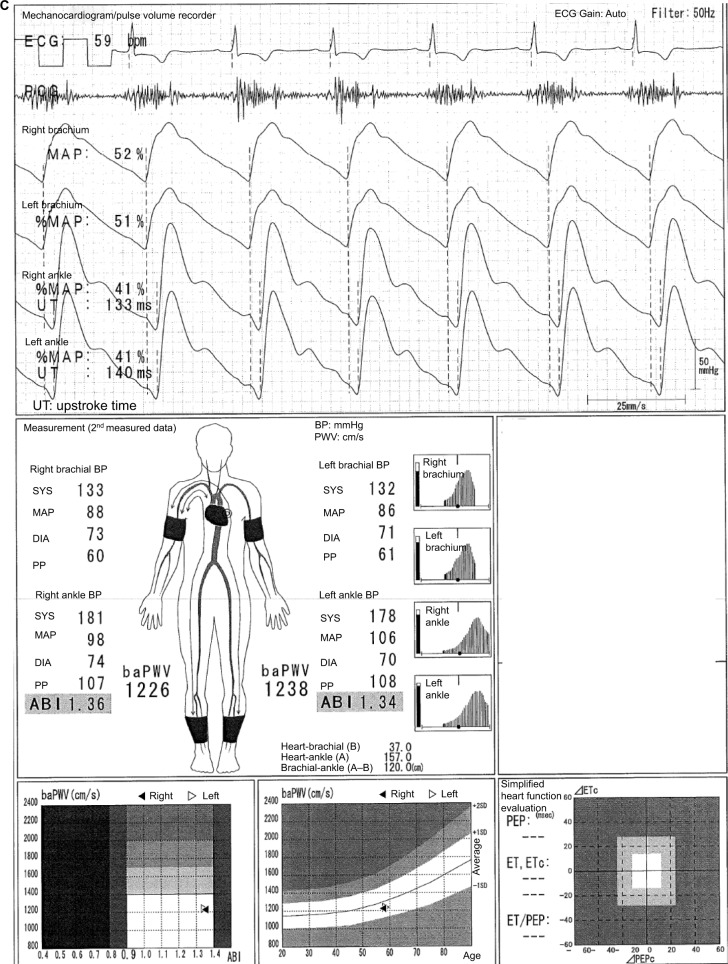
In the case shown in Figure 1, the difference between a normal 64-year-old male participant and a patient with disease can be easily identified. The ABI is 1.26 on both sides, and no laterality is observed. Furthermore, clear dicrotic waves are noted on the ankle pulse waveforms with normal upstroke time (UT) and percent mean arterial pressure (%MAP).1,4,5 As such, the forms of both ankles are normal, and arteriosclerosis obliterans (ASO) of the lower extremities (LE) are not observed. Therefore, the baPWVs of both sides are accurate, and a higher value of 1224 cm/s is within the normal range (standard deviation value of the population without any atherosclerotic risk factors). Laterality is not observed as well. Therefore, this is an ideal result for measuring indices using the Vascular Profiler.
Figure 1.
Normal participants.
Note: See the text for a full explanation of this figure.
Abbreviations: ABI, ankle-brachial index; baPWV, brachial-ankle pulse wave velocity; BP, blood pressure; DIA, diastolic; ECG, electrocardiogram; ET, ejection time; MAP, mean arterial pressure; %MAP, percent mean arterial pressure; PCG, phonocardiogram; PEP, pre-ejection period; PP, pulse pressure; SYS, systolic; UT, upstroke time.
Underestimation of the baPWV in individuals with ASO
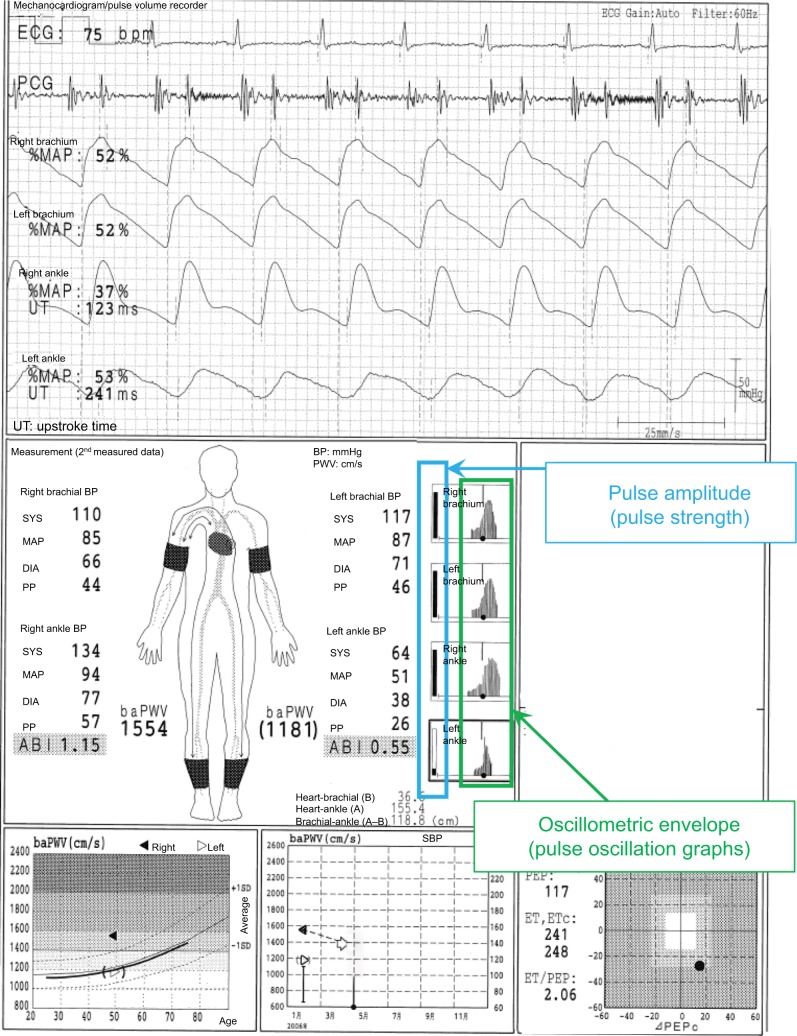
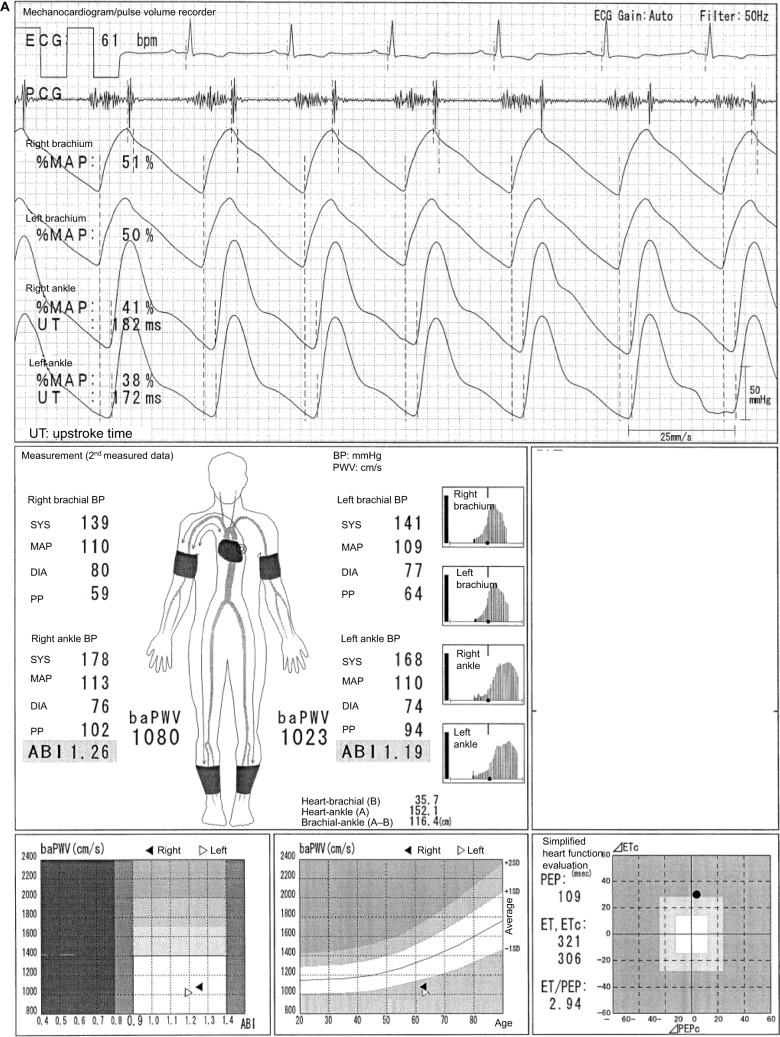
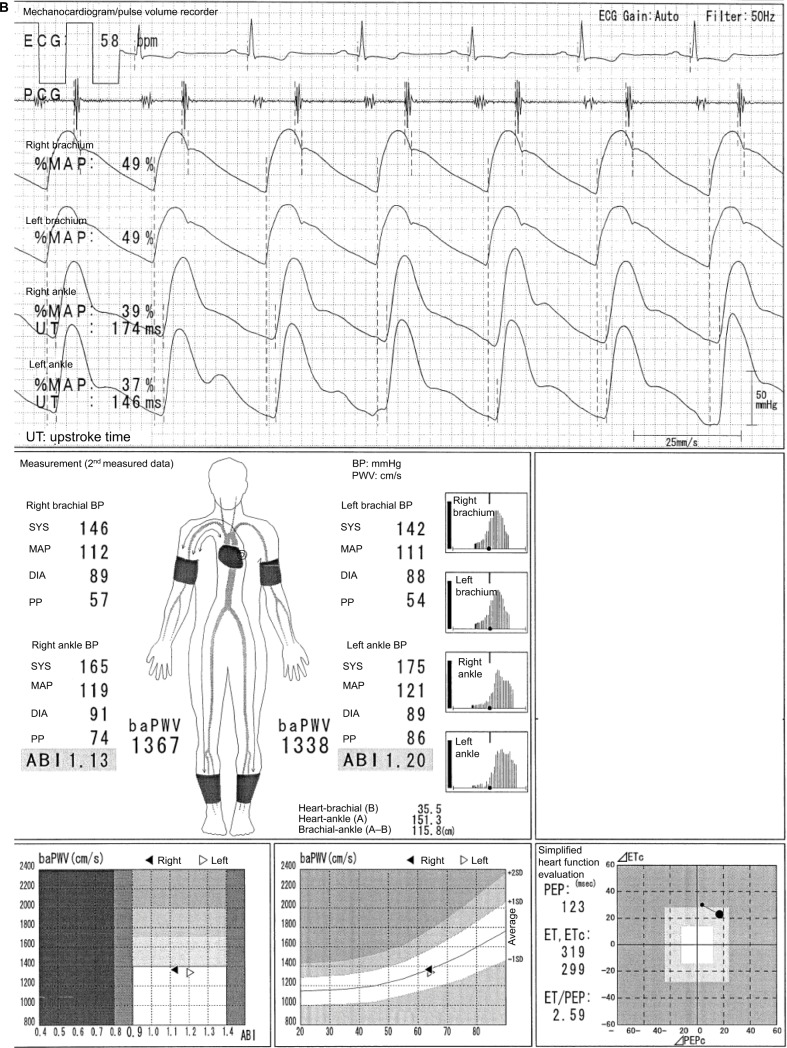
This typical case of a 49-year-old male individual (Figure 2) shows a laterality between the normal lower limb and that with ASO in pulse waveforms, pulse amplitudes, oscillometric envelope, baPWV, and so on. The pulse waveform of the right ankle is normal, and its aspect is similar to that of both ankles as shown in Figure 1. On the contrary, the left ABI (0.55) is not normal, and this result is consistent with the abnormally higher UT and %MAP.1,4,5 The pulse waveform of the left ankle is blunted and triangular, and no clear dicrotic waves are observed. When the Vascular Profiler is used, the height of the pulse waveform is drawn according to pulse pressure, and a pulse pressure of 10 mmHg is expressed as one grid. Thus, a low left ankle pulse waveform is observed because it reflects a pulse pressure of 26 mmHg and is averagely drawn using 2.6 grids in heights. The pulse amplitudes of the right ankle and both arms are perfect, and a strong pulse is observed. On the other hand, the pulse amplitude of the left ankle is significantly low and clearly indicates a weak pulse. The width of the oscillometric envelope is significantly narrow only in the left ankle. This also indicates a decrease in BP and pulse pressure caused by stenosis. The width of the oscillometric envelope reflects a pulse pressure as a whole. Furthermore, the baPWV of the left side is remarkedly lower than that of the normal right side because of the stenosis.6 In addition, the value of the left baPWV is expressed within a parenthesis, which means it should be cautiously interpreted. Moreover, a baPWV of 1554 cm/s in the right side is accurately measured. Nevertheless, this value should not be used, at least as a prognostic predictive marker. ASO in the left leg indicates a prognostically high-risk condition, and this significantly overweighs the significance of the baPWV as a prognostic predictor. In other words, a baPWV of 1554 cm/s indicates a prognostically middle-risk condition, but an ABI of 0.55 indicates a high-risk condition. Thus, they are inconsistent.1,7,8 It would be marginally possible to use a baPWV of 1554 cm/s as a marker of arterial stiffness and/ or function. Nevertheless, the exclusion criteria for patients with ASO have been generally accepted recently when using baPWV for any purpose.1 Moreover, in this case, a remeasurement is not necessary because the BPs and ABIs are accurate based on the information in the oscillometric envelopes.
Figure 2.
Underestimation of the baPWV in individuals with ASO.
Note: See the text for a full explanation of this figure.
Abbreviations: ABI, ankle-brachial index; ASO, arteriosclerosis obliterans; baPWV, brachial-ankle pulse wave velocity; BP, blood pressure; DIA, diastolic; ECG, electrocardiogram; ET, ejection time; PCG, phonocardiogram; MAP, mean arterial pressure; %MAP, percent mean arterial pressure; PEP, pre-ejection period; PP, pulse pressure; SYS, systolic; UT, upstroke time.
In this case, a pseudo-underestimation phenomenon of baPWV caused by ASO was explained. The fundamental requirement in PWV measurement is the absence of a significant physical obstacle in the measuring pathway. Therefore, a similar phenomenon of inaccurate baPWV estimation, which is similar with this case, can be considered in patients with aortic diseases, such as aortic aneurism and dissection.
Pseudo-normalized ABI due to LE arterial calcification
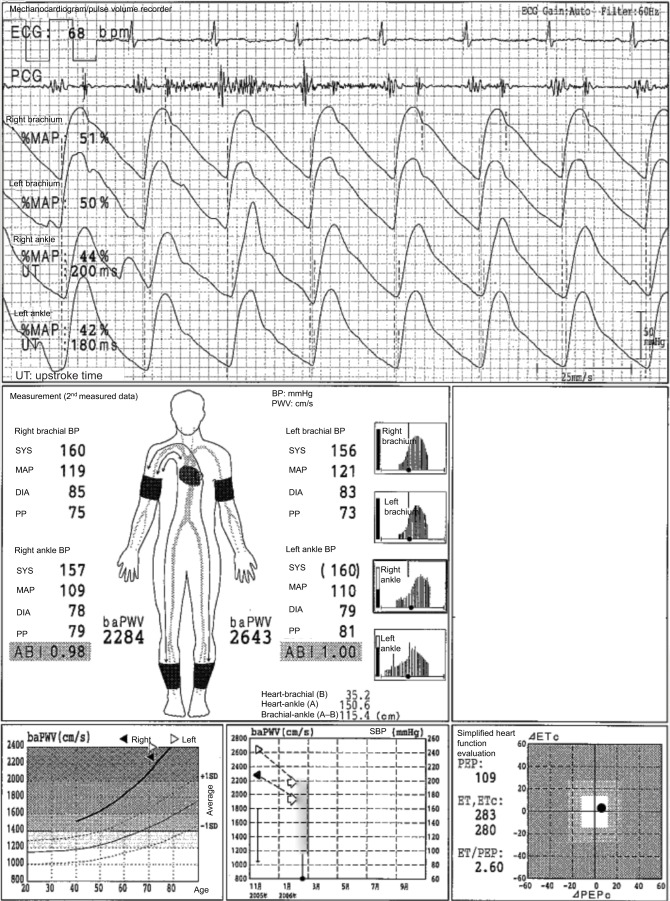
In Figure 3, the case of a 71-year-old male individual with diabetes mellitus is presented. The ABIs are 0.98 on the left side and 1.00 on the right side, but this patient had LE-ASO in both sides. This is a typical case of pseudo-normalized ABI due to a strong arterial calcification of the lower limbs. The ABIs range from borderline to normal, but the pulse waveforms of both ankles are triangular. The UTs are more than 180 ms, and the %MAPs are more than 40% on both sides. Thus, ASO in both sides can be strongly suspected. Furthermore, the baPWVs are significantly high, reaching more than 2000 cm/s, even though they are likely underestimated due to ASOs. As a result, arterial calcification and the pseudo-overestimation of ABI will likely occur. In fact, the toe-brachial index (TBI) is 0.36 on the right side and 0.43 on the left side. Thus, LE-ASO on both sides is confirmed in this patient.1,9
Figure 3.
Pseudo-normalized ABI due to LE arterial calcification.
Note: See the text for a full explanation of this figure.
Abbreviations: ABI, ankle-brachial index; baPWV, brachial-ankle pulse wave velocity; BP, blood pressure; DIA, diastolic; ECG, electrocardiogram; ET, ejection time; LE, lower extremities; MAP, mean arterial pressure; %MAP, percent mean arterial pressure; PCG, phonocardiogram; PEP, pre-ejection period; PP, pulse pressure; SPB, systolic blood pressure; SYS, systolic; UT, upstroke time.
False-positive LE-ASO due to an inaccurate measurement of the ankle BP and possibly unnoticed upper extremity ASO (UE-ASO)
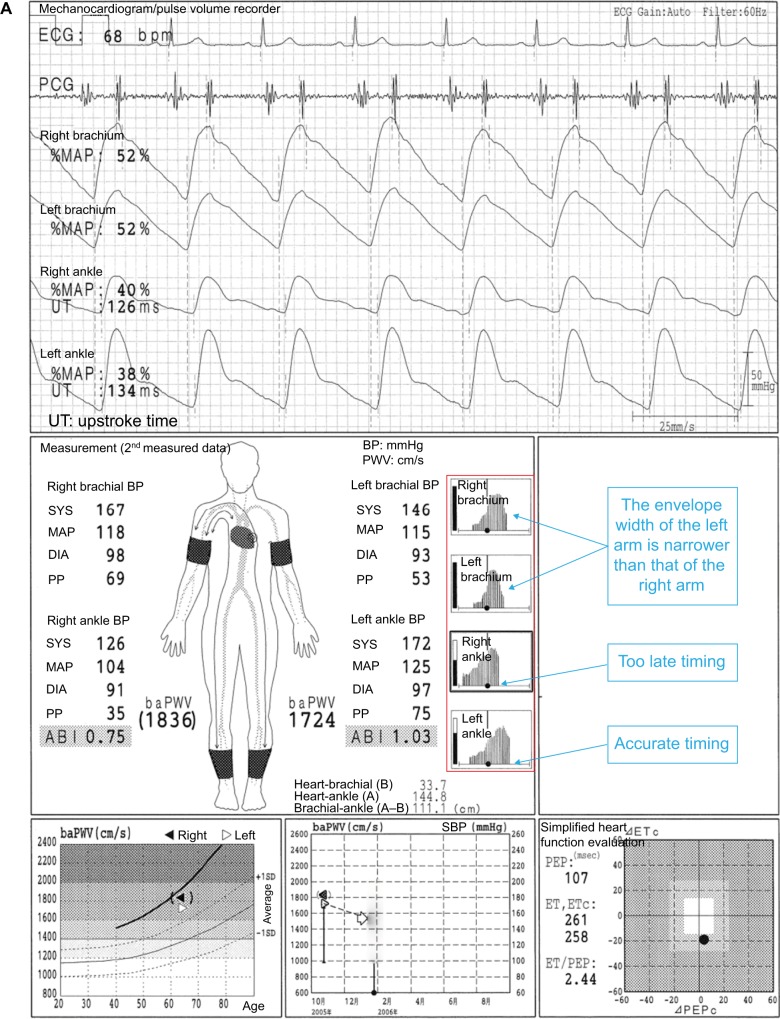
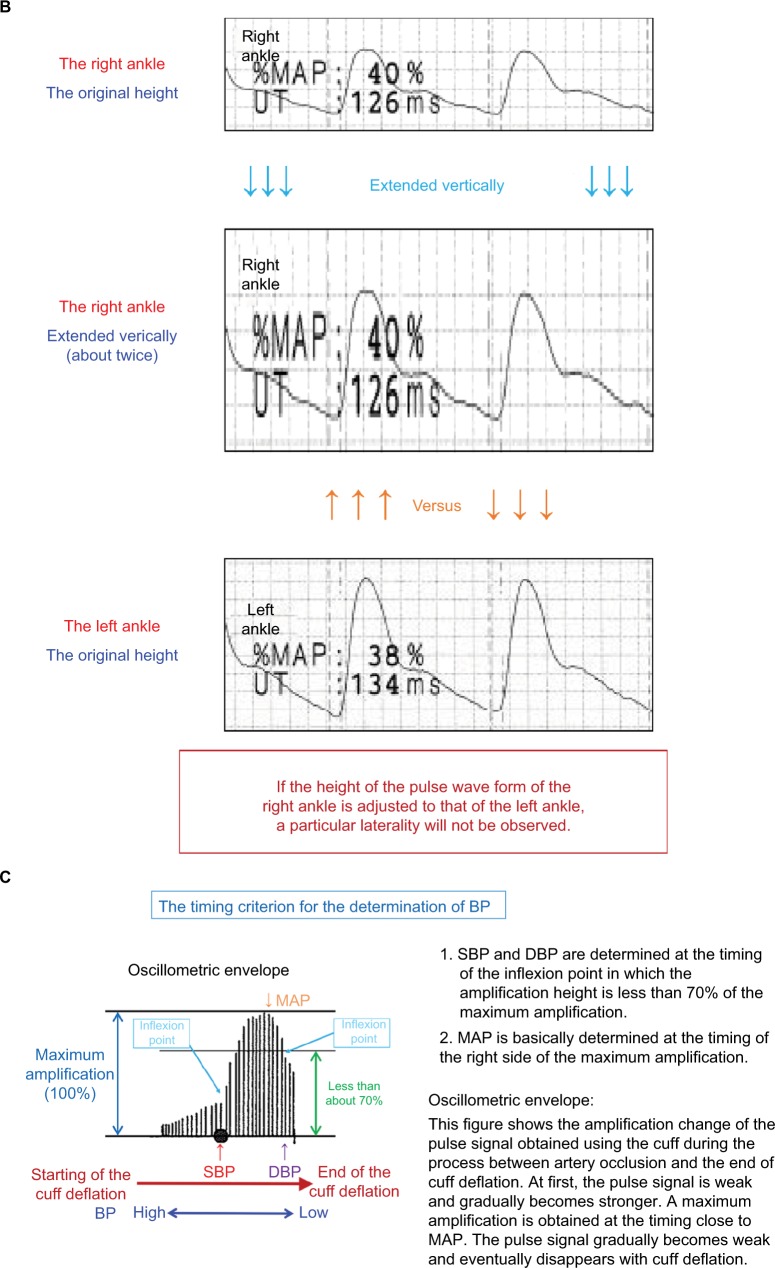
In Figure 4A, the case of a 64-year-old male individual is presented. The ABI in the right side is 0.75, but this is a result of an inaccurate measurement of the right ankle systolic BP (SBP). Thus, this does not indicate LE-ASO. On the other hand, an arterial stenosis of the left UE is most likely to occur. To focus on the oscillometric envelope of the right ankle, the determined timing of the SBP is remarkedly close to that of the maximum pulse amplitude. In other words, the timing of the SBP is too late, which means that the SBP is close to the mean BP. The right ankle SBP has to be determined much earlier (an equivalent timing of the oscillometric envelope in the left ankle), but this is mistaken for some reasons. The aspects of the oscillometric envelopes of both ankles are similar. The UTs and %MAPs of both ankles are normal, and no laterality is observed. Furthermore, dicrotic waves are clearly observed in the pulse waveforms of the right ankle, and no laterality is observed in the baPWVs. In general, the baPWV in the right side should be significantly lower than that of the left side in an individual who presents with ASO in the right LE. A pulse pressure as low as 35 mmHg causes a diminished waveform in the right ankle (due to a falsely measured SBP, that is, the height is averagely expressed in 3.5 grids). Figure 4B shows the comparison of the ankle pulse waveforms with the adjusted heights, and no obvious laterality is observed. The pulse amplitude is lower in the right ankle than in the left ankle. However, the following results are consistent and considered more important: 1) There is no significant laterality in the shape of the oscillometric envelopes; 2) no significant sign of stenosis is observed in the pulse waveform of the right ankle; and 3) the baPWV in the right side is not lower than that of the left side. Improper cuff positioning is considered to have caused the inaccurate measurement. Thus, in this case, a remeasurement with a firmly placed and appropriately positioned cuff should be conducted.
Figure 4.
False-positive LE-ASO due to an inaccurate measurement of the ankle BP and possibly unnoticed upper extremity ASO (UE-ASO).
Notes: (A) The case of a 64-year-old male individual. (B) Comparison of the ankle pulse waveforms with the adjusted heights. (C) Details on the oscillometric envelope of the device. See the text for a full explanation of this figure.
Abbreviations: ABI, ankle-brachial index; ASO, arteriosclerosis obliterans; baPWV, brachial-ankle pulse wave velocity; BP, blood pressure; DBP, diastolic blood pressure; DIA, diastolic; ECG, electrocardiogram; ET, ejection time; LE, lower extremities; MAP, mean arterial pressure; %MAP, percent mean arterial pressure; PCG, phonocardiogram; PEP, pre-ejection period; PP, pulse pressure; SBP, systolic blood pressure; SYS, systolic; UE, upper extremities; UT, upstroke time.
The pulse amplitudes in both arms are perfect. However, there are lateralities of 21 mmHg in the SBP and 5 mmHg in the diastolic BP (DBP), and the width of the left arm oscillometric envelope is somewhat narrower. Thus, an ASO in the left UE is suspected. There are no clear literalities in the pulse waveforms and %MAPs in both arms. Nevertheless, there are frequently cases in which a dynamic change is not observed in the brachial pulse waveform unless the UE-ASO causes a quite large decrease in the brachial BPs. As a reference, details on the oscillometric envelope of this device are depicted in Figure 4C.
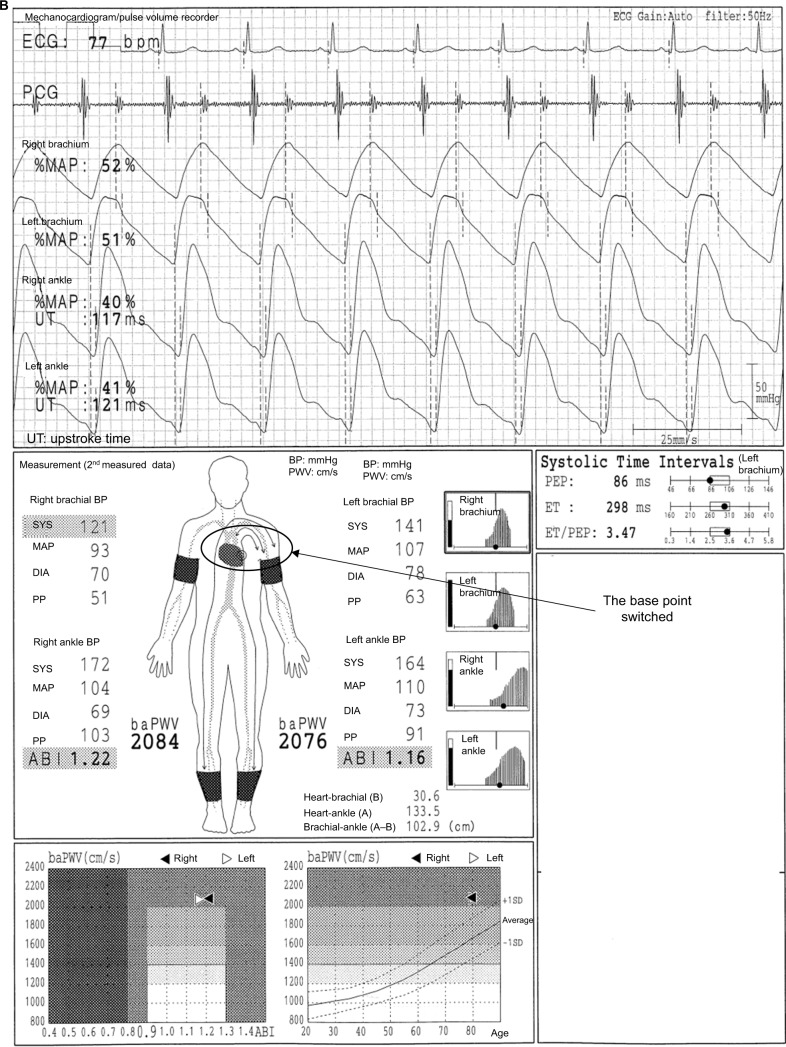
Overestimation of the baPWV and inaccurate measurement of the ejection time (ET) caused by ASO of the right UE
In Figure 5A, we present the case of an 80-year-old female individual. The baPWVs on both sides exceed 3400 cm/s. However, the values are significantly inaccurate. The SBP is lower by 19 mmHg, and the DBP is lower by 3 mmHg in the right arm than those of the left arm, and the pulse waveform of the right arm is blunted. The UT of the right arm pulse waveform is evidently longer than that of the left arm (no value is recorded in the result sheet). Moreover, a lower pulse amplitude and narrower oscillometric envelope are observed in the right arm than in the left arm. Therefore, a right UE-ASO likely exists. However, the brachial base of the baPWV measurement remains in the right arm. In this condition, the pulse transit time between the heart and the right arm becomes longer because of the stenosis, and the right arm pulse waveform slightly shifts to the right side. Therefore, the pulse transit time between the right brachium and the ankles, which is the denominator used to calculate the baPWV, becomes shorter. As a result, the baPWVs are overestimated. The Vascular Profiler has an automatic program that validates the existence of ASO in the right upper limb and that switches the base of the baPWV measurement. Unfortunately, the condition does not match the algorithm in this case. The automatic program requires both of the following: 1) the pulse amplitude of the right arm should be lower than that of the left arm (a slight difference is acceptable); and 2) the inter-arm BP differences must not be less than 16 mmHg in SBP and 5 mmHg in DBP, or 20 mmHg in SBP only. Figure 5B shows the remeasurement results with the base of the arm automatically switched by the program. The baPWV is approximately 2080 cm/s, which is accurate. Moreover, the values of the ET and the ET/PEP (pre-ejection period) have changed. The total systolic interval corresponds to the time between the onset of the Q wave of the electrocardiogram and that of the II sound of the phonocardiogram. Moreover, the total systolic interval is equal to the sum of the ET and PEP (ET+PEP). The ET corresponds to the time interval between the onset and dicrotic notch of the brachial pulse waveform. The PEP corresponds to the time of QII – ET. Thus, the total systolic intervals are both 384 ms in the first and second measurements (290 ms + 94 ms = 384 ms, Figure 5A; 298 ms + 86 ms = 384 ms, Figure 5B). The ET and ET/PEP increased (290ms→298ms and 3.09→3.47, respectively). Therefore, when there is ASO in the UE artery, the indices related to the systolic time intervals will be affected. In addition, there are several cases in which the conditions do not match the requirement in switching the brachial base. For the maneuver in such a situation, the cuffs of the arms can be set oppositely. Nevertheless, BPs and the pulse waveforms of the arms show those of the opposite side if this maneuver will be carried out. Thus, this must be reflected in the result sheet and/or medical record.
Figure 5.
Overestimation of the baPWV and inaccurate measurement of the ET caused by ASO of the right UE.
Notes: (A) The case of an 80-year-old female individual. (B) The remeasurement results with the base of the arm automatically switched by the program. See the text for a full explanation of this figure.
Abbreviations: ABI, ankle-brachial index; ASO, arteriosclerosis obliterans; baPWV, brachial-ankle pulse wave velocity; BP, blood pressure; DIA, diastolic; ECG, electrocardiogram; ET, ejection time; MAP, mean arterial pressure; %MAP, percent mean arterial pressure; PCG, phonocardiogram; PEP, pre-ejection period; PP, pulse pressure; SYS, systolic; UE, lower extremities; UT, upstroke time.
This case is considerably rare in Japan but would be more common in Western countries because of the higher prevalence of cardiovascular diseases, including peripheral arterial disease.10–14 Furthermore, a similar phenomenon will likely occur in the carotid-femoral PWV (cfPWV) when there is a carotid artery stenosis in the cfPWV pathway. Therefore, caution is needed in interpreting cfPWV especially when a carotid stenosis is suspected. And the prevalence of carotid arterial diseases will be higher in western countries than in Japan, with a similar prevalence of cardiovascular diseases.10–14
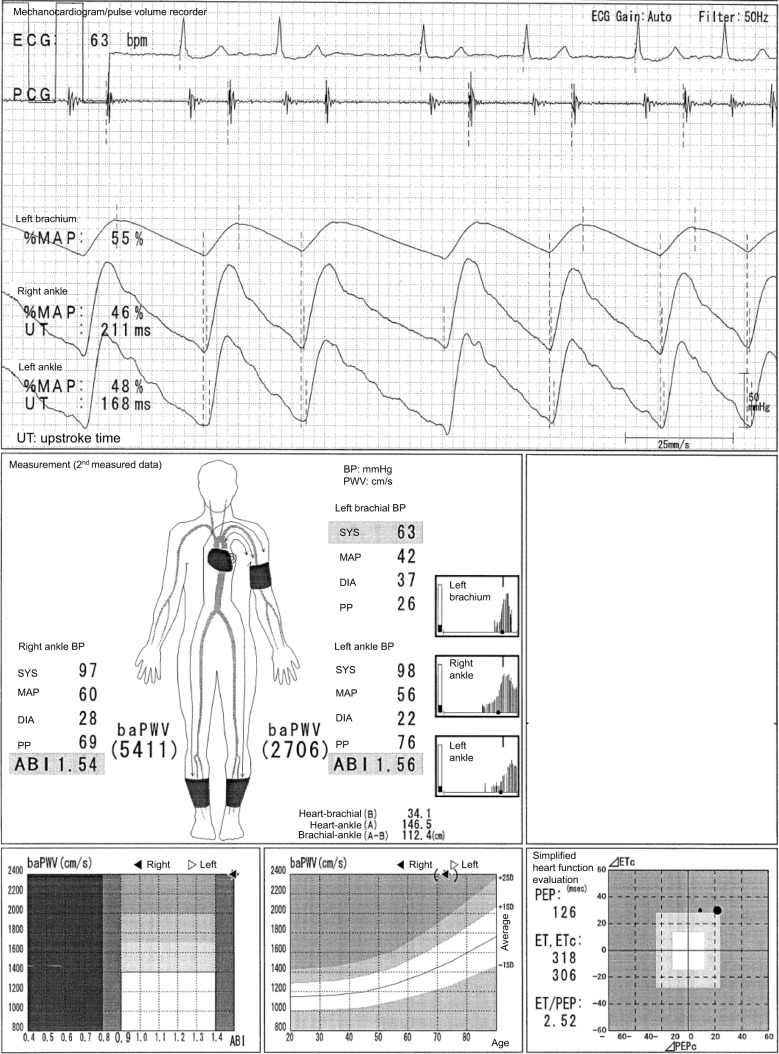
Overestimation of ABI and baPWV caused by ASO in the brachium for blood pressure measurement (non-hemodialysis access side) in hemodialysis patients
In Figure 6, the case of a 73-year-old male individual with a hemodialysis access in the right arm is presented. The ASOs in the left arm and both legs are clearly observed regardless of ABIs, and the progression of calcification in the LE and aortic stiffness also exists because of hemodialysis. However, both ABIs and baPWVs are not reliable at all. For this reason, all the pulse waveforms are triangular. All the pulse amplitudes are extremely low, and the %MAPs on both ankles are more than 45%. In addition, the oscillometric envelope of the left arm is significantly narrow, and a left brachial SBP of 63 mmHg, which is the denominator used to calculate the ABI, is remarkedly low. As a result, the ABIs are overestimated. Moreover, the baPWVs are also overestimated, which is similar to the phenomenon shown in Figure 5A (this effect seems to far more exceed the possible underestimation of baPWVs caused by LE-ASOs in this case). Therefore, the ABIs and baPWVs are never accurate regardless of the remeasurements that are carried out. Nevertheless, for the significance of the measurement, stenotic diseases can be easily considered using the pulse waveforms. Moreover, the absolute values of the BPs could be used as a reference value, considering the influence of the LE arterial calcification because the timing of BP measurement in each oscillometric envelope is acceptable.
Figure 6.
Overestimation of ABI and baPWV caused by ASO in the brachium for blood pressure measurement (non-hemodialysis access side) in hemodialysis patients.
Note: See the text for a full explanation of this figure.
Abbreviations: ABI, ankle-brachial index; ASO, arteriosclerosis obliterans; baPWV, brachial-ankle pulse wave velocity; BP, blood pressure; DIA, diastolic; ECG, electrocardiogram; ET, ejection time; MAP, mean arterial pressure; %MAP, percent mean arterial pressure; PCG, phonocardiogram; PEP, pre-ejection period; PP, pulse pressure; SYS, systolic; UT, upstroke time.
Herein, one of the pitfalls in using ABI is presented, that is, the BP and pulse waveforms in the left arm are not within the normal range. On the other hand, there are several cases in which the change in the brachial pulse waveform is not remarkable, although a stenosis exists. Thus, stenosis cannot be easily identified in such a situation. For example, the pulse waveform of the left brachium is similar to that of the right brachium (Figure 4A). Let us suppose that the right arm is not measured as shown in Figure 4A. Furthermore, the laterality cannot be validated using the information provided, including that of the pulse waveforms of both arms in hemodialysis patients, because BP is generally measured only in the contralateral side of the hemodialysis access. However, even if the decrease in SBP caused by UE-ASO is not significant, for example, 10 mmHg, the interpretation of the ABI could significantly change (i.e. 135÷140=0.96 and 135÷130=1.04). Thus, the only way which could avoid these pitfalls (and not perfectly) is to carry out measurement in both arms. BP measurement in the arm with hemodialysis access is generally contraindicated, and it is also explained in the device manual. Nevertheless, there is important information on this issue according to an oral presentation of the Japanese Association of Cardiovascular Intervention and Therapeutics in 2013.15 A total of 169 hemodialysis patients who agreed to have their BP measured in both arms with the Vascular Profiler were recruited. BP measurements were obtained in both arms in 147 patients (19 patients declined, and three patients had unsuccessful measurement due to involuntary movements). Forty patients (27.2%) had an inter-arm difference of ≧10 mmHg in their SBP. Among them, 15 patients underwent further examination as requested (ultrasound imaging). Stenosis and occlusion in the UE were confirmed in 12 out of 15 patients, and these were observed in the side where the BP reading was usually obtained (non-hemodialysis access side) in five patients. In addition, failed hemodialysis access was not observed in 150 patients. Thus, they conclude that measurements must be obtained in both arms in hemodialysis patients, particularly when LE-ASO is suspected, because the likelihood of measuring appropriate ABIs could be improved.15 Therefore, regarding the increased ABI in the hemodialysis patients with ASO, the phenomenon explained above is causing this to a certain extent as well as the LE arterial calcification,
Hemodialysis patients are highly recommended to undergo periodic ABI measurement. However, the pitfalls may likely be overlooked even if information from the pulse waveforms is provided at the same time as using the device because only one arm is generally used in measuring the BP of hemodialysis patients. Therefore, full attention in confirming all the information is always required when interpreting the measurements in hemodialysis patients.
Moreover, this case also implies that the measurement of the ABI would be more difficult in hemodialysis individuals when the conventional Doppler method is used. There is no information from the pulse waveform regarding the Doppler method (except the Doppler waveform). And it is impossible for the Doppler method to synchronize the SBP measurement of all the limbs in the same way that the Vascular Profiler performs. The statement document of the American Heart Association (2012) points outs that there were two studies that obtained a left ABI of 0.03 significantly lower than the right ABI (in the general population, not hemodialysis cohort).16 Moreover, according to a meta-analysis on the prevalence of laterality in brachial BPs, a significant increase in the odds ratio of the inter-arm difference (i.e. SBP ≧ 10 mmHg) was observed when the measuring process was compared (sequential versus simultaneous).17 This information implies that ABI measurement using the Doppler method is more prone to accidental errors than the synchronized measurement with the information from the pulse waveforms.
Increased ABI and decreased baPWV in patients with aortic regurgitation
In Figure 7A, the case of a 76-year-old female individual is presented. The features of her disease are as follows: 1) high ABI (i.e. >1.3) and pulse pressure in the UE and LE;18 2) low baPWV for the patient’s age and/or BP (in the absence of LE-ASO), and in the result sheet of the Vascular Profiler; 3) a high pulse waveform of the ankle and an oscillometric envelope with a significantly long width (reflecting a wider pulse pressure); 4) a tendency of lower UT and %MAP; and 5) continuous noise of the phonocardiogram depending on the case and the setting. These are considered as compensatory mechanisms. First, the high ABI is a result of the altered condition in the heart systole and the systemic arteries to rapidly increase the peripheral BP during early systole to compensate for the regurgitation during the late systole. Second, the low baPWV is primarily a result of the dilated aorta due to an increased blood mass in the aorta and arteries compared to the normal condition for the compensation of the regurgitation and of the concurrent tendency of decreased DBP. The wider the internal diameter of the artery, the lower the PWV. After the patient underwent an operation to replace the aortic valve, the ABIs and baPWV (both ABI=1.19; the average baPWV is 1457 cm/s) and the other parameters normalized, except for the electrocardiogram results and systolic function (Figure 7B).
Figure 7.
Increased ABI and decreased baPWV in patients with aortic regurgitation.
Notes: (A) The case of a 76-year-old female individual. (B) After the patient had undergone an operation to replace the aortic valve. See the text for a full explanation of this figure.
Abbreviations: ABI, ankle-brachial index; baPWV, brachial-ankle pulse wave velocity; BP, blood pressure; DIA, diastolic; ECG, electrocardiogram; ET, ejection time; MAP, mean arterial pressure; %MAP, percent mean arterial pressure; PCG, phonocardiogram; PEP, pre-ejection period; PP, pulse pressure; SYS, systolic; UT, upstroke time.
Decreased baPWV in a patient with aortic valve stenosis
Figure 8A shows the case of a 63-year-old male individual. The features of his disease are as follows: 1) a major noise in the systole on phonocardiogram; 2) low baPWV for the patient’s age and/or BP (in the absence of LE-ASO); and 3) prolongation tendency in ET and UT. The exact cause of the decreased baPWV cannot be fully elucidated. Nevertheless, the considerable mechanisms are as follows: 1) the energy of the systole does not adequately propagate because of the aortic valve abnormality and the turbulence in the ascending aorta; 2) the characteristics of the systemic arteries, including the aorta, could be temporally altered, such as the aortic regurgitation described previously. Figure 8B shows the result of the post-aortic valve replacement. baPWV increased to approximately 300 cm/s, which is within the reference range, although there is a slight increase in the brachial DBP. In addition, the heart noise disappeared. Figure 8C presents a typical case in which both features of the regurgitation and stenosis of the aortic valve are clearly observed (in a 58-year-old male individual). The following similarities are observed: 1) baPWV tends to be low; 2) ABI and pulse pressure tend to be high; 3) the width of the oscillometric envelope is wide; and 4) there is a strong noise during systole on phonocardiogram.
Figure 8.
Decreased baPWV in a patient with aortic valve stenosis.
Notes: (A) The case of a 63-year-old male individual. (B) The result of the post-aortic valve replacement. (C) A typical case in which both features of the regurgitation and stenosis of the aortic valve are clearly observed (in a 58-year-old male individual). See the text for a full explanation of this figure.
Abbreviations: ABI, ankle-brachial index; baPWV, brachial-ankle pulse wave velocity; BP, blood pressure; DIA, diastolic; ECG, electrocardiogram; ET, ejection time; MAP, mean arterial pressure; %MAP, percent mean arterial pressure; PCG, phonocardiogram; PEP, pre-ejection period; PP, pulse pressure; SYS, systolic; UT, upstroke time.
The difference in the oscillometric envelopes in various diseases
The oscillometric envelope varies with different conditions and diseases. In summary, when stenotic diseases exist, an oscillometric envelope with a narrow width is observed. Moreover, when severe LE arterial calcification or aortic regurgitation exists, an oscillometric envelope with a wider width is noted. However, the shape is different. The information in the oscillometric envelope will support the validity of the BP readings and ABI (see Figure S1).
The categorization of the errors in ABI and baPWV measured by the Vascular Profiler
The phenomena discussed in this article and other phenomena are summarized in Table S1, except a low ABI caused by ASO. Furthermore, a low ABI and a borderline ABI measured by the Vascular Profiler are frequently observed in healthy young individuals in Japan.19–21 For example, the left ABI of female students (age; 9–17 years old, mean 14.7) is 1.03±0.09 (range; 0.73–1.33).19 The prevalence of ABI of <1.0 among medical university students was 18.1% in males and 25.6% in females.20 The prevalence of ABI of ≤0.9 and age showed a J-curve relationship among women in the general population.21 In this study, the prevalence of ABI of ≤0.9 was 0.9% in the group under 40 years old, which decreased to 0.2% in the group of 60–69 years old, then increased to 1.6% in the group over 70 years old.21 These phenomena are likely caused by a weak reflected wave of the flexible artery at the ankle. This information is also included in Table S1. This categorization would also help us to understand the results of the Vascular Profiler, and also to judge the validity of the indices.
Discussion
Implication of the all presented cases
Several representative cases of the pitfalls in using the indices measured with the Vascular Profiler have been presented. An underestimation of baPWV in individuals with LE-ASO is generally known.1,6–8,22,23 However, several cases with a combination of pitfalls are widely observed. Moreover, a low baPWV does not always necessarily indicate a good cardiovascular condition. A recent study reported that a low baPWV and a high baPWV predict a worse prognosis in patients with heart failure and preserved ejection function.24 Therefore, when using baPWV in cohorts with severe disease conditions in both clinical and research settings, diseases such as ASO and arrhythmia, should not only be excluded but a specific exclusion criteria should also be considered.8
Inaccurate and falsely elevated ABI and its implication
Numerous studies that validated the ABI measured using the Vascular Profiler have been conducted, and positive associations between the use of the Doppler method and diagnostic imaging were noted.4,25–30 Nevertheless, measurements are sometimes not accurately performed (Figure 4A). Therefore, the oscillometric envelope (the timing of SBP determination) must be confirmed. Furthermore, the cause of the falsely elevated ABIs in hemodialysis patients is not only the LE arterial calcification but also the masked UE-ASO (Figure 6).15 The cut-off values for the ABIs measured using the Vascular Profiler for LE-ASO diagnosed via imaging modalities or clinical symptoms in Japanese hemodialysis patients ranged from 1.00 to 1.10.31–33 Ohtake et al suggested increasing the ABI cut-off value to 1.10 in hemodialysis patients.32 Considering the previous studies on the prognostic predictability of ABI in hemodialysis patients, an ABI cut-off value of 1.10 is used in predicting the all-cause mortality in 2013.34 In this study, the area under the receiver operating characteristic curve was 0.79. The sensitivity was 0.90, and the specificity was 0.62. Furthermore, a similar study was also conducted in 2003.26 In this study, the patients with an ABI of <0.90 or 0.90–0.99 (abnormal and borderline ABI) had the worst prognosis. However, those with a low–normal ABI of 1.00–1.09 also had a worse prognosis than those with a reference ABI of 1.10–1.29 (hazard ratio [HR], 1.92 and 95% confidence interval [CI], 1.02–3.59 for all-cause mortality; HR, 2.82 and 95% CI, 1.22–6.54 for cardiovascular mortality, after a multivariate adjustment using the Cox proportional hazards model). Ono et al suggested that those with an ABI of 0.90–1.10 and with abnormal ABIs should be carefully monitored.26 This study still has the largest hemodialysis cohort that used the device (1010 patients).26 In the largest cohort study of patients with diabetes (3981 participants), the adjusted HRs and p values for the all-cause and cardiovascular mortality among those with an abnormal ABI of ≤0.90 and those with a borderline ABI of 0.91–0.99 were similar to those with a reference ABI of 1.00–1.40.35 Moreover, in the largest cohort of high-risk patients and those with various cardiovascular diseases (4756 participants), those with a borderline ABI of 0.91–0.99 had a more significant adjusted HRs for the all-cause and cardiovascular mortality than those with a normal ABI of 1.00–1.40.36 Thus, the results were similar in cohorts with diabetes and those who are at high risk of cardiovascular diseases.35,36 On the contrary, in the general population with 2954 participants, the significance of the adjusted HR for cardiovascular events in those with an abnormal ABI of ≤0.90 was clearly presented in the Hisayama study. However, no difference was observed in those with a borderline ABI of 0.91–0.99 compared to those with a normal ABI of 1.00–1.40 even in the Kaplan–Meier analysis.37 The ABI in all the prognostic studies described above was measured using the Vascular Profiler. The ABI threshold must be modified based on patient characteristics and its use (evaluation of ASO or prognosis prediction). In addition, the ABI threshold should be increased to 1.00–1.10, particularly in high-risk cohorts, participants with diabetes, and those undergoing hemodialysis. At the same time, this problem must be recognized, particularly when performing a meta-analysis of the data along with heterogeneous populations38–40 because the exclusion of individuals with LE-ASO is essential to appropriately assess baPWV.1,6–8,22,23
Application to other indices and devices
Lastly, the above-mentioned phenomena will likely occur when other indices are measured using devices, such as CAVI, VaSera,41–45 and other similar devices in other countries.46–50 The contents of this article would be useful in interpreting the results and pitfalls of the four-limb BP/ABI/PWV measurements.
Conclusion
The pitfalls of using ABIs and baPWVs measured using the Vascular Profiler were discussed. Moreover, the need of an appropriate setting in using the patient exclusion criteria when assessing baPWV and of adjusting the cut-off value of ABI based on patient characteristics was also discussed. And the content is expectedly applied to enhance the literacy of related indices and of other similar devices.
Supplementary materials
The difference in the oscillometric envelope in various diseases.
Note: See the article text for a full explanation of this figure.
Abbreviations: BP, blood pressure; ASO, arteriosclerosis obliterans; %MAP, percent mean arterial pressure; UT, upstroke time; R-ABI, right ankle-brachial index; L-ABI, left ankle-brachial index.
Table S1.
The categorization of the errors in ABI and baPWV as measured by the Vascular Profiler
| ABI | baPWV | |
|---|---|---|
| Underestimation or lower value | A low and a borderline ABI in young individuals including children, men under 30 years old, women under 40 years old, especially, a low and a borderline ABI are frequently observed among healthy children and university students of both genders | 1. Obstruction such as arterial stenosis in the measurement pathway from the heart to the ankle 2. Moderate to severe aortic valve diseases |
| Overestimation or high value | 1. Arterial calcification in the lower limb 2. Severe aortic valve regurgitation 3. Low brachial systolic BP due to arterial stenosis in the upper limb (particularly hemodialysis patients) 4. Too narrow cuff width for the ankle 5. Too loose ankle cuff setting |
Arterial stenosis in the brachial base of the baPWV measurement (from the heart to the brachium) |
| Measurement error | Inaccurate timing determination of systolic BP in the oscillometric envelope | Inaccurate determination of the upstroke in the pulse waveform |
Abbreviations: ABI, ankle-brachial index; baPWV, brachial-ankle pulse wave velocity; BP, blood pressure.
Acknowledgments
The author would like to thank all the medical practitioners who provided the case results for their courtesy.
Footnotes
Disclosure
Dai Ato wrote this article, which is as an academic activity based on the guaranteed right of freedom in an academy for Japanese (Article 23) and on the supreme law provided in Article 98 of the Constitution of Japan. The author is a former employee of Fukuda Colin (formerly Omron Colin, Nippon Colin) Co., Ltd., which is one of the distributors of the Vascular Profiler (form PWV/ABI, BP-203RPE series). The author reports no other conflicts of interest in this work.
References
- 1.Yamashina A, Kario K, Kohara K, et al. [Guidelines for noninvasive vascular function test] (JCS2013). [Article in Japanese] Available from: http://www.j-circ.or.jp/guideline/pdf/JCS2013_yamashina_h.pdf.
- 2.Shimamoto K, Ando K, Fujita T, et al. Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension The Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension. Hypertens Res. 2014;37(4):253–390. doi: 10.1038/hr.2014.20. [DOI] [PubMed] [Google Scholar]
- 3.Vlachopoulos C, Xaplanteris P, Aboyans V, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis. 2015;241(2):507–532. doi: 10.1016/j.atherosclerosis.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto T, Ichihashi S, Iwakoshi S, Kichikawa K. Combination of pulse volume recording (PVR) parameters and ankle-brachial index (ABI) improves diagnostic accuracy for peripheral arterial disease compared with ABI alone. Hypertens Res. 2016;39(6):430–434. doi: 10.1038/hr.2016.13. [DOI] [PubMed] [Google Scholar]
- 5.Sawayama T. Up-stroke time prolongation as potential early diagnostic marker of peripheral artery disease – a longitudinal study. Japanese J Clin Physiol. 2016;46(2):79–85. Japanese. [Google Scholar]
- 6.Yokoyama H, Shoji T, Kimoto E, et al. Pulse wave velocity in lower limb arteries among diabetic patients with peripheral arterial disease. J Atheroscler Thromb. 2003;10(4):253–258. doi: 10.5551/jat.10.253. [DOI] [PubMed] [Google Scholar]
- 7.Munakata M. Brachial-ankle pulse wave velocity: background, method, and clinical evidence. Pulse. 2016;3(3–4):195–204. doi: 10.1159/000443740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ato D, Takami T. Brachial-ankle pulse wave velocity, mortality, and cardiovascular events. J Cardiovasc Disord. 2015;2(1):1009. [Google Scholar]
- 9.Norgren L, Hiatt WR, Dormandy JA, TASC II Working Group Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 10.Miura K, Nakagawa H, Ohashi Y, et al. Japan Arteriosclerosis Longitudinal Study (JALS) Group Four blood pressure indexes and the risk of stroke and myocardial infarction in Japanese men and women: a meta-analysis of 16 cohort studies. Circulation. 2009;119(14):1892–1898. doi: 10.1161/CIRCULATIONAHA.108.823112. [DOI] [PubMed] [Google Scholar]
- 11.Clark CE, Taylor RS, Shore AC, Ukoumunne OC, Campbell JL. Association of a difference in systolic blood pressure between arms with vascular disease and mortality: a systematic review and meta-analysis. Lancet. 2012;379(9819):905–914. doi: 10.1016/S0140-6736(11)61710-8. [DOI] [PubMed] [Google Scholar]
- 12.Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 13.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116(9):1509–1526. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 14.Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118(4):535–546. doi: 10.1161/CIRCRESAHA.115.307611. [DOI] [PubMed] [Google Scholar]
- 15.Takizawa C, Ito M, Iwasa M, et al. [Clinical significance of ABI by measuring bilateral arms in hemodialysis patients] Japanese Ass Cardiovasc Intervent Therapeut. 2013;5(Suppl I):736. (1884-0027). Japanese. [Google Scholar]
- 16.Aboyans V, Criqui MH, Abraham P, et al. American Heart Association Council on Peripheral Vascular Disease. Council on Epidemiology and Prevention. Council on Clinical Cardiology. Council on Cardiovascular Nursing. Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126(24):2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 17.Verberk WJ, Kessels AG, Thien T. Blood pressure measurement method and inter-arm differences: a meta-analysis. Am J Hypertens. 2011;24(11):1201–1208. doi: 10.1038/ajh.2011.125. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto T, Umebashi K, Tomizono M, Takanaga M, Hashimoto E, Sasaki Y. Evaluation of aortic regurgitation by ankle brachial pressure index (ABI) test. Japanese J Med Technol. 2016;65(1):18–24. Japanese. [Google Scholar]
- 19.Niboshi A, Hamaoka K, Sakata K, Inoue F. Characteristics of brachial-ankle pulse wave velocity in Japanese children. Eur J Pediatr. 2006;165(9):625–629. doi: 10.1007/s00431-006-0135-y. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe Y, Masaki H, Yunoki Y, et al. Ankle-brachial index, toe-brachial index, and pulse volume recording in healthy young adults. Ann Vasc Dis. 2015;8(3):227–235. doi: 10.3400/avd.oa.15-00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishida A, Miyagi M, Kinjo K, Ohya Y. Age- and sex-related effects on ankle-brachial index in a screened cohort of Japanese: the Okinawa Peripheral Arterial Disease Study (OPADS) Eur J Prev Cardiol. 2014;21(6):712–718. doi: 10.1177/2047487312462822. [DOI] [PubMed] [Google Scholar]
- 22.Motobe K, Tomiyama H, Koji Y, et al. Cut-off value of the ankle-brachial pressure index at which the accuracy of brachial-ankle pulse wave velocity measurement is diminished. Circ J. 2005;69(1):55–60. doi: 10.1253/circj.69.55. [DOI] [PubMed] [Google Scholar]
- 23.Ato D, Sawayama T. Factors associated with high brachial-ankle pulse wave velocity in non-hypertensive and appropriately treated hypertensive patients with atherosclerotic risk factors. Vasc Health Risk Manag. 2017;13:383–392. doi: 10.2147/VHRM.S144923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tokitsu T, Yamamoto E, Oike F, et al. Clinical significance of brachial-ankle pulse-wave velocity in patients with heart failure with preserved left ventricular ejection fraction. J Hypertens. 2018;36(3):560–568. doi: 10.1097/HJH.0000000000001589. [DOI] [PubMed] [Google Scholar]
- 25.Nukumizu Y, Matsushita M, Sakurai T, Kobayashi M, Nishikimi N, Komori K. Comparison of Doppler and oscillometric ankle blood pressure measurement in patients with angiographically documented lower extremity arterial occlusive disease. Angiology. 2007;58(3):303–308. doi: 10.1177/0003319707302673. [DOI] [PubMed] [Google Scholar]
- 26.Ono K, Tsuchida A, Kawai H, et al. Ankle-brachial blood pressure index predicts all-cause and cardiovascular mortality in hemodialysis patients. J Am Soc Nephrol. 2003;14(6):1591–1598. doi: 10.1097/01.asn.0000065547.98258.3d. [DOI] [PubMed] [Google Scholar]
- 27.Guo X, Li J, Pang W, et al. Sensitivity and specificity of ankle-brachial index for detecting angiographic stenosis of peripheral arteries. Circ J. 2008;72(4):605–610. doi: 10.1253/circj.72.605. [DOI] [PubMed] [Google Scholar]
- 28.Cortez-Cooper MY, Supak JA, Tanaka H. A new device for automatic measurements of arterial stiffness and ankle-brachial index. Am J Cardiol. 2003;91(12):1519–1522. A9. doi: 10.1016/s0002-9149(03)00416-8. [DOI] [PubMed] [Google Scholar]
- 29.Koji Y, Tomiyama H, Ichihashi H, et al. Comparison of ankle-brachial pressure index and pulse wave velocity as markers of the presence of coronary artery disease in subjects with a high risk of atherosclerotic cardiovascular disease. Am J Cardiol. 2004;94(7):868–872. doi: 10.1016/j.amjcard.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 30.Richart T, Kuznetsova T, Wizner B, Struijker-Boudier HA, Staessen JA. Validation of automated oscillometric versus manual measurement of the ankle-brachial index. Hypertens Res. 2009;32(10):884–888. doi: 10.1038/hr.2009.125. [DOI] [PubMed] [Google Scholar]
- 31.Ogata H, Kumata-Maeta C, Shishido K, et al. Detection of peripheral artery disease by duplex ultrasonography among hemodialysis patients. Clin J Am Soc Nephrol. 2010;5(12):2199–2206. doi: 10.2215/CJN.09451209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohtake T, Oka M, Ikee R, et al. Impact of lower limbs’ arterial calcification on the prevalence and severity of PAD in patients on hemodialysis. J Vasc Surg. 2011;53(3):676–683. doi: 10.1016/j.jvs.2010.09.070. [DOI] [PubMed] [Google Scholar]
- 33.Tsuyuki K, Kohno K, Ebine K, et al. Usefulness of exercise-ankle brachial pressure index test and its diagnostic criteria in patients on maintenance hemodialysis. J Jpn Coll Angiol. 2015;55:21–25. Japanese. [Google Scholar]
- 34.Otani Y, Otsubo S, Kimata N, et al. Effects of the ankle-brachial blood pressure index and skin perfusion pressure on mortality in hemodialysis patients. Intern Med. 2013;52(21):2417–2421. doi: 10.2169/internalmedicine.52.0410. [DOI] [PubMed] [Google Scholar]
- 35.Natsuaki C, Inoguchi T, Maeda Y, et al. Association of borderline ankle-brachial index with mortality and the incidence of peripheral artery disease in diabetic patients. Atherosclerosis. 2014;234(2):360–365. doi: 10.1016/j.atherosclerosis.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka S, Kaneko H, Kano H, et al. The predictive value of the borderline ankle-brachial index for long-term clinical outcomes: an observational cohort study. Atherosclerosis. 2016;250:69–76. doi: 10.1016/j.atherosclerosis.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 37.Kojima I, Ninomiya T, Hata J, et al. A low ankle brachial index is associated with an increased risk of cardiovascular disease: the Hisayama study. J Atheroscler Thromb. 2014;21(9):966–973. doi: 10.5551/jat.22608. [DOI] [PubMed] [Google Scholar]
- 38.Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, Ioakeimidis N, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: a systematic review and meta-analysis. Hypertension. 2012;60(2):556–562. doi: 10.1161/HYPERTENSIONAHA.112.194779. [DOI] [PubMed] [Google Scholar]
- 39.Tomiyama H, Matsumoto C, Shiina K, Yamashina A. Brachial-ankle PWV: current status and future directions as a useful marker in the management of cardiovascular disease and/or cardiovascular risk factors. J Atheroscler Thromb. 2016;23(2):128–146. doi: 10.5551/jat.32979. [DOI] [PubMed] [Google Scholar]
- 40.Ohkuma T, Ninomiya T, Tomiyama H, et al. Collaborative Group for J-BAVEL (Japan Brachial-Ankle Pulse Wave Velocity Individual Participant Data Meta-Analysis of Prospective Studies) Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta-analysis. Hypertension. 2017;69(6):1045–1052. doi: 10.1161/HYPERTENSIONAHA.117.09097. [DOI] [PubMed] [Google Scholar]
- 41.Kato A, Takita T, Furuhashi M, Maruyama Y, Miyajima H, Kumagai H. Brachial-ankle pulse wave velocity and the cardio-ankle vascular index as a predictor of cardiovascular outcomes in patients on regular hemodialysis. Ther Apher Dial. 2012;16(3):232–241. doi: 10.1111/j.1744-9987.2012.01058.x. [DOI] [PubMed] [Google Scholar]
- 42.Cameron JD, Asmar R, Struijker-Boudier H, et al. Current and future initiatives for vascular health management in clinical practice. Vasc Health Risk Manag. 2013;9:255–264. doi: 10.2147/VHRM.S42947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Townsend RR, Wilkinson IB, Schiffrin EL, et al. American Heart Association Council on Hypertension Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66(3):698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagayama D, Imamura H, Sato Y, et al. Inverse relationship of cardio-ankle vascular index with BMI in healthy Japanese subjects: a cross-sectional study. Vasc Health Risk Manag. 2017;13:1–9. doi: 10.2147/VHRM.S119646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maliha G, Townsend RR. A study of the VaSera arterial stiffness device in US patients. J Clin Hypertens. 2017;19(7):661–668. doi: 10.1111/jch.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li RY, Cao ZG, Zhang JR, Li Y, Wang RT. Decreased serum bilirubin is associated with silent cerebral infarction. Arterioscler Thromb Vasc Biol. 2014;34(4):946–951. doi: 10.1161/ATVBAHA.113.303003. [DOI] [PubMed] [Google Scholar]
- 47.Zheng Y, Li Z, Shu H, Liu M, Chen Z, Huang J. Relationship between sum of the four limbs’ pulse pressure and brachial-ankle pulse wave velocity and atherosclerosis risk factors in Chinese adults. Biomed Res Int. 2015;2015:434516. doi: 10.1155/2015/434516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng Q, Dong SY, Wang ML, Xiang H, Zhao XL. Association of EZSCAN values with arterial stiffness in individuals without diabetes or cardiovascular disease. PLoS One. 2014;9(3):e90854. doi: 10.1371/journal.pone.0090854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chae YM, Park JK. The relationship between brachial ankle pulse wave velocity and complement 1 inhibitor. J Korean Med Sci. 2009;24(5):831–836. doi: 10.3346/jkms.2009.24.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu M, Wang S, Wang D, et al. Combined moderate and high intensity exercise with dietary restriction improves cardiac autonomic function associated with a reduction in central and systemic arterial stiffness in obese adults: a clinical trial. Peer J. 2017;5:e3900. doi: 10.7717/peerj.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The difference in the oscillometric envelope in various diseases.
Note: See the article text for a full explanation of this figure.
Abbreviations: BP, blood pressure; ASO, arteriosclerosis obliterans; %MAP, percent mean arterial pressure; UT, upstroke time; R-ABI, right ankle-brachial index; L-ABI, left ankle-brachial index.
Table S1.
The categorization of the errors in ABI and baPWV as measured by the Vascular Profiler
| ABI | baPWV | |
|---|---|---|
| Underestimation or lower value | A low and a borderline ABI in young individuals including children, men under 30 years old, women under 40 years old, especially, a low and a borderline ABI are frequently observed among healthy children and university students of both genders | 1. Obstruction such as arterial stenosis in the measurement pathway from the heart to the ankle 2. Moderate to severe aortic valve diseases |
| Overestimation or high value | 1. Arterial calcification in the lower limb 2. Severe aortic valve regurgitation 3. Low brachial systolic BP due to arterial stenosis in the upper limb (particularly hemodialysis patients) 4. Too narrow cuff width for the ankle 5. Too loose ankle cuff setting |
Arterial stenosis in the brachial base of the baPWV measurement (from the heart to the brachium) |
| Measurement error | Inaccurate timing determination of systolic BP in the oscillometric envelope | Inaccurate determination of the upstroke in the pulse waveform |
Abbreviations: ABI, ankle-brachial index; baPWV, brachial-ankle pulse wave velocity; BP, blood pressure.