Abstract
Purpose
The size of the perivitelline space and the incidence of polyspermy were observed in ovulated and cultured oocytes from rabbits and hamsters with or without treatment by 4‐methylumbelliferone (MU), an inhibitor of hyaluronic acid (HA) synthase, in order to examine the relationship between the incidence of polyspermy and the size of the perivitelline space. The amount of HA in the medium with MU‐treated hamster oocytes was measured and compared with that in the medium with untreated oocytes.
Methods
The perivitelline space of oocytes with 1st polar bodies was measured by use of a micrometer under a microscope, and the incidence of polyspermy was observed in the oocytes after insemination. The amount of HA in the medium was examined using an enzyme‐linked immunosorbent assay. The distribution of cortical granules was observed by staining with FITC‐conjugated LCA.
Results
In both rabbit and hamster, the mean size of the perivitelline space was significantly smaller and the incidence of polyspermy was significantly higher in the cultured and MU‐treated oocytes than in the ovulated and untreated oocytes. The mean amount of HA in the medium with MU‐treated oocytes (8.96 pg) was significantly smaller than that in the medium with untreated oocytes (21.77 pg). The distribution of cortical granules did not differ among the ovulated, cultured, and MU‐treated oocytes.
Conclusions
These findings suggest that the size of the perivitelline space is closely related to the incidence of polyspermy, and that the oocyte itself synthesizes and secretes the HA involved in the enlargement of the perivitelline space.
Keywords: Hamster oocyte, Hyaluronic acid, Perivitelline space, Polyspermy, Rabbit oocyte
Introduction
The perivitelline space is the gap between the plasma membrane and the zona pellucida of oocytes. According to recent reports, polyspermy is significantly less frequent in porcine and mouse oocytes with larger perivitelline space than in oocytes with smaller perivitelline space [1, 2, 3, 4]. This suggests there may be a relationship between the size of the perivitelline space and the incidence of polyspermy in porcine and mouse oocytes. It remains unclear, however, whether such a relationship can be expected in oocytes in which the vitelline block and zona reaction differ in intensity from those of porcine and mouse oocytes.
It is known that glycosaminoglycans, including hyaluronic acid (HA), synthesized in cumulus cells stimulated by FSH and LH are secreted [5, 6], and the extracellular matrix of the cumulus cells is occupied by the secreted HA [7, 8, 9, 10]. HA, a molecule with a capacity to retain large volumes of water [11], is also found in the perivitelline space [11, 12, 13, 14]. Therefore, when HA accumulates in the perivitelline space, it enlarges the space by absorbing water.
In an investigation of the secretory source of HA in the perivitelline space, Salustri et al. [15] used (3H) glucosamine as a metabolic precursor to examine HA production in mouse cumulus–oocyte complexes (COCs), cumulus cells, and oocytes after 18 h of culture. They confirmed the presence of HA in the medium with COCs or cumulus cells, but not in the medium with oocytes. In recent experiments using an enzyme‐linked immunosorbent assay (ELISA), Ueno et al. [16] measured HA in the medium with mouse denuded oocytes (DOs) cultured for 14 h. As a result, Ueno et al. [16] found significantly less HA in a medium containing mouse DOs in which HA synthesis was inhibited than in a medium containing untreated mouse DOs. Ueno et al. [16] observed a significantly smaller perivitelline space and a significantly higher incidence of polyspermy in mouse DOs in which HA synthesis was inhibited, in comparison with untreated oocytes. From these findings, Ueno et al. [16] suggested that the oocyte itself synthesizes and secretes the HA involved in the enlargement of the perivitelline space. There have been no reports, however, of the synthesis and secretion of HA in mammalian oocytes other than mouse oocytes.
In this study we observed the size of the perivitelline space and the incidence of polyspermy after insemination in ovulated and cultured oocytes from rabbits and hamsters. We also examined changes in the size of the perivitelline space and the incidence of polyspermy in rabbit and hamster oocytes cultured in medium containing 4‐methylumbelliferone (MU), an inhibitor of HA synthase [17, 18, 19]. By means of these investigations we sought a relationship between the incidence of polyspermy and the size of the perivitelline space in oocytes in which the intensities of the block to polyspermy differ from those of porcine and mouse oocytes. We also measured the amount of HA in the medium with MU‐treated hamster oocytes by using an ELISA, a method different from that used by Salustri et al. [15], in order to examine the potential involvement of oocytes in the synthesis and secretion of HA and the role of the HA secreted by the oocytes in enlargement of the perivitelline space.
Materials and methods
Animals
Mature rabbits (Japanese white) and mature hamsters (Mesocricetus auratus) were used in this study. The animals were housed in autoclaved cages in an air‐conditioned room (24°C) under controlled‐lighting conditions (14L/10D) and given standard chow (Oriental Yeast, Tokyo, Japan) with tap water ad libitum. They received humane care, as outlined in the Guide for the Care and Use of Laboratory Animals (Niigata University Animal Care Committee). The estrous cycle of the female hamsters was checked by examination of the post‐estrous discharge [20]. The day on which the post‐estrous discharge appeared in the vagina was designated as day 1 (the day of ovulation) of the cycle. Only female hamsters with a 4‐day estrous cycle were used in the study. Each cycling female hamster was subcutaneously injected with 30 IU PMSG (Peamex®; Sankyo Yell Yakuhin, Tokyo, Japan) at 10:00 on day 1. As most hamsters ovulate 8 h after the onset of estrous [21], the ages of the oocytes were determined by reference to the onset of estrous.
Collection and culture of rabbit oocytes
To observe ovulated oocytes, 50 IU hCG (Gonatropin®; Teikoku Hormone Manufacturing, Tokyo, Japan) was intravenously injected into female rabbits, and COCs were collected from oviducts 14 h after the hCG injection. To observe oocytes matured in vitro, COCs were collected from antral follicles and cultured for 14 h in BO medium [22] containing 10 IU/ml PMSG (Peamex®) at 39°C in a CO2 incubator (5% CO2 in air).
To observe oocytes in which HA synthesis was inhibited, COCs collected from antral follicles were cultured at 39°C for 14 h in BO medium containing 0.25 mm MU (Wako Pure Chemical Industries, Osaka, Japan) in a CO2 incubator. The MU had previously been dissolved in dimethyl sulfoxide (DMSO) and then diluted with the culture medium. The concentration of DMSO in the culture medium was adjusted to 0.1% (v/v), and COCs cultured for 14 h in the medium containing 0.1% DMSO were used as controls.
At 14 h after culture or hCG injection, collected COCs were immersed in the culture medium containing 0.1% hyaluronidase (Sigma‐Aldrich, MO, USA), and cumulus cells were dispersed from COCs by pipetting.
Collection and culture of hamster oocytes
To observe ovulated oocytes, COCs were collected from oviducts of PMSG‐treated hamsters 2 h after ovulation. To observe oocytes matured in vitro, COCs were collected from antral follicles at 17:00 on day 3 and cultured in mTALP3 medium [23] supplemented with 5% fetal bovine serum (FCS; Gibco BRL, NY, USA), without hypotaurine and epinephrine, at 37°C in a CO2 incubator (5% CO2 in air). After culture for 16 h and 2 h after ovulation, the cumulus cells were dispersed from COCs by pipetting in the culture medium containing 0.1% hyaluronidase (Sigma‐Aldrich).
To observe oocytes in which HA synthesis was inhibited, COCs collected from antral follicles at 17:00 on day 3 were immersed in mTALP3 medium containing 0.1% hyaluronidase (Sigma‐Aldrich), and cumulus cells were dispersed from COCs by pipetting. These DOs were cultured for 16 h in mTALP3 medium containing 0.25 mm MU (Wako Pure Chemical Industries) at 37°C in a CO2 incubator. DOs cultured for 16 h in the medium containing 0.1% DMSO were used as controls.
Observation of maturation and size of the perivitelline space in ovulated and cultured oocytes
After ovulation or culture, the number of oocytes with 1st polar bodies in the perivitelline space (maturation rate) was examined. Only DOs with 1st polar bodies in the perivitelline space were selected, and the size of each part of oocytes was measured using a micrometer under a microscope. The size of perivitelline space was calculated by the method described in Fig. 1.
Figure 1.
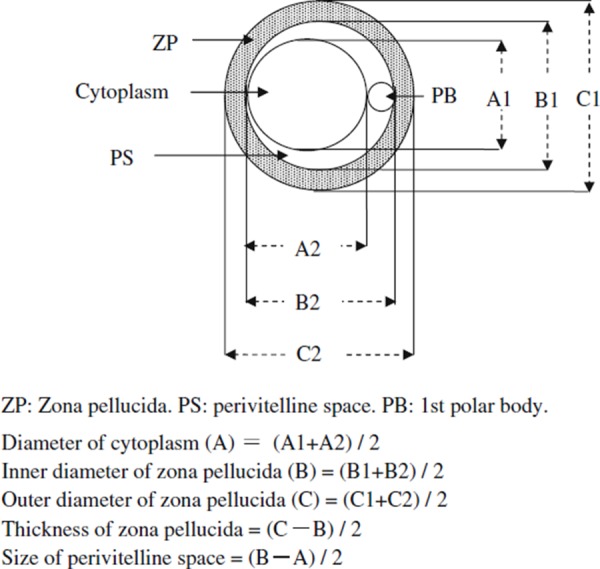
Calculation method for the size of each part of the oocyte
Observation of the incidence of polyspermy in oocytes
After ovulation or culture, only DOs with 1st polar bodies in the perivitelline space were selected and inseminated. A sperm suspension was prepared by minutely cutting caudal epididymides of mature rabbit and hamster males in the culture medium. To induce capacitation, rabbit sperm was cultured for 9–10 h in BO medium at 39°C in a CO2 incubator. Hamster sperm was cultured for 3 h in mTALP3 medium containing 0.055 μg/ml hypotaurine (Sigma‐Aldrich) and 0.01 μg/ml epinephrine (Sigma‐Aldrich) at 37°C in a CO2 incubator. A small volume of the sperm suspension was introduced into 100‐μl droplets of the culture medium to adjust the final concentration of spermatozoa to 2 × 106/ml. Rabbit and hamster DOs were introduced into the droplets of each sperm suspension and cultured for 6–12 h at 39 or 37°C, respectively, in a CO2 incubator. Inseminated DOs were observed under a phase‐contrast microscope. DOs containing 2 or more pronuclei in their cytoplasm were judged to be fertilized, and those containing 3 or more pronuclei were judged to be polyspermic.
Measurement of HA level in the medium with hamster oocytes
We only used hamster DOs for the HA measurement, because rabbit oocytes have been shown to be unable to mature in vitro without cumulus cells.
COCs collected from antral follicles of PMSG‐treated hamsters at 17:00 on day 3 were immersed in mTALP3 medium containing 0.1% hyaluronidase (Sigma‐Aldrich). The cumulus cells were then dispersed from the oocytes by pipetting, and 40–50 DOs were cultured in 60 μl mTALP3 medium at 37°C in a CO2 incubator (5% CO2 in air).
The culture medium containing DOs was collected at 0 and 16 h after culture and stored at −20°C until the HA measurement. The ELISA used by Kongtawelert and Ghosh [24] was used to quantify the HA. Activated polyvinyl chloride immunoassay plates (Nunc, Roskilde, Denmark) were precoated with poly‐l‐lysine (Sigma‐Aldrich) by addition of 100 μl/well of a solution containing 50 μg/ml in distilled water. After incubation for 1 h at 37°C, the solution was flicked out, the plates were air‐dried, and 100 μl/well of bovine vitreous humor HA (MP Biomedicals, OH, USA; 25 μg/ml), which had been dissolved in PBS [25], was added to the precoated plates. After an overnight incubation at room temperature and three washes with PBS containing 0.05% (v/v) Tween 20 (PBS–Tween 20), 100 μl PBS containing 1% (w/w) BSA (Sigma‐Aldrich) was added to each well. Next, after incubation at 37°C for 1 h followed by five washes with PBS–Tween 20, the plates were air‐dried, wrapped in polyethylene film, and stored at 4°C until the HA measurement.
Frozen media containing DOs immediately after collection and cultured for 16 h were thawed and centrifuged at 1,500 rpm for 10 min. The supernatant was collected and diluted twice with mTALP3 medium. Sixty microliters of these diluted samples or 60 μl mTALP3 medium containing HA at 0, 0.195, 0.39, 0.78, 1.56, 3.12, 6.24 or 12.48 ng/ml (standard solution) was pipetted into small tubes with 60 μl biotinylated HA binding protein (Seikagaku, Tokyo, Japan; 0.175 μg/ml distilled water) and incubated at 37°C for 90 min. One hundred microliters of this reaction mixture was then applied to HA‐coated and BSA‐blocked plates and incubated at 37°C for 1 h. After five washes with PBS‐Tween 20, 100 μl of the appropriate dilution (1:5,000) of alkaline phosphatase‐conjugated avidin (Sigma‐Aldrich) was added to each well. After incubation for 1 h at 37°C, the plates were washed five times with PBS–Tween 20 and air‐dried. Next, 100 μl alkaline phosphatase substrate (ρ‐nitrophenyl phosphate; Sigma‐Aldrich) was added and plates were incubated at 37°C for 15 min. The reaction was stopped by the addition of 80 μl 0.2 m NaOH. The absorbance at 405 nm was determined using a microplate reader (Bio‐Rad, CA, USA).
A standard curve for HA (Fig. 2) was constructed from the value of standard solution, and the amount of HA in the medium was determined by applying the value of the medium to the standard curve. Triplicate results were averaged for this assay.
Figure 2.
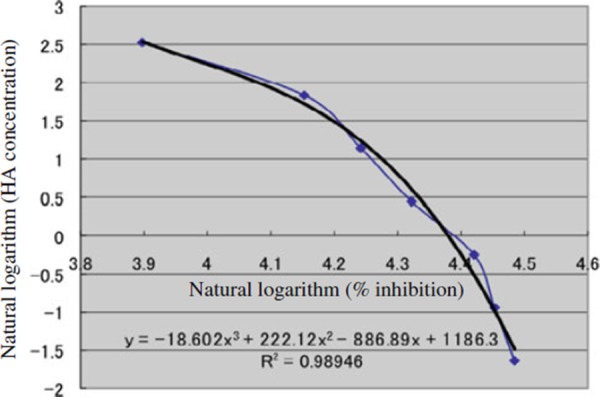
A standard curve for HA measurement. The actual curve (filled circles) using standard solution and its calibrated curve
The amount of HA was also measured in a medium containing DOs in which HA synthesis was inhibited, in order to clarify the ability of these oocytes to synthesize and secrete HA. A group of 40–50 DOs cultured for 16 h in 60 μl mTALP3 medium containing 0.25 mm MU (Wako Pure Chemical Industries) at 37°C in a CO2 incubator. The concentration of DMSO in the culture medium was adjusted to 0.1% (v/v), and the medium containing DOs cultured for 16 h with 0.1% DMSO was used as control. The medium containing the DOs was collected after culture and stored at −20°C until analysis. The amount of HA in the medium with untreated or MU‐treated DOs was determined using the ELISA method mentioned above. Triplicate results were averaged for this assay.
Observation of cortical granules
After ovulation or culture, DOs with 1st polar bodies in the perivitelline space were selected and stained with LCA conjugated with FITC (E‐Y Laboratories, CA, USA) according to the method of Ducibella et al. [26]. The DOs were immersed in PBS containing 0.2% pronase (Sigma‐Aldrich) to dissolve the zonae pellucidae. The naked oocytes were washed in PBS and fixed in PBS containing 3.7% paraformaldehyde for 30 min at room temperature, washed 3 times in a blocking solution composed of 3 mg BSA, 7.51 mg glycine (Wako Pure Chemical Industries), and 1 ml PBS, immersed in PBS containing 0.1% Triton X‐100 (Nacalai Tesque, Kyoto, Japan) for 5 min at 20°C, placed in the blocking solution again, and finally immersed in a staining solution composed of 10 μg LCA, 0.1 μl Triton X‐100, 3 mg BSA, and 1 ml PBS for 30 min at 20°C. The stained oocytes were thoroughly washed in PBS containing 0.01% Triton X‐100 and 0.3% BSA, placed on glass slides, and photographed under a reflected‐light fluorescence microscope (Nikon, Tokyo, Japan).
Statistical analysis
The size of each part including perivitelline space was statistically analyzed by one‐way analysis of variance (ANOVA). The rates of oocyte maturation, the rates of fertilization and polyspermy in oocytes after insemination, and the percentages of oocytes with cortical granules just beneath the plasma membrane were statistically analyzed by use of the chi‐squared test. The amount of HA was statistically analyzed by use of Student's t test. A value of p < 0.05 was considered to be statistically significant.
Results
Rate of maturation and size of the perivitelline space in rabbit oocytes
All (30/30) of the ovulated oocytes and 68.3% (28/41) of cultured oocytes held 1st polar bodies in their perivitelline spaces (Table 1). The percentage of oocytes with 1st polar bodies in the perivitelline space (maturation rates) was significantly higher in ovulated oocytes than in cultured oocytes.
Table 1.
Maturation and size of each part in rabbit oocytes
| Oocytes | No. of oocytes cultured | No. (%) of oocytes matured | Diameter of cytoplasm (μm) | Inner diameter of zona pellucida (μm) | Thickness of zona pellucida (μm) | Size of perivitelline space (μm) |
|---|---|---|---|---|---|---|
| Immediately after collection from antral follicles | 30 | – | 125.4 ± 1.01A,a | 125.6 ± 0.96 A | 13.08 ± 0.33 A | 0.12 ± 0.07c |
| Ovulated | 30 | 30 (100.0) A | 99.54 ± 0.60b | 122.6 ± 0.73b | 14.20 ± 0.28 A | 11.51 ± 0.34 A |
| Cultured | 41 | 28 (68.3)b | 92.61 ± 0.88c | 112.0 ± 0.89c | 13.48 ± 0.31 A | 9.68 ± 0.44b |
| MU‐treated | 42 | 28 (66.7) A | 98.66 ± 0.57 A | 113.1 ± 0.78b | 16.09 ± 0.31 A | 7.13 ± 0.39b |
| Control | 47 | 30 (63.8) A | 98.13 ± 0.77 A | 115.6 ± 0.78 A | 16.15 ± 0.36 A | 8.75 ± 0.45 A |
AMean ± SE
The oocytes with cumulus cells were cultured for 14 h in BO medium with 0.25 mm MU (MU‐treated) or in BO medium without MU (control)
Values with different superscripts in the same column in each group of experiments are significantly different (p < 0.05)
When rabbit COCs were cultured in the medium containing 0.25 mm MU, 66.7% of the oocytes held 1st polar bodies in the perivitelline space. This was not significantly different from the percentage (63.8%) for control oocytes cultured in the medium without MU (Table 1).
The perivitelline space could barely be seen in 10% (3/30) of oocytes immediately after collection from antral follicles (Fig. 3a) and could not be seen in 90% (27/30) of the oocytes. The mean size of the perivitelline space was 0.12 μm in oocytes immediately after collection from antral follicles and increased significantly to 11.51 μm in ovulated oocytes (Fig. 3b). The mean size of the perivitelline space in ovulated oocytes was significantly larger than that in cultured oocytes (9.68 μm, Fig. 3c).
Figure 3.
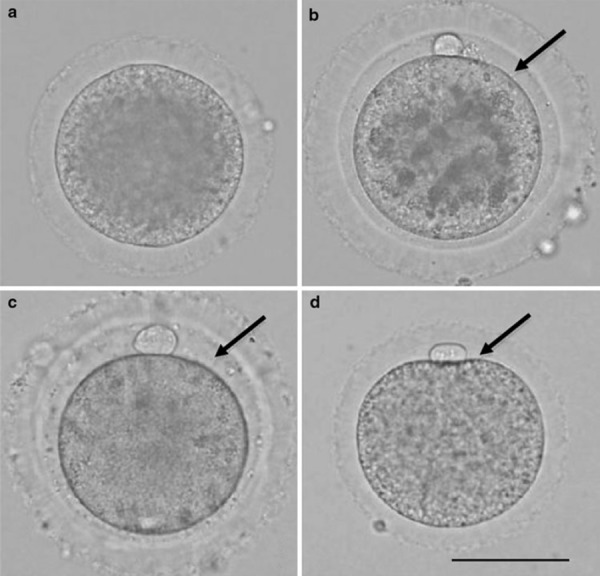
Rabbit oocytes. Scale bar shows 60 μm. Arrows indicate the perivitelline space. a Oocyte immediately after collection from antral follicle. b Oocyte immediately after collection from oviduct. c Oocyte cultured for 14 h in BO medium. d Oocyte cultured for 14 h in BO medium with 0.25 mm 4‐methylumbelliferone
The mean size of the perivitelline space in the MU‐treated oocytes (Fig. 3d) was 7.13 μm, which was significantly smaller than that in the control oocytes (8.75 μm).
Rate of maturation and size of the perivitelline space in hamster oocytes
The maturation rate for ovulated hamster oocytes was 100%, which was significantly higher than 84.6% for cultured oocytes (Table 2). The perivitelline space could barely be seen in half (27/54) of oocytes immediately after collection from antral follicles (Fig. 4a) and could not be seen in the other half. The mean size of the perivitelline space was 0.30 μm in oocytes immediately after collection from antral follicles and increased significantly to 7.08 μm in ovulated oocytes (Fig. 4b). The mean size of perivitelline space in ovulated oocytes was significantly larger than that in cultured oocytes (3.30 μm, Fig. 4c).
Table 2.
Maturation and size of each part in hamster oocytes
| Oocytes | No. of oocytes cultured | No. (%) of oocytes matured | Diameter of cytoplasm (μm) | Inner diameter of zona pellucida (μm) | Thickness of zona pellucida (μm) | Size of perivitelline space (μm) |
|---|---|---|---|---|---|---|
| Immediately after collection from antral follicles | 54 | – | 81.83 ± 0.78A,a | 82.44 ± 0.73b | 11.48 ± 0.29b | 0.30 ± 0.07c |
| Ovulated | 33 | 33 (100.0) A | 69.91 ± 0.45c | 84.05 ± 0.52 A | 12.75 ± 0.22 A | 7.08 ± 0.26 A |
| Cultured | 39 | 33 (84.6)b | 72.78 ± 0.74b | 79.39 ± 0.65c | 12.82 ± 0.22 A | 3.30 ± 0.19b |
| MU‐treated | 44 | 27 (61.3) A | 74.70 ± 0.81 A | 80.86 ± 0.63 A | 12.44 ± 0.28 A | 2.85 ± 0.26b |
| Control | 53 | 31 (58.5) A | 71.21 ± 0.49b | 80.16 ± 0.46 A | 11.67 ± 0.24b | 4.48 ± 0.23 A |
AMean ± SE
The oocytes without cumulus cells were cultured for 16 h in mTALP3 medium with 0.25 mm MU (MU‐treated) or in mTALP3 medium without MU (control)
Values with different superscripts in the same column in each group of experiments are significantly different (p < 0.05)
Figure 4.
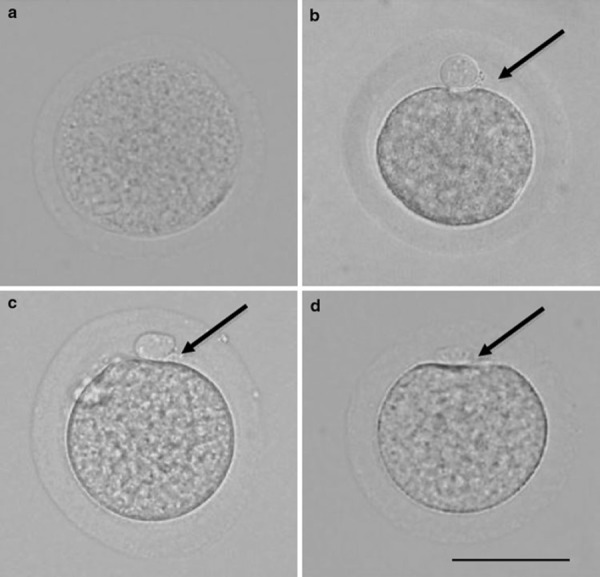
Hamster oocytes. Scale bar shows 60 μm. Arrows indicate the perivitelline space. a Oocyte immediately after collection from antral follicle. b Oocyte immediately after collection from oviduct. c Oocyte cultured for 16 h in mTALP3 medium. d Oocyte cultured for 16 h in mTALP3 medium with 0.25 mm 4‐methylumbelliferone
The maturation rate did not differ between DOs cultured with and without MU (Table 2), whereas the mean size of the perivitelline space was significantly smaller in MU‐treated DOs (2.85 μm, Fig. 4d) than in control DOs cultured with no MU (4.48 μm).
Incidence of polyspermy in rabbit oocytes
The rates of fertilization and polyspermy in rabbit oocytes after insemination are shown in Table 3. The fertilization rates did not differ between ovulated (70.3%) and cultured (70.0%) oocytes, or between MU‐treated (71.2%) and control (69.6%, Fig. 5a) oocytes. However, the incidence of polyspermy was significantly lower in ovulated (0%) and control (7.7%) oocytes than in cultured (19.0%) and MU‐treated (29.8%, Fig. 5b) oocytes.
Table 3.
Incidence of polyspermy in rabbit oocytes
| Oocytes | No. of oocytes inseminated | No. (%) of oocytes fertilized | ||
|---|---|---|---|---|
| Total | Monospermy | Polyspermy | ||
| Ovulated | 37 | 26 (70.3)a | 26 (100.0)a | 0 (0.0)b |
| Cultured | 30 | 21 (70.0)a | 17 (81.0)b | 4 (19.0)a |
| MU‐treated | 66 | 47 (71.2)a | 33 (70.2)b | 14 (29.8)a |
| Control | 56 | 39 (69.6)a | 36 (92.3)a | 3 (7.7)b |
The oocytes with cumulus cells were cultured for 14 h in BO medium with 0.25 mm MU (MU‐treated) or in BO medium without MU (control)
Values with different superscripts in the same column in each group of experiments are significantly different (p < 0.05)
Figure 5.
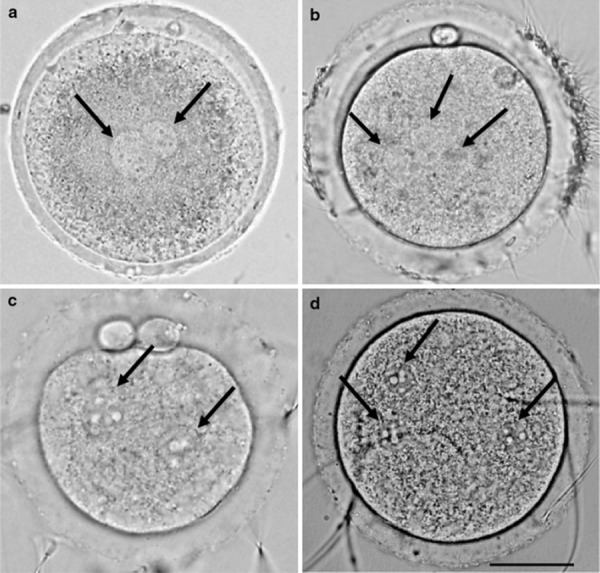
Fertilized rabbit and hamster oocytes. Scale bar shows 30 μm. Arrows indicate pronuclei. a Monospermic rabbit oocyte cultured without 4‐methylumbelliferone. b Polyspermic rabbit oocyte cultured with 0.25 mm 4‐methylumbelliferone. c Monospermic hamster oocyte cultured without 4‐methylumbelliferone. d Polyspermic hamster oocyte cultured with 0.25 mm 4‐methylumbelliferone
Incidence of polyspermy in hamster oocytes
The fertilization rates in ovulated and MU‐treated hamster oocytes were 72.4 and 66.7% (Table 4), respectively. These rates were not significantly different from those in cultured (73.3%) and control (63.4%) oocytes. However, the incidence of polyspermy was significantly lower in ovulated (0%) and control (11.5%, Fig. 5c) oocytes than in cultured (13.6%) and MU‐treated (39.3%, Fig. 5d) oocytes, respectively.
Table 4.
Incidence of polyspermy in hamster oocytes
| Oocytes | No. of oocytes inseminated | No. (%) of oocytes fertilized | ||
|---|---|---|---|---|
| Total | Monospermy | Polyspermy | ||
| Ovulated | 29 | 21 (72.4)a | 21 (100.0)a | 0 (0.0)b |
| Cultured | 30 | 22 (73.3)a | 19 (86.4)b | 3 (13.6)a |
| MU‐treated | 42 | 28 (66.7)a | 17 (60.7)b | 11 (39.3)a |
| Control | 41 | 26 (63.4)a | 23 (88.5)a | 3 (11.5)b |
The oocytes without cumulus cells were cultured for 16 h in mTALP3 medium with 0.25 mm MU (MU‐treated) or in mTALP3 medium without MU (control)
Values with different superscripts in the same column in each group of experiments are significantly different (p < 0.05)
Amount of HA in the medium with hamster oocytes
As shown in Table 5, HA was not detected from the culture medium containing hamster DOs immediately after collection from antral follicles. On the other hand, 24.69 pg HA per DO was detected in the medium after 16 h of culture.
Table 5.
Amount of hyaluronic acid in the medium with denuded hamster oocytes
| Oocytes | pg/DO |
|---|---|
| Immediately after collection | ND |
| Cultured for 16 h in mTALP3 medium | 24.69 ± 3.42 A |
| Cultured for 16 h in mTALP3 medium with 0.25 mm MU | 8.96 ± 0.63b |
| Cultured for 16 h in mTALP3 medium without MU | 21.77 ± 1.32 A |
ND not detectable
AValues are expressed as means ± SE from three independent assays
Values with different superscripts in the same column in each group of experiments are significantly different (p < 0.05)
The mean amount of HA per DO in the medium containing MU‐treated DOs (8.96 pg) was significantly smaller than the amount in the control medium with untreated DOs (21.77 pg).
Distribution of cortical granules in rabbit and hamster oocytes
In oocytes from rabbits and hamsters, the distribution of cortical granules did not differ between ovulated and cultured, or between MU‐treated (Fig. 6a, c) and untreated (Fig. 6b, d) (Table 6). Most of the cortical granules in those oocytes were distributed immediately beneath the plasma membrane (Fig. 6a–d). In the remaining oocytes from rabbits and hamsters, the cortical granules were distributed in the cortical cytoplasm and also immediately beneath the plasma membrane.
Figure 6.
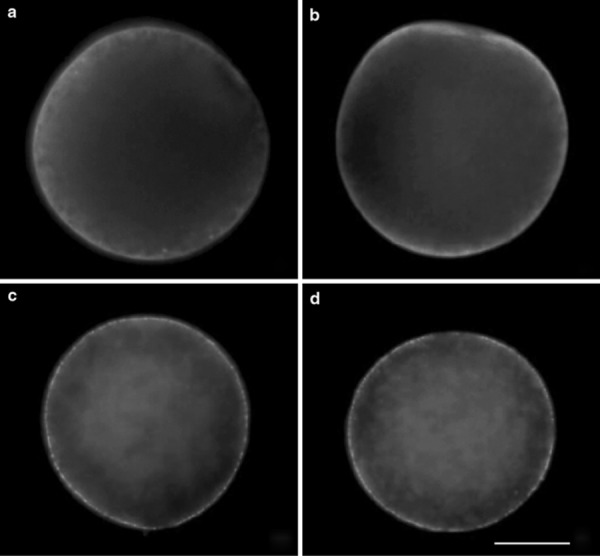
Cortical granules in rabbit and hamster oocytes. Scale bar shows 30 μm. Cortical granules are densely distributed immediately beneath the plasma membrane of every oocyte. a Rabbit oocyte cultured for 14 h in BO medium with 0.25 mm 4‐methylumbelliferone. b Rabbit oocyte cultured for 14 h in BO medium without 4‐methylumbelliferone. c Hamster oocyte cultured for 16 h in mTALP3 medium with 0.25 mm 4‐methylumbelliferone. d Hamster oocyte cultured for 16 h in mTALP3 medium without 4‐methylumbelliferone
Table 6.
Distribution of cortical granules in rabbit and hamster oocytes
| Oocytes | No. of rabbit oocytes examined | No. (%) of oocytes with cortical granules immediately beneath the plasma membrane | No. of hamster oocytes examined | No. (%) of oocytes with cortical granules immediately beneath the plasma membrane |
|---|---|---|---|---|
| Ovulated | 30 | 22 (73.3)a | 27 | 21 (77.7)a |
| Cultured | 23 | 17 (73.9)a | 24 | 18 (75.0)a |
| MU‐treated | 23 | 17 (73.9)a | 30 | 25 (83.3)a |
| Control | 27 | 20 (74.1)a | 32 | 27 (84.4)a |
The rabbit oocytes with cumulus cells and hamster oocytes without cumulus cells were cultured in BO or mTALP3 medium with 0.25 mm MU (MU‐treated), or in BO or mTALP3 medium without MU (control)
Values with different superscripts in the same column in each group of experiments are significantly different (p < 0.05)
Discussion
These investigations with rabbit and hamster oocytes confirmed that the size of the perivitelline space is significantly larger in ovulated oocytes than in cultured oocytes. In earlier studies with porcine and mouse oocytes [2, 4], the perivitelline space was reported to be significantly larger in ovulated oocytes than in oocytes cultured in vitro. From the results of this study, we consider that a significantly enlarged perivitelline space may be a factor common to ovulated mammalian oocytes. Although the reason why ovulated oocytes have larger perivitelline space is unclear, Okada [27] and Chung [28] reported that oviductal secretions are involved in the enlargement of perivitelline space in ovulated oocytes. Buhi [29, 30] reported that the substances in the perivitelline space of oocytes change after ovulation. In this study with rabbit and hamster oocytes, all of the oocytes matured in vivo were collected from oviducts and were therefore affected by oviduct secretions.
The reason for the high incidence of polyspermy after insemination in oocytes with small perivitelline space is unclear. Proteins and HA are present in the perivitelline space of oocytes before ovulation [11, 12, 13, 14, 31], and secretions from oviducts [27, 28, 29, 30, 32, 33, 34, 35] are also present after ovulation. When the perivitelline space enlarges, the substances in the perivitelline space increase in quantity. Therefore, it is suggested that the increased substances physically obstruct the movement and attachment of sperm to the plasma membrane of oocytes [12, 14]. HA has also been reported to have an inhibitory effect on membrane fusion [36, 37, 38]. Therefore, it is considered that large amounts of HA in the enlarged perivitelline space may prevent the membrane fusion of sperm and oocytes, resulting in the lower incidence of polyspermy in oocytes with larger perivitelline spaces.
Recently, Ueno et al. [16] have reported that HA was detected in the medium with mouse DOs cultured for 14 h by the ELISA method. No reports, however, have clarified whether the oocytes of animals other than mice have the ability to synthesize and secrete HA. In this study it was confirmed that HA was not detected in the medium with hamster DOs immediately after collection from antral follicles but was detected in the medium with DOs after culture. It was also confirmed that the amount of HA was significantly smaller in the medium with MU‐treated DOs in which HA synthesis was inhibited than in the control medium with untreated DOs. When we examined the size changes of the perivitelline space of oocytes in which HA synthesis was inhibited, the perivitelline spaces of MU‐treated oocytes were significantly smaller than those of the control oocytes. These findings strongly suggested that the oocyte itself synthesizes and secretes the HA involved in the enlargement of the perivitelline space.
As previously mentioned, Salustri et al. [15] detected HA in the medium with COCs or cumulus cells, but not in the medium with oocytes. The results of this study with hamster DOs and our former study with mouse DOs [16] differ from the results of Salustri et al. [15]. The methods used for HA detection may have been responsible for the discrepancy. We believe that the ELISA method used in our study is more sensitive than the method used by Salustri et al. [15].
In this study we confirmed that rabbit and hamster oocytes with smaller perivitelline space show higher incidence of polyspermy after insemination. The intensities of block to polyspermy in rabbit and hamster oocytes differ from those of porcine and mouse oocytes. Specifically, rabbit oocytes possess a weak zona reaction and strong vitelline block, and hamster oocytes possess a strong zona reaction and weak vitelline block, compared with those of porcine and mouse oocytes [39, 40, 41]. Our results suggest that the size of the perivitelline space is closely related to the incidence of polyspermy in mammalian oocytes irrespective of the intensities of block to polyspermy. Because it was also confirmed that the distribution of cortical granules in rabbit and hamster oocytes with smaller perivitelline space is similar to those with larger perivitelline space, we consider that the higher incidence of polyspermy in oocytes with small perivitelline space is not caused by the deficiency of cortical granules in those oocytes.
References
- 1. Funahashi H, Cantley TC, Stumpf TT, Terlouw SL, Day BN. Use of low‐salt culture medium for in vitro maturation of porcine oocytes is associated with elevated oocyte glutathione levels and enhanced male pronuclear formation after in vitro fertilization. Biol Reprod, 1994, 51, 633–639 10.1095/biolreprod51.4.633 [DOI] [PubMed] [Google Scholar]
- 2. Wang WH, Abeydeera LR, Prather RS, Day BN. Morphologic comparison of ovulated and in vitro‐matured porcine oocytes, with particular reference to polyspermy after in vitro fertilization. Mol Reprod Dev, 1998, 49, 308–316 10.1002/(SICI)1098‐2795(199803)49:3<308::AID‐MRD11>3.0.CO;2‐S [DOI] [PubMed] [Google Scholar]
- 3. Kitagawa T, Niimura S. Relationship between the size of perivitelline space and the incidence of polyspermy in porcine oocytes. Bull Facul Agric Niigata Univ, 2006, 59, 21–26 [Google Scholar]
- 4. Ueno S, Niimura S. Size of perivitelline space and incidence of polyspermy in mouse oocytes matured in vivo and in vitro. J Mamm Ova Res, 2008, 25, 44–49 10.1274/jmor.25.44 [Google Scholar]
- 5. Eppig JJ. The relationship between cumulus cell‐oocyte coupling, oocyte meiotic maturation, and cumulus expansion. Dev Biol, 1982, 89, 268–272 10.1016/0012‐1606(82)90314‐1 [DOI] [PubMed] [Google Scholar]
- 6. Ball GD, Wieben ED, Byers AP. DNA, RNA, and protein synthesis by porcine oocyte–cumulus complexes during expansion. Biol Reprod, 1985, 33, 739–744 10.1095/biolreprod33.3.739 [DOI] [PubMed] [Google Scholar]
- 7. Eppig JJ. FSH stimulates hyaluronic acid synthesis by oocyte–cumulus cell complexes from mouse preovulatory follicles. Nature, 1979, 281, 483–484 10.1038/281483a0 [DOI] [PubMed] [Google Scholar]
- 8. Eppig JJ. Regulation of cumulus oophorus expansion by gonadotropins in vivo and in vitro. Biol Reprod, 1980, 23, 545–552 10.1095/biolreprod23.3.545 [DOI] [PubMed] [Google Scholar]
- 9. Ball GD, Bellin ME, Ax RE, First NL. Glycosaminoglycans in bovine cumulus‐oocyte complexes: morphology and chemistry. Mol Cell Endocrinol, 1982, 28, 113–122 10.1016/0303‐7207(82)90045‐4 [DOI] [PubMed] [Google Scholar]
- 10. Salustri A, Yanagishita M, Hascall VC. Mouse oocytes regulate hyaluronic acid synthesis and mucification by FSH‐stimulated cumulus cells. Dev Biol, 1990, 138, 26–32 10.1016/0012‐1606(90)90173‐G [DOI] [PubMed] [Google Scholar]
- 11. Talbot P, Dandekar P. Perivitelline space: does it play a role in blocking polyspermy in mammals?. Microsc Res Tech, 2003, 61, 349–357 10.1002/jemt.10348 [DOI] [PubMed] [Google Scholar]
- 12. Talbot P, Dicarlantonio G. Ultrastructure of opossum oocyte investing coats and their sensitivity to trypsin and hyaluronidase. Dev Biol, 1984, 103, 159–167 10.1016/0012‐1606(84)90017‐4 [DOI] [PubMed] [Google Scholar]
- 13. Dandekar P, Talbot P. Perivitelline space of mammalian oocytes: extracellular matrix of unfertilized oocytes and formation of a cortical granules envelope following fertilization. Mol Reprod Dev, 1992, 31, 135–143 10.1002/mrd.1080310208 [DOI] [PubMed] [Google Scholar]
- 14. Dandekar P, Aggeler J, Talbot P. Structure, distribution and composition of the extracellular matrix of human oocytes and cumulus masses. Hum Reprod, 1992, 7, 391–398 [DOI] [PubMed] [Google Scholar]
- 15. Salustri A, Yanagishita M, Underhill CB, Laurent TC, Hascall VC. Localization and synthesis of hyaluronic acid in the cumulus cells and mural granulosa cells of the preovulatory follicle. Dev Biol, 1992, 151, 541–551 10.1016/0012‐1606(92)90192‐J [DOI] [PubMed] [Google Scholar]
- 16. Ueno S, Yoshida N, Niimura S. Amount of hyaluronan produced by mouse oocytes and role of hyaluronan in enlargement of the perivitelline space. J Reprod Dev, 2009, 55, 496–501 10.1262/jrd.20226 [DOI] [PubMed] [Google Scholar]
- 17. Nakamura T, Funahashi M, Takagaki K, Munakata H, Tanaka K, Saito Y, Endo M. Effect of 4‐methylumbelliferone on cell‐free synthesis of hyaluronic acid. Biochem Mol Biol Int, 1997, 43, 263–268 [DOI] [PubMed] [Google Scholar]
- 18. Itano N. Abnormal hyaluronan synthesis and cancer progression. Trends Glycosci Glycotechnol, 2004, 16, 199–210 [Google Scholar]
- 19. Kakizaki I, Kojima K, Takagaki K, Endo M, Kannagi R, Ito M, Maruo Y, Sato H, Yasuda T, Mita S, Kimata K, Itano N. A novel mechanism for the inhibition of hyaluronan biosynthesis by 4‐methylumbelliferone. J Biol Chem, 2004, 279, 33281–33289 10.1074/jbc.M405918200 [DOI] [PubMed] [Google Scholar]
- 20. Harvey EB, Yanagimachi R, Chang MC. Onset of estrus and ovulation in the golden hamster. J Exp Zool, 1961, 146, 231–235 10.1002/jez.1401460303 [DOI] [PubMed] [Google Scholar]
- 21. Orsini MW. The external vaginal phenomena characterizing the stages of the estrous cycle, pregnancy, pseudopregnancy, lactation, and the anestrous hamster, Mesocricetus auratus Waterhouse. Proc Anim Care Panel, 1961, 11, 193–206 [Google Scholar]
- 22. Brackett BG, Oliphant G. Capacitation of rabbit spermatozoa in vitro. Biol Reprod, 1975, 12, 260–274 10.1095/biolreprod12.2.260 [DOI] [PubMed] [Google Scholar]
- 23. Takano H, Yanagimachi R, Urch UA. Evidence that acrosin activity is important for the development of fusibility of mammalian spermatozoa with the oolemma: inhibitor studies using the golden hamster. Zygote, 1993, 1, 79–91 10.1017/S0967199400001325 [DOI] [PubMed] [Google Scholar]
- 24. Kongtawelert P, Ghosh P. A method for the quantitation of hyaluronan (Hyaluronic acid) in biological fluids using a labeled avidin–biotin technique. Anal Biochem, 1990, 185, 313–318 10.1016/0003‐2697(90)90300‐X [DOI] [PubMed] [Google Scholar]
- 25. Dulbecco R, Vogt M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med, 1954, 99, 167–182 10.1084/jem.99.2.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ducibella T Wassarman PM. Mammalian egg cortical granule and the cortical reaction. Elements of mammalian fertilization, 1991. Boca Raton: CRC Press; 205–231 [Google Scholar]
- 27. Okada A, Inomata K, Nagae T. Spontaneous cortical granule release and alteration of zona pellucida properties during and after meiotic maturation of mouse oocytes. Anat Rec, 1993, 237, 518–526 10.1002/ar.1092370412 [DOI] [PubMed] [Google Scholar]
- 28. Chung SO. Volume changes during the preimplantation stages of mouse egg development. Yonsei Med J, 1973, 14, 63–89 [DOI] [PubMed] [Google Scholar]
- 29. Buhi WC, Alvarez IM, Kouba AJ. Secreted proteins of the oviduct. Cells Tissues Organs, 2000, 166, 165–179 10.1159/000016731 [DOI] [PubMed] [Google Scholar]
- 30. Buhi WC. Characterization and biological roles of oviduct‐specific, oestrogen‐dependent glycoprotein. Reproduction, 2002, 123, 355–362 10.1530/rep.0.1230355 [DOI] [PubMed] [Google Scholar]
- 31. Talbot P, Dicarlantonio G. The oocyte–cumulus complex: ultrastructure of the extracellular components in hamsters and mice. Gamete Res, 1984, 10, 127–142 10.1002/mrd.1120100205 [Google Scholar]
- 32. Kapur RP, Johnson LV. An oviductal fluid glycoprotein associated with ovulated mouse ova and early embryos. Dev Biol, 1985, 112, 89–94 10.1016/0012‐1606(85)90122‐8 [DOI] [PubMed] [Google Scholar]
- 33. Kapur RP, Johnson LV. Selective sequestration of an oviductal fluid glycoprotein in the perivitelline space of mouse oocytes and embryos. J Exp Zool, 1986, 238, 249–260 10.1002/jez.1402380215 [DOI] [PubMed] [Google Scholar]
- 34. Buhi WC, O'Brien B, Alvares IM, Erdos G, Dubois D. Immunogold localization of porcine oviductal secretory proteins within the zona pellucida, perivitelline space, and plasma membrane of oviductal and uterine oocytes and early embryos. Biol Reprod, 1993, 48, 1274–1283 10.1095/biolreprod48.6.1274 [DOI] [PubMed] [Google Scholar]
- 35. Buhi WC, Alvarez IM, Kouba AJ. Oviductal regulation of fertilization and early embryonic development. J Reprod Fert Suppl, 1997, 52, 285–300 [PubMed] [Google Scholar]
- 36. Vollet JJ, Roth LE. Cell fusion by nascent membrane induction and divalent cation treatment. Cryobiology, 1974, 9, 249–262 [Google Scholar]
- 37. Kujawa MJ, Tepperman K. Culturing chick muscle cells on glycosaminoglycan substrates: attachment and differentiation. Dev Biol, 1983, 99, 277–286 10.1016/0012‐1606(83)90277‐4 [DOI] [PubMed] [Google Scholar]
- 38. Orkin RW, Knudson W, Toole BP. Loss of hyaluronate dependent coat during myoblast fusion. Dev Biol, 1985, 107, 527–530 10.1016/0012‐1606(85)90333‐1 [DOI] [PubMed] [Google Scholar]
- 39. Austin CR The mammalian egg, 1961. Oxford: Blackwell Scientific Publications; [Google Scholar]
- 40. Cherr GN, Drobnis EZ Dunber BS. Orand MG. Fertilization in the golden hamster. A comparative overview of mammalian fertilization, 1991. New York: Plenum Press; 217–244 [Google Scholar]
- 41. Yanagimachi R Knobil E. Neil JD. Mammalian fertilization. The physiology of reproduction, 1994. 2 New York: Raven Press; 189–317 [Google Scholar]


