Abstract
Purpose
The mechanism underlying primordial follicle activation is poorly understood. In this study, in‐vitro culture and subsequent xenotransplantation were conducted to determine whether testosterone promotes the activation of porcine primordial follicles.
Methods
Prepubertal porcine ovarian cortical strips containing primordial follicles were cultured in the presence of testosterone for 7 days, and subsequently transplanted to immunodeficient mice for 2 months. After culture and transplantation, development of follicles was examined histologically. The presence of androgen receptors in oocytes was assessed by use of western blot and immunohistochemical analyses.
Results
Testosterone at 10−6 m induced the primordial follicle transition to the intermediate (19 ± 4%) and primary (3 ± 1%) stages after 7‐day culture, while 56 ± 5% of primordial follicles remained in the initial pool. Higher concentrations, above 10−5 m, or lower concentrations, below 10−6 m, did not induce follicle transition to the primary stage. After 7‐day culture with 10−6 m testosterone, ovarian cortical strips were transplanted to immunodeficient mice. Some of the follicles developed to the secondary (15 ± 3%) and antral (10 ± 3%) stages, whereas 44 ± 7% of primordial follicles remained in the initial pool. In the culture experiment, estradiol‐17β (10−7–10−5 m) had no significant effect on follicle activation. The androgen receptor antagonist, cyproterone acetate, inhibited the stimulatory effect of testosterone on primordial follicle activation, suggesting an androgen receptor‐mediated action of testosterone. Western blot and immunohistochemical analyses revealed that androgen receptors were present in the oocytes of primordial follicles.
Conclusions
These results suggest that testosterone at 10−6 m promotes the activation of porcine primordial follicles in vitro through the androgen receptors in the oocytes.
Keywords: Androgen receptor, Pig, Primordial follicle activation, Testosterone, Xenografting
Introduction
In mammals, oocytes sequestered in primordial follicles remain quiescent in the ovary until recruited into the growing pool [1]. Folliculogenesis is the process by which the oocyte develops with the somatic cells and matures to a fertilizable egg [2]. The development of the follicle involves the integration of signals from both inside and outside the ovary [3]. The process of the gradual exit of primordial follicles from the non‐growing follicle pool, and their transition to the intermediate or primary follicle stage, is regarded as primordial follicle activation [4]. However, the mechanism regulating primordial follicle activation is poorly understood. Elucidation of the mechanisms regulating primordial follicle activation, and preservation of them for a future stockpile, are important for successful assisted reproductive technology [5].
Primordial follicle activation seems to be directed primarily by signals in the ovary, whereas endocrine hormones from the pituitary and other organs are necessary for folliculogenesis to proceed beyond the primary or secondary stage [6]. In the ovary, cross‐talk between the oocytes and somatic cells, for example granulosa cells and thecal cells, occurs at every stage in follicle development [5]. Primordial follicle activation is associated with oocyte growth, and when activation occurs the surrounding pre‐granulosa cells differentiate to the cuboidal shape to form intermediate follicles (having both cuboidal and pre‐granulosa cells) [7] or primary follicles (having a complete layer of cuboidal granulosa cells) [6]. Subsequently, the follicle develops to secondary, antral, and further advanced follicle stages [6, 8, 9].
In vitro culture is an important technique for studying the mechanism of primordial follicle activation, however, success in the culture of primordial follicles as a method of oocyte growth has been limited to mice. Eppig and O'Brien [10] reported the first successful production of a baby mouse derived from cultured primordial follicles. Several studies with farm animals and primates have shown the transition of primordial follicles to the primary stage during culture of cortical strips from caprine [11], bovine [12], baboon [13], and human ovaries [14]. In these studies, however, primordial follicles hardly developed to the secondary stage even when the tissues were cultured for 20 days [13, 15].
Xenografting of fresh and frozen–thawed ovarian tissues is an alternative method for the growth of oocytes in the primordial follicles of large animals [16, 17]. Cross‐species transplantation of ovaries from large mammals, including humans [16, 18], marmosets [19] and sheep [20], to recipient severe combined immunodeficient (SCID) mice or nude mice results in the development of antral follicles. The duration of ovarian xenografting depends on the time course necessary for the follicular development of the donors. Primordial follicles developed to the antral stage 45–75 days after xenografting of the ovarian tissues from 20‐day‐old piglets [21]. However, the development of primordial follicles from cows [22] and prepubertal pigs [23] was not initiated 6–8 weeks after xenografting. Primordial follicles from prepubertal pigs developed to the antral stage after 6 months in xenografts [24].
Ovarian steroids, which include progesterone, androgen, and estrogen, act via specific receptors and are essential for folliculogenesis and ovulation [2, 25]. In females, estrogens are produced by locally expressed p450 aromatase from follicular androgens in the ovary [25]. Therefore, production of estrogen is dependent on the production of androgens, for example androstenedione and testosterone [25]. Androgens are produced during follicular development and are present at high concentrations in follicular fluid [26]. In the pig, it was reported that total androgen concentration (5.2 × 10−8 m) in small follicles was higher than the estrogen concentration (2.94 × 10−8 m) [27], and that total testosterone concentrations in the follicular fluids of small and large antral follicles were around 4.8 × 10−8 m and 1.35 × 10−7 m, respectively [28]. There are some reports in primates [29, 30] and cows [31] showing that exposure of the fetal ovary to androgens may cause an increase in the number of primary or secondary follicles.
The objective of this study was to investigate the possible role of testosterone in primordial follicle activation in the pig. We examined the activation of primordial follicles in cultured ovarian cortical strips by treatment with testosterone and determined the development of activated follicles by xenografting.
Materials and methods
Collection of ovarian cortical strips containing primordial follicles
Ovaries were collected from 6‐month‐old crossbred gilts at a slaughterhouse in Kobe, Japan. Because pigs reach puberty at 6–7 months, ovaries without the corpus luteum were collected from prepubertal gilts. The ovaries were washed once in 0.2% (w/v) cetyltrimethylammonium bromide (Wako Pure Chemical Industries, Osaka, Japan) and Dulbecco's phosphate‐buffered saline (PBS) supplemented with 0.1% (w/v) polyvinyl alcohol (PVA; Sigma, MO, USA) 3 times. Cortical strips, from randomly selected ovaries, with a thickness of 0.5 mm (approximately) were dissected with surgical blades (No. 11; Feather Safety Razor, Osaka, Japan). The strips were examined under dissection and inverted microscopes, and strips containing primary and secondary follicles were avoided. Primordial follicles were identified as those having oocytes with diameters within the range 30–35 µm and by oocytes containing a large spherical nucleus surrounded by small lipid droplets [24]. Ovarian strips (approximately, 2 mm × 1 mm × 0.5 mm) that contained primordial follicles were selected, and cut into two pieces (each approximately 1 mm × 1 mm × 0.5 mm); one part was fixed immediately for histological examination to assess follicle number and morphology, and the other part was washed 3 times and immersed in HEPES‐buffered TCM‐199 (pH 7.4; Nissui Pharmaceutical, Tokyo, Japan) before culture. The HEPES‐buffered TCM‐199 contained 25 mm HEPES (Sigma), 10 mm NaHCO3, 0.1% PVA, and 0.08 mg/ml kanamycin sulfate (Sigma) in TCM 199.
In vitro culture
In each experiment, a group of 4 cortical stripes (1 mm × 1 mm × 0.5 mm) was cultured on a cellulose acetate floating membrane filter (pore size 0.45 μm and diameter 25 mm; Advantec, Toyo Roshi, Tokyo, Japan) in an organ culture dish (# 3037; BD Falcon, NJ, USA) for 7 days under a humidified atmosphere of 5% CO2 and 95% air at 38.5°C. The basic culture medium was alpha minimum essential medium (α‐MEM; Invitrogen, NY, USA) supplemented with 0.08 mg/ml kanamycin sulfate, 2.2 mg/ml NaHCO3, 50 μm 2‐mercaptoethanol (Nakalai Tesque, Kyoto, Japan), 0.1 mg/ml sodium pyruvate (Sigma), 1% (v/v) insulin–transferrin–selenium (ITS; Invitrogen), and 5% (v/v) bovine plasma (Nippon Biotest Laboratories, Tokyo, Japan) after heat inactivation. During the culture period, half of the total medium was changed every 2 days. In the experimental groups the basic culture medium was supplemented with 10−8, 10−7, 10−6, 10−5, or 10−4 m testosterone (Nakalai Tesque), or 10−7, 10−6 or 10−5 m estradiol‐17β (Sigma). In the inhibitory experiments, 10−7 or 10−6 m cyproterone acetate (CPTA; Sigma) was used with 10−6 m testosterone. All the steroids and inhibitor were dissolved in absolute ethanol and 1 µl of the solution was added to each 1 ml of culture medium to obtain the final concentrations just before use. As the vehicle, 1 µl ethanol was added to the control groups.
Assessment of follicular development
The ovarian cortical strips before and after culture were fixed in 3% (w/v) paraformaldehyde in PBS, then dehydrated, embedded in methacrylate resin (JB‐4; Polyscience, Niles, IL, USA), serially sectioned by 5 μm, and stained with hematoxylin and eosin. The numbers of different stages of follicles were recorded. The follicles were counted in every section in which the oocyte nucleus was seen. Double counting in adjacent sections was avoided. The follicles were classified into 3 categories according to the number and morphology of granulosa cell layers: primordial follicles with single layer of flattened granulosa cells surrounding the oocyte, intermediate follicles with a single layer containing a mixture of flat and cuboidal granulosa cells, and primary follicles with a single layer of cuboidal granulosa cells. Degenerated follicles were identified by the staining properties of the oocyte cytoplasm and nucleus—pale cytoplasm and dark pyknotic nucleus. Furthermore, follicles having a shrunken oocyte, extensive cytoplasmic vacuolations, and disintegrated granulosa cell layer were also regarded as degenerated follicles.
Xenografting
This study was approved by the Institutional Animal Care and Use Committee and carried out according to the Kobe University Animal Experimentation Regulations (permission numbers: 19‐05‐09 and 21‐05‐05). Six to 8‐week‐old female SCID mice were purchased from Clea Japan (Tokyo, Japan). Before xenografting, the mice were anaesthetized and the left kidney exteriorized through a dorsal–horizontal incision. A small hole was torn in the kidney capsule using fine forceps. Four to six pieces of porcine ovarian strips were inserted underneath the capsule. The surgery was performed at room temperature and the mice were kept on a warming plate (37°C) for 24 h. The mice were housed in filter‐topped cages in a positive pressure room, with free access to clean water and balanced feed pellets. The light cycle of the room was set at 12/12 h L/D. Ovarian strips from prepubertal pigs either fresh for control or cultured for 7 days were xenografted into the SCID mice for 2 months.
Histological examination for xenografted tissues
Mouse kidney tissues containing ovarian grafts and ovarian cortical strips before xenografting were fixed, stained, and examined histologically as described above. The follicles were classified into five categories according to the number and morphology of granulosa cell layers: primordial, intermediate, and primary follicles as described above and secondary follicles with two or more layers of cuboidal granulosa cells, and antral follicle having an antral cavity. Degenerated follicles were identified by the same method as described previously.
Immunohistochemistry for androgen receptor
Fresh cryostat sections of porcine ovarian cortical strips 5 µm thick were prepared on aminopropylsilane (APS)‐coated slides, and then air dried and fixed in 1% paraformaldehyde in PBS at room temperature for 15 min. To prevent non‐specific antibody binding, blocking was done by use of 5% (w/v) bovine serum albumin (BSA; Wako Pure Chemical Industries) for 1 h and immunostaining was then performed with rabbit polyclonal anti‐androgen receptor antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA; # sc‐815) overnight at 4°C in a humidified chamber. After washing with PBS, the sections were reacted with Alexa Fluor 488‐labeled goat anti‐rabbit immunoglobulin antibody (1:2000; Molecular Probes, Eugene, OR, USA; # A11008) for 1 h, and then counterstained with propidium iodide (PI; 100 µg/ml; Sigma) for 15 min. After washing 3 times with PBS, the sections were mounted with Vectashield Mounting Medium (Vector Laboratories, Burlingame, CA, USA) and observed under a fluorescence microscope (U‐LH100HGPO; Olympus, Tokyo, Japan). Some sections were incubated without the primary antibody as negative controls. The pictures were analyzed by ImageJ 1.42q software (Wayne Rasband, National Institute of Health, USA), which visualized the merged pictures, to confirm the exact localization of the androgen receptor.
Western blot analysis for androgen receptor
Fresh ovarian cortical strips (1 mm × 1 mm × 1 mm) containing primordial follicles (15–20 strips, weighing 20 mg), were collected and washed 3 times in PBS and homogenized by means of a glass homogenizer kept on ice in a radioimmune precipitation assay (RIPA) lysis buffer containing 50 mm Tris–HCl (pH 7.4), 150 mm NaCl, 1 mm EDTA, 0.1% (w/v) sodium dodecyl sulfate (SDS), 0.5 mm p‐aminophenyl methane sulfonyl fluoride hydrochloride (p‐APMSF; Wako Pure Chemical Industries), and 1% (v/v) Triton X‐100. The lysates were transferred to an Eppendorf tube to be sonicated by Vibra cell™ (Sonics and Materials, Danbury, CT, USA) with three short bursts of 10 s at 20% amplitude followed by intervals of 1 min on ice. After centrifugation at 11000g for 10 min at 4°C, the supernatants were collected and protein concentrations were measured using a Bradford assay containing bicinchoninic acid (Bio‐Rad Laboratories, Hercules, CA, USA). The protein samples were then transferred to Eppendorf tubes and an equal volume of 2‐times‐concentrated SDS sample buffer [32] was added. The samples were boiled for 5 min and kept at −20°C until use. The specificity of the antibody was tested with two porcine tissues (testis and ovary) that have been reported to be positive, and one tissue (spleen) that has been reported to be negative for androgen receptors in pigs, mice, and humans [33, 34]. The porcine testis and spleen samples were prepared for western blotting as described above.
Oocytes were collected from primordial follicles for western blot analysis. Ovarian cortical slices were minced into 1 mm square pieces. After 3 washes in 25 mm HEPES‐buffered TCM‐199, the tissues were incubated for 2.5 h with gentle agitation in TCM‐199 containing 2.5 mg/ml pronase (actinase E; Kaken Pharmaceutical, Tokyo, Japan) at 38.5°C under an atmosphere of 5% CO2 in humidified air. After washing 3 times in HEPES‐buffered TCM‐199, the tissues were gently pipetted for 15–20 min in HEPES‐buffered TCM‐199 containing 10% (v/v) fetal calf serum (FCS; Dainippon Pharmaceutical, Osaka, Japan). Oocytes with diameters ranging from 30 to 35 µm were picked up using a fine glass pipette. After washing three times in PBS–PVA, each group of 800 oocytes was transferred into an Eppendorf tube with 3 µl PBS–PVA. The samples were prepared and stored at −20°C for western blotting.
The samples were run on 10% SDS‐polyacrylamide gel electrophoresis and the proteins were transferred to hydrophobic polyvinylidene difluoride membranes (Immobilon; Millipore, Bedford, MA, USA). The membranes were cut into two pieces: one part contained >50 kDa and the other 50–25 kDa. The membranes were blocked with 10% (v/v) FCS in PBS containing 0.1% (v/v) Tween 20 (PBS–Tween) for 1 h and incubated overnight at 4°C with rabbit polyclonal anti‐androgen receptor antibody (1:1000; Santa Cruz Biotechnology; # sc‐815) in PBS–Tween containing 10% FCS. After washing 3 times in PBS–Tween, the membranes were treated with horseradish peroxidase‐conjugated (HRP) goat anti‐rabbit immunoglobulin (1:2000; GE Healthcare UK, Little Chalfont, Buckinghamshire, UK) for 1 h at room temperature. For the control, the membranes containing 50–25 kDa were probed with mouse monoclonal anti‐β‐actin antibody (1:10000; Sigma; # A2228) and subsequently with HRP‐conjugated goat anti‐mouse immunoglobulin antibody (1:20000; DakoCytomation Denmark, Glostrup, Denmark; # P0447). After washing 3 times in PBS–Tween, the peroxidase activity was visualized using western blotting luminol reagent (Chemi‐Lumi One; Nakalai Tesque).
Data and statistical analysis
All the culture experiments were repeated at least three times, and data were pooled and analyzed. For the immunofluorescence data, one representative picture of the results from three independent experiments was presented. The quantitative results were presented as the mean ± SEM. The data were analyzed using nonparametric ANOVA followed by Dunn's multiple comparisons among multiple groups as appropriate. For statistical comparison between two groups, a t test was used. All percentile data were transformed to Arc sin values before analysis. Differences at P < 0.05 were regarded as statistically significant.
Results
Effects of testosterone on primordial follicle activation
Ovarian cortical strips before culture contained almost all primordial follicles (Fig. 1a), whereas after 7‐day culture with 10−6 m testosterone, primordial follicles had developed to intermediate (19 ± 4%) or primary follicles (3 ± 1%, Fig. 1c, d), and 56 ± 5% remained in the primordial stage (Fig. 2a). Higher concentrations above 10−5 m, or lower concentrations below 10−6 m did not induce follicle transition to the primary stage (Fig. 2a). At 10−6 m, testosterone reduced the percentage of degenerated follicles (22 ± 4%) compared with the control 0 m group (56 ± 1%). However, lower concentrations, below 10−6 m, or higher concentrations, above 10−5 m, increased the number of degenerated follicles. 10−6 and 10−5 m testosterone increased the percentages of healthy primordial follicles compared with the control (P < 0.05).
Figure 1.
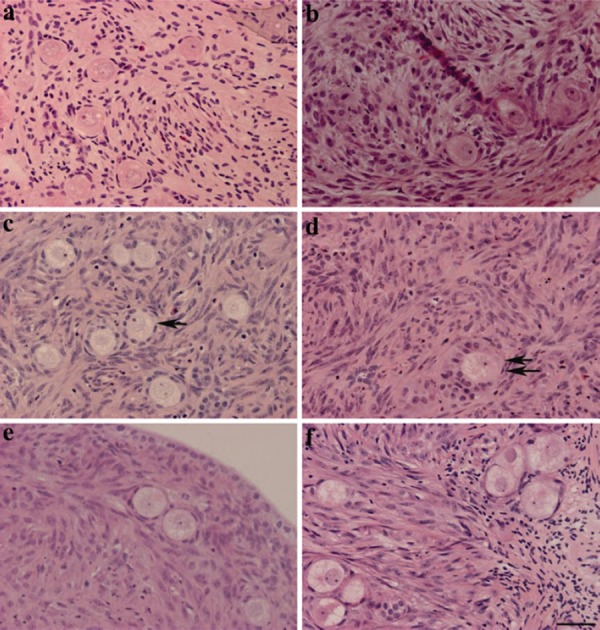
Testosterone‐induced primordial follicle activation in porcine ovarian strips. The ovarian strips were fixed in paraformaldehyde and stained with hematoxylin and eosin. The ovarian strips before culture (a) contained mainly healthy primordial follicles. 10−6 m testosterone treatment for 7 days yielded intermediate follicles (arrow in c) and primary follicles (double arrow in d). Ovarian strips treated with estradiol‐17β (e), cyproterone acetate + testosterone (f), and control treated with vehicle (b), in which the primordial follicles were intact, had not started to develop after 7 days. Scale bar represents 40 µm
Figure 2.
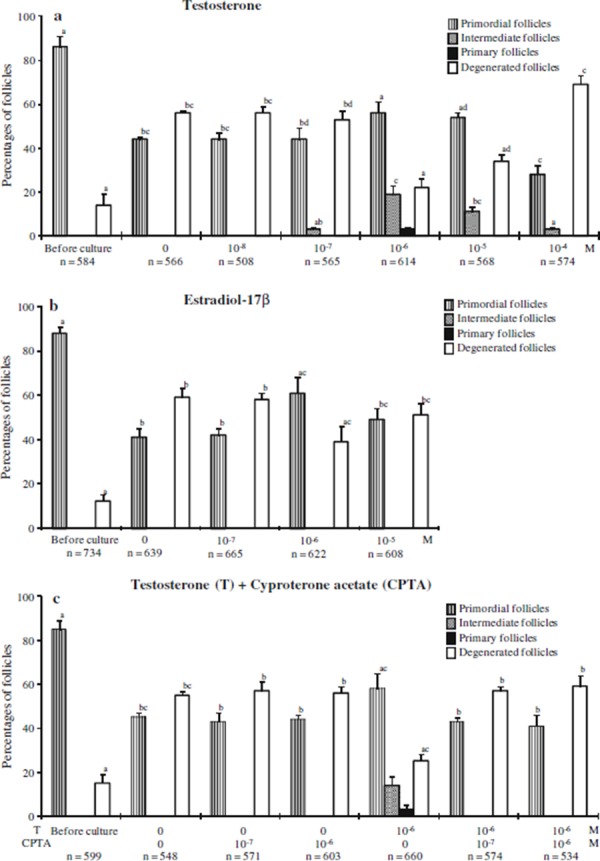
Distribution of different types of follicles in ovarian strips before and after culture with various concentrations of a testosterone (T), b estradiol‐17β, and c cyproterone acetate (CPTA). The ovarian strips were fixed and stained with H&E, and the number of different stages of the follicles were recorded. “n” shows the total number of follicles examined. Error bars indicate SEM. Columns with different letters in the same category of follicles are significantly different (P < 0.05)
Because testosterone can be aromatized to estradiol [25], the effect of estradiol was examined. In cortical strips treated with 10−7, 10−6, or 10−5 m estradiol‐17β, there were only primordial and degenerated follicles, without any intermediate or primary follicles (Fig. 1e). There was an increase in the percentage of primordial follicles and a decrease in degenerated follicles in cortical strips cultured with 10−6 m estradiol‐17β (Fig. 2b). However, there was no dose‐dependent relationship with the percentages of primordial follicles in the estradiol‐17β‐treated groups.
An androgen receptor antagonist, cyproterone acetate, was used to determine whether the stimulatory effect of testosterone on primordial follicle activation was mediated by androgen receptor. Cyproterone acetate inhibited the activation of primordial follicles and increased the percentages of degenerated follicles (Figs. 1f, 2c).
Development of primordial follicles in xenografts after testosterone treatment
Histological examination confirmed that the strips dissected from ovaries contained only primordial follicles and a few degenerated follicles before culture (Table 1). The oocyte diameters were 35 µm or less and each oocyte was surrounded by a single layer of 5–7 flattened cells. After 7 days of culture, ovarian cortical strips were xenografted to SCID mice. Two months after grafting, the fresh ovarian cortical strips contained only primordial and degenerated follicles, and none of them developed to the advanced stages (Fig. 3a; Table 1). In the grafts cultured without testosterone, none of the primordial follicles were activated (Table 1). In the grafts cultured with testosterone at 10−6 m, some follicles developed to the primary (11 ± 3%), secondary (15 ± 3%) and antral (10 ± 3%) stages (Fig. 3c–e). However, 44 ± 7% of primordial follicles in the grafts remained in the primordial stage (Fig. 3d). Abnormally enlarged antral cavities of the developing follicles in the grafts were filled with blood (Fig. 3e).
Table 1.
Effect of testosterone treatment and subsequent grafting on the development of porcine primordial follicles
| Concentration of testosterone | Day of culture | Xenografting | Number of strips examined d | Number of SCID mice | Number (%) of oocytes per tissue | Number (%) of follicles per tissue | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤35 µm | ≥36 µm | Primordial | Intermediate | Primary | Secondary | Antral | Degenerated | |||||
| 0 | 0 | Before | 16 | – | 35 ± 6 (87 ± 2)a | 0 ± 0 | 35 ± 6 (87 ± 2)a | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 5 ± 1 (13 ± 2)a |
| After | 16 | 4 | 33 ± 5 (87 ± 5)a | 0 ± 0 | 33 ± 5 (87 ± 5)a | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 5 ± 2 (13 ± 5)a | ||
| 0 | 7 | Before | 16 | – | 14 ± 2 (42 ± 2)b | 0 ± 0 | 14 ± 2 (42 ± 2)b | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 19 ± 3 (58 ± 2)b |
| After | 16 | 4 | 15 ± 4 (75 ± 5)ac | 0 ± 0 | 15 ± 4 (75 ± 5)ac | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 5 ± 2 (25 ± 5)bc | ||
| 10−6 m | 7 | Before | 16 | – | 26 ± 5 (56 ± 5)bc | 10 ± 2 (23 ± 5)a | 26 ± 5 (56 ± 5)bc | 8 ± 2 (16 ± 4) | 3 ± 1 (6 ± 2)a | 0 ± 0 | 0 ± 0 | 10 ± 2 (22 ± 2)c |
| After | 16 | 4 | 17 ± 3 (44 ± 7)b | 14 ± 2 (35 ± 4)b | 17 ± 3 (44 ± 7)b | 0 ± 0 | 5 ± 1 (11 ± 3)b | 6 ± 1 (15 ± 3) | 4 ± 1 (10 ± 3) | 8 ± 1 (20 ± 3)ac | ||
a–cValues with different superscripts in the same column are significantly different (P < 0.05)
dOvarian strips (approximately 1 mm × 1 mm × 0.5 mm) containing primordial follicles were cultured with 10−6 m testosterone for 7 days and xenografted to SCID mice for 2 months. Each value represents the mean ± SEM
Figure 3.

Development of porcine primordial follicles in xenografts. Ovarian cortical strips containing primordial follicles were cultured for 7 days before xenografting to SCID mice. After 2 months, the grafts were fixed and stained with H&E. In the grafts of fresh ovarian tissues (a) or vehicle‐treated strips (b), none of the follicles developed beyond the primordial stage. In the grafts treated with 10−6 m testosterone, the follicles developed to the secondary (c) and antral stages which contained growing oocytes (d, e). “r” indicates the renal tissue of SCID mice. The scale bars represent 100 µm
Expression of androgen receptors in oocytes
Because the results suggest a receptor‐mediated effect of testosterone on the transition of primordial follicles to the intermediate or primary stage, we examined the expression of the androgen receptor in the oocytes. In the western blots a band of androgen receptor protein was detected in the oocytes collected from primordial follicles (Fig. 4a). When the specificity was examined with the porcine tissues, the relevant bands were observed in the ovary and testis but were faint in the spleen. The androgen receptor signals were observed in oocytes, granulosa cells, and some of the interstitial cells of the ovarian tissues (Fig. 4b). There was strong cytoplasmic staining for the androgen receptor in oocytes in primordial follicles (Fig. 4b, b).
Figure 4.
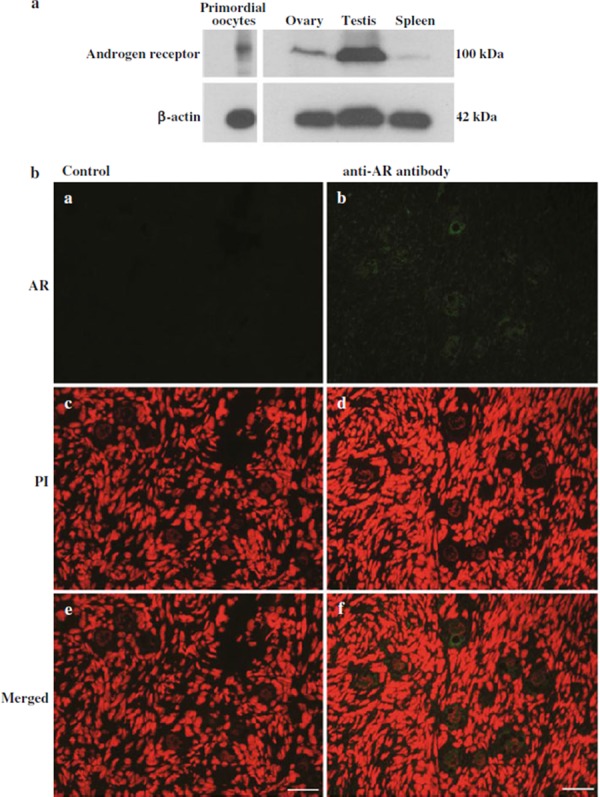
a Expression of the androgen receptor protein in porcine oocytes in primordial follicles was examined by western blot. A total number of 800 oocytes or tissue extracts from porcine ovary, testis, and spleen were run on SDS‐polyacrylamide gels. In each sample a single band of ~100 kDa was detected by anti‐androgen receptor antibody. b Immunolocalization of the androgen receptor protein in oocytes in primordial follicles. Cryosections were treated with rabbit anti‐androgen receptor antibody and Alexa Fluor 488‐labeled anti‐rabbit immunoglobulin antibody (green) and counterstained by PI (red). Serial sections were incubated without primary antibody as negative control. e, f were merged pictures. Scale bars represent 40 µm
Discussion
A proportion of porcine primordial follicles developed in ovarian cortical strips cultured with 10−6 m testosterone, and further developed to the antral stage in the xenografts after 2 months. These results indicate a role of testosterone in primordial follicle activation in pigs. When newborn mouse ovaries were cultured, primordial follicles developed to the secondary stage [10, 35], but this has not been observed for large domestic species and primates. In cultured cortical strips from fetal bovine or primate ovaries, a few follicles developed to the secondary stage [12, 13, 15, 31]. However, there are no reports on the culture of porcine primordial follicles in vitro.
In the ovary, oocytes in the primordial follicles remain dormant for years until stimulated to develop. In large mammalian species, follicular development is estimated to take about 60–90 days to complete, with the early stage proceeding much more slowly than the later stage [24]. It has been difficult to culture enzymatically isolated primordial follicles from large mammals, including pigs, because of the lack of a proper long‐term culture method, because there is limited or no growing potential after isolation [36, 37], or for both reasons. Xenografting of ovarian tissues or follicles has been used as a method to study oocyte growth and follicular development [16, 17]. However, primordial follicles from prepubertal porcine ovaries had not started to develop even after 2 months [24], and primordial follicles from cows did not develop even after 6–8 weeks [22]. In our study, the result of grafting fresh ovarian cortical strips from prepubertal pigs was consistent with a previous report [24]. In contrast, both Kaneko et al. [21] and Moniruzzaman et al. [24] reported the development of primordial follicles to the antral stage 45–75 days after xenografting ovarian tissues from infant pigs. The mechanism regulating the activation of primordial follicles in prepubertal pigs may be different from that of infant pigs [24]. In our study, it is suggested that some of the primordial follicles treated with 10−6 m testosterone were activated and these activated follicles developed to the antral stage in the xenografted environment within 2 months. During the testosterone treatment in vitro, primordial follicles from prepubertal pigs may be modified similar to the activated state of primordial follicles in infant pigs.
The factors involved in transition of primordial follicles to the growth phase, especially in large domestic species, are not well defined. In this study, the numbers of intermediate and primary follicles were increased by 10−6 m testosterone and this primordial follicle activation was inhibited by an antagonist to the androgen receptor. Moreover, androgen receptors were expressed in the oocytes in primordial follicles. These results suggest a role of testosterone in the primordial follicle activation in the pig. This finding is consistent with the results of studies showing that treatment with 10−5 m testosterone stimulated primordial follicle activation in cultured mouse ovaries [38], and that prenatal testosterone treatment induced follicle recruitment and later increased the number of growing follicles in sheep, while reducing the number of primordial follicles [39].
The most effective dose of testosterone (10−6 m; 288 µg/l) in this study was tenfold higher than the intra‐follicular androgen concentration of preovulatory porcine follicles [40]. At 10−6 m, testosterone gave the highest percentages of intermediate follicles and primary follicles, and yielded significantly lower numbers of degenerated follicles than the other concentrations. Although there have been some reports suggesting that androgens were atretogenic for antral follicles [41], an anti‐apoptotic role has been reported for prostate cancer cells, in which androgens induced expression of the cellular Fas/FasL‐associated death domain protein‐like inhibitory protein (c‐FLIP) gene, a potent inhibitor of Fas/FasL‐mediated apoptosis [42]. Although the number of intermediate follicles was increased by testosterone treatment, estradiol‐17β had no effect on primordial follicle activation. This result is consistent with that by Britt et al. [43], who reported that estradiol‐17β affects late follicular development whereas primordial follicle activation was thought to be independent of estradiol‐17β.
Testosterone exerts its biological effects on target cells by directly binding to androgen receptors [44]. In our study the androgen receptor was localized in oocytes in porcine primordial follicles. The androgen receptor protein has been reported to be present in the oocytes, granulosa cells, and thecal cells of preantral and antral follicles in porcine [45] and ovine ovaries [46]. Expression of mRNA of the androgen receptor has also been demonstrated in human intermediate‐stage follicles [47]. Because the androgen receptor antagonist blocked the effect of testosterone on primordial follicle activation in our study, it is thought that testosterone possibly activated the primordial follicles via the androgen receptor.
It is not known what factors regulate primordial follicle activation in pigs. Recently, we reported that the knockdown of FOXO3 in primordial oocytes induces primordial follicle activation in pigs [24]. In the mouse, testosterone induces activation of primordial follicles by redistribution of intra‐oocyte Foxo3a [38]. In our study, testosterone might have induced porcine primordial follicle activation by a similar mechanism including FOXO3 inactivation.
In summary, we showed that testosterone induces the activation of primordial follicles in pigs and the activation enhances their development to the antral stage in xenografts. Oocytes in porcine primordial follicles have androgen receptors, and testosterone activates primordial follicles through the androgen receptors.
Acknowledgments
We are grateful to the staff of the Kobe Meat Inspection Office for supplying pig ovaries. This study was supported in part by the Grant‐in‐Aid for Scientific Research from the Japan Society for the Promotion of Science to TM and MM.
References
- 1. Reynaud K, Driancourt MA. Oocyte attrition. Mol Cell Endocrinol, 2000, 163, 1–8 10.1016/S0303‐7207(99)00246‐4 [DOI] [PubMed] [Google Scholar]
- 2. Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev, 1996, 17, 121–155 [DOI] [PubMed] [Google Scholar]
- 3. Fortune JE, Cushman RA, Kito WS. The primordial follicle to primary follicle transition. Mol Cell Endocrinol, 2000, 163, 53–60 10.1016/S0303‐7207(99)00240‐3 [DOI] [PubMed] [Google Scholar]
- 4. Picton HM. Activation of follicle development: the primordial follicle. Theriogenology, 2001, 55, 1193–1210 10.1016/S0093‐691X(01)00478‐2 [DOI] [PubMed] [Google Scholar]
- 5. Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science, 2002, 296, 2178–2180 10.1126/science.1071965 [DOI] [PubMed] [Google Scholar]
- 6. Picton HM, Harris SE, Muruvi W, Chambers EL. The in vitro growth and maturation of follicles. Reproduction, 2008, 136, 703–715 10.1530/REP‐08‐0290 [DOI] [PubMed] [Google Scholar]
- 7. Da Silva‐Buttkus P, Jayasooriya GS, Mora JM, Mobberley M, Ryder TA, Baithun M et al. Effect of cell shape and packing density on granulosa cell proliferation and formation of multiple layers during early follicle development in the ovary. J Cell Sci, 2008, 121, 3890–3900 10.1242/jcs.036400 [DOI] [PubMed] [Google Scholar]
- 8. Lintern‐Moore S, Moore GP. The initiation of follicle and oocyte growth in the mouse ovary. Biol Reprod, 1979, 20, 773–778 10.1095/biolreprod20.4.773 [DOI] [PubMed] [Google Scholar]
- 9. Hirshfield AN. Theca cells may be present at the outset of follicular growth. Biol Reprod, 1991, 44, 1157–1162 10.1095/biolreprod44.6.1157 [DOI] [PubMed] [Google Scholar]
- 10. Eppig JJ, O'Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod, 1996, 54, 197–207 10.1095/biolreprod54.1.197 [DOI] [PubMed] [Google Scholar]
- 11. Silva JR, Hurk R, Matos MH, dos Santos RR, Pessoa C, Moraes MO et al. Influences of FSH and EGF on primordial follicles during in vitro culture of caprine ovarian cortical tissue. Theriogenology, 2004, 61, 1691–1704 10.1016/j.theriogenology.2003.09.014 [DOI] [PubMed] [Google Scholar]
- 12. Wandji SA, Srsen V, Voss AK, Eppig JJ, Fortune JE. Initiation in vitro of growth of bovine primordial follicles. Biol Reprod, 1996, 55, 942–948 10.1095/biolreprod55.5.942 [DOI] [PubMed] [Google Scholar]
- 13. Fortune JE, Kito S, Wandji SA, Srsen V. Activation of bovine and baboon primordial follicles in vitro. Theriogenology, 1998, 49, 441–449 10.1016/S0093‐691X(97)00416‐0 [DOI] [PubMed] [Google Scholar]
- 14. Hovatta O, Silye R, Abir R, Krausz T, Winston RM. Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long‐term culture. Hum Reprod, 1997, 12, 1032–1036 10.1093/humrep/12.5.1032 [DOI] [PubMed] [Google Scholar]
- 15. Wandji SA, Srsen V, Nathanielsz PW, Eppig JJ, Fortune JE. Initiation of growth of baboon primordial follicles in vitro. Hum Reprod, 1997, 12, 1993–2001 10.1093/humrep/12.9.1993 [DOI] [PubMed] [Google Scholar]
- 16. Oktay K, Newton H, Mullan J, Gosden RG. Development of human primordial follicles to antral stages in SCID/hpg mice stimulated with follicle stimulating hormone. Hum Reprod, 1998, 13, 1133–1138 10.1093/humrep/13.5.1133 [DOI] [PubMed] [Google Scholar]
- 17. Oktay K, Newton H, Gosden RG. Transplantation of cryopreserved human ovarian tissue results in follicle growth initiation in SCID mice. Fertil Steril, 2000, 73, 599–603 10.1016/S0015‐0282(99)00548‐8 [DOI] [PubMed] [Google Scholar]
- 18. Weissman A, Gotlieb L, Colgan T, Jurisicova A, Greenblatt EM, Casper RF. Preliminary experience with subcutaneous human ovarian cortex transplantation in the NOD‐SCID mouse. Biol Reprod, 1999, 60, 1462–1467 10.1095/biolreprod60.6.1462 [DOI] [PubMed] [Google Scholar]
- 19. Candy CJ, Wood MJ, Whittingham DG. Follicular development in cryopreserved marmoset ovarian tissue after transplantation. Hum Reprod, 1995, 10, 2334–2338 [DOI] [PubMed] [Google Scholar]
- 20. Gosden RG, Boulton MI, Grant K, Webb R. Follicular development from ovarian xenografts in SCID mice. J Reprod Fertil, 1994, 101, 619–623 10.1530/jrf.0.1010619 [DOI] [PubMed] [Google Scholar]
- 21. Kaneko H, Kikuchi K, Noguchi J, Hosoe M, Akita T. Maturation and fertilization of porcine oocytes from primordial follicles by a combination of xenografting and in vitro culture. Biol Reprod, 2003, 69, 1488–1493 10.1095/biolreprod.103.017038 [DOI] [PubMed] [Google Scholar]
- 22. Senbon S, Ota A, Tachibana M, Miyano T. Bovine oocytes in secondary follicles grow and acquire meiotic competence in severe combined immunodeficient mice. Zygote, 2003, 11, 139–149 10.1017/S096719940300217X [DOI] [PubMed] [Google Scholar]
- 23. Moniruzzaman M, Miyano T. KIT‐KIT ligand in growth of porcine primordial follicles. J Reprod Dev, 2007, 53, 1273–1281 10.1262/jrd.19107 [DOI] [PubMed] [Google Scholar]
- 24. Moniruzzaman M, Lee J, Zengyo M, Miyano T. Knockdown of FOXO3 induces primordial oocyte activation in pigs. Reproduction, 2010, 139, 349–357 10.1530/REP‐09‐0207 [DOI] [PubMed] [Google Scholar]
- 25. Drummond AE. The role of steroids in follicular growth. Reprod Biol Endocrinol, 2006, 4, 16 10.1186/1477‐7827‐4‐16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McNatty KP. Hormonal correlates of follicular development in the human ovary. Aust J Biol Sci, 1981, 34, 249–268 [DOI] [PubMed] [Google Scholar]
- 27. Eiler H, Nalbandov AV. Sex steroids in follicular fluid and blood plasma during the estrous cycle of pigs. Endocrinology, 1977, 100, 331–338 10.1210/endo‐100‐2‐331 [DOI] [PubMed] [Google Scholar]
- 28. Chang SC, Jones JD, Ellefson RD, Ryan RJ. The porcine ovarian follicle: 1. Selected chemical analysis of follicular fluid at different developmental stages. Biol Reprod, 1976, 15, 321–328 10.1095/biolreprod15.3.321 [DOI] [PubMed] [Google Scholar]
- 29. Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest, 1998, 101, 2622–2629 10.1172/JCI2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome?. Hum Reprod Update, 2005, 11, 357–374 10.1093/humupd/dmi013 [DOI] [PubMed] [Google Scholar]
- 31. Yang MY, Fortune JE. Testosterone stimulates the primary to secondary follicle transition in bovine follicles in vitro. Biol Reprod, 2006, 75, 924–932 10.1095/biolreprod.106.051813 [DOI] [PubMed] [Google Scholar]
- 32. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 1970, 227, 680–685 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- 33. Burek M, Duda M, Knapczyk K, Koziorowski M, Słomczyńska M. Tissue‐specific distribution of the androgen receptor (AR) in the porcine fetus. Acta Histochem, 2007, 109, 358–365 10.1016/j.acthis.2007.03.003 [DOI] [PubMed] [Google Scholar]
- 34. Takeda H, Chodak G, Mutchnik S, Nakamoto T, Chang C. Immunohistochemical localization of androgen receptors with mono‐ and polyclonal antibodies to androgen receptor. J Endocrinol, 1990, 126, 17–25 10.1677/joe.0.1260017 [DOI] [PubMed] [Google Scholar]
- 35. O'Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod, 2003, 68, 1682–1686 10.1095/biolreprod.102.013029 [DOI] [PubMed] [Google Scholar]
- 36. Hirao Y, Nagai T, Kubo M, Miyano T, Miyake M, Kato S. In vitro growth and maturation of pig oocytes. J Reprod Fertil, 1994, 100, 333–339 10.1530/jrf.0.1000333 [DOI] [PubMed] [Google Scholar]
- 37. Miyano T, Hirao Y. In vitro growth of oocytes from domestic species. J Mam Ova Res, 2003, 20, 78–85 10.1274/jmor.20.78 [Google Scholar]
- 38. Yang JL, Zhang CP, Li L, Huang L, Ji SY, Lu CL et al. Testosterone induces redistribution of forkhead box‐3a and down‐regulation of growth and differentiation factor 9 messenger ribonucleic acid expression at early stage of mouse folliculogenesis. Endocrinology, 2010, 151, 774–782 10.1210/en.2009‐0751 [DOI] [PubMed] [Google Scholar]
- 39. Smith P, Steckler TL, Veiga‐Lopez A, Padmanabhan V. Developmental programming: differential effects of prenatal testosterone and dihydrotestosterone on follicular recruitment, depletion of follicular reserve, and ovarian morphology in sheep. Biol Reprod, 2009, 80, 726–736 10.1095/biolreprod.108.072801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ainsworth L, Tsang BK, Downey BR, Marcus GJ, Armstrong DT. Interrelationships between follicular fluid steroid levels, gonadotropic stimuli, and oocyte maturation during preovulatory development of porcine follicles. Biol Reprod, 1980, 23, 621–627 10.1095/biolreprod23.3.621 [DOI] [PubMed] [Google Scholar]
- 41. Cheng G, Weihua Z, Mäkinen S, Mäkelä S, Saji S, Warner M, Gustafsson JA, Hovatta O. A role for the androgen receptor in follicular atresia of estrogen receptor beta knockout mouse ovary. Biol Reprod, 2002, 66, 77–84 10.1095/biolreprod66.1.77 [DOI] [PubMed] [Google Scholar]
- 42. Gao S, Lee P, Wang H, Gerald W, Adler M, Zhang L, Wang YF, Wang Z. The androgen receptor directly targets the cellular Fas/FasL‐associated death domain protein‐like inhibitory protein gene to promote the androgen‐independent growth of prostate cancer cells. Mol Endocrinol, 2005, 19, 1792–1802 10.1210/me.2004‐0445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Britt KL, Saunders PK, McPherson SJ, Misso ML, Simpson ER, Findlay JK. Estrogen actions on follicle formation and early follicle development. Biol Reprod, 2004, 71, 1712–1723 10.1095/biolreprod.104.028175 [DOI] [PubMed] [Google Scholar]
- 44. Quigley CA, Bellis A, Marschke KB, el‐Awady MK, Wilson EM, French FS. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev, 1995, 16, 271–321 [DOI] [PubMed] [Google Scholar]
- 45. Słomczyñska M, Tabarowski Z. Localization of androgen receptor and cytochrome P450 aromatase in the follicle and corpus luteum of the porcine ovary. Anim Reprod Sci, 2001, 65, 127–134 10.1016/S0378‐4320(00)00225‐6 [DOI] [PubMed] [Google Scholar]
- 46. Juengel JL, Heath DA, Quirke LD, McNatty KP. Oestrogen receptor alpha and beta, androgen receptor and progesterone receptor mRNA and protein localisation within the developing ovary and in small growing follicles of sheep. Reproduction, 2006, 131, 81–92 10.1530/rep.1.00704 [DOI] [PubMed] [Google Scholar]
- 47. Rice S, Ojha K, Whitehead S, Mason H. Stage‐specific expression of androgen receptor, follicle‐stimulating hormone receptor, and anti‐Müllerian hormone type II receptor in single, isolated, human preantral follicles: relevance to polycystic ovaries. J Clin Endocrinol Metab, 2007, 92, 1034–1040 10.1210/jc.2006‐1697 [DOI] [PubMed] [Google Scholar]


