Abstract
In preovulatory follicles, each oocyte is surrounded by numerous layers of cumulus cells, forming the cumulus cell–oocyte complex. An LH surge induces meiotic resumption of the oocyte to progress to metaphase II. Because the expression of LH receptors is not detected in the oocyte and is minimal (negligible) in cumulus cells as compared with granulosa cells, secondary factors from granulosa cells are required to induce the ovulation process. One of the key factors secreted from granulosa cells is an EGF‐like factor that activates the EGFR–ERK1/2 pathway in cumulus cells. The activated ERK1/2 pathway is not only involved in gene expression but also essential for the close of gap‐junctional communication among cumulus cells and between cumulus cells and the oocyte. Closing gap‐junctional communication decreases the amount of cGMP and/or cAMP to transfer into the oocyte, which requires activation of phosphodiesterase type III (PDE3) in the oocyte. PDE3 brakes down cAMP to decrease PKA activity in the oocyte. This decrease in PKA activity induces activation of CDK1 to resume meiosis from the germinal vesicle stage. Thus, the functions of cumulus cells that are regulated by granulosa cell‐secreted factors are essential for oocyte meiotic resumption and maturation with developmental competence.
Keywords: Cumulus cells, EGF‐like factor, Oocyte maturation, Granulosa cells
Introduction
After a surge of LH, oocytes resume meiosis, complete germinal vesicle breakdown (GVBD), and progress to the metaphase II (MII) stage. Because LH receptors (LHCGRs) are dominantly expressed in granulosa cells, but not in cumulus cells or oocytes [1], the LH surge dramatically changes the expression pattern of genes in granulosa cells [2]. Factors secreted from granulosa cells act on cumulus cells to induce meiotic resumption and maturation of the oocyte with developmental competence [3]. Thus, cumulus cells play a critical role in oocyte maturation; however, there is less information about the mechanisms by which cumulus cells differentiate and how these changes in cumulus cells affect oocyte maturation at the molecular level. The mechanisms underlying how cumulus cells regulate oocyte meiotic resumption are the focus of this review.
Dynamic changes in kinase activities in the oocyte
Meiosis of oocytes to the metaphase II stage is dependent on the activation of maturation‐promoting factor (MPF), which is composed of p34cdc2 kinase (CDK1) and cyclin B [4, 5, 6]. The activation of MPF disappears the nuclear membrane and condenses chromosomes to induce meiotic resumption in amphibian oocytes [7, 8] and mammalian oocytes [9, 10, 11]. Association with cyclin B is required for activation of CDK1, as well as for dephosphorylation of Thr 14 and Tyr 15 residues [12, 13, 14]. The phosphorylation status of these residues is regulated by the activity of two key enzymes: a Wee1 kinase and Cdc25 phosphatase [13, 14, 15, 16, 17].
Wee1B, which is a member of the Wee1 kinase family, is selectively expressed in oocytes [18]. Phosphorylation of Wee1B by PKA enhances its kinase enzyme activity, which phosphorylates CDK1 in oocytes [18]. Thus, the cAMP‐PKA pathway phosphorylates Wee1B to suppress CDK1 activity in order to keep meiotic arrest at the GV stage in mouse oocytes. It has been shown that three Cdc25 families, Cdc25A, Cdc25B and Cdc25C, are expressed in mouse oocytes, whereas only Cdc25B is essential for the induction of meiosis in mice [19]. Interestingly, Cdc25B is also phosphorylated by PKA, and its phosphorylation decreases the enzyme activity of Cdc25B, suggesting that the elevation of cAMP levels activates Wee1B, but decreases Cdc25B activity to markedly increase the phosphorylated inactive form of CDK1 in oocytes arrested at the GV stage (Fig. 1a) [20, 21]. The phosphorylated (active) form of Wee1B is localized in germinal vesicles, whereas the phosphorylated Cdc25B (inactive form) is localized in the cytoplasm. The decrease in cAMP level is a turning point to change the localization of two key enzymes: dephosphorylated Wee1B (inactive form) moves to the cytoplasm, and dephosphorylated Cdc25B (active form) enters the nucleus from the cytoplasm (Fig. 1b).
Figure 1.
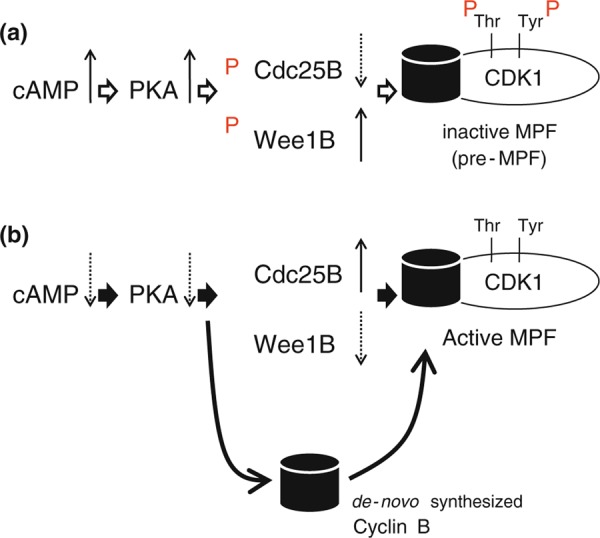
The regulation of MPF activation by cAMP–PKA dependent manner
In pig oocytes, the activation of CDK1 is induced concomitantly with the reduction in cAMP level [22, 23]. To examine the role of decreasing cAMP level in oocytes before GVBD, the cAMP level and CDK1 activity were analyzed in the oocyte after 28 h of culture in the presence of various concentrations of a phosphodiesterase inhibitor, IBMX. Addition of IBMX to the maturation medium produced a significant increase in the cAMP content of oocytes and a significant decline in the proportion of oocytes exhibiting GVBD and CDK1 activity; both results were observed to be dose‐dependent. Thus, a fall in cAMP level is required for activation of CDK1 and then induces meiotic resumption in porcine oocytes, as well as in mouse oocytes. Additionally, Wee1B and Cdc25C are expressed in porcine oocytes and play important roles in the regulation of meiotic resumption [24]. When denuded oocytes were cultured, activation of CDK1 was detected at a much earlier time point as compared with oocytes of COCs [25]. Therefore, cumulus cells regulate oocyte meiotic resumption via a cAMP‐dependent mechanism.
Regulation of the cAMP level in oocytes
A recent mutant mice model showed that regulation of the cAMP level in oocytes is dependent on a G protein‐coupling receptor family member, GPR3/GPR12 [26, 27]. In Gpr3 mutant mice, oocyte meiotic resumption was spontaneously exhibited before ovulation stimuli in small antral follicles [26]. Although the endogenous ligands for GPR3 remain unclear, it has been reported that sphingosine 1 phosphate (S1P) is potentially acted as a ligand for GPR3 [27]. When S1P was added to the medium, the cAMP level was increased in oocytes and spontaneous meiotic resumption was suppressed in mice, suggesting that the mouse oocyte produces cAMP to prevent meiotic resumption before ovulation stimuli [27].
Although the oocyte produces cAMP, regulation of the cAMP level in oocytes is dependent on cumulus cells. When oocytes were removed from cumulus cell layers and the denuded oocytes were cultured, the level of cAMP was quickly decreased [28, 29, 30]. Recently, Dr. Eppig and collaborators clearly showed that granulosa cells expressed and secreted natriuretic peptide precursor type C (NPPC) and acted on cumulus cells to produce cGMP [31]. In human, follicular fluid contains NPPC in preovulatory follicles, and its level is dramatically decreased after hCG stimulation [32]. cGMP is transferred to oocytes via gap junctional communication. One of the functions of cGMP is to decrease PDE3 activity, which increases the level of cAMP in oocytes [33]. The mechanisms are shown in Fig. 2.
Figure 2.
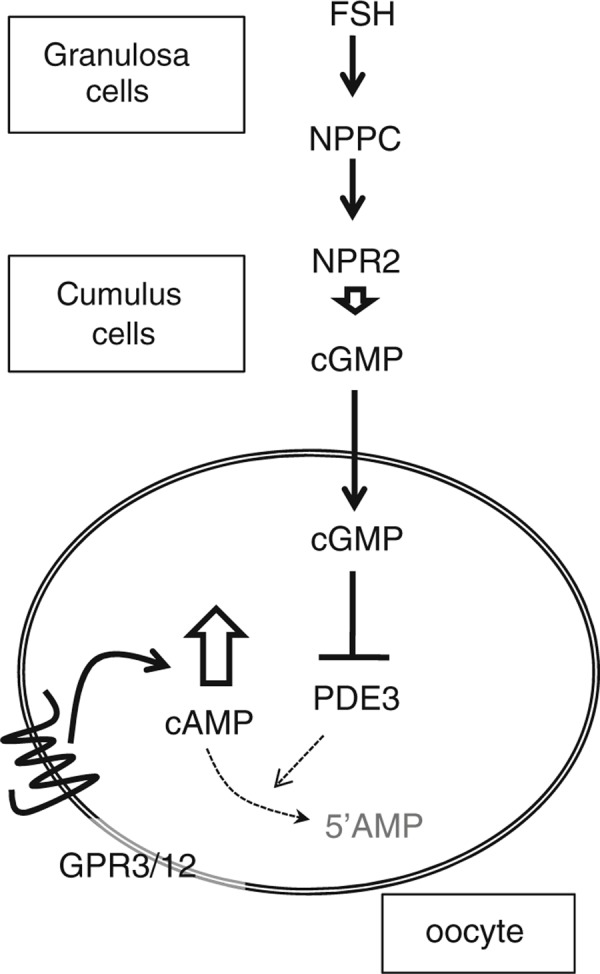
Model describing the expression and roles of natriuretic peptide precursor type C (NPPC) and its receptor, NPR2 in the maintenance of oocyte meiotic arrest at the GV stage in mice, NPPC is secreted from FSH‐stimulated granulosa cells, and then acts on cumulus cells to stimulate cGMP production. The cGMP is transferred to oocyte via gap junctional communication, which suppresses PDE3 activity that converts cAMP to 5′AMP. The cAMP is produced in oocyte via GPR3/12 dependent manner. The pathways increases cAMP level in oocyte during follicular development stage
Close of gap junctional communication
How is the level of cAMP decreased in oocytes during meiotic maturation? In oocytes, cAMP is changed to 5′AMP before oocytes begin exhibiting meiotic resumption, as described above. In amphibian oocytes, progesterone or insulin‐like growth factor 1 (IGF1) activates the PI 3‐kinase–PKB pathway that phosphorylates phosphodiesterase type III (PDE3) to increase enzyme activity [34, 35, 36]. Activated PDE3 changes cAMP to 5′AMP (Fig. 2). The PI 3‐kinase–PKB pathway is also activated in mouse oocytes before meiotic resumption [37]. This signaling pathway potentially upregulates PDE3 enzyme activity in oocytes; however, PI 3‐kinase inhibitors do not block meiotic resumption of mouse oocytes [38]. These results suggest that, although the PI 3‐kinase–PKB pathway is involved in the cAMP degradation pathway, this regulation is not the primary switch for oocyte maturation but may work as an amplifying loop to decrease cAMP levels during oocyte meiotic resumption in mammalians. Other initial factors are required for the initial reduction of cAMP level and the induction of oocyte meiotic resumption.
The other possibility is that the amount of cAMP and/or cGMP transferred from cumulus cells to oocytes is decreased. It has been reported that disruption of gap junctions within cumulus cells induces the meiotic resumption of mouse, rat and porcine oocytes due to blockage of the conduction of meiosis inhibitory signals from the outer layers of cumulus cells to oocytes [39, 40, 41, 42, 43]. Gap junctions, the specialized regions in opposite membranes between neighboring cells, are channels that pass low‐molecular‐weight substances and ions in order to enhance cellular interactions [44]. These channels are formed by hexametric structures consisting of connexin molecules (connexon) in numerous tissues [44]. Porcine ovarian follicles have been reported to express five members of the connexin gene family: connexin‐26, connexin‐30.3, connexin‐32, connexin‐43, and connexin‐60 [45, 46]. The connexin‐43 (Cx‐43) protein is dominantly expressed in cumulus cells of porcine COCs [47, 48, 49, 50]. Cx‐43 is also expressed in bovine cumulus cells of COCs, and Cx‐37 selectively consists of gap junctional communications between cumulus cells and oocytes [51].
ERK1/2 is required for the close of gap junctional communication in cumulus cells
Although the ERK1/2 pathway in oocytes is not essential for the resumption of meiosis, when COCs or intact follicles are cultured in vitro a MEK inhibitor significantly suppresses this process, presumably by blocking the activity or ERK1/2 in cumulus cells and granulosa cells [24, 52]. Moreover, EGF‐like factors can induce oocytes to resume meiosis and reach metaphase II stage, indicating that the activated EGF receptor pathway in cumulus cells is required for the resumption of meiosis [53, 54]. Collectively, these results indicate that the EGF‐like factor/EGFR/ERK1/2 pathway in cumulus cells is critical for oocyte maturation. This hypothesis has been supported recently by observation of a total block of oocyte maturation in mutant mice lacking ERK1/2 in somatic cumulus/granulosa cells but not in the oocyte [55].
One of the targets of ERK1/2 in cumulus cells is Cx‐43. Cx‐43 has numerous phosphorylated sites, and phosphorylation of these residues plays a key role in regulatory mechanisms governing the assembly of connexons into gap junctions in the plasma membrane, and gating the gap junction that is formed [56, 57, 58]. In particular, phosphorylation of serine on Cx‐43 is stimulated by ERK1/2 in rat liver cells [57, 59]. In fact, the phosphorylation of Cx‐43 is induced in an ERK1/2‐dependent manner in mice and rat COCs in culture [60], which results in a reduction of Cx‐43 and then the close of gap junctional communication among the cumulus cells [61]. In porcine COCs, at least three Cx‐43‐positive bands were detected by immunoblotting, and the upper bands disappeared after treatment with phosphatase, indicating that Cx‐43 is phosphorylated in cumulus cells of porcine COCs [47]. Time‐dependent changes were examined when porcine COCs were cultured with FSH up to 28 h. The staining intensities of the fast (43 kDa) and moderate (45 kDa) migrating bands were significantly increased at the 8‐h culture point as compared with those of cumulus cells separated from COCs immediately after collection [47]. However, after culture for 16 h and up to 28 h, significant reductions in the intensity of the 43‐kDa of Cx‐43 band were noted. In contrast, the intensity of the phosphorylated forms of 45‐ and 47‐kDa Cx‐43 were increased at 16 h and up to 28 h. Thus, within the first 4 h of culture, a large amount of Cx‐43 was synthesized in all layers of the cumulus cells, while after 16 h of culture Cx‐43 expression disappeared in cumulus cells, with increasing levels of phosphorylated Cx‐43.
In conclusion, the transfer from cumulus cells to oocytes is shut down after ovulation stimuli via ERK1/2‐induced phosphorylation of Cx‐43, because gap junctional communication is closed by this phosphorylation. The reduction in cGMP induces the activation of PDE3 to degenerate cAMP produced in a GPR3‐dependent manner and/or transferred from cumulus cells, and the pathways are essential for exhibiting meiotic resumption (Fig. 3).
Figure 3.
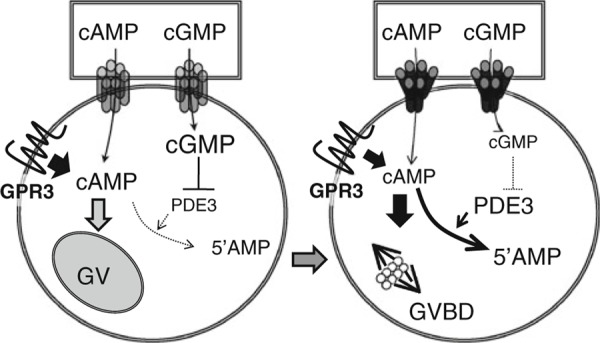
A schematic diagram with regard to the induction of meiotic resumption of porcine COCs. The close of gap junctional communication reduces the amount of cAMP and cGMP transferred from cumulus cells to oocyte, which results in the induction of meiotic resumption of oocyte
How to activate the ERK1/2 pathway in cumulus cells
In mice, the EGFR‐RAS pathway is a key signaling pathway to induce the phosphorylation of ERK1/2 in cumulus cells [62, 63]. The EGF receptor (EGFR, ERBB1) is a member of the EGF receptor superfamily that is expressed in cumulus cells but not in oocytes and, based on a specific receptor tyrosine kinase inhibitor, is known to affect oocyte maturation in LH‐stimulated preovulatory follicle cultures [53, 64, 65]. Specifically, the EGF‐like factors amphiregulin (Areg), betacellulin (Btc) and epiregulin (Ereg) are transiently expressed after LH stimulation in granulosa cells and act by binding to ERBB1 expressed on cumulus cells [53, 66, 67]. We also showed the expression of EGF‐like factors in cumulus cells during the ovulation process [67]. Because cumulus cells have low or undetectable levels of LH receptor [1], other stimulatory factors are required to induce the expression of EGF‐like factors in cumulus cells. In the mouse Areg gene promoter region, a putative cAMP‐responsive element (CRE) site is observed [68, 69]. Mutation of this region decreases the promoter activity in a luciferase promoter assay using primary culture of mouse granulosa cells, and the CRE sequence binds to phosphorylated CRE‐binding protein (CREB) at 2 h after LH stimulation [69]. The expression of Areg is directly regulated by the cAMP–PKA–CREB cascade in granulosa cells and cumulus cells during the ovulation process. It has been well known that cumulus cells express the G protein‐coupled receptor subtypes EP2 (PTGER2) or EP4 (PTGER4), which, when activated, stimulate adenylate cyclase to produce cAMP [70]. EP2 and EP4 are receptors for prostaglandin E2 (PGE2), which is converted from arachidonic acid by the rate‐limiting enzyme PTGS2 [71, 72, 73]. Using an in vivo approach, we documented that Areg and Ereg expression levels were markedly reduced in COCs and granulosa cells of Ptgs2‐null mice [67]. Thus, the initial induction of EGF‐like factor expression is directly induced by LH via the cAMP–PKA–CREB pathway, and expression is maintained in a PGE2 production‐dependent manner (Fig. 4).
Figure 4.
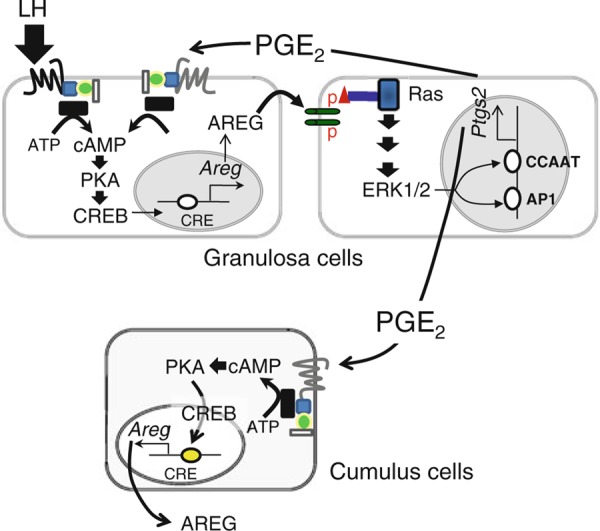
Schematic showing potential paracrine and autocrine pathways by which expression of amphioregulin (Areg) Ptgs2 expression is regulated in granulosa cells and cumulus cells of ovulating follicles. The proposed sequence is as follows: (1) LH binds to its cognate receptor localized to granulosa cells stimulating cAMP production to activate PKA–CREB pathway. (2) As a consequence, Areg mRNAs are induced rapidly providing ligand that (3) bind EGF‐receptors on granulosa cells (autocrine) as well as cumulus cells (paracrine) leading to activation of ERK1/2 and induced expression of Ptgs2 in both cell types. (4) Increased production of prostaglandins (PGs; PGE) then provides ligands that bind EP2 on granulosa cells and cumulus cells (paracrine and autocrine) that (like the FSH and LH receptors) activate cAMP–PKA–CREB pathway. (5) By the PGE/EP2 pathway, Areg mRNA can be induced in both cell types
Neuregulin is a novel inducer of oocyte developmental competence
In our microarray analysis of rat COCs, we found the expression of neuregulin (NRG1), another EGF‐like factor that acted on ErbB2/3 hetero‐dimers expressed in cumulus cells. Activation of the hetero‐dimer was also observed in cumulus cells and granulosa cells during the oocyte maturation (ovulation) process [74]. The murine Nrg1 gene has three promoter and transcriptional start sites [75], and the type I and type III sites are expressed in granulosa cells during ovulation [74]. Expression of type III Nrg1 was dramatically increased in granulosa cells by hCG, whereas that of type I was not changed. The type III Nrg1 promoter region has three putative C/EBP binding sites and a CRE site. Based on the transfection of specific promoter‐reporter constructs in granulosa cells, the most distal C/EBP‐binding site appears to play a critical role in the increase in promoter activity after stimulation. Because we have shown recently that the ERK1/2–C/EBPb pathway is essential for inducing cell fate decisions in granulosa cells and cumulus cells of preovulatory follicles [50], it is clear that Nrg1 expression is also dependent on this pathway.
To clarify the roles of NRG1 in oocyte maturation, we carried out in vitro maturation of COCs with or without NRG1. When mouse COCs were cultured with NRG1, spontaneous meiotic resumption of oocytes was suppressed. The induction of meiosis initiated by AREG was also delayed by NRG1 in the presence of 4 mM hypoxanthine. The matured oocyte cultured with both NRG1 and AREG had significantly higher developmental competence as compared with oocytes cultured with AREG alone. Thus, the AREG–EGFR–ERK1/2 pathway accelerates meiotic resumption, but it controls timing via activation of the NRG1–ErbB2/3–PKB pathway that works as a brake, and both pathways are required to regulate the timing of meiotic progression that has an impact on oocyte developmental competence (Fig. 5).
Figure 5.
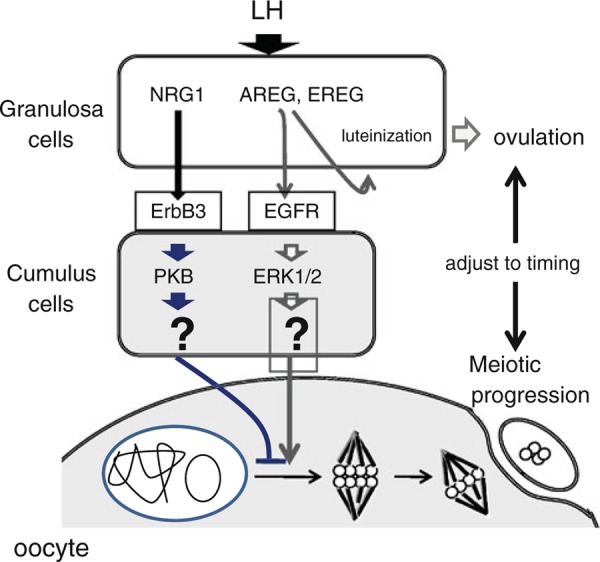
Schematic showing the roles of AREG that acts on EGFR and NRG1 that binds to ErbB3 to increase ERK1/2 or PKB pathway, respectively, in the regulation of meiotic progression in mice ovulating follicles
Conclusion
In preovulatory follicles, oocytes are surrounded by numerous layers of cumulus cells, which form the cumulus cell–oocyte complex (COC). Although after stimulation of ovulation by an LH surge the oocyte resumes meiosis by progressing to metaphase II, expression of LH receptors is not detected in the oocyte and is minimal (negligible) in cumulus cells as compared with granulosa cells. However, cumulus cells express members of the EGF receptor family (ErbB family) that respond to specific ligands, and members of the EGF‐like factor family are secreted by granulosa cells during the ovulation process. Via these intermediary steps, cumulus cells mediate LH signaling from granulosa cells to induce oocyte meiotic resumption. One of the key signaling pathways in cumulus cells is the EGFR–ERK1/2 pathway that regulates gap‐junctional communication among cumulus cells and between cumulus cells and the oocyte. Closing gap‐junctional communication by ERK1/2 decreases the level of cGMP and/or cAMP to transfer into the oocyte, which requires activation of PDE3 in the oocyte. PDE3 brakes down cAMP to decrease PKA activity in the oocyte. Because the PKA pathway activates Wee1B and downregulates both Cdc25C activity and cyclin B synthesis to decrease CDK1 activity, the decrease in PKA activity caused by PDE3 induces activation of CDK1 to resume meiosis from the GV stage. Thus, control of the cAMP level in oocytes by cumulus cells is essential for oocyte meiotic resumption.
Acknowledgments
Dr. JoAnne S. Richards, Dr. Yasuhisa Yamashita, and Dr. Ikko Kawashima have provided outstanding assistance and good suggestions. This work was supported in part by grants‐in‐aid for Scientific Research Nos. 21688019, 21028015 and 21248032 from the Japan Society for the Promotion of Science (JSPS).
Reference
- 1. Peng XR, Hsueh AJ, LaPolt PS, Bjersing L, Ny T. Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology, 1991, 129, 3200–3207 10.1210/endo‐129‐6‐3200 [DOI] [PubMed] [Google Scholar]
- 2. Richards JS. Hormonal control of gene expression in the ovary. Endocr Rev, 1994, 15, 725–751 [DOI] [PubMed] [Google Scholar]
- 3. Cross PC, Brinster RL. In vitro development of mouse oocytes. Biol Reprod, 1970, 3, 298–307 [DOI] [PubMed] [Google Scholar]
- 4. Masui Y, Markert CL. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool, 1971, 177, 129–146 10.1002/jez.1401770202 [DOI] [PubMed] [Google Scholar]
- 5. Lohka M, Hayes MK, Maller J. Purification of maturation‐promoting factor, an intercellular regulator of early events. Proc Natl Acad Sci USA, 1988, 85, 3009–3013 10.1073/pnas.85.9.3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dunphy WG, Brizuela L, Beach D, Newport J. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell, 1988, 54, 423–431 10.1016/0092‐8674(88)90205‐X [DOI] [PubMed] [Google Scholar]
- 7. Swenson KI, Farrell KM, Ruderman JV. The clam embryo protein cyclin A induces entry into M phase and the resumption of meiosis in Xenopus oocytes . Cell, 1986, 47, 861–870 10.1016/0092‐8674(86)90801‐9 [DOI] [PubMed] [Google Scholar]
- 8. Gautier J, Minshull J, Lohka M, Grotzer M, Hunt T, Maller JL. Cyclin is a component of maturation‐promoting factor from Xenopus. Cell, 1990, 60, 487–494 10.1016/0092‐8674(90)90599‐A [DOI] [PubMed] [Google Scholar]
- 9.Choi T, Aoki F, Mori M, Yamashita M, Nagahama Y, Kohmoto K. Activation of p34cdc2 protein kinase activity in meiotic and mitotic cell cycles in mouse oocytes and embryos. Development. 1991;113:789–95. [DOI] [PubMed]
- 10. Naito K, Hawkins C, Yamashita M, Nagahama Y, Aoki F, Kohmoto K, Toyoda Y, Moor RM. Association of p34cdc2 and cyclin B1 during meiotic maturation in porcine oocytes. Dev Biol, 1995, 168, 627–634 10.1006/dbio.1995.1107 [DOI] [PubMed] [Google Scholar]
- 11. Tatemoto H, Horiuchi T. Requirement for protein synthesis during the onset of meiosis in bovine oocytes and its involvement in maturation‐promoting factor. Mol Reprod Dev, 1995, 41, 47–53 10.1002/mrd.1080410108 [DOI] [PubMed] [Google Scholar]
- 12. Bondt HL, Rosenblatt J, Jancarik J, Jones HD, Morgan DO, Kim SH. Crystal structure of cyclin‐dependent kinase 2. Nature, 1993, 363, 595–602 10.1038/363595a0 [DOI] [PubMed] [Google Scholar]
- 13. Kumagai A, Dunphy WG. Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell, 1992, 70, 139–151 10.1016/0092‐8674(92)90540‐S [DOI] [PubMed] [Google Scholar]
- 14. Nebreda AR, Gannon JV, Hunt T. Newly synthesized protein(s) must associate with p34cdc2 to activate MAP kinase and MPF during progesterone‐induced maturation of Xenopus oocytes . EMBO J, 1995, 14, 5597–5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morgan DO. Principles of CDK regulation. Nature, 1995, 374, 131–134 10.1038/374131a0 [DOI] [PubMed] [Google Scholar]
- 16. Okamoto K, Nakajo N, Sagata N. The existence of two distinct Wee1 isoforms in Xenopus: implications for the developmental regulation of the cell cycle. EMBO J, 2002, 21, 2472–2484 10.1093/emboj/21.10.2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leise W 3rd, Mueller PR. Multiple Cdk1 inhibitory kinases regulate the cell cycle during development. Dev Biol, 2002, 249, 156–173 10.1006/dbio.2002.0743 [DOI] [PubMed] [Google Scholar]
- 18. Han SJ, Chen R, Paronetto MP, Conti M. Wee1B is an oocyte‐specific kinase involved in the control of meiotic arrest in the mouse. Curr Biol, 2005, 15, 1670–1676 10.1016/j.cub.2005.07.056 [DOI] [PubMed] [Google Scholar]
- 19. Oh JS, Han SJ, Conti M. Wee1B, Myt1, and Cdc25 function in distinct compartments of the mouse oocyte to control meiotic resumption. J Cell Biol, 2010, 188, 199–207 10.1083/jcb.200907161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu S, Wolgemuth DJ. The distinct and developmentally regulated patterns of expression of members of the mouse Cdc25 gene family suggest differential functions during gametogenesis. Dev Biol, 1995, 170, 195–206 10.1006/dbio.1995.1207 [DOI] [PubMed] [Google Scholar]
- 21. Han SJ, Conti M. New pathways from PKA to the Cdc2/cyclin B complex in oocytes: Wee1B as a potential PKA substrate. Cell Cycle, 2006, 5, 227–231 10.4161/cc.5.3.2395 [DOI] [PubMed] [Google Scholar]
- 22. Shimada M, Terada T. Roles of cAMP in regulation of both MAP kinase and p34(cdc2) kinase activity during meiotic progression, especially beyond the MI stage. Mol Reprod Dev, 2002, 62, 124–131 10.1002/mrd.10075 [DOI] [PubMed] [Google Scholar]
- 23. Shimada M, Nishibori T, Isobe N, Kawano N, Terada T. Luteinizing hormone receptor formation in cumulus cells surrounding porcine oocytes and its role during meiotic maturation of porcine oocytes. Biol Reprod, 2003, 68, 1142–1149 10.1095/biolreprod.102.010082 [DOI] [PubMed] [Google Scholar]
- 24. Shimaoka T, Nishimura T, Kano K, Naito K. Critical effect of pigWee1B on the regulation of meiotic resumption in porcine immature oocytes. Cell Cycle, 2009, 8, 2375–2384 10.4161/cc.8.15.9073 [DOI] [PubMed] [Google Scholar]
- 25. Shimada M, Zeng WX, Terada T. Inhibition of phosphatidylinositol 3‐kinase or mitogen‐activated protein kinase kinase leads to suppression of p34(cdc2) kinase activity and meiotic progression beyond the meiosis I stage in porcine oocytes surrounded with cumulus cells. Biol Reprod, 2001, 65, 442–448 10.1095/biolreprod65.2.442 [DOI] [PubMed] [Google Scholar]
- 26. Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ, Jaffe LA. The Gs‐linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science, 2004, 306, 1947–1950 10.1126/science.1103974 [DOI] [PubMed] [Google Scholar]
- 27. Hinckley M, Vaccari S, Horner K, Chen R, Conti M. The G‐protein‐coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev Biol, 2005, 287, 249–261 10.1016/j.ydbio.2005.08.019 [DOI] [PubMed] [Google Scholar]
- 28. Mattioli M, Galeati G, Barboni B, Seren E. Concentration of cyclic AMP during the maturation of pig oocytes in vivo and in vitro. J Reprod Fertil, 1994, 100, 403–409 10.1530/jrf.0.1000403 [DOI] [PubMed] [Google Scholar]
- 29. Downs SM, Eppig JJ. Cyclic adenosine monophosphate and ovarian follicular fluid act synergistically to inhibit mouse oocyte maturation. Endocrinology, 1984, 114, 418–427 10.1210/endo‐114‐2‐418 [DOI] [PubMed] [Google Scholar]
- 30. Downs SM, Coleman DL, Eppig JJ. Maintenance of murine oocyte meiotic arrest: uptake and metabolism of hypoxanthine and adenosine by cumulus cell‐enclosed and denuded oocytes. Dev Biol, 1986, 117, 174–183 10.1016/0012‐1606(86)90359‐3 [DOI] [PubMed] [Google Scholar]
- 31. Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science, 2010, 330, 366–369 10.1126/science.1193573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kawamura K, Cheng Y, Kawamura N, Takae S, Kawagoe Y, Mulders S, Terada Y, Hsueh AJ. Pre‐ovulatory LH/hCG surge decreases C‐type natriuretic peptide secretion by ovarian granulosa cells to promote meiotic resumption of pre‐ovulatory oocytes. Hum Reprod, 2011, 26, 3094–3101 10.1093/humrep/der282 [DOI] [PubMed] [Google Scholar]
- 33. Richard FJ, Tsafriri A, Conti M. Role of phosphodiesterase type 3A in rat oocyte maturation. Biol Reprod, 2001, 65, 1444–1451 10.1095/biolreprod65.5.1444 [DOI] [PubMed] [Google Scholar]
- 34. Chuang LM, Myers MG Jr, Backer JM, Shoelson SE, White MF, Birnbaum MJ, Kahn CR. Insulin‐stimulated oocyte maturation requires insulin receptor substrate 1 and interaction with the SH2 domains of phosphatidylinositol 3‐kinase. Mol Cell Biol, 1993, 13, 6653–6660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Muslin AJ, Klippel A, Williams LT. Phosphatidylinositol 3‐kinase activity is important for progesterone‐induced Xenopus oocyte maturation. Mol Cell Biol, 1993, 13, 6661–6666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Conti M, Andersen CB, Richard F, Mehats C, Chun SY, Horner K, Jin C, Tsafriri A. Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol, 2002, 187, 153–159 10.1016/S0303‐7207(01)00686‐4 [DOI] [PubMed] [Google Scholar]
- 37. Han SJ, Vaccari S, Nedachi T, Andersen CB, Kovacina KS, Roth RA, Conti M. Protein kinase B/Akt phosphorylation of PDE3A and its role in mammalian oocyte maturation. EMBO J, 2006, 25, 5716–5725 10.1038/sj.emboj.7601431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoshino Y, Yokoo M, Yoshida N, Sasada H, Matsumoto H, Sato E. Phosphatidylinositol 3‐kinase and Akt participate in the FSH‐induced meiotic maturation of mouse oocytes. Mol Reprod Dev, 2004, 69, 77–86 10.1002/mrd.20150 [DOI] [PubMed] [Google Scholar]
- 39. Larsen WJ, Wert SE, Brunner GD. A dramatic loss of cumulus cell gap junctions is correlated with germinal vesicle breakdown in rat oocytes. Dev Biol, 1986, 113, 517–521 10.1016/0012‐1606(86)90187‐9 [DOI] [PubMed] [Google Scholar]
- 40. Larsen WJ, Wert SE, Brunner GD. Differential modulation of rat follicle cell gap junction populations at ovulation. Dev Biol, 1987, 122, 61–71 10.1016/0012‐1606(87)90332‐0 [DOI] [PubMed] [Google Scholar]
- 41. Isobe N, Maeda T, Terada T. Involvement of meiotic resumption in the disruption of gap junctions between cumulus cells attached to pig oocytes. J Reprod Fertil, 1998, 113, 167–172 10.1530/jrf.0.1130167 [DOI] [PubMed] [Google Scholar]
- 42. Isobe N, Terada T. Effect of the factor inhibiting germinal vesicle breakdown on the disruption of gap junctions and cumulus expansion of pig cumulus–oocyte complexes cultured in vitro. Reproduction, 2001, 121, 249–257 10.1530/rep.0.1210249 [PubMed] [Google Scholar]
- 43. Sasseville M, Gagnon MC, Guillemette C, Sullivan R, Gilchrist RB, Richard FJ. Regulation of gap junctions in porcine cumulus–oocyte complexes: contributions of granulosa cell contact, gonadotropins, and lipid rafts. Mol Endocrinol, 2009, 23, 700–710 10.1210/me.2008‐0320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grazul‐Bilska AT, Reynolds LP, Redmer DA. Gap junctions in the ovaries. Biol Reprod, 1997, 57, 947–957 10.1095/biolreprod57.5.947 [DOI] [PubMed] [Google Scholar]
- 45. Itahana K, Morikazu Y, Takeya T. Differential expression of four connexin genes, Cx‐26, Cx‐30.3, Cx‐32, and Cx‐43, in the porcine ovarian follicle. Endocrinology, 1996, 137, 5036–5044 10.1210/en.137.11.5036 [DOI] [PubMed] [Google Scholar]
- 46. Itahana K, Tanaka T, Morikazu Y, Komatu S, Ishida N, Takeya T. Isolation and characterization of a novel connexin gene, Cx‐60, in porcine ovarian follicles. Endocrinology, 1998, 139, 320–329 10.1210/en.139.1.320 [DOI] [PubMed] [Google Scholar]
- 47. Shimada M, Maeda T, Terada T. Dynamic changes of connexin‐43, gap junctional protein, in outer layers of cumulus cells are regulated by PKC and PI 3‐kinase during meiotic resumption in porcine oocytes. Biol Reprod, 2001, 64, 1255–1263 10.1095/biolreprod64.4.1255 [DOI] [PubMed] [Google Scholar]
- 48. Shimada M, Nishibori M, Yamashita Y, Ito J, Mori T, Richards JS. Down‐regulated expression of A disintegrin and metalloproteinase with thrombospondin‐like repeats‐1 by progesterone receptor antagonist is associated with impaired expansion of porcine cumulus–oocyte complexes. Endocrinology, 2004, 145, 4603–4614 10.1210/en.2004‐0542 [DOI] [PubMed] [Google Scholar]
- 49. Shimada M, Terada T. Phosphatidylinositol 3‐kinase in cumulus cells and oocytes is responsible for activation of oocyte mitogen‐activated protein kinase during meiotic progression beyond the meiosis I stage in pigs. Biol Reprod, 2001, 64, 1106–1114 10.1095/biolreprod64.4.1106 [DOI] [PubMed] [Google Scholar]
- 50.Shimada M, Terada T. FSH and LH induce progesterone production and progesterone receptor synthesis in cumulus cells: a requirement for meiotic resumption in porcine oocytes. Mol Hum Reprod. 2002;8:612–8. [DOI] [PubMed]
- 51. Nuttinck F, Peynot N, Humblot P, Massip A, Dessy F, Fléchon JE. Comparative immunohistochemical distribution of connexin 37 and connexin 43 throughout folliculogenesis in the bovine ovary. Mol Reprod Dev, 2002, 57, 60–66 10.1002/1098‐2795(200009)57:1<60::AID‐MRD9>3.0.CO;2‐6 [DOI] [PubMed] [Google Scholar]
- 52. Su YQ, Wigglesworth K, Pendola FL, O'Brien MJ, Eppig JJ. Mitogen‐activated protein kinase activity in cumulus cells is essential for gonadotropin‐induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology, 2002, 143, 2221–2232 10.1210/en.143.6.2221 [DOI] [PubMed] [Google Scholar]
- 53. Park JY, Su YQ, Ariga M, Law F, Jin SL, Conti M. EGF‐like growth factors as mediators of LH action in the ovulatory follicle. Science, 2004, 303, 682–684 10.1126/science.1092463 [DOI] [PubMed] [Google Scholar]
- 54. Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology, 2005, 146, 77–84 10.1210/en.2004‐0588 [DOI] [PubMed] [Google Scholar]
- 55. Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science, 2009, 324, 938–941 10.1126/science.1171396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Musil LS, Cunningham BA, Edelman GM, Goodenough DA. Differential phosphorylation of the gap junction protein connexin 43 in junctional communication‐competent and ‐deficient cell lines. J Cell Biol, 1990, 111, 2077–2088 10.1083/jcb.111.5.2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hill CS, Oh SY, Schmidt SA, Clark KJ, Murray AW. Lysophosphatidic acid inhibits gap‐junctional communication and stimulates phosphorylation of connexin‐43 in WB cells: possible involvement of the mitogen‐activated protein kinase cascade. Biochem J, 1994, 303, 475–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lau AF, Kurata WE, Kanemitsu MY, Loo LW, Warn‐Cramer BJ, Eckhart W, Lampe PD. Regulation of connexin 43 function by activated tyrosine protein kinases. J Bioenerg Biomembr, 1996, 28, 359–367 10.1007/BF02110112 [DOI] [PubMed] [Google Scholar]
- 59. Warn‐Cramer BJ, Lampe PD, Kurata WE, Kanemitsu MY, Loo LW, Eckhart W, Lau AF. Characterization of the mitogen‐activated protein kinase phosphorylation sites on the connexin‐43 gap junction protein. J Biol Chem, 1996, 271, 3779–3786 10.1074/jbc.271.7.3779 [DOI] [PubMed] [Google Scholar]
- 60. Sela‐Abramovich S, Chorev E, Galiani D, Dekel N. Mitogen‐activated protein kinase mediates luteinizing hormone‐induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology, 2005, 146, 1236–1244 10.1210/en.2004‐1006 [DOI] [PubMed] [Google Scholar]
- 61. Granot I, Dekel N. Phosphorylation and expression of connexin‐43 ovarian gap junction protein are regulated by luteinizing hormone. J Biol Chem, 1994, 269, 30502–30509 [PubMed] [Google Scholar]
- 62. Fan HY, Shimada M, Liu Z, Cahill N, Noma N, Wu Y, Gossen J, Richards JS. Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development, 2008, 135, 2127–2137 10.1242/dev.020560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fan HY, Richards JS. Minirevew: physiological and pathological actions of RAS in the ovary. Mol Endocrinol, 2010, 24, 286–298 10.1210/me.2009‐0251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hsieh M, Lee D, Panigone S, Horne K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M. Luteinizing hormone‐dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol, 2007, 27, 1914–1924 10.1128/MCB.01919‐06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hsieh M, Thao K, Conti M. Genetic dissection of epidermal growth factor receptor signaling during luteinizing hormone‐induced oocyte maturation. PloS One, 2011, 6, e21574 10.1371/journal.pone.0021574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Espey L, Richards JS. Temporal and spatial patterns of ovarian gene transcription following an ovulatory dose of gonadotropin in the rat. Biol Reprod, 2002, 67, 1662–1670 10.1095/biolreprod.102.005173 [DOI] [PubMed] [Google Scholar]
- 67. Shimada M, Hernandez‐Gonzalez I, Gonzalez‐Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor‐like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol, 2006, 20, 1352–1365 10.1210/me.2005‐0504 [DOI] [PubMed] [Google Scholar]
- 68. Shao J, Evers BM, Sheng H. Prostaglandin E2 synergistically enhances receptor tyrosine kinase‐dependent signaling system in colon cancer cells. J Biol Chem, 2004, 279, 14287–14293 10.1074/jbc.M313276200 [DOI] [PubMed] [Google Scholar]
- 69. Fan HY, O'Connor A, Shitanaka M, Shimada M, Liu Z, Richards JS. Beta‐catenin (CTNNB1) promotes preovulatory follicular development but represses LH‐mediated ovulation and luteinization. Mol Endocrinol, 2010, 24, 1529–1542 10.1210/me.2010‐0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fujino H, Salvi S, Regan JW. Differential regulation of phosphorylation of the cAMP response element‐binding protein after activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. Mol Pharmacol, 2005, 68, 251–259 [DOI] [PubMed] [Google Scholar]
- 71. Sirois J, Simmons DL, Richards JS. Hormonal regulation of messenger ribonucleic acid encoding a novel isoform of prostaglandin endoperoxide H synthase in rat preovulatory follicles. Induction in vivo and in vitro. J Biol Chem, 1992, 267, 11586–11592 [PubMed] [Google Scholar]
- 72. Sirois J, Richards JS. Purification and characterization of a novel, distinct isoform of prostaglandin endoperoxide synthase induced by human chorionic gonadotropin in granulosa cells of rat preovulatory follicles. J Biol Chem, 1992, 267, 6382–6388 [PubMed] [Google Scholar]
- 73. Sirois J, Levy LO, Simmons dL, Richards JS. Characterization and hormonal regulation of the promoter of the rat prostaglandin endoperoxide synthase 2 gene in granulosa cells. Identification of functional and protein‐binding regions. J Biol Chem, 1993, 268, 12199–12206 [PubMed] [Google Scholar]
- 74. Noma N, Kawashima I, Fan HY, Fujita Y, Kawai T, Tomoda Y, Mihara T, Richards JS, Shimada M. LH‐induced neuregulin 1 (NRG1) type III transcripts control granulosa cell differentiation and oocyte maturation. Mol Endocrinol, 2011, 25, 104–116 10.1210/me.2010‐0225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Meyer D, Yamaai T, Garratt A, Riethmacher‐Sonnenberg E, Kane D, Theill LE, Birchmeier C. Isoform‐specific expression and function of neuregulin. Development, 1997, 124, 575–586 [DOI] [PubMed] [Google Scholar]


