Abstract
Purpose
Zona pellucida (ZP)‐free eggs are often used for studies such as evaluating the interaction of sperm‐oolemma. To acquire ZP‐free eggs, the most commonly used methods employ acidified Tyrode's solution, enzymatic digestion with a trypsin‐like enzyme, or mechanical methods using micropipettes. However, acidified Tyrode's solution and trypsin‐like enzymes often damage the oolemma, especially when many eggs are treated at once for mass sample analyses. The mechanical method requires skill, and it is time‐consuming to prepare many ZP‐free eggs. Therefore, in this study, to establish an easy, reliable method for preparing ZP‐free eggs, we examined the ZP digestion method originally reported by Zuccotti et al. (J Reprod Fertil 93:515–520, 1991) that uses collagenase.
Methods
Mouse unfertilized eggs were treated with collagenase and acidified Tyrode's solution to compare the ZP‐free rates, the effect on the oolemma, and the two‐cell development rates of ZP‐free eggs by in vitro fertilization. The effects on the oolemma were gauged by observing the polarity of the transmembrane protein localization of enhanced green fluorescence protein tagged CD9 protein (CD9‐EGFP) and using differential interference contrast microscopy.
Results
Collagenase removed the ZP and the cumulus cells from the cumulus oocyte complex. The collagenase method had no influence on the localization of CD9‐EGFP, resulting in a high two‐cell development rate. Additionally, the collagenase method could exclude low quality eggs with hardened ZP, since collagenase could not digest the hardened ZP.
Conclusions
The one‐step collagenase method is an easy preparation method for large numbers of high‐quality ZP‐free eggs.
Keywords: Collagenase, Fertilization in vitro, Ovum, Reproductive technique, Zona pellucida
Introduction
The zona pellucida (ZP) is an extracellular matrix around mammalian eggs that is involved in important steps such as species‐specific recognition, acrosome reaction, and polyspermy block in fertilization [1, 2, 3]. However, to evaluate the steps of sperm‐oolemma interaction, such as sperm binding and gamete fusion, ZP removal is very important.
Commonly used ZP‐removal methods reported in the literature include mechanical methods, enzymatic methods using a trypsin‐like enzyme, and methods using acidified Tyrode's solution. In general, these methods require removal of cumulus cells by hyaluronidase, which has few deleterious effects. In the mechanical methods, tools such as small bore pipettes [4], microdissection scissors [5] or a piezoelectric driven micromanipulator [6] are used to shave or cut the ZP. Mechanical methods are thought to cause less damage to the oocyte, but it is time consuming to prepare a large number of eggs this way. Enzymatic methods often use α‐chymotrypsin, pronase, or trypsin [7, 8, 9]. Enzymatic methods using such trypsin‐like enzymes are able to treat large numbers of eggs at once, but the excess treatment greatly reduces the sperm‐egg fusion rate [4, 10, 11, 12]. There are two acidic buffer methods; one is to immerse the eggs in the acidified Tyrode's solution, and the other is to slowly blow acidified Tyrode's solution on the ZP in neutral buffer. With the former method, the execution of procedures is important, requiring considerable skill in preparing the large number of zona‐free eggs, and it often requires recovery incubation since the oolemma becomes fragile, which affects the distribution of membrane proteins [5]. The latter method causes less damage since it exposes only the ZP to the acidic pH, but this method requires very careful handling.
During the study in which we needed to prepare large numbers of ZP‐free eggs, we found that collagenase was very effective in treating the eggs, especially for oolemma, yet its effect on oocytes was mild. ZP removal using collagenase is not commonly used today, although Zuccotti et al. [13] reported the effectiveness nearly 20 years ago.
Based on this knowledge, we aimed to establish an easy, reliable method for ZP‐free egg preparation and to evaluate its advantages, especially when we need to process large numbers of eggs.
Materials and methods
Animals
Female ICR mice (8 weeks old) and male ICR mice (16 weeks old) were purchased from Takasugi Experimental Animal Supply Co., Ltd. (Saitama, Japan). Transgenic mice expressing ZP3 promoter CD9‐EGFP were provided by Dr. Kenji Miyado [14]. Animals were kept in an air‐conditioned room (12 h light/dark cycle, 25°C) with free access to food and water. Studies were conducted according to the guidelines for the care and use of laboratory animals of the Chiba University Graduate School of Medicine.
Chemicals
Pregnant mare serum gonadotropin (PMSG) and human chorionic gonadotropin (hCG) were purchased from ASKA Pharmaceutical Co., Ltd. (Tokyo, Japan). Hyaluronidase and Hoechst 33342 were purchased from Sigma‐Aldrich (St. Louis, MO). Collagenase was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Egg collection (for young and aged eggs)
Female mice were hormonally treated to induce eggs by intraperitoneal injection of PMSG (5 IU) followed by hCG (5 IU) 48 h later. Eggs were collected in TYH‐HEPES (Table 1) from oviducts at 13–15 h after hCG injection, which were referred to as young eggs. Eggs collected from oviducts at 26 h after hCG injection were referred to as post‐ovulatory aged eggs [15, 16, 17]. Cumulus cells were removed by treatment with 5 min incubation in TYH‐HEPES containing 0.05% hyaluronidase. Cumulus cell‐free eggs were washed twice in TYH‐HEPES and used in the experiment described below.
Table 1.
TYH‐HEPES medium formulation
| Component | Concentration (g/l) |
|---|---|
| NaCl | 6.976 |
| KCl | 0.356 |
| CaCl22H2O | 0.251 |
| KH2PO4 | 0.162 |
| MgSO47H2O | 0.293 |
| NaHCO3 | 0.427 |
| Na pyruvate | 0.055 |
| Glucose | 1.000 |
| Streptomycin | 0.050 |
| Penicillin | 0.060 |
| Bovine serum albumin | 4.000 |
| Hepes (pH7.4) | 4.766 |
| NaOH | 0.468 |
ZP removal of cumulus‐free eggs
Cumulus cell‐free eggs were transferred to TYH‐HEPES medium containing 0.05, 0.1, 0.2, or 1 mg/ml collagenase (250 IU/mg) and incubated for 5–10 min. ZP‐free eggs were washed twice in TYH‐HEPES.
One‐step collagenase zona‐removal method
Cumulus‐oocyte complexes were collected from the oviducts of superovulated females at 13–15 h after hCG injection. Cumulus‐oocyte complexes were rinsed once in fresh TYH‐HEPES and transferred to TYH‐HEPES containing 1 mg/ml collagenase. After 5–10 min incubation, eggs were removed from loosened cumulus cells by gentle pipetting. Zona‐free eggs were transferred to fresh medium twice to remove the enzymes. From the results described later, 0.1 mg/ml was the minimum concentration required for removing ZP from cumulus‐free eggs. When we applied 0.1 mg/ml to the one‐step method, we noticed that the removal efficiency of cumulus cells and ZP was influenced by the amount of eggs and cumulus cells. Therefore, to stably remove cumulus cells and ZP from a large amount of eggs, we used tenfold concentration 1 mg/ml, which had no influence on the two‐cell development rate.
Acidified Tyrode's solution
ZPs of cumulus cell‐free eggs were removed by brief (approximately 30 s) incubation in acidified Tyrode's solution and then washed twice in TYH‐HEPES to recover the eggs.
Evaluation of CD9 localization after acidified Tyrode's and collagenase ZP removal
CD9‐EGFP female mice were superovulated, and the eggs were collected 13–15 h after hCG injection. ZP‐free eggs were obtained by acidified Tyrode's solution treatment and the one‐step collagenase method. The eggs were incubated in 2.5 μg/ml Hoechst 33342 for 10 min and then observed with a UPlanApo 40 × NA 0.85 dry objective lens in an Olympus BX50 (Olympus Co., Tokyo, Japan) microscope equipped with an imaging system composed of appropriate filters for fluorescence and a RETIGA Exi FAST 1394 CCD camera (Qimaging, Surrey, BC, Canada). Data acquisition and storage were controlled with SlideBook 4 software (Intelligent Imaging Innovations, Denver, CO).
Evaluation of zona‐free oocytes after IVF
Cauda epididymal sperm were allowed to swim for an hour in 1 ml TYH medium [18] at 37°C, with 5% CO2. Motile, swimming sperm were inseminated in zona‐free eggs at a final concentration of 1 × 104 sperm/ml. Eggs were observed with an IX71 inverted microscope (Olympus Co., Tokyo, Japan) equipped with a LUCPlanFL N 40 × NA 0.60 dry objective lens (Olympus) and the same components described above. To rule out the parthenogenetic activation, we separated the fertilized eggs to new culture medium after confirming the presence of a fertilization cone, second polar body, and pronuclei. Numbers of two‐cell embryos were counted until 30 h after insemination. We did not track the further development ratio because ZP‐free two‐cell embryos easily aggregate when two or more embryos come in contact.
Statistical analysis
Comparison of zona removal rates and fertilization rates were performed using Fisher's exact test. Values of P < 0.05 were considered statistically significant.
Results
Evaluation of the optimum concentration of collagenase required to remove ZP
We examined various concentrations of collagenase to determine the optimum concentration of collagenase for ZP removal; young eggs were treated with 0.05, 0.1, 0.2, or 1.0 mg/ml of collagenase for 10 min, and the resulting ZP‐free eggs were counted. The overall results were compared with the results obtained by acidified Tyrode's solution treatment. The zona removal rate of 0.1–1 mg/ml collagenase concentration ranged from 75 to 80%, whereas that of 0.05 mg/ml collagenase concentration decreased significantly to about 10%. By contrast, the ZP‐removal rate of acidified Tyrode's solution treatment was 93% (Table 2).
Table 2.
Comparison of zona removal rate between collagenase and acidified Tyrode's solution treatment
| Method of zona removal | Number of eggs | |
|---|---|---|
| Total | Zona removal rate (%) | |
| Collagenase (mg/ml) | ||
| 0.05 | 105 | 12 (11)a |
| 0.10 | 74 | 58 (78)b |
| 0.20 | 77 | 61 (79)b |
| 1.00 | 761 | 575 (75.5)b |
| Acidified Tyrode's solution | 202 | 189 (93.5)c |
Values with the same superscript are not significantly different. a–b, a–c, b–c: P < 0.01
Comparison of the effects of acidified Tyrode's solution and collagenase ZP‐free methods on oocyte
Effect observed by differential interference contrast (DIC) microscopy
Collagenase treatment caused no abnormalities in ZP‐free eggs (Fig. 1a). By contrast, eggs with acidified Tyrode's solution treatment had a seemingly normal surface (Fig. 1b), but some eggs apparently became fragile, resulting in membrane rupture during the egg recovery incubation (Fig. 1c).
Figure 1.
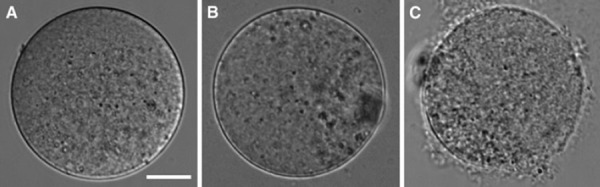
Effect of collagenase and acidified Tyrode's solution treatment on oolemma observed by DIC. a Collagenase treatment. b, c Acidified Tyrode's solution treatment. Collagenase treatment caused little or no damage to oolemma that have a smooth surface (a), while acidified Tyrode's solution treatment caused undesired significant damage to oolemma in various ways; one has a smooth surface (b), and the other has some damaged membrane that ruptured, leading to egg collapse (c). The dark area on right side of b and left side of c are the polar bodies that are out of focus. Bar 20 μm
Effect observed by membrane protein CD9 localization
In general, enhanced green fluorescence protein tagged CD9 protein (CD9‐EGFP) did not localize to the oolemma overlying the meiotic II spindle in ZP‐intact eggs before ZP removal (Fig. 2a). Collagenase treatment did not affect CD9‐EGFP, showing normal localization (Fig. 2b) that was identical to zona‐intact eggs (Fig. 2a). In contrast, acidified Tyrode's solution treatment affected CD9‐EGFP localization, showing membrane redistribution of CD9‐EGFP in the whole oolemma, even in the oolemma overlying the meiotic spindle (Fig. 2c). Such a loss of polarity was typically seen in images with some differences between the collagenase treatment and acidified Tyrode's solution treatment (Fig 2e–f).
Figure 2.
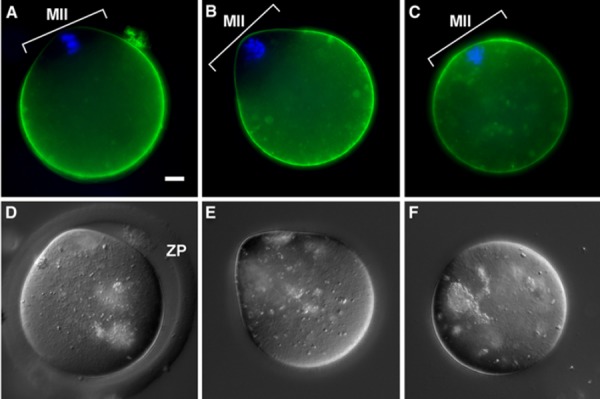
Effect of collagenase and acidified Tyrode's solution treatment on egg membrane molecule distribution observed by CD9‐EGFP localization. a–c Fluorescent image with CD9‐EGFP transgenic mouse eggs to trace the damage of oolemma. Green CD9‐EGFP. Blue Hoechst 33342 staining for chromosomes. d–f DIC images. a, d Control (ZP intact without cumulus cells after hyaluronidase treatment but before ZP removal treatment). b, e Collagenase treatment; one‐step collagenase method. c, f Acidified Tyrode's solution treatment. In the control (a) and collagenase treatment (b), CD9 is not localized (normal) on the oolemma overlying the MII chromosome area (MII), whereas in acidified Tyrode's solution treatment (c), CD9 was diffusely distributed even on the oolemma overlying the MII chromosome area; no polarization of CD9 was found; however, polarity was apparent in the control (a) and collagenase treatment (b). ZP Zona pellucida. Bar 10 μm
Two‐cell embryo development rate in ZP‐free in vitro fertilization
ZP‐free eggs after 1 or 0.1% collagenase and acidified Tyrode's solution treatment were subjected to insemination to evaluate the ability of these treated eggs to develop to two‐cell embryos. The development rates of the treated ZP‐free eggs were 79.9, 76, and 60%, respectively (Table 3). There was no significant difference in the two‐cell development rate between 0.1 and 1 mg/ml collagenase‐treated eggs.
Table 3.
Developmental rate of zona‐free eggs to two‐cell embryos; comparison between collagenase and acidified Tyrode's solution treatment
| Method of zona removal | Number of eggs | |
|---|---|---|
| Total | Two‐cell embryo rate (%) | |
| Collagenase (mg/ml) | ||
| 1.0 | 294 | 235 (79.9)a |
| 0.1 | 112 | 85 (76)a |
| Acidified Tyrode's solution | 108 | 65 (60)b |
Values with the same superscript are not significantly different. a–b: P < 0.05
Effect of egg quality on ZP‐removal rate
Collagenase could not dissolve the ZP of fragmented eggs. In contrast, acidified Tyrode's solution treatment dissolved the fragmented eggs’ ZP (Fig. 3). To test the ZP removal rate of the low quality eggs, we intentionally collected the post‐ovulatory aged eggs (aged eggs). The ZP removal rates of young and aged eggs with the collagenase treatment were 80.2 and 17%, respectively (Table 4). Neither the degree of collagenase concentration nor the treatment time removed the collagenase‐resistant ZP. In contrast, acidified Tyrode's solution treatment had a high ZP‐removal rate with no significant difference between young and aged eggs (94.8 and 93%, respectively) (Table 4).
Figure 3.
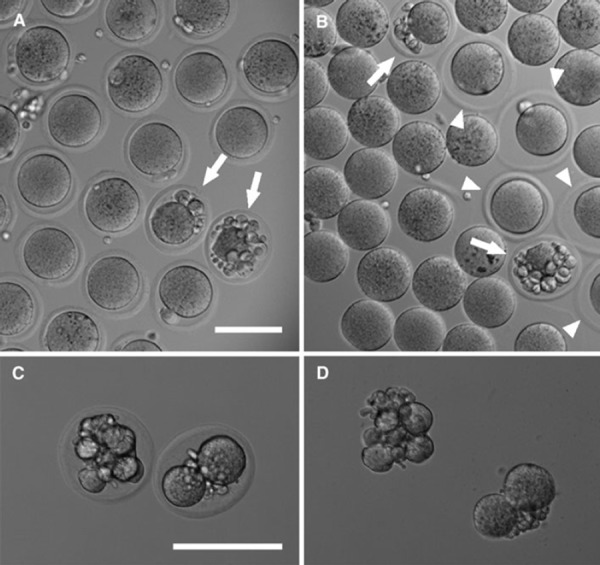
Effect of collagenase and acidified Tyrode's solution on the fragmented eggs. a Before collagenase treatment (control). b After collagenase treatment. c, d Two fragmented eggs before and after acidified Tyrode's solution treatment. Eggs shown in d are the same as those in c; images were taken immediately after eggs shown in c were transferred into acidified Tyrode's solution (d). a, b The ZP of the fragmented eggs (arrows in a and b) and some eggs that have normal morphology (arrowheads in b) were not removed by collagenase treatment. (c, d) The ZP of these two fragmented eggs became completely dissolved by acidified Tyrode's solution. Bars 100 μm
Table 4.
Comparison of zona removal rate between collagenase and acidified Tyrode's solution treatment; effect of post‐ovulatory aging
| Method of zona removal | Status of post‐ovulatory eggs | Number of eggs | |
|---|---|---|---|
| Total | Zona removal rate (%) | ||
| Collagenase (0.1 mg/ml) | Young | 227 | 182 (80.2)a |
| Aged | 175 | 30 (17)b | |
| Acidified Tyrode's solution | Young | 174 | 165 (94.8)c |
| Aged | 28 | 26 (93)c | |
Values with same superscript are not significantly different. a–b, a–c, b–c: P < 0.01
Discussion
In this study, we evaluated the collagenase method for ZP removal of unfertilized eggs originally reported by Zuccotti et al. [13] and successfully developed a simple one‐step collagenase ZP‐removal method: the incubation of a large number of cumulus oocyte complexes at once in 1 mg/ml collagenase for 10 min.
There were several intriguing findings in the development of this method. First, concerning the damage caused by the zona‐removal treatment, the oolemma after collagenase and the acidified Tyrode's solution treatment were seemingly normal (Fig. 1a, b), but some acidified Tyrode's solution‐treated eggs became fragile and ruptured during the resting incubation (Fig. 1c). Also, there was latent damage to the oolemma when localization of a vital transmembrane protein, membrane‐fusion‐related protein CD9, was examined using CD9‐EGFP eggs. In general, CD9 is localized in the oolemma of the microvillar region except the specific region overlying the meiotic spindle [19, 20]. However, our experiments showed that acidified Tyrode's solution treatment caused abnormal redistribution of CD9‐EGFP on the entire surface of the eggs, including the region overlying the meiotic spindle, while the collagenase treatment showed normal CD9 localization in ZP‐free eggs (Fig. 2). It is also reported that the distribution of integrin α6β1 is abnormally changed by acidified Tyrode's solution treatment [21]. These data suggest that although acidified Tyrode's solution treatment may not have damaged the surface molecules, it could perturb cytoskeleton organization, eventually causing nonphysiological action during fertilization. In contrast, the collagenase treatment was so mild that tenfold concentration did not affect or impair the fusibility of gametes and oocyte development at least up to the two‐cell stage (Table 4). Strictly speaking, it is known that the commercially available collagenase used in this experiment contains several other enzyme contaminants [13]. However, the results show that collagenase has no effect on the oocytes. Perhaps the possible deleterious enzymes contained in the crude collagenase are at a very low concentration or have only mild activity that can be inhibited by the albumin in the medium. Thus, it is suggested that collagenase treatment is safer than the other enzymes used for zona removal, such as α‐chymotrypsin, trypsin, and pronase, which readily damage the oolemma [4, 10, 11, 12, 13].
Another benefit of collagenase treatment in zona removal is that collagenase cannot remove the hardened ZP in eggs, whereas acidified Tyrode's solution treatment can remove all ZP regardless of aging or the egg's quality (Table 4; Fig. 3). The ZP of aged eggs becomes resistant to some proteases, a process that is called zona hardening [4, 22, 23, 24, 25, 26]. Zona hardening is caused by the exudation of the cortical granule that crosslinks the zona pellucida [27, 28]. Cortical granules are released spontaneously upon post‐ovulatory aging and intense mechanical stress [15, 17]. The difference in the zona‐removal rate by 0.1 mg/ml collagenase and acidified Tyrode's solution in Table 2 (78 and 93%, respectively) indicates that there are zona hardened eggs (about 15%) in the group prepared by the commonly used method with hormonal treatment. We think that these zona hardened eggs are latent low quality eggs.
It is reported that post‐ovulatory aging not only affects spontaneous cortical granule exocytosis, but also affects the ability to establish a membrane block to polyspermy [24, 29]. Thus, the collagenase ZP removal method is a mild method with no observable harm to the oocytes that can simultaneously discriminate the spontaneously activated low quality oocytes from normal, young oocytes [16].
In conclusion, we have established a one‐step collagenase ZP removal method that causes less damage and is more simplistic compared to commonly used methods, especially the acidified Tyrode's solution method. This simple and safe method will be quite useful in experiments that require a large number of high quality ZP‐free eggs (Fig. 4).
Figure 4.
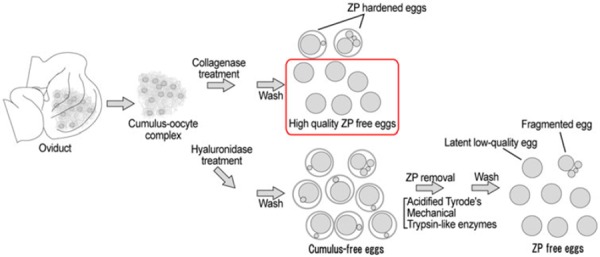
Advantage of the one‐step collagenase method and comparison of zona removal procedures. The one‐step collagenase method does not need hyaluronidase treatment. Other methods such as acidified Tyrode's solution, Trypsin‐like enzyme, and mechanical methods first require hyaluronidase treatment to denude eggs from cumulus cells. The one‐step collagenase method does not remove the zona pellucida (ZP) of the zona hardened eggs such as aged eggs, fragmented eggs, and latent low quality eggs. Thus, the ZP‐free eggs prepared by the one‐step collagenase method are high‐quality eggs that can be used for various purposes. The other methods remove most of the ZP from the eggs including low quality eggs. With the other methods, fragmented eggs can be discriminated, but latent low quality eggs will be hardly indistinguishable from normal eggs once they become ZP free
Acknowledgments
This work was mainly supported by a grant from Grant‐in‐Aid for Scientific Research on Innovation Areas to KT (221112504) and in part by Grant‐in‐Aid for Scientific Research (B) to KT (20659025, 22390033) and Grant‐in‐Aid for Scientific Research (C) to CI (21592079). The authors would like to thank Ms. K. Kamimura, Mr. T. Mutoh, and Ms. A. Kujirai for their excellent technical assistance.
References
- 1. Toshimori K Dynamics of the mammalian sperm head: modifications and maturation events from spermatogenesis to egg activation, 2009. Berlin: Springer; [PubMed] [Google Scholar]
- 2. Wassarman PM. The biology and chemistry of fertilization. Science, 1987, 235, 553–560 10.1126/science.3027891 [DOI] [PubMed] [Google Scholar]
- 3.Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill JD, editors. The physiology of reproduction, 2nd ed. New York: Raven press; 1994. p. 1189–317.
- 4. Wolf DP, Inoue M, Stark RA. Penetration of zona‐free mouse ova. Biol Reprod, 1976, 15, 213–221 10.1095/biolreprod15.2.213 [DOI] [PubMed] [Google Scholar]
- 5. Ziyyat A, Rubinstein E, Monier‐Gavelle F, Barraud V, Kulski O, Prenant M, Boucheix C, Bomsel M, Wolf JP. CD9 controls the formation of clusters that contain tetraspanins and the integrin alpha 6 beta 1, which are involved in human and mouse gamete fusion. J Cell Sci, 2006, 119, 416–424 10.1242/jcs.02730 [DOI] [PubMed] [Google Scholar]
- 6. Yamagata K, Nakanishi T, Ikawa M, Yamaguchi R, Moss SB, Okabe M. Sperm from the calmegin‐deficient mouse have normal abilities for binding and fusion to the egg plasma membrane. Dev Biol, 2002, 250, 348–357 10.1006/dbio.2002.0803 [PubMed] [Google Scholar]
- 7. Bowman P, McLaren A. The reaction of the mouse blastocyst and its zona pellucida to enzymes in vitro. J Embryol Exp Morphol, 1970, 24, 331–334 [PubMed] [Google Scholar]
- 8. Chang MC, Hunt DM. Effects of proteolytic enzymes on the zona pellucida of fertilized and unfertilized mammalian eggs. Exp Cell Res, 1956, 11, 497–499 10.1016/0014‐4827(56)90128‐8 [DOI] [PubMed] [Google Scholar]
- 9. Hoshi K, Saito A, Suzuki M, Hayashi K, Yanagimachi R. Effects of agents used for removal of zona pellucida on human sperm penetration into zona‐free hamster egg. Nippon Sanka Fujinka Gakkai Zasshi, 1982, 34, 2229–2234 [PubMed] [Google Scholar]
- 10. Boldt J, Howe AM, Preble J. Enzymatic alteration of the ability of mouse egg plasma membrane to interact with sperm. Biol Reprod, 1988, 39, 19–27 10.1095/biolreprod39.1.19 [DOI] [PubMed] [Google Scholar]
- 11. Kellom T, Vick A, Boldt J. Recovery of penetration ability in protease‐treated zona‐free mouse eggs occurs coincident with recovery of a cell surface 94 kD protein. Mol Reprod Dev, 1992, 33, 46–52 10.1002/mrd.1080330107 [DOI] [PubMed] [Google Scholar]
- 12. Komorowski S, Szczepanska K, Maleszewski M. Distinct mechanisms underlie sperm‐induced and protease‐induced oolemma block to sperm penetration. Int J Dev Biol, 2003, 47, 65–69 10.1387/18 [PubMed] [Google Scholar]
- 13. Zuccotti M, Urch UA, Yanagimachi R. Collagenase as an agent for dissolving the zona pellucida of hamster and mouse oocytes. J Reprod Fertil, 1991, 93, 515–520 10.1530/jrf.0.0930515 [DOI] [PubMed] [Google Scholar]
- 14. Miyado K, Yoshida K, Yamagata K, Sakakibara K, Okabe M, Wang X, Miyamoto K, Akutsu H, Kondo T, Takahashi Y, Ban T, Ito C, Toshimori K, Nakamura A, Ito M, Miyado M, Mekada E, Umezawa A. The fusing ability of sperm is bestowed by CD9‐containing vesicles released from eggs in mice. Proc Natl Acad Sci USA, 2008, 105, 12921–12926 10.1073/pnas.0710608105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abbott AL, Xu Z, Kopf GS, Ducibella T, Schultz RM. In vitro culture retards spontaneous activation of cell cycle progression and cortical granule exocytosis that normally occur in in vivo unfertilized mouse eggs. Biol Reprod, 1998, 59, 1515–1521 10.1095/biolreprod59.6.1515 [DOI] [PubMed] [Google Scholar]
- 16. Dalo DT, McCaffery JM, Evans JP. Ultrastructural analysis of egg membrane abnormalities in post‐ovulatory aged eggs. Int J Dev Biol, 2008, 52, 535–544 10.1387/ijdb.072549dd [DOI] [PubMed] [Google Scholar]
- 17. Xu Z, Abbott A, Kopf GS, Schultz RM, Ducibella T. Spontaneous activation of ovulated mouse eggs: time‐dependent effects on M‐phase exit, cortical granule exocytosis, maternal messenger ribonucleic acid recruitment, and inositol 1,4,5‐trisphosphate sensitivity. Biol Reprod, 1997, 57, 743–750 10.1095/biolreprod57.4.743 [DOI] [PubMed] [Google Scholar]
- 18. Toyoda Y, Yokoyama M, Hoshi T. Studies on the fertilization of mouse eggs in vitro. I. In vitro fertilization of eggs by fresh epididymal sperm. Jpn J Anim Reprod, 1971, 16, 147–151 [Google Scholar]
- 19. Kaji K, Oda S, Shikano T, Ohnuki T, Uematsu Y, Sakagami J, Tada N, Miyazaki S, Kudo A. The gamete fusion process is defective in eggs of Cd9‐deficient mice. Nat Genet, 2000, 24, 279–282 10.1038/73502 [DOI] [PubMed] [Google Scholar]
- 20. Runge KE, Evans JE, He ZY, Gupta S, McDonald KL, Stahlberg H, Primakoff P, Myles DG. Oocyte CD9 is enriched on the microvillar membrane and required for normal microvillar shape and distribution. Dev Biol, 2007, 304, 317–325 10.1016/j.ydbio.2006.12.041 [DOI] [PubMed] [Google Scholar]
- 21. Barraud‐Lange V, Naud‐Barriant N, Bomsel M, Wolf J‐P, Ziyyat A. Transfer of oocyte membrane fragments to fertilizing spermatozoa. FASEB J, 2007, 21, 3446–3449 10.1096/fj.06‐8035hyp [DOI] [PubMed] [Google Scholar]
- 22. Aonuma S, Okabe M, Kawai Y, Kawaguchi M. The change of solubility properties of zona pellucida to proteases related to fertilizability of mouse ova in vitro. Chem Pharm Bull (Tokyo), 1978, 26, 405–410 [DOI] [PubMed] [Google Scholar]
- 23. Dodson MG, Minhas BS, Curtis SK, Palmer TV, Robertson JL. Spontaneous zona reaction in the mouse as a limiting factor for the time in which an oocyte may be fertilized. J In Vitro Fert Embryo Transf, 1989, 6, 101–106 10.1007/BF01130735 [DOI] [PubMed] [Google Scholar]
- 24. Gardner AJ, Evans JP. Mammalian membrane block to polyspermy: new insights into how mammalian eggs prevent fertilisation by multiple sperm. Reprod Fertil Dev, 2006, 18, 53–61 10.1071/RD05122 [DOI] [PubMed] [Google Scholar]
- 25. Gianfortoni JG, Gulyas BJ. The effects of short‐term incubation (aging) of mouse oocytes on in vitro fertilization, zona solubility, and embryonic development. Gamete Res, 1985, 11, 59–68 10.1002/mrd.1120110107 [Google Scholar]
- 26. Krzanowska H. Rapidity of removal in vitro of the cumulus oophorus and the zona pellucida in different strains of mice. J Reprod Fertil, 1972, 31, 7–14 10.1530/jrf.0.0310007 [DOI] [PubMed] [Google Scholar]
- 27. Schmell ED, Gulyas BJ. Ovoperoxidase activity in ionophore treated mouse eggs. II. Evidence for the enzyme's role in hardening the zona pellucida. Gamete Res, 1980, 3, 279–290 10.1002/mrd.1120030310 [Google Scholar]
- 28. Vincent C, Pickering SJ, Johnson MH. The hardening effect of dimethylsulphoxide on the mouse zona pellucida requires the presence of an oocyte and is associated with a reduction in the number of cortical granules present. J Reprod Fertil, 1990, 89, 253–259 10.1530/jrf.0.0890253 [DOI] [PubMed] [Google Scholar]
- 29. Wortzman GB, Evans JP. Membrane and cortical abnormalities in post‐ovulatory aged eggs: analysis of fertilizability and establishment of the membrane block to polyspermy. Mol Hum Reprod, 2005, 11, 1–9 10.1093/molehr/gah125 [DOI] [PubMed] [Google Scholar]


