Abstract
Purpose
The aim of this study was to investigate the survival and development of premature follicles and oocytes from a vitrified‐transplanted ovary in a murine experimental model.
Methods
The 14‐day‐old mice were unilaterally ovariectomized and the separated ovaries were vitrified by cryotop. After 2 weeks the ovaries were warmed and autotransplanted into the gluteus superfiscialis muscle. After 3 weeks, these ovaries (vit‐trans), the ovaries from the opposite side (OPP), and 7‐week fresh mouse ovaries as sham and control group (7 week‐fresh), were recovered and examined histologically and by TUNEL test.
Results
All 4 vitrified‐autotransplanted ovaries had developing follicles. Primordial, primary, preantral and antral follicles were found in all three groups (7 week‐fresh, OPP and vit‐trans). The rate of apoptosis by TUNEL test was similar in all groups and no significant difference was found between vitrified‐transplanted ovarian tissue and controls.
Conclusions
These data demonstrate successful autotransplantation of vitrified whole mouse ovaries, manifested by the presence of all stages of folliculogenesis. According to the results of this experiment, heterotopic autotransplantation of whole cryopreserved ovary provides the opportunity for follicle development at all stages. However, further experiments are required to improve the efficiency of autotransplantation of cryopreserved ovaries to obtain better results.
Keywords: Autotransplantation, Back muscle, Mouse, Ovary, Vitrification
Introduction
Recent advances in cancer therapy have resulted in an increasing number of long‐term cancer survivors [1]. Premenopausal women who undergo high‐dose chemotherapy and radiotherapy for cancer problems face ovarian failure, which may cause infertility due to premature ovarian failure (POF) [2]. The major reason is the sensitivity of ovaries to cytotoxic treatments, especially to alkylating agents which are known to be high risk for gonadal failure [3]. Moreover, infertility may occur in mutant or transgenic animals. Therefore, there appears to be a need to maintain fertility in cancer patients and experimental animal lines. The cryopreservation of ovarian tissue is a potentially useful technology for preservation of genetic resources of experimental, domestic, and wild animals [4]. The first description of vitrification of ovaries with demonstrated function after thawing was reported by Parrot in 1960 [5]. Although there are several fertility preservation methods available, according to the American Society for Reproductive Medicine (ASRM) embryo cryopreservation is still the only accepted method for fertility preservation in patients with POF [6]. To continue the treatment the patients have to be at pubertal age and be capable of bearing an ovarian cycle, therefore in order to conserve ovarian cycle we have to postpone cancer treatment. Furthermore, they must also have a partner or use donor sperm [7]. Autotransplantation, xenografting and follicular cultures [8] have been suggested as experimental strategies for restoring ovarian functions in female cancer survivors to make use of their own cryopreserved ovarian tissue [9]. Cryopreservation/transplantation of ovarian tissue and in vitro maturation of follicles/oocytes are two emerging techniques. These treatments offer more alternatives and allow more suitable choices for preserving fertility according to the patient's specific situation. For these technologies to be recognized and carried out routinely, they must be safe, easy to perform and deliver successful results [10, 11, 12]. According to Varghese et al. [10] the advantages of cryopreservation and transplantation of ovarian tissue are: immediate harvesting (treatment can start without delay), no male partner/gamete donor is needed, allows complete gonadotoxic and or ionizing radiation treatment without exposure, allows endocrine function to be resumed, allows resumption and preservation of reproductive function, and is suitable for prepubertal girls. The disadvantages include: requires surgical procedures for tissue harvesting and transfer, possibility of reintroducing malignant cells, and low success rate. The biggest advantage of autotransplantation is being able to avoid immunosuppressant treatment. Autotransplantation of cryopreserved ovarian tissue has proven to be an effective method not only to restore endocrine function [13, 14, 15] but also to restore fertility in humans [16]. In 2002, the first successful autografting of cryopreserved ovarian tissue was performed on sheep [17]. In 2004, a live birth by fresh ovarian tissue transplantation in a monkey was reported [18]. Two live births after orthotopic transplantation of cryopreserved human ovarian tissue were also reported in 2004 [16, 19], and in 2010 a seventh live birth was reported by Roux et al. [20]. The orthotopic sites for transplantations were in the cortex of ovary [14] and the peritoneum beneath the ovarian hilum [3, 16, 21]; the heterotopic sites were arm muscle, subcutaneous tissue of abdomen, peritoneum, rectus muscle, colic omentum and mesoarium [22]. However, the best site for transplantation still remains unknown. The transplantation of cryopreserved intact sheep ovaries by artery and vein anastomosis was reported by Arav et al. [23]. In 2006, Imhof et al. [24] were successful in the pregnancy and live birth of a sheep by orthotopic microvascular re‐anastomosis of whole cryopreserved ovaries. Since the transplantation of fresh and cryopreserved‐thawed ovarian cortical strips faces limitations like ischemic injury and delays in neovascularization, intact ovarian tissue grafting followed by cryopreservation is believed to be a potentially better option for fertility preservation [25]. According to the above‐mentioned studies, the aim of this study was to investigate the survival and folliculogenesis of vitrified‐transplanted whole mouse ovaries with regard to the site of transplantation.
Materials and methods
All chemicals were purchased from Sigma (Germany), except those mentioned below.
Mice
Animal experiments were carried out according to the Declaration Helsinki and the Guiding Principles in the Care and Use of Animals (DHEW publication, NIH, 80‐23).
Female Naval Medical Research Institute (NMRI) mice (Pasteur Institute, Tehran, Iran) were house and bred at Royan Institute Resource and were kept at a temperature of 20–25°C and 50% humidity in light controlled condition (12L:12D photocycle) and provided with sterile food and water.
Ovary preparation for vitrification
After anesthesia of 14‐day‐old mice by interperitoneal (i.p.) injection of ketamine–xylazine mixture (100 mg/kg ketamine and 10 mg/kg xylazine), the left ovaries were removed by a surgical incision on the skin and peritoneum over the ovary site. The surrounding tissue that enveloped the ovary was then dissected in Minimum Essential Medium Alpha (MEM‐α) medium (Gibco, USA) medium by insulin needles. The incisions in the peritoneum and skin were sutured separately by 6‐0 absorbable suture (Ethicon, Belgium).
Vitrification method
The ovaries were immersed in an equilibration solution composed of 7.5% ethylene glycol (EG) and 7.5% dimethyl sulfoxide (DMSO) in HEPES‐buffered tissue culture medium (TCM) 199 (pH 7.2–7.4) containing 20% serum substitute supplement (albumin 20%, Octapharma) for 15 min at room temperature, and then in a vitrification solution for 30 min at 4°C. The vitrification solution was composed of 15% EG, 15% DMSO, and 0.5 M sucrose in HEPES‐buffered TCM 199 (pH 7.2–7.4) containing 20% serum substitute supplement. The ovarian tissues were placed on a polyester sheet of cryotop (Kiazato, Japan) and the cryotops were immediately plunged into liquid nitrogen [26, 27].
Warming method
After 2 weeks, the cryotops were removed from the liquid nitrogen tank. The sheets on which ovaries were loaded were placed directly into a warming solution composed of 1 M sucrose in HEPES‐buffered TCM 199 (pH 7.2–7.4) with 20% HSA (human serum albumin, Octapharma) for 10 min at room temperature. The detached ovaries from the sheet were transferred and incubated in MEM‐α medium (Gibco) supplemented with 10% fetal bovine serum (Sigma) and an antibiotic solution that was composed of 100 IU of penicillin G, 100 μg of amphotricin B (Gibco) for 30 min.
Autologous heterotopic transplantation of intact ovary
The cryopreserved ovary was autografted immediately after the warming procedure into the gluteal muscle of the same 4‐week‐old mouse. As mentioned before, the mice were anesthetised by i.p. injection of ketamine–xylazine mixture (100 mg/kg ketamine and 10 mg/kg xylazine). A single surgical incision of the skin gave access to the gluteal muscle. A 2‐mm deep incision was made and the ovary was placed in the packet‐like muscular site. Finally, the muscle and skin incisions were sutured (5‐0 non‐absorbable for muscle and 5‐0 absorbable for skin (Ethicon.
After 3 weeks, the mice were killed by cervical dislocation and the ovaries removed from the transplantation site.
Histological examination
Ovaries in all groups were fixed in Bouin's fixative overnight. They were then dehydrated in ascending concentrations of ethanol (70–100%) by an automated tissue processor, embedded in paraffin, and cut into 6‐μm serial sections. The sections were stained by hematoxylin and eosin (H&E) and TUNEL staining (In Situ Cell Death Detection Kit (POD), Roche, Germany). Healthy follicles were counted in selected sections on each H&E slide using a light microscope (Nikon). To avoid double counting of the same follicle and to ensure inclusion of the largest cross‐sections of follicles, counts were performed based on visible nucleolus within the oocyte. Primordial follicles were counted only when the oocyte had a definite nuclear membrane [12].
Statistical analysis
Follicle and blood vessel counts are presented as mean ± SE. Kolmogorov–Smirnov test of normality were performed to choose the appropriate statistical test. Kruskal–Wallis and Mann–Whitney tests were used to compare the significance of difference between mean numbers of primordial, primary, preantral and antral follicles present in sections from the vit‐trans, OPP and 7 week‐fresh groups. A P value less than 0.05 was considered significantly different. All analysis was performed by SPSS statistical analysis program.
Results
Morphological features of graft site
The graft sites appeared more vascularized than the muscular tissue and were easily distinguished from the surrounding muscular tissue. The muscle was tightly attached to the ovary with good vascularization at the site. The ovarian tissue was easily detectable in the muscular tissue (Fig. 1). Ovaries from the vit‐trans group were mostly smaller than ovaries from the 7 week‐fresh and OPP groups. OPP ovaries were more follicular on surface shape than 7 week‐fresh ovaries (Fig. 3) with no significant difference in size.
Figure 1.
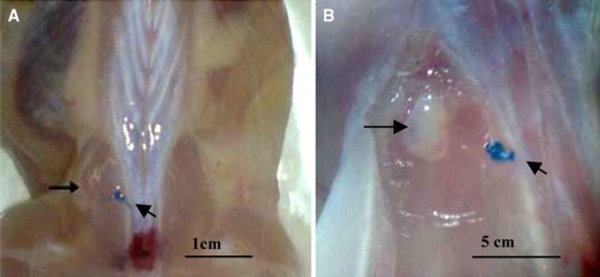
The transplantation ovary site. The arrows show the transplanted ovary at the site. The diagonal arrows show the non‐absorbable near the transplantation site. Scale bar: 1 cm (a), 5 mm (b)
Figure 3.
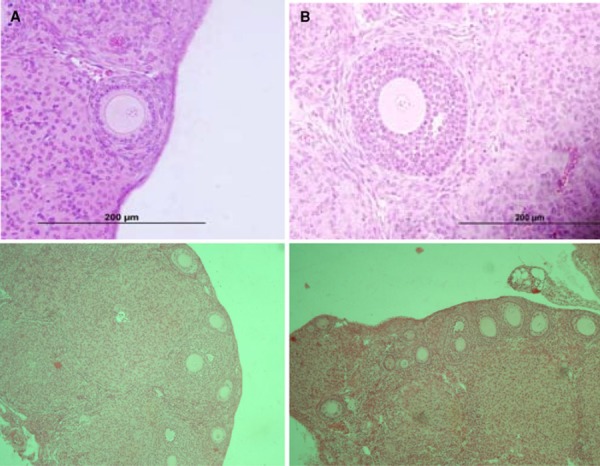
H&E sections of Opp group ovaries. a Primary follicle. b Antral follicle. Scale bar: 200 µm (a, b)
The follicles and histological analysis
Four mice were successfully ovariectomized and their ovaries were vitrified and autotransplanted. Three weeks after transplantation all of the ovaries were recovered successfully (100% recovery rate). In all of the recovered ovaries, follicles at all stages were detectable by histological examination (Figs. 2, 3, 4). No blood vessel was found at the center of the grafted ovaries as the follicle concentration was observed only at the surface (Fig. 5). All grafts contained some primordial, growing, and antral follicles. The vit‐trans group contained growing follicles, from small preantral follicles to preovulatory follicles. All of the ovaries in the vit‐trans group had at least one ovulatory follicle (Fig. 4). No significant difference was observed in follicle morphological features between the control group (7 week‐fresh) and the OPP and vit‐trans groups (Figs. 2, 3, 4). Functional blood vessels containing red blood cells were observed in the vit‐trans group. It is not clear if these blood vessels were newly‐formed ones or previous vessels which retained their function (Fig. 5); however, these blood vessels provided a good blood supply to the vit‐trans tissue. There was little morphological difference between some cases of the vit‐trans group and the control group with regard to corpora lutea. This can be explained by the physical pressures that the grafted ovaries received in muscle tissue.
Figure 2.
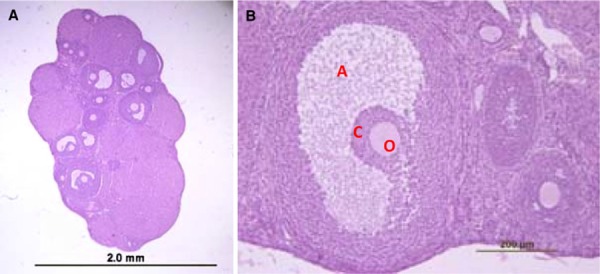
7th week‐fresh group ovary and follicles. a Section from intact ovary of a 7 weeks old mouse, after staining with H&E several antral follicles are visible within the section. b Preovulatory follicle (A antrum, C cumulus cells, O oocyte). Scale bar: 2.0 mm (a), 200 µm (b)
Figure 4.
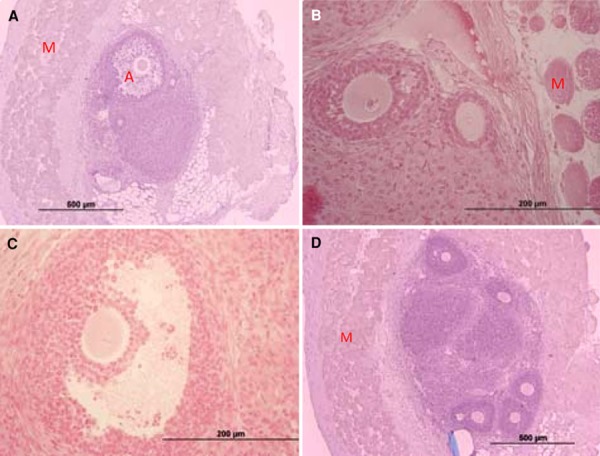
H&E sections of Vit‐trans group ovaries. a An overview of the vitrified‐transplanted ovarian tissue within the gluteus muscle fibers. An antral follicle is visile at the top of the image. b Preantral follicle near the borderline between muscular and ovarian tissue. c Antral follicle. d Several follicles within the transplanted ovarian tissue surrounded by gluteus muscle fibers. The unabsorbable suture is visible at the bottom of the section. (A antrum, M skeletal muscle fibers). Scale bar: 200 µm(a, d), 500 µm (b, c)
Figure 5.
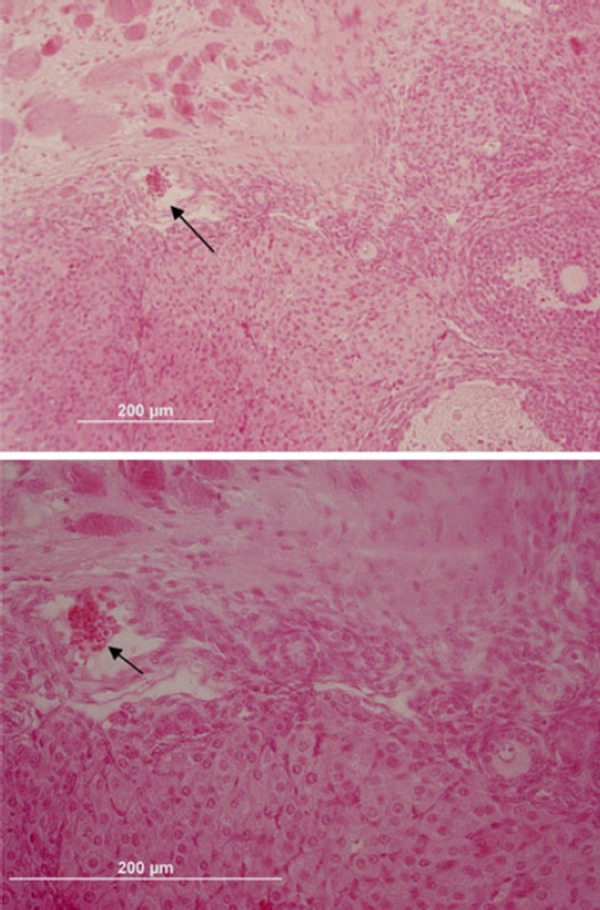
H&E staining of grafted ovaries. Functional blood vessel containing RBCs in the surface of the grafted ovary is shown by arrow
Follicle count
A mean total of 192.25 follicles were counted in the serial sections from the vit‐trans group; the numbers for the 7 week‐fresh and OPP groups were 721.25 and 560.25, respectively. Follicles were classified according to developmental stage as primordial (with one layer of flat granulosa cells), primary (with one layer of cuboidal granulosa cells), preantral (two or more layers of granulosa cells with no cavity within), antral (within cavity of antrum forming). Multiple growing follicles, including antral stage, were observed in grafts from all specimens. In the vit‐trans group, the number of follicles was dramatically reduced compared with the 7 week‐fresh and OPP groups (Table 1). In the primordial stage the 7 week‐fresh and OPP groups had significantly more primordial follicles than the vit‐trans group (359.75 ± 23.50 for 7 week‐fresh and 359.25 ± 24.36 for OPP groups compared with 71.25 ± 11.45 for the vit‐trans group, P < 0.05); however, there was no significant difference between the 7 week‐fresh group and the OPP group. There was a significant difference between all the groups in the primary stage; the 7 week‐fresh group had more than the OPP group and the OPP group had more than the vit‐trans group. No significant difference was found in the preantral follicle count between the vit‐trans and OPP groups, but there was a significant difference in the preantral follicle count between the vit‐trans and OPP groups compared with the 7 week‐fresh group (18.25 ± 4.00 for vit‐trans, 25.75 ± 4.32 for OPP in comparison to 106.25 ± 7.47 for 7 week‐fresh, P < 0.05). The antral follicle count showed a significant difference between the vit‐trans and OPP groups (4.25 ± 1.79 for vit‐trans and 11.50 ± 2.10 for OPP). Although the 7 week‐fresh antral follicle count is a little lower than the OPP group, it is not significantly different to the antral follicle count in the vit‐trans group (Table 1). Apoptotic cells were not detected in the areas containing primordial, primary, and small preantral follicles (data have not shown), but apoptosis was detected in some granulosa cells of antral and preovulatory follicles of all the groups (Figs. 6, 7, 8).
Table 1.
Number of follicles in vitrified‐transplanted ovaries versus age‐matched mice fresh ovaries and opposite ovaries of the same mice
| Group | Stage | |||
|---|---|---|---|---|
| Primordial | Primary | Preantral | Antral | |
| Vit‐trans | 71.25 ± 11.45a,b | 98.50 ± 11.01a,b | 18.25 ± 4.00a | 4.25 ± 1.79a |
| 7 week‐fresh | 359.75 ± 23.50a | 244.00 ± 24.87a,c | 106.25 ± 7.47a,b | 11.25 ± 2.86 |
| OPP | 359.25 ± 24.36b | 163.75 ± 9.63b,c | 25.75 ± 4.32b | 11.50 ± 2.10a |
Values (mean ± SE) with the same superscript letter within the same column are significantly different (P < 0.05)
Vit‐trans is the vitrified‐transplanted group as experimental group
OPP is the opposite ovary of the transplanted one from the same mouse and used as first control group
7 week‐fresh is the age‐matched group of mice and used as second control group
Figure 6.
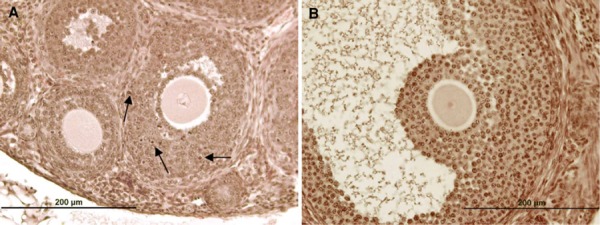
TUNEL staining sections of 7 week‐fresh group. The arrows show the apoptotic cells. Scale bar: 200 µm (a, b)
Figure 7.
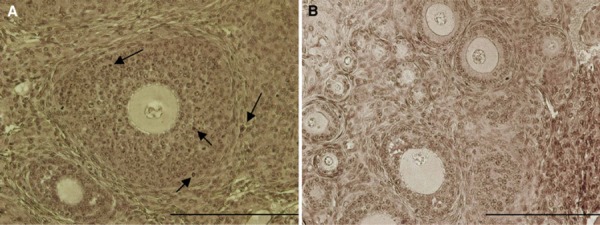
TUNEL staining sections of Opp group. The arrows show the apoptotic cells. Scale bar: 200 µm (a, b)
Figure 8.
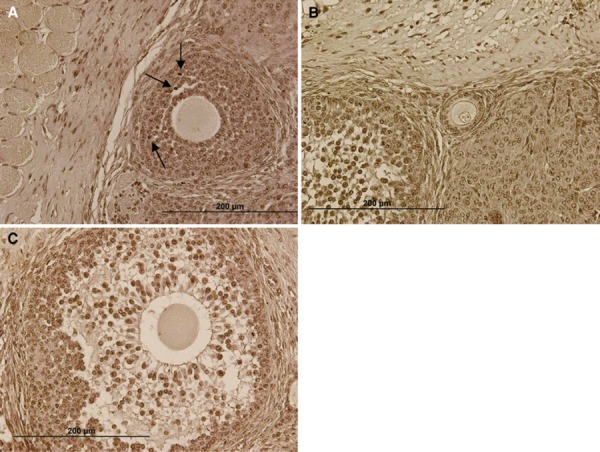
TUNEL staining sections of vit‐trans group. The arrows show the apoptotic cells. Scale bar: 200 µm (a–c)
Discussion
In spite of the many developments in recent years in human ovarian tissue cryopreservation and transplantation, more improvement is needed to achieve ideal results. Therefore, several animal models have been developed to optimize cryopreservation and transplantation methods to preserve whole ovarian tissue. It has been shown that whole ovarian vitrification and transplantation is a promising approach to save fertility. However, it was reported that the vitrification protocol significantly affects the survival of follicles. In this research we intended to evaluate the application of an efficient vitrification protocol with subsequent transplantation with regard to the best site for transplantation on the follicular structure of transplanted ovaries. In the experimental group massive primordial follicle activation occurred and follicles grew to antral size. Growing follicles, including antral stage, were observed in all transplants 3 weeks after transplantation. The ovary is well suited to transplantation and is well surrounded by muscular tissue. It seems that the transplantation time was sufficient to allow the angiogenic factors to work and to induce neovascularization. It has been demonstrated that primordial follicles can tolerate episodes of ischemic conditions for 3 h at 4°C [15]; however, after ovarian tissue cryopreservation followed by transplantation, the number of primordial follicles drops considerably due to hypoxia, and has been estimated at 50–60% [28] and even > 90% [25] of the population. The growing stages [29] or stromal cells [15] are even more susceptible to the effects of oxygen deprivation. Any growing follicles present in the graft within days after the transplantation procedures are therefore presumed to be derived from primordial follicles present at the time of transplantation [25, 30]. Ovarian grafts have been transplanted onto a number of different sites, including the bursal cavity, the kidney capsule, and subcutaneous sites [6]. Transplantation to the kidney capsule became popular because it was thought that the excellent blood supply would enhance graft survival [6]. Graft recovery and oocyte yield are significantly higher from the bursal cavity and kidney capsule than from subcutaneous sites [31]. Orthotopic transplantation of vitrified‐warmed ovine ovaries or hemi‐ovaries resulted in the successful development of oocytes which were then developed either to early parthenogenetic embryos [23] or to live births [17, 24, 32]. During histological evaluation of the H&E‐stained sections in this study, the follicles were classified and the proportions of different classes were examined as well as the morphological features of the recovered ovaries and their follicles. During the visual evaluation, very few atretic follicles (Fig. 8c) were observed in the grafted ovaries; however, using TUNEL test, apoptosis in the granolusa cells in the follicles of the grafted ovaries was not higher than in the 7 week‐fresh and OPP groups. This confirmed conservation of the healthy follicles in the grafted ovaries by vitrification and transplantation techniques applied in this research. Through our previous study we demonstrated that the gluteal muscle is a suitable site for ovarian transplantation [12]. In this research the effect of cryopreservation method was also evaluated. Cryoprotectants are required in any cryopreservation technique to prevent excessive intracellular ice crystal formation [33], but high cryoprotectant concentrations are essential for vitrification because of their viscosity, thus facilitating vitrification at practically attainable cooling rates [34]. Several authors have stressed the importance of fine‐tuning the cryoprotocol to the cell types being preserved [35], and significantly different results were achieved with the same cooling technique by varying cryoprotectant types or concentrations [36, 37]. Through previous studies it was shown that vitrification is the best technique [38]. The present study demonstrated that ovarian folliculogenesis was mostly restored and continued after heterotopic autotransplantation of vitrified‐warmed whole murine ovaries while transplanted to gluteal muscle. This study also reports the survival and development of autotransplanted murine follicles after cryopreservation of the whole ovary by vitrification using the cryotop method. Previous studies, however, evaluated whole ovarian transplantation, transplantation to the gluteal muscles [12] and vitrification [4, 26, 27] through separate experiments.
Acknowledgments
This study was supported by the grants from Royan Institute of the Islamic Republic of Iran. The authors are very grateful to Mrs. Mansoureh Safe for English editing, Mrs. Shabani for statistical analysis and especially to Dr. Naghi for his thorough editing of the manuscript and revision of the English version.
References
- 1. Blatt J. Pregnancy outcome in long‐term survivors of childhood cancer. Med Pediatr Oncol, 1999, 33 (1) 29–33 10.1002/(SICI)1096‐911X(199907)33:1<29::AID‐MPO6>3.0.CO;2‐2 [DOI] [PubMed] [Google Scholar]
- 2. Meirow D et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med, 2005, 353 (3) 318–321 10.1056/NEJMc055237 [DOI] [PubMed] [Google Scholar]
- 3.Meirow D, Levron J, Hardan I, Zalel Y, Bider D, Dor J. IVF and ovarian tissue cryopreservation as fertility preservation procedures in a patient recently exposed to chemotherapy. Fertil Steril. 2004;82:58.
- 4. Migishima F et al. Successful cryopreservation of mouse ovaries by vitrification. Biol Reprod, 2003, 68 (3) 881–887 10.1095/biolreprod.102.007948 [DOI] [PubMed] [Google Scholar]
- 5.Parrot DM. The fertility of mice with orthotopic ovarian grafts derived from frozen tissue. J Reprod Fertil. 1960;1:230–41. [DOI] [PubMed]
- 6. Donnez J et al. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update, 2006, 12 (5) 519–535 10.1093/humupd/dml032 [DOI] [PubMed] [Google Scholar]
- 7. Maltaris T et al. The effect of cancer treatment on female fertility and strategies for preserving fertility. Eur J Obstet Gynecol Reprod Biol, 2007, 130 (2) 148–155 10.1016/j.ejogrb.2006.08.006 [DOI] [PubMed] [Google Scholar]
- 8. Scott JE et al. Human ovarian tissue cultures: extracellular matrix composition, coating density and tissue dimensions. Reprod Biomed Online, 2004, 9 (3) 287–293 10.1016/S1472‐6483(10)62143‐8 [DOI] [PubMed] [Google Scholar]
- 9. Bedaiwy MA, Falcone T. Harvesting and autotransplantation of vascularized ovarian grafts: approaches and techniques. Reprod Biomed Online, 2007, 14 (3) 360–371 10.1016/S1472‐6483(10)60880‐2 [DOI] [PubMed] [Google Scholar]
- 10. Varghese AC et al. Cryopreservation/transplantation of ovarian tissue and in vitro maturation of follicles and oocytes: challenges for fertility preservation. Reprod Biol Endocrinol, 2008, 6, 47 10.1186/1477‐7827‐6‐47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eimani H et al. Survival rate of preantral follicles derived from vitrified neonate mouse ovarian tissue by Cryotop and conventional methods. Biofactors, 2007, 31 (2) 117–126 10.1002/biof.5520310202 [DOI] [PubMed] [Google Scholar]
- 12. Eimani H et al. Comparative study between intact and non‐intact intramuscular auto‐grafted mouse ovaries. Reprod Biomed Online, 2009, 18 (1) 53–60 10.1016/S1472‐6483(10)60424‐5 [DOI] [PubMed] [Google Scholar]
- 13. Oktay K et al. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA, 2001, 286 (12) 1490–1493 10.1001/jama.286.12.1490 [DOI] [PubMed] [Google Scholar]
- 14. Radford JA et al. Orthotopic reimplantation of cryopreserved ovarian cortical strips after high‐dose chemotherapy for Hodgkin's lymphoma. Lancet, 2001, 357 (9263) 1172–1175 10.1016/S0140‐6736(00)04335‐X [DOI] [PubMed] [Google Scholar]
- 15. Kim SS et al. Quantitative assessment of ischemic tissue damage in ovarian cortical tissue with or without antioxidant (ascorbic acid) treatment. Fertil Steril, 2004, 82 (3) 679–685 10.1016/j.fertnstert.2004.05.022 [DOI] [PubMed] [Google Scholar]
- 16.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez‐Madrid B, van Langendonckt A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–10. [DOI] [PubMed]
- 17. Salle B et al. Normal pregnancies and live births after autograft of frozen‐thawed hemi‐ovaries into ewes. Fertil Steril, 2002, 77 (2) 403–408 10.1016/S0015‐0282(01)02960‐0 [DOI] [PubMed] [Google Scholar]
- 18. Lee DM et al. Live birth after ovarian tissue transplant. Nature, 2004, 428 (6979) 137–138 10.1038/428137a [DOI] [PubMed] [Google Scholar]
- 19.Oktay K, Tilly J. Livebirth after cryopreserved ovarian tissue autotransplantation. Lancet. 2004;364(9451):2091–2 (author reply 2092–3). [DOI] [PubMed]
- 20.Roux C et al. Live birth after ovarian tissue autograft in a patient with sickle cell disease treated by allogeneic bone marrow transplantation. Fertil Steril. 2010;93(7): 2413.e15–9. [DOI] [PubMed]
- 21.Tryde Schmidt KL, Yding Andersen C, Starup J, Loft A, Byskov AG and Nyboe AA. Orthotopic autotransplantation of cryopreserved ovarian tissue to a woman cured of cancer—follicular growth, steroid production and oocyte retrieval. Reprod Biomed Online. 2004;8:448–53. [DOI] [PubMed]
- 22. Oktay K et al. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet, 2004, 363 (9412) 837–840 10.1016/S0140‐6736(04)15728‐0 [DOI] [PubMed] [Google Scholar]
- 23. Arav A et al. Oocyte recovery, embryo development and ovarian function after cryopreservation and transplantation of whole sheep ovary. Hum Reprod, 2005, 20 (12) 3554–3559 10.1093/humrep/dei278 [DOI] [PubMed] [Google Scholar]
- 24. Imhof M et al. Orthotopic microvascular reanastomosis of whole cryopreserved ovine ovaries resulting in pregnancy and live birth. Fertil Steril, 2006, 85 (Suppl 1) 1208–1215 10.1016/j.fertnstert.2005.11.030 [DOI] [PubMed] [Google Scholar]
- 25. Aubard Y et al. Orthotopic and heterotopic autografts of frozen‐thawed ovarian cortex in sheep. Hum Reprod, 1999, 14 (8) 2149–2154 10.1093/humrep/14.8.2149 [DOI] [PubMed] [Google Scholar]
- 26. Hasegawa A et al. Pup birth from mouse oocytes in preantral follicles derived from vitrified and warmed ovaries followed by in vitro growth, in vitro maturation, and in vitro fertilization. Fertil Steril, 2006, 86 (4 Suppl) 1182–1192 10.1016/j.fertnstert.2005.12.082 [DOI] [PubMed] [Google Scholar]
- 27. Vajta G et al. Open Pulled Straw (OPS) vitrification: a new way to reduce cryoinjuries of bovine ova and embryos. Mol Reprod Dev, 1998, 51 (1) 53–58 10.1002/(SICI)1098‐2795(199809)51:1<53::AID‐MRD6>3.0.CO;2‐V [DOI] [PubMed] [Google Scholar]
- 28. Baird DT et al. Long‐term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196°C. Endocrinology, 1999, 140 (1) 462–471 10.1210/en.140.1.462 [DOI] [PubMed] [Google Scholar]
- 29.Krohn PL. Transplantation of the ovary. In: Zuckerman S, Weir BJ (eds) The ovary. London: Academic Press; 1989.
- 30. Hernandez‐Fonseca HJ et al. Time course of follicular development after bovine ovarian tissue transplantation in male non‐obese diabetic severe combined immunodeficient mice. Fertil Steril, 2005, 83 (Suppl 1) 1180–1187 10.1016/j.fertnstert.2004.07.985 [DOI] [PubMed] [Google Scholar]
- 31. Yang HY et al. Graft site and gonadotrophin stimulation influences the number and quality of oocytes from murine ovarian tissue grafts. Reproduction, 2006, 131 (5) 851–859 10.1530/rep.1.00916 [DOI] [PubMed] [Google Scholar]
- 32. Bordes A et al. Normal gestations and live births after orthotopic autograft of vitrified‐warmed hemi‐ovaries into ewes. Hum Reprod, 2005, 20 (10) 2745–2748 10.1093/humrep/dei155 [DOI] [PubMed] [Google Scholar]
- 33. Coticchio G et al. Oocyte cryopreservation: a biological perspective. Eur J Obstet Gynecol Reprod Biol, 2004, 115 (Suppl 1) S2–S7 10.1016/j.ejogrb.2004.01.006 [DOI] [PubMed] [Google Scholar]
- 34. Rubinsky B. Principles of low temperature cell preservation. Heart Fail Rev, 2003, 8 (3) 277–284 10.1023/A:1024734003814 [DOI] [PubMed] [Google Scholar]
- 35.Dinnyes A, Liu J, Nedambale TL. Novel gamete storage. Reprod Fertil Dev. 2000;19:719–31. [DOI] [PubMed]
- 36. Cai XY et al. Cryoloop vitrification of rabbit oocytes. Hum Reprod, 2005, 20 (7) 1969–1974 10.1093/humrep/deh805 [DOI] [PubMed] [Google Scholar]
- 37. Newton H, Illingworth P. In vitro growth of murine pre‐antral follicles after isolation from cryopreserved ovarian tissue. Hum Reprod, 2001, 16 (3) 423–429 10.1093/humrep/16.3.423 [DOI] [PubMed] [Google Scholar]
- 38. Özmen B, Al‐Hassani S. Techniques for ovarian tissue, whole ovary, oocyte and embryo cryopreservation. J Reprod Infertil, 2010, 11 (1) 3–15 [PMC free article] [PubMed] [Google Scholar]


