Abstract
The substrate specificity of tryptophan (Trp) decarboxylase (TDC) for Trp and tyrosine (Tyr) decarboxylase (TYDC) for Tyr was used to modify the in vivo pools of these amino acids in transgenic tobacco. Expression of TDC and TYDC was shown to deplete the levels of Trp and Tyr, respectively, during seedling development. The creation of artificial metabolic sinks for Trp and Tyr also drastically affected the levels of phenylalanine, as well as those of the non-aromatic amino acids methionine, valine, and leucine. Transgenic seedlings also displayed a root-curling phenotype that directly correlated with the depletion of the Trp pool. Non-transformed control seedlings could be induced to display this phenotype after treatment with inhibitors of auxin translocation such as 2,3,5-triiodobenzoic acid or N-1-naphthylphthalamic acid. The depletion of aromatic amino acids was also correlated with increases in the activities of the shikimate and phenylpropanoid pathways in older, light-treated transgenic seedlings expressing TDC, TYDC, or both. These results provide in vivo confirmation that aromatic amino acids exert regulatory feedback control over carbon flux through the shikimate pathway, as well as affecting pathways outside of aromatic amino acid biosynthesis.
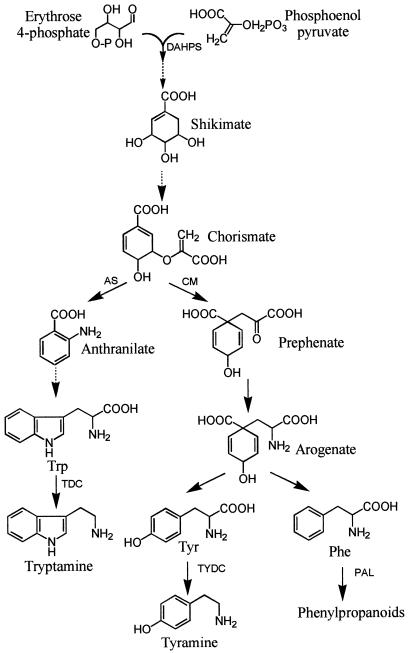
The aromatic amino acids (Phe, Trp, and Tyr), which are derived from the shikimate pathway (Fig. 1), are required as building blocks for protein synthesis. They are also required for the production of a large variety of secondary metabolites including quinones, indole derivatives, and alkaloids, as well as phenylpropanoid compounds such as phenolic esters, flavonoids, coumarins, anthocyanins, suberin, and lignin. The biosynthesis of these aromatic secondary metabolites is regulated by development (Whetten and Sederoff, 1995) and environmental stimuli such as light, pathogens, and wounding (Weaver and Herrmann, 1997). Expression of 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase (DAHPS, EC 4.1.2.15) (Keith et al., 1991; Henstrand et al., 1992; Suzuki et al., 1995) and Phe ammonia-lyase (PAL, EC 4.3.1.5) (Rubery and Northcote, 1968; Elkind et al., 1990; Fukasawa-Akada et al., 1996) are under tight transcriptional control. It is believed that DAHPS and PAL can flexibly regulate carbon flux through the shikimate (Herrmann, 1995; Schmid and Amrhein, 1995) and phenylpropanoid pathways (Bate et al., 1994), respectively. For example, a functional analysis of the PAL2 promoter in tobacco suggested that putative Myb protein binding sites could mediate tissue-specific expression of PAL (Hatton et al., 1995). However, it is unclear how coordinate regulation of DAHPS and PAL mRNA transcripts and their corresponding enzyme activities (Weaver and Herrmann, 1997), can alter the pool of aromatic amino acids in planta in response to different internal and external stimuli.
Figure 1.
The shikimate pathway and its ramifications leading to aromatic amino acids. The enzymes indicated are DAHPS, chorismate mutase (CM), anthranilate synthase (AS), TDC, TYDC, and PAL.
Results obtained by treatment of plants with glyphosate, a herbicide that inhibits 5-enolpyruvylshikimate 3-phosphate synthase (EC 2.5.1.19) (Sammons et al., 1995), suggest that regulation of shikimate pathway enzymes control the supply of aromatic amino acids (Holländer and Amrhein, 1980; Binarová et al., 1994). Glyphosate was reported to attenuate the elicitor-stimulated production of shikimate pathway-derived phytoalexins in Cassia obtusifolia (Sharon et al., 1992) and in soybean leaves (Johal and Rahe, 1988). Glyphosate treatment also induced mRNA transcription of Trp pathway enzymes and two stress-inducible enzymes unrelated to amino acid biosynthesis in Arabidopsis (Zhao et al., 1998). The specific regulatory control exerted by aromatic amino acids on the shikimate pathway can, however, not be clearly deduced from experiments using glyphosate, since this inhibitor may also affect other processes (Holländer and Amrhein, 1980; Binarová et al., 1994). The present study takes advantage of the significant substrate specificity of Trp decarboxylase (TDC, EC 4.1.1.28) for Trp and Tyr decarboxylase (TYDC, EC 4.1.1.25) for Tyr to modify the in vivo pool of aromatic amino acids in transgenic tobacco plants. This report also describes how the creation of artificial metabolic sinks for Trp, Tyr, or both affects the shikimate and phenylpropanoid pathways.
MATERIALS AND METHODS
TDC Tobacco Line
The T-201-1 tobacco line transformed with a full-length TDC cDNA gene isolated from Catharanthus roseus (De Luca et al., 1989) and expressed under the regulatory control of the cauliflower mosaic virus (CaMV) 35S promoter was obtained from Songstad et al. (1990).
TYDC Vector Construction
TYDC transgenic lines were obtained by transformation of tobacco with Agrobacterium tumefaciens cells containing a Papaver somniferum TYDC2 cDNA (Facchini and De Luca, 1994). The CaMV 35S promoter was isolated after digestion of the pBI-221D vector (CLONTECH Laboratories, Palo Alto, CA) with XbaI and HindIII (Promega, Madison, WI). The CaMV 35S promoter was then ligated to the pBI-102 vector (St-Pierre et al., 1996) previously digested with XbaI and HindIII to generate the pBI-102-35S vector containing the neomycin phosphotransferase II insert for kanamycin and streptomycin resistance. The TYDC fragment encoding a full-length open reading frame was obtained by XbaI digestion of the pBI-TYDC2i vector (Facchini and De Luca, 1994) and ligated to the pBI-102-35S construct. The resulting binary pBI-TYDC2 vector was then electroporated into A. tumefaciens cells (strain LBA 4404, CLONTECH Laboratories).
TYDC Tobacco Transformation
Seeds of tobacco (Nicotiana tabacum cv Xanthi; W.H. Perron, Laval, Québec, Canada) were sterilized and germinated in Magenta vessels containing 1% (w/v) agar and Murashige and Skoog (MS) basal medium (Murashige and Skoog, 1962). Transformation experiments with A. tumefaciens containing the TYDC2 gene were conducted according to the method of Horsch et al. (1985), and the transgenic TYDC2-expressing seedlings were transferred to a greenhouse to obtain seeds from mature plants. Progeny from TYDC2 and T-201-1 transgenic tobacco lines were then crossed to generate lines expressing both TDC and TYDC activities. The present study focuses on transgenic tobacco lines having the highest TDC and TYDC activities.
Northern Hybridization
Total RNA from 10-d-old tobacco seedlings was extracted following the procedure described in Jones et al. (1985). Samples containing 10 μg of RNA were separated by electrophoresis on a 1% (w/v) agarose gel containing formaldehyde and were transferred by capillarity in 10× SSC to nitrocellulose membranes (Schleicher & Schuell, Keene, NH). The 32P-labeled probes containing full-length TDC and TYDC fragments were produced by random priming (Feinberg and Vogelstein, 1984) and used for hybridization. Labeled blots were washed three times for 30 min and at 62°C with 4×, 2×, and 0.5× SSC.
Growth of Tobacco Seedlings for Physiological Studies
Transgenic and non-transformed control seeds were sterilized as above and were grown at 25°C in Magenta vessels containing 0.5 strength MS medium and 1% (w/v) agar. Seedlings were grown in darkness or exposed to light (16-h photoperiod, 10 μmol photons m−2 s−1) after 4 d of dark growth. Seedlings were harvested after 4, 6, 8, and 11 d for analysis of tryptamine, tyramine, chlorogenic acid, nicotine, and amino acids.
Analysis of Amines, Chlorogenic Acid, and Amino Acids
Fifteen tobacco seedlings were sonicated for 30 s in a 1.5-mL tube in the presence of 200 μL of methanol at a duty cycle of 75% and an output control of 6 (Branson, model 250 Sonifier). After incubating for 60 min at 60°C, the samples were centrifuged at 10,000g for 5 min. Twenty microliters of the supernatant was used for HPLC analysis of Trp, tryptamine, tyramine, and chlorogenic acid; another 50 μL was used for HPLC measurement of amino acids according to the method of Yao et al. (1995). HPLC data for Trp, tryptamine, tyramine, and chlorogenic acid were collected on a chromatograph (Waters, Milford, MA) equipped with a 600E system controller, a 717+ autosampler, and a 991 photodiode array detector. The mobile phase and column used were as in Yao et al. (1995).
Tyr was analyzed separately since the above procedure gave a poor resolution for this amino acid. Approximately 0.2 g of tobacco seedlings was sonicated as above in 5 mL of 100 mm Tris-HCl, pH 10.2, incubated for 60 min at 60°C, and centrifuged at 10,000g for 10 min. The supernatant was adjusted to pH 3.5 with 5 n HCl and extracted twice with 5 mL of ethyl acetate. The organic fractions were pooled and evaporated at 40°C under vacuum, then redissolved in 200 μL of methanol. Twenty microliters of the methanol fraction was analyzed with the same HPLC system as above using a scanning fluorescence detector (model 470, Waters) equipped with a 5-μL flow cell. The fluorescence detector was set for excitation and emission wavelengths of 274 and 304 nm, respectively, using an attenuation factor of 1×, a gain of 100×, and an emission bandwidth of 18 nm.
For nicotine determination, 15 tobacco seedlings were sonicated for 15 s in 250 μL of 28% (v/v) ethanol containing 5% (w/v) NH4OH. The homogenate was further incubated at 50°C for 60 min and centrifuged at 10,000g for 10 min. Twenty microliters of supernatant was used for HPLC analysis of nicotine using a 3.9- × 300-mm C18 reversed-phase column (Waters). The mobile phase was water/methanol (3:2, v/v) adjusted to pH 7.3 with phosphoric acid at a flow rate of 0.5 mL min−1, and the eluate was monitored at 256 nm. The UV fluorescence or absorption spectra and retention times of each metabolite was measured and compared with authentic standards (Sigma). Three consistent replicate analyses were performed, giving sds not exceeding 17% of mean values.
Enzyme Assays
Twenty-five seedlings were homogenized on ice in 1 mL of Tris-HCl (200 mm, pH 7.6) containing 4 mm EDTA and 25 mm mercaptoethanol. The homogenate was centrifuged at 14,000g for 10 min and desalted on a PD-10 column (Pharmacia Biotech, Uppsala). TDC, TYDC, and PAL activities were determined as described in De Luca et al. (1988), Facchini and De Luca (1994), and Zucker (1965), respectively. For measurement of DAHPS activity, approximately 0.1 g of seedlings was homogenized in 2 mL of 50 mm potassium phosphate, pH 7.0, with 0.25 m 1,3-propanediol and 0.5 mm phosphoenolpyruvate. The homogenate was centrifuged and desalted as above, and the DAHPS assay was performed as described in Method A of Schoner and Herrmann (1976).
Root-Curling Assay
Some transgenic tobacco lines exhibited a distinct root morphology characterized by the formation of an arch above agar medium and a delayed root penetration into agar. This phenotype was referred to as root-curling and its frequency and intensity were higher under low levels of nitrogen availability. This phenotype was exploited to assess the effects of indole-3-acetic acid (IAA) and auxin translocation inhibitors on its frequency. Fifty to 75 seeds were uniformly spread in a 150-mm diameter Petri dish containing 50 mL of 0.5 strength MS medium lacking nitrogen (ammonium nitrate was removed and potassium nitrate was replaced by an equimolar amount of potassium chloride) with 1% (w/v) agar. The root-curling phenotype was measured in the presence of 1 μm IAA or 1 μm concentrations of the auxin translocation inhibitors 2,3,5-triiodobenzoic acid (TIBA) and napthylphthalamic acid (NPA). Seedlings were germinated in darkness for 4 d and were exposed to light for a further 4 d. Seedlings were evaluated for root curling at d 8, and five replicates (one Petri dish per replicate) were performed for each treatment. Seedlings from each replicate were pooled and analyzed for Trp content as described above.
Temperature Effects on Germination Rate
Sterilized tobacco seeds were added to Petri dishes containing 0.5 strength MS medium with 1% (w/v) agar and germinated for 4 d in darkness at 14°C, 24°C, 30°C, or 34°C before evaluating the germination rate. Seedlings with roots longer than 2 mm were considered to have germinated.
RESULTS
TDC and TYDC Transgenic Tobacco Lines
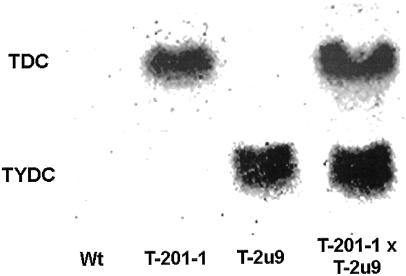
A total of 35 independent TYDC transgenic lines were produced and the TYDC activities measured in most of these lines were between one to five times the value of untransformed controls. Only two independently transformed lines (T-2u9 and T-2-36) had a TYDC activity approximately 50 times higher than the untransformed control line (data not showed). Transformation of tobacco with heterologous TDC and TYDC genes was confirmed by northern-blot analysis (Fig. 2), measurement of enzyme activities (Fig. 3, A and B), and accumulation of tryptamine and tyramine (Fig. 4, A and B). Untransformed control seedlings had undetectable or low levels of TDC and TYDC transcripts, whereas the T-201-1, T-2u9, and T-201-1 × T-2u9 lines, respectively, expressed TDC, TYDC, or both sets of transcripts (Fig. 2) along with the corresponding enzyme activities (Fig. 3, A and B).
Figure 2.
TDC and TYDC transcripts in different tobacco genotypes. Ten micrograms of total RNA extracted from 10-d-old seedlings were subjected to RNA-blot analysis using 32P-labeled pTDC5 (De Luca et al., 1989) and cTYDC2 (Facchini and De Luca, 1994) inserts (specific activity, 2 × 108 to 5 × 108 cpm μg−1).
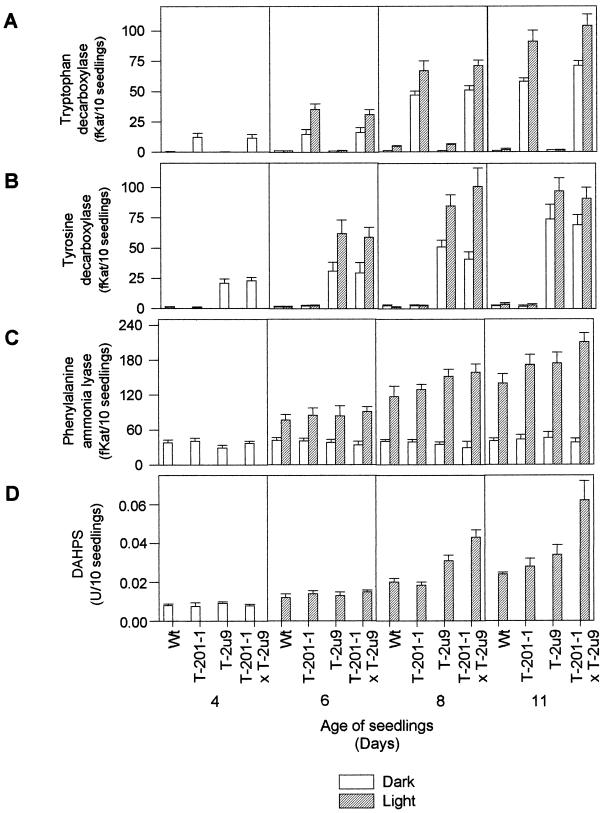
Figure 3.
TDC, TYDC, PAL, and DAHPS enzymatic activities in different genotypes of tobacco seedlings. DAHPS activity was only determined for light treatments. Three replicates were performed for each treatment, and bars represent sd.
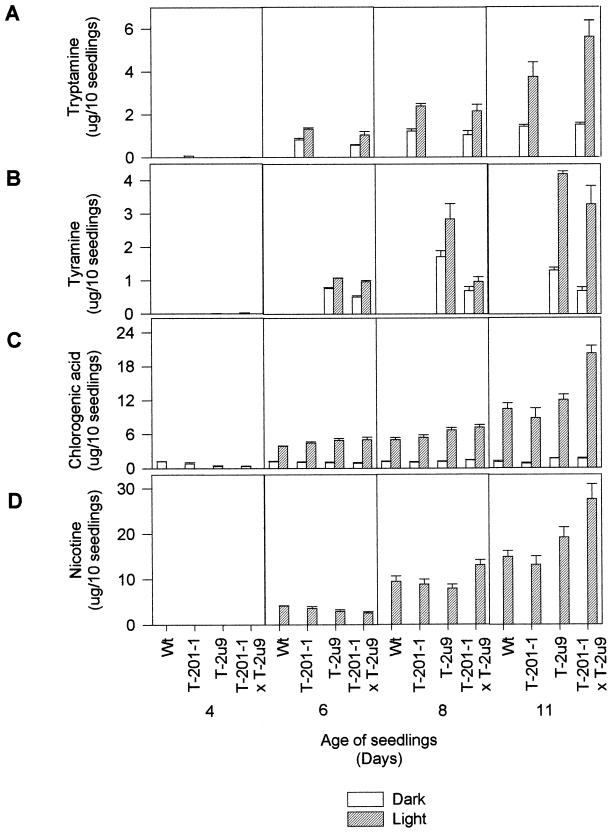
Figure 4.
Tryptamine, tyramine, chlorogenic acid, and nicotine levels in different genotypes of tobacco seedlings. Nicotine was only determined for light treatments. Three replicates were performed for each treatment, and bars represent sd.
TDC and TYDC activities reached their highest levels in older seedlings exposed to light, and the level of enzyme activities correlated well with the accumulation pattern of tryptamine and tyramine (Figs. 3, A and B, and 4, A and B). Tobacco lines expressing TDC or TYDC accumulated up to 3.8 and 4 μg/10 seedlings of tryptamine and tyramine, respectively, in 11-d-old light-grown seedlings (Fig. 4, A and B). The T-201-1 × T-2u9 line, which expressed both enzyme activities, accumulated up to 5.5 and 3.1 μg/10 seedlings of tryptamine and tyramine, respectively (Fig. 4, A and B). The additive effects of combined TDC and TYDC expression resulted in an almost doubling of total enzyme activities (Fig. 3, A and B) and aromatic amine levels in the cross (Fig. 4, A and B). Similar crosses between line T-201-1 and two other TYDC-expressing lines produced similar additive profiles of tryptamine and tyramine expression (data not showed). A correlation between TDC activity, tryptamine production, and TDC mRNA levels had already been established in 13 different TDC transgenic tobacco lines by Songstad et al. (1990). No growth rate differences were observed in transgenic lines compared with the untransformed control line.
Previous kinetic studies with cloned TYDC produced in Escherichia coli showed that dopa was a better substrate than Tyr (Facchini and De Luca, 1996). Therefore, transgenic plants were also analyzed for the presence of dopamine, but none was detected in the T-2u9 or T-201-1 × T-2u9 lines (data not shown). This suggests that dopa probably does not occur at significant levels in tobacco seedlings.
Reduction of Trp and Tyr Pools Affects the Phe Pool
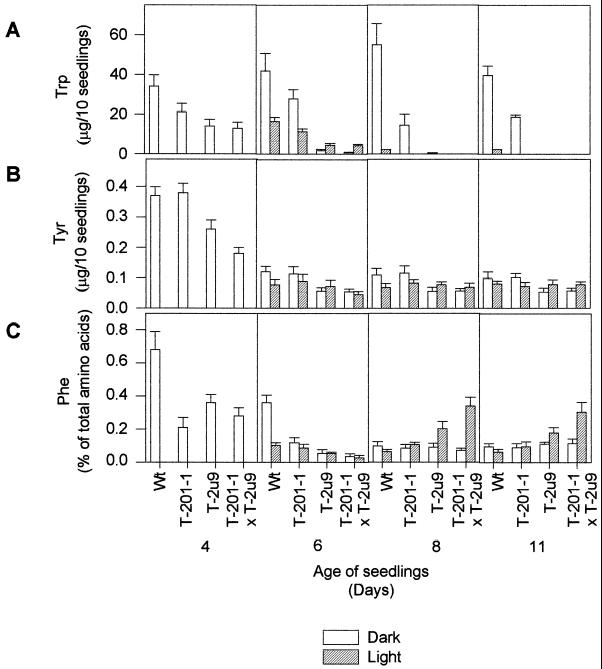
Expression of TDC and TYDC in transgenic tobacco seedlings caused a significant decrease in Trp (Fig. 5A), Tyr (Fig. 5B), and Phe (Fig. 5C) pools. Four-day-old etiolated transgenic TDC × TYDC seedlings contained 55%, 50%, and 60% less Trp, Tyr, and Phe, respectively, than non-transformed control seedlings (Fig. 5, A–C, d 4). Eight-day-old etiolated wild-type seedlings contained 3.5, 92, and 50 times higher levels of Trp than those found in the T-201-1, T-2u9, and in the cross, respectively (Fig. 5A, d 8). The most important attenuation of the Trp pool occurred in the TYDC transgenic lines, even though TYDC does not accept Trp as a substrate. The Tyr level, which was less affected by the light regime than that of Trp and Phe, was typically decreased by 10% to 50% in etiolated seedlings of the two lines transformed with TYDC, T-2u9, and T-201-1 × T-2u9 compared with the control (Fig. 5B). Line T-201-1 had similar levels of Tyr compared with the wild type for all treatments investigated (Fig. 5B). The pool of Phe was two to seven times lower in 4- and 6-d-old etiolated seedlings of all transgenic lines compared with non-transformed controls, whereas no differences were found in 8- and 11-d-old etiolated seedlings (Fig. 5C). It is noteworthy that 8- and 11-d-old TYDC or TDC × TYDC seedlings exposed to light had 80% to 500% higher levels of Phe than control or TDC-expressing seedlings (Fig. 5C, d 8 and 11). These data point out the significant effects caused by light on aromatic amino acid pools, and show how the transformation of tobacco with aromatic amino acid decarboxylases successfully perturbs this amino acid balance.
Figure 5.
Aromatic amino acid levels in different genotypes of tobacco seedlings. Three replicates were performed for each treatment, and bars represent sd.
Transgenic Tobacco Seedlings with Increased DAHPS and PAL Activities Have Higher Chlorogenic Acid Levels
Expression of TDC and TYDC in transgenic tobacco also had important consequences for DAHPS (Fig. 3D) and PAL (Fig. 3C) activities during light-activated seedling development. PAL and DAHPS activities were 35% and 145% higher, respectively, in 11-d-old light grown TDC × TYDC seedlings than in comparable light-treated control seedlings (Fig. 3, C and D; d 11). It is relevant that the significant increase of DAHPS activity only occurred in the cross, compared with the modest 10% and 20% increases observed in TDC- or TYDC-expressing plants. These data suggest that expression of both TDC and TYDC in the same plant significantly activates both the shikimate and phenylpropanoid pathways in light-grown seedlings (Fig. 3). It is well established that light activates the plastid-localized shikimate pathway (Della-Cioppa et al., 1986; Schmid et al., 1992; Weaver and Herrmann, 1997), as well as the phenylpropanoid pathway (Hahlbrock and Scheel, 1989). Light treatment of etiolated seedlings did activate the accumulation of chlorogenic acid in control and transgenic seedlings (Fig. 4C), but the pool of chlorogenic acid was increased by almost 100% in the cross compared with the non-transformed control (Fig. 4C, d 11).
Transgenic Tobacco Seedlings Display Altered Amino Acid Accumulation and Enhanced Nicotine Accumulation
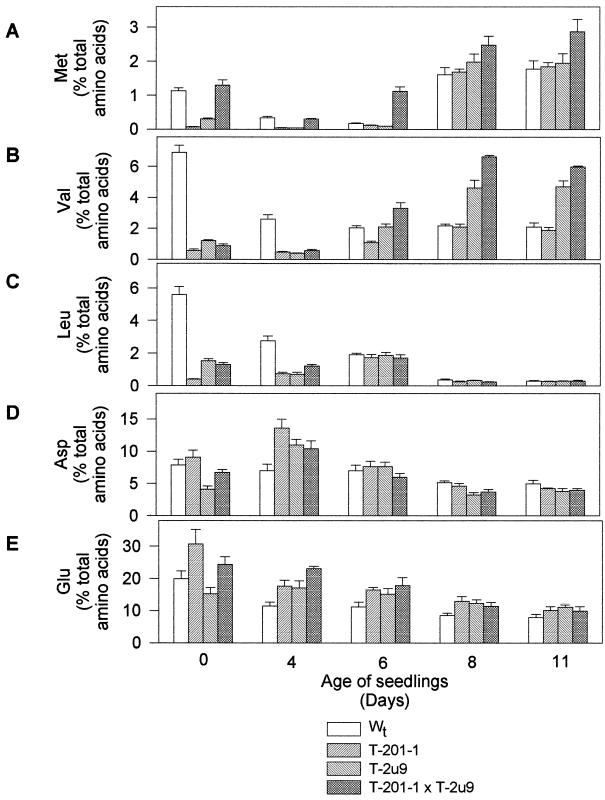
Expression of TDC and TYDC also affected the concentrations of amino acids in transgenic tobacco seeds compared with non-transformed controls (Fig. 6). Aromatic amino acids, which were repeatedly measured in different sets of dry seeds, were not detectable in all tobacco lines investigated (data not shown), but the concentrations of Met, Val, and Leu were four to 15 times lower in seeds of TDC- and TYDC-expressing tobacco than in wild type. However, Met levels were equal to those of non-transformed seeds (Fig. 6, A–C, d 0) in tobacco expressing both genes. These results indicate that the expression of TDC and/or TYDC in tobacco plants will affect amino acid pools occurring in transgenic seeds. Some variations in the pools of these amino acids were also observed during germination. Interestingly, the Met and Val levels increased more rapidly in the transgenic cross compared with nontransformed controls during seedling growth (Fig. 6, A and B).
Figure 6.
Non-aromatic amino acid levels in different genotypes of tobacco seedlings. Three replicates were performed for each treatment, and bars represent sd.
Light treatment of etiolated seedlings also activated the accumulation of nicotine in control and transgenic seedlings (Fig. 4D). The nicotine content in 11-d-old transgenic seedlings from the TDC × TYDC cross was almost 100% higher than that found in non-transformed controls (Fig. 4D).
Transformed Tobacco Displays Temperature-Sensitive Germination and Root-Curling Phenotypes
The germination rate of transgenic and control tobacco seeds was measured at 14°C, 24°C, 30°C, and 34°C (Table I). While the germination of control and T-201-1 seeds was not affected by the growth temperature, T-2u9 and T-201-1 × T-2u9 seeds showed decreased germination at 30°C, and severely decreased germination at 34°C (Table I). However, the temperature-sensitive germination of seeds could be eliminated by returning them to growth at 24°C (data not showed).
Table I.
Effects of temperature on germination of different tobacco genotypes
| Temperature | Germination Rate
|
|||
|---|---|---|---|---|
| Wild type | T-201-1 | T-2u9 | T-201-1 × T-2u9 | |
| °C | % | |||
| 14 | >95 | >95 | >95 | >95 |
| 24 | >95 | >95 | >95 | >95 |
| 30 | >95 | 94.3 (4.7) | 75.7 (5.7) | 78.1 (4.1) |
| 34 | 91.4 (3.8) | 90.2 (5.1) | 13.4 (3.9) | 11.2 (2.9) |
Three replicates were performed for each treatment and values provided in parentheses represent sd.
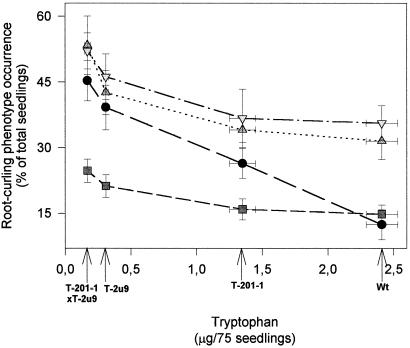
When tobacco seedlings were grown on MS medium lacking nitrogen, the T-201-1, T-2u9, and T-201-1 × T-2u9 lines displayed the root-curling phenotype (Fig. 7). The frequency of root curling appeared to increase as a result of the decreased Trp concentration observed in the different transgenic lines. The root-curling phenotype, which was particularly evident in TYDC and TDC × TYDC lines, could be attenuated by treatment with IAA (Fig. 7) or L-Trp (data not showed). Treatment of nontransformed control lines with TIBA or NPA, which are inhibitors of auxin transport, increased the frequency of root curling by 2- to 3-fold, whereas no significant effects were noted in TYDC and TDC × TYDC lines (Fig. 7).
Figure 7.
Relationship between root curling and Trp content in different genotypes of tobacco seedlings. Seeds were germinated in the presence of IAA (1 μm), 2,3,5-triiodobenzoic acid (1 μm), or N-1-naphthylphthalamic acid (1 μm). Five replicates were performed for each treatment and bars represent sd. , TIBA; ▿, NAA; ●, control; ░⃞, IAA.
Mature Tobacco Plants Expressing TDC, TYDC, or Both Genes Accumulate Less Nicotine
Leaves from 72-d-old flowering tobacco plants were harvested and analyzed for nicotine, tyramine, tryptamine, and chlorogenic acid levels (Table II). In contrast to the results obtained for seedlings (Fig. 4D), expression of TDC, TYDC, or both significantly affected the ability of transgenic mature plants to accumulate nicotine. The level of nicotine in leaves of 72-d-old tobacco plants was reduced by 19% to 45% in TDC or TYDC expressing lines compared with wild type (Table II). Adult TDC × TYDC tobacco plants also failed to show the additive biosynthesis of tryptamine and tyramine observed in seedlings (Fig. 4, A and B). For example, the level of tyramine in 72-d-old TDC × TYDC plants was 3.6 times lower than for the TYDC line (Table II). These results suggest that the expression of TDC or TYDC genes in older tobacco plants effectively redirects precursors into aromatic amines rather than into nicotine. The data also suggest that the availability of shikimate pathway substrates may become a limiting factor in the formation of aromatic amines in mature transgenic tobacco.
Table II.
Levels of tryptamine, tyramine, nicotine, and chlorogenic acid in different genotypes of 72-d-old tobacco plants grown under greenhouse conditions
| Genotype | Nicotine | Tyramine | Tryptamine | Chlorogenic Acid |
|---|---|---|---|---|
| μg g−1 dry wt | ||||
| Wild type | 1,047 (110) | 95 (12) | NDa | 20,833 (2,108) |
| T-201-1 | 844 (192) | 53 (17) | 10,244 (1,194) | 16,933 (2,520) |
| T-2u9 | 571 (66) | 4,605 (795) | ND | 13,153 (2,052) |
| T-201-1 × T-2u9 | 580 (69) | 1,263 (151) | 8,042 (907) | 23,489 (2,005) |
Three replicates were performed for each treatment and values provided in parentheses represent sd.
ND, Not detectable.
DISCUSSION
Expression of TDC, TYDC, or Both in Tobacco Alters the Amino Acid Balance of Seeds and Etiolated Seedlings
The amino acids required for seed germination and etiolated seedling growth are essentially supplied by the degradation of storage proteins (King and Gifford, 1997). Transgenic tobacco seedlings expressing TDC, TYDC, or both displayed variable altered aromatic and non-aromatic amino acids pools compared with the wild type (Figs. 5 and 6). Similar alterations of amino acid levels occurred in potato tubers from TDC-expressing transgenic plants (Yao et al., 1995) and in glyphosate-treated tobacco cell cultures (Dyer et al., 1988). These results clearly indicate that altering flux through the shikimate pathway can seriously affect the balance of both aromatic and non-aromatic amino acids. The present study also shows that amino acid pool variations were most apparent in transgenic seeds and in etiolated seedlings, whereas they were attenuated in older, light-treated transgenic seedlings (Figs. 5 and 6). The light-mediated increases of PAL and DAHPS enzyme activities (Fig. 3, C and D) may account for the capacity of light-exposed TDC or TYDC transgenic tobacco seedlings to better compensate for the artificial Trp and Tyr sinks introduced in these transgenic lines compared with wild type.
Expression of TDC, TYDC, or Both in Tobacco Alters Carbon Flux through the Shikimate Pathway
The biosynthesis of aromatic amino acids is in part regulated by feedback allosteric control (Bentley, 1990). A number of cellular metabolites, including Phe, Tyr, and Trp (Bentley, 1990; Poulsen and Verpoorte, 1991; Benesova and Bode, 1992), can feedback activate or inhibit DAHPS or the branch point enzymes anthranilate synthase and chorismate mutase (Fig. 1). Experimental evidence for feedback control of tobacco DAHPS by any of the three aromatic amino acids is lacking, but there have been studies documenting the activation of DAHPS by Tyr and Trp in carrot and potato (Suzich et al., 1985; Pinto et al., 1988). The increase in DAHPS activity observed in older light-treated TYDC expressing seedlings compared with the non-transformed controls (Fig. 3D) supports the hypothesis that decreasing the level of Tyr, Trp, and Phe activates this shikimate pathway enzyme. The additive increase in DAHPS activity in seedlings expressing both TDC and TYDC was coupled to a rise in the Phe pool, a rise in PAL activity, and an almost doubling of chlorogenic acid levels. These combined results provide clear evidence that decreasing aromatic amino acid pool sizes will enhance metabolic flux through the shikimate pathway in developing seedlings.
The present report confirms and extends previous studies performed with TDC-transformed canola (Chavadej et al., 1994) and potato plants (Yao et al., 1995), which also described some interesting metabolic alterations. Canola plants that expressed TDC produced transgenic seeds with an indole glucosinolate content that was 3% of that in untransformed seeds (Chavadej et al., 1994). This dramatic effect showed that the expression of TDC could be used to redirect Trp into tryptamine rather than into indole glucosinolates. In transgenic potato tubers, the expression of TDC decreased the pools of Phe and affected their ability to accumulate wound-induced phenylpropanoids (Yao et al., 1995).
Root-Curling and Temperature-Sensitive Seed Germination Phenotypes in Transgenic Tobacco Seedlings
The root-curling phenotype observed in transgenic seedlings was directly correlated with the depletion of Trp content, and root curling could be attenuated or amplified by the addition of IAA or an inhibitor of IAA translocation, respectively (Fig. 7). It is suggested that the lower Trp pool size occurring in TDC and TYDC transgenic tobacco seedlings may affect the biosynthesis of IAA or compounds with auxin-related activity. Trp is a precursor to IAA (Müller et al., 1998), although a number of alternative Trp-independent pathways have also been proposed (Normanly, 1997). It is noteworthy that the root-curling phenotype was transient, since roots eventually re-established their typical positive gravitropic growth in older seedlings. A possible explanation for these results may come from stable isotope labeling studies, which revealed that the biosynthesis of IAA in coleoptiles of 3-d-old maize seedlings relies on a Trp-dependent pathway, whereas those from 6- to 10-d-old seedlings rely on a Trp-independent pathway (Bartel, 1997). A correlation between an attenuated Trp pool size and an altered wavy root growth pattern has also been reported in Arabidopsis anthranilate synthase mutants (Rutherford et al., 1998). However, our transgenic tobacco lines did not express the wavy root phenotype observed in the Arabidopsis mutants.
Tobacco seeds expressing both TDC and TYDC genes did not germinate when grown at 34°C, whereas they germinated normally upon returning them to growth at 24°C. The reasons for this phenotype are not clear, but the altered amino acid balance observed in transgenic seeds suggests that a number of other undetermined biochemical changes may be responsible for the inability of these seeds to germinate at higher temperatures.
Depletion of Trp and Tyr Pools Affects Metabolite Biosynthesis in Other Pathways
Recent studies have shown that glyphosate, which inhibits the shikimate pathway, and acifluorfen, which induces oxidative stress in Arabidopsis, activated several genes involved in the biosynthesis of Trp and the Trp-derived phytoalexin camalexin. The stress-inducible genes for glutathione S-transferase and chalcone synthase were also activated by these treatments (Zhao and Last, 1996, 1998). Based on these results, it was suggested that regulation of the Trp pathway enzymes under amino acid deprivation was largely a stress response to trigger-increased biosynthesis of secondary metabolites. The results presented in Figures 4 and 5 demonstrate that the coordinated depletion of aromatic amino acids in tobacco expressing both TDC and TYDC leads to a doubling of nicotine and chlorogenic acid and the accumulation of tryptamine and tyramine. Although the expression of stress-related genes (such as glutatione S-transferase and chalcone synthase) have not been monitored, there is no visible evidence that TDC- and TYDC-expressing tobacco plants undergo a stress response as a result of decreased aromatic amino acid pools. In addition, mature transgenic tobacco accumulates reduced levels of nicotine, which is not consistent with this hypothesis.
The dramatic results presented in this report suggest that pathway engineering in plants remains highly variable, as a range of subtle homeostatic mechanisms become involved when attempts are made to perturb cell physiology (Westerhoff, 1995). The present study, which illustrates some of the complex cross-pathway metabolic interactions involved, emphasizes the need to better understand the regulation of biosynthesis and partitioning of metabolites to improve our ability to perform rational metabolic engineering.
Footnotes
This work was supported by a research grant (to V.D.L.) and a postdoctoral grant (to G.G.) from the Natural Sciences and Engineering Research Council of Canada.
LITERATURE CITED
- Bartel B. Auxin biosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:51–56. doi: 10.1146/annurev.arplant.48.1.51. [DOI] [PubMed] [Google Scholar]
- Bate NJ, Weiting JO, Meromi A, Meromi N-H, Doerner PW, Dixon RA, Lamb CJ, Elkind Y. Quantitative relationship between phenylalanine ammonia-lyase levels and phenylpropanoid accumulation in transgenic tobacco identifies a rate-determining step in natural products synthesis. Proc Natl Acad Sci USA. 1994;91:7608–7612. doi: 10.1073/pnas.91.16.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesova M, Bode R. Chorismate mutase isoforms from seeds and seedlings of Papaver somniferum. Phytochemistry. 1992;31:2983–2987. [Google Scholar]
- Bentley R. The shikimate pathway: a metabolic tree with many branches. CRC Crit Rev Biochem Mol Biol. 1990;25:307–384. doi: 10.3109/10409239009090615. [DOI] [PubMed] [Google Scholar]
- Binarová P, Cvikrová M, Havlicky T, Eder J, Plevkovà J. Changes of shikimate pathway in glyphosate tolerant alfalfa cell lines with reduced embryogenic ability. Biol Plant. 1994;36:65–73. [Google Scholar]
- Chavadej S, Brisson N, McNeil JN, De Luca V. Redirection of tryptophan leads to production of low indole glucosinolate canola. Proc Natl Acad Sci USA. 1994;91:2166–2170. doi: 10.1073/pnas.91.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Cioppa G, Bauer SsC, Klein BK, Shah DM, Fraley RT, Kishore GM. Translocation of the precursor of 5-enolpyruvylshikimate 3-phosphate synthase into chloroplasts of higher plants in vitro. Proc Natl Acad Sci USA. 1986;83:6873–6877. doi: 10.1073/pnas.83.18.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca V, Alvarez Fernandez F, Campbell D, Kurz WGW. Developmental regulation of enzymes of indole alkaloid biosynthesis in Catharanthus roseus. Plant Physiol. 1988;86:447–450. doi: 10.1104/pp.86.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca V, Marineau C, Brisson N. Molecular cloning and analysis of cDNA encoding a plant tryptophan decarboxylase: comparison with animal dopa decarboxylases. Proc Natl Acad Sci USA. 1989;86:2582–2586. doi: 10.1073/pnas.86.8.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer WE, Weller SC, Bressan RA, Herrmann KM. Glyphosate tolerance in tobacco (Nicotiana tabacumL.) Plant Physiol. 1988;88:661–666. doi: 10.1104/pp.88.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkind Y, Edwards R, Mavandad M, Hedrick SA, Ribak O, Dixon RA, Lamb CJ. Abnormal plant development and down-regulation of phenylpropanoid biosynthesis in transgenic tobacco containing a heterologous phenylalanine ammonia-lyase gene. Proc Natl Acad Sci USA. 1990;87:9057–9061. doi: 10.1073/pnas.87.22.9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchini PJ, De Luca V. Differential and tissue-specific expression of a gene family for tyrosine/dopa decarboxylase in opium poppy. J Biol Chem. 1994;269:26684–26690. [PubMed] [Google Scholar]
- Facchini PJ, De Luca V. Expression in Escherichia coliand partial characterization of two tyrosine/dopa decarboxylases from opium poppy. Phytochemistry. 1996;38:1119–1126. doi: 10.1016/0031-9422(94)00814-a. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1984;137:266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fukasawa-Akada T, Kung S-D, Watson JC. Phenylalanine ammonia-lyase gene structure, expression, and evolution in Nicotiana. Plant Mol Biol. 1996;30:711–722. doi: 10.1007/BF00019006. [DOI] [PubMed] [Google Scholar]
- Hahlbrock K, Scheel D. Physiology and molecular biology of phenylpropanoid metabolism. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:347–369. [Google Scholar]
- Hatton D, Sablowski R, Yung M-H, Smith C, Schuch W, Bevan M. Two classes of cis sequences contribute to tissue-specific expression of a PAL2promoter in transgenic tobacco. Plant J. 1995;7:859–876. doi: 10.1046/j.1365-313x.1995.07060859.x. [DOI] [PubMed] [Google Scholar]
- Henstrand JM, McCue KF, Brink K, Handa AK, Herrmann KM, Conn EE. Light and fungal elicitor induce 3-deoxy-d-arabino-heptulosonate 7-phosphate synthases mRNA in suspension cultured cells of parsley (Petroselinum crispumL.) Plant Physiol. 1992;98:761–763. doi: 10.1104/pp.98.2.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann KM. The shikimate pathway as an entry to aromatic secondary metabolism. Plant Physiol. 1995;107:7–12. doi: 10.1104/pp.107.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holländer H, Amrhein N. The site of inhibition of the shikimate pathway by glyphosate: inhibition by glyphosate of phenylpropanoid synthesis in buckwheat (Fagopyrum esculentumMoench.) Plant Physiol. 1980;66:823–839. doi: 10.1104/pp.66.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT. A simple and general method for transferring genes into plants. Science. 1985;277:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Johal GS, Rahe JE. Glyphosate, hypersensitivity and phytoalexin accumulation in the incompatible bean anthracnose host-parasite interaction. Physiol Mol Plant Pathol. 1988;32:267–281. [Google Scholar]
- Jones JDG, Dunsmuir P, Bedbrook J. High level expression of introduce chimeric gene. EMBO J. 1985;4:2411–2418. doi: 10.1002/j.1460-2075.1985.tb03949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B, Dong X, Ausubel FM, Fink GR. Differential induction of 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase genes in Arabidopsis thalianaby wounding and pathogenic attack. Proc Natl Acad Sci USA. 1991;88:8821–8825. doi: 10.1073/pnas.88.19.8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JE, Gifford DJ. Amino acid utilization in seeds of loblolly pine during germination and early seedling growth. Plant Physiol. 1997;113:1125–1135. doi: 10.1104/pp.113.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A, Hillebrand H, Weiler EW. Indole-3-acetic acid is synthesized from l-tryptophan in roots of Arabidopsis thaliana. Planta. 1998;206:362–369. doi: 10.1007/s004250050411. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Normanly J. Auxin metabolism. Physiol Plant. 1997;100:431–442. [Google Scholar]
- Pinto JEBP, Dyer WE, Weller SC, Herrmann KM. Glyphosate induces 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase in potato (Solanum tuberosumL.) cells grown in suspension culture. Plant Physiol. 1988;87:891–893. doi: 10.1104/pp.87.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen C, Verpoorte R. Roles of chorismate mutase, isochorismate synthase and anthranilate synthase in plants. Phytochemistry. 1991;30:377–386. [Google Scholar]
- Rubery PH, Northcote DH. Site of phenylalanine ammonia-lyase activity and the synthesis of lignin during xylem differentiation. Nature. 1968;219:1230–1232. doi: 10.1038/2191230a0. [DOI] [PubMed] [Google Scholar]
- Rutherford R, Gallois P, Masson PH. Mutations in Arabidopsis thalianagenes involved in the tryptophan biosynthesis pathway affect root waving on tilted agar surfaces. Plant J. 1998;16:145–154. doi: 10.1046/j.1365-313x.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- Sammons RD, Gruys KJ, Anderson KS, Johnson KA, Sikorski JA. Reevaluating glyphosate as a transition-state inhibitor of EPSP synthase: identification of an EPSP synthase-center-dot-EPSP-center-dot-glyphosate ternary complex. Biochemistry. 1995;34:6433–6440. doi: 10.1021/bi00019a024. [DOI] [PubMed] [Google Scholar]
- Schmid J, Amrhein N. Molecular organization of the shikimate pathway in higher plants. Phytochemistry. 1995;39:737–749. [Google Scholar]
- Schmid J, Schaller A, Leibinger U, Boll W, Amrhein N. The in vitrosynthesized tomato shikimate kinase precursor is enzymatically active and is imported and processed to the mature enzyme by chloroplasts. Plant J. 1992;2:375–383. [PubMed] [Google Scholar]
- Schoner R, Herrmann KM. 3-Deoxy-d-arabino-heptulosonate 7-phosphate synthase: purification, properties, and kinetics of the tyrosine-sensitive isoenzyme from Escherichia coli. J Biol Chem. 1976;18:5440–5447. [PubMed] [Google Scholar]
- Sharon A, Amsellem Z, Gressel J. Glyphosate suppression of an elicited defense response. Plant Physiol. 1992;98:654–659. doi: 10.1104/pp.98.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songstad DD, De Luca V, Brisson N, Kurz WGW, Nessler CL. High levels of tryptamine accumulation in transgenic tobacco expressing tryptophan decarboxylase. Plant Physiol. 1990;94:1410–1413. doi: 10.1104/pp.94.3.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre B, Bertrand C, Camirand A, Cappadocia M, Brisson N. The starch phosphorylase gene is subjected to different modes of regulation in starch-containing tissues of potato. Plant Mol Biol. 1996;30:1087–1098. doi: 10.1007/BF00019544. [DOI] [PubMed] [Google Scholar]
- Suzich JA, Dean JFD, Herrmann KM. 3-Deoxy-d-arabinoheptulosonate 7-phosphate synthase from carrot root (Daucus carota) is a hysteretic enzyme. Plant Physiol. 1985;79:765–770. doi: 10.1104/pp.79.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Fukuda Y, Shinshi H. Studies on elicitor-signal transduction leading to differential expression of defense genes in cultured tobacco cells. Plant Cell Physiol. 1995;36:281–289. [Google Scholar]
- Weaver LM, Herrmann KM. Dynamics of the shikimate pathway in plants. Trends Plant Sci. 1997;2:346–350. [Google Scholar]
- Westerhoff HV. Subtlety in control-metabolic pathway. Trends Biotechnol. 1995;13:242–244. doi: 10.1016/S0167-7799(00)88955-6. [DOI] [PubMed] [Google Scholar]
- Whetten R, Sederoff R. Lignin biosynthesis. Plant Cell. 1995;7:1001–1013. doi: 10.1105/tpc.7.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K, De Luca V, Brisson N. Creation of an artificial metabolic sink for tryptophan modifies the accumulation of phenylpropanoid compounds in potato and causes altered susceptibility to Phytophtora infestans. Plant Cell. 1995;7:1787–1799. doi: 10.1105/tpc.7.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Last RL. Coordinate regulation of the tryptophan biosynthetic pathway and indolic phytoalexin accumulation in Arabidopsis. Plant Cell. 1996;8:2235–2244. doi: 10.1105/tpc.8.12.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Williams CC, Last RL. Induction of Arabidopsis tryptophan pathway enzymes and camalexin by amino acid starvation, oxidative stress, and an abiotic elicitor. Plant Cell. 1998;10:359–370. doi: 10.1105/tpc.10.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. Induction of phenylalanine deaminase by light and its relation to chlorogenic acid synthesis in potato tuber tissue. Plant Physiol. 1965;40:779–784. doi: 10.1104/pp.40.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]