Abstract
Short-term Al treatment (90 μm Al at pH 4.5 for 1 h) of the distal transition zone (DTZ; 1–2 mm from the root tip), which does not contribute significantly to root elongation, inhibited root elongation in the main elongation zone (EZ; 2.5–5 mm from the root tip) to the same extent as treatment of the entire maize (Zea mays) root apex. Application of Al to the EZ had no effect on root elongation. Higher genotypical resistance to Al applied to the entire root apex, and specifically to the DTZ, was expressed by less inhibition of root elongation, Al accumulation, and Al-induced callose formation, primarily in the DTZ. A characteristic pH profile along the surface of the root apex with a maximum of pH 5.3 in the DTZ was demonstrated. Al application induced a substantial flattening of the pH profile moreso in the Al-sensitive than in the Al-resistant cultivar. Application of indole-3-acetic acid to the EZ but not to the meristematic zone significantly alleviated the inhibition of root elongation induced by the application of Al to the DTZ. Basipetal transport of exogenously applied [3H]indole-3-acetic acid to the meristematic zone was significantly inhibited by Al application to the DTZ in the Al-sensitive maize cv Lixis. Our results provide evidence that the primary mechanisms of genotypical differences in Al resistance are located within the DTZ, and suggest a signaling pathway in the root apex mediating the Al signal between the DTZ and the EZ through basipetal auxin transport.
The initial effect of Al toxicity is the inhibition of root elongation, a dramatic effect occurring within minutes after application. It is generally accepted that the root apex plays the major role in Al perception and response (for recent reviews, see Delhaize and Ryan, 1995; Horst, 1995; Kochian, 1995; Taylor, 1995; Rengel, 1996). This is well demonstrated by the fact that: (a) Al accumulation as an indicator of Al sensitivity occurs in the distal parts of the root apex (Delhaize et al., 1993a; Llugany et al., 1994; Sivaguru and Horst, 1998); (b) Al-resistance mechanisms, such as the release of Al-complexing organic compounds, are confined mainly to the root apex (Horst et al., 1982; Delhaize et al., 1993b; Pellet et al., 1995); and (c) callose formation, as a sensitive marker of Al sensitivity, is induced primarily in apical cells of the outer cortex (Wissemeier et al., 1987; Zhang et al., 1994; Wissemeier and Horst, 1995; Sivaguru and Horst, 1998). However, the question of the primary target of the Al effect remained open until recently.
Ryan et al. (1993) showed that the root tip was most Al sensitive. Sivaguru and Horst (1998) presented evidence that the distal transition zone (DTZ) is the most Al-susceptible zone of the primary root of the Al-sensitive maize (Zea mays) cv Lixis. Subsequently, Sivaguru et al. (1999a) and Horst et al. (1999) showed that Al leads to alterations in the organization of microtubules and actin microfilaments, which were most severe in the DTZ. Although it is still a matter of debate whether Al affects the root symplastically or apoplastically, evidence increases that the apoplast plays the major role in Al perception (Horst, 1995). Al binds strongly to the cell wall of root epidermal and cortical cells (Delhaize et al., 1993a), and Horst et al. (1999) demonstrated the role of pectin content in different apical root zones of maize for the Al response. Apart from negative charge properties of the cell wall and its cation exchange capacity, the exudation of organic compounds complexing Al (Delhaize et al., 1993b; Basu et al., 1994; Pellet et al., 1995) and the maintenance of higher apoplastic pH (Degenhardt et al., 1998) may play an additional or even more important role in the response of different species and genotypes to Al.
In this study we extend our former findings about the spatial sensitivity of the primary root of maize on genotypical differences between the Al-sensitive maize cv Lixis and the Al-resistant cv ATP-Y. We paid particular attention to the relationship between root surface pH and root-growth dynamics as affected by Al. Furthermore, we considered the role of indole-3-acetic acid (IAA) in Al toxicity, testing the hypothesis that Al might influence basipetal IAA transport in the root cortex, which is considered to contribute to the regulation of root growth and development (Hasenstein and Evans, 1988; Kerk and Feldman, 1994; Ruegger et al., 1997).
MATERIALS AND METHODS
Plant Material, Growth Conditions, and Experimental Treatments
Seeds of the maize (Zea mays L.) cv ATP-Y, which is classified as Al resistant, and cv Lixis, which is classified as Al sensitive by Llugany et al. [1994]; Horst et al. 1997), were soaked in tap water for 8 h. Selected seeds of equal size and shape were germinated in filter paper rolls moistened with nutrient solution for 3 d in a growth chamber under controlled environment conditions with 70% relative air humidity, 30°C/28°C day/night temperature, a day/night cycle of 16 h/8 h, and a 300 μm m−2 s−1 photon flux density.
All experiments were conducted with intact plants. Uniform seedlings with primary root lengths of about 7 to 10 cm were selected for the experiments. Seedlings were adapted to low pH by stepwise lowering of pH over 24 h before the beginning of the experiments.
Local Al treatment was provided by agarose blocks containing nutrient solution and 0.6% (w/v) low-gelling-temperature agarose (Fluka, Deisenhofen, Germany) (1.2% for the electrophysiological experiments and measurements of the partial root elongation). The nutrient solution had the following composition: CaSO4, 250 μm; KNO3, 400 μm; MgSO4, 100 μm; FeEDDHA, 20 μm; MnSO4, 1 μm; ZnSO4, 0.2 μm; CuSO4, 0.2 μm; KH2PO4, 10 μm; H3BO3, 8 μm; (NH4)6Mo7O24, 0.1 μm; and NH4NO3, 200 μm (pH 4.3).
For the study on the short-term effects of Al, the treatment duration was 1 h in all experiments at 30°C temperature in a growth chamber, except for the electrophysiological experiments, which were conducted under a 21°C to 23°C ambient temperature. The Al supply was a 90 μm monomeric Al obtained from an Al atomic spectroscopy standard solution (AlCl3·6H2O, 1,000 mg L−1, Fluka). To achieve a final monomeric Al concentration of 90 μm in the agarose gel, 300 μm Al was added to the cooled solution (measured by means of the aluminon method according to Kerven et al. [1989]; see also Sivaguru and Horst, 1998).
Determination of Callose in 1-mm Root Segments
Al was applied to specific apical root zones using the polyvinylchloride (PVC) block system previously described by Sivaguru and Horst (1998). After the Al treatment, 2-cm root tips were fixed in 96% (v/v) ethanol to avoid the formation of wound callose. The segments were then dissected, blotted dry, and transferred to Eppendorf cups containing 1 mL of 1 m NaOH. Each sample containing two 1-mm root segments was ultrasonicated (Bandelin Sonopuls, Bandelin Electronics, Berlin) for 40 s. Subsequently, the cups containing the samples were heated in an 80°C water bath for 20 min to solubilize the callose from the disintegrated cell walls, and were then centrifuged for 12 min at 12,000 min−1 at room temperature. Callose concentrations in the supernatant were quantified fluorometrically (Hitachi f2000, Hitachi, Tokyo; excitation at 393 nm and emission at 484 nm) according to the method described by Kauss (1989) using aniline blue. The Al-induced callose content was calculated from the callose content of Al-treated segments minus the callose content in segments from control roots not treated with Al.
Determination of Al in 1-mm Root Segments
After three brief rinses of the excised root tips in ultrapure water (18.3 MΩ, E-pure, D4642, Barnstead, Dubuque, IA), individual 1-mm root segments were dissected under ultrapure water from fresh root tips within 30 min after the Al treatment period. The root segments were placed into Eppendorf cups (two segments each) containing 500 μL of ultrapure water, frozen, and kept at −20°C until analysis. For the Al analysis the samples were transferred into 10-mL Teflon cups, and the ultrapure water was evaporated in a heating block at 120°C. The samples were dissolved in 1 mL of ultrapure concentrated HNO3 and wet oxidized at 190°C until the acid had completely evaporated. The ash was dissolved in 500 μL of ultrapure HNO3 (1:30 in ultrapure water). The samples were analyzed for Al using a graphite furnace atomic absorption spectrometer (UNICAM 939 QZ, Analytical Technology, Cambridge, UK) with Zeeman background compensation. Instrumental adjustments were optimized for the highest sensitivity. The Al contents of Al-treated root segments were corrected for mean Al contents from blanks and root segments not treated with Al (control).
Electrophysiology
A modified electrophysiological setup was used (Peters and Felle, 1999). It was turned by an angle of 90°, allowing undisturbed positive gravitropism of the root. The seedling itself remained intact. The electrical setup for the fabrication and application of ion-sensitive microelectrodes has been described by Felle and Bertl (1986) and Felle (1994, 1998). pH-selective micropipettes were pulled on a Getra instrument (vertical) from borosilicate tubing with solid filament (Hilgenberg, Malsfeld, Germany). Tip diameters were 3 to 4 μm. The tips were blunt and heat-polished. Before filling, the micropipette (apical 4 cm) was bent in a 30° angle, allowing measurement in the modified setup. To give the ion-sensitive sensor in the tip sufficient firmness to stay in place for repeated use, the cocktail (Fluka) was dissolved in a mixture of 40 mg of PVC per mL of tetrahydrofuran (THF) at a ratio of 30:70 (v/v). After evaporation of the THF, the remaining firm gel was topped with the undiluted sensor cocktail, followed by the reference solution (0.5 m KCl and 1 mm 2-([N-morpholino])-ethanesulfonic acid [MES], pH 6.0).
The micropipettes were connected through a Ag/AgCl half-cell to a high-impedance amplifier (FD 223, World Precision Instruments, Sarasota, FL). Signals were recorded on a chart recorder (L 2200, Linseis, Selb, Germany). Experiments were carried out under constant perfusion (1 mL min−1) in a specially designed plexiglass chamber at room temperature (22°C−23°C). The measurement solution consisted of distilled water containing 200 μm each of CaCl2 and KCl, and 1 mm MES. The pH was adjusted to 4.5. The pH profiles were measured at a distance of 20 μm from the root surface and in 200-μm intervals for the first 5 apical mm. Control measurements were carried out 15 min after the roots had been attached to the measurement cuvette. Only roots showing the specific basic pH profile were selected for further experiments. The Al treatment lasted 60 min at a concentration of 90 μm. Either the whole root or specific 1-mm root zones were treated with Al using a specially designed adjustable agarose block in plexiglass that was movable along the root in the plexiglass cuvette.
Partial Elongation of 1-mm Root Zones
The experimental setup for the electrophysiological experiments allowed simultaneous measurement of pH profiles and elongation growth of specific 1-mm root zones. Before the seedlings were attached to the measurement cuvette the root was marked with ink (Bruynzeel Permanent fine, Bruynzeel, The Netherlands) dots in 1-mm intervals. The distances between the dots and the width of the dots were measured at the beginning and during the course of the experiment, and then the elongation rate of the specific root zone was calculated. The ink used did not detach during the course of the experiment and did not affect root elongation or disturb the pH measurements, as confirmed in preliminary experiments. The precision of the measurements was 20 μm at a 64-fold magnification against a scale. Due to the vertical setup of the measurement cuvette, root bending did not occur.
Exogenous Application of IAA and Determination of Root Elongation Rate
The setup for the determination of the effect of exogenous IAA application on Al-induced inhibition of root growth was a modification of the PVC block system described by Sivaguru and Horst (1998) for the specific purposes of the experiments conducted. This included a smoothly moveable tray on which the PVC plates to which the plants were attached could be moved along the binocular (20-fold magnification; Stemi SV8, Zeiss, Oberkochen, Germany). Gravity-induced curvature did not complicate the measurements due to the vertical attachment of the seedlings to the PVC plates. Ninety micromolar monomeric Al or 10 μm of the IAA transport inhibitor 2,3,5-triiodobenzoic acid (TIBA) was applied via agarose blocks to the 1- to 2-mm apical root zone, while agarose blocks (1.2% [w/v] agarose) containing nutrient solution and 10−7 m IAA were positioned either around the meristematic zone (MZ) (0–1 mm) or the main elongation zone (EZ) (2.5–3.5 mm). This IAA concentration has been selected as most enhancing root elongation from a range of preliminary experiments in which IAA concentrations have been varied from 10−9 to 10−3 m (data not shown).
Exogenous Application of [3H]IAA and Localization in Specific Root Segments
Al (90 μm monomeric) or 10 μm of the IAA transport inhibitors TIBA or N-1-naphthylphthalamic acid (NPA) was applied to the DTZ of primary roots of intact plants in the PVC system as described before. [3H]IAA (777 GBq/mmol, Amersham Pharmacia Biotech, Freiburg, Germany) was applied to the MZ in 64-μL agarose blocks (1.2% [w/v] with nutrient solution) for 30 min. Then, 2 × 10−8 m tritiated IAA was mixed with untritiated IAA (Sigma, Deisenhofen, Germany) to reach a final IAA concentration of 10−7 m. After application, the roots were carefully removed from the system and rinsed in distilled water for 3 s from the base to the tip. Afterward, the segments were cut and frozen in 4 mL of distilled water in separate scintillation vials overnight. After reaching room temperature again, 8 mL of scintillation cocktail (Lumasafe Plus, Lumac LSC B.V., Groningen, The Netherlands) was added. Radioactivity was determined in a liquid scintillation counter (RackBeta 1217, LKB Wallac OY, Turku, Finland) allowing 10 min of integration time per specimen.
RESULTS
Effect of Al on Root Growth
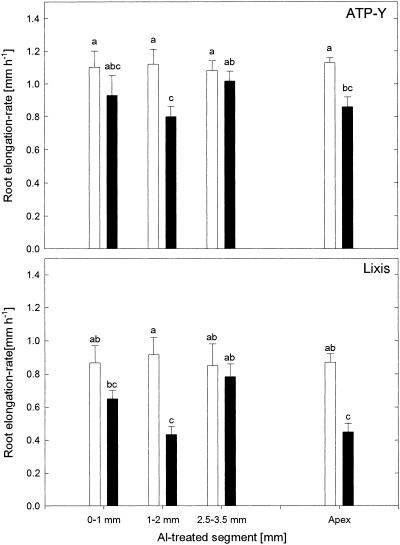
When Al was applied to the entire root apex, the root-elongation rate was significantly inhibited in both cultivars (Fig. 1). However, growth inhibition was more severe in cv Lixis (44.4%) than in cv ATP-Y (18.2%), reflecting the greater Al resistance of cv ATP-Y. Al treatment of the 1- to 2-mm apical root zone (the DTZ) led to a slightly stronger inhibition of root elongation than the treatment of the whole root apex (cv Lixis: 55.5%; cv ATP-Y: 27.3%). Furthermore, the genotypical differences in Al resistance were maintained. Al treatment of the 0- to 1-mm root zone significantly reduced root elongation only in the Al-sensitive cv Lixis; treatment of the 2.5- to 3.5-mm root zone did not affect root elongation in either cultivar.
Figure 1.
Effect of Al application (90 μm Almono in 0.6% [w/v] agarose gel) for 1 h to the entire root apex or to specific 1-mm root segments on the root elongation rate of primary roots of the maize cv ATP-Y (Al-resistant) and cv Lixis (Al-sensitive). Values are means of five independent replicates ± sd. The results shown are representative of three independent experiments. Different letters indicate significant differences at P < 0.05 (Tukey's test). White bars, 0 μm Al; black bars, 90 μm Al.
Effect of Local Al Application on Al Content and Callose Formation
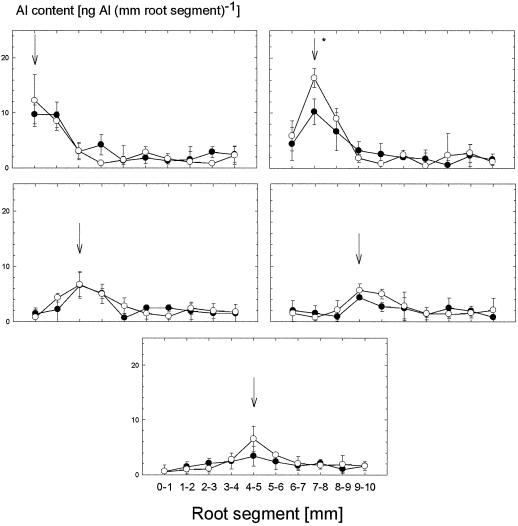
The particular Al sensitivity of the 1- to 2-mm apical root zone and the higher Al sensitivity of cv Lixis compared with cv ATP-Y was related to significantly higher Al accumulation (Fig. 2) and Al-induced callose formation (Fig. 3) when Al was applied to this apical root zone. Al application to the 0- to 1-mm zone also led to enhanced Al accumulation and callose formation; however, the cultivars did not differ significantly. Al application to the more basal root zones only led to slight or no increases in Al and callose contents, respectively.
Figure 2.
Al contents of specific 1-mm segments of the primary root apex of the Al-sensitive maize cv Lixis (○) and the Al-resistant cv ATP-Y (●). Ninety micromolar Almono was applied for 1 h in agarose gel to specific 1-mm root zones, as indicated by arrows. Values are means of three independent replicates ± sd. The results shown are representative of two independent experiments. Stars indicate significant genotypical differences at P < 0.05 (Tukey's test).
Figure 3.
Al-induced callose formation in specific 1-mm segments of the primary root apex of the Al-sensitive maize cv Lixis (○) and the Al-resistant cv ATP-Y (●). Ninety micromolar Almono was applied for 1 h in agarose gel to specific 1-mm root zones, as indicated by arrows. Values are means of five independent replicates ± sd. The results shown are representative of three independent experiments. Stars indicate significant genotypical differences at P < 0.05 (Tukey's test).
Effect of Al on Partial Elongation of Specific 1-mm Root Zones
For the measurement of the effect of Al application to the entire root apex or to individual 1-mm root zones on the partial elongation rate of individual apical root zones, a specially developed cuvette was used, which also allowed the simultaneous measurement of pH profiles along the root apex. In both maize cultivars the main EZ was 2.5 to 5 mm behind the root apex (Fig. 4). When Al was applied to the entire root apex, root elongation was inhibited over the entire EZ. The higher Al resistance of cv ATP-Y was expressed as a less-severe inhibition over the entire zone. The application of Al only to the 1- to 2-mm apical root zone, a zone that only slightly contributes to root elongation, produced the same inhibition of root elongation. The genotypical differences in Al sensitivity were similarly expressed. Application of Al to the 0- to 1-mm and the 2.5- to 3.5-mm root zones induced significantly less or no inhibition of root elongation, respectively.
Figure 4.
Effect of Al supply (90 μm Almono, 1 h) to the entire root apex or specific 1-mm apical root zones on partial elongation rates of apical 1-mm root segments of the primary roots of the maize cv ATP-Y (Al-resistant) and cv Lixis (Al-sensitive). Values are means of five independent measurements ± sd. The results shown are representative of two independent experiments. Different letters indicate significant differences at P < 0.05 (Tukey's test). ●, Control (Al); ○ 0 to 1 mm zone; ▾, 1 to 2 mm zone; ▿, 2.5 to 3.5 mm zone; ▪, entire apex.
Effect of Al on the pH Profile along the Root Surface
Roots of both maize cultivars growing in the absence of Al at pH 4.5 in the bulk solution developed a typical and stable pH profile at the root surface along the 5-mm root apex (Fig. 5). The pH increased steeply, from 4.8 at the root tip to about 5.3 at a 1-mm distance from the tip, dropped to the bulk solution pH at 3 mm from the tip, and then rose again to pH 4.7 in the 4- to 5-mm zone. Al application for only 15 min induced a substantial flattening of the pH profile in both cultivars. Longer Al treatment increased this effect only in the Al-sensitive cv Lixis. The application of Al to the 1- to 2-mm apical root zone induced a similar flattening of the pH profile along the root apex (Fig. 6). The flattening was more pronounced in cv Lixis, especially in the 1- to 2-mm root zone. Application of Al to the 0- to 1-mm or the 2.5- to 3.5-mm zones did not affect the pH profiles in either cultivar.
Figure 5.
Effect of Al application to the entire root apex on the root surface (distance 20 μm) pH profiles along the primary root of the maize cv ATP-Y (Al-resistant) and cv Lixis (Al-sensitive). Ninety micromolar Almono was supplied in a solution containing KCl and CaCl2 at 200 μm each for 15 (▾), 30 (▪), and 60 (♦) min. ●, Control (−Al) The bulk solution pH was adjusted to 4.5. The values are means of three independent replicates ± sd. The results shown are representative of three independent experiments.
Figure 6.
Effect of Al application (90 μm Almono in agarose gel) for 1 h to specific root zones on the root surface (distance 20 μm) pH profiles along the primary roots of the maize cv ATP-Y (●; Al-resistant) and cv Lixis (○; Al-sensitive). The pH of the bulk solution containing KCl and CaCl2 at 200 μm was adjusted to 4.5. Values are means of three independent replicates ± sd. The results shown are representative of three independent experiments. The shaded areas indicate the zone of Al application.
Effect of Exogenous IAA Application on Al-Induced Inhibition of Root Growth
Application of IAA to the 0- to 1-mm MZ as well as to the 2.5- to 3.5-mm EZ significantly enhanced root elongation in both maize cultivars (Fig. 7A). Al applied to the 1- to 2-mm zone (the DTZ) reduced the root elongation rate in cv Lixis significantly more than in cv ATP-Y, as shown above. When IAA was applied to the MZ of Al-treated roots, absolute root elongation was significantly enhanced only in the Al-resistant cv ATP-Y. Root elongation relative to the IAA-treated control without Al was not significantly affected in either cultivar (Table I). However, when IAA was applied to the EZ, Al-induced inhibition of root elongation was significantly reduced (Fig. 7B, Table I). TIBA treatment of the DTZ led to an inhibition of root elongation comparable to that caused by Al in both cultivars (Fig. 7, Table I). Genotypical differences did not occur. The TIBA effect could be compensated significantly in both cultivars by IAA application to the EZ.
Figure 7.
Effect of exogenous IAA supply (0.1 μm in 1.2% [w/v] agarose blocks containing nutrient solution) for 1 h to different apical root zones (A, MZ 0–1 mm; B, EZ 2.5–3.5 mm) on Al-induced inhibition of elongation of primary roots of the maize cv ATP-Y (Al-resistant) and cv Lixis (Al-sensitive). Ninety micromolar monomeric Al (gray bars) or 10 μm TIBA (black bars) in 0.6% (w/v) agarose gel was applied to the 1-to 2-mm root zone (DTZ). White bars, Control (−Al). Values are means of five independent replicates ± sd. The results shown are representative of three independent experiments. Different letters indicate significant differences at P < 0.05 (Tukey's test).
Table I.
Effect of exogenous application of 10−7 m IAA in agarose blocks to the apical root zones MZ (0–1 mm) or EZ (2.5–3.5 mm) on inhibition of root elongation induced by application of 90 μm monomeric AI or 10 μm TIBA to the DTZ (1–2 mm) relative to control roots treated only with nutrient solution in agarose blocks
| IAA Application Zone | Treatment to DTZ | Inhibition of Root Elongation
|
|||
|---|---|---|---|---|---|
| ATP-Y
(AI-resistant)
|
Lixis (AI-sensitive)
|
||||
| −IAA | +IAA | −IAA | +IAA | ||
| % | |||||
| MZ | 90 μm AI | 41.8 ± 6.5b | 34.2 ± 3.7b | 55.1 ± 5.3a | 60.8 ± 4.9a |
| 10 μm TIBA | 45.9 ± 11.5a | 46.1 ± 6.8a | 47.3 ± 9.6a | 55.4 ± 5.8a | |
| EZ | 90 μm AI | 42.3 ± 3.7b | 31.6 ± 2.3c | 52.8 ± 5.3a | 38.4 ± 3.8b |
| 10 μm TIBA | 48.3 ± 11.1a | 25.9 ± 4.6b | 45.0 ± 10.0a | 21.1 ± 7.4b | |
Values are the means of five independent replicates ± sd. Different letters indicate significant differences at P < 0.05 (Tukey test).
Effect of Al on Uptake and Distribution of [3H]IAA
Within 30 min of treatment, basipetal [3H]IAA transport did not go beyond the first 10 mm of the root apex in either cultivar. The application of either TIBA or NPA to the DTZ led to a significant decrease in basipetal transport of [3H]IAA. Total (Fig. 8) and relative contents (Fig. 9) in the EZ (2–5 mm) were significantly lower than in the nontreated control roots. In contrast to the EZ, in the MZ and DTZ (0–2 mm), [3H]IAA contents consistently accumulated. Roots treated with Al showed effects similar to those treated with the IAA transport inhibitors: accumulation in more apical root zones and lower contents of [3H]IAA in the EZ especially in the Al-sensitive cultivar. The effects of Al and IAA transport inhibitors applied to the DTZ on basipetal [3H]IAA transport from the MZ to the EZ are illustrated in Figure 9.
Figure 8.
Effect of Al on uptake and basipetal distribution of [3H]IAA in the apex of primary roots of the maize cv ATP-Y (Al-resistant) and cv Lixis (Al-sensitive). Application of 90 μm monomeric Al, 10 μm NPA, or 10 μm TIBA in nutrient solution, pH 4.3, in 0.6% (w/v) agarose gel to the DTZ for 30 min. Control roots were treated only with nutrient solution in agarose blocks, pH 4.3. [3H]IAA (0.1 μm in 1.2% [w/v] agarose blocks containing nutrient solution) was applied to the MZ for 30 min. Values are means of five independent replicates ± sd. The results shown are representative of three independent experiments. Different letters indicate significant differences at P < 0.05 (Tukey's test).
Figure 9.
Effect of Al on [3H]IAA distribution relative to entire IAA uptake into the apex of primary roots of the maize cv ATP-Y (Al-resistant) and cv Lixis (Al-sensitive). Application of 90 μm monomeric Al, 10 μm NPA, or 10 μm TIBA in nutrient solution, pH 4.3, in 0.6% (w/v) agarose gel to the DTZ for 30 min. Control roots were treated only with nutrient solution in agarose blocks, pH 4.3. [3H]IAA (0.1 μm in 1.2% [w/v] agarose blocks containing nutrient solution) was applied to the MZ for 30 min. Values are means of five independent replicates ± sd. The results shown are representative of three independent experiments. Different letters indicate significant differences at P < 0.05 (Tukey test).
DISCUSSION
The setup for the determination of partial elongation of specific 1-mm root zones revealed that the main EZ is located within 2.5 to 5 mm from the root tip, with a maximum at 4 mm (Fig. 4), thus confirming the findings of Pilet et al. (1983), Collings et al. (1992), Evans and Ishikawa (1997), and Blancaflor et al. (1998). The 1- to 2-mm zone contributed only very little to root elongation, so it appears justified to classify this zone as DTZ. In this root zone, cells are changing their mitotic mode and undergo a preparatory phase for rapid elongation (Baluška et al., 1996). This zone is most responsive to a variety of environmental stimuli such as gravitropism and auxin (Meuwly and Pilet, 1991; Ishikawa and Evans, 1993), as well as thigmomorphism (Ishikawa and Evans, 1990).
In the present study we confirmed our previous results (Sivaguru and Horst, 1998; Sivaguru et al., 1999a) and verify and substantiate that the DTZ is the most Al-sensitive apical root zone in maize. Application of Al to the DTZ inhibited root elongation similarly to Al application to the whole root apex. The application of Al to the 0- to 1-mm root zone (the MZ) was significantly less inhibitory, and application to the adjacent EZ (2.5–3.5 mm from the root tip) was not inhibitory at all (Figs. 1 and 4). The lower Al sensitivity of the MZ may be due to protection of the MZ through root-cap mucilage, which strongly binds Al (Archambault et al., 1996), thus protecting the MZ from Al injury (Horst et al., 1982). The low Al sensitivity of the EZ is not yet understood, but appears to be in agreement with the much lower Al sensitivity of stationary phase compared with log phase cells (Sivaguru et al., 1999b).
The cultivar differences in Al resistance were only clearly expressed when Al was applied to either the whole root apex or to the DTZ (Figs. 1 and 4), clearly indicating that this root zone plays an outstanding role in the expression of Al toxicity and Al resistance mechanisms in maize roots.
The high Al sensitivity of the DTZ and genotypical differences in Al sensitivity were characterized by higher Al accumulation and Al-induced callose formation (Figs. 2 and 3). This is in agreement with results showing a positive relationship between Al sensitivity and Al accumulation in root tips (Rinćon and Gonzales, 1992; Delhaize et al., 1993a; Llugany et al., 1994) and Al-induced callose formation (Wissemeier et al., 1987; Zhang et al., 1994; Horst et al., 1997). Our results are consistent with the view that Al resistance requires exclusion of Al from the apoplast and Al sensitivity is due to enhanced binding of Al to Al-sensitive binding sites of the apoplast (Blamey et al., 1992; Grauer and Horst, 1992; Horst et al., 1997).
Externally applied Al rapidly binds to the cell walls of root cells (Zhang and Taylor, 1989; Blamey et al., 1990; Delhaize et al., 1993a), where the main binding sites are the negative charges on carboxylic groups of the pectic matrix. These charges yield an electrical potential gradient determining binding and distribution of ions in the apoplast (Kinraide, 1993). Horst et al. (1999) showed that the spatial Al sensitivity of the root apex is positively correlated with the pectin contents of the root zones, with the exception of the MZ, but those results might have been due to contamination of the MZ with mucilage. The role of pectin content and its degree of methylation (and, thus, negative charge) on Al toxicity and resistance were further confirmed by N. Schmohl and W.J. Horst (unpublished results). Binding of Al may lead to impairment of the physical properties of the cell wall, leading to changes in extensibility and permeability (Blamey et al., 1993; Pritchard, 1994). It may also lead to mechanical stress, which could transduce a signal to the cytoskeleton via transmembrane proteins assumed to connect the cell wall and cytoskeleton (Nick, 1999), inducing severe disintegration of the microtubule and actin network (Blancaflor et al., 1998; Horst et al., 1999; Sivaguru et al., 1999a).
Our results do not support the recently expressed view that Al accumulation in root tip vacuoles is a major mechanism of Al resistance (Vázquez et al., 1999). However, we do not exclude the possibility that longer-term adaptation to Al supply will also require such a tolerance mechanism. However, in a normally growing root (2 mm h−1), the cells within the DTZ will not be exposed to Al for more than 1 h.
Cell wall pH is difficult to measure directly (Degenhardt et al., 1998; Peters et al., 1998), so it has been frequently calculated indirectly from pH changes in the incubation medium. However, these measurements cannot be expected to yield results that can be directly referred to as cell wall or apoplastic pH (Peters et al., 1998, and refs. therein). Using ion-sensitive microelectrodes, as proposed by Felle and Bertl (1986) and Felle (1994, 1998), we measured root surface pH directly in the 20-μm distance from the surface and related it to root growth in specific zones of the primary root. The pH profile we found is in agreement with the findings of Pilet et al. (1983), Collings et al. (1992), and Felle (1998) in the maize root apoplast and is consistent for both cultivars. It is particularly remarkable, however, that the roots seem to be capable of maintaining this pattern within a vast range of bulk solution pH from 7.9 (Felle, 1998) over 6.8 (Pilet et al., 1983) to 4.5 (our results). The finding that the roots maintained this pH profile even after 24 h of adaptation to low pH shows that it is not a transient effect due to seedling manipulation in the experimental setup. Effects of gravistimulation were also excluded by the vertical setup. It is of particular interest that the maize root is capable of either acidifying or alkalizing specific root zones depending on the pH of the surrounding medium, because the root response involved must therefore be understood as a powerful adaptation mechanism to transient changes in medium pH. This view is in agreement with findings by Yan et al. (1998) showing that maize roots are capable of coping with extremely low external pH if carefully adapted.
Miyasaka et al. (1989), Ryan et al. (1992), and Collings et al. (1992) explained the pH pattern along the rhizoplane as resulting from endogenous ionic currents traversing the root tip. Collings et al. (1992) discussed the role of the currents observed in transducing the gravitropic stimulus from the root cap to the EZ. By removing K+, Na+, Ca2+, or Cl− ions from the bathing solution or by applying the Ca2+ channel blocker lanthanum, they could demonstrate that the currents measured were primarily composed of protons, a finding that is in accordance with Ryan et al. (1992). Ca2+ influx and Cl− efflux contribute to these currents to a lesser extent (Iwabuchi et al., 1989; Ryan et al., 1992).
At pH 4.5, an increase in rhizosphere pH of 0.1 to 0.2 units leads to considerable decrease of phytotoxic Al3+ activity (Martell and Motekaitis, 1989; Kinraide, 1991), and the capability of roots to increase rhizosphere pH has been suggested as a possible mechanism of Al resistance (Miyasaka et al., 1989; Degenhardt et al., 1998). This is difficult to reconcile with the particularly high Al uptake and Al sensitivity of the DTZ and the highest pH at the root surface observed in this study (Figs. 2 and 5). Rather, it appears that the pH increase primarily led to less competition of Al3+ with H+ for apoplastic binding sites, thus enhancing Al toxicity, as suggested by Grauer and Horst (1992) and Kinraide (1993). In this context it is important to consider that Al application to the entire root apex or specifically to the DTZ led to a flattening of the alkalization peak (1–2 mm) in both cultivars after 15 min of application and remained relatively constant for the succeeding 45 min. This may explain why in the presence of Al, the pH increase in the DTZ was not sufficient to inactivate Al3+ especially in the Al-sensitive cultivar, where Al application led to a greater flattening of the pH peak in the DTZ than in the Al-resistant cultivar. However, we believe that the genotypical differences are the consequence rather than the cause for the differences in Al resistance, a conclusion shared by Miyasaka et al. (1989) after measuring ion fluxes with lower spatial resolution in the rhizosphere of two wheat cultivars differing in Al resistance.
The alkalization peak in the DTZ may be due to: (a) enhanced anion uptake, e.g. an H+/anion symport, which is in agreement with the findings of Collings et al. (1992) and Ryan et al. (1992) comparing two wheat cultivars differing in Al resistance; (b) enhanced respiration and thus bicarbonate production; and (c) enhanced release of organic acid anions, which at the low ambient pH in the apoplast will bind free protons. However, the release of organic anions appears unlikely to account for alkalization in the Al-sensitive cultivar, because it is well established that this will lead to Al resistance through complexation, as shown for malate in wheat (Delhaize et al., 1993b; Basu et al., 1994; Ryan et al., 1995a, 1995b), citrate in maize (Pellet et al., 1995), and oxalate in buckwheat (Zheng et al., 1998).
The capacity of the Al-resistant cultivar to better maintain the alkalization peak in the DTZ may be explained by two factors: (a) the physiological properties for maintenance of ionic currents are less affected by Al in the Al-resistant than in the Al-sensitive cultivar (Miyasaka et al., 1989) or (b) an Al exclusion mechanism is switched on in the Al-resistant cultivar promoting release of organic acid anions. They might bind free protons in the rhizosphere and hence lead to an increase in surface pH. At the prevailing cytoplasmic pH (>7.0) both malate and citrate are mainly present in dissociated form as anions. Since the external concentration of these anions is much lower and because of the negative membrane potential, transport of these anions into the external solution is a thermodynamically passive process along a steep electrochemical gradient. Hence, gating of anion channels permeable for these anions would allow considerable efflux within a short time (Schmidt and Schroeder, 1994; Papernik and Kochian, 1997; Ryan et al., 1997). Even though there is agreement about the role of adequate cell wall pH in root elongation (Rayle and Cleland, 1992; Zieschang et al., 1993; Kutschera, 1994; Felle, 1998), a causal relation between the changes in pH profile observed and Al-induced inhibition of root growth remains a matter of debate (Ryan et al., 1992).
The finding that Al applied to the DTZ, which does not contribute significantly to root elongation, severely inhibits cell elongation in the EZ not in contact with Al (Fig. 4) strongly suggests a signaling pathway mediating the Al signal between DTZ and EZ. It is clearly established that coordinated auxin transport is involved in the regulation of root growth, morphology, and the gravitropic growth response (Hasenstein and Evans, 1988; Kaufmann et al., 1995; Evans and Ishikawa, 1997; Ruegger et al., 1997; Müller et al., 1998). Auxin is transported from auxin-synthesizing shoot tissues via the phloem toward the root apical meristems, where it is proposed to be unloaded from the central stele into cortical and epidermal cells and then translocated basipetally to the EZ (Hasenstein and Evans, 1988; Estelle, 1998). Inhibition of basipetal auxin flow has been implicated in severe effects on root growth and morphology. These include swelling of root tips through uncontrolled periclinal divisions (Blancaflor and Hasenstein, 1995; Ruegger et al., 1997) as a consequence of auxin accumulation in the MZ and DTZ and suppression of the gravitropic response (Müller et al., 1998; Hasenstein et al., 1999) due to insufficient auxin accumulation and thus growth inhibition on the lower side of the bending root.
Similar effects on growth and morphological changes have been reported in Al-treated roots (Blancaflor et al., 1998; Sivaguru et al., 1999a). Although Hasenstein and Evans (1988) clearly demonstrated inhibition of basipetal transport of [3H]IAA in maize roots by Al, the implications of these results in the understanding of Al rhizotoxicity have not been followed up on to date. The results presented in this study confirm those findings by showing that local application of Al, as well as TIBA or NPA, to the DTZ led to reduced [3H]IAA contents in the EZ, while contents relative to control roots were elevated in the MZ/DTZ (Figs. 8 and 9). The involvement of Al/auxin transport interaction in the expression of Al-induced inhibition of root elongation is further substantiated by the result that exogenous application of IAA in root-growth-stimulating doses to the EZ alleviated inhibition of root elongation caused by Al or TIBA applied to the DTZ (Fig. 7; Table I). The stronger inhibition of [3H]IAA transport by Al in the Al-sensitive compared with the Al-resistant cultivar supports this assumption.
Although the results presented here suggest an involvement of auxin-transport inhibition in the expression of Al toxicity, the mechanism by which Al affects root apical basipetal IAA transport needs to be further elucidated.
In conclusion, the results shown convey substantial evidence that the DTZ of the maize primary root plays an outstanding role in the Al response, and that the primary mechanisms of genotypical differences in Al resistance are located within the DTZ. Furthermore, they provide circumstantial evidence for the existence of a signaling pathway in the root apex mediating the Al signal between DTZ and EZ through alterations in basipetal auxin transport. The nec-essary elucidation of the physiological processes responsible for Al sensitivity and for genotypical differences in Al resistance of the DTZ and of the Al signal is the subject of our ongoing research.
ACKNOWLEDGMENTS
The maize cv Lixis was kindly donated by Force Limagrain (Montpellier, France), and cv ATP-Y by Dr. Charles Thé (Institut de la Recherche Agronomique pour le Development, Cameroon). We also thank Rũdiger Sachse (Zentrum für Strahlenschutz and Radioökologie, Hannover, Germany) for his assistance on the Wallac RackBeta.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft within the Special Research Program 717 (“The Apoplast of Higher Plants”).
LITERATURE CITED
- Archambault DJ, Zhang G, Taylor GJ. Accumulation of Al in root mucilage of an Al-resistant and an Al-sensitive cultivar of wheat. Plant Physiol. 1996;112:1471–1478. doi: 10.1104/pp.112.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluška F, Volkmann D, Barlow PW. Specialized zones of development in roots. View from the cellular level. Plant Physiol. 1996;112:3–4. doi: 10.1104/pp.112.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu U, Godbold D, Taylor GJ. Aluminum resistance in Triticum aestivumassociated with enhanced exudation of malate. J Plant Physiol. 1994;144:747–753. [Google Scholar]
- Blamey FPC, Asher CJ, Kerven GL, Edwards DG. Factors affecting aluminum sorption by calcium pectate. Plant Soil. 1993;149:87–94. [Google Scholar]
- Blamey FPC, Edmeades DC, Wheeler DM. Role of cation-exchange capacity in differential aluminum tolerance of Lotusspecies. J Plant Nutr. 1990;13:729–744. [Google Scholar]
- Blamey FPC, Edmeades DC, Wheeler DM. Empirical models to approximate calcium and magnesium amelioration effects and genetic differences in aluminium tolerance in wheat. Plant Soil. 1992;144:281–287. [Google Scholar]
- Blancaflor EB, Hasenstein KH. Time course and auxin sensitivity of cortical microtubule reorientation in maize roots. Protoplasma. 1995;185:72–82. doi: 10.1007/BF01272755. [DOI] [PubMed] [Google Scholar]
- Blancaflor EB, Jones DL, Gilroy S. Alterations in the cytoskeleton accompany aluminum-induced growth inhibition and morphological changes in primary roots of maize. Plant Physiol. 1998;118:159–172. doi: 10.1104/pp.118.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collings DA, White RG, Overall RL. Ionic current changes associated with the gravity-induced bending response in roots of Zea maysL. Plant Physiol. 1992;100:1417–1426. doi: 10.1104/pp.100.3.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt J, Larsen PB, Howell SH, Kochian LV. Aluminum resistance in the Arabidopsis mutant alr-104 is caused by an aluminum-induced increase in rhizosphere pH. Plant Physiol. 1998;117:19–27. doi: 10.1104/pp.117.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Craig S, Beaton CD, Bennet RJ, Jagadish VC, Randall PJ. Aluminum tolerance in wheat (Triticum aestivumL.). I. Uptake and distribution of aluminum in root apices. Plant Physiol. 1993a;103:685–693. doi: 10.1104/pp.103.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR. Aluminum toxicity and tolerance in plants. Plant Physiol. 1995;107:315–321. doi: 10.1104/pp.107.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Randall PJ. Aluminum tolerance in wheat (Triticum aestivumL.). II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol. 1993b;103:695–702. doi: 10.1104/pp.103.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estelle M. Polar auxin transport: new support for an old model. Plant Cell. 1998;10:1775–1778. doi: 10.1105/tpc.10.11.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans ML, Ishikawa H. Cellular specifity of the gravitropic motor response in roots. Planta. 1997;203:115–122. doi: 10.1007/pl00008099. [DOI] [PubMed] [Google Scholar]
- Felle HH. The H+/Cl− symporter in root hair cells of Sinapsis alba. Plant Physiol. 1994;106:1131–1136. doi: 10.1104/pp.106.3.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle HH. The apoplastic pH of Zea maysroot cortex as measured with pH-sensitive microelectrodes: aspects of regulation. J Exp Bot. 1998;49:987–995. [Google Scholar]
- Felle HH, Bertl A. The fabrication of H+-selective liquid-membrane microelectrodes for use in plant cells. J Exp Bot. 1986;37:1416–1428. [Google Scholar]
- Grauer UE, Horst WJ. Modeling cation amelioration of aluminium toxicity. Soil Sci Soc Am J. 1992;56:166–172. [Google Scholar]
- Hasenstein KH, Blancaflor EB, Lee JS. The microtuble cytoskeleton does not integrate auxin transport and gravitropism in maize roots. Physiol Plant. 1999;105:729–738. doi: 10.1034/j.1399-3054.1999.105418.x. [DOI] [PubMed] [Google Scholar]
- Hasenstein KH, Evans ML. Effects of cations on hormone transport in primary roots of Zea mays. Plant Physiol. 1988;86:890–894. doi: 10.1104/pp.86.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst WJ. The role of the apoplast in aluminium toxicity and resistance of higher plants: a review. Z Pflanzenernähr Bodenk. 1995;158:419–428. [Google Scholar]
- Horst WJ, Püschel A-K, Schmohl N. Induction of callose formation is a sensitive marker for genotypic aluminium sensitivity in maize. Plant Soil. 1997;192:23–30. [Google Scholar]
- Horst WJ, Schmohl N, Kollmeier M, Baluska F, Sivaguru M. Does aluminium inhibit root growth of maize through interaction with the cell wall—plasma membrane—cytoskeleton continuum? Plant Soil. 1999;215:163–174. [Google Scholar]
- Horst WJ, Wagner A, Marschner H. Mucilage protects root meristems from aluminium injury. Z Pflanzenphysiol. 1982;109:95–103. [Google Scholar]
- Ishikawa H, Evans ML. Electrotropism of maize roots: role of the root cap and relationship by gravitropism. Plant Physiol. 1990;94:913–918. doi: 10.1104/pp.94.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Evans ML. The role of the distal elongation zone in the response of maize roots to auxin and gravity. Plant Physiol. 1993;102:1203–1210. doi: 10.1104/pp.102.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi A, Yano M, Shimuzu H. Development of extracellular electric pattern around Lepidiumroots: its possible role in root growth and gravitropism. Planta. 1989;183:381–390. [Google Scholar]
- Kaufmann PB, Wu LL, Brock TG, Kim D. Hormones and the orientation of growth. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 547–571. [Google Scholar]
- Kauss H. Fluorimetric measurement of callose and other 1,3-β-glucans. In: Linskens HF, Jackson JF, editors. Modern Methods of Plant Analysis: 10 Plant Fibers. Berlin: Springer-Verlag; 1989. pp. 127–137. [Google Scholar]
- Kerk N, Feldman L. The quiescent center in roots of maize: initiation, maintenance and role in organization of the root apical meristem. Protoplasma. 1994;183:100–106. [Google Scholar]
- Kerven GL, Edwards DG, Asher CJ, Hallman PS, Kokot S. Aluminum determination in soil solution. II. Short term calorimetric procedures for the measurement of inorganic monomeric aluminium in the presence of organic acid ligands. Aust J Soil Res. 1989;27:91–102. [Google Scholar]
- Kinraide TB. Identity of the rhizotoxic aluminium species. Plant Soil. 1991;134:167–178. [Google Scholar]
- Kinraide TB. Aluminum enhancement of plant growth in acid rooting media: a case of reciprocal alleviation of toxicity by two toxic cations. Physiol Plant. 1993;88:619–625. doi: 10.1111/j.1399-3054.1993.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Kochian LV. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:237–260. [Google Scholar]
- Kutschera U. The current status of the acid-growth hypothesis. New Phytol. 1994;126:549–569. [Google Scholar]
- Llugany M, Massot N, Wissemeier AH, Poschenrieder C, Horst WJ, Barceló J. Aluminum tolerance of maize cultivars as assessed by callose production and root elongation. Z Pflanzenernähr Bodenk. 1994;157:447–451. [Google Scholar]
- Martell AE, Motekaitis RJ. Coordination chemistry and specification of Al(III) in aqueous solution. In: Lewis TE, editor. Environmental Chemistry and Toxicology of Aluminum. Chelsea, MI: Lewis Publishers; 1989. pp. 3–17. [Google Scholar]
- Meuwly P, Pilet EP. Local treatment with indole-3-acetic acid influences differential growth responses in Zea maysL. roots. Planta. 1991;185:58–64. doi: 10.1007/BF00194515. [DOI] [PubMed] [Google Scholar]
- Miyasaka SC, Kochian LV, Shaff JE, Foy CD. Mechanisms of aluminum tolerance in wheat. An investigation of genotypic differences in rhizosphere pH, K+, and H+transport, and root-cell membrane potentials. Plant Physiol. 1989;91:1188–1196. doi: 10.1104/pp.91.3.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A, Guan C, Gälweiler L, Tänzler P, Huijser P, Marchant A, Parry G, Bennett A, Wisman E, Palme K. AtPIN2 defines a locus of Arabidopsisfor root gravitropism control. EMBO J. 1998;17:6903–6911. doi: 10.1093/emboj/17.23.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick P. Signals, motors, morphogenesis: the cytoskeleton in plant development. Plant Biol. 1999;1:169–179. [Google Scholar]
- Papernik LA, Kochian LV. Possible involvement of Al-induced electrical signals in Al tolerance in wheat. Plant Physiol. 1997;115:657–667. doi: 10.1104/pp.115.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellet DM, Grunes DL, Kochian LV. Organic acid exudation as an aluminum-tolerance mechanism in maize (Zea maysL.) Planta. 1995;196:788–795. [Google Scholar]
- Peters WS, Felle HH. The correlation of profiles of surface pH and relative elemental growth rates in maize roots. Plant Physiol. 1999;121:905–912. doi: 10.1104/pp.121.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters WS, Lüthen H, Böttger M, Felle H. The temporal correlation of changes in apoplast pH and growth rate in maize coleoptile segments. Aust J Plant Physiol. 1998;25:21–25. [Google Scholar]
- Pilet PE, Versel JM, Mayor G. Growth distribution and surface pH patterns along maize roots. Planta. 1983;158:398–402. doi: 10.1007/BF00397731. [DOI] [PubMed] [Google Scholar]
- Pritchard J. The control of cell expansion in roots. New Phytol. 1994;127:3–26. doi: 10.1111/j.1469-8137.1994.tb04255.x. [DOI] [PubMed] [Google Scholar]
- Rayle DL, Cleland R. The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiol. 1992;99:1271–1274. doi: 10.1104/pp.99.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengel Z. Uptake of aluminium in plant cells. New Phytol. 1996;134:389–406. [Google Scholar]
- Rinćon M, Gonzales RA. Aluminum partitioning in intact roots of aluminum-tolerant and aluminum-sensitive wheat (Triticum aestivumL.) cultivars. Plant Physiol. 1992;99:1021–1028. doi: 10.1104/pp.99.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M. Reduced naphthylphthalamic acid binding in the tir3mutant of Arabidopsis is associated with a regulation in polar auxin transport and diverse morphological defects. Plant Cell. 1997;9:745–757. doi: 10.1105/tpc.9.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ. Characterization of Al-stimulated efflux of malate from the apices of Al-tolerant wheat roots. Planta. 1995a;196:103–110. [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ. Malate efflux from root apices and tolerance to aluminum are highly correlated in wheat. Aust J Plant Physiol. 1995b;22:531–536. [Google Scholar]
- Ryan PR, DiTomaso JM, Kochian LV. Aluminum toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. J Exp Bot. 1993;44:437–446. [Google Scholar]
- Ryan PR, Shaff JE, Kochian LV. Aluminum toxicity in roots: correlations among ionic currents, ion fluxes, and root elongation in aluminum-sensitive and aluminum-tolerant wheat cultivars. Plant Physiol. 1992;99:1193–1200. doi: 10.1104/pp.99.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Skerrett M, Findlay GP, Delhaize E, Tyerman SD. Aluminum activates an anion channel in the apical cells of wheat roots. Proc Natl Acad Sci USA. 1997;94:6547–6552. doi: 10.1073/pnas.94.12.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Schroeder JI. Anion selectivity of slow anion channels in the plasma membrane of guard cells. Plant Physiol. 1994;106:383–391. doi: 10.1104/pp.106.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaguru M, Baluška F, Volkmann D, Felle HH, Horst WJ. Impacts of aluminum on the maize cytoskeleton: short-term effects on the distal part of the transition zone. Plant Physiol. 1999a;119:1–10. doi: 10.1104/pp.119.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaguru M, Horst WJ. The distal part of the transition zone is the most aluminium-sensitive apical root zone of Zea maysL. Plant Physiol. 1998;116:155–163. [Google Scholar]
- Sivaguru M, Yamamoto Y, Matsumoto H. Differential impacts of aluminium on microtubules depends on the growth phase in suspension cultured tobacco cells. Physiol Plant. 1999b;107:110–119. [Google Scholar]
- Taylor GJ. Overcoming barriers to understanding the cellular basis of aluminum resistance. Plant Soil. 1995;171:89–103. [Google Scholar]
- Vázquez MD, Poschenrieder C, Corrales I, Barceló J. Change in apoplastic aluminum during the initial growth response to aluminum by roots of a tolerant maize variety. Plant Physiol. 1999;119:435–444. doi: 10.1104/pp.119.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissemeier AH, Horst WJ. Effect of calcium supply on aluminium-induced callose formation, its distribution and persistence in roots of soybean (Glycine max(L.) Merr.) J Plant Physiol. 1995;145:470–476. [Google Scholar]
- Wissemeier AH, Klotz F, Horst WJ. Aluminium-induced callose synthesis in roots of soybean (Glycine maxL.) J Plant Physiol. 1987;129:487–492. [Google Scholar]
- Yan F, Feuerle R, Schäffer S, Fortmeier H, Schubert S. Adaptation of active proton pumping and plasmalemma ATPase activity of corn roots to low root medium pH. Plant Physiol. 1998;117:311–319. doi: 10.1104/pp.117.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Hoddinott J, Taylor GJ. Characterisation of 1–3-β-glucan (callose) synthesis in roots of Triticum aestivumin response to aluminium toxicity. J Plant Physiol. 1994;144:229–234. [Google Scholar]
- Zhang G, Taylor GJ. Kinetics of aluminum uptake by excised roots of aluminum-tolerant and aluminum-sensitive cultivars of Triticum aestivumL. Plant Physiol. 1989;91:1094–1099. doi: 10.1104/pp.91.3.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SJ, Ma JF, Matsumoto H. High aluminum resistance in buckwheat. 1. Al-induced specific secretion of oxalic acid from root tips. Plant Physiol. 1998;117:745–751. doi: 10.1104/pp.117.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieschang HE, Köhler K, Sievers A. Changing proton concentrations at the surfaces of gravistimulated Phleumroots. Planta. 1993;190:546–554. [Google Scholar]