Abstract
Rationale and Objectives
To investigate whether quantitative kinetic analysis of lesions and background parenchyma (BP) in breast MRI can elucidate differences between BRCA carriers and sporadic controls with high risk for breast cancer.
Materials and Methods
59 BRCA and 59 control cases (49 benign, 10 malignant) were examined in this study. Principal component analysis was applied for quantitative analysis of dynamic signal in background parenchyma (B) and lesion (L) in terms of initial enhancement ratio (IER) and delayed enhancement ratio (DER).
Results
Control B-IER, B-DER, L-IER, and L-DER were higher than BRCA cases in all women and women with benign lesions; statistically significant differences in B-IER and B-DER (all women: p = 0.02 and p = 0.02, respectively; benign only: p = 0.005 and p = 0.005, respectively). In the control cohort, B-IER and B-DER were higher in the premenopausal women than in the postmenopausal women (p = 0.013 and 0.003, respectively), but not in the BRCA cohort; this led to significant differences in B-IER and B-DER between control and BRCA groups in the premenopausal women (p = 0.01 and 0.01, respectively), but not in the postmenopausal women.
Conclusion
Results suggest possible differences in the vascular properties of BP between BRCA carriers and non-carriers and its association with menopausal status.
Keywords: BRCA 1/2 mutation carrier, Background parenchymal enhancement (BPE), Principal component analysis (PCA), Dynamic Contrast Enhanced Magnetic resonance imaging (DCE-MRI)
INTRODUCTION
BRCA 1 and BRCA 2 genes are involved in maintaining genome integrity by engaging in DNA repair and cell cycle checkpoint control, and are known as tumor suppressor genes [1]. BRCA gene mutations are relatively common, affecting about 1 in 400 in the general population [2, 3]. Lifetime breast cancer risk in female BRCA mutation carriers is approximately 85% in BRCA 1 mutation carriers and about 45% in BRCA 2 mutation carriers [4]. These women develop aggressive interval tumors that are often high grade, triple negative breast cancers [5]. Therefore, the American Cancer Society recommends annual screening breast MRI, beginning at age 30, in all BRCA mutation carriers [6, 7].
Recent studies have shown that the background parenchymal enhancement (BPE) from dynamic contrast-enhanced MRI (DCE-MRI) correlates with breast cancer risk [8, 9]. BPE refers to the enhancement of the normal-appearing fibroglandular tissue and is assessed on the contrast-enhanced image at the first time point after contrast agent injection [10]. BPE reflects the vascularity of the breast parenchymal tissue and is sensitive to hormonal changes [11]. BPE has been shown to vary with the menstrual cycle, increase with hormone replacement therapy and decrease with menopause, anti-estrogen therapies, and bilateral salpingooopherectomy [12–15]. To date, no study has investigated possible differences between BPE of BRCA mutation carriers and non-mutation carriers and the effect of menopausal status on the BPE of these cohorts. Assessment of BPE kinetic properties of BRCA mutation carriers in comparison with non-mutation carriers may help better understand the role of BRCA in breast physiology and improve diagnostic accuracy of MRI in this population with an increased risk of familial breast cancer.
Therefore, the purpose of our study was to investigate whether quantitative kinetic analysis of lesions and background parenchyma (BP) can elucidate differences between high-risk BRCA mutation carriers and sporadic controls, given the biological differences in the BRCA population.
MATERIALS AND METHODS
Patient Data
After institutional review board approval, a Health Insurance Portability and Accountability Act compliant retrospective review of 830 subjects who each underwent a bilateral contrast-enhanced breast MRI and subsequent MRI-guided breast biopsy between March 2008 and August 2015 was performed. Included in this study were BRCA 1 and/or 2 mutation carriers with mass, focus or an area of non-mass-like enhancement (NME) on MRI that was occult on mammography and MRI-directed ultrasound. None of the BRCA 1 and/or 2 mutation carriers was on anti-estrogen therapy. The control group consisted of high-risk patients (>20% lifetime risk using the Tyrer Cuzick or BRCAPRO risk models) without known BRCA gene mutation who underwent MRI-guided biopsy. The controls were matched with BRCA patients for similar pathologic (benign or malignant) and lesion type (mass, focus or NME), age and year of MRI-guided biopsy (within 5 years of age and 2 years of biopsy).
A total of 59 MRI-guided biopsies in the BRCA cohort and 59 biopsies in the control group were selected. Only patients who underwent MRI guided biopsy were included in the study in order to provide pathologic correlation for the index lesion. The BRCA and control cases were stratified into benign and malignant cases (BRCA: n = 10 malignant and n = 49 benign cases; controls: n = 10 malignant and n = 49 benign cases), as summarized in Table 1. The mean ± standard deviation (SD) ages of BRCA benign and malignant patients were 40.33 ± 10.93 years (range 25-69 years) and 53.1 ± 10.03 years (range 44-61years), respectively. The ages of benign and malignant control patients were 41.94 ± 10.46 years (range 25-64 years) and 55.2 ± 10.63 years (range 45-63 years), respectively. 65.3 % of benign cases in the BRCA cohort were premenopausal, while 57.1% of benign control cases were premenopausal women. The pre-menopausal women in both groups underwent their MRI-guided biopsies during similar weeks of their menstrual cycle. Of the 32 benign premenopausal BRCA cases, 75% were biopsied during week 2 of the menstrual cycle. Similarly, of the 28 benign premenopausal control cases, 82% were biopsied during week 2.
Table 1.
Patient characteristics
| BRCA CASES
|
CONTROL CASES
|
|||
|---|---|---|---|---|
| Malignant | Benign | Malignant | Benign | |
| No. of patients | 10 | 49 | 10 | 49 |
| Age (years) | 53.1 ± 10.03 | 40.33 ± 10.93 | 55.2 ± 10.63 | 41.94 ± 10.46 |
| Estrogen receptor status | ||||
| Positive | 4 (40%) | N/A | 10 (100%) | N/A |
| Negative | 6 (60%) | N/A | 0 | N/A |
| Progesterone receptor status | ||||
| Positive | 4 (40%) | N/A | 10 (100%) | N/A |
| Negative | 6 (60%) | N/A | 0 | N/A |
| Pre-menopausal | 2 (20%) | 32 (65.3%) | 5 (50%) | 28 (57.1%) |
| Menopausal | 8 (80%) | 17 (34.7%) | 5 (50%) | 21 (42.9%) |
Clinical indications for MRI in the BRCA group included high-risk screening (n = 49), personal history of breast cancer (n = 2), history of ovarian cancer (n = 7), and nipple discharge (n = 1). Indications for MRI in the control cohort included strong family history (n = 23), personal history of breast cancer (n = 13), personal history of high-risk lesion (n = 15) and newly diagnosed breast cancer (n = 7) and nipple discharge (n = 1). Benign BRCA cases (n = 49) included benign tumors (fibroadenoma and papilloma, n = 6); inflammatory change (n = 7) and normal fibroglandular tissue (n = 36). Benign control cases (n = 49) also included benign tumors (fibroadenoma and papilloma, n = 9); inflammatory change (n = 4) and normal fibroglandular tissue (n = 36). Of the 98 benign concordant lesions, 91 lesions underwent follow-up imaging with a mean of 32 months, range 18 – 65 months. The remaining 7 lesions underwent subsequent surgical excision and were found to be benign. BRCA malignant cases (n = 10) included invasive ductal carcinoma (IDC, n = 2; 1 intermediate grade and 1 poorly differentiated), invasive lobular carcinoma (ILC, n = 1, intermediate grade), ductal carcinoma in-situ (DCIS, n = 5; 2 intermediate grade and 3 high grade), and mixed pathology (IDC and DCIS, n = 2). Malignant control cases (n = 10) included DCIS (n = 8; 3 intermediate grade and 5 high grade), ILC (n = 1, intermediate grade), and invasive ductal carcinoma (n = 1, intermediate grade). Four of 10 BRCA tumors were estrogen receptor (ER) positive, and all 10 cancers in the control group were ER positive tumors.
MRI Data Acquisition
DCE-MRI was performed as part of standard MRI-guided core biopsy protocol. The original diagnostic MRI where the index lesion was seen was performed 5-7 days prior to the MRI-guided core biopsy. Unilateral breast MRI data were acquired using a whole body 3T Tim Trio system (Siemens, Erlangen, Germany) and a 7-element breast coil (In Vivo, Orlando, FL). DCE-MRI data were acquired using a sagittal 3D volume interpolated breathhold exam (VIBE) sequence with TR/TE = 4.01 ms/1.52 ms, resolution 1.4 × 0.9 × 1.5 mm, and fat suppression for five consecutive frames (duration 40 sec each). After the first frame, a single dose of Gd-DTPA (Magnevist, Bayer, Germany) contrast agent with a dose of 0.1 mM/kg body weight was injected at 2 mL/sec, followed by saline flush with a power injector (Medrad, Indiana, PA).
Analysis of BPE pattern
The BPE was initially assigned one of four categories in accordance with the BIRADS MRI lexicon classification system: minimal, mild, moderate, or marked. A combination of the pre-, initial post-contrast T1-weighted fat-saturated and subtraction images were used to assess BPE category [11, 16].
In addition, quantitative analysis of dynamic signal enhancement in the background parenchyma was performed on biopsy MRI protocol with the principal component analysis (PCA) method used in earlier studies [17, 12]. The PCA method is a semi-automatic method that effectively segments tissue with a similar pattern of enhancement, thus eliminating the need to manually select regions with fibroglandular tissue (FGT) for assessment of background parenchyma kinetics. In brief, the PCA method effectively decomposes DCE-MRI images of one slice without a suspicious lesion into eigenvalues, eigenvectors, and projection coefficient maps. The primary principal component (i.e., one with the largest eigenvalue) usually captures the major portion of signal variance from the most enhancing tissue throughout that particular slice. The subsequent principal components correspond essentially to noise. Hence, it was assumed that the primary principal component represents the contrast enhancement kinetics of the BP.
Fig.1 shows an example of measured DCE-MRI images of one slice (Fig.1a) and the principal components (Fig.1b and c) for a sagittal slice without a suspicious lesion. The primary principal component (i.e., with the largest eigenvalue) usually captures the major portion of signal variance from the most enhancing tissue as shown by the first projection coefficient map in Fig.1b. The subsequent principal components correspond essentially to noise as shown by the projection maps in Fig.1b. Hence, it was assumed that the primary principal component represents the contrast enhancement kinetics of the background parenchyma (BP). Each eigenvector (Fig.1c) was scaled so that the sum of the squares of its elements was equal to the corresponding eigenvalue. Then the scaled eigenvector of primary principal component was used to measure initial enhancement ratio (IER) and delayed enhancement ratio (DER) of the BP; IER defined as the percent increase between the first and third frames (pre-contrast and 80 second post-contrast scans, respectively), and DER as the percent increase between the first and fifth frames (pre-contrast and 160 second post-contrast scans, respectively) as shown in Fig.1. IER and DER were chosen to quantify wash-in and delayed contrast enhancement characteristics, respectively.
Figure 1.
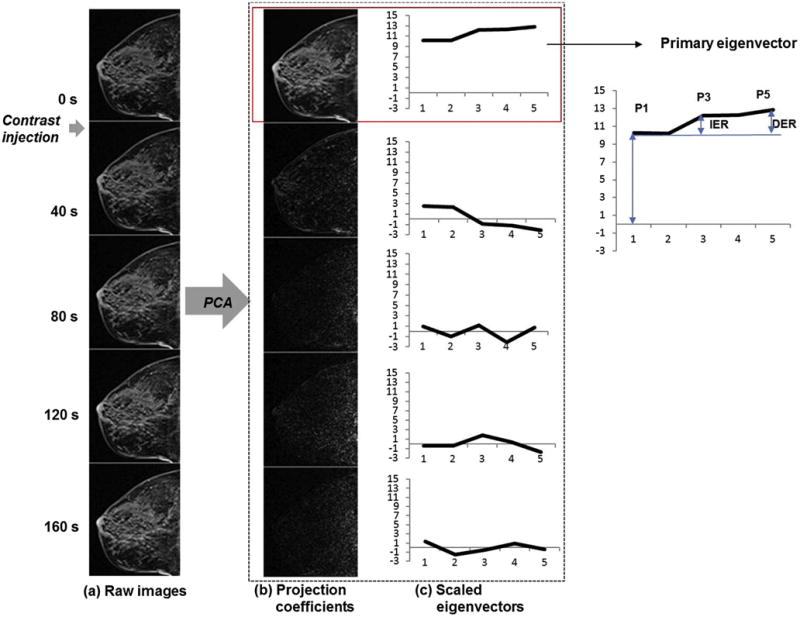
Representative example of the PCA-based analysis of DCE-MRI data. (a) Fat-suppressed T1-weighted images with contrast injection during the second image. Signal intensity range was kept constant to show the contrast enhancement. (b) Projection coefficient maps of the principal components in the descending order of eigenvalues. (c) The principal components used to compute the projection coefficient maps shown in (b). Plotted are the eigenvectors scaled so that the sum of their squares are equal to the corresponding eigenvalues.
The PCA method was applied to three randomly selected sagittal slices without any suspicious lesion or benign pathology. The slices were chosen from both the lateral and medial aspects of the breast. The regions of interest (ROIs) were drawn to include the entire breast tissue to the level of the pectoralis muscle, including the skin and nipple. In our previous study [12], we measured the reproducibility of the PCA based method for two operators. Each operator randomly selected 3 sagittal slices for the PCA analysis; we found that the intraclass correlation coefficient was 0.98 (95% CI: 0.96-0.99). The IER and DER measurements based on the primary eigenvectors from the three slices were averaged for each subject in the final data analysis and referred to as B-IER and B-DER. Data analysis was conducted using a custom-made software tool implemented in Interactive Data Language (IDL) (Exelis VIS, Boulder, CO), one of the commonly used computer program languages. IDL includes a graphical user interface to draw the ROIs and perform the PCA method using the DCE-MRI data. Figure 2 provides examples of the PCA method applied to BRCA and control premenopausal and postmenopausal cases. The primary eigenvectors were used to measure IER and DER of the BP.
Figure 2.
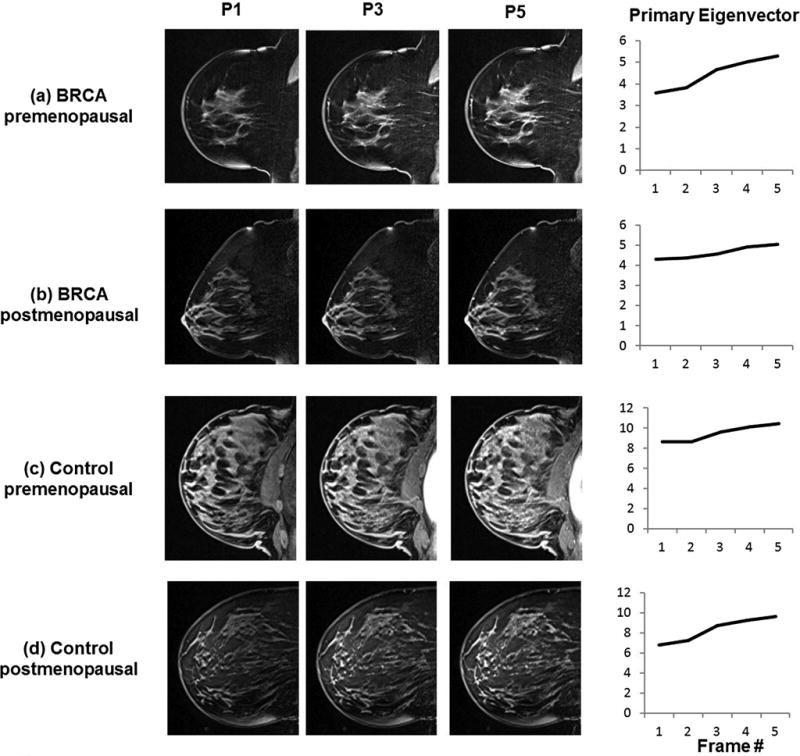
Representative examples of the DCE images at 3 time points used to measure IER and DER for (a) BRCA premenopausal case, (b) BRCA postmenopausal case, (c) control premenopausal case, and (d) control postmenopausal case. The corresponding scaled primary eigenvectors are shown for each case to demonstrate how the eigenvectors represent background parenchymal enhancement.
Analysis of breast lesions
The conventional analysis of the breast MRI was performed according to the ACR Breast MRI BI-RADS Lexicon [18]. Lesion types were classified as a mass, NME or a focus. The lesion type and features (shape, margin and internal enhancement of a mass or focus, distributor and internal enhancement of NME), time-signal enhancement curve (Type 1 progressive enhancement, Type 2 plateau curve or a Type 3 washout curve) and conventional BPE categories were obtained from the original radiology report. The time signal intensity curves were assessed using DynaCAD (Invivo, Pewaukee, WI), a commercially available computer-aided evaluation system [19].
Regions of interest were selected in consensus by two readers (fellowship-trained breast imager with 2 and 12 years of experience) and drawn manually around each biopsied lesion on all slices containing the abnormal finding. This was performed after review of the associated reports and images. The regions of interest (ROIs) included the enhancing component of the lesion, excluding the surrounding fat and non-enhancing fibroglandular tissue. A corresponding signal enhancement curve analysis was performed according to a recent study [12]. The contrast enhancement curve based on the average value of the lesion ROI was characterized in terms of two parameters, IER and DER as defined above for analysis of BPE kinetics. The IER and DER values of a lesion (L) were referred to as L-IER and L-DER.
Statistical Analysis
Two-way analysis of covariance (ANCOVA) was used to compare BRCA carriers to non-carriers while accounting for the fact that carriers were 1-1 matched to non-carries in terms of age and scan date and adjusting for the potential confounding effects of menopausal status and whether or not tumor was present. To further account for the influence of tumor presence and menopausal status, the analyses to compare carriers to non-carriers were also stratified by these factors. In all analyses, the indicator variable to identify subjects that were one-to-one matched to each other was included in the analysis as a blocking factor.
Linear regression models were used to test whether the difference between BRCA carriers and non-carriers in terms of each imaging measure was dependent on menopausal status or age (i.e., whether the magnitude of difference tended to increase or decrease with age or menopausal status) and to compare BRCA carriers and non-carriers in terms of each imaging measure adjusted for the potential confounding effects of age, menopausal status, LMP and whether or not tumor was present.
For analyses including pre-menopausal women, week of cycle was included in the analysis as a classification factor. In all analyses, the error variance was allowed to differ across comparison groups in order to remove the unnecessary assumption of variance homogeneity.
The association between lesion type and conventional radiographic measures was determined by using the Fisher exact test (2 groups) or the χ2 test (≥ 3 groups). All statistical tests were conducted at the two-sided 5% significance level using SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
Table 2 shows the radiographic characteristics of the patients included in this study in terms of conventional assessment of BPE, mammographic density, and lesion kinetic curve type on the basis of BI-RADS criteria as well as lesion size [20]. For all BRCA and control cohorts, 70-82% of cases demonstrated mild and moderate BPE, while 60-88% of all cases had mammographic density categorized as heterogeneously dense or extremely dense. Only 20% of malignant BRCA lesions and no malignant control lesion demonstrated type III kinetic curves, whereas 88% of benign BRCA lesions and 92% of benign control lesions demonstrated type I or type II kinetic curves. Similarly, 12.2% of benign BRCA lesions and 8.2% of benign control lesions demonstrated type III kinetic curves. The lesion types (NME, mass, focus) were evenly distributed when comparing malignant and benign BRCA and control cases, respectively. None of the conventional radiographic measures assessed in this study was significantly associated with lesion malignancy in either the BRCA or control cohort in this study.
Table 2.
Radiographic characteristics. The p-values are from Fisher exact test (2 groups) or the χ2 test (≥ 3 groups).
| BRCA CASES
|
CONTROL CASES
|
|||||
|---|---|---|---|---|---|---|
| Malignant | Benign | p | Malignant | Benign | p | |
| No. of patients | 10 | 49 | 10 | 49 | ||
| BPE | 0.33 | 0.39 | ||||
| None | 3 (30%) | 5 (10.2%) | 2 (20%) | 4 (8.2%) | ||
| Mild | 4 (40%) | 20 (40.8%) | 4 (40%) | 18 (36.7%) | ||
| Moderate | 3 (30%) | 20 (40.8%) | 4 (40%) | 18 (36.7%) | ||
| Marked | 0 | 4 (8.2%) | 0 | 9 (18.4%) | ||
| Mammographic density | 0.10 | 0.15 | ||||
| Predominantly fatty | 0 | 0 | 0 | 1 (2.0%) | ||
| Scattered FGT | 4 (40%) | 6 (12.2%) | 3 (30%) | 10 (20.4%) | ||
| Heterogeneously dense | 4 (40%) | 28 (57.2%) | 7 (70%) | 21 (42.9%) | ||
| Extremely Dense | 2 (20%) | 15 (30.6%) | 0 | 17 (34.7%) | ||
| Lesion kinetic curve | 0.08 | 0.47 | ||||
| Type I | 1 (10%) | 24 (49%) | 4 (40%) | 24 (48.9%) | ||
| Type II | 7 (70%) | 19 (38.8%) | 6 (60%) | 21 (42.9%) | ||
| Type III | 2 (20%) | 6 (12.2%) | 0 | 4 (8.2%) | ||
| Lesion size | 0.30 | 0.65 | ||||
| ≥2cm | 5 (50%) | 16 (32.7%) | 4 (40%) | 16 (32.7%) | ||
| <2cm | 5 (50%) | 33 (67.3%) | 6 (60%) | 33 (67.3%) | ||
| Lesion type | 0.42 | 0.37 | ||||
| NME | 8 (80%) | 29 (59.2%) | 8 (80%) | 28 (57.1%) | ||
| Mass | 2 (20%) | 17 (34.7%) | 2 (20%) | 18 (36.8%) | ||
| Focus | 0 | 3 (6.1%) | 0 | 3 (6.1%) | ||
Comparisons of quantitative contrast enhancement measures between BRCA and control group are shown in Figure 3. Control cases demonstrated higher percent enhancement than BRCA cases in terms of all four measures, L-IER, L-DER, B-IER, and B-DER, when compared in all women (Fig.3a and b) and in women with benign lesions (Fig.3c and d). The differences in B-IER and B-DER between BRCA and control groups were statistically significant for both subsets (all women: p = 0.02, p = 0.02, respectively; benign only: p = 0.005, p = 0.005, respectively).
Figure 3.
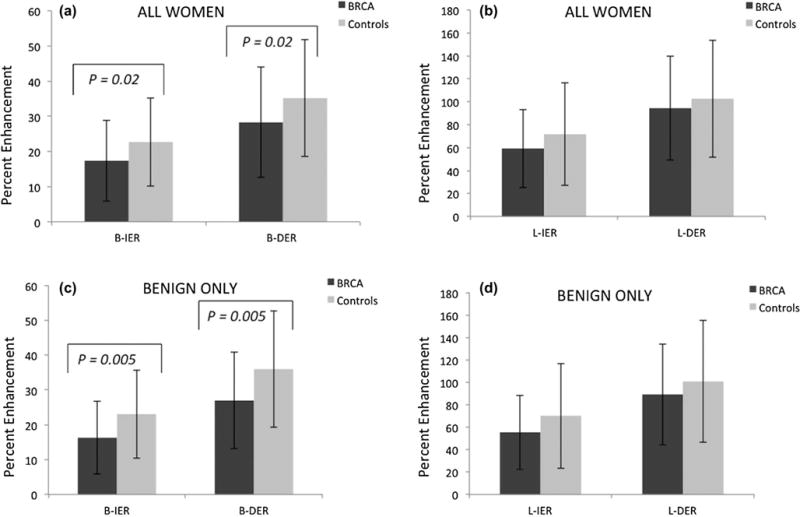
(a) Mean B-IER and B-DER for all BRCA women and all controls. (b) Mean L-IER and L-DER for all BRCA women and all controls. (c) Mean B-IER and B-DER for benign BRCA and control cases only. (d) Mean L-IER and L-DER for benign BRCA and control cases only.
We evaluated the effect of menopausal status on the quantitative contrast enhancement in BRCA and control groups. We separated the BRCA and control groups into premenopausal and postmenopausal categories as shown in Figure 4. In premenopausal women, BPE enhancement kinetics of control cases were significantly higher than BRCA cases in terms of B-IER (p = 0.01) and B-DER (p = 0.01), as shown in Figure 4a. The differences in B-IER and B-DER between BRCA and control cases were noticeably smaller in the post-menopausal women than in the pre-menopausal women (Fig 4a and 4c). Similar trend was also observed with the lesion kinetics as shown in Fig 4b and 4d. Within the BRCA group, there was no significant difference between premenopausal and postmenopausal BPE and lesion enhancement kinetics. Conversely, in the control group, the lesion and BPE enhancement kinetics were significantly higher in the premenopausal group than the postmenopausal group, achieving statistical significance for B-IER and B-DER (p = 0.013 and 0.003, respectively).
Figure 4.
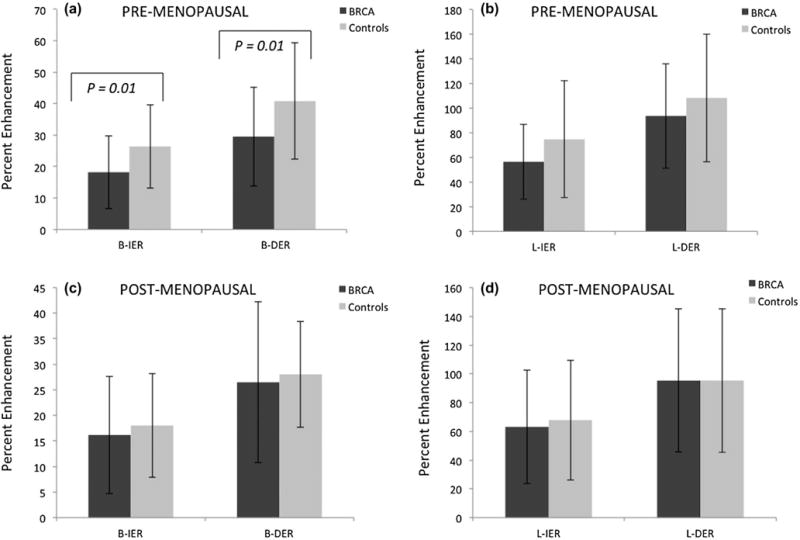
(a) Mean B-IER and B-DER for pre-menopausal BRCA patients and controls. (b) Mean L-IER and L-DER for pre-menopausal BRCA patients and controls. (c) Mean B-IER and B-DER for post-menopausal BRCA patients and controls. (d) Mean L-IER and L-DER for post-menopausal BRCA patients and controls.
Furthermore, we assessed whether the difference between BRCA carriers and non-carriers, in terms of each imaging measure, was dependent on menopausal status or age. The correlation of age with each imaging parameter was weak (r < 0.2) and did not achieve statistical significance (p > 0.06). The difference between BRCA carriers and controls in terms of each imaging parameter was not significantly dependent on age (p > 0.4 for BRCA1, p > 0.3 for BRCA2). This implies that the magnitude of difference between carriers and non-carriers neither increases nor decreases with age.
Also, no differences in the enhancement parameters were observed among the premenopausal women in BRCA positive and controls in different phases of the menstrual cycle. In fact, irrespective of whether or not the analysis accounted for the effect of tumor and/or carrier status, last menstrual period (LMP) had no significant main effect on any outcome measure (p > 0.26) whereas menopausal status had a significant effect on B-DER and B-IER (p < 0.035) but no significant effect on L-DER or L-IER (p >0 .75).
DISCUSSION
Our study demonstrated that the initial and delayed enhancement ratios were higher in the lesion and background parenchyma among women in the control group compared to the respective BRCA cohort. Interestingly, we found significant difference in BPE kinetics between premenopausal and postmenopausal women in the control cohort, but not in the BRCA cohort. Our results suggest that the BRCA gene mutation has multifactorial and complex clinical and biologic implications.
Recent studies have suggested the need for a better understanding of BPE and the observed effects of hormonal fluctuations on BPE [15]. Since BPE is suggested as a potential risk factor and biomarker for breast cancer [15, 11], it is important to elucidate the biologic factors of BPE and its relationship with the BRCA gene. High levels of BPE may be an indicator that high-risk women, including BRCA gene mutation carriers, may benefit from chemoprevention [21].
BRCA 1 and BRCA 2 mutation carriers are widespread and account for about 20-25% of hereditary breast cancers [22] and about 5-10% of all breast cancers [23]. Breast cancers associated with BRCA 1 and BRCA 2 mutations tend to develop at younger ages than sporadic breast cancers. Cells with BRCA gene mutation are more likely to develop genetic alterations that lead to aggressive cancers that are often occult on mammography. [24]. Obdeijin et al found that MRI detected breast cancers at an earlier stage with a more favorable prognosis. Several studies have suggested that BRCA carriers should undergo annual screening and radiologic surveillance with MRI only and eliminate the mammogram given the greater sensitivity of breast MRI to detect breast cancer [7, 25, 26].
BRCA 1/2 gene mutation in young women is associated with more aggressive breast cancer and the absence of estrogen receptors, progesterone receptors and HER-2 receptors, referred to as “triple-negative” [27]. In our study, five of seven BRCA malignant lesions were ER negative, consistent with this observation. Recent studies have demonstrated that both triple receptor negativity and expression of CK5 (indicating a basal phenotype) and p-cadherin are associated with BRCA 1 mutations. BRCA 2 related breast cancers are most often of luminal type and seem phenotypically more difficult to recognize [28]. Despite these histological features of BRCA tumors, there is scant information on the effect of the BRCA gene mutation on breast physiology and enhancement kinetics. In addition, conventional analyses are not helpful in differentiating malignancies from benign lesions in this patient population. There is significant overlap in the kinetic curve types between benign and malignant lesions on breast MRI (9). Our BRCA cohort had 10 malignancies leading to a positive predictive value of biopsy of 16.9%. This low cancer yield remains a diagnostic dilemma in BRCA mutation carriers who undergo annual screening MRI and often unnecessary biopsies.
When examining the conventional measures of BPE, we found no statistically significant difference between the BRCA and high-risk control populations. However, our quantitative analysis showed that the matched controls demonstrated greater contrast enhancement in BP than BRCA cases for “all women” and “benign only” cases. As shown in Figure 3, statistical significance was achieved for BPE kinetic parameters, BIER, and B-DER, for “all women” and “benign only”. Quantitative kinetic analysis of BPE may underscore subtle changes in BPE between controls and BRCA carriers not appreciable with conventional measures. These findings suggest that the BRCA gene has multifactorial and complex clinical and biologic implications in BRCA mutation carriers.
In our study, matched controls also demonstrated greater contrast enhancement in lesions (i.e., L-IER and L-DER) than BRCA cases for “all women” and “benign only” cases. Perhaps, the histological and molecular features of BRCA lesions affect the contrast enhancement kinetics to a lesser degree than of matched-controls. These disparities in tumor characteristics may also affect the surrounding background parenchyma, altering the contrast enhancement characteristics of BPE as seen in our study. To date, there has been no report on whether BPE, particularly its contrast kinetic characteristics, is associated with malignancy in BRCA mutation carriers. Several studies have suggested the correlation with elevated BPE and breast cancer risk in high-risk women [11, 15, 21]. If further studies corroborate these findings, breast MRI may serve as a non-invasive imaging biomarker for this high-risk population with clinical implications related to chemoprevention [21].
Numerous studies have demonstrated a negative correlation between BPE and age so that as age increases and women become postmenopausal, BPE decreases. Although the exact biological basis for BPE is not entirely understood, current literature suggests that BPE is related to the effect of hormonal changes on breast tissue and is sensitive to estrogen and estradiol [29–32, 14]. A recent paper by Kang et al. demonstrated that the degree of parenchymal enhancement in premenopausal women was significantly higher than that of postmenopausal women in both the early and delayed phases of contrast enhancement [33]. We also examined the effect of menopausal status on the relationship between BRCA and control groups. We found that the BPE was higher in BRCA and control premenopausal patients compared to the respective postmenopausal cohort, consistent with Kang et al [33]. Within our control group, the control lesion and BPE kinetics were higher in the premenopausal group than the postmenopausal group (achieving statistical significant for B-IER and B-DER), behaving as expected. Interestingly, BRCA lesion and BPE kinetics did not significantly differ between the premenopausal and postmenopausal cohort.
Our findings of lack of difference in BP and lesion enhancement between pre- and post-menopausal groups of BRCA carriers may reflect a biologic difference in BRCA patients who have lost their tumor suppression genes and cannot repair genetic alterations. Although the precise mechanism for oncogenic and angiogenic activity of BRCA tumors is not well defined, our data suggests that BRCA cases may not undergo the normal variation in breast composition and vascularity, as seen in sporadic high risk controls. As a result, breast cancers associated with BRCA 1 and BRCA 2 mutations tend to develop at younger ages than sporadic breast cancers [34]. We postulate that there may be a dysregulation in the normal response to physiologic hormonal changes in pre-menopausal BRCA carriers, altering the local environment and surrounding BPE. Studies have shown that BPE reflects a more systemic milieu than just lesion-specific characteristics [21]. Recent studies have used varying quantitative analyses to better quantify BPE [35–37]. In a paper by Kim et al, higher background parenchymal signal enhancement ratio (SER) around DCIS on preoperative MRI was a significant factor associated with worse ipsilateral breast tumor recurrence [36]. Additional work needs to be done to understand how vascularity and molecular features differ in BRCA patients compared to sporadic controls with subsequent local and systemic influence on BPE.
Limitations of the study include its retrospective approach and relatively small sample size of the malignant cases. Perhaps a larger sample size of malignant cases would further elucidate the differences between the BRCA and control cohorts. The small number of malignancies in our cohort limits our ability to see if the BPE kinetic measures could differentiate between benign and malignant lesions. Low temporal resolution (40 s/frame) of the DCE-MRI data was another limiting factor in characterization of contrast enhancement curve. A high temporal resolution of 10 s/frame or higher can be possible for either localized area or a low spatial resolution image acquisition, but not in typical clinical setting where high spatial resolution – approximately 1 mm isotropic voxel size – is required for the entire breast volume. A higher temporal resolution data would have enabled use of pharmacokinetic model analyses, which provide more physiologically relevant parameters than the heuristic measures used in this study [38, 39]. Future prospective study with fast data acquisition and pharmacokinetic model analysis is warranted.
CONCLUSIONS
In conclusion, our results demonstrate that the vascularity of the breast parenchyma of BRCA mutation carriers may respond differently to hormonal level changes and reflect the underlying genetic alterations. These results suggest that the etiology of BPE is multifactorial and may be an imaging biomarker in high-risk groups such as BRCA mutation carriers. We suggest that contrast enhancement kinetics play an important role in investigating critical differences in between BRCA carriers and non-carriers. Our findings and conclusions need to be validated with a larger sample size.
Acknowledgments
Grant support: NIH 1 R01 CA160620
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Donovan PJ, Livingston DM. BRCA1 and BRCA2: breast/ovarian cancer susceptibility gene products and participants in DNA double-strand break repair. Carcinogenesis. 2010;31(6):961–7. doi: 10.1093/carcin/bgq069. [DOI] [PubMed] [Google Scholar]
- 2.Whittemore AS, Gong G, John EM, McGuire V, Li FP, Ostrow KL, et al. Prevalence of BRCA1 mutation carriers among U.S. non-Hispanic Whites. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2004;13(12):2078–83. [PubMed] [Google Scholar]
- 3.Group ABCS. Prevelance and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Br J Cancer. 2000;(83):1301–8. doi: 10.1054/bjoc.2000.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. American journal of human genetics. 2003;72(5):1117–30. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilliland FD, Joste N, Stauber PM, Hunt WC, Rosenberg R, Redlich G, et al. Biologic characteristics of interval and screen-detected breast cancers. Journal of the National Cancer Institute. 2000;92(9):743–9. doi: 10.1093/jnci/92.9.743. [DOI] [PubMed] [Google Scholar]
- 6.Smith RA, Saslow D, Sawyer KA, Burke W, Costanza ME, Evans WP, 3rd, et al. American Cancer Society guidelines for breast cancer screening: update 2003. CA: a cancer journal for clinicians. 2003;53(3):141–69. doi: 10.3322/canjclin.53.3.141. [DOI] [PubMed] [Google Scholar]
- 7.Gillman JT, Hildegard, Moy Linda. Role of T1-Weighted Fat-Suppressed Screening Breast MRI in the High-Risk Population. Imaging in Medicine [Google Scholar]
- 8.Bluemke DA, Gatsonis CA, Chen MH, DeAngelis GA, DeBruhl N, Harms S, et al. Magnetic resonance imaging of the breast prior to biopsy. JAMA: the journal of the American Medical Association. 2004;292(22):2735–42. doi: 10.1001/jama.292.22.2735. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez RL, DeMartini WB, Eby PR, Kurland BF, Peacock S, Lehman CD. BI-RADS lesion characteristics predict likelihood of malignancy in breast MRI for masses but not for nonmasslike enhancement. AJR American journal of roentgenology. 2009;193(4):994–1000. doi: 10.2214/AJR.08.1983. [DOI] [PubMed] [Google Scholar]
- 10.Mahoney MC, Gatsonis C, Hanna L, DeMartini WB, Lehman C. Positive predictive value of BI-RADS MR imaging. Radiology. 2012;264(1):51–8. doi: 10.1148/radiol.12110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King V, Brooks JD, Bernstein JL, Reiner AS, Pike MC, Morris EA. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology. 2011;260(1):50–60. doi: 10.1148/radiol.11102156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amarosa AR, McKellop J, Klautau Leite AP, Moccaldi M, Clendenen TV, Babb JS, et al. Evaluation of the kinetic properties of background parenchymal enhancement throughout the phases of the menstrual cycle. Radiology. 2013;268(2):356–65. doi: 10.1148/radiol.13121101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King V, Gu Y, Kaplan JB, Brooks JD, Pike MC, Morris EA. Impact of menopausal status on background parenchymal enhancement and fibroglandular tissue on breast MRI. European radiology. 2012;22(12):2641–7. doi: 10.1007/s00330-012-2553-8. [DOI] [PubMed] [Google Scholar]
- 14.King V, Kaplan J, Pike MC, Liberman L, David Dershaw D, Lee CH, et al. Impact of tamoxifen on amount of fibroglandular tissue, background parenchymal enhancement, and cysts on breast magnetic resonance imaging. The breast journal. 2012;18(6):527–34. doi: 10.1111/tbj.12002. [DOI] [PubMed] [Google Scholar]
- 15.Price ER, Brooks JD, Watson EJ, Brennan SB, Comen EA, Morris EA. The impact of bilateral salpingo-oophorectomy on breast MRI background parenchymal enhancement and fibroglandular tissue. European radiology. 2014;24(1):162–8. doi: 10.1007/s00330-013-2993-9. [DOI] [PubMed] [Google Scholar]
- 16.DeMartini WB, Liu F, Peacock S, Eby PR, Gutierrez RL, Lehman CD. Background parenchymal enhancement on breast MRI: impact on diagnostic performance. AJR American journal of roentgenology. 2012;198(4):W373–80. doi: 10.2214/AJR.10.6272. [DOI] [PubMed] [Google Scholar]
- 17.Eyal E, Badikhi D, Furman-Haran E, Kelcz F, Kirshenbaum KJ, Degani H. Principal component analysis of breast DCE-MRI adjusted with a model-based method. Journal of magnetic resonance imaging: JMRI. 2009;30(5):989–98. doi: 10.1002/jmri.21950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breast Imaging Reporting and Data Sysmte (BI-RADS) 4th. Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 19.Ko ES, Choi HY, Lee BH, Noh WC, Kim RB. Central washout sign in computer-aided evaluation of breast MRI: preliminary results. Acta radiologica. 2011;52(3):256–63. doi: 10.1258/ar.2010.100187. [DOI] [PubMed] [Google Scholar]
- 20.Morris E. Diagnostic breast MR imaging: current status and future directions. Radiol Clin North Am. 2007;45(5):863–80, vii. doi: 10.1016/j.rcl.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Weinstein S. Invited commentary. Radiographics: a review publication of the Radiological Society of North America, Inc. 2014;34(1):247–9. doi: 10.1148/rg.341135172. [DOI] [PubMed] [Google Scholar]
- 22.Easton DF. How many more breast cancer predisposition genes are there? Breast cancer research: BCR. 1999;1(1):14–7. doi: 10.1186/bcr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campeau PM, Foulkes WD, Tischkowitz MD. Hereditary breast cancer: new genetic developments, new therapeutic avenues. Human genetics. 2008;124(1):31–42. doi: 10.1007/s00439-008-0529-1. [DOI] [PubMed] [Google Scholar]
- 24.Mote PA, Leary JA, Avery KA, Sandelin K, Chenevix-Trench G, Kirk JA, et al. Germline mutations in BRCA1 or BRCA2 in the normal breast are associated with altered expression of estrogen-responsive proteins and the predominance of progesterone receptor A. Genes, chromosomes & cancer. 2004;39(3):236–48. doi: 10.1002/gcc.10321. [DOI] [PubMed] [Google Scholar]
- 25.Obdeijn IM, Winter-Warnars GA, Mann RM, Hooning MJ, Hunink MG, Tilanus-Linthorst MM. Should we screen BRCA1 mutation carriers only with MRI? A multicenter study. Breast cancer research and treatment. 2014;144(3):577–82. doi: 10.1007/s10549-014-2888-8. [DOI] [PubMed] [Google Scholar]
- 26.Sardanelli F, Podo F, Santoro F, Manoukian S, Bergonzi S, Trecate G, et al. Multicenter surveillance of women at high genetic breast cancer risk using mammography, ultrasonography, and contrast-enhanced magnetic resonance imaging (the high breast cancer risk italian 1 study): final results. Investigative radiology. 2011;46(2):94–105. doi: 10.1097/RLI.0b013e3181f3fcdf. [DOI] [PubMed] [Google Scholar]
- 27.Zakhartseva LM, Gorovenko NG, Podolskaya SV, Anikusko NF, Lobanova OE, Pekur KA, et al. Breast cancer immunohistochemical features in young women with BRCA 1/2 mutations. Experimental oncology. 2009;31(3):174–8. [PubMed] [Google Scholar]
- 28.Heerma van Voss MR, van der Groep P, Bart J, van der Wall E, van Diest PJ. Lympho-vascular invasion in BRCA related breast cancer compared to sporadic controls. BMC cancer. 2010;10:145. doi: 10.1186/1471-2407-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuhl CK, Bieling HB, Gieseke J, Kreft BP, Sommer T, Lutterbey G, et al. Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: normal contrast medium enhancement and cyclical-phase dependency. Radiology. 1997;203(1):137–44. doi: 10.1148/radiology.203.1.9122382. [DOI] [PubMed] [Google Scholar]
- 30.Hussain Z, Roberts N, Whitehouse GH, Garcia-Finana M, Percy D. Estimation of breast volume and its variation during the menstrual cycle using MRI and stereology. The British journal of radiology. 1999;72(855):236–45. doi: 10.1259/bjr.72.855.10396212. [DOI] [PubMed] [Google Scholar]
- 31.Delille JP, Slanetz PJ, Yeh ED, Kopans DB, Halpern EF, Garrido L. Hormone replacement therapy in postmenopausal women: breast tissue perfusion determined with MR imaging–initial observations. Radiology. 2005;235(1):36–41. doi: 10.1148/radiol.2351040012. [DOI] [PubMed] [Google Scholar]
- 32.Reston VA, Radiology ACo, editor. Breast Imaging Reporting and Data System (BI-RADS) 4th. 2003. [Google Scholar]
- 33.Kang SS, Ko EY, Han BK, Shin JH, Hahn SY, Ko ES. Background parenchymal enhancement on breast MRI: influence of menstrual cycle and breast composition. Journal of magnetic resonance imaging: JMRI. 2014;39(3):526–34. doi: 10.1002/jmri.24185. [DOI] [PubMed] [Google Scholar]
- 34.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, et al. SEER Cancer Statistics Review, 1975-2010. National Cancer Institute; Bethesda, MD: 2013. [Google Scholar]
- 35.Scaranelo AM, Carrillo MC, Fleming R, Jacks LM, Kulkarni SR, Crystal P. Pilot study of quantitative analysis of background enhancement on breast MR images: association with menstrual cycle and mammographic breast density. Radiology. 2013;267(3):692–700. doi: 10.1148/radiol.13120121. [DOI] [PubMed] [Google Scholar]
- 36.Kim SA, Cho N, Ryu EB, Seo M, Bae MS, Chang JM, et al. Background parenchymal signal enhancement ratio at preoperative MR imaging: association with subsequent local recurrence in patients with ductal carcinoma in situ after breast conservation surgery. Radiology. 2014;270(3):699–707. doi: 10.1148/radiol.13130459. [DOI] [PubMed] [Google Scholar]
- 37.Kajihara M, Goto M, Hirayama Y, Okunishi S, Kaoku S, Konishi E, et al. Effect of the menstrual cycle on background parenchymal enhancement in breast MR imaging. Magnetic resonance in medical sciences: MRMS: an official journal of Japan Society of Magnetic Resonance in Medicine. 2013;12(1):39–45. doi: 10.2463/mrms.2012-0022. [DOI] [PubMed] [Google Scholar]
- 38.Huang W, Li X, Morris EA, Tudorica LA, Seshan VE, Rooney WD, et al. The magnetic resonance shutter speed discriminates vascular properties of malignant and benign breast tumors in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(46):17943–8. doi: 10.1073/pnas.0711226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. Journal of magnetic resonance imaging: JMRI. 1999;10(3):223–32. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]


