Abstract
AAV9 vector provides efficient gene transfer in all segments of the renal nephron, with minimum expression in non-renal cells, when administered retrogradely via the ureter. It is important to restrict the transgene expression to the desired cell type within the kidney, so that the physiological endpoints represent the function of the transgene expressed in that specific cell type within kidney. We hypothesized that segment-specific gene expression within the kidney can be accomplished using the highly efficient AAV9 vectors carrying the promoters of genes that are expressed exclusively in the desired segment of the nephron in combination with administration by retrograde infusion into the kidney via the ureter. We constructed AAV vectors carrying eGFP under the control of: kidney-specific cadherin (KSPC) gene promoter for expression in the entire nephron; Na+/glucose co-transporter (SGLT2) gene promoter for expression in the S1 and S2 segments of the proximal tubule; sodium, potassium, 2 chloride co-transporter (NKCC2) gene promoter for expression in the thick ascending limb of Henle's loop (TALH); E-cadherin (ECAD) gene promoter for expression in the collecting duct (CD); and cytomegalovirus (CMV) early promoter that provides expression in most of the mammalian cells, as control. We tested the specificity of the promoter constructs in vitro for cell type-specific expression in mouse kidney cells in primary culture, followed by retrograde infusion of the AAV vectors via the ureter in the mouse. Our data show that AAV9 vector, in combination with the segment-specific promoters administered by retrograde infusion via the ureter, provides renal nephron segment-specific gene expression.
Keywords: Renal gene transfer, AAV, Promoters
1. Introduction
Adeno-associated viral (AAV) vectors have become one of the most attractive tools for in vivo gene transfer in translational, as well as basic research studies [1]. AAV as a wild-type virus or as a gene-transfer vector does not cause any disease in humans. Recent advances in AAV vector production methods offer preparation of clinical grade AAV vectors, consistently and cost effectively [2–4]. AAV vectors provide sustained long-term gene expression with minimal immunological consequences [5,6]. Systemic administration of AAV9 vector transduces various organs such as liver, heart, lung and skeletal muscle efficiently but provides very low transduction in kidney [6–11].
Several AAV serotypes and routes of administration in combination with kidney specific promoter have been attempted to improve AAV vector-directed gene transfer to the kidney. Systemic administration of AAV1 carrying G6PAse-α provided gene expression in hepatocytes and kidneys that alleviated metabolic abnormalities in a mouse model of type 1α glycogen storage disease [12]. AAV9 carrying kidney-specific cadherin (KSPC) promoter expressing hepatocyte growth factor provided gene expression in the mouse kidney and liver after systemic administration in COL4A3-deficient mice, which attenuated tubulointerstitial fibrosis and repressed fibrotic markers [13]. In both studies, in spite of low renal transduction by AAV9 or AAV1 vector, the therapeutic benefits were achieved largely due to superior hepatic gene expression that acted in an endocrine fashion on the kidney. Subsequently, Ito et al., 2008 [14] demonstrated that the intra pelvic injection of AAV2 vector provides expression predominantly in the renal medulla. To improve the transduction in kidney epithelial cells Chung et al., 2011 [15] administered AAV vectors by retrograde ureteral infusion and showed that AAV8 and AAV9 transduce kidney cells efficiently when compared with other AAV serotypes tested. In previous studies we analyzed the magnitude and distribution of gene expression to show that administration of AAV9 vector by retrograde infusion via the ureter provides efficient gene transfer to renal proximal tubule (PT), distal convoluted tubule, and collecting duct (CD) cells with minimum off-target gene expression [11]. Within the kidney, AAV9 administered by retrograde infusion via the ureter provided efficient gene transfer to renal cells including PT, distal convoluted tubule, collecting duct cells, and podocytes [11]. Moreover, retrograde infusion via the ureter circumvented the extra-renal gene expression by AAV9 vector in spite of bearing the ubiquitously active CMV promoter. The renal nephron consists of several segments, each with distinct function to orchestrate overall fluid and electrolyte balance [16]. The distinct function of each nephron segment is attributed to the unique gene expression profiles exhibited by the epithelial cells in the specific nephron segments. Therefore, it is important to express the gene of interest in the desired cell type within the kidney, so that the renal functional parameters measured will represent the function of the gene expressed in the desired cell type within kidney. We hypothesized that use of the promoter of the gene that is expressed exclusively in the desired nephron segment such as, KSPC [17], SGLT2 [18], NKCC2 [19], and E-CAD [20], provides preferential gene expression in entire nephron, PT, TALH, and CD, respectively.
In this study we generated AAV vectors encoding eGFP gene under the control of nephron segment specific promoters KSPC, SGLT2, NKCC2, or ECAD and determined the distribution of gene expression within the kidney following retrograde ureteral infusion of AAV9 vector carrying the promoter constructs.
2. Methods
AAV vectors
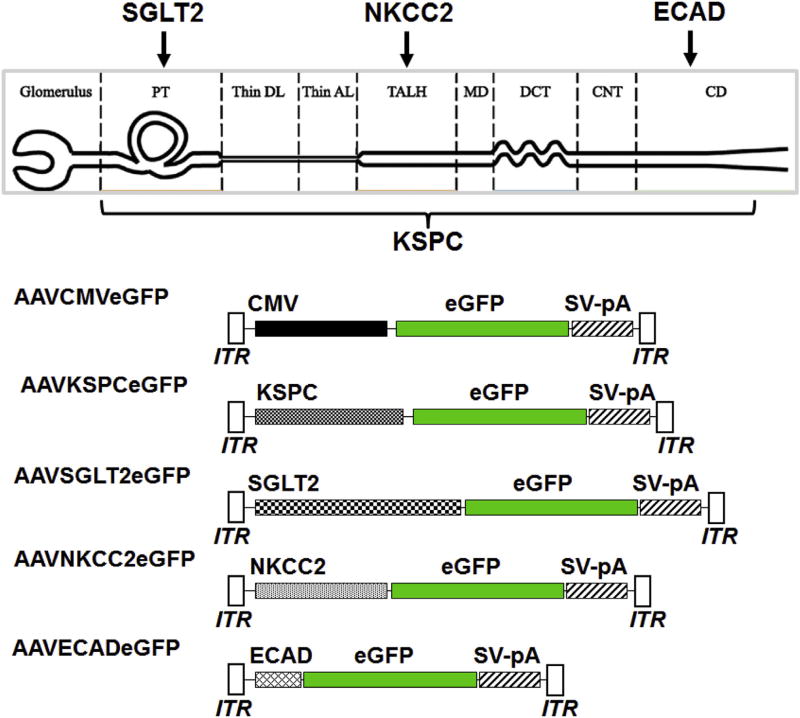
The AAV vector pAAVCMVeGFP, harboring the cytomegalovirus (CMV) promoter driving the expression of eGFP [11] was used to generate the sets of AAV vectors carrying kidney-specific promoters encoding eGFP (Fig. 1). The kidney specific promoters were PCR-amplified from the mouse genomic DNA using the primers (Table 1) with appropriate restriction sites at the 5′ and 3'ends:1.3 kb KSPC promoter (GeneBank: AF118228.1) with NotI and HindIII; 1.1 kb SGLT2 promoter (GeneBank: AJ292928.1) with BsrgI and KpnI; 469 bp NKCC2 promoter (GeneBank: U45313.1) with XbaI and HindIII; and 1.25 kb ECAD promoter (GeneBank: AY566874.1) with XbaI and HindIII restriction sites at 5′ and 3′ ends respectively. The PCR amplicons were then inserted in place of CMV promoter in pAAVCMVeGFP using standard molecular biology protocols to create the plasmids pAAVKSPCeGFP, pAAVAGLT2eGFP pAAVNKCC2eGFP or pAAVECADeGFP (Fig. 1). The 1.1 kb SGLT2 promoter is similar to the one described before [18], except that it contains only 400 bp of the 5′ untranslated region and includes an HNF1a binding site, crucial for the transcription form the SGLT2 promoter [21]; the remaining sequence includes the first exon, first intron, and part of the second exon with the starting codon ATG present in the first exon, mutated to AGG to inactivate the gene expression from the endogenous starting codon. Reporter gene expression from each of the promoters was confirmed by transient transfection into HEK 293 cells before proceeding to packaging into AAV9 for in vivo studies. Recombinant AAV vector genomes were packaged into AAV9 capsids by triple transfection method in HEK 293 cells [22,23]. AAV vectors were purified by ammonium sulfate fractionation and iodixanol gradient centrifugation. Titers of the AAV vectors (viral genome particles/ml; vgp/ml) were determined by quantitative real-time PCR [23,24].
Fig. 1.
Diagrammatic representation of the segments of the nephron modified from Nguyen et al., 2012 [42] and choice of promoters for segment-specific gene expression and AAV vector promoter constructs. PT = proximal tubule, DL = descending limb of the loop of Henle, AL = ascending limb of the loop of Henle, TALH = thick ascending limb of the loop of Henle, MD = macula densa, DCT = distal convoluted tubule, CNT =connecting tubule, CD = collecting duct, CMV = cytomegalovirus, eGFP = enhanced green fluorescent protein, SV-pA = Simian virus 40 polyadenylation signal sequence, ITR = inverted terminal repeat sequence, KSPC = kidney-specific cadherin, SGLT2 = sodium-glucose cotransporter 2, NKCC2 = sodium potassium 2-chloride cotransporter, ECAD = e cadherin.
Table 1.
Primers used for PCR amplification of each promoter. The restriction sites are shown in bold. Additional 6 nucleotides (shown in lower case) are added at the 5'end the primers for efficient digestion by the restriction enzymes.
| Promoter | Primers |
|---|---|
| KSPC | Fw: 5'atctgaGCTAGCAGCTTGCTCTGCCATGGGAAGG |
| Rv: 5'atctgaAAGCTTGCAAATTTGGCTTAGGTGGGGCGAG | |
| SGLT2 | Fw: 5'atctgaTGTACAAGAGAAAGTAGAAAATATTTGGGT |
| Rv: 5'atctgaAAGCTTGGTACCTATTGGTTCTGAACACAGACTG | |
| NKCC2 | Fw: 5'atctgaTCTAGAAATATGTGAGGCCCTGGGTTCG, |
| Rv: 5′atctgaAAGCTTATCCAGCAGCCTTCTTCTGAGC | |
| ECAD | Fw: 5'atctgaTCTAGAAGCTTGCTCTGCCATGGGAAGG |
| Rv: 5'atctgaAAGCTTCAGACGCCGAGCAAACACTG |
Preparation of primary cultures of mouse PT and CD cells
Mouse PT and CD cells were isolated from the mouse kidney using the Miltenyi Biotec's Magnetic-Activated Cell Sorting (MACS) technology. We used biotinylated Lotus tetragonolobyus lectin (Vector #B-1325) for the separation of PT cells and Dolichos biflorus agglutinin (Vector #B-1035) for CD cells. The purity of the cells prepared was assessed by immnuofluorescence staining using an antibody to SGLT2 (a marker protein of PT cells, S1 and S2 segments) and an antibody to α-ENaC (a marker protein of CD cells).
Animal procedure
All animal protocols used in this study were conducted in accordance with the National Institute of Health approved by the Institutional Animal Care and Use Committee at the George Washington University. C57BL/6 mice (8–10 weeks old weighing ~ 20 g) were purchased from the Jackson Laboratory (Bar Harbor, ME). Infusion of AAV vectors by retrograde infusion via the ureter was carried out as described previously [11]. The mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (50 mg/kg) and placed in a supine position and the legs taped down on a heated board to maintain their body temperature at 37°C. For all procedures, the depth of anesthesia was monitored by foot-pinch reflex. An abdominal incision was made between the point of the xyphoid cartilage and the navel and the ureter was located and gently dissected out. The distal portion of the ureter closest to the bladder and the renal artery supplying the target kidney were clamped off with micro-venous clips. Using a tuberculin syringe fitted with a 33-gauge needle, the ureter was then punctured. The needle was temporarily and snugly ligated in place using a 6-0 silk suture to prevent leakage. After the urine was gently aspirated out, the tuberculin syringe was replaced with another containing approximately 100 µL of the AAV vector (10^11 viral genome particles) and the solution was slowly retrogradely injected towards the kidney via the ureter. The needle was withdrawn and a micro-venous clip was placed proximal to the injection site on the ureter to prevent leakage. The arterial and the ureteral clips were maintained for 30 min to attain maximum exposure to the infusion. The arterial and ureteral clips were then sequentially removed and the ureter was inspected for any evidence of leakage. The abdominal contents were replaced in reverse order and the incision site was closed using a double layer of 6-0 silk sutures for the muscle and skin.
Immunofluorescence
eGFP expression in mouse kidneys was detected by immunofluorescence analysis, as described previously [11]. Two weeks following vector administration, the kidneys were collected and fixed in 3.8% para-formaldehyde for 1 h at 4 °C. After washing with PBS (3 times, 5 min each), the tissues were equilibrated with 30% sucrose in PBS overnight. Six µm cryosections were immunostained for eGFP using a chicken anti-GFP antibody (Aves Labs Inc. Tigard, Oregon). Biotinylated Lotus tetragonolobus agglutinin, which binds strongly to the brush border membrane of the renal PT cells and weakly to the intercalated cells of the CD, was used to distinguish PTs and CDs from other nephron segments. Nuclei were stained with 4′,6-diamidino-2-phenylindole.
3. Results and discussion
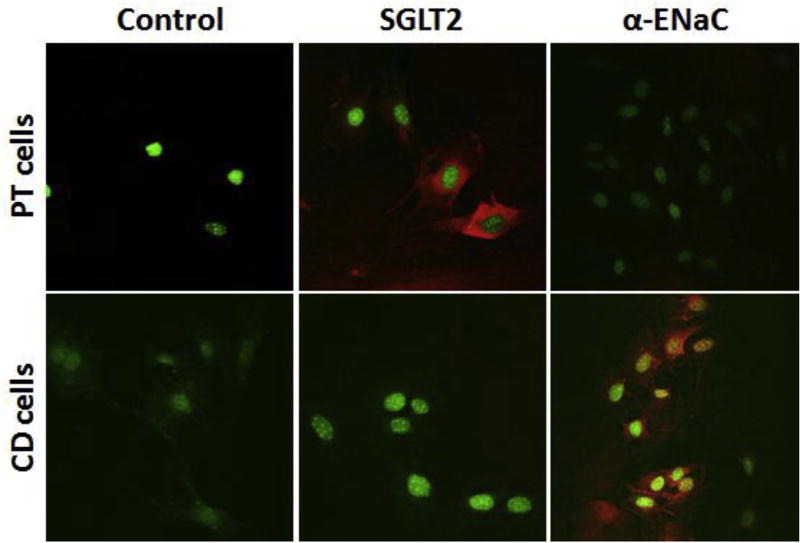
Renal PT and CD cell preparations were validated by immunohistochemical analyses using antibodies to SGLT2, a marker for S1 and S2 segments of renal PT cells, and α-ENaC, a marker for CD cells. Imunohistochemical analyses showed that the renal PT cells were positive for the expression of SGLT2 and negative for the expression of α-ENaC (Fig. 2). By contrast, the CD cell preparations were positive for the expression of α-ENaC and negative for SGLT2 (Fig. 2). These results validated the identity of the mouse PT and CD cell preparations.
Fig. 2.
Immunofluorescence staining showing the expression of marker protein for proximal tubule or collecting duct cells: Mouse proximal tubule (PT) cells or collecting duct (CD) cells were freshly isolated from the mouse kidney via the Miltenyi Biotec's Magnetic-Activated Cell Sorting (MACS) technology. We used biotinylated Lotus tetragonolobus lectin antibody (Vector #B-1325) for the separation of PT cells and Dolichos biflorus agglutinin antibody (Vector #B-1035) for separation of CD cells. The purity of the cells prepared was assessed by immunofluorescence staining using the antibody to the SGLT2 (a marker protein of PT cells, S1 and S2 segments) and the antibody to α-ENaC (marker protein of CD cells). Red stain indicates the presence of SGLT2 or α-ENaC in PT or CD cells respectively. DAPI-stained nuclei are shown in green.
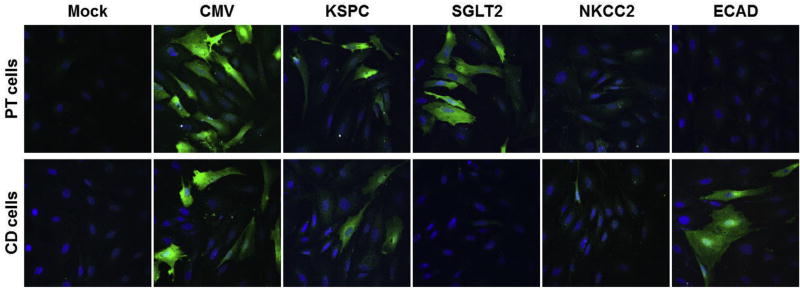
We used freshly prepared mouse PT and CD cells to test the expressions from each of the promoter constructs. We transduced primary PT or CD cells with each of the AAV vector promoter constructs. Three days following AAV transduction, the cells were prepared for immunofluorescence staining to detect the expression of eGFP. Results show that the CMV promoter provided strong GFP expression in PT and CD cells, as anticipated (Fig. 3). The KSPC promoter provided expression in both PT and CD cells. SGLT2 promoter construct provided expression in PT cells but not in CD cells. Expression from NKCC2 promoter was neglible in PT and CD cells, as expected because NKKC2 is strongly expressed in the thick ascending loop of Henle [25]. ECAD promoter provided expression in the CD but not in the PT cells.
Fig. 3.
Confocal microscopy images showing AAV9-mediated eGFP expression in the primary cultures of mouse proximal tubule and collecting duct cells. Mouse renal proximal tubule (PT) cells or collecting duct (CD) cells were isolated from mouse kidney and then transduced with AAV9 vector carrying enhanced green fluorescence protein (eGFP) under the control of CMV, KSPC, SGLT2, NKCC2 or ECAD promoters. Three days after the cells were fixed. eGFP expression was detected by immunofluorescence staining (green), using chicken anti-GFP antibody. DAPI-stained nuclei are stained blue.
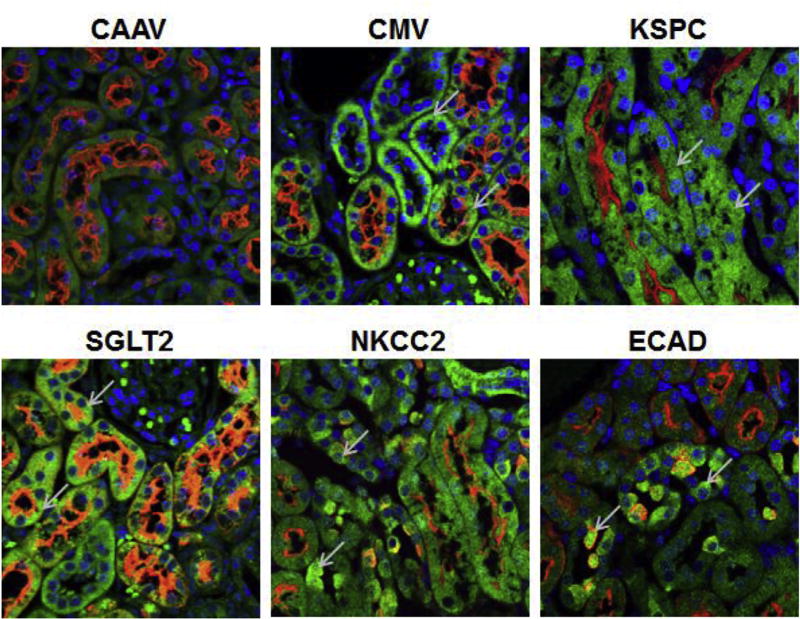
To test the specificity of gene expression from the four promoters in vivo, we injected each of the AAV vector type into the mouse kidney by retrograde ureteral infusion (8 week old C57Bl/6 mice, 10^11 vgp/mouse). Two weeks following the vector infusion, kidney sections were prepared for immunofluorescence staining. Results show that all four AAV vectors provided gene expression in the kidney (Fig. 4). Double immunofluorescence staining, using the antibodies against the eGFP and biotinylated LTA, was used to demonstrate the regions of AAV-mediated gene expression by the each of the promoter constructs.
Fig. 4.
Confocal microscopy images showing AAV9-mediated eGFP expression in mouse kidney sections. C57Bl/6 mice were injected with AAV9 vector carrying eGFP under the control of CAAV, CMV, KSPC, SGLT2, NKCC2, or ECAD promoter (1e10 + 11 vgp/mouse) by retrograde infusion via the ureter. Two weeks following vector administration, the kidneys were harvested for sectioning and immunofluorescence staining to detect eGFP expression. Examples of eGFP positive cell (green) in each section are indicated by the arrows, Brush border of renal proximal tubule and intercalating cells of collecting duct are stained with Lotus tetragonolobus agglutinin (red). Cell nuclei are stained with DAPI (blue). The definitions of the abbreviations are in Fig. 1 legend.
Recently we reported that systemic administration of AAV9 vector in mice provides preferential transduction in liver, pancreas, skeletal muscle and heart but not in kidneys. However, retrograde infusion of AAV9 via the ureter provides efficient transduction in all segments of the nephron from CD cells to PT cells [11]. In order to achieve gene expression in the desired cell type in the nephron segment, we incorporated panels of promoters of the genes that are known to preferentially express in specific segments of the nephron.
Cadherins are calcium-dependent cell adhesion molecule, which also mediate signal transduction, morphogenesis, and differentiation [26,27]. Over 100 different types of cadherins are expressed in vertebrates [28], two classical type I cadherins, epithelial cadherin (ECAD) and neural cadherin (NCAD) [20], and an atypical Ksp cadherin (KSPC) are expressed in the kidney [17]. Within the kidney NCAD is expressed predominantly in the PT epithelial cells, whereas ECAD is expressed in the distal convoluted tubule and CD cells [20,29]. KSPC is expressed in all segments of the nephron, from proximal to distal tubules epithelial cells, and in the parietal epithelium of Bowman's capsule; however, it is not expressed in renal interstitial cells, blood vessels, and glomerular tufts [17,20,30,31]. Transgenic mice carrying 1.3 kb KSPC promoter provided uniform distribution of kidney specific gene expression [31]. Systemic administration of AAV9 carrying 1.3 kb KSPC promoter provided gene expression in kidney and liver [32]. And hence we anticipated that retrograde infusion via the ureter of AAV9 vector carrying KSPC promoter would provide expression in the entire nephron, whereas ECAD promoter would provide expression in the CD cells. Immunofluorescence analyses showed that the KSPC promoter provided the GFP expression in LTA positive PT cells, LTA negative cells as well as the intercalating cells in the CDs that were lightly positive for LTA. GFP expression was not observed in the liver with the AAV9 carrying KSPC promoter administered by retrograde infusion via the ureter (data not shown). ECAD promoter provided the GFP expression only in the intercalated cells in the CDs that were lightly positive for LTA, but not in any other segment of the nephron (Fig. 4).
Sodium-dependent co-transporters specific for glucose, SGLT1 and SGLT2 are responsible for the majority of the glucose reabsorption in the kidney [33–35]. SGLT1 and SGLT2 are expressed in the apical side of the renal PT [36]. SGLT2 is a high capacity, low affinity glucose transporter responsible for 90% of the glucose reabsorption in the S1 and S2 segments of the PT, whereas SGLT1 controls high affinity low capacity reabsorption of remaining glucose in the S3 segment of the PT [36,37]. SGLT2 is expressed at a high level in the kidney but very low level in the other organs [36]. Rubera et al., 2004 [18] showed that the 1.1 kb SGLT2 promoter together with the splice region that includes first exon, first intron and part of the second intron are necessary for kidney expression from the SGLT2 promoter. Pontoglio et al., 2000 [21] later showed that the 400 bp promoter region that includes HNF1α binding site along with the splice region is adequate for kidney PT specific gene expression. We used the SGLT2 promoter that contains 400 bp 5′ untranslated region along with the first exon, first intron and part of the second exon with the start codon mutated to avoid the translation from the first exon 1, in combination with AAV9 to achieve PT specific gene expression using AAV9 vectors. Immunofluorescence analyses of the mouse kidneys that were infused with AAV9 carrying eGFP with the SGLT2 promoter showed the expression only in the LTA-positive renal PT cells (Fig. 4). GFP expression from SGLT2 promoter was not detected in LTA-negative cells.
Na-K-Cl cotransporters NKCC1 and NKCC2 isoforms mediate coupled transport of Na+, K+ and Cl-ions in the ratio of 1:1:2 [38]. NKCC1 is expressed in several organs including brain, salivary glands, heart, lung, liver, kidney, colon, and skeletal muscle [38–40], whereas NKCC2 is expressed predominantly in the apical membrane of TALH [19,38,41]. Deletion analyses of NKCC2 promoter regions showed that the 469 bases upstream of the NKCC2 transcription start site provided maximal TALH specific gene expression [19]. Incorporation of 469 base NKCC2 promoter in the AAV vector delivered via the ureter by retrograde infusion provided eGFP expression in the epithelial cells in the TALH of the nephron (Fig. 4).
These results show that AAAV9 administered retrogradely via the ureter can be engineered with the appropriate promoter to achieve expression in the desired segment of the nephron, hence can be very useful tools to study the role of gene expression in a segment of interest in the nephron in vivo.
Supplementary Material
Acknowledgments
The work was funded by grants from the National Kidney Foundation NKF-MD-Mini and US National Institutes of Health, R01DK090918, P01HL074940, P01HL068686, R01HL092196, R37HL023081, and R01DK039308.
Footnotes
Conflicts of interest
The authors have declared that no conflict of interest exists.
Transparency document
Transparency document related to this article can be found online at https://doi.org/10.1016/j.bbrc.2018.01.169.
References
- 1.Asokan A, Schaffer DV, Samulski RJ. The AAV vector toolkit: poised at the clinical crossroads. Mol. Ther. 2012;20:699–708. doi: 10.1038/mt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotin RM. Large-scale recombinant adeno-associated virus production. Hum. Mol. Genet. 2011;20:R2–R6. doi: 10.1093/hmg/ddr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan Z, Qiao C, Hu P, Li J, Xiao X. A versatile adeno-associated virus vector producer cell line method for scalable vector production of different serotypes. Hum. Gene Ther. 2011 May;22(5):613–624. doi: 10.1089/hum.2010.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jungmann A, Leuchs B, Katus HA, Rommelaere J, Müller OJ. Protocol for efficient generation and characterization of adeno-associated viral (AAV) vectors. Hum. Gene Ther. Meth. 2017 Sep 21; doi: 10.1089/hgtb.2017.192. https://doi.org/10.1089/hum.2017.192 [Epub ahead of print] [DOI] [PubMed]
- 5.Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999 Sep;6(9):1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez YJ, Wang J, Kearns WG, et al. Latent adeno-associated virus infection elicits humoral but not cell-mediated immune responses in a nonhuman primate model. J. Virol. 1999;73:8549–8558. doi: 10.1128/jvi.73.10.8549-8558.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, Chen C, Li J, Xiao X. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat. Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- 8.Inagaki K, Fuess S, Storm TA, Gibson GA, Mctiernan CF, Kay MA, Nakai H. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol. Ther.: J. Am. Soc. Gene Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bostick B, Ghosh A, Yue Y, Long C, Duan D. Systemic AAV-9 transduction in mice is influenced by animal age but not by the route of administration. Gene Ther. 2007;14:1605–1609. doi: 10.1038/sj.gt.3303029. [DOI] [PubMed] [Google Scholar]
- 10.Konkalmatt PR, Beyers RJ, O'Connor DM, Xu Y, Seaman ME, French BA. Cardiac-selective expression of extracellular superoxide dismutase after systemic injection of adeno-associated virus 9 protects the heart against postmyocardial infarction left ventricular remodeling. Circ. Cardiovasc. Imag. 2013;6:478–486. doi: 10.1161/CIRCIMAGING.112.000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konkalmatt PR, Asico LD, Zhang Y, Yang Y, Drachenberg C, Zheng X, Han F, Jose PA, Armando I. Renal rescue of dopamine D2 receptor function reverses renal injury and high blood pressure. JCI Insight. 2016 Jun 2;1(8) doi: 10.1172/jci.insight.85888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh A, et al. Long-term correction of murine glycogen storage disease type Ia by recombinant adeno-associated virus-1-mediated gene transfer. Gene Ther. 2006;13(4):321–329. doi: 10.1038/sj.gt.3302650. [DOI] [PubMed] [Google Scholar]
- 13.Schievenbusch S, Strack I, Scheffler M, Nischt R, Coutelle O, Hösel M, Hallek M, Fries JW, Dienes HP, Odenthal M, Büning H. Combined paracrine and endocrine AAV9 mediated expression of hepatocyte growth factor for the treatment of renal fibrosis. Mol. Ther. 2010 Jul;18(7):1302–1309. doi: 10.1038/mt.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito K, et al. Adeno-associated viral vector transduction of green fluorescent protein in kidney: effect of unilateral ureteric obstruction. BJU Int. 2008;101(3):376–381. doi: 10.1111/j.1464-410X.2007.07313.x. [DOI] [PubMed] [Google Scholar]
- 15.Chung DC, et al. Adeno-associated virus-mediated gene transfer to renal tubule cells via a retrograde ureteral approach. Nephron Extra. 2011;1(1):217–223. doi: 10.1159/000333071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skorecki Karl, Chertow Glenn M, Marsden Philip A, Taal Maarten W, Yu Alan SL, Luyckx V. Brenner & Rector's the Kidney. ten. Elsevier; Philadelphia, PA: 2016. [Google Scholar]
- 17.Thomson RB, Igarashi P, Biemesderfer D, Kim R, Abu-Alfa A, Soleimani M, Aronson PS. Isolation and cDNA cloning of Kspcadherin Kspcadherin, a novel kidney-specific member of the cadherin multigene family. J. Biol. Chem. 1995;270:17594–17601. doi: 10.1074/jbc.270.29.17594. [DOI] [PubMed] [Google Scholar]
- 18.Rubera I, Poujeol C, Bertin G, Hasseine L, Counillon L, Poujeol P, Tauc M. Specific Cre/lox recombination in the mouse proximal tubule. J. Am. Soc. Nephrol. 2004;15:2050–2056. doi: 10.1097/01.ASN.0000133023.89251.01. [DOI] [PubMed] [Google Scholar]
- 19.Igarashi P, Whyte DA, Li K, Nagami GT. Cloning and kidney cell-specific activity of the promoter of the murine renal Na-K-C1 cotransporter gene. J. Biol. Chem. 1996;271:9666–9674. doi: 10.1074/jbc.271.16.9666. [DOI] [PubMed] [Google Scholar]
- 20.Prozialeck WC, Lamar PC, Appelt DM. Differential expression of E-cadherin, N-cadherin and beta-catenin in proximal and distal segments of the rat nephron. BMC Physiol. 2004;4:10. doi: 10.1186/1472-6793-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pontoglio M, Prié D, Cheret C, Doyen A, Leroy C, Froguel P, Velho G, Yaniv M, Friedlander G. HNF1alpha controls renal glucose reabsorption in mouse and man. EMBO Rep. 2000 Oct;1(4):359–365. doi: 10.1093/embo-reports/kvd071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan M, Schallhorn A, Wurm FM. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 1996;24(4):596–601. doi: 10.1093/nar/24.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prasad K-MR, Xu Y, Yang Z, et al. Robust cardiomyocyte-specific gene expression following systemic injection of AAV: in Vivo Gene Delivery Follows a Poisson Distribution. Gene Ther. 2011;18:43–52. doi: 10.1038/gt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ried MU, Girod A, Leike K, et al. Adeno-associated virus capsids displaying immunoglobulin-binding domains permit antibody-mediated vector retargeting to specific cell surface receptors. J. Virol. 2002;76:4559–4566. doi: 10.1128/JVI.76.9.4559-4566.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer LG, Schnermann J. Integrated control of Na transport along the nephron. Clin. J. Am. Soc. Nephrol. 2015 Apr 7;10(4):676–687. doi: 10.2215/CJN.12391213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu. Rev. Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- 27.Geiger B, Ayalon O. Cadherins. Annu. Rev. Cell Biol. 1992;8:307–332. doi: 10.1146/annurev.cb.08.110192.001515. [DOI] [PubMed] [Google Scholar]
- 28.Gul IS, Hulpiau P, Saeys Y, van Roy F. Evolution and diversity of cadherins and catenins. Exp. Cell Res. 2017 Sep 1;358(1):3–9. doi: 10.1016/j.yexcr.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Lee SY, Han SM, Kim JE, Chung KY, Han KH. Expression of E-cadherin in pig kidney. J. Vet. Sci. 2013;14(4):381–386. doi: 10.4142/jvs.2013.14.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomson RB, Ward DC, Quaggin SE, Igarashi P, Muckler ZE, Aronson PS. cDNA cloning and chromosomal localization of the human and mouse isoforms of Ksp-cadherin. Genomics. 1998;51:445–451. doi: 10.1006/geno.1998.5402. [DOI] [PubMed] [Google Scholar]
- 31.Shao X, Johnson JE, Richardson JA, Hiesberger T, Igarashi P. A minimal Kspcadherin promoter linked to a green fluorescent protein reporter gene exhibits tissue-specific expression in the developing kidney and genitourinary tract. J. Am. Soc. Nephrol. 2002 Jul;13(7):1824–1836. doi: 10.1097/01.asn.0000016443.50138.cd. [DOI] [PubMed] [Google Scholar]
- 32.Schievenbusch S, Strack I, Scheffler M, Nischt R, Coutelle O, Hösel M, Hallek M, Fries JW, Dienes HP, Odenthal M, Büning H. Combined paracrine and endocrine AAV9 mediated expression of hepatocyte growth factor for the treatment of renal fibrosis. Mol. Ther. 2010 Jul;18(7):1302–1309. doi: 10.1038/mt.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hediger MA, Coady MJ, Ikeda TS, Wright EM. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. Nature. 1987;330:379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- 34.Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for d-glucose. J. Clin. Invest. 1994;93:397–404. doi: 10.1172/JCI116972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mackenzie B, Panayotova-Heiermann M, Loo DD, Lever JE, Wright EM. SAAT1 is a low affinity Na+/glucose cotransporter and not an amino acid transporter. A reinterpretation. J. Biol. Chem. 1994;269:22488–22491. [PubMed] [Google Scholar]
- 36.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol. Rev. 2011;91:733–794. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 37.Hediger MA, Coady MJ, Ikeda TS, Wright EM. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. Nature. 1987;330:379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- 38.Payne JA, Xu JC, Haas M, Lytle CY, Ward D, Forbush B., 3rd Primary structure, functional expression, and chromosomal localization of the bumetanide-sensitive Na-K-Cl cotransporter in human colon. J. Biol. Chem. 1995 Jul 28;270(30):17977–17985. doi: 10.1074/jbc.270.30.17977. [DOI] [PubMed] [Google Scholar]
- 39.Xu JC, Lytle C, Zhu TT, Payne JA, Benz E, Jr, Forbush B., 3rd Molecular cloning and functional expression of the bumetanide-sensitive Na-K-Cl cotransporter. Proc. Natl. Acad. Sci. U. S. A. 1994 Mar 15;91(6):2201–2205. doi: 10.1073/pnas.91.6.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delpire E, Rauchman MI, Beier DR, Hebert SC, Gullans SR. Molecular cloning and chromosome localization of a putative basolateral Na(+)-K(+)-2Cl- cotransporter from mouse inner medullary collecting duct (mIMCD-3) cells. J. Biol. Chem. 1994 Oct 14;269(41):25677–25683. [PubMed] [Google Scholar]
- 41.Kaplan MR, Plotkin MD, Lee WS, Xu ZC, Lytton J, Hebert SC. Apical localization of the Na-K-Cl cotransporter, rBSC1, on rat thick ascending limbs. Kidney Int. 1996 Jan;49(1):40–47. doi: 10.1038/ki.1996.6. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen MT, Yang LE, Fletcher NK, Lee DH, Kocinsky H, Bachmann S, Delpire E, McDonough AA. Effects of K+-deficient diets with and without NaCl supplementation on Na+, K+, and H2O transporters' abundance along the nephron. Am. J. Physiol. Ren. Physiol. 2012 Jul 1;303(1):F92–F104. doi: 10.1152/ajprenal.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.