Abstract
OBJECTIVES
Approximately 4% to 12% of pregnant women have asthma; few studies have examined the effects of maternal asthma medication use on birth defects. We examined whether maternal asthma medication use during early pregnancy increased the risk of selected birth defects.
METHODS
National Birth Defects Prevention Study data for 2853 infants with 1 or more selected birth defects (diaphragmatic hernia, esophageal atresia, small intestinal atresia, anorectal atresia, neural tube defects, omphalocele, or limb deficiencies) and 6726 unaffected control infants delivered from October 1997 through December 2005 were analyzed. Mothers of cases and controls provided telephone interviews of medication use and additional potential risk factors. Exposure was defined as maternal periconceptional (1 month prior through the third month of pregnancy) asthma medication use (bronchodilator or anti-inflammatory). Associations between maternal periconceptional asthma medication use and individual major birth defects were estimated by using adjusted odds ratios (aOR) and 95% confidence intervals (95%CI).
RESULTS
No statistically significant associations were observed for maternal periconceptional asthma medication use and most defects studied; however, positive associations were observed between maternal asthma medication use and isolated esophageal atresia (bronchodilator use: aOR = 2.39, 95%CI = 1.23, 4.66), isolated anorectal atresia (anti-inflammatory use: aOR = 2.12, 95%CI = 1.09, 4.12), and omphalocele (bronchodilator and anti-inflammatory use: aOR = 4.13, 95%CI = 1.43, 11.95).
CONCLUSIONS
Positive associations were observed for anorectal atresia, esophageal atresia, and omphalocele and maternal periconceptional asthma medication use, but not for other defects studied. It is possible that observed associations may be chance findings or may be a result of maternal asthma severity and related hypoxia rather than medication use.
Keywords: anti-inflammatory, asthma, birth defects, bronchodilator, medication
Asthma is one of the most frequent obstructive pulmonary diseases experienced during pregnancy, affecting ~4% to 12% of pregnant women.1,2 Current clinical guidelines recommend that women with asthma maintain asthma medication use during pregnancy.2 Approximately 3% of pregnant women reportedly use asthma medications.3 These medications act in 2 ways: as bronchodilators or anti-inflammatories. To date, pregnancy safety data for many asthma medications are sparse.
Animal studies found that ~10% of circulating maternal albuterol (a bronchodilator) is transferred to the fetus, but the disposition in the fetal lungs is comparable to that in maternal lungs.4 Several previous studies have examined the effects of maternal asthma or asthma medication use during pregnancy on a number of maternal and fetal outcomes; however, few have examined major birth defects as primary outcomes. One case study and several epidemiologic studies have suggested increased risks of orofacial clefts, cardiovascular defects, nervous system (excluding spina bifida) defects, respiratory system defects, digestive system defects, anal atresia, gastroschisis, and all malformations combined associated with asthma or asthma medication use during pregnancy,3,5–10 whereas other studies did not identify associations between maternal asthma and/ or asthma medication use and birth defects.11–20 In particular, a recent study in the United Kingdom found a small, increased risk of birth defects among children of women with asthma; however, no association was identified between birth defects and asthma medication.21
Birth defects are the leading cause of infant morbidity and mortality. As maternal asthma is a common disease during pregnancy, investigation of the safety of maternal asthma medication use during pregnancy is an important public health issue. Results reported in previous studies of asthma medication use during pregnancy and birth defects were limited in part because of small numbers of cases for specific birth defects. By using data from a population-based, multicenter, case-control study, we examined whether maternal use of asthma medications during early pregnancy increased the risk of selected birth defects after controlling for other potential confounders.
METHODS
Data from the National Birth Defects Prevention Study (NBDPS) were analyzed. The NBDPS is an ongoing, multi-center, population-based, case-control study being conducted in 10 US states (Arkansas, California, Georgia, Iowa, Massachusetts, New Jersey, New York, North Carolina, Texas, and Utah) to investigate genetic and environmental risk factors for major structural birth defects. Methods for the NBDPS have been previously described.22 Each participating center obtained appropriate institutional review board approvals.
Case deliveries included in the current analyses were live births, stillbirths, or elective terminations with estimated dates of delivery (EDDs) from October 1, 1997, through December 31, 2005, and diagnosed with 1 or more of the following birth defects: neural tube defects (further divided into 2 groups, anencephaly and spina bifida), esophageal atresia, small intestinal atresia, anorectal atresia, limb deficiencies, diaphragmatic hernia, and omphalocele. We selected these specific defects because there were 200 or more case infants for each defect. Other major defect groups were studied by other investigators using the same dataset. Case deliveries were identified from the birth defects surveillance system in each participating state and reports from medical records were reviewed by clinical geneticists using criteria developed for the NBDPS to assign diagnosis.23 Each case was further classified as isolated (no additional major defect) or multiple (1 or more additional major defects in a different organ system); diagnoses attributed to a chromosomal or single-gene etiology were excluded. Control infants included in the current analyses were nonmalformed, live births with EDDs during the same time period as case infants, randomly selected from either birth certificates or from birth hospitals. Annually, each center recruits 100 control mothers, which provides sufficient sample size for a 1:1 case:control ratio for any 1 birth defect included in the NBDPS. Case and control infants who were adopted, in foster care, or whose mother did not speak English or Spanish were excluded from the NBDPS.
Systematic data collection procedures were used to recruit case and control families.22 Exposure information was collected by telephone interview with case and control mothers. The interview was conducted in English or Spanish with a median time to interview of 10 months for cases and 8 months for controls from the EDD; most interviews (78%) were completed within 12 months. Information collected included maternal health (eg, chronic disease); medication used; pregnancy history and complications; nutritional/behavioral factors (eg, diet, vitamin use, caffeine, tobacco, alcohol); exposures at home and work; and demographics. To improve recall and better define exposure to medications, mothers were asked for the specific names, dosage, and dates of prescribed medications and over-the-counter medicines, as well as the health conditions and symptoms related to use of that specific medicine. A pregnancy calendar was used to aid maternal recall on timing and frequency of the medication use, such as date, month, or trimester of pregnancy and dosage. Information on medication use was collected for 3 months before pregnancy through the end of the pregnancy.
The Boston University Slone Epidemiology Center Drug Dictionary was used to match maternal reports of medication use to the active ingredient to identify mothers who used asthma medications. Exposure was defined as reported asthma medication use at least once during the periconceptional period (1 month prior through the third month of pregnancy). Some mothers reported asthma medication was taken “as needed.” To reduce misclassification, mothers who reported using asthma medication “as needed,” but with an unknown time period of use, were excluded from the analysis. Examination of route of administration (oral versus inhaled) was not conducted, as such information was not available for all mothers. Asthma medication use was divided into 3 groups: anti-inflammatories, bronchodilators, and both anti-inflammatories and bronchodilators.
The main analyses focused on infants with isolated birth defects. Associations between maternal characteristics and each defect were evaluated by using χ2 tests. Variables that were significantly associated (P <.05) with both a defect and asthma medication use or that were cited as risk factors for a defect in prior literature were examined as potential confounders and included in multivariable analyses. For each defect, logistic regression was used to estimate adjusted odds ratios (aORs) to assess the association between maternal asthma medication and each birth defect studied. Potential confounders examined included infant gender, maternal age, BMI, parity, race/ethnicity, education, alcohol use, smoking, folic acid–containing vitamin use, fever, and crack/cocaine use. The latter 5 variables were specific to the periconceptional period. Individual logistic regression models were conducted for bronchodilator use only, anti-inflammatory use only, and both bronchodilator and anti-inflammatory use.
Additional analyses that included both isolated and multiple defects were performed for defects that were found to be associated with asthma medication in the previously described analysis of isolated cases only (ie, esophageal atresia, anorectal atresia, and omphalocele). Further, for each of these defects (isolated cases only), aORs were estimated for mothers who used asthma medication during the periconceptional period and those who used asthma medication during the second and/or third trimester only compared with non-users to assess if the effects found were biologically plausible. We also conducted an analysis limited to case infants with multiple defects consistent with VACTERL, an association of defects that includes Vertebral defects, Anorectal atresia, Cardiac defects, Tracheo-esophageal fistula, Esophageal atresia, Renal defects, and Limb defects (VATER is an association that includes Vertebral defects, Anorectal atresia, Tracheo-esophageal fistula, Esophageal atresia, and Renal defects).
RESULTS
There were a total of 2853 isolated cases for the 7 types of selected defects after excluding 30 cases because of incomplete maternal reports for all medication questions. Isolated and multiple cases included in the additional analysis described previously numbered 440 for esophageal atresia, 642 for anorectal atresia, 272 for omphalocele, and 145 with VACTERL association (data not shown). Eligible control infants numbered 6789. Of these, 59 were excluded because of incomplete maternal reports for all medication questions and 4 were excluded because of maternal reports of terbutaline use (medication also used to prevent preterm labor). Overall, the average participation rates were 71% for the mothers of infants with birth defects included in this analysis and 67% for the control mothers.
Compared with controls (6726 infants), case infants were more likely to be boys. Case mothers were more likely than control mothers to be of Hispanic ethnicity, have a high school education or less, and report a fever in the first trimester (Table 1). All other proportions were similar among case and control infants and mothers. As shown in Table 2, reported maternal periconceptional bronchodilator and anti-inflammatory medication use was similar between cases and controls, except for fluticasone and beclomethasone. Albuterol (cases = 2.75%; controls = 2.11%) was the most frequently reported bronchodilator, and fluticasone (cases = 1.22%; controls = 0.77%) was the most frequently reported anti-inflammatory used. All other medications were reportedly used by a very small proportion (<0.5%) of cases or controls.
TABLE 1.
Selected Infant and Maternal Characteristics Among Selected, Isolated Casesa and Controls, National Birth Defects Prevention Study, 1997–2005
| Totalb | Cases (%) n = 2853 |
Controls (%) n = 6726 |
|
|---|---|---|---|
| Infant sex | |||
| Malec | 4898 | 1498 (53.31) | 3400 (50.59) |
| Female | 4633 | 1312 (46.69) | 3321 (49.41) |
| Maternal age at delivery, y | |||
| <20 | 1029 | 312 (10.947) | 717 (10.66) |
| 20–34 | 7171 | 2115 (74.13) | 5056 (75.17) |
| ≥35 | 1379 | 426 (14.93) | 953 (14.17) |
| BMI | |||
| <18.5 (underweight) | 488 | 129 (4.76) | 359 (5.56) |
| 18.5–24 (normal weight) | 5007 | 1402 (51.73) | 3605 (55.83) |
| 25–29 (overweight) | 2065 | 614 (22.66) | 1451 (22.47) |
| ≥30 (obese)c | 1607 | 565 (20.85) | 1042 (16.14) |
| Parity (number of previous live births) | |||
| 0 Child | 3858 | 1159 (40.65) | 2699 (40.17) |
| ≥1 Child | 5712 | 1692 (59.35) | 4020 (59.83) |
| Race/Ethnicity | |||
| Black non-Hispanic | 1028 | 266 (9.35) | 762 (11.38) |
| Hispanicc | 2256 | 764 (26.84) | 1492 (22.28) |
| Others | 621 | 189 (6.64) | 432 (6.45) |
| White non-Hispanic | 5638 | 1627 (57.17) | 4011 (59.89) |
| Education, y | |||
| ≤12c | 4043 | 1271 (44.82) | 2772 (41.52) |
| >12 | 5470 | 1565 (55.18) | 3905 (58.48) |
| Alcohol used | |||
| Yes | 3478 | 1012 (35.76) | 2466 (36.99) |
| No | 6018 | 1818 (64.24) | 4200 (63.01) |
| Cigarette smokingd | |||
| Yes | 1791 | 524 (18.44) | 1267 (18.94) |
| No | 7741 | 2318 (81.56) | 5423 (81.06) |
| Folic acid–containing vitamine | |||
| Yes | 4874 | 1456 (51.03) | 3418 (50.82) |
| No | 4705 | 1397 (48.97) | 3308 (49.18) |
| Cocaine or crack used | |||
| Yes | 68 | 22 (0.78) | 46 (0.69) |
| No | 9461 | 2816 (99.22) | 6645 (99.31) |
| Feverc,d | |||
| Yes | 1082 | 357 (12.51) | 725 (10.78) |
| No | 8497 | 2496 (87.49) | 6001 (89.22) |
| Vasoactive medications used | |||
| Yes | 2478 | 761 (26.67) | 1717 (25.53) |
| No | 7101 | 2092 (73.33) | 5009 (74.47) |
Defects include diaphragmatic hernia, esophageal atresia, small intestinal atresia, anorectal atresia, neural tube defects, omphalocele, or limb deficiencies with no additional major defect (isolated).
Total may not add up because of missing observations.
P < .05 in χ2 test.
During the periconceptional period.
During the period 1 month prior through the first month of pregnancy.
TABLE 2.
Maternal Periconceptional Asthma Medication Reports Among Selected, Isolateda Cases and Controls, National Birth Defects Prevention Study, 1997–2005
| Medication | Cases n (%)b |
Controls n (%)b |
|---|---|---|
| Bronchodilators (n = 243) | ||
| Albuterol | 77 (2.75) | 139 (2.11) |
| Salmeterol | 13 (0.48) | 23 (0.36) |
| Pirbuterol | 3 (0.11) | 3 (0.05) |
| Theophylline | 3 (0.11) | 3 (0.05) |
| Ipratropium bromide | 2 (0.07) | 4 (0.06) |
| Other bronchodilatorc | 0 (0) | 5 (0.07) |
| Anti-inflammatories (n = 187) | ||
| Fluticasoned | 34 (1.22) | 51 (0.77) |
| Beclomethasoned | 12 (0.43) | 11 (0.17) |
| Prednisone | 10 (0.36) | 19 (0.29) |
| Triamcinolone | 9 (0.33) | 14 (0.21) |
| Montelukast | 4 (0.14) | 14 (0.21) |
| Budesonide | 3 (0.11) | 9 (0.14) |
| Cromolyn sodium | 2 (0.07) | 9 (0.14) |
| Zafirlukast | 0 (0.00) | 5 (0.08) |
| Methylprednisolone | 1 (0.04) | 3 (0.05) |
| Other anti-inflammatoryc | 1 (0.04) | 3 (0.05) |
Defects include diaphragmatic hernia, esophageal atresia, small intestinal atresia, anorectal atresia, neural tube defects, omphalocele, or limb deficiencies with no additional major defect (isolated).
Total percentage is not equal to 100% because some people took more than one medication.
Other bronchodilator includes ephedrine, epinephrine, metaproterenol. Other anti-inflammatory includes flunisolide, prednisolone, nedocromil.
Statistical difference between the cases and controls in the use of asthma medication.
As shown in Table 3, a statistically significant association was found between isolated esophageal atresia and bronchodilator use only (aOR = 2.39; 95% confidence interval [CI] = 1.23, 4.66). Of note, although there were fewer exposed cases and CIs were wide, the aORs for esophageal atresia and anti-inflammatory use only and use of both types of medication were also elevated, but were not statistically significant. In addition, the risk of isolated anorectal atresia associated with anti-inflammatory only use was significantly increased (aOR = 2.12; 95%CI = 1.09, 4.12). Further, the risk of isolated omphalocele (aOR=4.13; 95%CI = 1.43, 11.95) was significantly elevated for users of both bronchodilator and anti-inflammatory medications. No other defects were significantly associated with any medication group. We also found that mothers who used multiple bronchodilators were at higher risk for delivering a child with one of the defects studied than those who used 1 bronchodilator (data not shown).
TABLE 3.
Odds of Asthma Medication Use During the Periconceptional Perioda Among Mothers of Selected Cases and Controls, National Birth Defects Prevention Study, 1997–2005
| Birth Defects | Total | Bronchodilator Use | Anti-inflammatory Use | Both | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| nb (% exposed) | Adjusted ORc (95% CI) | nb (% exposed) | Adjusted ORc (95% CI) | nb (% exposed) | Adjusted ORc (95% CI) | ||
| Isolated cases only | |||||||
| Neural tube defects | 1067 | 30 (2.85) | 1.20 (0.79, 1.81) | 18 (1.71) | 1.04 (0.62, 1.74) | 6 (0.59) | 0.98 (0.41, 2.32) |
| Anencephaly | 317 | 9 (2.86) | 1.43 (0.72, 2.86) | 7 (2.22) | 1.43 (0.62, 3.31) | 1 (0.33) | — |
| Spina bifida | 646 | 17 (2.69) | 1.02 (0.60, 1.75) | 8 (1.26) | 0.77 (0.37, 1.58) | 4 (0.66) | 1.03 (0.37, 2.91) |
| Esophageal atresia | 178 | 10 (5.65) | 2.39 (1.23, 4.66) | 6 (3.45) | 1.61 (0.69, 3.76) | 3 (1.83) | 2.93 (0.88, 9.75) |
| Small intestinal atresia | 227 | 6 (2.68) | 0.88 (0.36, 2.19) | 6 (2.68) | 1.64 (0.71, 3.82) | 1 (0.47) | — |
| Anorectal atresia | 285 | 6 (2.14) | 0.88 (0.38, 2.01) | 10 (3.53) | 2.12 (1.09, 4.12) | 1 (0.38) | — |
| Limb deficiency | 533 | 19 (3.61) | 1.45 (0.89, 2.36) | 12 (2.27) | 1.12 (0.60, 2.11) | 7 (1.38) | 2.02 (0.89, 4.56) |
| Diaphragmatic hernia | 409 | 7 (1.75) | 0.70 (0.32, 1.50) | 11 (2.72) | 1.49 (0.79, 2.80) | 4 (1.03) | 1.54(0.54, 4.34) |
| Omphalocele | 154 | 6 (4.05) | 1.61 (0.71, 3.79) | 4 (2.63) | 1.27 (0.46, 3.53) | 4 (2.76) | 4.13 (1.43, 11.95) |
| Isolated and multipled | |||||||
| Esophageal atresia | 440 | 17 (3.88) | 1.53 (0.92, 2.57) | 12 (2.76) | 1.29 (0.70, 2.38) | 5 (1.21) | 1.70 (0.66, 4.37) |
| Anorectal atresia | 642 | 14 (2.20) | 0.91 (0.52, 1.58) | 14 (10.45) | 1.28 (0.72, 2.25) | 4 (0.65) | 1.05 (0.37, 2.96) |
| Omphalocele | 272 | 10 (3.77) | 1.50 (0.77, 2.89) | 9 (3.35) | 1.70 (0.85, 3.44) | 5 (1.97) | 2.92 (1.12, 7.58) |
Periconceptional period is defined as 1 mo before pregnancy through the end of the third month of pregnancy.
Total excludes subjects missing exposure information.
Confounders adjusted for include age, parity, race/ethnicity, education, alcohol use, smoking, gender, folic acid use, fever in first trimester.
Only those defects that were found to have an association with asthma medication were looked at in additional analyses.
Because many infants with esophageal atresia, anorectal atresia, and omphalocele had multiple, unrelated anomalies, additional analyses of these types of birth defects, including infants with multiple defects were conducted; only the positive association between use of both bronchodilator and anti-inflammatory medications and omphalocele persisted when both isolated and multiple defects were included in the analysis (Table 3). Infants with VATER or VACTERL association were considered as a separate group. However, only 1 VACTERL case was exposed to any asthma medication (data not shown), so further analysis was not conducted.
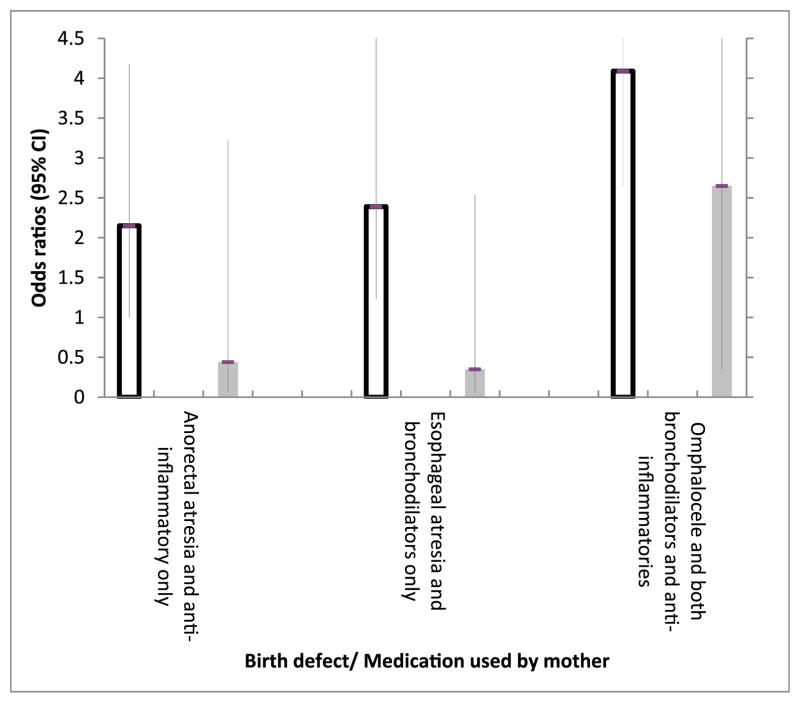
To examine if the significant results in Table 3 are biologically plausible and to assess the potential impact of biases, we conducted a stratified analysis by timing of maternal medication use (1 month before through the third month and fourth through ninth month of pregnancy) (Fig 1), because the exposure effect on malformations should occur only within the periconceptional period when major fetal organs are largely developed. The observed positive associations persisted only among those who reportedly used the medications during the periconceptional period, but not among those who took medication after the periconceptional period only. Of note, 60% to 67% of mothers of children with esophageal atresia, anorectal atresia, and omphalocele used bronchodilators throughout their pregnancy (data not shown).
FIGURE 1.
Association between start of maternal asthma medication use and selected birth defects, 1997–2005.
DISCUSSION
By using data from a large, population-based, case-control study, we found no significant associations between maternal reports of bronchodilator or anti-inflammatory use during the periconceptional period and most birth defects studied. Positive associations were observed, however, between maternal reports of asthma medication use and the occurrence of isolated esophageal atresia, isolated anorectal atresia, and omphalocele for particular categories of asthma medication use. These associations were observed only for reported periconceptional use when these birth defects develop,24 which support the biological plausibility of our findings. Given the low baseline prevalence of these defects, if the observed association proved to be causal, the absolute risks of asthma medications on these rare defects would be small. For instance, in 2003, the prevalence rates of anorectal atresia and omphalocele were 4.6 and 1.2 cases per 10 000 births, respectively25; thus, even with a fourfold risk from asthma medication, the risk of having these defects would still be 0.18% and 0.05% respectively.
Our findings of no association between maternal periconceptional asthma medication use and most of the selected defects studied are consistent with many of the previous, related studies.11–20 Our findings of a positive association for anorectal atresia are supported by the work of Källén and Olausson,3 who found a significantly increased risk of anal atresia for infants exposed to anti-asthmatic drugs. When stratified on inhaled corticosteroid use, those exposed to corticosteroids showed a higher risk of anal atresia, which is consistent with our findings for anti-inflammatory use and anorectal atresia. Our findings of positive associations for esophageal atresia, anorectal atresia, and omphalocele are not directly comparable with previous studies with positive findings with other defects,7,8,26 but are supported by work by Demissie et al,5 who reported increased risks for all congenital malformations among women who used anti-asthmatic drugs during pregnancy. Furthermore, Blais et al10 reported an increased risk for digestive system defects and “other” musculoskeletal defects among women who were defined as having an asthma diagnosis and at least one prescription for asthma medication in the 2 years before or during pregnancy.
The positive associations found in our study were related to maternal bronchodilator use only, anti-inflammatory use only, or use of both types of medications. This raises a critical question of whether maternal asthma severity, rather than maternal use of asthma medications, is associated with the increased risks identified. Asthma status may be adversely altered by pregnancy,2 and previous evidence5,9,27 suggested that asthma rather than asthma medication was associated with fetal growth restriction. With the interview information available for analysis, we were unable to distinguish between the effects of asthma and those of asthma medications; however, we did observe that mothers with possible indicators of uncontrolled asthma or severe asthma episodes (eg, use of multiple bronchodilators) were at higher risk for delivering a child with one of the defects studied than those who used 1 bronchodilator (data not shown). This is further supported by our findings that nearly two-thirds of mothers of infants with esophageal atresia, anorectal atresia, or omphalocele who used bronchodilators, did so for the entire pregnancy. When regular use of bronchodilators is required, an activated inflammatory process is implied28; thus, use of bronchodilators throughout pregnancy might indicate that these mothers had frequent or ongoing inflammatory exacerbations during pregnancy.
A potential mechanism for the effect of asthmatic episodes or poorly controlled asthma on the fetus is fetal hypoxia.29 Fetal hypoxia can be greater than maternal hypoxia and uterine blood flow is also affected by maternal hypoxia.30 Albuterol (an asthma medication most commonly used in this study), with doses equal to the maximum recommended daily inhalation dose for adults, was found to have teratogenic effects in an animal study.31 It is unclear if the 3 malformations with positive associations in this study or the development of certain organs are more sensitive to the effects of hypoxia or medication than others. Blais et al10 found that maternal asthma was associated with an increased risk of digestive system defects and defects in the group “other musculoskeletal defects,” which contained omphalocele. Asthma treatment guidelines for pregnant women recommend the reduction of airway inflammation to decrease airway hyperresponsiveness.32 Guidelines for the management of persistent asthma include long-term controller medications (eg, inhaled corticosteroids) and short-term reliever medications (eg, bronchodilators)33,34 to maintain adequate oxygen supply for both the mother and fetus.
The NBDPS provides one of the largest sample sizes to date to study the effects of asthma medications on several specific birth defects. Systematic case review and classification reduces heterogeneity within case groups. Population-based selection of cases and controls and use of a systematic recruitment protocol reduces the potential for selection bias. In particular, a comparison between controls and all live births at each study center showed that maternal characteristics in the 2 groups were similar.35 Further, by using a detailed interview, the NBDPS collects data on potential confounders, which allowed adjustment in multivariable models.
As mentioned, a limitation of the NBDPS interview was the lack of information on maternal asthma/asthma severity, as well as dose and route of administration of asthma medications, which may misclassify different levels of risk. To reduce potential misclassification of mothers without asthma, we restricted our analysis to the 2 medication groups most commonly used for asthma treatment. With revisions made to the NBDPS interview to collect information on asthma in pregnancy, analyses of future participants will permit examination of asthma status in addition to medication use. Future data from the NBDPS will include date of asthma diagnosis, symptoms during pregnancy, and specific medications used to treat the asthma symptoms. Another limitation was limited power to examine associations between rare exposures, such as use of both types of asthma medications and birth defect groupings, such as VATER/ VACTERL. Because of the limited power and the number of exposure-outcome combinations analyzed, some of the positive associations identified should not be interpreted as strong evidence of increased risks, as they could be because of chance. We conducted the Woolf test and Dersimonian-Laird test to examine the homogeneity of the odds ratios and a Mantel-Haenszel test to assess if there was a significantly common trend between asthma medications and birth defects. Although it is possible that the ORs observed are chance findings or random variations, a common significant exposure-disease association was also found after controlling for birth defect type. This may indicate a common teratogenic pathway of asthma medications on birth defects or an effect of maternal asthma rather than medication. To estimate potential selection bias, we compared the demographic compositions between the control participants in this study and the target population in each center, and found them to be similar. The participating rate of cases was also similar to that of controls, suggesting that selection bias is less likely. In addition, participation rates among mothers of infants with birth defects varied only slightly according to the type of birth defect, ranging from 70% to 72% with the exception of anencephaly (68%); however, we still cannot rule out the possibility of selection bias with 30% nonparticipation. Another limitation may be recall bias because of time to interview or because mothers of affected children may be more likely to report their exposures than mothers of controls; however, a series of strategies, such as asking specific names, dosage, and dates of prescribed medication, have been used to minimize recall biases. In addition, median time to interview was within 8 to 10 months for both cases and controls. In addition, because asthma is a chronic disease and long-term controller medications are typically used on a regular basis, recall of asthma medication use is probably better than recall of medications taken for acute conditions on a short-term basis; however, the positive associations we found could also be a result of recall bias or chance.
CONCLUSIONS
This study found no significant associations between maternal periconceptional use of asthma medications and risks of neural tube defects, some musculoskeletal defects (limb deficiency and diaphragmatic hernia), and small intestinal atresia. It suggests, however, that maternal asthma medication use may increase the risk of esophageal atresia, anorectal atresia, and omphalocele. Although the magnitudes of the associations were moderate (ORs ranged from 2.14 to 4.26), we cannot disentangle the effects of asthma severity and related hypoxia from those attributable to medication use, nor can we rule out the possibility that these findings are attributable to bias or chance. The current clinical guidelines and specific recommendations for aggressive asthma management during pregnancy should remain unchanged. Additional studies with detailed data on asthma severity and treatment and a large sample are recommended to clarify whether our findings are attributable to maternal use of asthma medication, asthma severity, or chance alone.
WHAT’S KNOWN ON THIS SUBJECT
Asthma is a common obstructive pulmonary disease experienced during pregnancy. Clinical guidelines recommend women with asthma maintain asthma medication use during pregnancy. Epidemiologic studies suggest an association between several types of defects and asthma or asthma medication use during pregnancy.
WHAT THIS STUDY ADDS
Data from a large, population-based, multicenter, case-control study was used. This provides the opportunity to study specific birth defects with minimal heterogeneity in case groups, as well as control for a variety of potential confounders.
Acknowledgments
This study was supported by a cooperative agreement from the Centers for Disease Control and Prevention, grant U50/ CCU223184. Coding of drug information in the study used the Slone Epidemiology Center Drug Dictionary, under license from the Slone Epidemiology Center at Boston University.
We thank the participating families, staff, and scientists from all NBDPS sites (Arkansas, California, Georgia, Iowa, Massachusetts, New Jersey, North Carolina, New York, Texas, and Utah).
We thank Adrienne Hoyt for data management and analytic support. We thank Dr Greg DiRienzo for his assistance with statistical testing.
ABBREVIATIONS
- aOR
adjusted odds ratio
- CI
confidence interval
- EDD
estimated dates of delivery
- NBDPS
National Birth Defects Prevention Study
- VACTERL
an association of defects that includes Vertebral defects, Anorectal atresia, Cardiac defects, Tracheo-esophageal fistula, Esophageal atresia, Renal defects, and Limb defects
- VATER
an association that includes Vertebral defects, Anorectal atresia, Tracheo-esophageal fistula, Esophageal atresia, and Renal defects
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Dr Lin was the lead investigator on this project and was responsible for all aspects of the project, including study design, data analysis, and manuscript writing. Mr Munsie was the lead statistician on the project, and was responsible for the data analysis and manuscript writing. Dr Herdt-Losavio was an epidemiologist on the project, and was responsible for data analysis/interpretation and manuscript writing/review. Dr Druschel is the Principal Investigator of the National Birth Defects Prevention Study Center in New York; she was responsible for study design, acquisition of data, and manuscript revision/review. Dr Campbell was the data manager for the project, and was responsible for providing data, assisting with study design, data analysis, and manuscript review. Drs Browne, Romitti, and Bell were epidemiologists on the project, and were responsible for study design, data interpretation and manuscript review. Dr Olney was a clinical geneticist on the project, and was responsible for classifying birth defects, study design, and manuscript review.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Kwon HL, Belanger K, Bracken MB. Asthma prevalence among pregnant and childbearing-aged women in the United States: estimates from national health surveys. Ann Epidemiol. 2003;13(5):317–324. doi: 10.1016/s1047-2797(03)00008-5. [DOI] [PubMed] [Google Scholar]
- 2.National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program Asthma and Pregnancy Working Group. NAEPP Expert Panel Report. Managing asthma during pregnancy: recommendations for pharmacologic treatment—2004 update. J Allergy Clin Immunol. 2005;115(1):34–46. doi: 10.1016/j.jaci.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Källén B, Otterblad Olausson P. Use of anti-asthmatic drugs during pregnancy. 3. Congenital malformations in the infants. Eur J Clin Pharmacol. 2007;63(4):383–388. doi: 10.1007/s00228-006-0259-z. [DOI] [PubMed] [Google Scholar]
- 4.Physicians’ Desk Reference. 53. Montvale, NJ: Medical Economics Company; 2006. Proventil, brand of albuterol, USP; pp. 3067–3069. [Google Scholar]
- 5.Demissie K, Breckenridge MB, Rhoads GG. Infant and maternal outcomes in the pregnancies of asthmatic women. Am J Respir Crit Care Med. 1998;158(4):1091–1095. doi: 10.1164/ajrccm.158.4.9802053. [DOI] [PubMed] [Google Scholar]
- 6.Briggs GG, Freeman RA, Yaffe SJ. Michigan Medicaid Study. Drugs in Pregnancy and Lactation. 4. Baltimore, MD: Williams and Wilkins; 1994. [Google Scholar]
- 7.Lin S, Munsie JP, Herdt-Losavio ML, et al. National Birth Defects Prevention Study. Maternal asthma medication use and the risk of gastroschisis. Am J Epidemiol. 2008;168(1):73–79. doi: 10.1093/aje/kwn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin S, Herdt-Losavio ML, Gensburg L, Marshall E, Druschel C. Maternal asthma, asthma medication use and the risk of congenital heart defects. Birth Defects Res A Clin Mol Teratol. 2009;85(2):161–168. doi: 10.1002/bdra.20523. [DOI] [PubMed] [Google Scholar]
- 9.Blais L, Forget A. Asthma exacerbations during the first trimester of pregnancy and the risk of congenital malformations among asthmatic women. J Allergy Clin Immunol. 2008;121(6):1379–1384. e1. doi: 10.1016/j.jaci.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 10.Blais L, Kettani F-Z, Elftouh N, Forget A. Effect of maternal asthma on the risk of specific congenital malformations: a population-based cohort study. Birth Defects Res A Clin Mol Teratol. 2010;88(4):216–222. doi: 10.1002/bdra.20651. [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Wen SW, Demissie K, Marcoux S, Kramer MS. Maternal asthma and pregnancy outcomes: a retrospective cohort study. Am J Obstet Gynecol. 2001;184(2):90–96. doi: 10.1067/mob.2001.108073. [DOI] [PubMed] [Google Scholar]
- 12.Källén B, Rydhstroem H, Aberg A. Congenital malformations after the use of inhaled budesonide in early pregnancy. Obstet Gynecol. 1999;93(3):392–395. [PubMed] [Google Scholar]
- 13.Schatz M, Zeiger RS, Harden KM, et al. The safety of inhaled beta-agonist bronchodilators during pregnancy. J Allergy Clin Immunol. 1988;82(4):686–695. doi: 10.1016/0091-6749(88)90984-0. [DOI] [PubMed] [Google Scholar]
- 14.Schatz M, Zeiger RS, Hoffman CP, et al. Perinatal outcomes in the pregnancies of asthmatic women: a prospective controlled analysis. Am J Respir Crit Care Med. 1995;151(4):1170–1174. doi: 10.1164/ajrccm/151.4.1170. [DOI] [PubMed] [Google Scholar]
- 15.Schatz M, Zeiger RS, Harden K, Hoffman CC, Chilingar L, Petitti D. The safety of asthma and allergy medications during pregnancy. J Allergy Clin Immunol. 1997;100(3):301–306. doi: 10.1016/s0091-6749(97)70241-0. [DOI] [PubMed] [Google Scholar]
- 16.Schatz M, Dombrowski MP, Wise R, et al. Maternal-Fetal Medicine Units Network, The National Institute of Child Health and Development; ; National Heart, Lung and Blood Institute. The relationship of asthma medication use to perinatal outcomes. J Allergy Clin Immunol. 2004;113(6):1040–1045. doi: 10.1016/j.jaci.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Alexander S, Dodds L, Armson BA. Perinatal outcomes in women with asthma during pregnancy. Obstet Gynecol. 1998;92(3):435–440. doi: 10.1016/s0029-7844(98)00191-4. [DOI] [PubMed] [Google Scholar]
- 18.Enriquez R, Griffin MR, Carroll KN, et al. Effect of maternal asthma and asthma control on pregnancy and perinatal outcomes. J Allergy Clin Immunol. 2007;120(3):625–630. doi: 10.1016/j.jaci.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 19.Sheiner E, Mazor M, Levy A, Wiznitzer A, Bashiri A. Pregnancy outcome of asthmatic patients: a population-based study. J Matern Fetal Neonatal Med. 2005;18(4):237–240. doi: 10.1080/14767050500260616. [DOI] [PubMed] [Google Scholar]
- 20.Tamási L, Somoskövi A, Müller V, et al. A population-based case-control study on the effect of bronchial asthma during pregnancy for congenital abnormalities of the offspring. J Asthma. 2006;43(1):81–86. doi: 10.1080/02770900500448803. [DOI] [PubMed] [Google Scholar]
- 21.Tata LJ, Lewis SA, McKeever TM, et al. Effect of maternal asthma, exacerbations and asthma medication use on congenital malformations in offspring: a UK population-based study. Thorax. 2008;63(11):981–987. doi: 10.1136/thx.2008.098244. [DOI] [PubMed] [Google Scholar]
- 22.Yoon PW, Rasmussen SA, Lynberg MC, et al. The national birth defects prevention study. Public Health Rep. 2001;116(suppl 1):32–40. doi: 10.1093/phr/116.S1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen SA, Olney RS, Holmes LB, et al. Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2003;67(3):193–201. doi: 10.1002/bdra.10012. [DOI] [PubMed] [Google Scholar]
- 24.Sadler TW. Langman’s Medical Embryology. 9. Philadelphia, PA: Lippincott Williams and Wilkins; 2004. [Google Scholar]
- 25.New York State Department of Health. Congenital Malformations Registry Summary Report. Albany, NY: New York State Department of Health; 2007. [Google Scholar]
- 26.Park JM, Schmer V, Myers TL. Cardiovascular anomalies associated with prenatal exposure to theophylline. South Med J. 1990;83(12):1487–1488. doi: 10.1097/00007611-199012000-00031. [DOI] [PubMed] [Google Scholar]
- 27.Bracken MB, Triche EW, Belanger K, Saftlas A, Beckett WS, Leaderer BP. Asthma symptoms, severity, and drug therapy: a prospective study of effects on 2205 pregnancies. Obstet Gynecol. 2003;102(4):739–752. doi: 10.1016/s0029-7844(03)00621-5. [DOI] [PubMed] [Google Scholar]
- 28.Lipworth BJ. Fortnightly review: modern drug treatment of chronic asthma. BMJ. 1999;318(7180):380–384. doi: 10.1136/bmj.318.7180.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy VE, Gibson PG, Smith R, Clifton VL. Asthma during pregnancy: mechanisms and treatment implications. Eur Respir J. 2005;25(4):731–750. doi: 10.1183/09031936.05.00085704. [DOI] [PubMed] [Google Scholar]
- 30.Management of Asthma during Pregnancy. Bethesda, MD: National Institutes of Health, National Heart, Lung, and Blood Institute, US Department of Health and Human Services; 1993. National Asthma Education Program. Report of the Working Group on Asthma and Pregnancy. Publication No. 93-3279A. [Google Scholar]
- 31.Ventolin (albuterol, USP) inhalation aerosol [package insert] Research Triangle Park, NC: GlaxoSmithKline RL-963; 2001. [Google Scholar]
- 32.Osur SL. The management of asthma and rhinitis during pregnancy. J Womens Health (Larchmt) 2005;14(3):263–276. doi: 10.1089/jwh.2005.14.263. [DOI] [PubMed] [Google Scholar]
- 33.NAEPP Expert Panel Report. Managing asthma during pregnancy: Recommendations for pharmacologic treatment—2004 Update. J Allergy Clin Immunol. 2005;115:34–46. doi: 10.1016/j.jaci.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 34.Whitty JE, Dombrowski MP. Respiratory diseases in pregnancy. In: Gabbe SG, Niebyl JR, Simpson JL, editors. Obstetrics: Normal and Problem Pregnancies. 4. Philadelphia, PA: Churchill Livingstone; 2002. pp. 1040–1046. [Google Scholar]
- 35.Cogswell ME, Bitsko RH, Anderka M, et al. National Birth Defects Prevention Study. Control selection and participation in an ongoing, population-based, case-control study of birth defects: the National Birth Defects Prevention Study. Am J Epidemiol. 2009;170(8):975–985. doi: 10.1093/aje/kwp226. [DOI] [PubMed] [Google Scholar]