Abstract
OBJECTIVE
To investigate whether antihypertensive classes and specific medications in early pregnancy increase the risk of severe hypospadias and to assess prior associations detected for late-treated and untreated hypertension in the National Birth Defects Prevention Study.
METHODS
Using telephone interviews from mothers of 2,131 children with severe hypospadias and 5,129 nonmalformed male control children for 1997–2009 births in a population-based case–control study, we estimated adjusted odds ratios (ORs) and 95% confidence intervals (CIs) with multivariable logistic regression. We adjusted P values to account for multiple testing.
RESULTS
Forty-eight (2.3%) case and 70 (1.4%) control mothers reported early pregnancy antihypertensive treatment, 45 (2.1%) case and 31 (0.6%) control mothers reported late treatment, and 315 (14.8%) case and 394 (7.7%) control mothers reported untreated hypertension. Selective β-blockers, centrally acting agents, renin–angiotensin system-acting agents, diuretics, and specific medications, methyldopa and atenolol, were not associated with hypospadias. Nonselective β-blockers (adjusted OR 3.22, 95% CI 1.47–7.05) were associated with hypospadias; however, P values adjusted for multiple testing were not statistically significant. We confirmed prior findings for associations between hypospadias and untreated hypertension (adjusted OR 2.09, 95% CI 1.76–2.48) and late initiation of treatment (adjusted OR 3.98, 95% CI 2.41–6.55). The increased risks would translate to severe hypospadias prevalences of 11.5, 17.7, and 21.9 per 10,000 births for women with untreated hypertension, nonselective β-blocker use, and late initiation of treatment, respectively.
CONCLUSION
Our study suggests a relationship between hypospadias and the severity of hypertension.
Maternal hypertensive disorders affect up to 10% of pregnancies1; however, data regarding specific risks of hypertension and its treatments on birth defects are limited.2,3 Altered placental perfusion resulting from maternal hypertension4–6 or treatment-induced iatrogenic hypotension is of particular concern.2,7 Because placental insufficiency is a proposed mechanism in the development of hypospadias,8–11 women with hypertension may be at greater risk of having a neonate with hypospadias.10,12–17 Women whose hypertension becomes evident in later gestation may also be at greater risk as a result of underlying abnormal placentation that is present before abnormal fusion of the urethral folds.
Prior studies have suggested an association between hypospadias and hypertensive disorders,10,12,13,17,18 β-blockers,19–21 and diuretics,22 whereas others have failed to show similar associations.9,15,17,21,23–28 Study limitations may explain these inconsistencies. Grouping antihypertensive treatments may obscure risks of specific classes or medications, separating the effects of maternal hypertension from those of the medication is difficult, and information on the type and severity of hypertension and on confounding resulting from to common comorbidities (eg, diabetes) is often lacking.29
Previously, we used National Birth Defects Prevention Study data to examine the associations among maternal hypertension, early or late antihypertensive treatment, and severe hypospadias in the offspring.16 Compared with mothers without hypertension, we observed the highest risk in mothers initiating treatment in later pregnancy, a moderate risk in mothers with untreated hypertension, and a slight risk in mothers treated during early pregnancy. Our current study incorporated 7 additional years of data to confirm prior findings and investigate specific antihypertensive classes and medications used in early pregnancy.
MATERIALS AND METHODS
The National Birth Defects Prevention Study is the largest population-based, case–control study of birth defects in the United States.30,31 The objective is to investigate environmental and genetic risk factors for more than 30 major structural birth defects. The study identifies cases of birth defects among liveborn neonates, fetal deaths (20 weeks of gestation or greater), and elective pregnancy terminations. Control neonates are live births without birth defects randomly selected from birth certificates or hospital discharge listings in the same population as the case neonates. Computer-assisted maternal telephone interviews are conducted within 24 months of delivery. Pregnancy dating is based on a hierarchy of information: early ultrasound scan, last menstrual period, late ultrasound scan, and neonatal examination. The National Birth Defects Prevention Study has institutional review board approvals at each site (Arkansas, California, Georgia, Iowa, Massachusetts, New Jersey, New York, North Carolina, Texas, Utah) and obtains informed consent from study participants.
We studied children with severe hypospadias identified from the population-based birth defects surveillance systems of participating National Birth Defects Prevention Study sites and male control neonates with estimated dates of delivery from October 1, 1997, through December 31, 2009. Participation was 64% among case and 63% among control mothers. To confirm case diagnoses, clinical geneticists reviewed data abstracted from childrens’ medical records, including clinicians’ and nurses’ notes, consultations (urology, endocrinology, and genetic), reports (operative, pathology, and autopsy), and results of radiographic studies. Each hypospadias case was required to meet specific eligibility criteria.16,31 Only those children with severe hypospadias (ie, subcoronal or penile, scrotal, or perineal meatal opening) diagnosed at the time of physical examination, surgery, or autopsy were included in the study. Children with coronal (“first-degree”) hypospadias, a female karyotype (46,XX), true mosaicism (46, XX/46,XY), a known or strongly suspected chromosome abnormality, a diagnosed single gene condition, certain hormonal profile or anatomical features consistent with an intersex condition, or an unconfirmed diagnosis were excluded.
Trained interviewers asked mothers about the diagnosis, timing, and treatment of “high blood pressure” for neonates with 1997–2005 estimated dates of delivery and “high blood pressure, toxemia, preeclampsia, or eclampsia” for those with 2006–2009 estimated dates of delivery. Antihypertensive medication name, start and stop dates, and frequency of use were collected for 3 calendar months before conception through the end of pregnancy; and medication use was categorized by pregnancy month. If the mother did not recall the medication name, a list of commonly prescribed anti-hypertensive medications was read to her.
We compared early pregnancy medication use (1 month preconception through pregnancy month 4) between mothers of cases and controls. This period encompassed the relevant timeframe for urethral closure (weeks 8–14 postconception),11 and it permitted capture of maternal medication use before pregnancy that may have continued while the pregnancy was unrecognized. We also considered late initiation of medication (pregnancy month 5 through birth). Lastly, we considered untreated hypertension (women reporting high blood pressure during pregnancy without antihypertensive medication use).
Medications were coded using the Slone Epidemiology Center Drug Dictionary and categorized into classes: centrally acting antiadrenergic agents, β-blockers, calcium channel blockers, diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and direct vasodilators. The angiotensin-converting enzyme inhibitors and angiotensin receptor blockers were combined into renin– angiotensin system-acting agents. We subdivided β-blockers (selective and nonselective), calcium channel blockers (dihydropyridine and nondihydropyridine), and diuretics (thiazide or thiazide-like, potassium-sparing, and loop). Mothers reporting antihypertensive medications for the treatment of other indications (eg, β-blockers for migraine headaches) were excluded from the analyses as were mothers with missing information on hypertension or antihypertensive medication use.
Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated separately for each exposure and severe hypospadias by unconditional multivariable logistic regression. We compared estimates from the original 1997–2002 data with estimates derived from the additional 7 years of data (2003–2009) to determine whether it was appropriate to combine results for the overall time period. To assess confounding, we examined categories of maternal age (younger than 20 years, 20–34, 35 or older), race or ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other), education (less than high school, high school, some college, college degree), prepregnancy body mass index (calculated as weight (kg)/[height (m)]2; under-weight [less than 18.5], normal weight [18.5 to less than 25], overweight [25 to less than 30], obese [30 or higher]), parity (0, 1, 2 or more), and study site (as listed previously). We dichotomized (yes, no) fertility treatment, periconceptional folic acid use (1 month preconception through pregnancy month 1), prepregnancy and gestational diabetes, early pregnancy nausea or vomiting, early pregnancy alcohol consumption, early pregnancy smoking, multiple birth, and history of hypospadias in a first-degree relative. We stratified analyses by low birth weight (less than 2,500 g, a proxy for placental dysfunction) and pre-term birth (less than 37 weeks of gestation, a proxy for severe hypertension).2,11 We plotted adjusted ORs with 95% CIs from stratified multivariable models, and we adjusted P values to account for multiple testing using a stepdown Bonferroni method.32
RESULTS
Of the completed maternal interviews for 2,142 children with severe hypospadias and 5,183 non-malformed male control children, we excluded interviews with missing information on hypertension or antihypertensive medication use (eight [0.4%] in the case group, 22 [0.4%] in the control group) or use of medications with antihypertensive properties for the treatment of other indications (three [0.1%] in the case group, 32 [0.6%] in the control group); thus, our analyses included 2,131 in the case group and 5,129 in the control group. Multiple characteristics were associated with the occurrence of hypospadias (Table 1). Overall, 408 (19.1%) case and 495 (9.7%) control mothers reported hypertension during pregnancy: 48 (2.3%) case and 70 (1.4%) control mothers reported early pregnancy treatment, 45 (2.1%) case and 31 (0.6%) control mothers reported late initiation of treatment, and 315 (14.8%) case and 394 (7.7%) control mothers reported untreated hypertension.
Table 1.
Distribution of Selected Characteristics of Children With Severe Hypospadias and Nonmalformed Control Children in the National Birth Defects Prevention Study, 1997–2009
| Characteristic | Children in the Case Group (n=2,131) |
Children in the Control Group (n=5,129) |
P |
|---|---|---|---|
| Maternal age (y) | <.001 | ||
| Younger than 20 | 144 (6.8) | 551 (10.7) | |
| 20–34 | 1,556 (73.0) | 3,877 (75.6) | |
| 35 or older | 431 (20.2) | 701 (13.7) | |
| Maternal race or ethnicity | <.001 | ||
| Non-Hispanic white | 1,531 (71.8) | 2,942 (57.4) | |
| Non-Hispanic black | 271 (12.7) | 565 (11.0) | |
| Hispanic | 182 (8.5) | 1,230 (24.0) | |
| Other | 144 (6.8) | 384 (7.5) | |
| Missing | 3 (0.1) | 8 (0.2) | |
| Maternal education | <.001 | ||
| Less than high school | 163 (7.7) | 867 (16.9) | |
| High school | 416 (19.5) | 1,236 (24.1) | |
| Some college | 570 (26.8) | 1,341 (26.2) | |
| College degree | 937 (44.0) | 1,570 (30.6) | |
| Missing | 45 (2.1) | 115 (2.2) | |
| Maternal prepregnancy BMI (kg/m2) | .387 | ||
| Underweight | 100 (4.7) | 279 (5.4) | |
| Normal weight | 1,121 (52.6) | 2,643 (51.5) | |
| Overweight | 480 (22.5) | 1,118 (21.8) | |
| Obese | 386 (18.1) | 858 (16.7) | |
| Missing | 44 (2.1) | 231 (4.5) | |
| Parity | <.001 | ||
| 0 | 1,144 (53.7) | 2,053 (40.0) | |
| 1 | 622 (29.2) | 1,672 (32.6) | |
| 2 or more | 357 (16.8) | 1,395 (27.2) | |
| Missing | 8 (0.4) | 9 (0.2) | |
| Maternal fertility treatment | <.001 | ||
| Yes | 214 (10.0) | 204 (4.0) | |
| No | 1,917 (90.0) | 4,925 (96.0) | |
| Oral contraceptive use | .166 | ||
| Yes | 252 (11.9) | 669 (13.1) | |
| No | 1,869 (88.1) | 4,448 (86.9) | |
| Periconceptional folic acid use | <.001 | ||
| Yes | 1,355 (63.6) | 2,644 (51.6) | |
| No | 738 (34.6) | 2,372 (46.3) | |
| Missing | 38 (1.8) | 113 (2.2) | |
| Prepregnancy diabetes | .001 | ||
| Yes | 28 (1.3) | 29 (0.6) | |
| No | 2,102 (98.6) | 5,094 (99.3) | |
| Missing | 1 (<0.1) | 6 (0.1) | |
| Gestational diabetes | .116 | ||
| Yes | 114 (5.4) | 230 (4.5) | |
| No | 2,016 (94.6) | 4,893 (95.4) | |
| Missing | 1 (<0.1) | 6 (0.1) | |
| Early pregnancy nausea or vomiting | <.001 | ||
| Yes | 1,319 (61.9) | 3,467 (67.6) | |
| No | 802 (37.6) | 1,640 (32.0) | |
| Missing | 10 (0.5) | 22 (0.4) | |
| Early pregnancy alcohol use | <.001 | ||
| Yes | 876 (41.1) | 1,860 (36.3) | |
| No | 1,197 (56.2) | 3,144 (61.3) | |
| Missing | 58 (2.7) | 125 (2.4) | |
| Early pregnancy smoking | .074 | ||
| Yes | 353 (16.6) | 941 (18.4) | |
| No | 1,734 (81.4) | 4,089 (79.7) | |
| Missing | 44 (2.1) | 99 (1.9) | |
| Multiple birth | <.001 | ||
| Yes | 174 (8.2) | 144 (2.8) | |
| No | 1,957 (91.8) | 4,958 (97.2) | |
| Preterm birth (less than 37 wk of gestation) | <.001 | ||
| Yes | 561 (26.3) | 484 (9.4) | |
| No | 1,568 (73.6) | 4,645 (90.6) | |
| Missing | 2 (0.1) | 0 (0.0) | |
| Low birth weight (less than 2,500 g) | <.001 | ||
| Yes | 556 (26.1) | 286 (5.6) | |
| No | 1,544 (72.5) | 4,773 (93.1) | |
| Missing | 31 (1.5) | 70 (1.4) | |
| Family history (1st-degree relative) | <.001 | ||
| Yes | 97 (4.6) | 10 (0.2) | |
| No | 2,034 (95.5) | 5,119 (99.8) |
BMI, body mass index.
Table 2 shows crude and adjusted estimates for the three study periods: 1997–2002, 2003–2009, and the combined 1997–2009 study period. Based on the comparability of the findings for the two individual study periods, we used the combined 1997–2009 study period in our analyses. For the 1997–2009 study period, the adjusted ORs were 1.56 (95% CI 1.05–2.33) for early pregnancy medication use, 3.98 (95% CI 2.41–6.55) for late initiation of treatment, and 2.09 (95% CI 1.76–2.48) for untreated hypertension.
Table 2.
Early Pregnancy Use of Antihypertensive Medication, Late Initiation of Antihypertensive Medication, Untreated Hypertension, and the Risk of Severe Hypospadias, the National Birth Defects Prevention Study, 1997–2002, 2003–2009, and Overall
| Exposure Group* and Study Period | No. of Cases and No. of Controls | OR | 95% CI | Adjusted OR† | 95% CI | P | Adjusted P‡ |
|---|---|---|---|---|---|---|---|
| Early use | |||||||
| 1997–2002 | 19/24 | 2.17 | 1.18–3.98 | 1.77 | 0.89–3.55 | .105 | 1.000 |
| 2003–2009 | 29/46 | 1.68 | 1.05–2.68 | 1.56 | 0.94–2.57 | .085 | 1.000 |
| Overall | 48/70 | 1.84 | 1.27–2.67 | 1.56 | 1.05–2.33 | .029 | .620 |
| Late initiation | |||||||
| 1997–2002 | 14/13 | 2.94 | 1.38–6.29 | 4.49 | 1.85–10.91 | .001 | .024 |
| 2003–2009 | 31/18 | 4.58 | 2.55–8.22 | 4.23 | 2.26–7.94 | <.001 | .004 |
| Overall | 45/31 | 3.90 | 2.46–6.19 | 3.98 | 2.41–6.55 | <.001 | .004 |
| Untreated hypertension | |||||||
| 1997–2002 | 120/157 | 2.08 | 1.61–2.68 | 2.09 | 1.55–2.81 | <.001 | .004 |
| 2003–2009 | 195/237 | 2.19 | 1.79–2.68 | 2.13 | 1.71–2.64 | <.001 | .004 |
| Overall | 315/394 | 2.15 | 1.84–2.52 | 2.09 | 1.76–2.48 | <.001 | .004 |
| No hypertension | |||||||
| 1997–2002 | 679/1,857 | Referent | Referent | ||||
| 2003–2009 | 1,045/2,777 | Referent | Referent | ||||
| Overall | 1,723/4,634 | Referent | Referent |
OR, odds ratio; CI, confidence interval.
Women taking medications used to treat hypertension who did not report hypertension were excluded from the analysis.
Adjusted for site, maternal age, race and ethnicity, parity, fertility treatment, prepregnancy diabetes, gestational diabetes, and multiple birth.
Adjusted using the stepdown Bonferroni method.32
The most commonly reported medication classes used in early pregnancy were β-blockers and centrally acting antiadrenergic agents (Fig. 1). Methyldopa, labetalol, and atenolol were the most commonly reported individual medications (Table 3). Nonselective β-blockers (adjusted OR 3.22, 95% CI 1.47-7.05), including the specific medications labetalol (adjusted OR 3.02, 95% CI 1.23–7.44) and propranolol (adjusted OR 4.22, 95% CI 0.87–20.51), were associated with hypospadias, whereas selective β-blockers, centrally acting agents, calcium channel blockers, renin–angiotensin system-acting agents, and diuretics were not. Methyldopa (adjusted OR 1.48, 95% CI 0.75–2.92) and atenolol (adjusted OR 0.86, 95% CI 0.262.81) also were not associated with hypospadias. As a result of small sample sizes (less than five), we could not reliably estimate the ORs for other classes or specific medications. Figure 2 presents results stratified by low birth weight and preterm birth for early antihypertensive medication users, late initiators of medications, and untreated hypertension. For each exposure group, adjusted ORs were higher among low-birth-weight and preterm births.
Fig. 1.
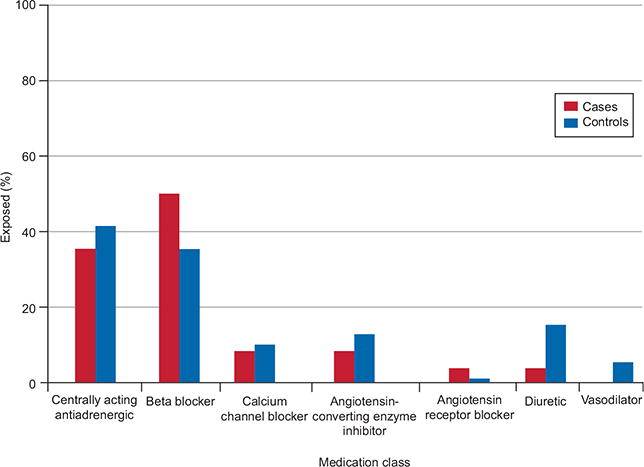
Proportion of mothers reporting specific classes of antihypertensive medications among those reporting early pregnancy use.
Van Zutphen. Maternal Hypertension and Hypospadias. Obstet Gynecol 2014.
Table 3.
Early Pregnancy Use of Specific Antihypertensive Medication Classes and Drugs and the Risk of Severe Hypospadias, the National Birth Defects Prevention Study, 1997–2009
| Exposure Group* | No. of Cases and No. of Controls | OR | 95% CI | Adjusted OR† | 95% CI | P | Adjusted P‡ |
|---|---|---|---|---|---|---|---|
| Centrally acting antiadrenergic | 17/29 | 1.58 | 0.86-2.88 | 1.42 | 0.74–2.71 | .294 | 1.000 |
| Methyldopa | 15/27 | 1.49 | 0.79–2.82 | 1.48 | 0.75–2.92 | .254 | 1.000 |
| Clonidine | 2/2 | NE | NE | NE | NE | ||
| Beta-blocker | 24/25 | 2.58 | 1.47–4.53 | 2.02 | 1.11–3.69 | .022 | .506 |
| Selective | 10/13 | 2.07 | 0.91–4.73 | 1.39 | 0.57–3.42 | .470 | 1.000 |
| Acebutolol | 1/0 | NE | NE | NE | NE | ||
| Atenolol | 5/10 | 1.35 | 0.46–3.94 | 0.86 | 0.26–2.81 | .802 | 1.000 |
| Bisoprolol | 1/0 | NE | NE | NE | NE | ||
| Metoprolol | 3/3 | 2.69 | 0.54–13.34 | 1.82 | 0.33–10.07 | .493 | 1.000 |
| Nonselective | 16/12 | 3.59 | 1.69–7.60 | 3.22 | 1.47–7.05 | .004 | .091 |
| Labetalol | 12/9 | 3.59 | 1.51–8.53 | 3.02 | 1.23–7.44 | .016 | .384 |
| Carvedilol | 1/0 | NE | NE | NE | NE | ||
| Nadolol | 1/0 | NE | NE | NE | NE | ||
| Propranolol | 4/3 | 3.59 | 0.80–16.04 | 4.22 | 0.87–20.51 | .075 | 1.000 |
| Calcium channel blocker | 4/7 | 1.54 | 0.45–5.26 | 1.07 | 0.28–4.05 | .922 | 1.000 |
| Dihydropyridine | 4/6 | 1.79 | 0.51–6.36 | 1.24 | 0.31–4.95 | .757 | 1.000 |
| Amlodipine | 2/4 | NE | NE | NE | NE | ||
| Nifedipine | 2/1 | NE | NE | NE | NE | ||
| Nisoldipine | 0/1 | NE | NE | NE | NE | ||
| Nondihydropyridine | 0/1 | NE | NE | NE | NE | ||
| Verapamil | 0/1 | NE | NE | NE | NE | ||
| Renin–angiotensin system-acting | 5/10 | 1.35 | 0.46–3.94 | 0.89 | 0.29–2.76 | .843 | 1.000 |
| ACE inhibitor | 3/9 | 0.90 | 0.24–3.32 | 0.59 | 0.15–2.31 | .444 | 1.000 |
| Benazapril | 1/2 | NE | NE | NE | NE | ||
| Enalapril | 1/0 | NE | NE | NE | NE | ||
| Fosinopril | 0/1 | NE | NE | NE | NE | ||
| Lisinopril | 0/4 | NE | NE | NE | NE | ||
| Quinapril | 1/0 | NE | NE | NE | NE | ||
| Trandolapril | 0/1 | NE | NE | NE | NE | ||
| Angiotensin receptor blocker | 2/1 | NE | NE | NE | NE | ||
| Losartan | 1/0 | NE | NE | NE | NE | ||
| Olmesartan | 1/0 | NE | NE | NE | NE | ||
| Valsartan | 0/1 | NE | NE | NE | NE | ||
| Diuretic | 5/11 | 1.22 | 0.42–3.52 | 1.09 | 0.35–3.39 | .885 | 1.000 |
| Thiazide or thiazide-like | 3/8 | 1.01 | 0.273.81 | 0.90 | 0.223.72 | .882 | 1.000 |
| Hydrochlorothiazide | 3/7 | 1.15 | 0.30–4.46 | 0.99 | 0.23–4.22 | .985 | 1.000 |
| Chlorthalidone | 0/1 | NE | NE | NE | NE | ||
| Potassium-sparing (triamterene) | 0/1 | NE | NE | NE | NE | ||
| Loop (furosemide) | 0/1 | NE | NE | NE | NE | ||
| Vasodilator (hydralazine) | 0/4 | NE | NE | NE | NE |
OR, odds ratio; CI, confidence interval; NE, not estimable; ACE, angiotensin-converting enzyme.
Women taking medications used to treat hypertension who did not report hypertension were excluded from the analysis.
Adjusted for site, maternal age, race and ethnicity, parity, fertility treatment, prepregnancy diabetes, gestational diabetes, and multiple birth.
Adjusted using the stepdown Bonferroni method.32
Fig. 2.
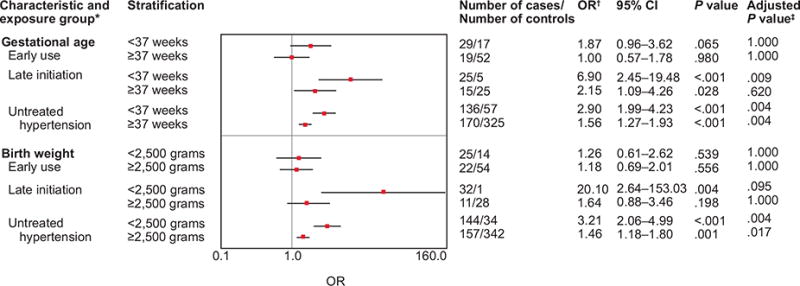
Associations between early pregnancy antihypertensive medication use, late initiation of antihypertensive medication, untreated hypertension, and hypospadias stratified by low birth weight and preterm birth.
*Women taking medications used to treat hypertension, who did not report hypertension, were excluded from the analysis.
†Adjusted for site, maternal age, race/ethnicity, parity, fertility treatment, pre-pregnancy diabetes, gestational diabetes, and multiple births.
‡Adjusted using the step-down Bonferroni method.32 OR, odds ratio; CI, confidence interval.
Van Zutphen. Maternal Hypertension and Hypospadias. Obstet Gynecol 2014.
Multiple testing adjustment for the associations presented in Table 2, Table 3, and Figure 2 revealed that the P values for associations between hypospadias and early antihypertensive medication use (Table 2), β-blocker, nonselective β-blocker, and labetalol use (Table 3) for the overall study period were no longer statistically significant at α=0.05. In Figure 2, P values for the associations between hypospadias and early medication use among non-Hispanic black mothers and late initiation among non-Hispanic white mothers, term births, and low-birth-weight births were no longer statistically significant.
DISCUSSION
We confirmed our earlier findings of associations between hypospadias and both untreated hypertension and late initiation of antihypertensive medications.16 Our findings agree with several studies that have suggested a relationship between preeclampsia or gestational hypertension and hypospadias10,12,13,17,18; however, others have not demonstrated this association.9,15,23,24 Inadequate spiral artery invasion of the placenta may affect uteroplacental perfusion early in gestation in women with gestational hypertension or preeclampsia,4–6 and placental insufficiency might affect fetal somatic and urethral development.8–11,17 Brouwers et al17 observed that low birth weight and preeclampsia showed the strongest associations with the proximal type of hypospadias (which corresponds to our severe hypospadias cases). Notably, we found the strongest associations for all categories of exposure among neonates with low birth weight (a proxy for placental dysfunction) and preterm births (a proxy of severe hypertension).2,11
Early pregnancy use of centrally acting antiadrenergic, selective β-locker, renin–angiotensin system-acting, and diuretic medications were not associated with hypospadias in our study. However, we detected an association for nonselective β-blockers: an appreciable and stable association for labetalol and a less stable elevated risk for propranolol. These results are in contrast with a study that found no associations between β-blockers and genital or urinary anomalies,26 but agree with a study that reported a greater than expected number of hypospadias cases in pregnancies exposed to propranolol.20 The association with labetalol might be confounded by the severity of the hypertension because labetalol is used for the urgent control of severe hypertension in pregnancy.2,3 Alternatively, the association might reflect a treatment-induced reduction in placental blood flow. Treatment-induced decreases in mean arterial pressure were associated with inadequate fetal growth in a meta-analysis of randomized controlled trials of pregnancy hypertension.7 Assuming these decreases are associated with reduced placental blood flow, maternal antihypertensive medication use during the time of urethral development might cause hypospadias. Notably, an in vitro study of human umbilical artery resistance showed that labetalol, hydralazine, and nifedipine significantly affected fetoplacental circulation, whereas α-methyldopa did not.33 These findings are consistent with both Doppler ultrasound studies, which showed that α-methyldopa does not affect uteroplacental or fetal hemodynamics33,34 and a sheep model of maternal hypertension in which labetalol and pindolol (another nonselective β-blocker) compromised uteroplacental perfusion.35 Beta-blockers also may affect urethral development by interfering with Leydig cell activity in the testis. Leydig cells begin production of testosterone by week 8 and influence sexual differentiation.36 Because β-2-type receptors play a role in Leydig cell activity, nonselective β-blockers may interfere with urethral development.37 Furthermore, β-blockers induced significant decreases in testosterone in male rats.38
The strength of our study is that we used data from the National Birth Defects Prevention Study, a large, population-based study with a standardized protocol for maternal interviews performed within 24 months of the estimated due date and strict definitions and clinical review for consistent case classification. Data on timing of medication use and maternal health and lifestyle factors permitted evaluation of specific medications during the window of urethral closure and evaluation of confounding and effect modification. Our inability to classify the type or severity of hypertension constrained our ability to evaluate confounding by indication.29 Reporting inaccuracy and recall bias resulting from retrospective ascertainment of medication exposures were minimized through a questionnaire design that elicited medication use by indication.29,39 Recall of prescription chronic hypertension medications is likely to be reasonably accurate.39 Differential recall among case mothers is not likely because we observed associations for some medications and not others. Correlated lifestyle or maternal characteristics also could bias our findings. Lastly, sample sizes for a number of medications were small, leading to imprecise estimates.
This study contributes new data on the risks and relative safety of maternal hypertension and its treatments during pregnancy. The findings of associations between nonselective β-blockers and hypospadias require confirmation. Severe hypospadias affects approximately 5.5 per 10,000 births40; thus, the increased risks for women with untreated hypertension, nonselective β-blocker use, or late initiation of treatment would translate to severe hypospadias prevalences of 11.5, 17.7, and 21.9 per 10,000 births, respectively, a modest increase relative to the baseline risk of 200–300 per 10,000 for any major birth defect. For severe hypertension, continuation of treatment during pregnancy is necessary for maternal and fetal health; however, there is no consensus on the necessity of medication use for mild to moderate hypertension during pregnancy.2,3 Our findings might be considered in a risk–benefit assessment for treatment choices in these women.
Acknowledgments
Supported by a cooperative agreement from the Centers for Disease Control and Prevention (Cooperative Agreement U01DD000487). The Slone Drug Dictionary under license from the Slone Epidemiology Center at Boston University, Boston, Massachusetts, was used to classify medications.
We thank the participants, staff, and scientists of the National Birth Defects Prevention Study from Arkansas, California, Georgia, Iowa, Massachusetts, New Jersey, New York, North Carolina, Texas, and Utah. We thank Adrian Michalski for replicating the analyses.
The findings and conclusions in this report have not been formally disseminated by the Centers for Disease Control and Prevention and should not be construed to represent any agency determination or policy.
Financial Disclosure
Dr. Mitchell receives research support from Novartis Vaccines and Diagnostics for research related to a meningitis vaccine and serves on Biogen-Idec pregnancy registry advisory committees for agents used to treat multiple sclerosis. During the period of study and until August 2012, he owned stock (value less than $20,000) in Johnson & Johnson. Dr. Werler serves on the advisory boards of studies supported by manufacturers of medications used to treat rheumatoid arthritis. These manufacturers may include antihypertensive medications in their product lines.
Footnotes
LEVEL OF EVIDENCE: II
Presented in a poster at the Society for Epidemiologic Research 43rd Annual Meeting, June 23–26, 2010, Seattle, Washington.
The other authors did not report any potential conflicts of interest.
References
- 1.Roberts CL, Ford JB, Algert CS, Antonsen S, Chalmers J, Cnattingius S, et al. Population-based trends in pregnancy hypertension and pre-eclampsia: an international comparative study. BMJ Open. 2011;1:e000101. doi: 10.1136/bmjopen-2011-000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chronic hypertension in pregnancy. Practice Bulletin No. 125. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2012;119:369–407. doi: 10.1097/AOG.0b013e318249ff06. [DOI] [PubMed] [Google Scholar]
- 3.Podymow T, August P. Update on the use of antihypertensive drugs in pregnancy. Hypertension. 2008;51:960–9. doi: 10.1161/HYPERTENSIONAHA.106.075895. [DOI] [PubMed] [Google Scholar]
- 4.Kliman HJ. Uteroplacental blood flow. The story of decidualization, menstruation, and trophoblast invasion. Am J Pathol. 2000;157:1759–68. doi: 10.1016/S0002-9440(10)64813-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayhew TM, Charnock-Jones DS, Kaufmann P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta. 2004;25:127–39. doi: 10.1016/j.placenta.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Culver JC, Dickinson ME. The effects of hemodynamic force on embryonic development. Microcirculation. 2010;17:164–78. doi: 10.1111/j.1549-8719.2010.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Dadelszen P, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: an updated metaregression analysis. J Obstet Gynaecol Can. 2002;24:941–5. doi: 10.1016/s1701-2163(16)30592-8. [DOI] [PubMed] [Google Scholar]
- 8.Gatti JM, Kirsch AJ, Troyer WA, Perez-Brayfield MR, Smith EA, Scherz HC. Increased incidence of hypospadias in small-for-gestational age infants in a neonatal intensive-care unit. BJU Int. 2001;87:548–50. doi: 10.1046/j.1464-410x.2001.00088.x. [DOI] [PubMed] [Google Scholar]
- 9.Hussain N, Chaghtai A, Herndon CD, Herson VC, Rosenkrantz TS, McKenna PH. Hypospadias and early gestation growth restriction in infants. Pediatrics. 2002;109:473–8. doi: 10.1542/peds.109.3.473. [DOI] [PubMed] [Google Scholar]
- 10.Sun G, Tang D, Liang J, Wu M. Increasing prevalence of hypospadias associated with various perinatal risk factors in Chinese newborns. Urology. 2009;73:1241–5. doi: 10.1016/j.urology.2008.12.081. [DOI] [PubMed] [Google Scholar]
- 11.Yinon Y, Kingdom JC, Proctor LK, Kelly EN, Salle JL, Wherrett D, et al. Hypospadias in males with intrauterine growth restriction due to placental insufficiency: the placental role in the embryogenesis of male external genitalia. Am J Med Genet A. 2010;152A:75–83. doi: 10.1002/ajmg.a.33140. [DOI] [PubMed] [Google Scholar]
- 12.Akre O, Lipworth L, Cnattingius S, Sparén P, Ekbom A. Risk factor patterns for cryptorchidism and hypospadias. Epidemiology. 1999;10:364–9. [PubMed] [Google Scholar]
- 13.Akre O, Boyd HA, Ahlgren M, Wilbrand K, Westergaard T, Hjalgrim H, et al. Maternal and gestational risk factors for hypospadias. Environ Health Perspect. 2008;116:1071–6. doi: 10.1289/ehp.10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Møller H, Weidner IS. Epidemiology of cryptorchidism and hypospadias. Epidemiology. 1999;10:352–4. [PubMed] [Google Scholar]
- 15.Aschim EL, Haugen TB, Tretli S, Daltveit AK, Grotmol T. Risk factors for hypospadias in Norwegian boys—association with testicular dysgenesis syndrome? Int J Androl. 2004;27:213–21. doi: 10.1111/j.1365-2605.2004.00473.x. [DOI] [PubMed] [Google Scholar]
- 16.Caton AR, Bell EM, Druschel CM, Werler MM, Mitchell AA, Browne ML, et al. Maternal hypertension, antihypertensive medication use, and the risk of severe hypospadias. Birth Defects Res A Clin Mol Teratol. 2008;82:34–40. doi: 10.1002/bdra.20415. [DOI] [PubMed] [Google Scholar]
- 17.Brouwers MM, van der Zanden LF, de Gier RP, Barten EJ, Zielhuis GA, Feitz WF, et al. Hypospadias: risk factor patterns and different phenotypes. BJU Int. 2010;105:254–62. doi: 10.1111/j.1464-410X.2009.08772.x. [DOI] [PubMed] [Google Scholar]
- 18.Sørensen HT, Pedersen L, Nørgaard M, Wogelius P, Rothman KJ. Maternal asthma, preeclampsia and risk of hypospadias. Epidemiology. 2005;16:806–7. doi: 10.1097/01.ede.0000181631.31713.b6. [DOI] [PubMed] [Google Scholar]
- 19.Briggs GG, Freeman RK, Yaffe SJ. Beta blockers. Drugs in Pregnancy and Lactation Update. 2003;16:10–5. [Google Scholar]
- 20.Czeizel A. Teratogenicity of ergotamine. J Med Genet. 1989;26:69–70. doi: 10.1136/jmg.26.1.69-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lennestål R, Otterblad Olausson P, Källén B. Maternal use of antihypertensive drugs in early pregnancy and delivery outcome, notably the presence of congenital heart defects in the infants. Eur J Clin Pharmacol. 2009;65:615–25. doi: 10.1007/s00228-009-0620-0. [DOI] [PubMed] [Google Scholar]
- 22.Briggs GG, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation. Baltimore (MD): Williams & Wilkins; 1998. [Google Scholar]
- 23.Heinonen OP, Slone D, Shapiro S. Birth defects and drugs in pregnancy. Littleton (MA): PSG Publishing Co; 1977. [Google Scholar]
- 24.Adams SV, Hastert TA, Huang Y, Starr JR. No association between maternal pre-pregnancy obesity and risk of hypospadias or cryptorchidism in male newborns. Birth Defects Res A Clin Mol Teratol. 2011;91:241–8. doi: 10.1002/bdra.20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006;354:2443–51. doi: 10.1056/NEJMoa055202. [DOI] [PubMed] [Google Scholar]
- 26.Davis RL, Eastman D, McPhillips H, Raebel MA, Andrade SE, Smith D, et al. Risks of congenital malformations and perinatal events among infants exposed to calcium channel and beta-blockers during pregnancy. Pharmacoepidemiol Drug Saf. 2011;20:138–45. doi: 10.1002/pds.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorensen HT, Czeizel AE, Rockenbauer M, Steffensen FH, Olsen J. The risk of limb deficiencies and other congenital abnormalities in children exposed in utero to calcium channel blockers. Acta Obstet Gynecol Scand. 2001;80:397–401. [PubMed] [Google Scholar]
- 28.Bánhidy F, Acs N, Puhó EH, Czeizel AE. Chronic hypertension with related drug treatment of pregnant women and congenital abnormalities in their offspring: a population-based study. Hypertens Res. 2011;34:257–63. doi: 10.1038/hr.2010.227. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell AA. Studies of drug-induced birth defects. In: Strom BL, Kimmel SE, Hennessy S, editors. Pharmacoepidemiology. 5th. New York (NY): Wiley-Blackwell; 2012. pp. 487–504. [Google Scholar]
- 30.Yoon PW, Rasmussen SA, Lynberg MC, Moore CA, Anderka M, Carmichael SL, et al. The National Birth Defects Prevention Study. Public Health Rep. 2001;116(suppl 1):32–40. doi: 10.1093/phr/116.S1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler-Noreuil KM, Moore CA, National Birth Defects Prevention Study Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2003;67:193–201. doi: 10.1002/bdra.10012. [DOI] [PubMed] [Google Scholar]
- 32.Holm S. A simple sequentially rejective Bonferroni test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 33.Houlihan DD, Dennedy MC, Ravikumar N, Morrison JJ. Anti-hypertensive therapy and the feto-placental circulation: effects on umbilical artery resistance. J Perinat Med. 2004;32:315–9. doi: 10.1515/JPM.2004.058. [DOI] [PubMed] [Google Scholar]
- 34.Montan S, Anandakumar C, Arulkumaran S, Ingemarsson I, Ratnam SS. Effects of methyldopa on uteroplacental and fetal hemodynamics in pregnancy-induced hypertension. Am J Obstet Gynecol. 1993;168:152–6. doi: 10.1016/s0002-9378(12)90905-9. [DOI] [PubMed] [Google Scholar]
- 35.Erkinaro T, Kavasmaa T, Ylikauma L, Mäkikallio K, Haapsamo M, Acharya G, et al. Placental and fetal hemodynamics after labetalol or pindolol in a sheep model of increased placental vascular resistance and maternal hypertension. Reprod Sci. 2009;16:749–57. doi: 10.1177/1933719109335068. [DOI] [PubMed] [Google Scholar]
- 36.Sadler TW. Langman’s medical embryology. 9th. Baltimore (MD): Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 37.Baumhäkel M, Schlimmer N, Kratz M, Hackett G, Jackson G, Böhm M. Cardiovascular risk, drugs and erectile function—a systematic analysis. Int J Clin Pract. 2011;65:289–98. doi: 10.1111/j.1742-1241.2010.02563.x. [DOI] [PubMed] [Google Scholar]
- 38.el-Sayed MG, el-Sayed MT, Elazab Abd el S, Hefeiz MH, el-Komy AA, Hassan E. Effects of some beta-adrenergic blockers on male fertility parameters in rats. Dtsch Tierarztl Wochenschr. 1998;105:10–2. [PubMed] [Google Scholar]
- 39.Werler MM, Pober BR, Nelson K, Holmes LB. Reporting accuracy among mothers of malformed and nonmalformed infants. Am J Epidemiol. 1989;129:415–21. doi: 10.1093/oxfordjournals.aje.a115145. [DOI] [PubMed] [Google Scholar]
- 40.Paulozzi LJ, Erickson JD, Jackson RJ. Hypospadias trends in two US surveillance systems. Pediatrics. 1997;100:831–4. doi: 10.1542/peds.100.5.831. [DOI] [PubMed] [Google Scholar]


