Abstract
There is growing evidence that over consumption of high-fat foods and insulin resistance may alter hippocampal-dependent cognitive function. To study the individual contributions of diet and peripheral insulin resistance to learning and memory, we used a transgenic mouse line that overexpresses ecto-nucleotide pyrophosphatase phosphodiesterase-1 in adipocytes, which inhibits the insulin receptor. Here, we demonstrate that a model of peripheral insulin resistance exacerbates high-fat diet induced deficits in performance on the Morris Water Maze task. This finding was then reviewed in the context of the greater literature to explore potential mechanisms including triglyceride storage, adiponectin, lipid composition, insulin signaling, oxidative stress, and hippocampal signaling. Together, these findings further our understanding of the complex relationship among peripheral insulin resistance, diet and memory.
1 Introduction
1.1 Importance of work
In humans, cognitive impairment has been shown to be comorbid with obesity and obesity-related complications such as insulin resistance [1]. Obesity is driven in part by over-consumption of diet high in fat [2]. Short-term exposure to high-fat diet has been correlated with decreased cognitive performance in humans [3–5]. In rodent models, cognitive impairment has been associated with both intake of a high-fat diet and insulin resistance [2]. Further elucidating the complex relationship between these factors provides valuable insight into the current obesity epidemic.
1.2 Importance of understanding mechanism
Obesity and obesity-related complications, including insulin resistance, diabetes, and metabolic syndrome, are difficult to disentangle in order to investigate downstream effects such as cognition. By studying obesity-related complications individually, it is possible to identify their contributions to cognitive impairment. While all obesity-related complications are key to understanding the relationship between human obesity and cognitive impairment, this review will focus on the interplay between an obesogenic high-fat diet and peripheral insulin resistance with particular emphasis on their impact on learning and memory.
1.3 Insulin resistance and ENPP1
Ecto-nucleotide pyrophosphatase phosphodiesterase-1 (ENPP1) is an endogenous transmembrane glycoprotein that inhibits insulin receptor signaling. We created a murine model with adipose tissue-specific over-expression of ENPP1 (AtENPP1-Tg). Adipocyte insulin resistance is characteristic in obese patients who also present with insulin resistance, metabolic syndrome and are at risk for developing diabetes [6–8]. The AtENPP1-Tg mouse has been shown to develop systemic insulin resistance independently of obesity and as direct consequence of increased adipocyte insulin resistance [9, 10]. Specifically, AtENPP1-Tg mice on 8 weeks of high-fat diet had a higher plasma glucose curve compared to WT mice on a glucose tolerance test and higher glucose on an insulin tolerance test [10]. This demonstrates that the adipose tissue specific inhibition of insulin receptor in our AtENPP1-Tg mouse line models aspects of human insulin resistance. The AtENPP1-Tg mouse model provides a platform to determine whether adipose tissue insulin resistance is directly responsible for cognitive dysfunction observed in insulin resistance and metabolic syndrome-related conditions such as type 2 diabetes.
2 Animal models
2.1 Methodological variability
The relationship between obesity and cognitive deficits is a rapidly growing area of research. This has resulted in the use of multiple animal models, and perhaps more importantly, lack of consensus on the use of a specific animal model. As both models of obesity and models of learning and memory have significant subtleties [11, 12], comparing results across animal strain, diet, and behavioral assay is difficult. Previous reviews examining these factors [11, 12] complement our focus on mechanisms that underlie the effects of high-fat diet and insulin resistance on behavior.
2.2 Diets that influence learning and memory
There is a rich field of studies investigating the effects of diet on learning and memory in humans and rodent models. In humans, a diet high in fat and high in sugar decreases performance on hippocampal-dependent memory tasks [13]. Diets high in fat have been associated with decreased performance on numerous cognitive tests [14] and increased risk for Alzheimer’s Disease [15]. Higher dietary saturated fatty acids in particular are associated with poor cognitive performance [16, 17]. In rodents, consumption of a diet containing a high percentage of fat (by kcal) negatively impacts performance on a variety of learning and memory tasks. Specifically, consumption of a 60% fat diet, but not a 41% fat diet, decreased memory retention assessed with a 14-Unit T-maze [18]. A 60% fat diet has also been shown to decrease spatial memory performance on Morris Water Maze [19–21]. Rodents maintained on a 45% fat diet have no difference in performance on Morris Water Maze, but do have deficits in procedural learning tested with operant training [22], novel object recognition [23], and the contextual memory based two-way active avoidance test [24]. Taken together, these studies suggest that a diet high in fat impairs human cognition, which is successfully modeled in rodents.
2.3 Insulin resistance influences learning and memory
As animals maintained on a high-fat diet develop peripheral insulin resistance [25, 26], insulin resistance itself has been studied for its effect on learning and memory. Diet-induced insulin resistance is thought to decrease cognitive performance on tasks [27] including on the Morris Water Maze [28]. However to study the interplay between diet and insulin resistance, we tested our AtENPP1-Tg model of peripheral insulin resistance on different diets to determine the effect on cognitive performance measured with the Morris Water Maze.
2.4 AtENPP1-Tg, diet, and learning and memory
2.41 Methods
Adult male and female C57B1/6J mice (n=105, age 3–5 months) were separated into groups based on genetic background (WT or AtENPP1-Tg). Mice were bred by our laboratory and group housed in a 12–12 light-dark cycle. Using a two by two design, mice were divided and given ad libitum access to either 17% fat regular chow (RC) (Teklad 7912) or 60% fat high-fat diet (HFD) (Research Diets D12492). Specifically, the groups were allocated as follows: RC WT 12 female and 14 male, RC AtENPP1-Tg 12 female and 12 male, HFD WT 13 female and 13 male, HFD AtENPP1-Tg 14 female and 15 male. The macronutrient profile for the RC diet was 17% fat, 58% carbohydrates, and 25% protein by energy while the HFD was 60% fat, 20% carbohydrates, and 20% protein. Animal use was completed in accordance with the Guide for the Care and Use of Laboratory Animals and with the approval of the University of Texas Medical Branch Institutional Animal Care and Use Committee. All behavior tests were conducted during the light cycle.
After 10 weeks, mice were weighed and HFD mice (WT 41.7 ± 1 and AtENPP1-Tg 39.3 ± 1 g) weighed significantly more than their RC counterparts (WT 27.8 ± 1 and AtENPP1-Tg 26.8 ± 1 g) regardless of genetic background (p < 0.05 each). This is consistent with previous studies where WT and AtENPP1-Tg mice did not have different body weights [29]. Hippocampal-dependent learning and memory were assessed using the Morris water maze protocol described previously [30]. Briefly, mice were trained on the Morris water maze task to find a visible platform in a pool of water four times a day for three consecutive days. Each individual trial allowed up to one minute to freely find the platform. Mouse performance was recorded to determine time to platform, swimming velocity, and mouse path to platform. These variables were averaged across the four trials each day for statistical analysis [30]. Significant differences were determined with IBM SPSS Statistics 20 software using repeated measures ANOVA with Tukey’s multiple comparisons test for post hoc analysis. Main effects included repeated days, diet, genetic background and sex.
2.42 Learning Morris Water Maze task
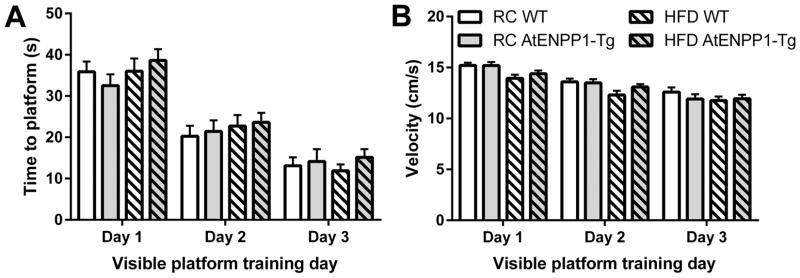
Visible platform performance tests data was averaged across the four sessions each day and the average of the individual mouse was used in data presentation and statistical analysis. During acquisition of the task, there was a significant main effect of repeated days on both latency to visible platform (F (2, 200) = 109, p < 0.01) and velocity (F (2, 200) = 71.1, p < 0.01) (Fig. 1), suggesting that the mice learned the task [31]. However, there were no significant main effects of genetic background or diet on the visible platform task, indicating that all groups acquired the Morris water maze task (e.g. no deficits in vision, mobility, or learning).
Fig. 1.
Acquisition of Morris water maze visible platform task. Average time to platform (A) and velocity (B) over repeated days is not different between groups. Values are mean ± SEM to find platform in 60 second trials.
2.43 Memory assayed by probe trial
Mice were then challenged to find a hidden platform in a goal quadrant four times a day for three consecutive days. On the following day, a probe trial was conducted where the hidden platform was removed and time spent in the goal quadrant was measured. Probe trial performance is commonly used to test rodent memory function [31]. The next day, mice were allowed to find a hidden platform and the cycle repeated to achieve three probe trial days. There was no significant main effect of sex (F (1, 102) = 0.02, p = 0.98) so mice were collapsed across sex for further analysis. Additionally, no significant main effects were observed on time to hidden platform, regardless of whether measured before the probe trials (when acquiring the task) or between the probe trials (reacquiring the task).
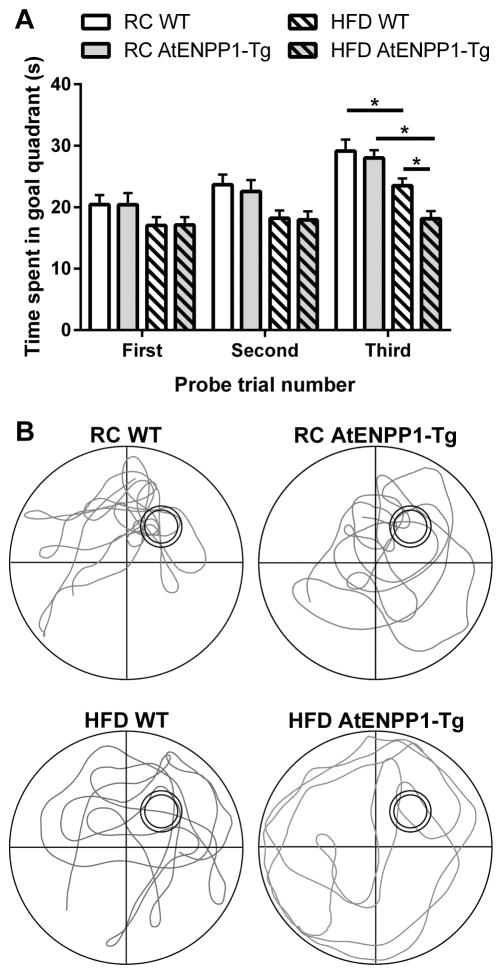
Significant differences in each probe trial (Fig. 2) were determined using two-way ANOVA with Tukey’s multiple comparisons test for post hoc analysis. The first probe trial had a significant main effect of diet (F (1, 100) = 4.64, p < 0.05) but no main effect of genetic background or interaction. Similarly, the second probe trial also had a significant main effect of diet (F (1, 100) = 10.85, p < 0.01) and no main effect of genetic background or interaction. The third probe trial had significant main effects of diet (F (1, 100) = 29.79, p < 0.01), genetic background (F (1, 100) = 5.24, p < 0.05), and main interaction (F (1, 100) = 4.24, p < 0.05). While there was no difference between WT and AtENPP1-Tg mice consuming a RC diet, mice consuming HFD spent significantly less time in the goal quadrant (RC WT vs HFD WT, p < 0.05). When fed a HFD, AtENPP1-Tg mice demonstrated decreased performance compared to WT (HFD WT vs HFD AtENPP1-Tg, p < 0.05).
Fig. 2.
AtENPP1-Tg exacerbates HFD induced deficits in Morris water maze probe trial performance. (A) Mean ± SEM time in goal quadrant during probe trial. * indicates p < 0.05. (B) Representative traces of mouse locomotion during the third probe trial. Double circle represents the trained location of the platform in the goal quadrant, which was removed for probe trial testing.
2.44 Peripheral insulin resistance and high-fat diet conclusions
Overall, these data suggest that the AtENPP1-Tg model of insulin resistance exacerbates hippocampal-dependent memory impairments caused by high-fat diet consumption. No effect of treatment was observed during acquisition of the hidden platform water maze task or reacquisition following probe trials, suggesting that the time to hidden platform task is less sensitive than the probe trials to our treatments. With this behavioral knowledge, we are now able to give greater context to previous work exploring possible underlying mechanisms, specifically those involved in high-fat diet and insulin resistance models.
3 Potential mechanisms
3.1 Triglyceride storage
In humans with insulin resistance, triglyceride storage is dysregulated and maintenance on a high-fat diet diverts triglyceride storage to areas other than adipose tissue [9]. One such area may be the hippocampus, where an excess of triglycerides contribute to cognitive impairment. Indeed, high-fat diet suppressed post-synaptic responses in the hippocampus in mice, and high-fat diet combined with overexpression of ENPP1 exerted a cumulative extinguishing effect on hippocampal synapses [9]. Another study demonstrated that intraventricular injection of the triglyceride triolein, but not palmitate, impaired performance on hippocampal-dependent memory tasks in mice [32]. Additionally, when hippocampal slices were exposed to triolein, long-term potentiation was impaired, emphasizing the negative impact of aberrant triglyceride storage on learning and memory.
3.2 Adiponectin
Adiponectin is an adipokine secreted by adipose tissue that regulates metabolic homeostasis and inflammation. In the context of obesity, plasma concentration of adiponectin is decreased despite increased adipose tissue mass [33]. In humans, plasma adiponectin level is negatively correlated with ENPP1 expression. However, this relationship does not exist with other adipo-cytokines such as leptin, interleukin-6, or tumor necrosis factor alpha [9]. Additionally, long-term exposure to a high-fat diet has been associated with decreased expression of adiponectin in both adipose tissue and serum [34]. High-fat diet inhibits adiponectin secretion through adipocyte dysregulation and increased expression of tumor necrosis factor alpha [35]. Because adiponectin promotes an anti-inflammatory state, a decrease in adiponectin, such as that observed with high-fat diet, creates a cellular environment amenable to increased concentration of pro-inflammatory cytokines, especially interleukin-1 (IL-1). IL-1 is of particular interest due to the enrichment of its receptor in the hippocampus [36], and neuroinflammation is known to alter cognition and synaptic plasticity [37]. Exogenous administration of IL-1 and endogenous release of IL-1 impaired long-term potentiation in the CA1 hippocampal region [38]. Overall, an obesogenic diet may contribute to impaired learning and memory through decreased adiponectin, and increased pro-inflammatory cytokines.
3.3 Lipid composition
Consumption of a high-fat diet causes dyslipidemia, which alters lipid composition in neurons [39]. Lipid composition in neuronal membranes is critical for basic physiological functions such as signaling and trafficking. In the hippocampus, changes in synaptic lipid composition in synapses may influence cognition. Triglyceride content in hippocampal synaptosomes was significantly increased in AtENPP1-Tg mice exposed to a 60% high-fat diet for 12 weeks, and phospholipid content in hippocampal synaptosomes was significantly decreased [29]. These findings were shown to cause systemic insulin resistance [10]. As such, elevated triglycerides in hippocampal synapses can impair synaptic transmission and cause deficits in learning and memory [29].
3.4 Insulin signaling
Insulin resistance is a major obesity-related complication. Insulin receptor signaling is critical for proper glucoregulation and is involved in the pathogenesis of type 2 diabetes. Long-term consumption of a high-fat diet interrupts insulin signaling and leads to weight gain, impaired glucose handling, and insulin resistance [22]. In addition to its peripheral effects, high-fat diet also contributes to central insulin resistance [40]. Hippocampal insulin resistance leads to cognitive impairment and is the result of inadequate insulin receptor signaling, as well as impaired insulin transport across the blood brain barrier [41]. Indeed, high-fat diet has been shown to block the ability of insulin to induce long-term depression in the CA1 hippocampus [42]. In AtENPP1-Tg mice maintained on a high-fat diet, both insulin receptor alpha and beta subunits were downregulated in the hippocampus [29]. However, loss of neural insulin receptor in a knockout mouse model has no effect on hippocampal-dependent memory as measured with Morris Water Maze, suggesting that insulin resistance alone is not sufficient to cause deficits in learning and memory [43].
3.5 Oxidative stress
Long-term consumption of a high-fat diet results in increased oxidation of free fatty acids [44–46]. Three weeks of maintenance on a 60% fat diet is sufficient to increase circulating free fatty acids, specifically polyunsaturated fatty acids [47]. Because polyunsaturated fatty acids possess reactive hydrogen atoms, they are especially vulnerable to peroxidation by reactive oxygen species (ROS). Lipid peroxidation results in the production of new fatty acid radicals that are self-perpetuating [48]. High-fat diet also increases neural ROS production and expression of COX-2, a marker of neuroinflammation [49]. Long-term consumption of a 60% fat diet, but not a 41% fat diet, increased hippocampal protein carbonyls, further indicating oxidative damage [18]. Additionally, fluctuating glucose concentrations resulting from insulin resistance induce oxidative stress [50]. Persistent oxidative stress in the hippocampus results in decreased neurogenesis, and reduced dendritic arborization in existing neurons which leads to impaired cognition [51]. Together, both high-fat diet and insulin resistance induce neurological oxidative stress, which in the hippocampus leads to deficits in learning and memory.
3.6 Hippocampal signaling and synaptic transmission
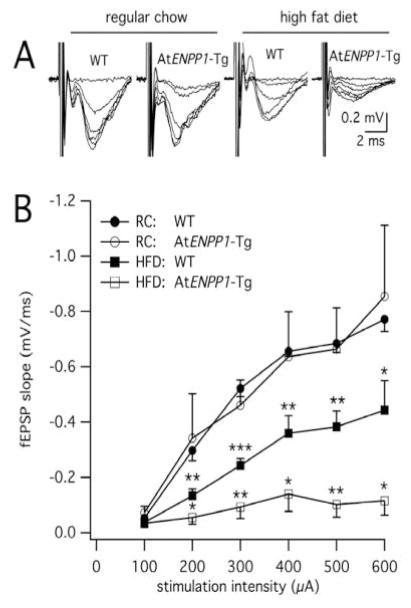
New memories are formed through synaptic adaptations in the hippocampal circuit in response to episodic learning-associated stimuli [52]. The CA1 area plays a fundamental role in memory consolidation and storage serving as the last integrative station in the hippocampal trisynaptic circuit. At the cellular level, memory formation occurs through complex structure-function modifications of synapses in the CA1 region that encompass formation of new spines [53, 54], increase in synaptic strength and modification of post-synaptic receptors and signaling pathways [55] that culminate in modified behavior [56, 57]. Changes in the ability of neurons to respond and adapt to synaptic stimuli are a sign of disrupted circuitry, which lead to memory deficits that in rodents are manifested by reduced performance on Morris Water Maze task [58]. Recent findings showed that AtENPP1-Tg mice maintained on a high-fat diet exhibit decreased synaptic transmission in the hippocampal CA1 area compared to wild type controls [29]. Electrophysiological field recordings of synaptic responses in the CA1 region of WT and AtENPP1-Tg mice (n=4–5 per group) under normal and high-fat diet revealed a significant suppression of basal synaptic function in the CA1 hippocampal region induced by the transgene and the diet (Fig. 3). These striking results correlate with altered lipid profiling, and aberrant composition of glutamate receptors in these animals [29] that might predispose synapses to altered plasticity and consequently behavioral deficits in CA1-associated memory formation (Fig. 2). Furthermore, a high-fat diet also decreases GABA levels in the hippocampus, consequently decreasing inhibitory tone which, in addition to affecting memory formation [59], may increase food intake and perpetuate obesity [60]. Indeed, a theory has been advanced proposing a feedforward cycle of hippocampal dysfunction promotes over-consumption of energy dense food that causes further hippocampal dysfunction [61]. This decrease in GABA inhibitory signaling seems to contradict our findings of decreased synaptic function (Fig. 3). However, a high-fat diet also decreases glutamatergic excitatory signaling as well [62]; which suggests that the sum of these two effects is the net inhibition presented here. Additionally, high-fat diet has been shown to impair neurogenesis in the hippocampus [63] and the effect of AtENPP1-Tg on neurogenesis remains an ongoing research interest. The data presented here suggest there are behavioral consequences to the loss of hippocampal CA1 basal synaptic transmission with a diet high in fat and peripheral insulin resistance.
Fig. 3.
Weakened hippocampal signaling in At-ENPP1-Tg mice fed a HFD. (A) CA1 extracellular field recordings show depressed signaling in WT mice fed a HFD, which was further augmented in At-ENPP1-Tg mice fed a HFD. Averages of these recordings are represented in (B) at increasing stimulation intensities. * indicates p<0.05, **p<0.01, ***p<0.001 relative to RC WT. Reproduced from Sallam [29] with permission from John Wiley and Sons.
4 Conclusions
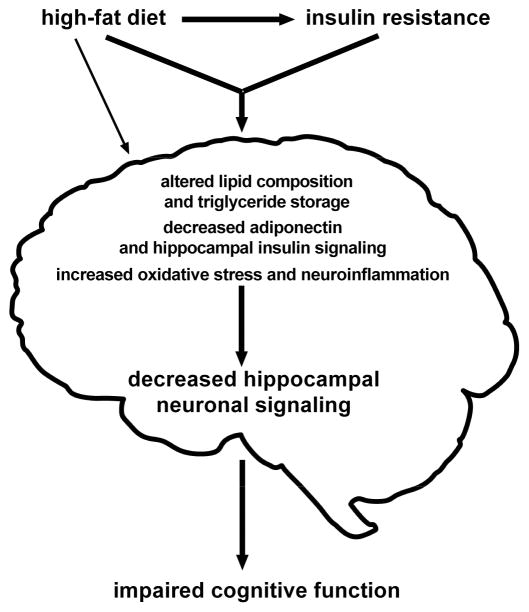
Animal models of obesity and its related complications demonstrate translational validity and mirror comparable human studies. Using the AtENPP1-Tg model in combination with a high-fat diet, we were able to study mechanistic changes that culminate in decreased spatial memory (Fig. 4). These insights compliment and extend the obesity literature by disentangling peripheral insulin resistance and diet in order to study them independently and jointly (e.g. diet induced insulin resistance models). In sum, there is strong evidence that both dietary fat and insulin resistance contribute to cognitive deficits through a variety of mechanisms that all together constitute peripherally-driven CNS deficits in the neurometabolic syndrome.
Fig. 4.
Graphical depiction of proposed mechanisms that mediate diet and insulin resistance-induced deficits in cognition.
Mice exposed to HFD showed reduced performance on Morris Water Maze.
Peripheral insulin resistance exacerbates HFD induced cognitive deficits.
Mechanisms converge on decreased hippocampal signaling, which alters memory.
Acknowledgments
We thank the Rodent In Vivo Assessment core at the University of Texas Medical Branch for sharing their behavioral expertise and Reyna Collura for graphical design assistance. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK106229, P30DK079638), National Institute on Drug Abuse (T32DA07287), Clinical and Translational Science Award (UL1TR001439 and KL2TR001441) and from the National Center for Advancing Translational Science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes Relat Metab Disord. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 2.Bray GA, Paeratakul S, Popkin BM. Dietary fat and obesity: a review of animal, clinical and epidemiological studies. Physiol Behav. 2004;83:549–555. doi: 10.1016/j.physbeh.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 3.Holloway CJ, Cochlin LE, Emmanuel Y, Murray A, Codreanu I, Edwards LM, Szmigielski C, Tyler DJ, Knight NS, Saxby BK, Lambert B, Thompson C, Neubauer S, Clarke K. A high-fat diet impairs cardiac high-energy phosphate metabolism and cognitive function in healthy human subjects. Am J Clin Nutr. 2011;93:748–755. doi: 10.3945/ajcn.110.002758. [DOI] [PubMed] [Google Scholar]
- 4.Edwards LM, Murray AJ, Holloway CJ, Carter EE, Kemp GJ, Codreanu I, Brooker H, Tyler DJ, Robbins PA, Clarke K. Short-term consumption of a high-fat diet impairs whole-body efficiency and cognitive function in sedentary men. FASEB J. 2011;25:1088–1096. doi: 10.1096/fj.10-171983. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Mckeown RE, Muldoon MF, Tang S. Cognitive performance is associated with macronutrient intake in healthy young and middle-aged adults. Nutr Neurosci. 2006;9:179–187. doi: 10.1080/10284150600955172. [DOI] [PubMed] [Google Scholar]
- 6.Maddux BA, Sbraccia P, Kumakura S, Sasson S, Youngren J, Fisher A, Spencer S, Grupe A, Henzel W, Stewart TA. Membrane glycoprotein PC-1 and insulin resistance in non-insulin-dependent diabetes mellitus. Nature. 1995;373:448–451. doi: 10.1038/373448a0. [DOI] [PubMed] [Google Scholar]
- 7.Dong H, Maddux BA, Altomonte J, Meseck M, Accili D, Terkeltaub R, Johnson K, Youngren JF, Goldfine ID. Increased hepatic levels of the insulin receptor inhibitor, PC-1/NPP1, induce insulin resistance and glucose intolerance. Diabetes. 2005;54:367–372. doi: 10.2337/diabetes.54.2.367. [DOI] [PubMed] [Google Scholar]
- 8.Frittitta L, Youngren JF, Sbraccia P, D’Adamo M, Buongiorno A, Vigneri R, Goldfine ID, Trischitta V. Increased adipose tissue PC-1 protein content, but not tumour necrosis factor-alpha gene expression, is associated with a reduction of both whole body insulin sensitivity and insulin receptor tyrosine-kinase activity. Diabetologia. 1997;40:282–289. doi: 10.1007/s001250050675. [DOI] [PubMed] [Google Scholar]
- 9.Chandalia M, Davila H, Pan W, Szuszkiewicz M, Tuvdendorj D, Livingston EH, Abate N. Adipose tissue dysfunction in humans: a potential role for the transmembrane protein ENPP1. J Clin Endocrinol Metab. 2012;97:4663–4672. doi: 10.1210/jc.2012-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan W, Ciociola E, Saraf M, Tumurbaatar B, Tuvdendorj D, Prasad S, Chandalia M, Abate N. Metabolic consequences of ENPP1 overexpression in adipose tissue. Am J Physiol Endocrinol Metab. 2011;301:E901–911. doi: 10.1152/ajpendo.00087.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francis H, Stevenson R. The longer-term impacts of Western diet on human cognition and the brain. Appetite. 2013;63:119–128. doi: 10.1016/j.appet.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Freeman LR, Haley-Zitlin V, Rosenberger DS, Granholm AC. Damaging effects of a high-fat diet to the brain and cognition: a review of proposed mechanisms. Nutr Neurosci. 2014;17:241–251. doi: 10.1179/1476830513Y.0000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francis HM, Stevenson RJ. Higher reported saturated fat and refined sugar intake is associated with reduced hippocampal-dependent memory and sensitivity to interoceptive signals. Behav Neurosci. 2011;125:943–955. doi: 10.1037/a0025998. [DOI] [PubMed] [Google Scholar]
- 14.Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Dietary fat intake and 6-year cognitive change in an older biracial community population. Neurology. 2004;62:1573–1579. doi: 10.1212/01.wnl.0000123250.82849.b6. [DOI] [PubMed] [Google Scholar]
- 15.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, Schneider J, Wilson RS. Dietary fats and the risk of incident Alzheimer disease. Arch Neurol. 2003;60:194–200. doi: 10.1001/archneur.60.2.194. [DOI] [PubMed] [Google Scholar]
- 16.Okereke OI, Rosner BA, Kim DH, Kang JH, Cook NR, Manson JE, Buring JE, Willett WC, Grodstein F. Dietary fat types and 4-year cognitive change in community-dwelling older women. Ann Neurol. 2012;72:124–134. doi: 10.1002/ana.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eskelinen MH, Ngandu T, Helkala EL, Tuomilehto J, Nissinen A, Soininen H, Kivipelto M. Fat intake at midlife and cognitive impairment later in life: a population-based CAIDE study. Int J Geriatr Psychiatry. 2008;23:741–747. doi: 10.1002/gps.1969. [DOI] [PubMed] [Google Scholar]
- 18.Morrison CD, Pistell PJ, Ingram DK, Johnson WD, Liu Y, Fernandez-Kim SO, White CL, Purpera MN, Uranga RM, Bruce-Keller AJ, Keller JN. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J Neurochem. 2010;114:1581–1589. doi: 10.1111/j.1471-4159.2010.06865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White CL, Pistell PJ, Purpera MN, Gupta S, Fernandez-Kim SO, Hise TL, Keller JN, Ingram DK, Morrison CD, Bruce-Keller AJ. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: contributions of maternal diet. Neurobiol Dis. 2009;35:3–13. doi: 10.1016/j.nbd.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pipatpiboon N, Pintana H, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. DPP4-inhibitor improves neuronal insulin receptor function, brain mitochondrial function and cognitive function in rats with insulin resistance induced by high-fat diet consumption. Eur J Neurosci. 2013;37:839–849. doi: 10.1111/ejn.12088. [DOI] [PubMed] [Google Scholar]
- 21.Jeon BT, Jeong EA, Shin HJ, Lee Y, Lee DH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes. 2012;61:1444–1454. doi: 10.2337/db11-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mielke JG, Nicolitch K, Avellaneda V, Earlam K, Ahuja T, Mealing G, Messier C. Longitudinal study of the effects of a high-fat diet on glucose regulation, hippocampal function, and cerebral insulin sensitivity in C57BL/6 mice. Behav Brain Res. 2006;175:374–382. doi: 10.1016/j.bbr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Kim HG, Jeong HU, Park G, Kim H, Lim Y, Oh MS. Mori folium and mori fructus mixture attenuates high-fat diet-induced cognitive deficits in mice. Evid Based Complement Alternat Med. 2015;2015:379418. doi: 10.1155/2015/379418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noble EE, Mavanji V, Little MR, Billington CJ, Kotz CM, Wang C. Exercise reduces diet-induced cognitive decline and increases hippocampal brain-derived neurotrophic factor in CA3 neurons. Neurobiol Learn Mem. 2014;114:40–50. doi: 10.1016/j.nlm.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pratchayasakul W, Kerdphoo S, Petsophonsakul P, Pongchaidecha A, Chattipakorn N, Chattipakorn SC. Effects of high-fat diet on insulin receptor function in rat hippocampus and the level of neuronal corticosterone. Life Sci. 2011;88:619–627. doi: 10.1016/j.lfs.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Manco M, Bertuzzi A, Salinari S, Scarfone A, Calvani M, Greco AV, Mingrone G. The ingestion of saturated fatty acid triacylglycerols acutely affects insulin secretion and insulin sensitivity in human subjects. Br J Nutr. 2004;92:895–903. doi: 10.1079/bjn20041268. [DOI] [PubMed] [Google Scholar]
- 27.McNeilly AD, Williamson R, Sutherland C, Balfour DJ, Stewart CA. High fat feeding promotes simultaneous decline in insulin sensitivity and cognitive performance in a delayed matching and non-matching to position task. Behav Brain Res. 2011;217:134–141. doi: 10.1016/j.bbr.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sallam HS, Tumurbaatar B, Zhang WR, Tuvdendorj D, Chandalia M, Tempia F, Laezza F, Taglialatela G, Abate N. Peripheral adipose tissue insulin resistance alters lipid composition and function of hippocampal synapses. J Neurochem. 2015;133:125–133. doi: 10.1111/jnc.13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dineley KT, Westerman M, Bui D, Bell K, Ashe KH, Sweatt JD. Beta-amyloid activates the mitogen-activated protein kinase cascade via hippocampal alpha7 nicotinic acetylcholine receptors: In vitro and in vivo mechanisms related to Alzheimer’s disease. J Neurosci. 2001;21:4125–4133. doi: 10.1523/JNEUROSCI.21-12-04125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cordner ZA, Tamashiro KL. Effects of high-fat diet exposure on learning & memory. Physiol Behav. 2015;152:363–371. doi: 10.1016/j.physbeh.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farr SA, Yamada KA, Butterfield DA, Abdul HM, Xu L, Miller NE, Banks WA, Morley JE. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008;149:2628–2636. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Turdi S, Park T, Morris NJ, Deshaies Y, Xu A, Sweeney G. Adiponectin corrects high-fat diet-induced disturbances in muscle metabolomic profile and whole-body glucose homeostasis. Diabetes. 2013;62:743–752. doi: 10.2337/db12-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnea M, Shamay A, Stark AH, Madar Z. A high-fat diet has a tissue-specific effect on adiponectin and related enzyme expression. Obesity (Silver Spring) 2006;14:2145–2153. doi: 10.1038/oby.2006.251. [DOI] [PubMed] [Google Scholar]
- 35.Hossain MM, Mukheem A, Kamarul T. The prevention and treatment of hypoadiponectinemia-associated human diseases by up-regulation of plasma adiponectin. Life Sci. 2015;135:55–67. doi: 10.1016/j.lfs.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Parnet P, Kelley KW, Bluthé RM, Dantzer R. Expression and regulation of interleukin-1 receptors in the brain. Role in cytokines-induced sickness behavior. J Neuroimmunol. 2002;125:5–14. doi: 10.1016/s0165-5728(02)00022-x. [DOI] [PubMed] [Google Scholar]
- 37.Hwang LL, Wang CH, Li TL, Chang SD, Lin LC, Chen CP, Chen CT, Liang KC, Ho IK, Yang WS, Chiou LC. Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity (Silver Spring) 2010;18:463–469. doi: 10.1038/oby.2009.273. [DOI] [PubMed] [Google Scholar]
- 38.Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18:2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nam M, Choi MS, Jung S, Jung Y, Choi JY, Ryu dH, Hwang GS. Lipidomic Profiling of Liver Tissue from Obesity-Prone and Obesity-Resistant Mice Fed a High Fat Diet. Sci Rep. 2015;5:16984. doi: 10.1038/srep16984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biessels GJ, Reagan LP. Hippocampal insulin resistance and cognitive dysfunction. Nat Rev Neurosci. 2015;16:660–671. doi: 10.1038/nrn4019. [DOI] [PubMed] [Google Scholar]
- 41.Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012;136:82–93. doi: 10.1016/j.pharmthera.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pipatpiboon N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. PPARγ agonist improves neuronal insulin receptor function in hippocampus and brain mitochondria function in rats with insulin resistance induced by long term high-fat diets. Endocrinology. 2012;153:329–338. doi: 10.1210/en.2011-1502. [DOI] [PubMed] [Google Scholar]
- 43.Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, Kondo T, Alber J, Galldiks N, Küstermann E, Arndt S, Jacobs AH, Krone W, Kahn CR, Brüning JC. Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci U S A. 2004;101:3100–3105. doi: 10.1073/pnas.0308724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 45.Unger RH. Lipid overload and overflow: metabolic trauma and the metabolic syndrome. Trends Endocrinol Metab. 2003;14:398–403. doi: 10.1016/j.tem.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Chinen I, Shimabukuro M, Yamakawa K, Higa N, Matsuzaki T, Noguchi K, Ueda S, Sakanashi M, Takasu N. Vascular lipotoxicity: endothelial dysfunction via fatty-acid-induced reactive oxygen species overproduction in obese Zucker diabetic fatty rats. Endocrinology. 2007;148:160–165. doi: 10.1210/en.2006-1132. [DOI] [PubMed] [Google Scholar]
- 47.Liu TW, Heden TD, Matthew Morris E, Fritsche KL, Vieira-Potter VJ, Thyfault JP. High-Fat Diet Alters Serum Fatty Acid Profiles in Obesity Prone Rats: Implications for In Vitro Studies. Lipids. 2015;50:997–1008. doi: 10.1007/s11745-015-4061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nowak JZ. Oxidative stress, polyunsaturated fatty acids-derived oxidation products and bisretinoids as potential inducers of CNS diseases: focus on age-related macular degeneration. Pharmacol Rep. 2013;65:288–304. doi: 10.1016/s1734-1140(13)71005-3. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X, Dong F, Ren J, Driscoll MJ, Culver B. High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp Neurol. 2005;191:318–325. doi: 10.1016/j.expneurol.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 50.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 51.Huang TT, Leu D, Zou Y. Oxidative stress and redox regulation on hippocampal-dependent cognitive functions. Arch Biochem Biophys. 2015;576:2–7. doi: 10.1016/j.abb.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Basu J, Siegelbaum SA. The Corticohippocampal Circuit, Synaptic Plasticity, and Memory. Cold Spring Harb Perspect Biol. 2015:7. doi: 10.1101/cshperspect.a021733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newpher TM, Ehlers MD. Spine microdomains for postsynaptic signaling and plasticity. Trends Cell Biol. 2009;19:218–227. doi: 10.1016/j.tcb.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 54.De Roo M, Klauser P, Garcia PM, Poglia L, Muller D. Spine dynamics and synapse remodeling during LTP and memory processes. Prog Brain Res. 2008;169:199–207. doi: 10.1016/S0079-6123(07)00011-8. [DOI] [PubMed] [Google Scholar]
- 55.Hell JW. CaMKII: claiming center stage in postsynaptic function and organization. Neuron. 2014;81:249–265. doi: 10.1016/j.neuron.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kheirbek MA, Tannenholz L, Hen R. NR2B-dependent plasticity of adult-born granule cells is necessary for context discrimination. J Neurosci. 2012;32:8696–8702. doi: 10.1523/JNEUROSCI.1692-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu X, Ramirez S, Tonegawa S. Inception of a false memory by optogenetic manipulation of a hippocampal memory engram. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130142. doi: 10.1098/rstb.2013.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 59.McQuail JA, Frazier CJ, Bizon JL. Molecular aspects of age-related cognitive decline: the role of GABA signaling. Trends Mol Med. 2015;21:450–460. doi: 10.1016/j.molmed.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandoval-Salazar C, Ramírez-Emiliano J, Trejo-Bahena A, Oviedo-Solís CI, Solís-Ortiz MS. A high-fat diet decreases GABA concentration in the frontal cortex and hippocampus of rats. Biol Res. 2016;49:15. doi: 10.1186/s40659-016-0075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hargrave SL, Jones S, Davidson TL. The Outward Spiral: A vicious cycle model of obesity and cognitive dysfunction. Curr Opin Behav Sci. 2016;9:40–46. doi: 10.1016/j.cobeha.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valladolid-Acebes I, Merino B, Principato A, Fole A, Barbas C, Lorenzo MP, García A, Del Olmo N, Ruiz-Gayo M, Cano V. High-fat diets induce changes in hippocampal glutamate metabolism and neurotransmission. Am J Physiol Endocrinol Metab. 2012;302:E396–402. doi: 10.1152/ajpendo.00343.2011. [DOI] [PubMed] [Google Scholar]
- 63.Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P, Erlanson-Albertsson C. High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol. 2006;13:1385–1388. doi: 10.1111/j.1468-1331.2006.01500.x. [DOI] [PubMed] [Google Scholar]