Abstract
Background
Age-related loss of muscle mass and function (sarcopenia) is linked to poor outcomes after surgery and trauma. Here we evaluate CT measured psoas muscle density and area using quick and simple tools available to the beside clinician. We hypothesize these measures will predict poor outcomes after blunt traumatic injury.
Methods
We conducted a retrospective cohort study of patients ages ≥ 45 years in the Ohio State University Trauma Registry in 2008 that received a CT abdomen/pelvis with intravenous contrast. Psoas Index (PI) and Hounsfield Unit average calculation (HUAC) were measured at the L3 level. 90-day mortality, complication, length of stay ≥ 7 days, dependent discharge were compared to PI and HUAC.
Results
151 patients met inclusion criteria. Patients were stratified into interquartile ranges based either on PI or HUAC values. After adjustment with sex-specific cutoffs, the lowest interquartile range of PI was associated with 90-day mortality (RR 5.95, p < 0.008) but did not reach significance in other outcomes. The lowest interquartile range of HUAC was associated with 90-day mortality (RR 5.95, p < 0.008) length of stay ≥ 7 days (RR 1.63, p = 0.048), complication risk (RR 2.30, p = 0.002), and dependent discharge 2.14, p = 0.015).
Conclusion
Psoas muscle density is a significant predictor of poor outcomes after traumatic injury. This objective, quick, and readily available measure of sarcopenia can identify patients requiring aggressive nutritional and physical therapy to improve prognosis, prevent recurrent traumatic injury, and aid in discharge planning.
Keywords: Sarcopenia, Frailty, Trauma
Introduction
Sarcopenia is marked by age-associated reduction of lean muscle, mass, composition, or contractility, leading to decreased muscle size and function(1, 2). The pathologic manifestation of sarcopenia, known as the frailty syndrome describes the general decline in physiologic reserve associated with loss of muscle mass and function that leaves the patient recursively vulnerable to disease and wasting(3, 4). Previous studies have shown that frailty is strongly associated with poor surgical and post-traumatic outcomes(4–6). However, an objective assessment of physiologic reserve to measure frailty has not yet been standardized. Many approaches rely on combining data from the patient’s past medical history with functional and mental status assessments to create a composite risk score(4–7). Unfortunately, these measures are time-consuming, reliant on the completeness of the medical record and quality of subjective data that are often not available in the emergent traumatic or surgical setting.
Thus, several groups have proposed using direct imaging measures of sarcopenia as an objective and easy to measure marker of physiologic reserve(8–13). Several studies have validated the computed tomography (CT) psoas muscle measurements at the L3 vertebral level as a predictor of poor outcomes in multiple settings including liver transplantation, and oncologic surgery(8–13). In addition, recent studies have found that CT psoas density, which focuses on measuring muscle quality and degree of age-related fatty muscular infiltration rather than gross mass, can be used to predict mortality in gastrointestinal oncologic surgery(10, 12).
Like major surgery, traumatic injury represents a significant physiologic insult which frail patients have a limited capacity to recover from. Thus, evaluating frailty in the emergent traumatic setting would be a valuable tool in identifying patients at increased risk of poor outcomes that may benefit from specific physical and nutritional regimens to mitigate risk, and improve physiologic reserve from future traumatic events. CT psoas based measurements of sarcopenia in trauma are attractive due to the ubiquitous use of CT scans in evaluating traumatic injuries, and the simplicity and speed of measurement. The objective of this study is to evaluate CT based psoas muscle area and density as prognostic markers for poor outcomes in blunt traumatic injury.
Methods
Data source and patient characteristics
We reviewed the Ohio State University Trauma Registry that found patients ages 45 and older with blunt mechanism of trauma that underwent evaluation between January 1st, 2008 and December 31st 2008 with CT abdomen/pelvis with venous contrast during initial trauma evaluation. For each patient record, detailed clinical variables were collected including age, sex, Body Mass Index (BMI), independent status before trauma, mechanism of trauma, presenting Glasgow Coma Scale (GCS) Score, Injury Severity Score (ISS), number of injuries sustained, number of medications, and number of comorbidities. Outcome data collected included 90-day mortality (from the Social Security Death Index), risk of complication, discharge to a dependent facility (long-term acute care hospital or skilled nursing facility), and length of stay ≥ 7 days.
CT image analysis and sarcopenia calculation
The right and left psoas muscles were traced at the L3 level with EasyViz® PACS (Picture Archiving and Communications System) imaging software (Karos Health, Waterloo ON, Figure 1). EasyViz® is an “ultra-thin” web-based PACS client installed enterprise-wide that is easily accessible to bedside clinicians. The psoas index (PI) was measured by summing the right psoas muscle area (RPA) and left psoas area (LPA), to obtain the total psoas area (TPA) then normalizing by the square of the height: PI = (RPA + LPA)/height2 in cm2/m2(13). Psoas density was measured as the Hounsfield Unit average calculation, and was determined by summing the product of the right mean psoas Hounsfield Unit density (RPHU) and RPA, with that of the product of the left mean psoas Hounsfield Unit density (LPHU) and LPA, then dividing by the TPA: HUAC = [(RPHU × RPA) + (LPHU × LPA)]/(TPA) in Hounsfield Units(10, 12). Patients were stratified based on the interquartile range (IQR) for our measures of sarcopenia into 4 groups where IQR1 corresponded to the lowest 25th percentile of values to IQR4 which corresponded to the highest 25th percentile of values. To adjust for sex-specific differences in psoas area, we separated our patients into quartiles with sex specific cutoffs, thereby defining the lowest 25th percentile of men and the lowest 25th percentile of women as sarcopenic. Our sarcopenia cutoff for HUAC was the lowest 25th percentile value (HUAC value of 38.5 HU).
Figure 1. CT Psoas Muscle Area and Density Measurement.
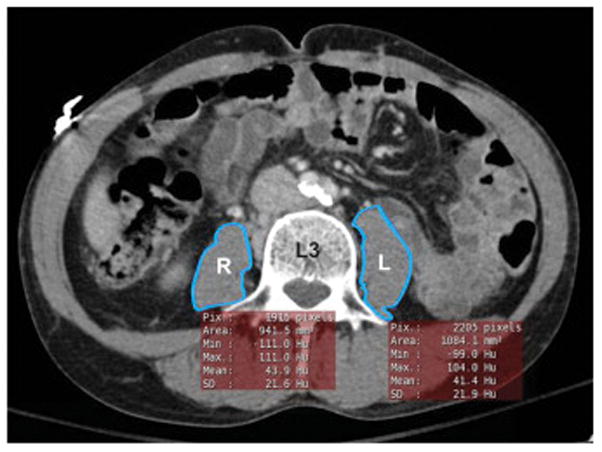
A representative CT venous phase abdomen pelvis with intravenous contrast. Quick tracing of the right (R) and left (L) psoas muscle at the L3 level using EasyViz® automatically generates area and mean density measurements.
Statistical Analysis
There was no missing data for any patients. Variables were compared using Student’s T test, Fischer’s Exact test, χ2 test, and Pearson correlation using STATA version 14.2 (StataCorp, College Station, TX). Statistical significance was defined by α = 0.05. The study was approved by the Ohio State University Institutional Review Board (2016E0305).
Results
Baseline patient characteristics
We stratified patients into interquartile ranges based on either their psoas area or psoas density (Supplementary Table 1). Our muscle density cutoff for sarcopenia was defined as the 25th percentile value of HUAC (sarcopenia by HUAC ≤ 38.5 HU). Given the significant sex-specific difference in PI, our muscle size specific cutoff was defined as the 25th percentile value of PI by sex (sarcopenia by PI, male ≤ 7.77 cm2/m2, female ≤ 4.75 cm2/m2). We categorized complications by a set list (Supplementary Table 2). 151 patients met our study criteria. Patient characteristics (Table 1) included mean age 58.8 years, 60.9% male, BMI 28.3, home independence before trauma 89.4%, mean ISS 10.4, GCS 13.4, Mechanism of fall 22.5%, number of injuries from trauma of 4.7, mean number of home medications 3.7, and pre-trauma comorbidities 2.9.
Table 1.
Characteristics of the Trauma Cohort by Psoas Density or Psoas Area
| Total Cohort n = 151 |
Non-Sarcopenic by Psoas Density n = 113 |
Sarcopenic by Psoas Density n = 38 |
p – value | Non-Sarcopenic by Psoas Area n = 113 |
Sarcopenic by Psoas Area n = 38 |
p - value | |
|---|---|---|---|---|---|---|---|
| Age (years) | 58.8 (11.3) | 56.5 (9.5) | 65.6 (13.2) | < 0.001* | 57.6 (10.8) | 52.2 (11.7) | 0.03* |
| Sex (# Male) | 92 (61.0%) | 72 (63.7%) | 20 (52.6%) | 0.23 | 69 (61.0%) | 23 (60.5%) | 0.95 |
| BMI (kg/m2) | 28.3 (6.9) | 27.6 (4.7) | 30.4 (10.8) | 0.03* | 29.1 (7.1) | 26.0 (5.3) | 0.02* |
| # Home Independence | 135 (89.4%) | 104 (92.0%) | 31 (81.6%) | 0.07 | 102 (86.4%) | 33 (86.8%) | 0.56 |
| Initial GCS | 13.4 (3.7) | 13.6 (3.4) | 12.6 (4.4) | 0.14 | 13.4 (3.7) | 13.4 (3.8) | 0.96 |
| Initial ISS | 10.4 (7.6) | 10.0 (7.1) | 11.4 (8.9) | 0.33 | 10.9 (7.5) | 8.6 (7.8) | 0.10 |
| Mechanism Fall | 34 (22.5%) | 21 (17.8%) | 13 (34.2%) | 0.05* | 25 (21.2%) | 9 (23.6%) | 0.84 |
| # Injuries | 4.7 (3.5) | 4.6 (3.3) | 4.9 (4.0) | 0.42 | 4.8 (3.5) | 4.3 (3.7) | 0.46 |
| # Medications | 3.7 (4.0) | 3.2 (4.9) | 5.2 (4.1) | 0.01* | 3.7 (3.8) | 3.7 (4.5) | 0.98 |
| # Comorbidities | 2.9 (2.4) | 2.3 (2.2) | 4.6 (2.3) | < 0.001* | 2.8 (2.3) | 3.1 (2.9) | 0.50 |
BMI; Body Mass Index, GCS (Glasgow Coma Scale); ISS (Injury Severity Score), values are means or total number in group, values in parentheses are standard deviation, or percentage of total group,
marks significance α = 0.05 level
Psoas density vs. psoas area as a marker of frailty
When we stratified patients by psoas density, we found that the sarcopenic group had significant covariates that we would expect to find with frailty. Patients were significantly older (Table 1, mean age 65.6 vs 56.5 years, p < 0.001), took more home medications (Table 1, 5.2 vs 3.2, p < 0.01) and had more comorbidities (Table 1, 4.6 vs 2.3, p < 0.001). There was also a significant increase in trauma caused by falls (34.2% vs 17.8%, p< 0.05) and BMI (30.4 vs 27.6, p < 0.03).
Initial analysis comparing sarcopenia by sex-adjusted PI found significant differences between men and women (Figure 2a, 9.01 vs 5.95 cm2/m2, p < 0.001). In contrast, average psoas density HUAC values between men and women, there was no sex-specific difference (Figure 2b, 43.6 vs 42.7 HU, p = 0.46). For our covariate analysis, after sex-specific adjustment of PI, the sarcopenic group remained significantly older (Table 1, mean age 62.2 vs 57.6 years, p < 0.03), however, other covariates associated with frailty were not significant.
Figure 2. Sex-Specific Differences in Sarcopenia by Psoas Area (PI) and Psoas Density (HUAC).
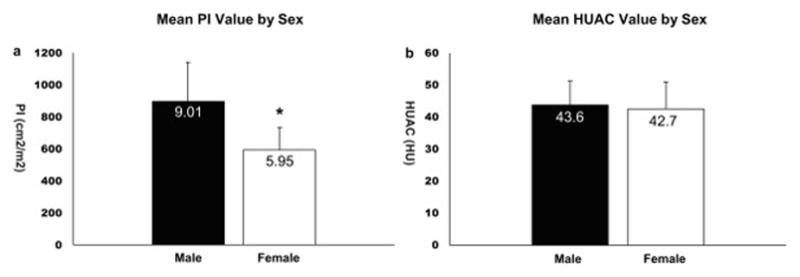
a. The average PI value between males and females is significantly different, (9.01 vs 5.95 cm2/m2, p < 0.001).
b. The average HUAC value between males and females is not significantly different, (43.6 HU vs 42.7 HU, p = 0.46).
PI; Psoas Index, HUAC; Hounsfield Unit average calculation, HU; Hounsfield Unit,
Error bars are standard deviation, * marks significance below a = 0.05
To validate psoas density as a marker of frailty, we tested it against other covariates of frailty, including age, number of medications, and number of comorbidities. We found that psoas density was negatively correlated with these other markers of frailty (Figure 3a, Age vs HUAC r = −0.37, p < 0.001, 3b, # of comorbidities vs HUAC r = −0.41, p < 0.001, 3c, # of medications vs. HUAC r = −0.33, p < 0.001), indicating that as muscle density decreases, patients tend to be older, with increasing comorbidities and medication use. In comparison, PI was significantly correlated with older age in women (Figure 4a, r = −0.41, p = 0.002), but not in men (Figure 4a, r = −0.18, p = 0.08), or our other markers of frailty for both sexes (Figure 4b, women r = −0.14, p = 0.30, men r = 0.04, p = 0.74, 4c, women r = −0.10, p = 0.44, men r = 0.12, p = 0.24). Our results suggest that psoas density covaries with multiple markers of frailty in contrast to psoas area, making density a better overall marker of frailty.
Figure 3. Psoas Density Correlates with Other Markers of Frailty.
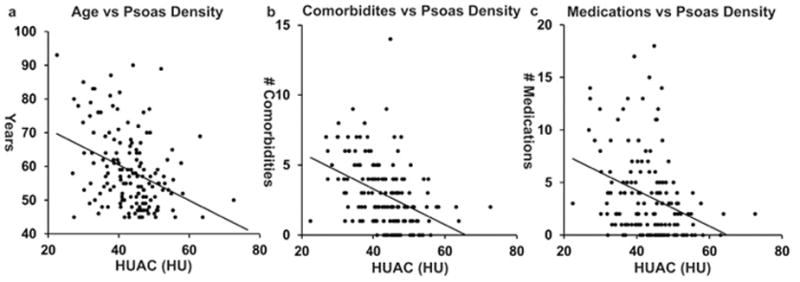
a. Age correlates significantly with psoas density (r = −0.37, p < 0.001).
b. Number of comorbidities correlated significantly with psoas (r = −0.41, p < 0.001).
c. Number of medications correlated significantly with psoas density (r = −0.33, p < 0.001).
HUAC; Hounsfield Unit average calculation, HU; Hounsfield Unit
Figure 4. Psoas Area Correlates with Age in Women, but No Other Markers of Frailty.
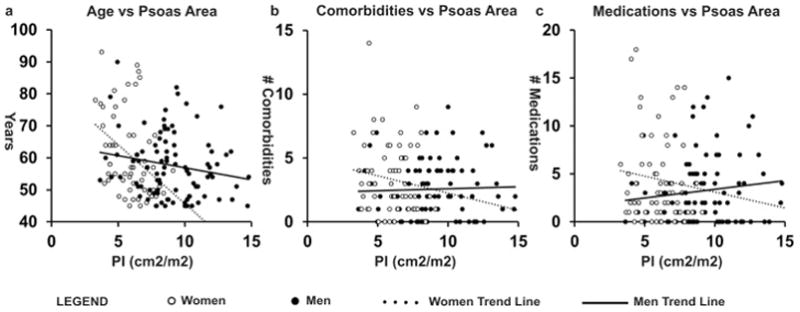
a. Age correlates significantly with PI in women (r = −0.41, p = 0.002), but not men (r = −0.18, p = 0.08). b. Number of comorbidities do not correlate with PI in women (r = −0.14, p = 0.30) or men (r = 0.04, p = 0.74). c. Number of medications do not correlate with PI in women (r = −0.10, p = 0.44) or men (r = 0.12, p = 0.24).
PI; Psoas Index
Outcomes by psoas density and psoas area measured sarcopenia
In terms of our cohort outcomes, (Table 2) the 90-day mortality was 6.0%. The average length of stay was 7.2 days with 31.7% of our cohort staying 7 or more days. The overall complication rate was 25.8%. After excluding our inpatient deaths, our dependent discharge to a skilled nursing facility or long term acute care hospital rate was 21.8%.
Table 2.
Outcome by Psoas Density or Psoas Area
| Total Cohort n = 151 |
Non-Sarcopenic by Psoas Density n = 113 |
Sarcopenic by Psoas Density n = 38 |
p – value | Non-Sarcopenic by Psoas Area n = 113 |
Sarcopenic by Psoas Area n = 38 |
p - value | |
|---|---|---|---|---|---|---|---|
| #90 Day Mortality | 9 (6.0%) | 3 (2.7%) | 6 (15.8%) | 0.008* | 3 (2.7%) | 6 (15.8%) | 0.008* |
| Length of Stay (Days) | 7.2 (9.2) | 6.5 (8.6) | 9.3 (10.3) | 0.11 | 7.4 (9.3) | 6.8 (8.9) | 0.41 |
| Length of Stay = 7 Days | 48 (31.7%) | 31 (27.4%) | 17 (44.7%) | 0.05* | 38 (33.6%) | 10 (26.3%) | 0.40 |
| Complication | 39 (25.8%) | 22 (19.5%) | 17 (44.7%) | 0.002* | 29 (25.6%) | 10 (26.3%) | 0.92 |
| Dependent Discharge | 33 (23.2%) | 21 (19.1%) | 12 (37.5%) | 0.03* | 29 (26.3%) | 4 (12.5%) | 0.10 |
Values are means, or total number in group, parentheses are standard deviation, or percentage of total group,
marks significance below α = 0.05
Patients with sarcopenia by HUAC had significantly increased probability of 90-day mortality (Table 2, 15.8% vs 2.7%, p = 0.008), length of stay ≥ 7 days (Table 2, 44.7% vs 27.4%, p < 0.05), complication risk (Table 2, 44.7% vs 19.5%, p = 0.002), and dependent discharge (Table 2, 37.5% vs 19.1%, p = 0.03). Patients with sarcopenia by sex-adjusted PI had significantly increased risk of mortality (Table 2, 15.8% vs 2.7%, p = 0.008) but no increased risk in our other outcome measures. When our cohort is grouped into quartiles by psoas density, there appeared to be a density-dependent effect. As psoas density decreases, the probability of 90-day mortality, length of stay ≥ 7 days, increased complication rate, and dependent discharge increased (Figure 5a). This effect was only noticeable with 90-day mortality when stratifying by sex-adjusted PI (Figure 5b).
Figure 5. Psoas Density is a Better Predictor of Poor Outcomes than Psoas Area.
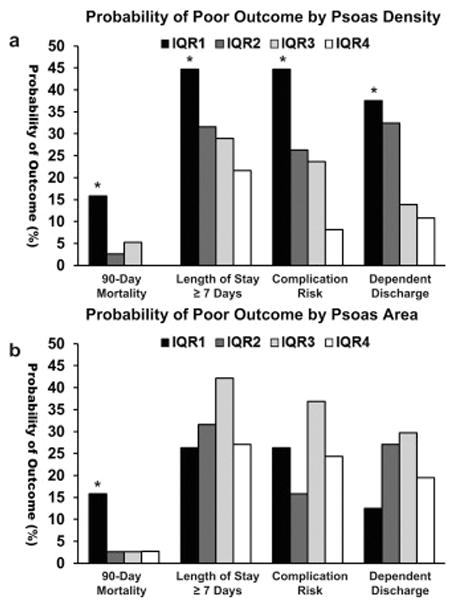
a. When the cohort is grouped into interquartile ranges by HUAC, those in the sarcopenia group (IQR1) have significantly increased probability of mortality (p = 0.008), length of stay = 7 (p = 0.047), complication (p = 0.002), and dependent discharge (p = 0.03).
Decreasing density from a higher quartile increases probability of poor outcome, indicating a density-dependent effect.
b. When the cohort is grouped into interquartile ranges by PI, those in the sarcopenia group have significantly increased probability of mortality only (p = 0.008).
IQR1; 1st – 25th percentile, IQR2; 26th – 50th percentile, IQR3; 51st – 75th percentile, IQR4; 76th – 100th percentile, HUAC; Hounsfield Unit Average Calculation, PI; Psoas Index, * marks significance below a = 0.05
In terms of relative risk, patients with sarcopenia by HUAC had increased 90-day mortality (Table 3, RR 5.95, p = 0.008), length of stay ≥ 7 days (Table 3, RR 1.63, p = 0.048), complication risk (Table 3, RR 2.30, p = 0.002), and dependent discharge (Table 3, 2.14, p = 0.015). Patients with sarcopenia by PI had increased relative risk of 90-day mortality (Table 5, RR 5.95, p = 0.008) but failed to reach significance in other outcomes. Taken together, our data shows that psoas density is a significant predictor of multiple poor outcomes after trauma, and is therefore a better predictor of poor outcomes compared to psoas area.
Table 3.
Relative Risk of Poor Outcome by Psoas Density, Psoas Size
| Psoas Density HUAC (IQR1 vs IQR2 - 4) | Relative Risk (95% CI) |
|---|---|
| 90 Day Mortality* | 5.95 (1.56 – 22.6) p = 0.008 |
| Length of Stay ≥ 7 days* | 1.63 (1.02 – 2.59) p = 0.048 |
| Complication* | 2.30 (1.37 – 3.85) p = 0.002 |
| Dependent Discharge* | 2.14 (1.18 – 3.88) p = 0.015 |
| Psoas Area PI (IQR1 vs IQR2 - 4) | |
| 90 Day Mortality* | 5.95 (1.56 – 22.6) p = 0.008 |
| Length of Stay ≥ 7 days | 0.78 (0.33 – 1.41) p = 0.402 |
| Complication | 1.03 (0.56 – 1.92) p = 0.915 |
| Dependent Discharge | 0.86 (0.18 – 1.25) p = 0.102 |
HUAC; Hounsfield Unit Average Calculation, IQR; Interquartile Range, PI; Psoas Index, CI; Confidence Interval.
marks significance below α = 0.05 level
Discussion
Frailty is recognized as a significant contributor to age-related morbidity and mortality. However, there remains no standardized definition or method to identify the representative pathologic loss of physiologic reserve. Current methods often rely on measures in the patient history and functional tests that are reliant on patient effort. The subjective nature and variability of these metrics, as well as the time-consuming nature in gathering them may limit their clinical utility(4–7). Therefore, there is significant interest in assessing sarcopenia with faster, objective measurements. In surgery and trauma, there is a growing body of literature showing that CT-based muscle measurements predict complications and mortality, with CT psoas area and density becoming increasingly utilized (8–14).
In contrast to previous studies, we directly compare CT psoas area to density in predicting poor outcomes after blunt trauma. We find that psoas density is an overall better predictive marker of outcomes for several reasons. Psoas density was significantly associated with more covariates of frailty, including age, number of comorbidities, and number of medications. Density predicted multiple poor outcomes, including mortality, inpatient length of stay, complications, and dependent discharge as compared to psoas area, which significantly predicted mortality after normalizing for height and sex, but not our other outcome measures. Interestingly, we found a trend in which decreasing psoas density increased the likelihood of poor outcome. Taken together, our data suggests that CT psoas density measured during initial trauma evaluation can identify patients at greatest risk of poor outcomes.
We hypothesize that the significant differences found between our measures of sarcopenia are because they are capturing different aspects of this condition. Sarcopenia is defined not only as age-related loss of skeletal muscle mass, but also loss of muscle strength and quality. Therefore, the psoas area, which many studies focus on as their sole measure of sarcopenia, may only be capturing the mass component, but not necessarily the functional component of this condition. Studies comparing muscle quantity to function support the premise that muscle mass differs from muscle quality. In a large-scale, prospective, longitudinal study comparing CT and dual-energy X-ray absorptiometry measurements of leg muscle mass against knee strength in a geriatric population, the researchers found that although loss of muscle quantity was associated with loss of strength, patients with preserved or even increased muscle mass had continued significant loss of strength, underscoring that muscle quantity does not necessarily measure muscle quality (15). In a retrospective cohort study comparing CT-measured psoas density and area to assess severity of hypercortisolism and central sarcopenia, the researchers found that 24-hour cortisol levels had a more significant correlation with density than area, suggesting that density is capturing relevant morphometric information that area is not(14).
Evidence suggests that CT muscle density directly measures muscle quality and function. In a study comparing CT muscle density of the midthigh to muscle fiber adiposity and triglyceride content from percutaneous biopsy specimens of the vastus lateralis, researchers found that there was a significant negative correlation between density and lipid content showing that density is measuring fatty infiltration found in poorer quality muscle(15). Studies have also shown that higher CT muscle attenuation in the elderly is associated with increased muscle strength after adjustment for muscle area, indicating that muscle density may account for muscle strength differences that are not otherwise attributed to muscle quantity(15).
CT psoas muscle attenuation is not simply a function of advanced age. In a recent study looking at the effects of age on the CT muscle density of multifidus muscle groups in an otherwise well cohort, elderly patients (60–89 years) did have decreased psoas density compared to the youngest age group (20–39 years, 41.4 HU elderly group vs 43.2 HU young group), however this difference was not statistically significant(16). The attenuation values are close to the average psoas density of our trauma cohort (43.3 HU). Furthermore, our cut-off value of sarcopenia (38.5 HU) is within the range calculated based on sensitivity analysis of mortality in a large cohort of patients undergoing elective gastrointestinal oncologic surgery (38.1–39.9 HU)(10). Given the similar values in these studies compared to our own, we speculate that our cut-off value of sarcopenia is more widely generalizable.
Much of the literature regarding sarcopenia is meant for prognostication in the elective setting, with the goal of pre-habilitating sarcopenic patients before surgical insult to prevent morbidity and improve survival(17). In contrast, we recognize that traumatic injury is a spontaneous event in which pre-habilitation is not possible, which may decrease its utility in improving outcomes. Nevertheless, the preponderance of evidence suggests that specific physical and nutritional interventions in the immediate in-patient setting in elderly patients may significantly improve their outcomes, and post-hospitalization quality of life. It is well known that immobility and poor nutrition during hospitalization leads to significant decline in muscle function with resulting disability, especially in frail patients(18, 19). Even after discharge, many patients fail to regain their previous level of function with standard nutrition and physical therapy regimens(20). However, certain specific protein diet and resistance exercise regimens have been shown to preserve skeletal muscle mass and improve muscle function(21, 22). Patients in the intensive care setting who have additional protein supplementation had reduced rates time on mechanical ventilation, and mortality (23, 24). Additional nutritional interventions, such as dietary supplementation have shown promise in combating sarcopenia. Beta-hydroxy-beta-methylbutyrate, an endogenous metabolite has been demonstrated to decrease muscle catabolism and preserve muscle mass in geriatric patients without adverse effect(25, 26). Interestingly, beta-hydroxy-beta-methylbutyrate has been shown to significantly decrease mortality when given to elderly patients in an acute, inpatient setting for acute exacerbations of chronic medical conditions, such as heart failure and chronic obstructive pulmonary disease(27).
Furthermore, there is evidence to suggest that frailty is in part, reversible with aggressive exercise and nutritional therapies. In a randomized control trial of elderly patients in a communal living facility, 6 months of exercise and nutritional interventions improved frailty scores, muscle strength, and gait speed—especially in patients undergoing both interventions(21). Given the high proportion of falls as the mechanism of trauma in the elderly, we conjecture that identification of frail patients with objective CT-based measures may help identify patients that would benefit most from post-discharge, aggressive rehabilitation that may serve to prevent future secondary traumatic injury(28).
Our study has several noted limitations. Our data came from a cohort of 151 patients that were derived from a single institution in a single year, which may not reflect the patient population or outcomes at other centers and should be externally validated at other centers in a prospective fashion. The inclusion of all blunt mechanism of traumatic injury spans a wide spectrum of physiologic insult as compared to other studies assessing outcomes after a more specific injury or surgical procedure. Some included patients may have received fluids, or in rare cases blood products which could contribute to edema and have an unknown impact on psoas muscle density. Lastly, our results were obtained in CT scans of the abdomen and pelvis with intravenous contrast measured during the venous phase. We chose this modality due to its use as the standard imaging modality in our institution for traumatic evaluation, as well as its overall broad use for multiple surgical indications. It is unknown how generalizable our results will be when considering other phase/non-contrast CT scans.
In our single-center retrospective cohort of patients who were evaluated in the trauma bay with blunt mechanism who received a CT scan of the abdomen/pelvis, psoas muscle density appears to be a good overall predictor of multiple clinically significant parameters. These include 90-day mortality, complication rate, length of stay, and dependent discharge. Psoas density correlates with other markers of physiologic reserve including co-morbidities, increased medication use, and advanced age and outperformed psoas area in these clinical parameters. Clinicians, dietitians, and therapists could use this tool to identify patients who would benefit from additional interventions to improve prognosis, aid in discharge planning, and prevent recurrent traumatic injury. Future studies will need to externally validate our findings, as well as determine if specific nutritional and physical therapy regimens can improve psoas density and decrease risk.
Supplementary Material
Supplementary Table 1. Ranges (IQR) by Psoas Density (HUAC) or Psoas Area (PI)
Supplementary Table 2. Standardized List of Complications
Acknowledgments
Funding Sources: TY is the recipient of National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number NIH T32AI 106704-01A1. WL is the recipient of an OSU College of Medicine Roessler Research Scholarship.
Abbreviations
- BMI
Body Mass Index
- CT
Computed Tomography
- GCS
Glasgow Coma Scale
- HU
Hounsfield Unit
- HUAC
Hounsfield Unit Average Calculation
- IQR
Interquartile Range
- ISS
Injury Severity Score
- LPA
Left Psoas Area
- LPHU
Left Psoas Hounsfield Unit
- PACS
Picture Archiving and Communications System
- PI
Psoas Index
- RPA
Right Psoas Area
- RPHU
Right Psoas Hounsfield Unit
Footnotes
Author Contributions: TY, WL, DE designed the research study. TY, WL, DE collected data. TY, DE analyzed data. TY created figures. TY, WL, DE prepared the manuscript. TY, DE prepared manuscript revisions.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenberg IH. Sarcopenia: Origins and Clinical Relevance. Journal of Nutrition. 1997;127:990–1. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 2.Linda P, Fried CMT, Waltson Jeremy, Newman Anne B, Hirsch Calvin, Gottdiener John, Seeman Teresa, Tracy Russell, Kop Willem J, Burke Gregory, McBurnie Mary Ann. Frailty in Older Adults: Evidence for a Phenotype. Journal of Gerontology. 2001;56A(3):146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Linda P, Fried LF, Darer Jonathan, Williamson Jeff D, Anderson Gerard. Untangling the Concepts of Disability, Frailty, and Comorbidity: Implications for Improved Targeting and Care. Journal of Gerontology. 2004;59(3):255–63. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 4.Farhat JS, Velanovich V, Falvo AJ, Horst HM, Swartz A, Patton JH, Jr, et al. Are the frail destined to fail? Frailty index as predictor of surgical morbidity and mortality in the elderly. J Trauma Acute Care Surg. 2012;72(6):1526–30. doi: 10.1097/TA.0b013e3182542fab. discussion 30–1. [DOI] [PubMed] [Google Scholar]
- 5.Fairchild B, Webb TP, Xiang Q, Tarima S, Brasel KJ. Sarcopenia and frailty in elderly trauma patients. World J Surg. 2015;39(2):373–9. doi: 10.1007/s00268-014-2785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):901–8. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 7.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211(2):271–8. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones KI, Doleman B, Scott S, Lund JN, Williams JP. Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis. 2015;17(1):O20–6. doi: 10.1111/codi.12805. [DOI] [PubMed] [Google Scholar]
- 10.Buettner S, Wagner D, Kim Y, Margonis GA, Makary MA, Wilson A, et al. Inclusion of Sarcopenia Outperforms the Modified Frailty Index in Predicting 1-Year Mortality among 1,326 Patients Undergoing Gastrointestinal Surgery for a Malignant Indication. J Am Coll Surg. 2016;222(4):397–407. e2. doi: 10.1016/j.jamcollsurg.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 11.Wojda TR, Cardone MS, Lo WD, Stawicki SPA, Evans DC. Ultrasound and Computer Tomography Imaging Technologies for Nutrition Assessment in Surgical and Critical Care Patient Populations. Curr Surg Rep. 2015;3(21) [Google Scholar]
- 12.Joglekar S, Asghar A, Mott SL, Johnson BE, Button AM, Clark E, et al. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J Surg Oncol. 2015;111(6):771–5. doi: 10.1002/jso.23862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izumi T, Watanabe J, Tohyama T, Takada Y. Impact of psoas muscle index on short-term outcome after living donor liver transplantation. Turk J Gastroenterol. 2016;27(4):382–8. doi: 10.5152/tjg.2016.16201. [DOI] [PubMed] [Google Scholar]
- 14.Miller BS, Ignatoski KM, Daignault S, Lindland C, Gauger PG, Doherty GM, et al. A quantitative tool to assess degree of sarcopenia objectively in patients with hypercortisolism. Surgery. 2011;150(6):1178–85. doi: 10.1016/j.surg.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Bret H, Goodpaster CLC, Visser Marojolein, Kelley David E, Scherzinger Ann, Harris Tamara B, Stamm Elizabeth, Newman Anne B. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. Journal of Applied Physiology. 2001;90:2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 16.Lee SH, Park SW, Kim YB, Nam TK, Lee YS. The fatty degeneration of lumbar paraspinal muscles on computed tomography scan according to age and disc level. Spine J. 2017;17(1):81–7. doi: 10.1016/j.spinee.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Levett DZ, Edwards M, Grocott M, Mythen M. Preparing the patient for surgery to improve outcomes. Best Pract Res Clin Anaesthesiol. 2016;30(2):145–57. doi: 10.1016/j.bpa.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Thomas M, Gill HA, Guo Zhenchao. The Deleterious Effects of Bed Rest Among Community-Living Older Persons. Journal of Gerontology. 2004;59A(7):755–61. doi: 10.1093/gerona/59.7.m755. [DOI] [PubMed] [Google Scholar]
- 19.Portegijs E, Buurman BM, Essink-Bot ML, Zwinderman AH, de Rooij SE. Failure to regain function at 3 months after acute hospital admission predicts institutionalization within 12 months in older patients. J Am Med Dir Assoc. 2012;13(6):569, e1–7. doi: 10.1016/j.jamda.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Hardy SE, Gill TM. Recovery from disability among community-dwelling older persons. JAMA. 2004;291(13):1596–602. doi: 10.1001/jama.291.13.1596. [DOI] [PubMed] [Google Scholar]
- 21.Ng TP, Feng L, Nyunt MS, Feng L, Niti M, Tan BY, et al. Nutritional, Physical, Cognitive, and Combination Interventions and Frailty Reversal Among Older Adults: A Randomized Controlled Trial. Am J Med. 2015;128(11):1225–36. e1. doi: 10.1016/j.amjmed.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Tieland M, van de Rest O, Dirks ML, van der Zwaluw N, Mensink M, van Loon LJ, et al. Protein supplementation improves physical performance in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13(8):720–6. doi: 10.1016/j.jamda.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Peter JM, Weijs SNS, de Groot Sabine DW, Driessen Ronald H, de Jong Evelien, Girbes Armand RJ, Strack van Schijndel Rob JM, Beishuizen Albertus. Optimal Protein and Energy Nutrition Decreases Mortality in Mechanically Ventilated, Critically Ill Patients: A Prospective Observational Cohort Study. Journal of Parenteral and Enteral Nutrition. 2012;36(1):60–8. doi: 10.1177/0148607111415109. [DOI] [PubMed] [Google Scholar]
- 24.Cathy Alberda LG, Jones Naomi, Jeejeebhoy Khursheed, Day Andrew G, Dhaliwal Rupinder, Heyland Daren K. The relationship between nutritional intak and clincal outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Medicine. 2009;35:1728–37. doi: 10.1007/s00134-009-1567-4. [DOI] [PubMed] [Google Scholar]
- 25.Deutz NE, Pereira SL, Hays NP, Oliver JS, Edens NK, Evans CM, et al. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin Nutr. 2013;32(5):704–12. doi: 10.1016/j.clnu.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Fitschen PJ, Wilson GJ, Wilson JM, Wilund KR. Efficacy of beta-hydroxy-beta-methylbutyrate supplementation in elderly and clinical populations. Nutrition. 2013;29(1):29–36. doi: 10.1016/j.nut.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Deutz NE, Matheson EM, Matarese LE, Luo M, Baggs GE, Nelson JL, et al. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin Nutr. 2016;35(1):18–26. doi: 10.1016/j.clnu.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Casey CM, Parker EM, Winkler G, Liu X, Lambert GH, Eckstrom E. Lessons Learned From Implementing CDC’s STEADI Falls Prevention Algorithm in Primary Care. Gerontologist. 2016 doi: 10.1093/geront/gnw074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Ranges (IQR) by Psoas Density (HUAC) or Psoas Area (PI)
Supplementary Table 2. Standardized List of Complications


