Abstract
Parkinson’s disease is defined by the loss of dopaminergic neurons in the substantia nigra and formation of Lewy body inclusions containing aggregated α-synuclein. Efforts to explain dopamine neuron vulnerability are hindered by the lack of dopaminergic cell death in α-synuclein transgenic mice. To address this, we manipulated dopamine levels in addition to α-synuclein expression. Nigra-targeted expression of mutant tyrosine hydroxylase with enhanced catalytic activity increased dopamine without damaging neurons in non-transgenic mice. In contrast, raising dopamine in mice expressing human A53T mutant α-synuclein induced progressive nigrostriatal degeneration and reduced locomotion. Dopamine elevation in A53T mice increased levels of potentially toxic α-synuclein oligomers, resulting in conformationally and functionally modified species. Moreover, in genetically tractable C. elegans models expression of α-synuclein mutated at the site of interaction with dopamine prevented dopamine-induced toxicity. The data suggest a unique mechanism linking two cardinal features of Parkinson’s disease, dopaminergic cell death and α-synuclein aggregation.
Introduction
Parkinson’s disease (PD) is a debilitating neurodegenerative disorder that is principally defined by the motor symptoms of resting tremor, rigidity, and bradykinesia. These symptoms occur primarily because of the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SN), which depletes dopamine at nigrostriatal synapses (1). A longstanding hypothesis to explain the vulnerability of this cell population states that dopamine induces neuronal injury via oxidative stress. The enzyme- or metal-catalyzed oxidation of dopamine generates electron-deficient quinones and reactive oxygen species that can induce cellular dysfunction (2–3). The SN in PD patients is reported to harbor oxidatively modified proteins, lipids, and nucleic acids (4–6). Efforts to study the effects of dysregulated dopamine in animal models have included ectopic dopamine administration or increased cytosolic dopamine by suppression of vesicular monoamine transporter (VMAT2) expression (7–9).
More recently, α-synuclein has emerged as a critical player in PD pathogenesis. Disturbances in α-synuclein protein folding or expression level, i.e. through mutations or extra copies of the gene that encodes it, SNCA, are known causes of PD (10–17). In both familial and sporadic cases, aggregated α-synuclein is present within Lewy bodies and Lewy neurites, which are the hallmark pathological lesions of the disease (18–19). α-Synuclein is a small highly conserved protein (140 residues) that is abundantly expressed in the nervous system primarily at presynaptic terminals (20). The precise physiological functions of α-synuclein remain unclear, though it is thought to play diverse roles in synaptic maintenance and plasticity, neurotransmitter release and homeostasis, and the regulation of synaptic vesicle pools (21–23). In disease, α-synuclein assembles into β-sheet rich amyloid-like fibrils, generating several intermediate oligomeric species of unknown toxicity (24).
Intriguingly, α-synuclein oligomers are kinetically stabilized by oxidized dopamine and other catecholamines in vitro (24–25), providing a possible link between dopamine- and α-synuclein-mediated toxicities. Extensive evidence from cultured cells and cell-free systems indicates that dopamine potently and substoichiometrically inhibits the fibrillization of α-synuclein, resulting in the kinetic arrest of soluble oligomer species. Dopamine oxidation is required for oligomer stabilization, suggesting α-synuclein interacts with dopamine-quinone and/or other oxidation products. The interaction is non-covalent, reversible, and dependent on residues E83 and the Y125EMPS129 motif in the C-terminus of α-synuclein (24–28). The toxicity of these oligomers is largely unexplored, though in vitro dopamine-incubated α-synuclein is reported to interfere with and resist degradation by chaperone-mediated autophagy (29), and to inhibit SNARE complex formation and neurotransmitter release (30). Importantly, it is unknown if dopamine is able to modify α-synuclein oligomerization in vivo and whether the resultant species are capable of driving neurodegeneration. These are the critical questions we aimed to answer in this study.
Results
Expression of TH-RREE elevates dopamine levels and causes hyperactivity in NonTg mice
To increase dopamine levels in vivo, we used a lentiviral vector containing the TH gene encoding human tyrosine hydroxylase (TH). TH is the rate-limiting enzyme in dopamine biosynthesis converting tyrosine to L-DOPA, which is then metabolized by aromatic amino acid decarboxylase to generate dopamine. A mutated TH enzyme that has R37R38 replaced by E37E38 (TH-RREE) is insensitive to feedback inhibition by dopamine and was used to achieve increased catalytic activity of TH (26–27, 31). The function of the vector was first tested on human neuroblastoma SH-SY5Y cells. This cell line has undetectable levels of endogenous TH, despite the expression of other enzymes required for synthesis and processing of dopamine. The cells were transduced after 5 days of differentiation with 20 µM retinoic acid and TH expression was confirmed by western blot and immunocytochemistry compared with empty vector (CtrlVect)-transduced controls (Supplementary Fig. 1a–c). The TH-RREE transduced cells had measurable intracellular levels of L-DOPA, dopamine, and DOPAC in the range of 25–200 fm/µg, whereas catecholamines were not detected in CtrlVect-transduced cells (Supplementary Fig. 1d), demonstrating that TH-RREE vector enhances catecholamine production.
We performed bilateral injections of TH-RREE vector into the SN of aged 10-month old) NonTg mice. This age was selected because the mice expressing A53T mutant human α-synuclein are asymptomatic and do not exhibit the characteristic motor phenotype derived from spinal cord degeneration (32). The typical age of onset of this phenotype in our colony is approximately 18 months. At 5 months post-injection (mpi), TH expression was increased in both SN and striatum in NonTg TH-RREE mice compared to age-matched NonTg CtrlVect mice (Fig. 1a–b). TH-RREE significantly elevated striatal concentrations of L-DOPA and dopamine by 36 and 52%, respectively, while DOPAC was not affected. Importantly, 5-HT levels did not change, indicating a specific effect on the catecholamine neurotransmitter system (Fig. 1c). Consistent with elevated striatal dopamine, NonTg TH-RREE mice exhibited hyperactivity in open field-testing compared with NonTg CtrlVect mice (Fig. 1d). There was no difference in rotarod performance between the NonTg injection groups (Fig. 1e). These data indicate that the TH-RREE vector significantly enhanced dopamine levels fully 5 months following injection.
Figure 1. TH-RREE lentiviral vector increases dopamine levels and causes hyperactivity in NonTg mice.
(a) TH protein levels were increased in the striatum of TH-RREE vector injected mice relative to empty vector-injected controls (CtrlVect) at 5 mpi. Densitometric analysis was conducted by normalizing TH to the GAPDH loading control. Blots are cropped; for full-length blots see Supplementary Fig. 11. (n = 3 mice per group, P = 0.0201, t = 3.742, d.f. = 4; two-tailed unpaired Student’s t test). (b) Increased TH expression was confirmed by immunohistochemistry in the SN (left panels) and striatum (right panels). Scale bar, 200 µm. (n = 4 mice per group). (c) TH overexpression significantly increased the steady state concentrations of striatal catecholamines L-DOPA and dopamine (DA), but did not alter DOPAC or 5-HT. (L-DOPA, n = 4 mice per group, P = 0.0235, t = 3.017, d.f. = 6; DA, n = 4 mice per group, P = 0.0137, t = 3.445, d.f. = 6; DOPAC, n = 5 mice for CtrlVect and n = 4 mice for TH-RREE, P = 0.2713, t = 1.194, d.f. = 7; 5-HT, n = 4 mice for CtrlVect and n = 3 mice for TH-RREE, P = 0.658, t = 0.4703, d.f. = 5; two-tailed unpaired Student’s t test). (d) TH-RREE injected NonTg mice exhibited greater locomotion as measured by open field activity. (n = 5 mice per group, P = 0.0399, t = 2.451, d.f. = 8; two-tailed unpaired Student’s t test). (e) There was no change in rotarod performance between the injection groups. (n = 3 mice per group, P = 0.9959, t = 0.005455, d.f. = 4; two-tailed unpaired Student’s t test). Box plots show median, 25th and 75th percentiles, minimum and maximum values. *P < 0.05.
Dopamine-induced neurodegeneration and motor deficit is dependent on α-synuclein expression
Bilateral injection of the TH-RREE vector into the SN of 10-month old A53T mutant α-synuclein mice also achieved TH overexpression (Supplementary Fig. 2). However, in contrast to NonTg mice, A53T TH-RREE mice exhibited striatal degeneration and neuronal loss in the SN at 5 mpi. To circumvent detection of vector-encoded TH, we used another marker of dopaminergic neurons, VMAT2, to evaluate neuronal loss. A53T TH-RREE mice had fewer VMAT2 positive cells in the SN compared with all other injection groups (Fig. 2a). We quantified the loss of neurons using unbiased stereological counting of Nissl cells, which revealed a significant 25% reduction in A53T TH-RREE mice compared to controls (Fig. 2b). The number of neurons quantified in age-matched A53T CtrlVect as well as NonTg CtrlVect and NonTg TH-RREE mice was typical for the mouse SN (33), indicating that neurodegeneration had not occurred in these mice (Fig. 2b).
Figure 2. Dopamine-induced neurodegeneration of the SN is dependent on α-synuclein.
(a) At 5 mpi, fewer VMAT2-positive cells (arrows) were present in the SN of A53T TH-RREE mice compared with all other injection groups. Scale bar, 100 µm. (n = 3 mice per group). (b) Unbiased stereological counting of Nissl-positive neurons in the SN revealed a significant 25% loss of cells in A53T TH-RREE mice. The data are presented as mean ± s.e.m. (n = 3 mice per group except n = 4 mice for A53T TH-RREE, PA53T TH-RREE versus A53T CtrlVect = 0.0049, PA53T TH-RREE versus NonTg CtrlVect = 0.0316, PA53T TH-RREE versus NonTg TH-RREE = 0.0253, F(3,9) = 8.810; one-way ANOVA with Tukey’s correction for multiple comparisons). (c–d) Histological analysis of VMAT2 staining in the striatum, with subtraction of background staining in the cortex, indicated severe dopaminergic denervation in A53T TH-RREE mice. Scale bar, 200 µm. (n = 3 mice per group, PA53T TH-RREE versus A53T CtrlVect = 0.0043, PA53T TH-RREE versus NonTg CtrlVect = 0.0163, PA53T TH-RREE versus NonTg TH-RREE = 0.0163, F(3,8) = 10.02; one-way ANOVA with Tukey’s correction for multiple comparisons). (e–f) Striatal DAT levels normalized to the actin loading control were increased in NonTg TH-RREE mice relative to NonTg CtrlVect, whereas DAT levels were decreased in A53T TH-RREE mice as compared to A53T CtrlVect. Blots are cropped; for full-length blots see Supplementary Fig. 11. (n = 3 mice per group except n = 4 mice for NonTg CtrlVect, PA53T TH-RREE versus A53T CtrlVect = 0.0211, PA53T CtrlVect versus NonTg CtrlVect = 0.0097, PNonTg TH-RREE versus NonTg CtrlVect = 0.0466, F(3,9) = 8.411; one-way ANOVA with Tukey’s correction for multiple comparisons). Box plots show median, 25th and 75th percentiles, minimum and maximum values. *P < 0.05, **P < 0.01.
The degeneration of cell bodies was accompanied by a significant 62% decrease in VMAT2 staining in the striatum of A53T TH-RREE mice, demonstrating a severe loss of dopaminergic nerve terminals (Fig. 2c–d). Denervation of the striatum was further corroborated by quantification of dopamine transporter (DAT) levels, which revealed a significant 55% decline in A53T TH-RREE mice compared with A53T CtrlVect. In contrast, the levels of DAT were two-fold higher in NonTg TH-RREE as compared to NonTg CtrlVect mice (Fig. 2e–f). DAT upregulation may serve to compensate for increased dopamine levels, and may not be apparent in A53T TH-RREE mice due to the substantial loss of dopaminergic terminals. The injury in A53T mice appears to be primarily presynaptic in that levels of D1 receptors remained unchanged (Supplementary Fig. 3a–b). Levels of dopamine- and cAMP-regulated phosphoprotein-32 (DARPP-32), which serves as an important signaling molecule in spiny projection neurons, were also unaffected (Supplementary Fig. 3a,c).
To further investigate the progressive degenerative phenotype in A53T TH-RREE mice, the total number of SN neurons was quantified at the earlier time point of 2.5 mpi. No difference was observed between A53T CtrlVect and TH-RREE mice, indicating that cell loss had not yet occurred (Fig. 3a). Despite the maintenance of neuronal cell bodies, striatal VMAT2 levels were decreased by 25% (Fig. 3b), revealing a milder loss of nerve terminals than at the later time point of 5 mpi (Fig. 2c–d). Consistent with progressive degeneration of synapses, we noted a time-dependent decline of striatal dopamine content in A53T TH-RREE mice. At 2.5 mpi, dopamine was increased significantly by 71% compared with age-matched A53T CtrlVect mice due to mutant TH expression (Fig. 3c). However, A53T TH-RREE mice subsequently exhibited a 37% drop in dopamine concentration between 2.5 and 5 mpi, whereas A53T CtrlVect mice showed no change during the same period (Fig. 3c).
Figure 3. Dopaminergic neurodegeneration in A53T TH-RREE mice is progressive and ultimately leads to locomotor deficit.
(a) At the early timepoint of 2.5 mpi, A53T TH-RREE mice did not yet exhibit loss of neuronal cell bodies in the SN. The data are presented as mean ± s.e.m. (n = 4 mice for CtrlVect and n = 3 mice for TH-RREE, P = 0.8453, t = 0.2055, d.f. = 5; two-tailed unpaired Student’s t test). (b) VMAT2 staining in the striatum revealed a modest loss of terminals suggesting that dopaminergic synapses had degenerated prior to overt cell death. Scale bar, 200 µm. (n = 4 mice per group, P = 0.0424, t = 2.569, d.f. = 6; two-tailed unpaired Student’s t test). (c) A53T TH-RREE mice exhibited an elevation of dopamine (DA) levels only transiently, with an initial increase of 71% at 2.5 mpi compared with age-matched A53T CtrlVect mice. Subsequently, however, A53T TH-RREE mice underwent a significant 37% drop in striatal dopamine between 2.5 and 5 mpi, which was not observed in A53T CtrlVect mice. (n = 3 mice per group except n = 5 mice for TH-RREE 2.5 mpi, PTH-RREE 2.5 mpi versus CtrlVect 2.5 mpi = 0.0092, PTH-RREE 2.5 mpi versus TH-RREE 5 mpi = 0.0187, F(3,10) = 7.460; one-way ANOVA with Tukey’s correction for multiple comparisons). (d) Levels of DOPAC in the striatum remained unchanged over time regardless of lentiviral treatment. (CtrlVect, n = 3 mice per group; TH-RREE, n = 6 mice for 2.5 mpi and n = 4 mice for 5 mpi, F(3,12) = 0.5481; one-way ANOVA with Tukey’s correction for multiple comparisons). (e) Consistent with a late-onset depletion of dopamine in the striatum, from 2.5 to 5 mpi A53T TH-RREE mice developed a reduction in locomotor activity that was not observed in CtrlVect mice. (n = 3 mice per group, PTH-RREE 5 mpi versus CtrlVect 5 mpi = 0.0152, PTH-RREE 5 mpi versus TH-RREE 2.5 mpi = 0.0488, PTH-RREE 5 mpi versus CtrlVect 2.5 mpi = 0.0213, F(3,8) = 7.107; one-way ANOVA with Tukey’s correction for multiple comparisons). (f) The motor deficit in A53T TH-RREE mice was not severe enough to affect coordination or balance, as rotarod performance remained intact. (n = 4 mice per group except n = 3 mice for TH-RREE 2.5 mpi and n = 3 mice for CtrlVect 5 mpi, F(3,10) = 0.07172; one-way ANOVA with Tukey’s correction for multiple comparisons). Box plots show median, 25th and 75th percentiles, minimum and maximum values. *P < 0.05, **P < 0.01.
The reduction in dopamine levels cannot be explained by increased conversion to DOPAC since levels of this metabolite remained unchanged from 2.5 to 5 mpi (Fig. 3d). We also did not detect an increase in dopamine-protein adducts by near infrared fluorescence (Supplementary Fig. 4). Rather, the loss of dopamine is likely due to the ongoing nigrostriatal degeneration documented in these mice. Concomitant with the decrease in dopamine levels, A53T TH-RREE mice exhibited a significant impairment in ambulatory activity that was not apparent at 2.5 mpi but emerged by 5 mpi (Fig. 3e). Performance on the rotarod remained intact, suggesting that balance and coordination were not disrupted (Fig. 3f). Collectively, the results show that enhanced production of dopamine promotes the progressive degeneration of dopaminergic neurons and a specific motor deficit in mice that express A53T α-synuclein, but not in NonTg mice.
Dopamine modifies α-synuclein oligomer conformations in A53T mice
The data indicate that increased steady state levels of dopamine result in neurotoxicity in an α-synuclein dependent manner. To investigate possible mechanisms, we examined the influence of dopamine on α-synuclein aggregation, and in particular, the kinetic stabilization of potentially toxic α-synuclein oligomers. The presence of Lewy body-like inclusions in circumscribed regions including the brainstem of aged A53T mice has been well-documented (32). α-Synuclein-positive inclusions were indeed detected in the brainstem of A53T TH-RREE mice at 5 mpi (age 15 months), similar to age-matched A53T CtrlVect mice (Supplementary Fig. 5a). In contrast, the SN is known to remain devoid of inclusions during the lifespan of A53T mice (32). Expression of TH-RREE vector did not induce α-synuclein inclusion formation or alter levels or conformations of detergent-insoluble α-synuclein in the SN at 5 mpi (Supplementary Fig. 5).
Examination of detergent-soluble α-synuclein extracted from SN of A53T mice at 5 mpi revealed significant alterations in the quantities and conformations of α-synuclein species as a result of dopamine elevation. A53T TH-RREE mice had a significant 22% reduction in monomeric human α-synuclein compared with A53T CtrlVect mice (Fig. 4a–b). Upon fractionation of the soluble SN extract by native size exclusion chromatography (SEC), we detected the presence of various oligomeric α-synuclein species of different molecular weights (Fig. 4c). Quantification of total oligomeric species revealed a significant increase in A53T TH-RREE mice, greater than two-fold above A53T CtrlVect mice (Fig. 4d). To characterize the oligomers, we used multiple α-synuclein antibodies and a combination of SDS-PAGE, immunoelectron microscopy, and biochemical assays. In A53T CtrlVect mice, oligomers had Stokes radii of up to 65 Å and ranged in molecular weight from 36 to 80 kD (Fig. 4c and Supplementary Fig. 6a–b), consistent with previous observations (34). However, in addition to these low Å species, larger species of up to 122 Å were detected in A53T TH-RREE mice. On 12% SDS-PAGE, these species migrated as dimers, trimers, and high molecular weight polymers stable to SDS and heat, consistent with the presence of oxidized/crosslinked species (35). The appearance of unique, high Å species in A53T TH-RREE mice was observed with multiple α-synuclein antibodies that recognize N- or C-terminal epitopes (Fig. 4c and Supplementary Fig. 6a–b). Moreover, analysis of quinone-associated protein by near-infrared fluorescence revealed that only the high Å oligomeric fractions from A53T TH-RREE mice were positive, supporting a direct interaction of dopamine and α-synuclein oligomers in these mice (Supplementary Fig. 6c).
Figure 4. Dopamine induces conformationally distinct α-synuclein oligomers in the mouse brain.
(a–d) The SN of A53T TH-RREE and A53T CtrlVect mice at 5 mpi was extracted with 1% Triton and the soluble fraction was analyzed by SDS-PAGE (a–b). The soluble extract was further subjected to native size exclusion chromatography and fractions corresponding to 31–38 Å, 41–65 Å, and 72–122 Å were pooled and analyzed by SDS-PAGE (c–d). Neural specific enolase (NSE) was used as a loading control. The quantification revealed a decrease in soluble α-synuclein monomer and a corresponding increase in α-synuclein oligomers in A53T TH-RREE compared with A53T CtrlVect. The oligomer species in A53T TH-RREE mice were present in higher Å fractions than in controls and had a range of molecular weights. Monomer α-synuclein and NSE blots are cropped; for full-length blots see Supplementary Fig. 11. (Monomer, n = 3 mice for CtrlVect and n = 5 mice for TH-RREE, P = 0.0401, t = 2.611, d.f. = 6; Oligomer, n = 3 mice for CtrlVect and n = 4 mice for TH-RREE, P = 0.0338, t = 2.900, d.f. = 5; two-tailed unpaired Student’s t test). (e) Immunoelectron microscopy on low (41–65) and high (72–122) Å fractions from A53T mice using Syn505 (N-terminal) and LB509 (C-terminal) α-synuclein antibodies, with 10 nm gold-conjugated secondary. Scale bar, 10 nm. (n = 3 mice per group). (f) Pooled oligomeric fractions (41–122 Å) from indicated mice were added to aggregation reactions with fresh recombinant α-synuclein, and fibril formation was monitored by Thioflavin T (ThioT). Prior immunodepletion of α-synuclein from A53T CtrlVect fractions was performed as a control. rfu, relative fluorescence units. The data are presented as mean ± s.e.m. (n = 3 mice per group except n = 4 mice for no seed and n = 4 mice for immunodepl; PA53T CtrlVect 20 hour versus no seed 20 hour = 0.0114, PA53T CtrlVect 20 hour versus A53T TH-RREE 20 hour = 0.0172, PA53T CtrlVect 20 hour versus A53T CtrlVect Immunodepl. 20 hour = 0.0292, PA53T CtrlVect 24 hour versus no seed 24 hour = 0.0158, PA53T CtrlVect 24 hour versus A53T TH-RREE 24 hour = 0.0019, PA53T CtrlVect 24 hour versus A53T CtrlVect Immunodepl. 24 hour = 0.0139, F(3,10) = 4.563; repeated measures two-way ANOVA with Tukey’s correction for multiple comparisons). Box plots show median, 25th and 75th percentiles, minimum and maximum values. *P < 0.05, **P < 0.01.
Further structural analysis of oligomers isolated from high (72–122) or low (41–65) Å SEC fractions was undertaken by immunoelectron microscopy. Oligomers typically appeared as clusters with >2 gold particles in both high and low Å fractions from A53T TH-RREE mice as well as low Å fractions from A53T CtrlVect mice. The species were labeled with α-synuclein antibodies directed at either the N- or C-terminus, indicating that both ends were exposed (Fig. 4e). Clusters of >2 gold particles were not observed in high Å fractions from A53T CtrlVect mice or fractions from A53T TH-RREE mice that were immunodepleted of α-synuclein prior to imaging. Similar to mouse-derived oligomers, oligomers generated from incubating purified recombinant human wild-type α-synuclein with dopamine in vitro migrated as 36 to >98 kD bands on SDS-PAGE and were recognized by both N- and C-terminally directed α-synuclein antibodies in both western blots and by immunoelectron microscopy (Supplementary Fig. 7c–d). Electron microscopy imaging of the recombinant oligomers revealed clusters of >2 gold particles as observed in oligomer fractions from mouse SN (Supplementary Fig. 7d).
Since there is growing evidence for prion-like spreading of α-synuclein pathology in disease (36), we investigated if dopamine promotes in vivo formation of oligomeric species with the capacity for templated propagation. α-Synuclein oligomers extracted from A53T TH-RREE or A53T CtrlVect mice (pooled 41–122 Å SEC fractions) were monitored in a seeding assay with fresh recombinant α-synuclein in vitro. In the case of A53T CtrlVect mice, oligomers efficiently seeded aggregation relative to the no-seed condition, as measured by Thioflavin T (Fig. 4f). Importantly, this effect was abolished by prior immunodepletion of α-synuclein from the SEC fractions. In contrast, the same fractions from TH-RREE mice were unable to act as seeds (Fig. 4f), suggesting that seeding-competent species were not retained following the interaction of α-synuclein with dopamine in vivo. We also did not detect dopamine-modified oligomers in the motor cortex of A53T TH-RREE mice (Supplementary Fig. 8), suggesting that these species do not spread trans-synaptically. Collectively, these data indicate that dopamine promotes the generation of conformationally and functionally modified α-synuclein oligomeric species in the mouse brain.
To further establish the link between dopamine-induced oligomers and toxicity, we exposed primary neuronal cultures to recombinant α-synuclein oligomers generated in the presence of dopamine in vitro. Two weeks post-treatment, exogenous α-synuclein from oligomer preparations had become internalized within neurons and localized to neurites (Supplementary Fig. 9a). At this time point, cells were evaluated for viability using Calcein AM and propidium iodide (PI) dyes. Exposure of neurons to 1 µM dopamine-incubated α-synuclein induced a significant 44% reduction in Calcein positive-PI negative cells relative to PBS treated controls (Supplementary Fig. 9b–c). Treatment with equivalent doses of monomeric α-synuclein, or dopamine that had been incubated under aggregation conditions without α-synuclein, did not reduce cell viability (Supplementary Fig. 9b–c). The observed neurotoxicity can therefore be attributed specifically to α-synuclein oligomers, consistent with our findings in vivo linking dopamine-modified species with severe nigrostriatal degeneration.
Disrupting the interaction of dopamine and α-synuclein mitigates neurotoxicity in C. elegans
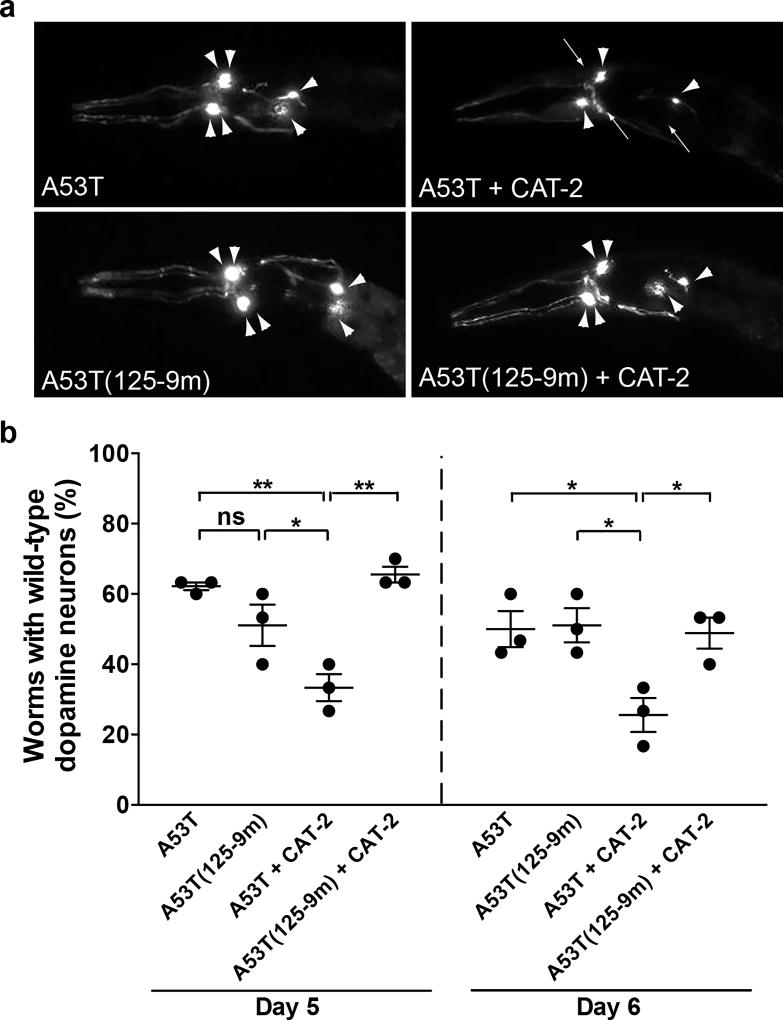
To gain further mechanistic insight into the synergistic toxicity of dopamine and α-synuclein, we took advantage of the genetic tractability of the nematode model system, Caenorhabditis elegans. Previous studies have shown that expressing human wild-type or PD-linked mutants of α-synuclein in dopaminergic neurons of C. elegans causes progressive age-dependent neurodegeneration (37–40). Overexpression of the TH homolog, CAT-2, also induces dopamine neuron loss in worms (38). To test the combined effect of these factors on neuronal health, we first generated transgenic worms expressing human A53T α-synuclein under control of the worm dopamine transporter (dat-1) promoter. C. elegans hermaphrodites have precisely eight dopamine neurons, which were visualized by co-expressing Pdat-1::GFP. Consistent with previous C. elegans models of A53T α-synuclein toxicity, we documented that 62% and 50% of A53T worms maintained normal dopamine neurons on days 5 and 6 post-hatching, respectively (Fig. 5). Notably, CAT-2 overexpression in A53T worms significantly exacerbated the degeneration of dopamine neurons, with only 33% and 26% of worms exhibiting normal neurons at days 5 and 6, respectively (Fig. 5). These data are consistent with our observations in the A53T TH-RREE mouse model and suggest that neuronal susceptibility to dopamine/α-synuclein toxicity is conserved.
Figure 5. C. elegans expressing A53T α-synuclein lacking the site of interaction with dopamine are resistant to dopamine neurotoxicity.
(a–b) Expression of A53T human α-synuclein in dopaminergic neurons of C. elegans resulted in 62% of worms with wild-type dopamine neurons at day 5 post-hatching, and 50% at day 6 post-hatching. Overexpression of CAT-2 in A53T worms significantly exacerbated toxicity at both timepoints, whereas co-expression of CAT-2 with A53T α-synuclein mutated at the site of interaction with dopamine [A53T(125-9m)] showed significant resistance to CAT-2 induced toxicity. Representative images from day 5 post-hatching are shown in (a), with intact neurons marked by arrowheads and degenerating or missing neurons by arrows. ns, not significant. The data are presented as mean ± s.e.m. (n = 30 worms per genotype per replicate, 3 independent replicates, PA53T + CAT-2 Day 5 versus A53T Day 5 = 0.0026, PA53T + CAT-2 Day 5 versus A53T(125-9m) Day 5 = 0.0393, PA53T + CAT-2 Day 5 versus A53T(125-9m) + CAT-2 Day 5 = 0.0013, F(3,8) = 15.19; PA53T + CAT-2 Day 6 versus A53T Day 6 = 0.029, PA53T + CAT-2 Day 6 versus A53T(125-9m) Day 6 = 0.0232, PA53T + CAT-2 Day 6 versus A53T(125-9m) + CAT-2 Day 6 = 0.0365, F(3,8) = 6.488; one-way ANOVA on each timepoint with Tukey’s correction for multiple comparisons). *P < 0.05, **P < 0.01.
We next investigated whether mutating α-synuclein at the site of interaction with dopamine rescues neurons from degeneration. Mutation of the Y125EMPS129 motif in the C-terminus of α-synuclein to F125AAFA129 abolishes the interaction with dopamine, and inhibits dopamine-mediated stabilization of oligomers (25, 27). Transgenic worms expressing A53T α-synuclein with the F125AAFA129 mutation [A53T(125-9m)] at similar levels as A53T α-synuclein, showed no difference in dopamine neuron degeneration compared with A53T worms (Fig. 5, Supplementary Fig. 10). However, A53T(12-9m) worms displayed complete resistance to CAT-2-induced neurodegeneration, with the percentage of worms exhibiting normal dopamine neurons restored to 66% and 49% on days 5 and 6, respectively (Fig. 5). These findings suggest that the specific interaction of dopamine with the C-terminus of α-synuclein is a critical contributor to the degeneration of dopamine neurons in vivo. The demonstration of this phenomenon in two in vivo platforms (worms and mice) strongly supports a role for dopamine-induced oligomers in the mechanism of neurotoxicity.
Discussion
In this study, we used a novel approach to enhance nigrostriatal dopamine levels in A53T mutant human α-synuclein transgenic mice. In this well-characterized mouse model, the SN does not develop α-synuclein inclusions and does not undergo neuronal cell loss (32). However, elevation of dopamine levels in the A53T mice induced significant nigrostriatal degeneration and a previously undescribed locomotor impairment. Neurodegeneration was not observed in NonTg mice receiving the same treatment, indicating that dopamine-induced toxicity is dependent on α-synuclein. We showed for the first time that dopamine promotes α-synuclein oligomerization in vivo, and furthermore that disrupting the ability of dopamine to stabilize/modify oligomers rescues neurons from dopamine toxicity. Taken together, these findings suggest that dopamine-induced α-synuclein oligomers may be a promising new target for PD treatment.
Dopamine has long been considered a contributor to the death of dopaminergic neurons in disease (3). At cytosolic pH, dopamine auto-oxidizes to form reactive quinone species, hydrogen peroxide, and other electrophiles (2). Due to the notion that dopamine may be neurotoxic, there has been widespread practice of ‘DOPA sparing’ that delays administration of the highly effective symptomatic therapy, L-DOPA, until more advanced stages of the disease. While there is presently no conclusive evidence indicating that L-DOPA accelerates PD progression, clinical trials have provided mixed results and the issue of L-DOPA toxicity is unfortunately unresolved (41–42). Despite the importance of investigating catecholamine toxicity, there are few studies in animal models. Single high dose injections of dopamine into the striatum of rats were shown to be acutely toxic in the short timeframe of one week (7). Other reports have shown that redistribution of endogenous dopamine to the cytosol is associated with toxicity in VMAT2 mutant mice (8–9). However, the long-term effects of increasing nigrostriatal dopamine levels have remained unknown.
The present study provides the first investigation of dopamine toxicity by chronic enhancement of dopamine synthesis in mice. By expressing mutant TH that is insensitive to feedback inhibition by dopamine (26–27, 31), striatal dopamine in NonTg mice was increased by over 50%. Surprisingly, this significant perturbation of dopamine homeostasis was insufficient to induce degeneration. NonTg TH-RREE mice maintained a normal number of SN neurons, intact synaptic contacts with the striatum, and robust motor coordination/balance. The excess dopamine was likely released into the synapse leading to the observed hyperactivity, consistent with other studies (43). It is possible the excess dopamine was well tolerated due to compensatory upregulation of metabolic enzymes and transporters; indeed, there was a significant increase in DAT levels in these mice.
α-Synuclein has been independently linked to PD through dominantly inherited mutations and multiplications of the SNCA gene (10–17), and by the presence of aggregated α-synuclein in Lewy bodies and Lewy neurite inclusions (18–19). Yet efforts to model PD by α-synuclein overexpression in transgenic mice has often resulted in a lack of inclusion pathology and neurodegenerative changes in the SN. Dopaminergic neurons are entirely spared in the A53T transgenic mouse model used in the present work (32) and we also document a normal complement of neuronal cell bodies and terminals and a lack of intraneuronal α-synuclein inclusions in the SN in A53T CtrlVect mice. This may be related to several factors including the fact that mouse α-synuclein naturally has a threonine at position 53. In vitro, mouse α-synuclein fibrillizes more rapidly than human wild-type or A53T α-synuclein, and yet aged mice do not spontaneously develop α-synuclein inclusions or a PD-like disorder (44). It is likely that mice have evolved protective mechanisms against α-synuclein aggregation, such as an enhanced ability to maintain α-synuclein in a physiological lipid- or protein-bound state, and that these mechanisms may counteract efforts to initiate disease by transgenic α-synuclein expression.
Our study offers another possible explanation for the challenges in producing SN neurodegeneration in α-synuclein transgenic mice: lack of dopamine dysregulation. In stark contrast to NonTg mice, elevation of dopamine levels in A53T mice resulted in progressive nigrostriatal degeneration with locomotor impairment, markedly improving this mouse as a model of PD. Similarly, co-expression of CAT-2 and A53T α-synuclein in C. elegans induced more severe dopamine neuron degeneration than A53T α-synuclein expression alone. These findings are consistent with a limited number of studies reporting toxicity associated with the convergence of dopamine and α-synuclein. In the case of VMAT2-deficient mice, loss of SN neurons was only observed in animals on a normal α-synuclein background, whereas identical reduction of VMAT2 in animals with Snca gene locus deletion did not result in cell loss (8–9, 45). In C. elegans expressing wild-type human α-synuclein, increasing cytosolic dopamine by mutation of the worm homolog of VMAT2 accelerated neurodegeneration. Conversely, protection from neuronal loss was reported for worms expressing wild-type human α-synuclein and a nonfunctional mutant of CAT-2 that prevented dopamine synthesis (40). Additionally, in rodent midbrain cultures, elevating cytosolic dopamine concentration reduced dopaminergic cell survival and this effect was dependent on α-synuclein (46).
The synergistic toxicity of dopamine and α-synuclein may be mediated by α-synuclein oligomers, which are thought to be neurotoxic species. Lentiviral vector delivery of artificial α-synuclein variants with enhanced oligomerization into the rat SN resulted in the selective loss of dopaminergic cells (47). Similarly, artificial mutants with impaired β-sheet structure and increased propensity to form oligomers induced degeneration of dopaminergic nerve terminals in worms and overt dopaminergic cell loss in flies (39). In addition, oligomer-containing extracts from A53T transgenic mice were toxic when applied to primary mouse cortical cultures (34). While it is known that oxidized dopamine kinetically stabilizes soluble α-synuclein oligomers in cell-free systems (24–25) and in SH-SY5Y cultures (26–27), the toxicity of these species is largely unexplored. Evidence from studies conducted in vitro suggests that dopamine-induced oligomers can block their own degradation and that of other substrates by chaperone-mediated autophagy (29), and can reduce neurotransmitter release by inhibition of SNARE complex formation (30). In human fetal dopaminergic cultures, reduced viability was linked to endogenous dopamine and the accumulation of soluble α-synuclein protein complexes (48).
Critically, the effects of dopamine on α-synuclein oligomerization in vivo have not been investigated. We therefore sought to determine if A53T TH-RREE mice had alterations in oligomer biochemical and/or functional properties. Soluble α-synuclein oligomers were extracted from the SN and enriched by SEC under non-denaturing conditions in order to preserve native conformations. Increasing dopamine resulted in greater total levels of α-synuclein oligomers detected by western blot. The oligomers included species with Stokes radii of up to 65 Å that were also detected in A53T CtrlVect mice, consistent with previous characterization of oligomers in aged A53T mice (34). Larger oligomers of up to 122 Å were uniquely observed in A53T TH-RREE mice, suggesting that dopamine is capable of modifying oligomer conformations. These species may reflect dopamine-induced remodeling of existing oligomers, and/or formation of oligomers from α-synuclein monomers de novo. Additionally, oligomers with larger Stokes radii may contain a greater number of monomer units than lower Å species, and/or occupy less compacted arrangements.
Multiple biochemical and imaging approaches were employed to characterize the oligomers extracted from the SN. In addition to well-established methods such as western blotting and SEC, immunoelectron microscopy was performed on SEC fractions to visualize mouse-derived α-synuclein oligomers. Currently, there is no accepted method of specifically imaging α-synuclein oligomers in brain tissue in situ. However, with our approach, we were able to identify and image oligomers of known Stokes radii. Oligomer species from both A53T TH-RREE and A53T CtrlVect mice were labeled with antibodies directed at the N- or C-terminus of α-synuclein, suggesting that epitopes at both ends of the protein were exposed. These epitopes were also exposed in soluble α-synuclein oligomers generated with dopamine in vitro, consistent with previous imaging by immunoelectron microscopy (25). Furthermore, LB509 and Syn505 antibodies are known to react robustly with α-synuclein in pathological inclusions in human disease brain (19, 32). The reactivity of both mouse-derived and recombinant oligomers with these antibodies suggests they may share disease-associated conformations.
Unlike the α-synuclein species extracted from A53T CtrlVect mice, oligomers from A53T TH-RREE mice were unable to act as seeds for α-synuclein fibrillization in vitro. Additionally, we did not detect dopamine-modified oligomers in cortical tissue from A53T TH-RREE mice. These data argue against both intracellular and inter-cellular prion-like propagation of these species. While α-synuclein aggregates that exhibit prion-like behavior have been shown in many contexts to be cytotoxic (34, 36), seeding ability is neither necessary nor sufficient for toxicity. In a comparison of different oligomer species generated in vitro, α-synuclein oligomers that were toxic to SH-SY5Y cultures were also incapable of seeding intracellular aggregation of α-synuclein. Conversely, seeding-competent oligomers were found to be non-toxic when applied to SH-SY5Y cells (49). While the spread of α-synuclein pathology in PD may primarily be driven by self-replicating seeding and cell-to-cell transmission, toxicity may depend on the interaction of aggregates with cell-type specific factors. In this regard, dopamine may act as an enhancer of oligomer toxicity. Moreover, dopamine-modified species may resist sequestration into non-toxic fibrils or inclusions, and may therefore be available to participate in pathological interactions that ultimately lead to neuron death.
While the mouse data clearly demonstrate an association between the neurodegenerative/locomotor disease phenotype and the presence of modified and more abundant α-synuclein oligomers, the functional evidence from our transgenic C. elegans models further establishes this link. It is well-documented that the Y125EMPS129 motif in α-synuclein is required for the stabilization of α-synuclein oligomers by dopamine in vitro. Mutation or deletion of this motif restores the ability of α-synuclein to fibrillize in the presence of dopamine (25), and the increase in oligomeric species in SH-SY5Y cells with elevated dopamine is prevented by expression of α-synuclein lacking this region (27). Worms expressing A53T α-synuclein specifically mutated in the Y125EMPS129 domain were entirely resistant to the neurotoxic effects of increasing dopamine levels. Therefore, preventing the interaction of dopamine and α-synuclein and thereby inhibiting its effects on α-synuclein oligomers, is neuroprotective. While we were not able to biochemically characterize α-synuclein oligomer species in these worms due to expression in only eight cells in the entire animal, our findings nonetheless support a key role of dopamine in neurodegeneration, specifically through its influence on α-synuclein.
Collectively, this work underscores the potential critical importance of the interaction of dopamine and α-synuclein in driving disease. In PD, dopaminergic terminals are thought to degenerate prior to cell bodies (50), suggesting that the disease may arise at the synapse. Increasing dopamine levels in A53T mice recapitulated the substantial loss of terminals that precedes overt nigral cell death, offering a new model of disease progression in PD. These mice also underwent an eventual decline in dopamine levels and developed an associated motor deficit, mimicking the depletion of striatal dopamine and resulting hypokinesia that occurs in PD. Our findings also demonstrate that dopamine modifies α-synuclein aggregation in vivo, resulting in oligomer conformations that are biochemically and structurally similar to neurotoxic oligomers induced by dopamine in vitro. Finally, we were able to rescue dopaminergic neurons from dopamine toxicity by specifically inhibiting its interaction with α-synuclein in C. elegans. Dopamine-modified α-synuclein species may mediate neurodegeneration by disrupting cellular membranes, as previously proposed for α-synuclein oligomers (49). Moreover, damage to synaptic vesicle membranes could lead to dopamine leakage and further induction of pathogenic α-synuclein species.
Online Methods
Animals
The mice (Mus musculus) used in this study were homozygous for expression of human A53T α-synuclein under the mouse PrP promoter (line M83). These mice undergo spinal cord degeneration at approximately 18 months of age in our colony, and the phenotype has been described previously (32). Any animals showing symptoms of spinal cord degeneration, i.e. hunched back, altered gait, or hindlimb paralysis, were excluded from the study. NonTg littermates were also used for experiments. No care was taken as to the sex of the animals. Mice were randomly assigned to the lentiviral vector injection groups and subsequent analyses, and the organization of experimental conditions was also random. Data collection and analyses were not performed blind to the conditions of the experiments except where otherwise indicated. All animal work was conducted according to National Institute of Health guide for the care and use of laboratory animals and in compliance with procedures approved by the Children’s Hospital of Philadelphia Institutional Animal Care and Use Committee.
Production of Lentiviral Vectors and Determination of Titer
Generation of human tyrosine hydroxylase-1 with mutation of R37R38 to E37E38 (TH-RREE) has been previously described (26). The coding sequence was removed from the pcDNA3.1 vector and was subcloned into the self-inactivating pTY-linker lentiviral expression vector at the Pme1 site downstream of the CMV promoter. Generation of replication-deficient pseudotyped HIV-derived lentiviral vectors was achieved by transient co-transfection of HEK293T cells with the expression vector along with the vectors carrying the additional transcripts required for encapsulation, packaging and envelope proteins. 24 hours after the cells were seeded into 150 mm dishes coated with poly-D-Lysine they were transiently transfected with a mixture containing 550 µg of the pTY-TH-RREE expression vector, 357.5 µg of CMVD82 packaging vector, and 192.5 µg of pVSV-G envelope vectors using the calcium phosphate transfection protocol. The culture medium was changed every day and the media containing the virus was collected 72 hours post-transfection, filtered through 0.45 µm membranes, and concentrated by ultracentrifugation at 50,000 g for 2 hours at 4°C. The viral pellet was subsequently re-suspended in 800 µL of DMEM medium to make a concentrated viral stock. An empty vector control virus containing all elements except for the gene encoding TH-RREE (CtrlVect) was generated in parallel and both viruses were obtained from the University of Pennsylvania Vector Core.
To determine vector functionality and viral titer, SH-SY5Y human neuroblastoma cells (American Type Culture Collection) were plated in 24-well plates at 5×104 cells/well in DMEM/F12 medium containing 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin. The cells were allowed to recover for 2 days and were then differentiated for 5 days with 20 µM retinoic acid. The cells were infected with different dilutions of CtrlVect or TH-RREE lentivirus and viral titers were calculated by TH immunocytochemistry as described previously (26) for 3 independent cell plating/infection experiments.
Stereotaxic Injection of Lentiviral Vector into the SN
A53T α-synuclein transgenic mice or non-transgenic littermates received bilateral lentiviral vector injections at 10 months of age. The mice were placed into an anesthesia chamber connected to an isofluorane delivery system to induce anesthesia. The mice were then placed into a stereotaxic head holder, an anesthesia mask was fitted, and an incision was made in the skin. Small bilateral holes were drilled into the skull according to the coordinates for the SN in the mouse brain (anterior-posterior −3.3 mm from bregma, mediolateral 1 mm) and a 30g Hamilton syringe was inserted slowly to 4 mm dorsoventral depth. 2 µl of the viral concentrate (5×107 IU/ml) was injected into the SN at a rate of 0.25 µl/min, and the needle was left in place for an additional 5 min before being slowly withdrawn, in order to minimize leakage of the injected fluid. The skin was sutured sterilely and the animals were monitored until they recovered from anesthesia.
Motor Function Testing
All behavioral tests were conducted 1 week prior to sacrifice. Mice were habituated to the testing room for 2 hours before testing. To measure open field activity, the mice were singly housed and the cage was placed inside a laser monitoring device consisting of an open, rectangular frame containing sensors (Opto-M3 activity meter, Columbus Instruments). The number of infrared beam breaks was quantified over a 12-hour period during the dark cycle from 6 pm to 6 am using Multi Device Interface software version 1.3 (Columbus Instruments). For each animal, data was collected for up to 3 consecutive days and averaged. Food and water were available ad libitum.
To assess motor coordination and balance, mice were tested on the Rotarod (Ugo Basile, model 7650). The mice were acclimated to the apparatus with four training sessions of 5 min each at 4 rpm, followed by 5 min of rest in the home cage. Testing was performed one hour later with two trials in which speed was accelerated from 4 to 40 rpm in 300 sec. For each mouse, the latency to fall off the rotarod within this time period was recorded for the two trials and averaged.
Immunohistochemistry and Stereological Cell Counts
For histological analysis, the mice were deeply anesthetized and perfused transcardially with saline followed by 4% PFA in 0.1 M phosphate buffer (pH 7.4). The brains were post-fixed overnight at 4°C in 4% PFA and then cryoprotected in 30% sucrose solution for 2 days at 4 °C. The brains were submerged in dry ice-chilled isopentane for 30 sec and then stored at −80 °C until sectioning. On the day of sectioning, brains were mounted on chucks with OCT (Tissue-Tek) and the brainstem, SN, and striatum were each sectioned using a cryostat (Leica, Jung Frigocut 2800N). Free-floating coronal sections of alternating 10 and 30 µm thickness were collected from each region and stored at 4°C in 0.1 M phosphate buffer containing 0.01% sodium azide.
The sections were quenched in 5% H2O2 in methanol for 30 min, followed by antigen retrieval by boiling in citrate solution pH 6 for 10 min. The tissue was blocked for 1 hour at RT with 5% NGS, 3% BSA in PBS containing 0.1% Triton X-100, and then incubated overnight at 4°C with primary antibody diluted in blocking buffer. Antibodies were used against VMAT2 (rabbit, 1:20000, Covance Custom Immunology Services; courtesy of Dr. Gary Miller at Emory University, Atlanta, GA) (43), TH (rabbit, 1:2000, Calbiochem 657012) (51), and Syn505 (mouse, 1:5000, courtesy of Dr. Virginia M. Lee at University of Pennsylvania, Philadelphia, PA) (32). Incubation with biotin-conjugated secondary antibodies was followed by avidin-biotin-peroxidase complex, each for 1 hour at RT (Vector Laboratories PK-6101, PK-6102) (34), and visualization by 3,3-diaminobenzidine (DAB) for 1 min (Vector Laboratories). In some cases, sections were counterstained with Cresyl Violet. The tissue was then mounted, dehydrated in an increasing alcohol series and cleared in xylenes, and coverslipped with Permount mounting medium. Slides were scanned by the Children’s Hospital of Philadelphia Pathology Core facility and images were accessed using Aperio Imagescope software version 11.2 (Leica Biosystems). Quantification of VMAT2 staining intensity in the striatum was performed using Image J software version 1.48 (National Institutes of Health).
Unbiased stereological quantification of neurons in the SN was performed as described previously (33). Briefly, 30 µm sections spaced 90 µm apart were selected in order to represent the full rostro-caudal axis of the SN. The sections were stained with Cresyl Violet and the Nissl-positive neurons were blindly counted using the optical fractionator method (StereoInvestigator version 11.02.1, MBF Bioscience).
Catechol Quantification by HPLC with Electrochemical Detection
Striatal tissues were homogenized by sonication in 10 volumes of 0.1 M perchloric acid containing 1 µM 3,4 dihydroxybenzylamine as an internal standard. The samples were centrifuged at 16,000 g for 10 min at 4°C and the supernatants were filtered through 0.22 µm filters. 50 µl of each sample was injected onto an Agilent 1100 series HPLC (Palo Alto, CA) controlled by ChemStation software version 1.04 (Agilent). Catechols were resolved on a reverse-phase C18 Luna column (150×4.6 mm, 5 µm; Phenomenex) and detected using a Coularray detector (ESA Biosciences, Chelmsford, MA) as previously described (26). Protein pellets were solubilized in 50 mM Tris pH 7.4 containing 2% SDS and protein concentration was determined using the BCA microassay kit (Pierce). Monoamine levels were normalized to protein concentration and expressed as femtomoles analyte /µg of protein.
Sequential Extraction and Native Size Exclusion Chromatography
Striatal or SN tissue from individual mice was homogenized in 10 volumes of lysis buffer: 1% Triton X-100 in 20 mM HEPES pH 7.4, 150 mM NaCl, 10% glycerol, 1 mM EGTA, and protease inhibitor cocktail (P2714, Sigma). The tissue was grinded with a mechanical homogenizer and centrifuged at 16,000 g for 10 min at 4°C. The pellet was further extracted by sonication in 2% SDS, 50 mM Tris pH 7.4 with protease inhibitor cocktail, boiling at 95°C for 10 min, and centrifugation at 16,000 g for 10 min. The resulting supernatant was designated the Triton-insoluble fraction. Protein concentration was determined using the BCA assay (Pierce).
For size exclusion chromatography (SEC), 500 µg of Triton-soluble SN tissue in a total volume of 270 µl was loaded onto a Superdex 200 HR10/30 column (GE Healthcare) connected to an Agilent 1100 series HPLC system (Palo Alto, CA) controlled by ChemStation software version 1.04 (Agilent). Mobile phase consisted of 25 mM HEPES and 150 mM NaCl, pH 7.25 and the flow rate was set to 0.3 mL/min. Fractions corresponding to 122–94, 94–81, 81–72, 72–65, 65–59, 59–54, 54–50, 50–45, 45–41, 41–38, 38–34, and 34–32 Å were each pooled and concentrated with 3,000 NMWL Ultracel Microcon filters (Millipore). Fractions 122–72, 65–41, and 38–32 were further combined to enhance the α-synuclein signal on SDS-PAGE. The SEC column was calibrated using globular protein standards (GE Healthcare).
Western Blotting
Proteins sequentially extracted or further fractionated by SEC were run on 10% or 12% SDS-PAGE gels and transferred to PVDF membranes. The membranes were blocked for 1 hour at RT in 5% (w/v) milk in 20 mM Tris pH 7.4, 150 mM NaCl and 0.1% Tween. Incubation with primary antibodies diluted in blocking buffer was overnight at 4°C. Antibodies used against synaptic proteins were TH (rabbit, 1:4000, Calbiochem 657012) (52), DAT (rat, 1:1000, Millipore MAB369) (36), D1 receptor (rat, 1:1000, Sigma D2944) (36), DARPP-32 (rabbit, 1:1000, Cell Signaling 2306S) (53). Antibodies directed against α-synuclein were LB509 (mouse, 1:1000) (32, 34), Syn505 (mouse, 1:1000) (54), and SNL-4 (rabbit, 1:1000) (55), all courtesy of Dr. Virginia M. Lee at University of Pennsylvania, Philadelphia, PA. Loading controls for Triton-soluble proteins were GAPDH (mouse, 1:10000, Abcam ab8245) (56), NSE (rabbit, 1:4000, Abcam ab53025) (57), and Actin (rabbit, 1:1000, Sigma A2066) (58). The loading control for Triton-insoluble proteins was Vimentin (mouse, 1:1000, BD Pharmingen 550513) (34). Membranes were incubated for 1 hour at RT with secondary antibodies conjugated to IRDye 680 or 800 (1:5000, Rockland) (34) and then scanned using an Odyssey Infrared Imaging System (Li-Cor). Quantification of protein levels was performed using ImageStudio software version 4.0.21 (Li-Cor) and was normalized to loading control levels. For α-synuclein oligomer bands, all individual immunobands >19 kD that were detected were quantified and the intensities summed, and the total was divided by the total NSE.
Near-infrared (nIRF) scanning of oxidized catechols was performed as described previously (59). Briefly, prior to transfer the SDS-PAGE gels were scanned in the 700 nm channel at intensity 10 on an Odyssey Infrared Imaging System (Li-Cor).
Immunoelectron Microscopy of SEC Fractions
SEC fractions from SN tissue corresponding to low (41–65) and high (72–122) Å Stokes radii or dopamine-incubated recombinant α-synuclein were applied to 300 mesh carbon-coated grids and blocked with 1% BSA in 20 mM Tris pH 7.4, 150 mM NaCl. The α-synuclein oligomers were labeled with the Syn505 antibody against the N-terminus or LB509 antibody against the C-terminus, followed by 10 nm gold conjugated secondary (Electron Microscopy Sciences) as previously described (25). Grids were negatively stained with 1% uranyl acetate and imaged at the University of Pennsylvania Electron Microscopy Resource Laboratory. Control samples were immunodepleted of α-synuclein (see below) prior to grid preparation or had primary antibody omitted.
In Vitro Aggregation and Seeding Assays
Recombinant human wild-type α-synuclein was expressed and purified as described previously (34). Purified α-synuclein was incubated at 6 mg/mL (415 µM) with or without equimolar dopamine at 37°C and shaking at 1400 rpm for up to 6 days. At indicated timepoints, fibrillar content of the reaction mixture was assayed by the addition of Thioflavin T (Sigma) to a final concentration of 25 µM. Fluorescence emission was measured at 482 nm during excitation at 450 nm. Sedimentation analysis was performed by centrifugation at 16,000 g for 10 min at 4°C. The supernatants and pellets were boiled in SDS sample buffer at 95°C for 10 min. α-Synuclein aggregates were resolved by SDS-PAGE and the gels were stained with Coomassie Blue R-250. Circular dichroism spectra were obtained using a Jasco J-810 spectropolarimeter at the Children’s Hospital of Philadelphia Protein Core facility. The protein was diluted to 20 µM in 0.05 M KH2PO4 pH 7.8. Spectra were corrected for baseline measurement of an equivalent volume of PBS diluted in KH2PO4 buffer. The n value reported equals the number of independent aggregation assays.
Seeding assays were performed by incubating 5 µg total protein from pooled oligomeric SEC fractions (41–122 Å) for each mouse with 425 µg fresh recombinant α-synuclein (300 µM final concentration) at 37°C and shaking at 1400 rpm. Aliquots at indicated timepoints were analyzed by Thioflavin T. To immunodeplete α-synuclein for control samples, pooled SEC fractions were incubated overnight at 4°C with LB509 antibody at a 1:5 ratio of antibody to total µg protein. Protein G-conjugated beads (Sigma) were equilibrated in 25 mM HEPES, 150 mM NaCl, pH 7.4 and incubated with the immunocomplexes for 1 hour at 4°C. α-Synuclein oligomers were then pulled down by centrifugation at 2000 g for 2 min, and the resulting supernatant was used for experiments.
C. elegans Models
α-Synuclein plasmids for microinjection into worms were generated using Gateway technology (Invitrogen, San Diego, CA). Plasmids containing sequences for human A53T α-synuclein or A53T α-synuclein with Y125EMPS129 mutated to F125AAFA129 [A53T(125-9m)] were generated previously (27). The coding sequences were PCR-amplified with addition of flanking attB1 and attB2 sites, and the resulting fragments were used to generate Gateway entry vectors by BP reaction with pDONR221. LR reactions were then performed with each entry vector and the previously constructed pDEST-DAT-1 destination vector containing the dat-1 promoter (38). All primer sequences are available upon request.
Nematodes were maintained using well-established methods (60). Constructs were injected into worms to create transgenic animals as previously described (61). Strains UA287 (baEx168 a,b,c [Pdat-1::A53T α-synuclein, Punc-54::tdTomato];vtIs7 [Pdat-1::GFP]) and UA288 (baEx169 a,b,c [Pdat-1::A53T(125-9m) α-synuclein, Punc-54::tdTomato];vtIs7 [Pdat-1::GFP]) were generated by injecting a solution of 50 ng/µl of either Pdat-1::A53T α-synuclein or Pdat-1::A53T(125-9m) α-synuclein into strain BY250 (vtIs7 [Pdat-1::GFP]) with a phenotypic marker (Punc-54::tdTomato, 50 ng/µl, for body wall muscle expression). Three independent stable lines were created for each group (a, b, c). To analyze CAT-2 overexpression in these same backgrounds, we removed the Pdat-1::GFP transgene from the UA287 and UA288 transgenic backgrounds by outcrossing to the N2 background. Then these two strains, which still contained the Punc-54::tdTomato phenotypic marker, were both crossed into UA57 (baIn4 [Pdat-1::CAT-2, Pdat-1::GFP]), generating UA296 (baEx168 a,b,c [Pdat-1::A53T α-synuclein, Punc-54::tdTomato];baIn4 [Pdat-1::CAT-2, Pdat-1::GFP] and UA297 (baEx169 a,b,c [Pdat-1::A53T(125-9m) α-synuclein, Punc-54::tdTomato];baIn4 [Pdat-1::CAT-2, Pdat-1::GFP].
For dopaminergic neurodegeneration analyses, transgenic hermaphroditic animals were scored as described previously (62). Briefly, on the day of analysis, the six anterior dopaminergic neurons [four CEP (cephalic) and two ADE (anterior deirid)] were examined in 30 randomly selected worms that also express the tdTomato marker in the body wall muscle cells. Each animal was considered normal when there was a full complement of six anterior DA neurons. However, if a worm exhibited any degenerative phenotype, such as a missing dendritic process, cell body loss, or a blebbing neuronal process, it was scored as degenerating. Three independent transgenic worm lines were analyzed per genetic background and an average of the total percentage of worms with normal neurons was reported in the study.
For label free quantification of α-synuclein expression by mass spectrometry, protein from GFP and tdTomato positive day 1 adult nematodes was extracted in a buffer containing 50 mM Tris, pH 7.5 with 8 M urea and 2% SDS. 50 µg of soluble protein underwent buffer exchange to PBS and concentrated using 10 kDa cutoff spin filters (Amicon). The concentrated protein was combined with 6× loading buffer and run on a 10% NuPAGE gel with MES running buffer (Invitrogen). The gel was fixed and stained using Novex Colloidal Blue staining kit (Invitrogen). Purified recombinant α-synuclein was used as a marker for sample bands of interest. Sample bands of interest with apparent molecular weight of 14 kDa were excised from the gel and digested with trypsin overnight. Trypsin digests were then analyzed by LC-MS/MS on a hybrid LTQ Orbitrap Elite mass spectrometer (ThermoFisher) coupled with a nanoLC Ultra (Eksigent). The mass spectrometer was set for parallel reaction monitoring for human α-synuclein sequence with the A53T mutation.
Primary Neuronal Cultures
Hippocampal neurons were provided by the University of Pennsylvania Neuron Culture Service Center. After dissection from C57BL/6 mouse embryos at day 18–19, cells were mechanically dissociated, trypsinized, and seeded at 100,000 cells per well into 24-well plates freshly coated with 50 µg/ml poly-D-lysine (Sigma). After plating, neurons were cultured for 2 hours in culture media containing 5% heat-inactivated fetal bovine serum, 1% Glutamax, 2% B-27 supplement, 100 U/ml penicillin, 100 µg/ml streptomycin in Neurobasal media (all from Invitrogen). After 2 hours, the media was changed to culture media without fetal bovine serum to discourage survival of glial cells. Twice per week, half of the media was replaced with fresh culture media.
Following one week in culture, neurons were treated with dopamine-incubated α-synuclein (from day 4–6 of in vitro aggregation) at a final concentration of 1 µM. Control conditions were equivalent doses of PBS, monomeric α-synuclein, or dopamine that had been incubated in parallel under aggregation conditions but without α-synuclein. Two weeks post-treatment, cell viability was assayed using calcein AM (Sigma) and propidium iodide (PI) (Sigma) incubated for 20 min at RT at final concentrations of 3 µM in PBS. Fluorescence images were obtained using MetaMorph software version 7.7 (Molecular Devices) and an inverted Olympus IX70 microscope equipped with an IX-FLA fluorescence observation attachment (Olympus Optical Co., Tokyo, Japan). For quantification of viable cells (calcein-positive and PI-negative), at least 10 random fields of view were blindly counted per treatment condition and averaged to obtain one replicate value. Independent culture experiments performed with separate plating/treatments were considered independent replicates and were reported as the n value.
To image human α-synuclein in treated neurons, cells were labeled in a two-stage protocol as previously described (63). Two weeks following treatment, cells were live-incubated in media containing Syn204 antibody (mouse IgG2a, 1:500) for 1 hour at 4°C to label extracellular α-synuclein. The cells were then fixed with 4% PFA for 20 min at RT, and permeabilized with 0.1% Triton X-100 in 5% NGS, 3% BSA in PBS for 1 hour at RT. Cells were incubated with LB509 (mouse IgG1, 1:500) overnight at 4°C to label both extracellular and intracellular α-synuclein. Secondary antibodies conjugated to Alexa Fluor (anti-mouse IgG1 488, 1:500, Thermo Fisher A21121; anti-mouse IgG2a 594, 1:500, Thermo Fisher A-21135) were incubated for 1 hour at RT. The staining was imaged by laser-scanning confocal microscopy (Olympus Fluoview) and the n value equals the number of independent culture experiments.
Statistics
All statistical analysis was done using Prism 6 software (GraphPad). Two-tailed unpaired Student’s t test was used for all comparisons between two groups. For comparisons with multiple groups, one-way ANOVA using Tukey’s post-hoc correction for multiple comparisons. Specifically for Thioflavin T assays with multiple groups measured over time, repeated-measures two-way ANOVA with Tukey’s or Bonferroni’s correction for multiple comparisons was used. No statistical methods were used to pre-determine sample sizes but our sample sizes are similar to those reported previously (34, 36). All experiments were performed at least twice, with measurements from the same samples treated as technical replicates. In the case of representative images, several images per sample were obtained. Readers are referred to the Life Sciences Reporting Summary available online for additional information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health AG013966 (H. Ischiropoulos), NS038690 (J. Wolfe), and NS087077 and NS052325 (R. Kalb). D. Mor was supported by National Institutes of Health Ruth L. Kirschstein National Research Service Award Individual Predoctoral Fellowship NS087779. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank T. Clarke and T. Pierson from the Wolfe lab for technical assistance with animals and for helpful advice on vector production. We thank H. Bennett from the Kalb lab for initial work on the dopamine neuron degeneration assay in C. elegans and input on worm breeding. We thank L. Spruce, H. Fazelinia and S. Seeholzer from the Children’s Hospital of Philadelphia Proteomic Core facility for mass spectrometry. We thank V. Lee (University of Pennsylvania, Philadelphia, Pennsylvania, USA) and G. Miller (Emory University, Atlanta, Georgia, USA) for generously providing α-synuclein and VMAT2 antibodies, respectively, and S. Przedborski (Columbia University, New York, NY, USA) for the use of stereology equipment and helpful feedback on the manuscript. Finally, we thank R. Lightfoot and members of the Ischiropoulos lab for productive discussions and technical support.
Footnotes
Author Contributions
D.E.M., E.T., J.R.M., R.G.K., and H.I. conceived and designed the experiments. D.E.M., E.T., H.K., N.S.G., M.J.D., S.D., P.G., J.L.G., and V.X.T. performed the experiments and analyzed the data. D.E.M. wrote the paper, with important contributions from J.R.M., E.T., J.H.W., R.G.K., K.A.C., G.A.C, and H.I.
Competing Financial Interests Statement
The authors declare no competing financial interests.
References
- 1.Ehringer H, Hornykiewicz O. Verteilung von Noradrenalin und Dopamin (3-Hydroxytyramin) im Gehirn des Menschen und ihr Verhalten bei Erkrankungen des extrapyramidalen Systems. Klin Wschr. 1960;38:1236–1239. [Google Scholar]
- 2.Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol. 1978;14:633–43. [PubMed] [Google Scholar]
- 3.Jenner P, Olanow CW. Oxidative stress and the pathogenesis of Parkinson's disease. Neurology. 1996;47:S161–70. doi: 10.1212/wnl.47.6_suppl_3.161s. [DOI] [PubMed] [Google Scholar]
- 4.Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, Jenner P, Marsden CD. Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J Neurochem. 1989;52:381–9. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Perry G, Smith MA, Robertson D, Olson SJ, Graham DG, Montine TJ. Parkinson’s disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am J Pathol. 1999;154:1423–1429. doi: 10.1016/S0002-9440(10)65396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 7.Hastings TG, Lewis DA, Zigmond MJ. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc Natl Acad Sci USA. 1996;93:1956–61. doi: 10.1073/pnas.93.5.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colebrooke RE, Humby T, Lynch PJ, McGowan DP, Xia J, Emson PC. Age-related decline in striatal dopamine content and motor performance occurs in the absence of nigral cell loss in a genetic mouse model of Parkinson's disease. Eur J Neurosci. 2006;24:2622–30. doi: 10.1111/j.1460-9568.2006.05143.x. [DOI] [PubMed] [Google Scholar]
- 9.Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, Colebrooke RE, Di Monte DA, Emson PC, Miller GW. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27:8138–48. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–47. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 11.Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, Przuntek H, Epplen JT, Schöls L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 12.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 13.Chartier-Harlin M-C, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destée A. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 14.Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atarés B, Llorens V, Gomez Tortosa E, del Ser T, Muñoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 15.Proukakis C, Dudzik CG, Brier T, MacKay DS, Cooper JM, Millhauser GL, Houlden H, Schapira AH. A novel α-synuclein missense mutation in Parkinson disease. Neurology. 2013;80:1062–4. doi: 10.1212/WNL.0b013e31828727ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lesage S, Anheim M, Letournel F, Bousset L, Honoré A, Rozas N, Pieri L, Madiona K, Dürr A, Melki R, Verny C, Brice A, French Parkinson's Disease Genetics Study Group G51D α-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann Neurol. 2013;73:459–71. doi: 10.1002/ana.23894. [DOI] [PubMed] [Google Scholar]
- 17.Pasanen P, Myllykangas L, Siitonen M, Raunio A, Kaakkola S, Lyytinen J, Tienari PJ, Pöyhönen M, Paetau A. Novel α-synuclein mutation A53E associated with atypical multiple system atrophy and Parkinson's disease-type pathology. Neurobiol Aging. 2014;35:2180. doi: 10.1016/j.neurobiolaging.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 18.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 19.Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am J Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- 20.Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T. The precursor protein of non-A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–75. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 21.George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15:361–72. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 22.Abeliovich A, Schmitz Y, Fariñas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–52. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 23.Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20:3214–20. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conway KA, Rochet JC, Bieganski RM, Lansbury PT. Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 25.Norris EH, Giasson BI, Hodara R, Xu S, Trojanowski JQ, Ischiropoulos H, Lee VM-Y. Reversible inhibition of alpha-synuclein fibrillization by dopaminochrome-mediated conformational alterations. J Biol Chem. 2005;280:21212–21219. doi: 10.1074/jbc.M412621200. [DOI] [PubMed] [Google Scholar]
- 26.Mazzulli JR, Mishizen AJ, Giasson BI, Lynch DR, Thomas SA, Nakashima A, Nagatsu T, Ota A, Ischiropoulos H. Cytosolic catechols inhibit alpha-synuclein aggregation and facilitate the formation of intracellular soluble oligomeric intermediates. J Neurosci. 2006;26:10068–10078. doi: 10.1523/JNEUROSCI.0896-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazzulli JR, Armakola M, Dumoulin M, Parastatidis I, Ischiropoulos H. Cellular oligomerization of alpha-synuclein is determined by the interaction of oxidized catechols with a C-terminal sequence. J Biol Chem. 2007;282:31621–31630. doi: 10.1074/jbc.M704737200. [DOI] [PubMed] [Google Scholar]
- 28.Herrera FE, Chesi A, Paleologou KE, Schmid A, Munoz A, Vendruscolo M, Gustincich S, Lashuel HA, Carloni P. Inhibition of alpha-synuclein fibrillization by dopamine is mediated by interactions with five C-terminal residues and with E83 in the NAC region. PLoS One. 2008;3(10):e3394. doi: 10.1371/journal.pone.0003394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez-Vicente M, Talloczy Z, Kaushik S, Massey AC, Mazzulli J, Mosharov EV, Hodara R, Fredenburg R, Wu D-C, Follenzi A, Dauer W, Przedborski S, Ischiropoulos H, Lansbury PT, Sulzer D, Cuervo AM. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest. 2008;118:777–788. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi BK, Choi MG, Kim JY, Yang Y, Lai Y, Kweon DH, Lee NK, Shin YK. Large α-synuclein oligomers inhibit neuronal SNARE-mediated vesicle docking. Proc Natl Acad Sci USA. 2013;110:4087–92. doi: 10.1073/pnas.1218424110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakashima A, Kaneko YS, Mori K, Fujiwara K, Tsugu T, Suzuki T, Nagatsu T, Ota A. The mutation of two amino acid residues in the N-terminus of tyrosine hydroxylase (TH) dramatically enhances the catalytic activity in neuroendocrine AtT-20 cells. J Neurochem. 2002;82:202–6. doi: 10.1046/j.1471-4159.2002.00921.x. [DOI] [PubMed] [Google Scholar]
- 32.Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM-Y. Neuronal alpha- synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 33.Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD, Naini A, Vila M, Jackson-Lewis V, Ramasamy R, Przedborski S. D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest. 2003;112:892–901. doi: 10.1172/JCI18797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsika E, Moysidou M, Guo J, Cushman M, Gannon P, Sandaltzopoulos R, Giasson BI, Krainc D, Ischiropoulos H, Mazzulli JR. Distinct region-specific alpha-synuclein oligomers in A53T transgenic mice: implications for neurodegeneration. J Neurosci. 2010;30:3409–3418. doi: 10.1523/JNEUROSCI.4977-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Souza JM, Giasson BI, Chen Q, Lee VM, Ischiropoulos H. Dityrosine cross-linking promotes formation of stable alpha -synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J Biol Chem. 2000;275:18344–9. doi: 10.1074/jbc.M000206200. [DOI] [PubMed] [Google Scholar]
- 36.Luk KC, et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–53. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lakso M, Vartiainen S, Moilanen A-M, Sirviö J, Thomas JH, Nass R, Blakely RD, Wong G. Dopaminergic neuronal loss and motor deficits in Caenorhabditis elegans overexpressing human alpha-synuclein. J Neurochem. 2003;86:165–172. doi: 10.1046/j.1471-4159.2003.01809.x. [DOI] [PubMed] [Google Scholar]
- 38.Cao S, Gelwix CC, Caldwell KA, Caldwell GA. Torsin-mediated protection from cellular stress in the dopaminergic neurons of Caenorhabditis elegans. J Neurosci. 2005;25:3801–12. doi: 10.1523/JNEUROSCI.5157-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karpinar DP, Balija MB, Kügler S, Opazo F, Rezaei-Ghaleh N, Wender N, Kim HY, Taschenberger G, Falkenburger BH, Heise H, Kumar A, Riedel D, Fichtner L, Voigt A, Braus GH, Giller K, Becker S, Herzig A, Baldus M, Jäckle H, Eimer S, Schulz JB, Griesinger C, Zweckstetter M. Pre-fibrillar alpha-synuclein variants with impaired beta-structure increase neurotoxicity in Parkinson's disease models. EMBO J. 2009;28:3256–68. doi: 10.1038/emboj.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao P, Yuan Y, Pehek EA, Moise AR, Huang Y, Palczewski K, Feng Z. Alpha-synuclein disrupted dopamine homeostasis leads to dopaminergic neuron degeneration in Caenorhabditis elegans. PloS One. 2010;5:e9312. doi: 10.1371/journal.pone.0009312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fahn S, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med. 2004;351:2498–508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 42.Olanow CW. Levodopa: effect on cell death and the natural history of Parkinson's disease. Mov Disord. 2015;30:37–44. doi: 10.1002/mds.26119. [DOI] [PubMed] [Google Scholar]
- 43.Lohr KM, et al. Increased vesicular monoamine transporter enhances dopamine release and opposes Parkinson disease-related neurodegeneration in vivo. Proc Natl Acad Sci USA. 2014;111(27):9977–82. doi: 10.1073/pnas.1402134111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rochet JC, et al. Inhibition of fibrillization and accumulation of prefibrillar oligomers in mixtures of human and mouse alpha-synuclein. Biochemistry. 2000;39:10619–26. doi: 10.1021/bi001315u. [DOI] [PubMed] [Google Scholar]
- 45.Specht CG, Schoepfer R. Deletion of the alpha-synuclein locus in a subpopulation of C57BL/6J inbred mice. BMC Neurosci. 2001;2:11. doi: 10.1186/1471-2202-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mosharov EV, et al. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62:218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, Tzitzilonis C, Soragni A, Jessberger S, Mira H, Consiglio A, Pham E, Masliah E, Gage FH, Riek R. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci USA. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu J, Kao SY, Lee FJ, Song W, Jin LW, Yankner BA. Dopamine-dependent neurotoxicity of alpha-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat Med. 2002;8:600–6. doi: 10.1038/nm0602-600. [DOI] [PubMed] [Google Scholar]
- 49.Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, Kretzschmar H, Hengerer B, Kostka M. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007;27:9220–9232. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng H-C, Ulane CM, Burke RE. Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol. 2010;67:715–725. doi: 10.1002/ana.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brichta L, Shin W, Jackson-Lewis V, Blesa J, Yap EL, Walker Z, Zhang J, Roussarie JP, Alvarez MJ, Califano A, Przedborski S, Greengard P. Identification of neurodegenerative factors using translatome-regulatory network analysis. Nat Neurosci. 2015;18:1325–33. doi: 10.1038/nn.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khasnavis S, Ghosh A, Roy A, Pahan K. Castration induces Parkinson disease pathologies in young male mice via inducible nitric-oxide synthase. J Biol Chem. 2013;288:20843–55. doi: 10.1074/jbc.M112.443556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qian Y, Forssberg H, Diaz Heijtz R. Motor Skill Learning Is Associated with Phase-Dependent Modifications in the Striatal cAMP/PKA/DARPP-32 Signaling Pathway in Rodents. PLoS One. 2015;10:e0140974. doi: 10.1371/journal.pone.0140974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waxman EA, Duda JE, Giasson BI. Characterization of antibodies that selectively detect alpha-synuclein in pathological inclusions. Acta Neuropathol. 2008;116:37–46. doi: 10.1007/s00401-008-0375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luk KC, Kehm VM, Zhang B, O'Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J Exp Med. 2012;209:975–86. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eales KL, Palygin O, O'Loughlin T, Rasooli-Nejad S, Gaestel M, Müller J, Collins DR, Pankratov Y, Corrêa SA. The MK2/3 cascade regulates AMPAR trafficking and cognitive flexibility. Nat Commun. 2014;5:4701. doi: 10.1038/ncomms5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun Y, Zhang H, Hu R, Sun J, Mao X, Zhao Z, Chen Q, Zhang Z. The expression and significance of neuronal iconic proteins in podocytes. PLoS One. 2014;9:e93999. doi: 10.1371/journal.pone.0093999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem. 2007;282:5641–52. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- 59.Mazzulli JR, Burbulla LF, Krainc D, Ischiropoulos H. Detection of Free and Protein-Bound ortho-Quinones by Near-Infrared Fluorescence. Anal Chem. 2016;88(4):2399–405. doi: 10.1021/acs.analchem.5b04420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berkowitz LA, Knight AL, Caldwell GA, Caldwell KA. Generation of stable transgenic C. elegans using microinjection. J Vis Exp. 2008;18:833. doi: 10.3791/833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamamichi S, Rivas RN, Knight AL, Cao S, Caldwell KA, Caldwell GA. Hypothesis-based RNAi screening identifies neuroprotective genes in a Parkinson’s disease model. Proc Natl Acad Sci USA. 2008;105:728–733. doi: 10.1073/pnas.0711018105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.