Abstract
Although endogenous glucocorticoids (GCs) are important regulators of bone integrity and the immune system, their role in bone repair after fracture—a process highly dependent on inflammation and bone formation—is unclear. Because most effects of GCs are mediated by the glucocorticoid receptor (GR), we used an inducible global GR knockout (GRgtROSACreERT2) mouse model to eliminate endogenous GC action in all cells contributing to bone repair. The healing process was analyzed by cytokine/chemokine multiplex analysis, flow cytometry, histology, gene-expression analysis, microcomputed tomography, and biomechanical analysis. We observed increased early systemic and local inflammatory responses, as well as a significantly higher number of T cells infiltrating the fracture callus. Later in the healing process, we found impaired endochondral ossification in the absence of the GR, leading to persistent cartilage in the calli of the GRgtROSACreERT2 mice, decreased bending stiffness, and a significantly lower proportion of healed bones. Collectively, our data show that the absence of the GR significantly impairs fracture healing associated with a defective cartilage-to-bone transition, underscoring an important role of GCs during fracture healing.—Rapp, A. E., Hachemi, Y., Kemmler, J., Koenen, M., Tuckermann, J., Ignatius, A. Induced global deletion of glucocorticoid receptor impairs fracture healing.
Keywords: inflammation, knockout mice, steroids, bone repair, endochondral ossification
Endogenous glucocorticoids (GCs) as major components of the stress response and circadian rhythms control multiple physiologic processes, in particular glucose and energy metabolism, the immune system, organ integrity, and bone metabolism (1, 2). Upon stimulation of the hypothalamic–pituitary–adrenocortical axis by stress, injury, or cytokines, GCs are released by the adrenal cortex. Most effects of GCs are mediated by the glucocorticoid receptor (GR), a member of the nuclear receptor superfamily that is almost ubiquitously expressed (3). The GR functions primarily as a ligand-dependent transcription factor that translocates into the nucleus after hormone binding. In the nucleus, the GR can act in different manners: as a homodimer, the GR binds to palindromic GR binding sites; as a monomer, it binds to so-called GR-binding half sites in the vicinity of lineage-specific transcription factors to transactivate or repress genes (4). In addition, the GR can influence gene expression as a monomer by a mechanism termed tethering via binding to transcription factors, including activator protein 1, NF-κB, and signal transducer and activator of transcription (5, 6).
An excess of exogenous GCs is known to negatively influence bone integrity because long-term medication leads to glucocorticoid-induced osteoporosis (GIO) (2, 7), whereas low-dose GC replacement therapy does not result in a significant reduction of bone mineral density (BMD) (8). GIO occurs mostly because of direct effects on cells of the osteoblast lineage, including a reduction of the osteoblast number and differentiation, as well as impaired osteoblast function. In addition, cells of the osteoclast lineage are affected in a complex manner: osteoclastogenesis is decreased, whereas the life span and resorptive capacity are enhanced (9, 10). Additionally, the RANKL/OPG ratio is altered in GIO (11). The role of endogenous GCs on bone is less clear; furthermore, investigation of the role of endogenous GCs on bone is complicated by the fact that a constitutive knockout of the GR is postnatally lethal because of atelectasis of the lung (12). However, analysis of GRnull fetuses revealed that embryonic skeletal development is normal in the absence of the GR (13). Postnatally, it was shown that GR signaling is necessary for physiologic bone metabolism because disruption of signaling specifically in osteoblasts resulted in decreased trabecular bone mass in GRRunx2Cre mice (13). Similarly, overexpression of the GC-inactivating enzyme 11β hydroxysteroid dehydrogenase 2 (11β-HSD2) in osteoblasts and osteocytes in Col2.3-11β-HSD2 transgenic mice led to diminished trabecular and cortical bone mass (1, 14, 15) and delayed cranial bone development (16).
Notably, although investigation of the role of endogenous GCs during bone-defect repair is limited, it is known that GCs are released upon traumatic insult and that the hypothalamic–pituitary–adrenocortical axis is stimulated by proinflammatory cytokines that are up-regulated during the early phase of fracture healing, including IL-6, IL-1β, and TNF-α (17). Knowledge of the role of GCs is predominantly derived from animal studies investigating the effect of exogenous GC. Here, the effects appear to depend on the duration of GC administration because long-term medication results in impaired healing (18), whereas short-term administration of synthetic GCs leads only to moderate, transient effects (19, 20). In contrast to the effects on bone development, intramembranous bone regeneration of a drill-hole defect was unaffected in Col2.3-11β-HSD2 mice (21). However, in mice with targeted tamoxifen-induced postnatal deletion of the GR in chondrocytes, endochondral bone healing was delayed (22).
Because the general role of endogenous GCs during fracture healing remains unclear, we aimed to unravel the effect of GR-mediated action of GCs. We discovered that the absence of the GR moderately affects systemic and local inflammation and furthermore impairs endochondral ossification in the late phase of fracture healing.
MATERIALS AND METHODS
Animal model and husbandry
Animal experiments were performed according to national and international guidelines for the Care and Use of Laboratory Animals, and were approved by the local Ethics Committee (Regierungspräsidium Tübingen, Germany).
Homozygous Nr3c1tm2Gsc mice (23) were cross-bred with hemizygous Gt(ROSA)26Sortm9(cre/ESR1)Arte mice to obtain Nr3c1tm2GscGt(ROSA)26Sortm9(cre/ESR1)Arte mice (hereafter referred to as GRflox when Cre negative and GRgtROSACreERT2 when Cre positive). All mice were maintained on a C57BL/6 background. Genotypes were determined by PCR using DNA from tail biopsy samples as previously described (13). The mice were housed in groups of up to 5 animals with a 14 h light–10 h dark cycle at 23°C and 55 ± 10% humidity. Standard rodent chow and water were available ad libitum. Six-week-old GRgtROSACreERT2 and GRflox littermate controls were injected intraperitoneally with tamoxifen (Sigma-Aldrich, St. Louis, MO, USA) in corn oil on 5 consecutive days (10 mg/kg per day).
When 14 wk old, the mice received a unilateral femur osteotomy as described previously (24). Briefly, under general anesthesia (2 vol% isoflurane, Forene; Abbott Laboratories, Chicago, IL, USA) the right femur was exposed, and the midshaft was osteotomized with an oscillating saw (0.4 mm thick). The osteotomy was stabilized using an external fixator (axial stiffness 3.2 N/mm; RISystem, Davos, Switzerland) that was fitted to the bone with 4 mini-Schanz screws. An analgesic was administered via the drinking water from 1 d before to 3 d after surgery (tramadol-hydrochloride, 25 mg/L; Grünenthal, Aachen, Germany). Before surgery, the mice received a single dose of antibiotics (clindamycin-2-dihydrogenphosphate, 45 mg/kg; Ratiopharm, Ulm, Germany).
Mice were euthanized by intracardial blood withdrawal under deep isoflurane anesthesia after 6 or 24 h, and after 3, 7, 14, or 28 d. After euthanasia, the tamoxifen-induced recombination of the loxP locus of the GR was confirmed by PCR. The following primers were used: GR 5′-GGCATGCACATTACTGGCCTTCT-3′; GR flox 5′-GTGTAGCAGCCAGCTTACAGGA-3′; and GR wild-type 5′-CCTTCTCATTCCATGTCAGCATGT-3′.
Cytokine and chemokine analysis
Blood serum and fracture hematomas collected 6 h after osteotomy were used for cytokine analysis. Blood was collected in microvettes (Sarstedt, Nümbrecht, Germany), then centrifuged at 800g for 5 min at 4°C, followed by a second centrifugation at 13,000g for 2 min. The obtained serum was stored at −80°C until use. The fracture hematoma was directly transferred into lysis buffer (10 mM Tris pH 7.5, 10 mM NaCl, 0.1 mM EDTA, 0.5 mM Triton X-100, 0.2 mM PMSF, all from Sigma-Aldrich) with protease inhibitor cocktail (Halt Protease and Phosphatase Inhibitor Cocktail; Thermo Fisher Scientific, Waltham, MA, USA) and minced. After 30 min incubation on ice, the samples were centrifuged at 18,000g for 30 min at 4°C. Protein concentration was determined using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. The lysates were stored at −80°C until use. Using a mouse multiplex cytokine and chemokine assay (ProcartaPlex; eBioscience, San Diego, CA, USA), the levels of the proinflammatory mediators TNF-α, IL-1β, IL-6, IFN-γ, monocyte chemotactic protein 1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α), and keratinocyte chemoattractant 1 (CXCL1) and the anti-inflammatory mediators IL-4, IL-10, and IL-13 were determined on a Bio-Plex 200 (Bio-Rad, Hercules, CA, USA). Using standard curves, the cytokine levels were calculated automatically with Bio-Plex Manager software.
Flow cytometry
At 24 h after osteotomy, blood and the fracture hematoma were analyzed for immune cells using flow cytometry. To remove erythrocytes from blood, lysis was performed twice for 5 min on 37°C using lysis buffer (150 mM NH4Cl, 10 mM KHCO3, 0.125 mM EDTA, all from Sigma-Aldrich). The hematoma between the bone ends was excised and pressed through a cell strainer to obtain a single-cell suspension. Cells of the innate and adaptive immune systems were stained using the following antibodies or respective isotypes: CD11b Alexa Fluor 700 (clone M1/70; eBioscience), Ly6G V450 (clone 1A8; BD Pharmingen, Heidelberg, Germany), F4/80 FITC (clone BM8; eBioscience), CD3ε PE-Cyanine 7 (clone 145-2C11; eBioscience), CD4 APC-eFluor 780 (clone GK1.5; eBioscience), CD8a APC (clone 53-6.7; eBioscience), and CD19 PE (clone 1D3; eBioscience). 7-AAD (Sigma-Aldrich) was used to distinguish between living and dead cells. A minimum of 10,000 events was measured with an LSRII flow cytometer (BD Biosciences, San Jose, CA, USA) and analyzed by FlowJo software (Treestar, Ashland, OR, USA).
Histomorphometry
Femurs collected after 28 d were fixed in 4% formalin for 48 h, dehydrated, and embedded in methyl methacrylate. At the other time points, femurs were fixed for 48 h, decalcified in 20% EDTA for 10 to 12 d, dehydrated, and embedded in paraffin. Sections 6 to 8 µm thick were stained with Giemsa stain or Safranin O/Fast Green. Evaluation of the callus composition was performed by light microscopy (Leica DMI6000 B, Software MetaMorph; Leica Microsystems, Buffalo Grove, IL, USA) under 50-fold magnification. At all the time points, the whole callus was analyzed for the amount of bone, cartilage, and fibrous tissue.
To determine the number of osteoblast and osteocytes, sections were stained with toluidine blue or Giemsa stain. For visualization of osteoclasts, tartrate-resistant acid phosphatase (TRAP) staining was performed using naphthol AS-MX phosphate and Fast Red TR-Salt (both Sigma-Aldrich) in 0.2 M acetate buffer at pH 5.0. Osteoblast and osteoclast numbers, and surface per bone surface were determined using the OsteoMeasure histomorphometry system (Osteometrics, Decatur, GA, USA). Analysis was performed according to the recommendations of the American Society for Bone and Mineral Research (25, 26).
Immunohistochemistry
For immunohistochemical analyses, paraffin was removed from paraffin-embedded sections and the sections rehydrated. After antigen retrieval in 0.1 M sodium citrate buffer (pH 6, 95°C, 20 min), nonspecific binding sites were blocked using 10% goat serum or bovine serum albumin in Tris-buffered saline, pH 7.6, with 0.1% Triton X-100 (Sigma-Aldrich) for 1 h at room temperature. Sections were incubated overnight at 4°C with the respective primary antibody or isotype for staining of Ly6G for polymorphonuclear neutrophils (PMNs; 1:300; Biolegend, San Diego, CA, USA), F4/80 for macrophages (1:500; Bio-Rad AbD Serotec, Puchheim, Germany), and CD31 for endothelial cells (1:10; Dianova, Hamburg, Germany). For staining of CD8-positive cytotoxic T cells (1:500; Bioss, Woburn, MA, USA), sections were incubated for 2 h at room temperature. Incubation with a biotinylated secondary antibody was performed at room temperature for 30 min to 1 h. For signal detection, either horseradish peroxidase–linked streptavidin and subsequent ACE single solution (both Zytomed Systems, Berlin, Germany) or Vectastain Elite ABC kit and NovaRed substrate (both Vector Laboratories, Burlingame, CA, USA) were applied. The sections were counterstained with hematoxylin and mounted.
Isolation and culture of bone marrow stromal cells and periosteal-derived osteoblasts, and subsequent RNA isolation
Long bones of tamoxifen-treated GRgtROSACreERT2 and GRflox animals were excised and cleaned from adhering muscle, and bone marrow was flushed using high-glucose DMEM with l-glutamine (Sigma-Aldrich) supplemented with 20% fetal bovine serum (Thermo Fisher Scientific) and 1% penicillin–streptomycin (Sigma-Aldrich), then plated. Cells were grown to subconfluence, detached with Accutase (Sigma-Aldrich), and reseeded with 3500 cells/cm2. Cells at passage 2 were collected for RNA isolation.
To isolate osteoblasts, cortical bone was minced, washed with PBS, and digested for 2 h in 0.1 w/v% collagenase A (Sigma-Aldrich) in AlphaMEM (Biochrom, Cambourne, United Kingdom) at 37°C. After digestion, bone chips were washed 2 times in PBS, transferred to a 6-well plate, and cultivated in α-MEM supplemented with 10% FBS Superior (Biochrom), 1% l-glutamine, 1% penicillin–streptomycin, and 0.5% amphotericin B (all Thermo Fisher Scientific). Osteoblasts were detached with trypsin and reseeded with 3000 cells/cm2. Cells at passage 2 were collected for RNA isolation. Total RNA was isolated using RNeasy Midi Kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s instruction.
RNA isolation and gene-expression analysis
RNA from calli was isolated on d 14 after fracture. The osteotomized femur was collected, placed on ice, and directly transferred into RNAlater (Sigma-Aldrich, St. Louis, MO, USA). The callus between the 2 inner pins was excised and minced. To further disrupt the calli, a mixer mill was used. RNA isolation was performed using the TRIzol Plus RNA Purification Kit (Ambion, Austin, TX, USA) according to the manufacturer’s protocol, with additional DNA digestion using RNAse-free DNAse I (Qiagen). cDNA was synthesized from 1 to 2 µg RNA using RevertAid H Minus reverse transcriptase (M-MulV; Fermentas, Waltham, MA, USA) with 5× reaction buffer at room temperature, random primers (200 ng/μl; Thermo Fisher Scientific), and 10 mM dNTPs following the manufacturer’s instructions. Real-Time quantitative PCR was performed in 10 μl reaction volume using SYBR Green I dye (Thermo Fisher Scientific) on an ABI ViiA-7 system (Applied Biosystems; Thermo Fisher Scientific). The mRNA abundance of collagen type 10 α1 chain (Col10a1), collagen type 2 α1 chain (Col2a1), Runx2 (Runx2), osteocalcin (Bglap), sex-determining region Y-box 9 (Sox9), osterix (Sp7), Trap, receptor activator of nuclear factor κB ligand (Rankl), and osteoprotegerin (Opg) was calculated relative to the expression of the reference gene Gapdh and the data analyzed using a model based on correction for exact PCR efficiencies. The primers used are listed in Supplemental Table 1.
Microcomputed tomography
Intact and osteotomized femurs collected after 28 d were scanned using a microcomputed tomography (µCT) device (SkyScan 1172; Bruker microCT, Kontich, Belgium) at a resolution of 8 µm at a peak voltage of 50 kV and 200 µA. Within each scan, 2 phantoms with defined hydroxyapatite content (250 and 750 mg/cm3) were scanned to determine the BMD. In intact femurs, a volume of interest of 1 mm length below the trochanter major was analyzed for cortical thickness, BMD, and second moment of inertia. Trabecular bone was analyzed in the distal femur 0.2 mm above the growth plate and in the proximal tibia. In fractured femurs, the former osteotomy gap was analyzed for BMD and trabecular parameters. To distinguish between mineralized and nonmineralized tissue, a fixed global threshold of ∼643 mg hydroxyapatite per cubic centimeter was applied (27).
Analogous to the standard clinical assessment of X-rays to determine the healing outcome, the number of bridged cortices was determined in 2 perpendicular axes from µCT scans. A fracture was considered to be healed when the osteotomy gap was bridged at 3 or more sites.
Biomechanical testing
Intact and osteotomized femurs collected after 28 d were analyzed for flexural rigidity by nondestructive 3-point bending (24). Briefly, the proximal end of the femur was fixed to an aluminum cup, which in turn was fixed to a hinge joint of the 3-point bending setup in the material testing machine (Z10; Zwick Roell, Ulm, Germany). The femur condyles rested unfixed on a bending support. The bending load was applied to the midshaft of intact bones or the middle of the callus up to a maximum load of 4 N. Flexural rigidity (EI) was calculated from the slope (k) of the linear region of the force–displacement curve. The distance of the load vector and the proximal (a) and distal (b) bending supports was considered in the calculation when the force was not applied exactly in the middle between the supports (l/2). For the calculation, the formula EI = k(a2b2)/3l was used.
Statistical analysis
Statistical analyses were performed by GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA). Data were analyzed for normal distribution by the Shapiro-Wilk normality test. Normally distributed variables were compared by Student’s t test, while the Mann-Whitney U test was performed to compare nonnormally distributed data. Data derived from immunostaining at different time points were analyzed by applying 2 separate 2-way ANOVAs. The first was applied to compare the genotypes at the different time points; the second was applied to analyze the effects within the genotypes over time. Because we applied a scoring system, cortical bridging was analyzed by contingency analysis by Fisher’s exact test. A values of P ≤ 0.05 was considered statistically significant.
RESULTS
Conditional deletion of GR moderately affects bone mass in intact skeleton
To eliminate the GR, we treated male GRgtROSACreERT2 mice and GRflox control mice with tamoxifen when aged 6 wk and analyzed the degree of recombination of the GRloxP locus after euthanasia when aged 18 wk. GRgtROSACreERT2 mice displayed an efficient recombination of the GRloxP locus on the gene level, resulting in a decreased GR expression level in the entire bone (Supplemental Fig. S1A), isolated bone marrow–derived stromal cells, and isolated periostal cells, which are the osteoprogenitor cells contributing to bone regeneration (Supplemental Fig. S1C, D).
Upon GR deletion, the body weight of GRflox and GRgtROSACreERT2 mice was not significantly different 12 wk after treatment (26.5 ± 1.84 g vs. 27.0 ± 2.0 g; n = 10/genotype), indicating that the tamoxifen-induced deletion of the GR when aged 6 wk did not influence growth or maturation.
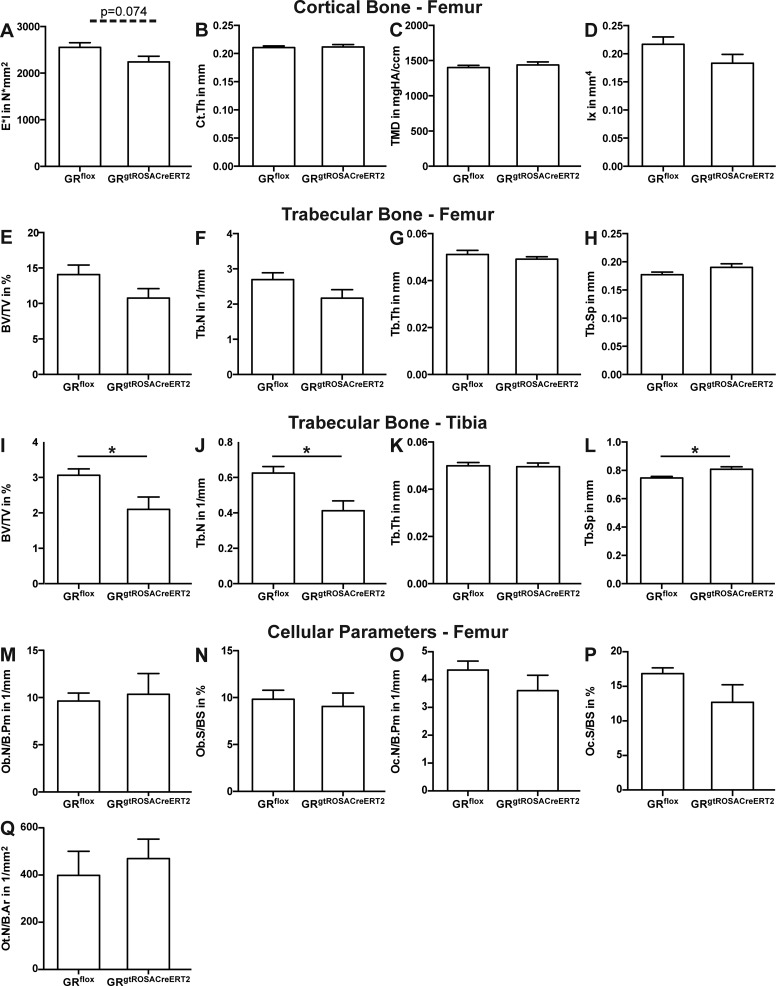
Biomechanical testing of the femur diaphysis revealed a slight but nonsignificant decrease of stiffness in the femur of GRgtROSACreERT2 mice (Fig. 1A). Cortical thickness, tissue mineralization, and second moment of inertia of the femur diaphysis were unaffected (Fig. 1B–D). Bone volume per tissue volume (BV/TV) ratio of cancellous bone in the distal femur of GRgtROSACreERT2 mice was not significantly decreased; there were no alterations in trabecular thickness, number, or spacing (Fig. 1E–H). However, in the proximal tibia, BV/TV, trabecular number, and trabecular spacing were significantly reduced (Fig. 1I, J, L). Osteoblast numbers and surface, osteoclast numbers and surface, and osteocyte numbers per bone area were not significantly altered in the distal femur in GRgtROSACreERT2 mice (Fig. 1M–Q). In summary, conditional deletion of the GR affected trabecular bone mass only in specific bones and had a minor impact on cortical bone.
Figure 1.
Bone mass is moderately affected by postnatal deletion of GR. GRflox and GRgtROSACreERT2 mice were treated with tamoxifen when aged 6 wk. Twelve weeks later, bone phenotype was analyzed. Bending stiffness was analyzed by 3-point bending test in femur diaphysis (A). Cortical thickness (Ct.Th, B), tissue mineral density (TMD, C), and second moment of inertia (Ix, D) of femur diaphysis were analyzed by µCT. Trabecular parameters bone volume per tissue volume (BV/TV, E), trabecular number (Tb.N, F), trabecular thickness (Tb.Th, G), and trabecular separation (Tb.Sp, H) in distal femur and proximal tibia (I–L) were analyzed by µCT. By histomorphometry, osteoblast number per bone perimeter (Ob.N/B.Pm, M), osteoblast surface per bone surface (Ob.S/BS, N), osteoclast number per bone perimeter (Oc.N/B.Pm, O), osteoclast surface per bone surface (Oc.S/BS, P), and osteocyte number per bone area (Ot.N/B.Ar, Q) were analyzed in distal femur. Data are presented as means ± sem; n = 7–9 per group (A–H); n = 4–7 per group (M–Q).
Conditional deletion of GR affects systemic and local inflammation after fracture
To evaluate the inflammatory response and the healing process after fracture, we performed femur osteotomy in GRgtROSACreERT2 and control mice 7 wk after the final tamoxifen injection. First, we analyzed the inflammatory response after fracture by multiplex cytokine/chemokine analysis of serum and fracture hematoma obtained 6 h after osteotomy and by flow cytometry of circulating immune cells in the blood and immune cells invading the fracture hematoma after 24 h.
Basal levels of the analyzed cytokines and chemokines in uninjured GRflox were below the detection limit. At 6 h after fracture, CXCL1 and IL-6 were up-regulated in both genotypes. While CXCL1 was not differentially regulated in GRflox and GRgtROSACreERT2 mice, IL-6 was significantly higher in GRgtROSACreERT2 mice (Table 1, top). All other cytokines and chemokines (TNF-α, IL-1β, IFN-γ, MCP-1, MIP-1α, IL-4, IL-10, and IL-13) were not detectable at this time point (data not shown).
TABLE 1.
Deletion of GR leads to increased inflammation
| Compartment | Cytokine/chemokine | GRflox, n = 5–7 | GRgtROSACreERT2, n = 6 | P |
|---|---|---|---|---|
| Serum (pg/ml) | CXCL1 | 235.6 ± 72.8 | 166.1 ± 23.5 | 0.945 |
| IL-6 | 162.0 ± 52.9 | 377.8 ± 95.3* | 0.035 | |
| Hematoma (pg/mg protein) | TNF-α | 2.7 ± 1.3 | 4.9 ± 0.7 | 0.166 |
| IL-1β | 3.2 ± 1.5 | 12.2 ± 2.7* | 0.021 | |
| IL-6 | 2243.9 ± 827.7 | 3456.7 ± 809.7 | 0.323 | |
| IFN-γ | 0.8 ± 0.4 | 0.9 ± 0.2 | 0.903 | |
| MCP-1 | 216.6 ± 117.2 | 188.2 ± 61.2 | 0.837 | |
| MIP-1α | 29.13 ± 5.65 | 33.62 ± 4.72 | 0.537 | |
| CXCL1 | 90.9 ± 18.4 | 211.5 ± 70.2 | 0.150 |
Cytokine and chemokine levels in serum and callus homogenates were determined by cytokine and chemokine multiplex analysis. Data are presented as means ± sem. *P ≤ 0.05.
Analysis of callus lysates collected 6 h after fracture revealed a significant increase in IL-1β levels in GRgtROSACreERT2 compared to GRflox mice, while other mediators, including CXCL1, IL-6, IFN-γ, MCP-1, MIP-1α, and TNF-α, were not differentially expressed (Table 1, bottom). Levels of anti-inflammatory cytokines IL-4, IL-10, and IL-13 were below the limit of detection (data not shown).
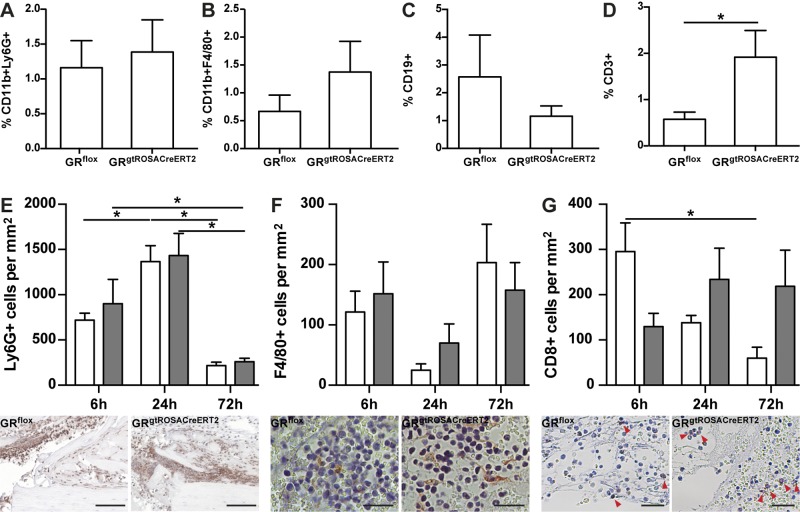
Flow cytometric analysis of immune cells in the blood revealed no significant alterations of circulatory PMNs (CD11b+Ly6G+), cells of the monocyte/macrophage lineage (CD11b+F4/80+), B cells (CD19+), or T cells (CD3+) 24 h after osteotomy in the absence of the GR, indicating no considerable systemic inflammation (Table 2). Analysis of immune cells invading the fracture hematoma 24 h after osteotomy by fluorescent-activated cell sorting revealed no significant differences in the fraction of PMNs (Fig. 2A), macrophages (Fig. 2B), or B cells (Fig. 2C). In contrast, the fraction of CD3+ cells was significantly increased in GRgtROSACreERT2 compared to GRflox mice (Fig. 2D).
TABLE 2.
Frequency of immune cells circulating in blood after fracture is not altered in absence of GR
| Characteristic | GRflox, n = 5 | GRgtROSACreERT2, n = 5 | P |
|---|---|---|---|
| PMNs (CD11b+Ly6G+) | 7.4 ± 1.0 | 7.0 ± 1.3 | 0.841 |
| Monocytes/macrophages (CD11b+F4/80+) | 3.1 ± 0.8 | 3.2 ± 0.3 | 0.841 |
| B cells (CD19+) | 36.18 ± 4.0 | 41.2 ± 3.1 | 0.690 |
| T cells (CD3+) | 22.7 ± 1.8 | 29.4 ± 1.2 | 0.095 |
| T helper (CD3+CD4+) | 55.7 ± 2.2 | 63.7 ± 2.1 | 0.116 |
| Cytotoxic T cells (CD3+CD8+) | 37.5 ± 1.8 | 31.5 ± 2.2 | 0.222 |
Immune-cell populations circulating in the blood were analyzed by flow cytometry. Data are presented as means ± sem.
Figure 2.
Deletion of GR moderately influences inflammatory phase after fracture. Immune-cell populations infiltrating hematoma, including CD11b+Ly6G+ (PMNs, A), CD11b+F4/80+ (monocyte/macrophage lineage, B) CD19+ cells (B cells; C), and CD3+ cells (T cells; D), were analyzed by flow cytometry. Immunohistochemical staining of Ly6G (E), F4/80 (F), and CD8 (G) was performed to confirm flow cytometry results. White bars, GRflox mice; gray bars, GRgtROSACreERT2 mice. Micrographs directly below respective diagrams depict respective immune-cell population 24 h after osteotomy. Scale bars, 50 µm (E); 25 µm (F, G). Data are presented as means ± sem. Flow cytometry n = 5–6 per genotype, immunohistochemistry n = 3–6 per group. *P ≤ 0.05.
Next, we stained sections of fracture calli obtained after 6, 24, and 72 h for immune-cell markers. In the peripheral callus, Ly6G+ cells were found as early as 6 h after osteotomy. As expected, the number of Ly6G+ cells significantly increased within the first 24 h and significantly declined by 72 h after osteotomy. We found no significant differences in the number of Ly6G+ cells between both genotypes (Fig. 2E). The numbers of F4/80+ cells were not significantly different between the genotypes 6, 24, or 72 h after fracture (Fig. 2F). After 6 h, we detected more CD8+ cells in GRflox compared to GRgtROSACreERT2 mice. While the number of CD8+ cells declined in GRflox after 24 h (not significant) and 72 h (significant), their number did not significantly decline in GRgtROSACreERT2 mice, indicating prolonged inflammation (Fig. 2G). Taken together, the fracture-induced inflammatory response was moderately increased in the absence of GR.
Conditional deletion of GR disturbs endochondral ossification
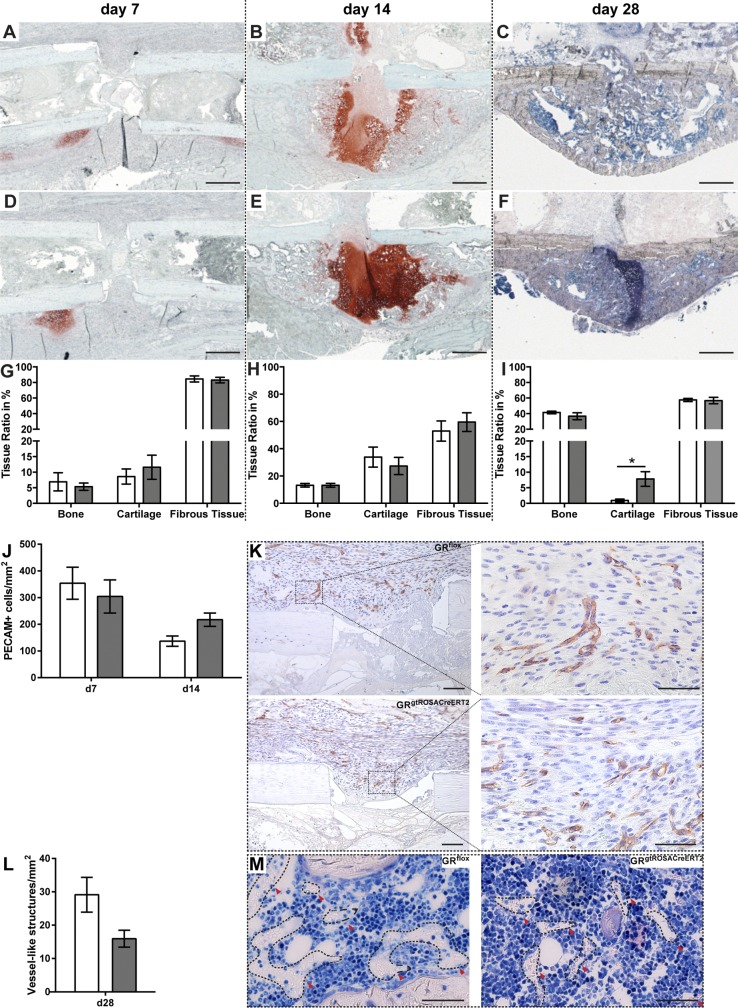
To assess the healing response in the absence of GR, we analyzed fracture calli at d 7, 14, and 28. Histomorphometric analysis of the fracture callus on d 7 and 14 revealed no significant alterations of the callus composition in the absence of the GR in the early and intermediate phases of fracture repair, indicating no sustainable influence of the elevated inflammation on bone formation (Fig. 3). However, after 28 d, we found significantly more residual cartilage in the calli of GRgtROSACreERT2 mice compared to the GRflox control, indicating delayed callus maturation (Fig. 3C, F, I). Because osteogenesis depends on angiogenesis, we stained sections derived from d 3, 7 and 14 for the endothelial cell marker CD31 (PECAM) and additionally counted erythrocyte-containing structures as a surrogate for vessels on d 28. On d 3, there were no PECAM-positive cells in the fracture hematoma. On d 7 and 14, we detected no significant differences in the number of PECAM-positive cells between GRflox and GRgtROSACreERT2 animals (Fig. 3J, K). The PECAM-positive cells often formed vessel-like structures (Fig. 3K). Also on d 28, there was no significant difference in the number of vessel-like structures between GR sufficient and GR-deficient mice, indicating effective revascularization of the callus (Fig. 3L, M).
Figure 3.
Absence of GR delays callus maturation in late phase of fracture healing but has no influence on revascularization. Callus composition on d 7 (A, D, G), 14 (B, E, H), and 28 (C, F, I) was analyzed by histomorphometry. On d 7 and 14 sections were stained with Safranin O/Fast Green, while on d 28 Giemsa stain was applied. Sections from d 7 and 14 were stained for CD31/PECAM (K), and positively stained cells were quantified (J). M depicts structures counted as “vessel” in Giemsa-stained sections from d 28. Data are presented as means ± sem. White bars, GRflox mice; gray bars, GRgtROSACreERT2 mice; n = 7–9 per group (G–I); n = 3–7 per genotype and time point (J). *P ≤ 0.05. Scale bars, 500 µm (A–F); 100 µm (K); 50 µm (magnified areas, K, M).
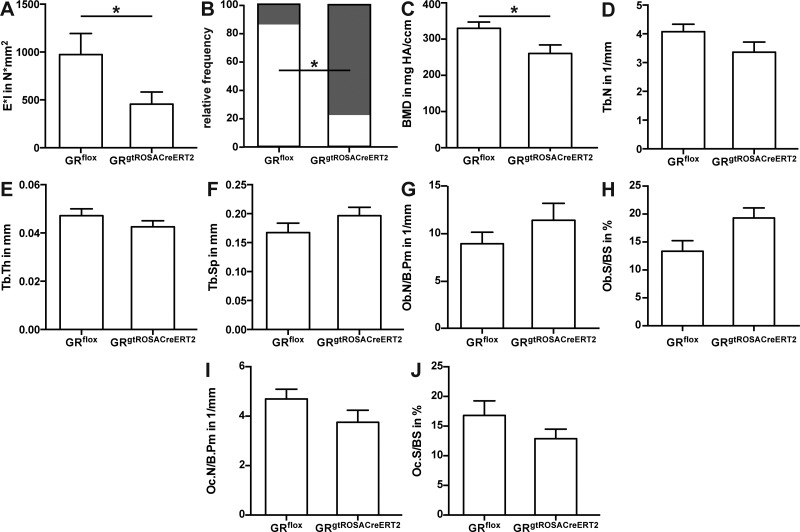
The higher cartilage content resulted in a significant reduction of flexural rigidity of the osteotomized femurs in GRgtROSACreERT2 mice (Fig. 4A). Analysis of cortical bridging revealed a significantly lower proportion of successfully healed (i.e., ≥3 cortices bridged) femurs in GRgtROSACreERT2 compared to GRflox mice (Fig. 4B). Confirming the histomorphometry, the BMD was significantly lower in GRgtROSACreERT2 compared to GRflox mice (Fig. 4C). Trabecular parameters in the callus were not significantly altered (Fig. 4D–F). At the cellular level, the numbers of osteoblasts per bone perimeter and osteoblast surface per bone surface were nonsignificantly elevated in GRgtROSACreERT2 mice (Fig. 4G, H), whereas osteoclast numbers and surface were nonsignificantly decreased (Fig. 4I, J).
Figure 4.
GR signaling is necessary for successful fracture repair. Bending stiffness of fracture calli was analyzed by 3-point bending test (A). Healing frequency was assessed from µCT data sets (B). White proportion, healed; gray proportion, not healed. BMD (C), trabecular number (Tb.N, D), trabecular thickness (Tb.Th, E), and trabecular spacing (Tb.Sp, F) were determined in former osteotomy by µCT. Osteoblast number per bone perimeter (Ob.N/B.Pm, G), osteoblast surface per bone surface (Ob.S/BS, H), osteoclast number per bone perimeter (Oc.N/B.Pm, I), and osteoclast surface per bone surface (Oc.S/BS, J) were assessed in callus by histomorphometry. Data are presented as means ± sem; n = 7–9 per group. All data are derived from d 28 after surgery. *P ≤ 0.05.
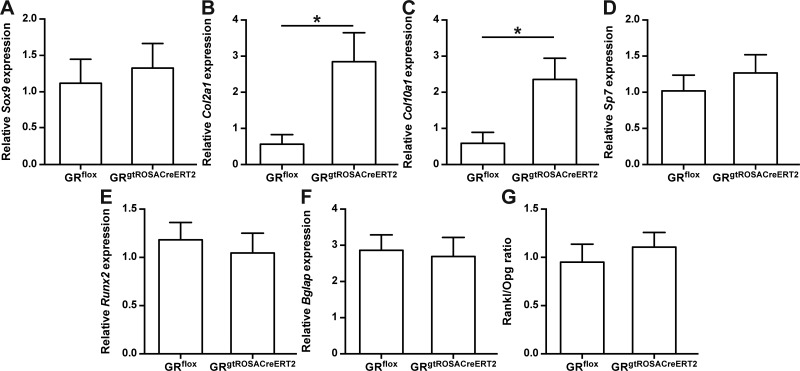
Conditional deletion of GR increases expression of chondrogenic markers during intermediate healing phase
Because only the late phase of fracture healing appeared to be significantly influenced by the absence of the GR, we investigated the intermediate phase of fracture healing more closely and performed gene-expression analysis of RNA isolated from calli on d 14 after fracture. The expression level of the early chondrogenic marker Sox9 was unaltered, whereas the late chondrogenic marker genes Col2a1 and Col10a1 were significantly increased in GRgtROSACreERT2 compared to GRflox mice (Fig. 5A–C). Expression of the osteogenic markers Runx2, Sp7, and Bglap was not significantly different in the absence of the GR (Fig. 5D–F). Regarding osteoclastogenesis, the ratio of Rankl to Opg was not different between the GRgtROSACreERT2 and GRflox mice (Fig. 5G). Together with the observations on d 28, these observations indicate a more advanced healing process in GRflox compared to GRgtROSACreERT2 mice.
Figure 5.
Absence of GR alters chondrogenic but not osteogenic marker gene expression in intermediate healing phase. Gene-expression levels of chondrogenic differentiation markers Sox9 (A), Col2a1 (B), and Col10a1 (C), osteogenic markers Sp7 (D), Runx2 (E), and Bglap (F), and Rankl/Opg ratio (G) were analyzed by quantitative real-time PCR on d 14. Data are presented as means ± sem; n = 5–7 per group. *P ≤ 0.05.
DISCUSSION
Physiologic levels of GCs are described to be important for physiologic bone homeostasis. Primary adrenal insufficiency patients, in whom the adrenal glands do not produce sufficient amounts of steroid hormones, display increased serum levels of collagen cross-links and alkaline phosphatase, indicating increased bone turnover as well as significantly decreased bone mass compared to primary adrenal insufficiency patients receiving low-dose GC substitution (8). Moreover, osteoblast-specific disruption of GR signaling in mice led to decreased bone mass in the trabecular (13) and cortical (1, 14, 15) compartments. In accordance with these experimental and clinical data, we found reduced trabecular bone mass in the femur and tibia of mice with a postnatal tamoxifen-induced conditional deletion of the GR after 6 wk, whereas the growth and maturation of these mice were not negatively affected by this GR deletion. However, we did not find any differences in osteoblast, osteoclast, or osteocyte numbers, although we cannot exclude the influence of the GR on other cells on bone mass.
While the role of endogenous GC signaling on bone integrity is clearly recognized, the effect on bone regeneration is less clear and minimally investigated. Most data on the effect of GCs on bone regeneration are derived from studies applying exogenous GCs. From such studies, it is evident that the duration of the application and dosing significantly influence the outcome, because short-term application of GCs did not result in impaired healing (19, 20), whereas healing considerably deteriorated after long-term application (18).
In the present study, we investigated the role of GR-mediated signaling of endogenous GCs and observed disturbed healing in mice with disrupted GC signaling. Consistent with the ability of GCs to restrict inflammatory processes, we found significantly increased serum levels of IL-6 and a significant increase of IL-1β locally in the fracture hematoma of GRgtROSACreERT2 mice in the early inflammatory phase after osteotomy, indicating an increased immune response. To our knowledge, to date, the early inflammatory phase of fracture healing has not been investigated in mice with disrupted GC signaling. However, in models of strong systemic inflammation, including cecal ligation and puncture, LPS, or TNF challenge, a lack of impairment of the GR in conditional knockout and knock-in mice aggravates the inflammatory response (28–30). In other models for sterile inflammation, including thioglycollate-induced peritonitis and carrageenan-induced pleura inflammation, higher numbers of invading inflammatory cells were found in lavage liquids of mice lacking 11β hydroxysteroid dehydrogenase 1 with reduced GC activity, also indicating elevated inflammation (31). In contrast, in local inflammatory responses, including contact allergy and arthritis, despite the failure to respond to exogenous GC action, GR-deficient mice are frequently not hyperresponsive to inflammation (32, 33), as also appears to be the case during fracture healing.
Notably, we found higher T-cell numbers in the hematoma of mice with disrupted GC-signaling, whereas PMNs, macrophages, and B cells were only moderately influenced. Immunostaining suggested normal clearance of PMNs because the kinetics of PMN numbers (i.e., an increase of PMNs within 24 h after fracture and a strong reduction of PMN numbers after 72 h) was similar in GRflox and GRgtROSACreERT2 mice. Therefore, the greater presence of T cells alone in GRgtROSACreERT2 mice was insufficient to provoke effects on the subsequent steps of the healing response because callus composition at 7 and 14 d was unaltered.
The unaltered callus composition further indicates normal intramembranous bone formation in distal callus areas as well as normal chondrocytic differentiation of mesenchymal precursors and differentiation of chondrocytes in the absence of GR-mediated actions of endogenous GCs. Studies suggested that at least in calvariae, endogenous GC signaling in mature osteoblasts is required to induce the expression of Wnt ligands, including Wnt7b and Wnt10b, whereby the lineage commitment of mesenchymal progenitors during intramembranous ossification is regulated (34). Consequently, mice with osteoblast-specific overexpression of 11β-HSD2 display delayed development of the cranial skeleton (16). Consistent with this, calvariae-derived primary osteoblasts from GRnull fetuses display reduced mineralization in vitro compared to wild type (13), and silencing of the GR using short hairpin RNA impairs osteogenic differentiation of human bone marrow–derived mesenchymal stem cells (35). In contrast to the impaired cranial development, intramembranous regeneration of a drill-hole defect in the tibia of mice with osteoblast-specific overexpression of 11β-HSD2 appeared to be normal. Therefore, the authors concluded that GC signaling might be redundant in intramembranous bone formation in long bones (21), which is consistent with the unaltered intramembranous ossification we observed.
At d 28, the late healing phase, we detected a higher proportion of remaining cartilage in the calli of GRgtROSACreERT2 mice, resulting in impaired fracture healing. Because endochondral ossification critically depends on vascularization (36), we determined whether revascularization is impaired in the absence of the GR. We found no evidence for defective revascularization during fracture healing because the number of CD31-positive cells and vessel-like structures were not significantly different during the time course of fracture healing in GRgtROSACreERT2 compared to wild-type mice. Therefore, the observed delay in healing must have other causes. It was reported that flexibly stabilized osteotomies in the tibial metaphysis displayed impaired healing in mice with chondrocyte-specific deletion of the GR compared to wild-type control (22). The authors detected larger cartilaginous calli during early healing stages, and decreased mineralization and inferior bone remodeling in the late healing phase (22). These results greatly resemble our findings. To determine whether there are differences at the molecular level in the intermediate healing phase that might explain the increased residual cartilage detected on d 28, we performed gene-expression analysis. We found an elevated expression of chondrogenic and osteogenic markers on d 14 in the absence of the GR. Considering the increased cartilage proportion in GRgtROSACreERT2 mice, this suggests ongoing regenerative processes in these mice, whereas these processes decline in GR-sufficient mice. Together with the observations of Tu et al. (22), our observations suggest a possible role for endogenous GCs in cartilage-to-bone transition in endochondral ossification. We also deleted the GR in other compartments of the bone beyond cartilage, which caused, for example, a higher infiltration of CD3+ cells at early stages. Because the ubiquitous deletion leads to similar effects as the cartilage-specific deletion shown by Tu et al., this suggests a predominant role of the GR in chondrocytes for normal bone healing.
In summary, our data show that the absence of the GR significantly impairs fracture healing; particularly during the late healing phase, the lack of the GR resulted in delayed endochondral bone regeneration and consequently in impaired callus maturation. Therefore, we conclude that the GR might be involved in cartilage-to-bone transformation during fracture healing.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank G. Strauss (Department of Pediatrics and Adolescent Medicine, Ulm University Medical Center) for the use of the fluorescence-activated cell sorting facility and her support. The authors acknowledge the excellent technical assistance of N. Möbius (Institute of Comparative Molecular Endocrinology, Ulm University), I. Baum, S. Schroth, U. Maile, and M. Tomo (Institute of Orthopaedic Research and Biomechanics, Ulm University Medical Centre). This work was financed by the German Research Foundation in the context of the Collaborative Research Centre 1149 Study, Danger Response, Disturbance Factors, and Regenerative Potential after Acute Trauma (CRC 1149, C02/INST 40/492-1). The authors declare no conflicts of interest.
Glossary
- 11β-HSD2
11β hydroxysteroid dehydrogenase 2
- Bglap
osteocalcin
- BMD
bone mineral density
- BV/TV
bone volume per tissue volume
- Col10a1
collagen type 10 α1 chain
- Col2a1
collagen type 2 α1 chain
- CXCL1
keratinocyte chemoattractant 1
- GC
glucocorticoid
- GIO
glucocorticoid-induced osteoporosis
- GR
glucocorticoid receptor
- GRflox
inducible global glucocorticoid receptor knockout, Cre-negative mouse
- GRgtROSACreERT2
inducible global glucocorticoid receptor knockout, Cre-positive mouse
- MCP-1
monocyte chemotactic protein 1
- µCT
microcomputed tomography
- MIP-1α
macrophage inflammatory protein 1α
- Opg
osteoprotegerin
- PMN
polymorphonuclear neutrophil
- Rankl
receptor activator of nuclear factor κB ligand
- Sox9
sex-determining region Y-box 9
- Sp7
osterix
- TRAP
tartrate-resistant acid phosphatase
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
A. E. Rapp, J. Tuckermann, and A. Ignatius designed the study; A. E. Rapp, Y. Hachemi, J. Kemmler, and M. Koenen conducted the experiments; A. E. Rapp, Y. Hachemi, J. Tuckermann, and A. Ignatius analyzed and interpreted the data; A. E. Rapp and Y. Hachemi drafted the article; J. Tuckermann and A. Ignatius revised the article; and all authors approved the final manuscript.
REFERENCES
- 1.Kalak R., Zhou H., Street J., Day R. E., Modzelewski J. R., Spies C. M., Liu P. Y., Li G., Dunstan C. R., Seibel M. J. (2009) Endogenous glucocorticoid signalling in osteoblasts is necessary to maintain normal bone structure in mice. Bone 45, 61–67 10.1016/j.bone.2009.03.673 [DOI] [PubMed] [Google Scholar]
- 2.Hartmann K., Koenen M., Schauer S., Wittig-Blaich S., Ahmad M., Baschant U., Tuckermann J. P. (2016) Molecular actions of glucocorticoids in cartilage and bone during health, disease, and steroid therapy. Physiol. Rev. 96, 409–447 10.1152/physrev.00011.2015 [DOI] [PubMed] [Google Scholar]
- 3.Hübner S., Dejager L., Libert C., Tuckermann J. P. (2015) The glucocorticoid receptor in inflammatory processes: transrepression is not enough. Biol. Chem. 396, 1223–1231 10.1515/hsz-2015-0106 [DOI] [PubMed] [Google Scholar]
- 4.Lim H. W., Uhlenhaut N. H., Rauch A., Weiner J., Hübner S., Hübner N., Won K. J., Lazar M. A., Tuckermann J., Steger D. J. (2015) Genomic redistribution of GR monomers and dimers mediates transcriptional response to exogenous glucocorticoid in vivo. Genome Res. 25, 836–844 10.1101/gr.188581.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baschant U., Tuckermann J. (2010) The role of the glucocorticoid receptor in inflammation and immunity. J. Steroid Biochem. Mol. Biol. 120, 69–75 10.1016/j.jsbmb.2010.03.058 [DOI] [PubMed] [Google Scholar]
- 6.Hübner S., Tuckermann J. (2012) Molecular mechanisms of the glucocorticoid receptor in steroid therapy—lessons from transgenic mice. Biomol. Concepts 3, 241–253 10.1515/bmc-2011-0033 [DOI] [PubMed] [Google Scholar]
- 7.Frenkel B., White W., Tuckermann J. (2015) Glucocorticoid-induced osteoporosis. Adv. Exp. Med. Biol. 872, 179–215 10.1007/978-1-4939-2895-8_8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koetz K. R., Ventz M., Diederich S., Quinkler M. (2012) Bone mineral density is not significantly reduced in adult patients on low-dose glucocorticoid replacement therapy. J. Clin. Endocrinol. Metab. 97, 85–92 10.1210/jc.2011-2036 [DOI] [PubMed] [Google Scholar]
- 9.Jia D., O’Brien C. A., Stewart S. A., Manolagas S. C., Weinstein R. S. (2006) Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology 147, 5592–5599 10.1210/en.2006-0459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conaway H. H., Henning P., Lie A., Tuckermann J., Lerner U. H. (2016) Activation of dimeric glucocorticoid receptors in osteoclast progenitors potentiates RANKL induced mature osteoclast bone resorbing activity. Bone 93, 43–54 10.1016/j.bone.2016.08.024 [DOI] [PubMed] [Google Scholar]
- 11.Piemontese M., Xiong J., Fujiwara Y., Thostenson J. D., O’Brien C. A. (2016) Cortical bone loss caused by glucocorticoid excess requires RANKL production by osteocytes and is associated with reduced OPG expression in mice. Am. J. Physiol. Endocrinol. Metab. 311, E587–E593 10.1152/ajpendo.00219.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tronche F., Kellendonk C., Reichardt H. M., Schütz G. (1998) Genetic dissection of glucocorticoid receptor function in mice. Curr. Opin. Genet. Dev. 8, 532–538 10.1016/S0959-437X(98)80007-5 [DOI] [PubMed] [Google Scholar]
- 13.Rauch A., Seitz S., Baschant U., Schilling A. F., Illing A., Stride B., Kirilov M., Mandic V., Takacz A., Schmidt-Ullrich R., Ostermay S., Schinke T., Spanbroek R., Zaiss M. M., Angel P. E., Lerner U. H., David J. P., Reichardt H. M., Amling M., Schütz G., Tuckermann J. P. (2010) Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab. 11, 517–531 10.1016/j.cmet.2010.05.005 [DOI] [PubMed] [Google Scholar]
- 14.Sher L. B., Woitge H. W., Adams D. J., Gronowicz G. A., Krozowski Z., Harrison J. R., Kream B. E. (2004) Transgenic expression of 11beta-hydroxysteroid dehydrogenase type 2 in osteoblasts reveals an anabolic role for endogenous glucocorticoids in bone. Endocrinology 145, 922–929 10.1210/en.2003-0655 [DOI] [PubMed] [Google Scholar]
- 15.Sher L. B., Harrison J. R., Adams D. J., Kream B. E. (2006) Impaired cortical bone acquisition and osteoblast differentiation in mice with osteoblast-targeted disruption of glucocorticoid signaling. Calcif. Tissue Int. 79, 118–125 10.1007/s00223-005-0297-z [DOI] [PubMed] [Google Scholar]
- 16.Zhou H., Mak W., Kalak R., Street J., Fong-Yee C., Zheng Y., Dunstan C. R., Seibel M. J. (2009) Glucocorticoid-dependent Wnt signaling by mature osteoblasts is a key regulator of cranial skeletal development in mice. Development 136, 427–436 10.1242/dev.027706 [DOI] [PubMed] [Google Scholar]
- 17.Dunn A. J. (2000) Cytokine activation of the HPA axis. Ann. N. Y. Acad. Sci. 917, 608–617 10.1111/j.1749-6632.2000.tb05426.x [DOI] [PubMed] [Google Scholar]
- 18.Waters R. V., Gamradt S. C., Asnis P., Vickery B. H., Avnur Z., Hill E., Bostrom M. (2000) Systemic corticosteroids inhibit bone healing in a rabbit ulnar osteotomy model. Acta Orthop. Scand. 71, 316–321 10.1080/000164700317411951 [DOI] [PubMed] [Google Scholar]
- 19.Li J., Wang X., Zhou C., Liu L., Wu Y., Wang D., Jiang H. (2012) Perioperative glucocorticosteroid treatment delays early healing of a mandible wound by inhibiting osteogenic differentiation. Injury 43, 1284–1289 10.1016/j.injury.2012.04.014 [DOI] [PubMed] [Google Scholar]
- 20.Aslan M., Simşek G., Yildirim U. (2005) Effects of short-term treatment with systemic prednisone on bone healing: an experimental study in rats. Dent. Traumatol. 21, 222–225 10.1111/j.1600-9657.2005.00300.x [DOI] [PubMed] [Google Scholar]
- 21.Weber A. J., Li G., Kalak R., Street J., Buttgereit F., Dunstan C. R., Seibel M. J., Zhou H. (2010) Osteoblast-targeted disruption of glucocorticoid signalling does not delay intramembranous bone healing. Steroids 75, 282–286 10.1016/j.steroids.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 22.Tu J., Henneicke H., Zhang Y., Stoner S., Cheng T. L., Schindeler A., Chen D., Tuckermann J., Cooper M. S., Seibel M. J., Zhou H. (2014) Disruption of glucocorticoid signaling in chondrocytes delays metaphyseal fracture healing but does not affect normal cartilage and bone development. Bone 69, 12–22 10.1016/j.bone.2014.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tronche F., Kellendonk C., Kretz O., Gass P., Anlag K., Orban P. C., Bock R., Klein R., Schütz G. (1999) Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 23, 99–103 10.1038/12703 [DOI] [PubMed] [Google Scholar]
- 24.Röntgen V., Blakytny R., Matthys R., Landauer M., Wehner T., Göckelmann M., Jermendy P., Amling M., Schinke T., Claes L., Ignatius A. (2010) Fracture healing in mice under controlled rigid and flexible conditions using an adjustable external fixator. J. Orthop. Res. 28, 1456–1462 10.1002/jor.21148 [DOI] [PubMed] [Google Scholar]
- 25.Parfitt A. M., Drezner M. K., Glorieux F. H., Kanis J. A., Malluche H., Meunier P. J., Ott S. M., Recker R. R. (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2, 595–610 10.1002/jbmr.5650020617 [DOI] [PubMed] [Google Scholar]
- 26.Dempster D. W., Compston J. E., Drezner M. K., Glorieux F. H., Kanis J. A., Malluche H., Meunier P. J., Ott S. M., Recker R. R., Parfitt A. M. (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 28, 2–17 10.1002/jbmr.1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan E. F., Mason Z. D., Chien K. B., Pfeiffer A. J., Barnes G. L., Einhorn T. A., Gerstenfeld L. C. (2009) Micro-computed tomography assessment of fracture healing: relationships among callus structure, composition, and mechanical function. Bone 44, 335–344 10.1016/j.bone.2008.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleiman A., Hübner S., Rodriguez Parkitna J. M., Neumann A., Hofer S., Weigand M. A., Bauer M., Schmid W., Schütz G., Libert C., Reichardt H. M., Tuckermann J. P. (2012) Glucocorticoid receptor dimerization is required for survival in septic shock via suppression of interleukin-1 in macrophages. FASEB J. 26, 722–729 10.1096/fj.11-192112 [DOI] [PubMed] [Google Scholar]
- 29.Vandevyver S., Dejager L., Van Bogaert T., Kleyman A., Liu Y., Tuckermann J., Libert C. (2012) Glucocorticoid receptor dimerization induces MKP1 to protect against TNF-induced inflammation. J. Clin. Invest. 122, 2130–2140 10.1172/JCI60006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wüst S., van den Brandt J., Tischner D., Kleiman A., Tuckermann J. P., Gold R., Lühder F., Reichardt H. M. (2008) Peripheral T cells are the therapeutic targets of glucocorticoids in experimental autoimmune encephalomyelitis. J. Immunol. 180, 8434–8443 10.4049/jimmunol.180.12.8434 [DOI] [PubMed] [Google Scholar]
- 31.Coutinho A. E., Gray M., Brownstein D. G., Salter D. M., Sawatzky D. A., Clay S., Gilmour J. S., Seckl J. R., Savill J. S., Chapman K. E. (2012) 11β-Hydroxysteroid dehydrogenase type 1, but not type 2, deficiency worsens acute inflammation and experimental arthritis in mice. Endocrinology 153, 234–240 10.1210/en.2011-1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuckermann J. P., Kleiman A., Moriggl R., Spanbroek R., Neumann A., Illing A., Clausen B. E., Stride B., Förster I., Habenicht A. J., Reichardt H. M., Tronche F., Schmid W., Schütz G. (2007) Macrophages and neutrophils are the targets for immune suppression by glucocorticoids in contact allergy. J. Clin. Invest. 117, 1381–1390 10.1172/JCI28034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baschant U., Frappart L., Rauchhaus U., Bruns L., Reichardt H. M., Kamradt T., Bräuer R., Tuckermann J. P. (2011) Glucocorticoid therapy of antigen-induced arthritis depends on the dimerized glucocorticoid receptor in T cells. Proc. Natl. Acad. Sci. USA 108, 19317–19322 10.1073/pnas.1105857108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou H., Mak W., Zheng Y., Dunstan C. R., Seibel M. J. (2008) Osteoblasts directly control lineage commitment of mesenchymal progenitor cells through Wnt signaling. J. Biol. Chem. 283, 1936–1945 10.1074/jbc.M702687200 [DOI] [PubMed] [Google Scholar]
- 35.Cárcamo-Orive I., Gaztelumendi A., Delgado J., Tejados N., Dorronsoro A., Fernández-Rueda J., Pennington D. J., Trigueros C. (2010) Regulation of human bone marrow stromal cell proliferation and differentiation capacity by glucocorticoid receptor and AP-1 crosstalk. J. Bone Miner. Res. 25, 2115–2125 10.1002/jbmr.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maes C. (2013) Role and regulation of vascularization processes in endochondral bones. Calcif. Tissue Int. 92, 307–323 10.1007/s00223-012-9689-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.