Abstract
Nontypeable Haemophilus influenzae (NTHi), one of the most common acute otitis media (OM) pathogens, is postulated to promote middle-ear epithelial remodeling in the progression of OM from acute to chronic. The goal of this study was to examine early quantitative proteomic secretome effects of NTHi lysate exposure in a human middle-ear epithelial cell (HMEEC) line. NTHi lysates were used to stimulate HMEEC, and conditional quantitative stable isotope labeling with amino acids in cell culture of cell secretions was performed. Mass spectrometry analysis identified 766 proteins across samples. Of interest, several heterogeneous nuclear ribonucleoproteins (hnRNPs) were regulated by NTHi lysate treatment, especially hnRNP A2B1 and hnRNP Q, known to be implicated in microRNA (miRNA) packaging in exosomes. After purification, the presence of exosomes in HMEEC secretions was characterized by dynamic light scattering (<100 nm), transmission electron microscopy, and CD63/heat shock protein 70 positivity. hnRNP A2B1 and hnRNP Q were confirmed to be found in exosomes by Western blot and proteomic analysis. Finally, exosomal miRNA content comprised 110 unique miRNAs, with 5 found to be statistically induced by NTHi lysate (miR-378a-3p + miR-378i, miR-200a-3p, miR-378g, miR30d-5p, and miR-222-3p), all known to target innate immunity genes. This study demonstrates that NTHi lysates promote release of miRNA-laden exosomes from middle-ear epithelium in vitro.—Val, S., Krueger, A., Poley, M., Cohen, A., Brown, K., Panigrahi, A., Preciado, D. Nontypeable Haemophilus influenzae lysates increase heterogeneous nuclear ribonucleoprotein secretion and exosome release in human middle-ear epithelial cells.
Keywords: otitis media, quantitative proteomic, infection, innate immunity, vesicle
Otitis media (OM) is one of the most common conditions of early childhood, accounting for a very high proportion of all pediatric physician office visits annually (1, 2) at a national health care cost estimated to be >$1 billion (3). In the first years of life, a majority of children, at some point, will experience OM (4, 5). Acute OM (AOM) is defined as acute onset of middle-ear effusion (MEE), along with signs and symptoms of middle-ear inflammation and infection. Nontypeable Haemophilus influenzae (NTHi) is 1 of the most common infectious pathogens in AOM (6, 7), and it is known to signal through TLR-2 and MAPK pathways to induce activation of a plurality of cytokines and chemokines in the middle-ear epithelium (8). This inflammation is thought to participate in the conversion of middle-ear mucosa to one permissive of chronic mucin secretion (9), but the mechanisms driving the remodeling of the middle-ear epithelium still have to be elucidated. We previously showed the predominance of neutrophil extracellular traps in chronic OM (COM) effusion (10). NETs are characterized by partial decompaction of nuclear DNA, which is then emitted as long filaments into the extracellular space. This DNA carries antibacterial proteins and histones (and specifically citrullinated histones) (11).
Stable isotope labeling with amino acids in cell culture (SILAC) is a highly accurate and quantitative mass spectrometry (MS)-based proteomics technique (12) that allows for global measurements of protein secretion by experimental condition. The proteome of MEEs has been assayed before (13), but to our knowledge, the response of human middle-ear epithelium to NTHi lysates has not been studied by this technique.
Exosomes are small vesicles released by cells ranging from 30 to 100 nm in diameter, containing proteins, along with nucleic acids, as mRNAs, microRNAs (miRNAs), or noncoding RNAs (14–16). Exosomes are found in biologic fluids targeting neighboring cells or being transported systemically. They are taken up by cells through various potential mechanisms, where their miRNAs have the ability to regulate gene expression (17). They are thought to play an important role in cell–cell communication and immune response. We recently reported the presence of miRNAs in MEEs from patients with COM, with a high abundance of miRNA (miR)-223 related to the presence of neutrophils in the middle ear (18). This specific miRNA is known to be part of the signature of activated neutrophils (19), but the cellular origin of the other miRNAs detected in middle-ear fluid could not be determined.
In efforts to determine specific host epithelial middle-ear cell responses to bacterial antigens, we hypothesized that the exposure of human middle-ear epithelial cell (HMEEC) to NTHi lysates would result in the secretion of key mediators of immune response, cell-to-cell communication, and epithelial remodeling. We chose to use lysates of bacteria instead of live bacteria to elicit a faster response of middle-ear epithelial cells and avoid cell death as a result of overgrowth of live bacteria. Although it cannot replicate the same effect of live bacteria, the use of transient NTHi lysate exposure has been used as a method by us and others to examine the effects of bacterial pathogen protein exposure on middle-ear epithelial cell early responses (20–24). Finally, stimulation with lysates has been postulated to be relevant clinically (20, 25), as bacteria content is released in the middle ear of children after antibiotic treatment for AOM. Moreover, we have previously shown that NTHi lysate preparations contain high amounts of outer-membrane proteins, adhesins (high molecular weight proteins 1 and 2), and lipoproteins, known to be implicated in biologic effects of NTHi bacteria in AOM (26).
MATERIALS AND METHODS
Bacteria culture and preparation of lysates
NTHi was grown on chocolate agar at 37°C in 5% CO2 overnight and inoculated in brain heart infusion (BHI; BD Laboratories, Franklin Lakes, NY, USA) and broth, supplemented with 10 mg/ml NAD (Sigma-Aldrich, St. Louis, MI, USA). After overnight incubation, bacteria were subcultured into 500 ml fresh BHI, supplemented with 10 mg/ml NAD; upon reaching log-phase growth, bacteria were centrifuged 10 min at 10,000 g at room temperature and suspended in Ham’s F-12 nutrient mix, supplemented with L-glutamine (Thermo Fisher Scientific, Waltham, MA, USA). Sonication was then performed 15 times for 15 s in 4 ml batches on ice. Then, the lysates were centrifuged at 10,000 g for 10 min at 4°C to remove the remaining, nonlysed bacteria, as well as debris of cells. Stock solutions of 3–8 mg/ml were portioned into 1-ml aliquots and stored at −20°C.
Cell lines and treatments
HMEEC-1 is a human immortalized middle-ear epithelial cell line described by Chun et al. (27), which was generously provided to us by Dr. David Lim’s laboratory (House Ear Clinic, Los Angeles, CA, USA). Cells were maintained in 1:1 of bronchial epithelial cell basal medium (Lonza, Walkersville, MD, USA) and DMEM (Thermo Fisher Scientific) and supplemented with the SingleQuots kit (Lonza) containing bovine pituitary extract, hydrocortisone, human epidermal growth factor, epinephrine, transferrin, insulin, triiodothyronine, retinoic acid, gentamycin, and amphotericin-B. Cells were deprived of SingleQuots, 4 h before bacterial lysate challenge in bronchial epithelial basal medium/DMEM with 1% penicillin-streptomycin (Thermo Fisher Scientific). After achieving confluence in a T-75 flask, HMEECs were treated with 200 μg/ml NTHi lysates vs. the same volume of PBS (<1%) during 24 h, rinsed 6 times with HBSS with calcium and magnesium (Thermo Fisher Scientific), and incubated in 1:1 of bronchial epithelial cell growth medium/DMEM with 1% penicillin-streptomycin for 24 or 48 h. Cell secretions were then collected, filtered with a 0.22-μm membrane, and concentrated with an Amicon-3K column (Millipore, Billerica, MA, USA). The proteins were then portioned into aliquots and stored at −80°C for secretome analysis. It was necessary to remove the SingleQuots from the medium to ensure that the purity of the measured proteins in the secretions was specific to the epithelium and not to the medium. Viability of the cells at these time points was confirmed both visually and with XTT viability assay (Trevigen, Gaithersburg, MD, USA). There was no loss of viability in these experimental conditions (data not shown).
SuperSILAC
The HMEEC-1 cell line was cultured in DMEM without arginine and lysine (Thermo Fisher Scientific) containing the SingleQuots kit (Lonza), supplemented with[13C]6, [15N]2-labeled lysine, and [13C]6-labeled arginine (Cambridge Isotope Laboratories, Andover, MA, USA) for 5 cell passages, until complete incorporation of the labeled amino acids [verified by liquid chromatography-tandem MS (LC-MS/MS), preliminary experiment]. Labeled cells were rinsed 6 times with HBSS with calcium and magnesium (Thermo Fisher Scientific), and the cell secretions were recovered. This was called the SuperSpike-in standard. In parallel, HMEECs, in 4 replicates, were cultured in regular medium and with 200 μg/ml NTHi lysates. Cell secretions were then collected, filtered with a 0.22 μm membrane, and concentrated with an Amicon-3K column (Millipore) to reduce the volume from 40 ml to 500 μl. The proteins were then aliquoted and stored at −80°C. Total protein (30 μg) in secretions from unlabeled cells (conditions of interest) was spiked with 30 μg total proteins in secretions from labeled cells (SuperSpike-in standard) and processed for SDS-PAGE. Coomassie blue staining and then in-gel digestion were performed cutting protein bands, and LC-MS/MS was carried out as described previously (13, 28). The overall protocol and the protein quantification calculation were performed as previously described (29). In brief, raw files were processed for protein identification and quantification using Integrated Proteomics Pipeline (IP2) v.1.01 software developed by Integrated Proteomics Applications (San Diego, CA, USA). Mass spectral data were uploaded into IP2 software and searched against the forward and reverse UniProt human database for tryptic peptides, allowing 2 missed cleavages, possible modification of oxidized methionine (15.99492 Da), and heavy arginine (6.0204 Da) and heavy lysine (8.0142 Da). IP2 uses the Sequest 2010 (06_10_13_1836) search engine (Scripps Research Institute, La Jolla, CA, USA; http://fields.scripps.edu/sequest/). Mass tolerance was set at ±30 ppm for MS and ±1.5 Da for MS/MS. Data were filtered, based on a 1% protein false discovery rate. All of the bands from each lane were summed in the analysis. Census software version 1.77, built into the IP2 platform, was used to determine the ratios of unlabeled and labeled peptide pairs using an extracted chromatogram approach. Then each treatment ratio was divided by the corresponding control ratio, thereby cancelling out the SuperSILAC common denominator and enabling comparison of the effect of NTHi lysate treatment over time. Proteins with a fold change ±1.2 were retained for further evaluation.
Western blotting
Cells were grown in T-75 flasks under the same conditions as above and treated identically with 200 µg/ml NTHi lysates. Secretions were collected for bicinchoninic acid assay (BCA; Thermo Fisher Scientific) to assay the total quantity of proteins. Western blotting, as described previously, was then performed (30). In brief, 30 μg total protein for each sample was separated by electrophoresis on NuPage Novex 4–12% Bis-Tris gels (Thermo Fisher Scientific). The MW marker Kaleidoscope was used as a standard (Bio-Rad Laboratories, Hercules, CA, USA). Proteins were then transferred to a nitrocellulose membrane (Thermo Fisher Scientific). Membranes were blocked with 5% nonfat dry milk in PBS with 0.05% Tween-20 and incubated with the following primary antibodies: mouse monoclonal anti-human heterogeneous nuclear ribonucleoprotein (hnRNP) A2B1 (ab6102; Abcam, Cambridge, United Kingdom) and mouse monoclonal anti-human hnRNP Q (sc-56703; Santa Cruz Biotechnology, Dallas, TX, USA) at 1/1000 in 10 ml of 2.5% milk solution in PBS with 0.05% Tween-20. Antibodies against exosomal markers CD63 and heat shock protein (HSP)70 were used at 1/5000 (rabbit antibodies; EXOAB-CD63A-1 and EXOAB-Hsp70A-1; System Biosciences, Mountain View, CA, USA). Secondary anti-mouse (sc-2005) or anti-rabbit (sc-2004) antibodies coupled to horseradish peroxidase (Santa Cruz Biotechnology) were used at a 1/10,000 dilution. Detection was performed with a SuperSignal West Dura Extended Duration Substrate Kit (Thermo Fisher Scientific), according to the manufacturer’s instructions.
Exosome isolation from HMEEC secretions
Methods previously reported for the isolation of exosomes from MEEs (18) were used to isolate exosomes from HMEEC secretions as follows. The whole secretions of HMEECs (40 ml per condition) were centrifuged at 10,000 g for 10 min at 4°C to remove debris, filtered with a 0.22-μm membrane to remove bigger vesicles, and concentrated with an Amicon-3K column (Millipore) to concentrate proteins >3 kDa. Secretions were then mixed with a 1/5 dilution of Exoquick-TC (System Biosciences) and incubated overnight at 4°C. The day after, the samples were centrifuged at 1500 g for 30 min, and the exosome pellet was reconstituted in 100 μl PBS, 1 time, and thawed for further experiments.
Exosome detection
The purified exosomes were assayed for protein concentration with BCA (Thermo Fisher Scientific). First, 15 μl purified exosomes were mixed with 85 μl RIPA buffer + 1% protease inhibitor cocktail (Sigma-Aldrich) and incubated 5 min at room temperature to liberate exosome proteins. Proteins (10 μl) were used for the BCA assay, according to the manufacturer’s instructions. The Exocet assay (System Biosciences) was used to quantify further purified exosomes. Western blot analysis of exosomal proteins was also performed with a 20-μl exosome pellet in PBS for every experiment and condition for the specific markers CD63 and HSP70 and for hnRNP A2B1 and Q.
Determination of exosome size by dynamic light scattering
The size of exosomes was evaluated by a Zetasizer Nano ZS system (Malvern Instruments, Malvern, United Kingdom) that uses the dynamic light scattering (DLS) technique, analyzing the velocity distribution of particle movement by measuring the dynamic fluctuations of scattered light intensity at a fixed angle caused by the Brownian motion of the particle. The particle’s hydrodynamic radius, or considered diameter, is then calculated with the Stokes–Einstein equation (Malvern Instruments). After exosome isolation with the Exoquick-TC, the pellet was reconstituted in 100 μl PBS. Purified exosomes (10 μl) were diluted in 990 μl filtered PBS, and vortexed for 1 min. The machine was set on 3 runs, with each 3 readings, completed within 1 min. The whole volume was then immediately put in a disposable cuvette for size measurements to avoid aggregation of the exosomes. Three independent measurements were performed for each sample and averaged by the software for the analysis.
Electron microscopy of isolated microvesicles
After exosome isolation with the Exoquick-TC, the pellet was reconstituted in 100 μl PBS, filtered through a 0.2-μm membrane. A 1/20 dilution (10 μl) of purified exosomes was then plated onto carbon-coated formvar grids (Electron Microscopy Sciences, Hatfield, PA, USA); fixed with a solution of 2% glutaraldehyde, 0.1 M cacodylate; stained with 4% aqueous uranyl acetate; and imaged with a Talos F200X Transmission Electron Microscope (FEI, Brno, Czech Republic) at 80 kV. The same protocol was performed with PBS as a negative control.
miRNA isolation and analysis
The miRNA content in purified exosomes was isolated with SeraMir columns (System Biosciences) using the protocol recommended by the supplier. The quality of the miRNA was then evaluated using the Agilent Small RNA Kit and the Agilent RNA 6000 Nano Kit for the total RNA (Agilent Technologies, Santa Clara, CA, USA).
Nanostring identification of miRNAs
The global miRNA profile in exosomes was determined using Nanostring human microarrays (human V2 miRNA array >800 probes; Nanostring Technologies, Seattle, WA, USA). To account for differences in hybridization and purification, data were normalized to the average counts for all control spikes in each sample using proprietary bioinformatics software (nSolver Analysis Software 2.5; Nanostring Technologies). In brief, we calculated a background level of expression for each sample using the mean level of the negative controls plus 2 sd of the mean. miRNAs expressing <2 sd from the mean were set to 0 expression. Those miRNAs that were considered non-0 expression were normalized using a scaling factor based on the top 100 expressing miRNAs across all samples. For each sample, the average of the geometric means of the top 100-expressing miRNAs across all samples was divided by the geometric mean of each sample (See website for further details; http://www.nanostring.com).
Label-free proteomic analysis of exosome proteins
Exosomes were isolated with the Exoquick-TC method previously described, and 30 μg total proteins were loaded in an SDS-PAGE. In-gel digestion was performed cutting protein bands, and the peptide mixtures from each lane were sequentially analyzed by LC-MS/MS using the nano-LC system (Easy nLC1000) connected to the Q Exactive mass spectrometer (Thermo Fisher Scientific). The platform is configured with nano-electrospray ion source (Easy-Spray; Thermo Fisher Scientific), Acclaim PepMap 100 C18 nanoViper trap column (3 μm particle size, 75 μm internal diameter × 20 mm length), and Easy-Spray C18 analytical column (2 μm particle size, 75 μm internal diameter × 500 mm length). The peptides were eluted at a flow rate of 300 nl/min using linear gradients of 2–25% acetonitrile (in aqueous phase and 0.1% formic acid) for 35 min, followed by 25–40% for 10 min, 40–60% for 1 min, and static flow at 60% for 14 min.
MS data were collected in a data-dependent manner, switching between 1 full-scan MS mode (mass:charge ratio 380–1600, resolution 70,000, AGC 3e6) and the 10 MS/MS mode (resolution 17,500), where MS/MS analysis of the top 10 target ions was performed once and dynamically excluded from the list for 20 s. The MS raw data sets were searched against the UniProt human database, which also included common contaminants using MaxQuant software (v.1.5.5.1; Max Planck Institute of Biochemistry, Martinsried, Germany). We used default parameters for the searches: first search peptide tolerance 20 ppm, main search peptide tolerance 4.5 ppm, maximum 2 missed cleavage; and the peptide and resulting protein assignments were allowed at 0.01 false discovery rate (thus, 99% confidence level).
Statistical analysis
The statistical difference between experimental and control groups for all experiments was determined by 2-tailed Student’s t tests and ANOVA test, followed by Dunnett’s test or Wilcoxon test. Significance level was set at P < 0.05. A paired 2-tailed Student’s t test was run for the 110 miRNAs detected by the Nanostring chip technique.
RESULTS
SuperSILAC analysis of HMEEC secretions
MS analysis identified 1731 proteins across samples; of these, 766 proteins were identified with a total peptide count (PC) of at least 20 across all 4 samples (Control vs. NTHi lysates at 24 and 48 h), set as a cutoff (Supplemental Table 1). Overall, 89 proteins were enriched >1.2-fold at 24 h, and 162 proteins were enriched >1.2-fold at 48 h by NTHi lysates. On the other hand, 30 and 37 proteins were found to be depleted >0.7-fold at 24 and 48 h, respectively, with NTHi lysates. The 30 overall most abundant proteins identified were not regulated by NTHi lysate treatment across time points. Several cytoskeletal, cytoskeletal-binding proteins, and extracellular matrix proteins demonstrated a high PC, such as actin, spectrin, filamin, plectin, and myosin-9. Three HSPs—HSP70, HSP71, and HSP90—along with the innate-immune protein complement C3, were found to be highly abundant across groups as well.
To understand better epithelial effects of NTHi lysates over time, we analyzed the most regulated proteins in HMEECs at each time point. Table 1 lists the 15 proteins most enriched in secretions at 24 (top panel) and 48 h (bottom panel). Neutrophil gelatinase-associated lipocalin, an innate-immunity protein, was found to be the most highly enriched at both time points, with induction factors of 5.87 and 5.98. Extracellular matrix proteins, as well as extracellular matrix remodeling proteins (fibronectin, laminins, serpin H1 and N-acetylglucosamine-6-sulfatase, interstitial collagenase, extracellular matrix protein 1), were enriched at one or both time points. Somewhat surprisingly, the typically intracellular heterogeneous ribonucleoproteins A2B1 (hnRNP A2B1) and K (hnRNP K) were enriched in secretions at 24 h (fold inductions of 1.99 and 1.78) and hnRNP Q at 48 h (fold induction 4.73). Given our recent publication identifying miRNA-laden exosomes within MEE samples (18) and the fact that hnRNPs are known to function in the packaging of exosomes (31), we elected to validate further this finding of the hnRNP presence in HMEEC in vitro secretions.
TABLE 1.
Most enriched proteins from HMEEC secretions in response to NTHi lysates sorted by fold inductions for the 24 h time point and the 48 h time point
| Locus | Description | 24 T/C | 48 T/C |
|---|---|---|---|
| Most enriched proteins at 24 h | |||
| P80188 | Neutrophil gelatinase-associated lipocalin | 5.87 | 5.98 |
| P09758 | Tumor-associated calcium signal transducer 2 | 5.47 | 1.56 |
| P03956 | Interstitial collagenase | 4.59 | 4.10 |
| P22626 | hnRNPs A2/B1 | 1.99 | 0.77 |
| P15586 | N-Acetylglucosamine-6-sulfatase | 1.94 | 2.03 |
| P55072 | Transitional endoplasmic reticulum ATPase | 1.91 | 1.85 |
| P61978 | hnRNP K | 1.78 | 1.04 |
| P38159 | RNA-binding motif protein, X chromosome | 1.75 | 1.38 |
| P50454 | Serpin H1 | 1.73 | 3.14 |
| P02751 | Fibronectin | 1.61 | 1.15 |
| Q16787 | Laminin subunit α-3 | 1.58 | 1.40 |
| Q13753 | Laminin subunit γ-2 | 1.52 | 1.39 |
| P24592 | Insulin-like growth factor-binding protein 6 | 1.52 | 1.31 |
| Q13751 | Laminin subunit β-3 | 1.49 | 1.39 |
| P00749 | Urokinase-type plasminogen activator | 1.48 | 1.32 |
| Most enriched proteins at 48 h | |||
| P80188 | Neutrophil gelatinase-associated lipocalin | 5.87 | 5.98 |
| O60506 | hnRNP Q | 1.04 | 4.73 |
| P03956 | Interstitial collagenase | 4.59 | 4.10 |
| P50454 | Serpin H1 | 1.73 | 3.14 |
| P15586 | N-Acetylglucosamine-6-sulfatase | 1.94 | 2.03 |
| P55072 | Transitional endoplasmic reticulum ATPase | 1.91 | 1.85 |
| Q9NZ08 | Endoplasmic reticulum aminopeptidase 1 | 0.61 | 1.83 |
| Q02818 | Nucleobindin-1 | 1.24 | 1.74 |
| P31431 | Syndecan-4 | 1.45 | 1.72 |
| Q16610 | Extracellular matrix protein 1 | 1.17 | 1.64 |
| P07602 | Prosaposin | 0.86 | 1.62 |
| Q9BQE3 | Tubulin α-1C chain | 0.84 | 1.62 |
| P07711 | Cathepsin L1 | 1.31 | 1.59 |
| Q6UVK1 | Chondroitin sulfate proteoglycan 4 | 1.01 | 1.58 |
| P09758 | Tumor-associated calcium signal transducer 2 | 5.47 | 1.56 |
T/C, treated over control.
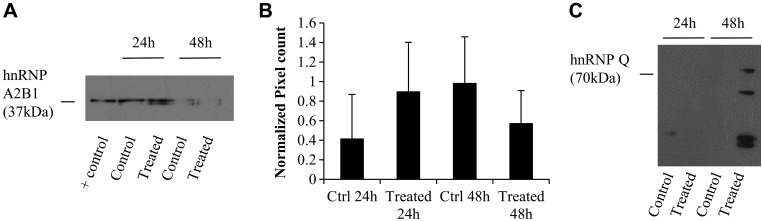
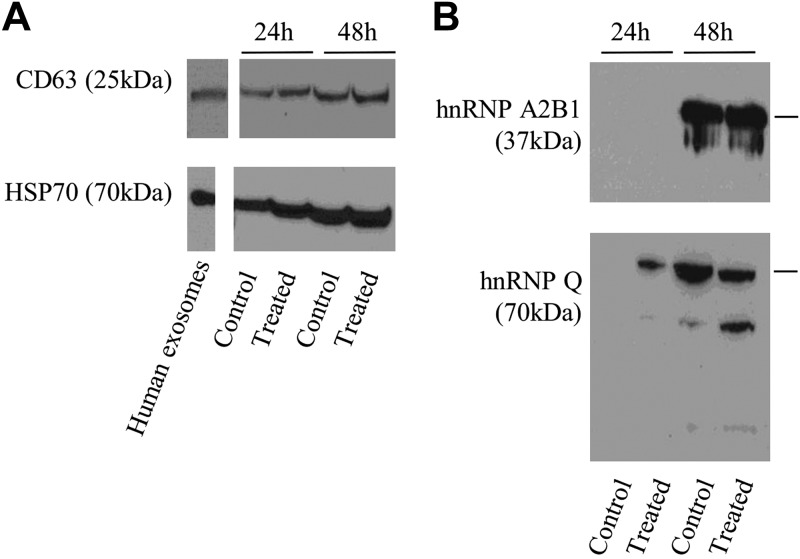
Western blot analysis of hnRNPs (Fig. 1) was performed to validate the SILAC findings for noted proteins of interest. In 3 independent biologic replicate experiments, we confirmed that hnRNP A2B1 was enriched at 24 h but depleted at 48 h (Fig. 1A shows a representative blot and Fig. 1B the mean of PC for the 3 experiments) and that hnRNP Q was enriched only at 48 h (Fig. 1C). Several noted bands smaller than 70 kDa (the MW of hnRNP Q) suggested cleavage of the protein-generating fragments.
Figure 1.
Western blot detection of hnRNP A2B1 and hnRNP Q in HMEEC secretions. Proteins (50 μg) were loaded in an SDS-PAGE and separated. The positive control consisted of 50 µg protein from a human exosomal protein suspension obtained commercially from System Biosciences. After electrophoresis, proteins were transferred to a nitrocellulose membrane, which was then exposed to antibodies anti-hnRNP A2B1 (37 kDa) (A) and the normalized pixel count for 3 independent Western blots (data not all shown) over positive control (B), or anti-hnRNP Q (70 kDa) (C). Note that the protein doublet for hnRNP A2B1 is present as a result of the proteins’ 2 isoforms (hnRNPB1 is 353 aa, 37.4 kDa; hnRNPA2 is 341 aa, 36 kDa). Multiple bands for hnRNP Q likely represent protein fragmentation.
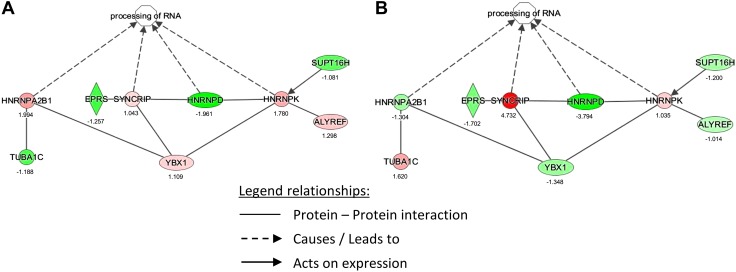
The dataset of regulated proteins by NTHi lysate treatment was analyzed using Ingenuity Pathway Analysis (IPA; v8.5; Ingenuity Systems, Redwood City, CA, USA), focusing on the detected hnRNP proteins. Figure 2 shows IPA results, indicating RNA processing activation involving hnRNPs at 24 h (Fig. 2A) and 48 h (Fig. 2B), along with a summary of the induction factors (Table 2). Given that hnRNPs are known to be involved in the packaging of miRNAs into exosomes (31, 32) and may be secreted within exosomes, we then investigated for the presence of exosomes and miRNAs in HMEEC secretions upon NTHi lysate exposure.
Figure 2.
IPA for hnRNP proteins identified in HMEEC secretions. Ratios of hnRNPs for treated-over-control conditions were manually entered in IPA and analyzed for regulated signaling pathways and networks, focusing on hnRNPs; 24 h (A), 48 h (B) Legend of molecules: HNRNP A2B1, heterogeneous ribonucleoprotein A2B1; HNRNPAD, heterogeneous ribonucleoprotein D; HNRNPK, heterogeneous ribonucleoprotein K = SYNCRIP, synaptotagmin binding, cytoplasmic RNA interacting protein; heterogeneous ribonucleoprotein Q; TUBA1C, tubulin α 1C chain; EPRS, glutamyl-prolyl-tRNA synthetase; YBX1, Y box binding protein 1; SUPT16H, suppressor of Ty 16 homolog; ALYREF, Aly/REF export factor. Red, upregulated; green, downregulated.
TABLE 2.
hnRNP protein fold inductions (treated over control)
| hnRNP | 24 h | 48 h |
|---|---|---|
| A2B1 | 1.994 | −1.304 |
| Q | 1.043 | 4.732 |
| D | −1.961 | −3.794 |
| K | 1.780 | 1.035 |
Characterization of exosomes in HMEEC secretions
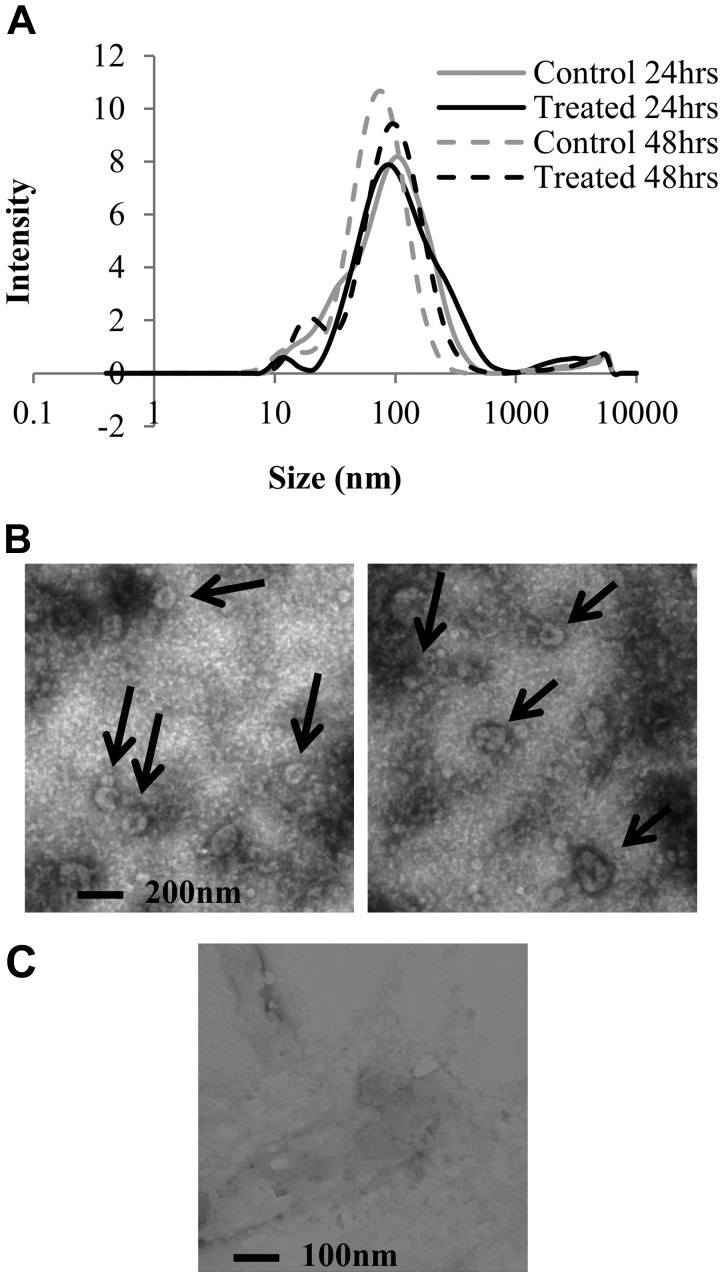
HMEEC secretions were collected after NTHi lysate vs. control treatment at 24 and 48 h and concentrated by column centrifugation. Exosomes were then isolated with the Exoquick-TC method, before being characterized by DLS (Fig. 3A and Table 3) and electron microscopy (EM; Fig. 3B). The DLS technique measures the hydrodynamic diameter of particles, which is the size of a hypothetical hard sphere that diffuses in the same fashion as that of the particle being measured. Results showed that a majority of purified vesicles had a diameter close to 100 nm with a mean size of 68.8–88.7 nm. In addition, the polydispersivity index, showing the heterogeneity of the size distribution, was between 0.36 and 0.44, a midrange value. Because the DLS measurements can be biased by the biologic nature of exosomes, we then used EM to visualize them. Figure 3B depicts isolated exosomes from HMEEC secretions, plated and stained on grids. The arrows point to vesicles between 100 and 150 nm of diameter, with the accumulation of the dark stain in the middle, typical of exosomes viewed by EM (33). PBS, used to dilute the exosome samples for this experiment, was also imaged (Fig. 3C) and showed some impurities but no vesicle shape.
Figure 3.
Evaluation of the size of exosomes from HMEEC secretions. Exosomes were purified with the Exoquick-TC method and resuspended in PBS. Samples were evaluated by DLS and EM. A) DLS results showed as a granulometric distribution; solid lines represent the 24 h time points and dotted lines the 48 h time point. Each line represents the mean of 3 runs. B) EM pictures of a representative treated sample. C) EM picture of saline only, without any exosome sample, showing expected background of EM image. Both techniques showed that the vesicles were mostly smaller than 100 nm, confirming the exosome nature of these.
TABLE 3.
Characterization of the average size and polydispersivity index of exosomes by DLS
| Control | Treated | |||
|---|---|---|---|---|
| Parameter | 24 h | 48 h | 24 h | 48 h |
| Average size (nm) | 88.70 | 69.23 | 84.85 | 68.82 |
| Polydispersivity index | 0.42 | 0.36 | 0.44 | 0.42 |
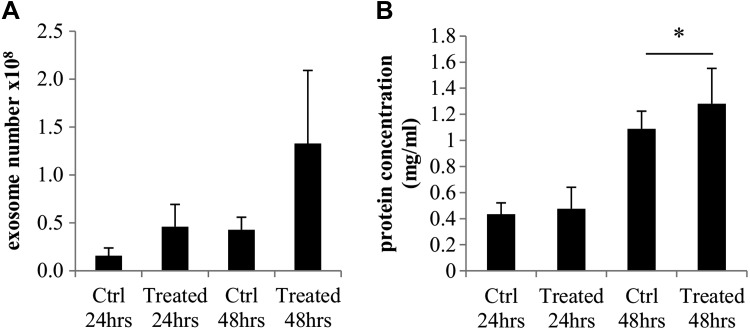
To confirm the biologic nature of the isolated vesicles, we used both the Exocet assay (as a direct measurement of acetyl-coenzyme A acetylcholinesterase activity, known to be enriched within exosomes) and the overall protein concentration in the isolated vesicles. Figure 4A shows the mean results of 4 independent experiments. At 24 h, controls contained 1.6 × 107 exosomes per sample, whereas NTHi lysate-treated samples contained 3-fold more exosomes (4.6 × 107 exosomes/sample), although this difference was not statistically significant. At 48 h, the controls showed 4.3 × 107 exosomes, whereas the NTHi lysate-treated samples showed 1.33 × 108 exosomes, a difference that also was not statistically significant. As a separate means of exosome sample quantification, whole-protein assays were also conducted with the same samples, with the same volume of exosomes per condition (10 μl). At 24 h, control exosome samples contained a mean 0.43 mg/ml, and NTHi lysate-treated exosome samples contained 0.48 mg/ml total protein. At 48 h, control exosome samples contained 1.09 mg/ml, whereas NTHi lysate-treated exosome samples contained 1.28 mg/ml total protein (P < 0.05; Fig. 4B).
Figure 4.
Exosome and protein assays of isolated vesicles. To confirm the exosome nature of the vesicles, an Exocet assay was performed, and the results are shown in A. A BCA protein assay was also performed and shown in B. In these experiments, the same volume of exosomes was used for each sample. *P < 0.05.
Finally, further characterization was performed by Western blot analysis. Results showed consistent presence of known exosomal markers CD63 and HSP70 proteins (Fig. 5A) in all of the samples. Notably, the intensity of the bands is consistent with the higher abundance of exosomes at 48 h. Much like in HMEEC secretions, hnRNP A2B1 and hnRNP Q were also detected in isolated exosomes from HMEEC secretions (Fig. 5B). As seen in Fig. 1B, hnRNP Q showed several bands with a lower MW than the theoretical one (70 kDa), likely as a result of degradation or cleavage of the protein.
Figure 5.
Western blot analysis of exosome markers (CD63, 25 kDa; HSP70, 70 kDa; A) and hnRNPs (hnRNP A2B1, 37 kDa; hnRNP Q 70 kDa; B) in exosomes from HMEEC secretions. The same volume of exosomes per sample was used.
miRNA characterization
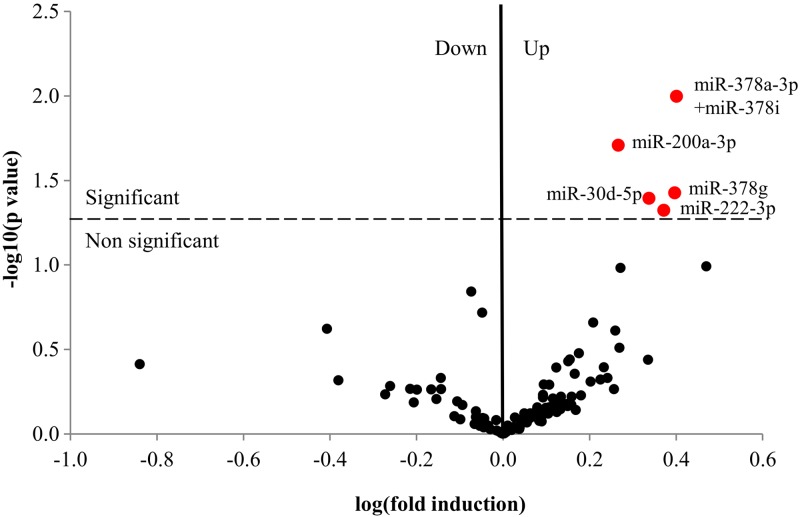
miRNAs were isolated from exosome pellets for each condition and characterized by a Nanonstring chip recognizing 800 cogent human miRNA targets. Overall, 123 miRNAs were detected in HMEEC secretions. The 5 most abundant miRNAs were miR-205-5p, miR-23a-3p, let-7a-5p, miR-21-5p, and miR-1246, but levels of these were not affected by NTHi lysate treatment. (Supplemental Table 2 shows the whole dataset of miRNAs detected in HMEEC secretion exosomes.) Five miRNAs were significantly induced by NTHi lysate treatment at 48 h, as shown in the volcano plot: miR-222-3p, miR-30d-5p, miR-378g, miR-200a-3p, and miR378a-3p + miR-378i (Fig. 6). Notably, no downregulated miRNA was statistically significant (all P > 0.05). Table 4 shows the count values, se, and fold inductions for the 5 significantly induced miRNAs.
Figure 6.
Volcano plot of miRNAs detected by Nanostring chip. HMEECs were subjected to the same NTHi lysate treatments as described before. Exosomes were purified; miRNAs were isolated with an affinity membrane and assayed with a Nanostring chip for 800 human miRNA targets at 48 h. miRNA datasets were filtered to remove the background noise analyzed for significant regulation by NTHi lysate treatment (110 miRNAs total). Ratios were calculated as the means of the miRNA count for treated over the mean of the miRNA count for controls. Statistical analysis was done using multiple t tests to calculate the P value. A Volcano plot was then used to represent the data as −log 10(P) = f[log(fold induction)]. The dotted line represents the limit of P = 0.05. The red dots are the statistically upregulated miRNAs of the dataset.
TABLE 4.
miRNAs significantly induced by NTHi lysate treatment
| miRNA | P | Mean control | Mean treated | se | Ratio T/C |
|---|---|---|---|---|---|
| hsa-miR-378a-3p + miR-378i | 0.0100 | 64.67 | 162.7 | 21.3 | 2.52 |
| hsa-miR-200a-3p | 0.0196 | 26 | 48 | 5.831 | 1.85 |
| hsa-miR-378g | 0.0374 | 42.67 | 106.3 | 20.76 | 2.49 |
| hsa-miR-30d-5p | 0.0403 | 48 | 104.3 | 18.83 | 2.17 |
| hsa-miR-222-3p | 0.0475 | 469.7 | 1104 | 224.2 | 2.35 |
hsa-miR, human miRNA.
Pathways predicted to be regulated by miRNAs in HMEEC secretion exosomes
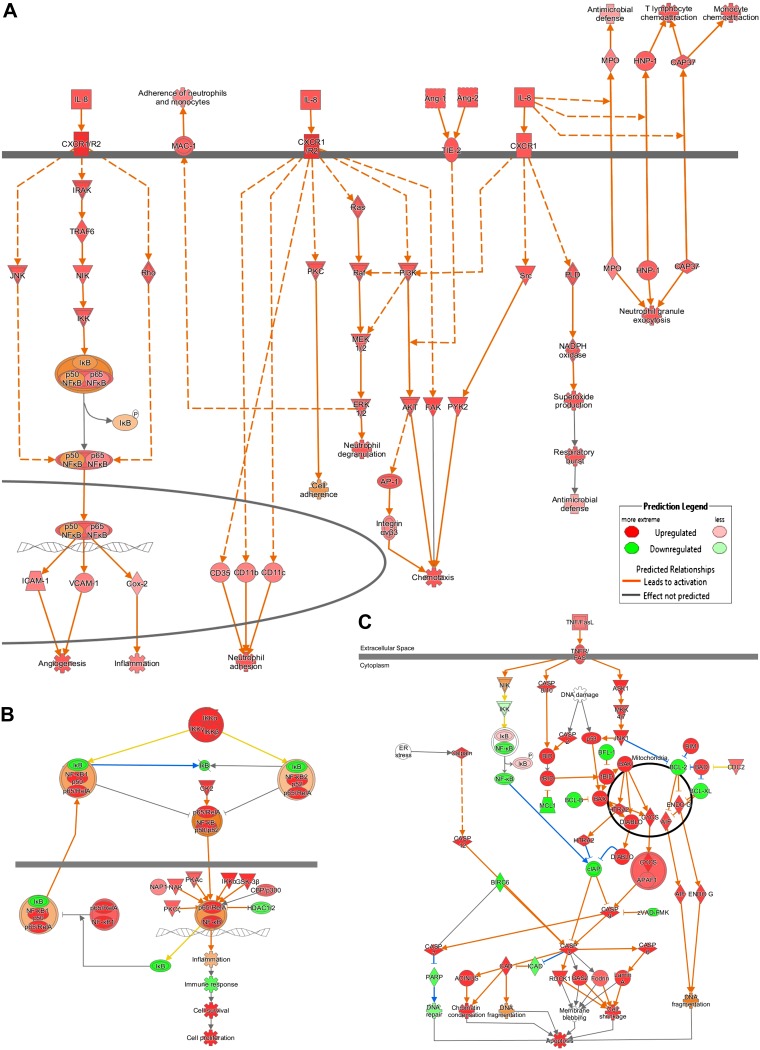
To understand better the molecular effects of the NTHi lysate treatment, the miRNA target predictor function of IPA was used to analyze the predicted effects of the 5 statistically induced miRNAs (Table 4). mRNA targets were narrowed to experimentally observed effects and to genes implicated in signaling pathways. With these settings, 73 mRNAs were predicted to be targeted. These mRNAs are implicated in inflammatory pathways, such as the IL-8 and NF-κB pathways, as well as in apoptosis (Fig. 7). IPA predicted the activation of IL-8-related pathways via the CXCR1/2 receptor, activating NF-κB, oxidative stress, inflammation, antimicrobial defense, chemotaxis, angiogenesis and neutrophil granule exocytosis, and neutrophil adhesion. The apoptosis pathway was predicted to be repressed to favor cell proliferation and survival.
Figure 7.
Signaling pathway predicted to be activated by the miRNA target predictor of IPA. The miRNA target predictor tool was used to predict the likelihood of activation or inhibition of signaling pathways. IL-8 (A), NF-κB (B), and apoptosis (C) pathways are shown in this figure.
A Core Analysis was then performed with the dataset of 73 mRNA targets, and the canonical pathways and upstream regulators were predicted (Tables 5 and 6). This dataset was related to molecular mechanisms of cancer, PI3K/AKT pathway, IL-8, leukocyte extravasation pathway, and others shown in Table 5.
TABLE 5.
Canonical pathways predicted to be impacted by miRNA targets
| Ingenuity canonical pathway | P |
|---|---|
| Molecular mechanisms of cancer | 3.9e−11 |
| PI3K/AKT signaling | 6.3e−10 |
| Sumoylation pathway | 7.0e−8 |
| TGF-β signaling | 4.8e−7 |
| ILK signaling | 4.0e−7 |
| p53 signaling | 8.9e−6 |
| Tight junction signaling | 8.9e−6 |
| Wnt/β-catenin signaling | 8.1e−6 |
| IL-8 signaling | 2.5e−6 |
| Leukocyte extravasation signaling | 1.5e−6 |
The 5 miRNAs of Table 2 were entered in IPA, and the miRNA target predictor was used to obtain another dataset of the targeted mRNAs. This other dataset (73 mRNAs) was analyzed by IPA doing a core analysis and canonical pathways were predicted. ILK, integrin-linked kinase.
TABLE 6.
Upstream regulators predicted to be regulated by miRNA targets
| Upstream regulator | Molecule type | P (overlap) |
|---|---|---|
| TP63 | Transcription regulator | 2.79e−20 |
| TP53 | Transcription regulator | 6.82e−19 |
| FOXO3 | Transcription regulator | 6.93e−19 |
| SP1 | Transcription regulator | 6.62e−18 |
| TGFB1 | Growth factor | 7.19e−18 |
| JUN | Transcription regulator | 1.59e−17 |
| ERK | Group | 1.1e−16 |
| TNF | Cytokine | 8.92e−16 |
| IL6 | Cytokine | 1.26e−15 |
| IGF1 | Growth factor | 1.73e−15 |
Upstream regulators were predicted with the same method as in Table 5.
Exosome protein characterization
HMEEC exosome samples were also subjected to SDS-PAGE and in-gel digestion to identify proteins semiquantitatively by MS using a label-free technique. Supplemental Table 3 shows 887 proteins detected for 4 samples with a minimum total PC of 10. The most abundant proteins were cytoskeletal and extracellular matrix proteins, with some immune proteins, such as complement C3. Consistent with previous results, hnRNPs were also detected by MS in the exosomes with higher PC abundance for the hnRNPs of interest: hnRNP K, hnRNP A2B1, and hnRNP Q (Table 7). To analyze further the diversity of proteins detected in exosomes, we performed IPA Core Analysis for these exosomal proteins (Table 8). Canonical pathways were generated, demonstrating RNA processing and vesicle formation, as expected. Finally, protein ubiquitination pathways, as well as epithelial remodeling pathways, were also predicted by IPA to be activated.
TABLE 7.
hnRNP proteins detected by MS in HMEEC exosomes
| Protein ID | Total PCs | Protein | Gene | Ctrl | Treated | SC (%) | ||
|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | |||||
| P61978 | 68 | hnRNP K | HNRNPK | 15 | 19 | 16 | 18 | 47.5 |
| P22626 | 61 | hnRNPs A2/B1 | HNRNPA2B1 | 11 | 17 | 13 | 20 | 63.2 |
| O60506 | 56 | hnRNP Q | SYNCRIP | 12 | 14 | 14 | 16 | 29.5 |
| P09651 | 55 | hnRNP A1 | HNRNPA1 | 9 | 16 | 13 | 17 | 41.4 |
| P14866 | 52 | hnRNP L | HNRNPL | 11 | 17 | 10 | 14 | 43.3 |
| O43390 | 51 | hnRNP R | HNRNPR | 11 | 14 | 14 | 12 | 29.9 |
| Q14103 | 35 | hnRNP D0 | HNRNPD | 7 | 7 | 7 | 14 | 32.1 |
| P51991 | 33 | hnRNP A3 | HNRNPA3 | 6 | 7 | 7 | 13 | 28 |
| P52272 | 22 | hnRNP M | HNRNPM | 4 | 9 | 2 | 7 | 15.3 |
Exosome samples were subjected to SDS-PAGE and in-gel digestion to analyze proteins by MS. For semiquantification, PCs were detected, and the percentage of sequence coverage (SC) was calculated. Ctrl, control.
TABLE 8.
Canonical pathways predicted to be activated by IPA for HMEEC exosome proteins
| Ingenuity canonical pathway | P | Ratio |
|---|---|---|
| Protein ubiquitination pathway | 1.2e−25 | 0.224 |
| tRNA charging | 1.2e−17 | 0.513 |
| EIF2 signaling | 3.9e−12 | 0.18 |
| Glycolysis I | 5.8e−8 | 0.44 |
| Caveolar-mediated endocytosis signaling | 2.5e−8 | 0.239 |
| Remodeling of epithelial adherens junctions | 9.4e−6 | 0.221 |
| Clathrin-mediated endocytosis signaling | 4.4e−6 | 0.132 |
| Actin cytoskeleton signaling | 2.8e−6 | 0.123 |
| Tight junction signaling | 5.1e−5 | 0.132 |
| Epithelial adherent junction signaling | 3.2e−5 | 0.137 |
| Cleavage and polyadenylation of pre-mRNA | 2.5e−5 | 0.5 |
The dataset of 883 exosome proteins was entered in IPA and a Core analysis was performed. EIF2, eukaryotic initiation factor 2.
DISCUSSION
A key component of OM progression from acute to chronic is epithelial remodeling during middle-ear inflammation or upon bacterial exposure (9, 34). Normally, middle-ear mucosa is comprised of a simple squamous epithelium, which during the acutely infected and inflamed state, has the capacity to grow and proliferate to many times its original thickness, into a thick, often pseudostratified, columnar epithelial complex (35, 36). The characterization and quantification of cell-secretion protein levels during the process of the bacterially induced mucosal response could help elucidate key mechanisms contributing to the progression of OM. In this report, we use a powerful proteomics technique, such as SILAC, to explore this question.
Results revealed that among expected extracellular matrix and immune-related proteins, our quantitative proteomics analysis also interestingly revealed the enrichment of a group of proteins implicated in RNA processing: the hnRNPs. Specifically, hnRNP A2B1 was shown to be enriched at 24 h of incubation but not at 48 h, which was confirmed by Western blot analysis in 3 independent experiments. This finding was surprising, as these proteins are mainly found in the intracellular compartment of cells and were not expected in their secretions. hnRNPs are known RNA-binding proteins involved in the process of nuclear RNA conversion to mature mRNA transcripts, acting as trans factors for gene-expression regulation (37). As a potential explanation as to the presence of hnRNPs in cell secretions, it has been reported that hnRNP A2B1 also functions to package miRNAs into exosomes (31). In addition, hnRNP Q (also called SYNCRIP) has been shown to be involved in hepatocyte exosomal miRNA sorting (32). This led us to investigate the presence of hnRNP-containing exosomes in HMEEC secretions.
Exosomes are membrane-formed vesicles with a size of ∼100 nm. They are endocytic vesicles transporting miRNAs, proteins, and mRNAs and function as putative mediators of intercellular communication. We recently were the first group to characterize their presence within human middle-ear fluid samples from patients with COM (18). In this study, we used multiple techniques to characterize the presence of exosomes in in vitro HMEEC secretions, allowing for the comparison of these with what we reported in the in vivo patient samples. First, exosomes were isolated with the broadly used Exoquick-TC method. Then, they were analyzed by DLS, confirming the size of the isolated vesicles to be lower than 100 nm for all of the conditions tested. Given that DLS is better suited for sizing of particles consisting of elemental compounds (metal, carbon) rather than biologic vesicles, we also visualized our preparations by EM. Transmission EM showed the presence of exosomal-sized vesicles (100 nm) with an accumulation of staining in the middle, a typical depression that occurs in exosomes during processing the grids in the electron microscope chamber (33). Finally, we also found the presence of CD63 and HSP70 in the isolated exosomes—typical markers for exosomes (38–40)—further validating the isolation techniques that we used. Finally, hnRNP A2B1 and hnRNP Q were also detected by MS within the HMEEC-secreted exosomes, consistent with the identification of these the same cells’ secretions through SILAC. Interestingly, the MS analysis in our HMEEC-secreted exosomes also revealed the presence of ubiquitin-related proteins. This may be important, as it has been shown that sumoylation of hnRNP is required for the hnRNP function of miRNA packaging into exosomes (31). Further experiments looking at exosomal hnRNP post-translational modifications would help elucidate whether hnRNP sumoylation is a common mechanism for miRNA processing in HMEEC.
Kesimer and Gupta (41) characterized exosomes released by primary tracheobronchial cells and showed that transmembrane proteins or proteins bound to exosomes may increase their overall size, as measured by DLS or visualized by EM. This may serve as a potential explanation for variability in the size of epithelial-secreted exosomes.
Next, we analyzed specific miRNA content in the HMEEC-secretion exosomes. miRNAs are small, noncoding RNAs that function in RNA silencing and post-transcriptional regulation of gene expression. miRNAs are abundant in epithelial cells at mucosal sites, and their expression can differ depending on tissues and development stage of cells (42). In our study, NTHi lysate-treated samples, at 48 h, showed the statistically significant enrichment of the following miRNAs relative to control: miR-378a-3p + miR-378i, miR-200a-3p, miR-378g, miR30d-5p, and miR-222-3p. The most statistically induced miRNA, miR-378a-3p + miR-378i, was also the most up-regulated one (fold induction, 2.52). Notably, another miR-378 was also induced by the treatment: miR-378g. miR-378a is reported to be implicated in colorectal cancer as a tumor suppressor (43) and also in metabolism and angiogenesis (44). miR-378i was also found in colorectal cancer samples (45) and in patients with lung cancer, pulmonary tuberculosis, or pneumonia (46). Its role in HMEEC-secretion exosomes is unclear and understudied. Another induced miRNA, miR-200a-3p, has been implicated in maintenance of epithelial integrity, inhibiting tumor cell motility and invasiveness (47). The induction of miR-222 was reported to be oncogenic, as it activates apoptosis and cell migration (48), making this specific miRNA a potential inductor of adverse biologic effects. Finally, we were unable to locate any studies analyzing epithelial effects of miR-30d-5p.
To our knowledge, our report is the first to characterize exosomal miRNA release from HMEECs upon NTHi lysate exposure. Previously, Samuels et al. (49) reported the induction of miR-146 in response to inflammatory stimuli (IL-1β and TNF-α) in HMEEC lysates, not secreted exosomes. Likewise, other miRNAs were regulated in HMEEC lysates in response to LPS challenge (50). None of the miRNAs characterized in these two studies were found in ours.
IPA was then used to perform a broad analysis based on published literature referencing the 5 induced miRNAs. IPA results showed that published mRNA targets of these miRNAs were involved in inflammation, cancer, cell growth, and acute response. The predicted upstream regulators were also related to these processes (LPS, TGF-β, TNF). Based on known miRNA functionality, these results indicate that identified miRNAs may function to limit epithelial immune and growth responses after initial bacterial exposure. This goes along with the work of other labs reporting on the immunomodulatory role of exosomal miRNA response to bacterial infection (51) and their broad role in innate immunity (52), as well as their potency to neutralize pathogens, as suggested by Kesimer et al. (53), showing that exosomes released by human tracheobronchial cells have neutralizing effects on the influenza virus. IPA results also demonstrated that the IL-8 signaling pathway was predicted to be activated, a common mediator implicated in OM early immune response (23, 24). In the future, the testing of the individual or combined effects of specific miRNAs on HMEECs, in presence and absence of bacterial inductor, would permit further elucidation of the role of miRNAs in OM.
Importantly, some of the miRNAs present in HMEEC secretions were also reported by us to be found in MEEs from patients with COM (18). These are listed in Supplemental Table 4. Overall, 20 miRNAs were found to be common to both HMEEC and MEE exosomes, of which 7 were specific to MEEs (not found in control blood samples). Notably, of the 7 common miRNAs between HMEECs and MEEs, only one was noted in HMEECs to be induced by NTHi lysate exposure, miR-222-3p. This miRNA has been postulated to induce apoptosis and cell migration (48). The most abundant miRNA in MEEs, miRNA-223, was not found in HMEEC exosomes, which reinforces our hypothesis that this miRNA is produced by the abundant neutrophils found in COM effusions (10).
Limitations to this study include the use of bacteria lysate instead of live bacteria, but as stated previously, in our model, the use of live NTHi was not feasible. Further studies will be needed to compare the effect of live bacteria on exosome production, as live whole bacteria are also likely to be relevant in host responses to infection. In addition, the last step of NTHi culture was performed without an iron source. Despite the minimal presence of iron from colony transfer and the growth medium, in these conditions, NTHi might express different proteins compared with iron-rich conditions, as suggested by Whitby et al. (54). In consequence, biologic effects could be impacted.
CONCLUSIONS
In summary, NTHi lysate treatment was found to induce the production of miRNA containing exosomes, along with miRNA packaging proteins hnRNPs by HMEECs. miRNAs induced by NTHi lysate treatment were mostly predicted to target immune response genes.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Drs. Mary C. Rose and Colberg-Poley (Center for Genetic Medicine) for their kind advice. This work was partially supported by U.S. National Institutes of Health (NIH) Core Grants 2R24HD050846-06 (National Center for Medical Rehabilitation Research), National Institute of Child Health and Human Development 5P30HD040677 (Intellectual and Developmental Disabilities Research Center), and UL1TR000075 (NIH National Center for Advancing Translational Sciences), and for the proteomics analysis supported by Award Number 1U54HD090257-01 from NIH, District of Columbia Intellectual and Developmental Disabilities Research Center Award (DC-IDDRC) Program. The EM analysis was carried out at the George Washington University Nanofabrication and Imaging Center (GWNIC) with the help of Christine Brantner and Anastas Popratiloff. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the NIH.
Glossary
- AOM
acute otitis media
- BCA
bicinchoninic acid assay
- COM
chronic otitis media
- DLS
dynamic light scattering
- EM
electron microscopy
- HMEEC
human middle-ear epithelial cell
- hnRNP
heterogeneous nuclear ribonucleoprotein
- HSP
heat shock protein
- IP2
Integrated Proteomics Pipeline
- IPA
Ingenuity Pathway Analysis
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- MEE
middle-ear effusion
- miRNA
microRNA
- MS
mass spectrometry
- NTHi
nontypeable Haemophilus influenzae
- OM
otitis media
- PC
peptide count
- SILAC
stable isotope labeling by amino acid in cell culture
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. Val and D. Preciado designed the research and wrote the paper; S. Val, A. Krueger, M. Poley, and A. Cohen performed research; K. Brown and A. Panigrahi contributed to new reagents or analytic tools; and S. Val, K. Brown, and A. Panigrahi analyzed data.
REFERENCES
- 1.Grubb M. S., Spaugh D. C. (2010) Treatment failure, recurrence, and antibiotic prescription rates for different acute otitis media treatment methods. Clin. Pediatr. (Phila.) 49, 970–975 10.1177/0009922810370363 [DOI] [PubMed] [Google Scholar]
- 2.Cherry D. K., Woodwell D. A. (2002) National Ambulatory Medical Care Survey: 2000 summary. Adv. Data, 1–32 [PubMed] [Google Scholar]
- 3.Ahmed S., Shapiro N. L., Bhattacharyya N. (2014) Incremental health care utilization and costs for acute otitis media in children. Laryngoscope 124, 301–305 10.1002/lary.24190 [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld R. M., Shin J. J., Schwartz S. R., Coggins R., Gagnon L., Hackell J. M., Hoelting D., Hunter L. L., Kummer A. W., Payne S. C., Poe D. S., Veling M., Vila P. M., Walsh S. A., Corrigan M. D. (2016) Clinical practice guideline: otitis media with effusion (update). Otolaryngol. Head Neck Surg. 154, 201–214 10.1177/0194599815623467 [DOI] [PubMed] [Google Scholar]
- 5.Lieberthal A. S., Carroll A. E., Chonmaitree T., Ganiats T. G., Hoberman A., Jackson M. A., Joffe M. D., Miller D. T., Rosenfeld R. M., Sevilla X. D., Schwartz R. H., Thomas P. A., Tunkel D. E. (2013) The diagnosis and management of acute otitis media. Pediatrics 131, e964–e999 10.1542/peds.2012-3488 [DOI] [PubMed] [Google Scholar]
- 6.Casey J. R., Pichichero M. E. (2004) Changes in frequency and pathogens causing acute otitis media in 1995–2003. Pediatr. Infect. Dis. J. 23, 824–828 10.1097/01.inf.0000136871.51792.19 [DOI] [PubMed] [Google Scholar]
- 7.Klein J. O. (2002) Strategies for decreasing multidrug antibiotic resistance: role of ototopical agents for treatment of middle ear infections. Am. J. Manag. Care 8(14 Suppl), S345–S352 [PubMed] [Google Scholar]
- 8.Lee H. Y., Takeshita T., Shimada J., Akopyan A., Woo J. I., Pan H., Moon S. K., Andalibi A., Park R. K., Kang S. H., Kang S. S., Gellibolian R., Lim D. J. (2008) Induction of beta defensin 2 by NTHi requires TLR2 mediated MyD88 and IRAK-TRAF6-p38MAPK signaling pathway in human middle ear epithelial cells. BMC Infect. Dis. 8, 87. 10.1186/1471-2334-8-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juhn S. K., Jung M. K., Hoffman M. D., Drew B. R., Preciado D. A., Sausen N. J., Jung T. T., Kim B. H., Park S. Y., Lin J., Ondrey F. G., Mains D. R., Huang T. (2008) The role of inflammatory mediators in the pathogenesis of otitis media and sequelae. Clin. Exp. Otorhinolaryngol. 1, 117–138 10.3342/ceo.2008.1.3.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Val S., Poley M., Brown K., Choi R., Jeong S., Colberg-Poley A., Rose M. C., Panchapakesan K. C., Devaney J. C., Perez-Losada M., Preciado D. (2016) Proteomic characterization of middle ear fluid confirms neutrophil extracellular traps as a predominant innate immune response in chronic otitis media. PLoS One 11, e0152865. 10.1371/journal.pone.0152865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D. S., Weinrauch Y., Zychlinsky A. (2004) Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 12.Hathout Y., Flippin J., Fan C., Liu P., Csaky K. (2005) Metabolic labeling of human primary retinal pigment epithelial cells for accurate comparative proteomics. J. Proteome Res. 4, 620–627 10.1021/pr049749p [DOI] [PubMed] [Google Scholar]
- 13.Preciado D., Goyal S., Rahimi M., Watson A. M., Brown K. J., Hathout Y., Rose M. C. (2010) MUC5B is the predominant mucin glycoprotein in chronic otitis media fluid. Pediatr. Res. 68, 231–236 10.1203/PDR.0b013e3181eb2ecc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simons M., Raposo G. (2009) Exosomes--vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 21, 575–581 10.1016/j.ceb.2009.03.007 [DOI] [PubMed] [Google Scholar]
- 15.Mathivanan S., Ji H., Simpson R. J. (2010) Exosomes: extracellular organelles important in intercellular communication. J. Proteomics 73, 1907–1920 10.1016/j.jprot.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 16.Sato-Kuwabara Y., Melo S. A., Soares F. A., Calin G. A. (2015) The fusion of two worlds: non-coding RNAs and extracellular vesicles--diagnostic and therapeutic implications (Review). Int. J. Oncol. 46, 17–27 10.3892/ijo.2014.2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janas T., Janas M. M., Sapoń K., Janas T. (2015) Mechanisms of RNA loading into exosomes. FEBS Lett. 589, 1391–1398 10.1016/j.febslet.2015.04.036 [DOI] [PubMed] [Google Scholar]
- 18.Val S., Jeong S., Poley M., Krueger A., Nino G., Brown K., Preciado D. (2017) Purification and characterization of microRNAs within middle ear fluid exosomes: implication in otitis media pathophysiology. Pediatr. Res. 81, 911–918 10.1038/pr.2017.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J., Liang Y., Han H., Qin H. (2013) Identification of a miRNA signature in neutrophils after traumatic injury. Acta Biochim. Biophys. Sin. (Shanghai) 45, 938–945 10.1093/abbs/gmt100 [DOI] [PubMed] [Google Scholar]
- 20.Moon S. K., Park R., Lee H. Y., Nam G. J., Cha K., Andalibi A., Lim D. J. (2006) Spiral ligament fibrocytes release chemokines in response to otitis media pathogens. Acta Otolaryngol. 126, 564–569 10.1080/00016480500452525 [DOI] [PubMed] [Google Scholar]
- 21.Watanabe T., Jono H., Han J., Lim D. J., Li J. D. (2004) Synergistic activation of NF-kappaB by nontypeable Haemophilus influenzae and tumor necrosis factor alpha. Proc. Natl. Acad. Sci. USA 101, 3563–3568 10.1073/pnas.0400557101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komatsu K., Jono H., Lim J. H., Imasato A., Xu H., Kai H., Yan C., Li J. D. (2008) Glucocorticoids inhibit nontypeable Haemophilus influenzae-induced MUC5AC mucin expression via MAPK phosphatase-1-dependent inhibition of p38 MAPK. Biochem. Biophys. Res. Commun. 377, 763–768 10.1016/j.bbrc.2008.10.091 [DOI] [PubMed] [Google Scholar]
- 23.Preciado D., Burgett K., Ghimbovschi S., Rose M. (2013) NTHi induction of Cxcl2 and middle ear mucosal metaplasia in mice. Laryngoscope 123, E66–E71 10.1002/lary.24097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Val S., Kwon H. J., Rose M. C., Preciado D. (2015) Middle ear response of Muc5ac and Muc5b mucins to nontypeable Haemophilus influenzae. JAMA Otolaryngol. Head Neck Surg. 141, 997–1005 10.1001/jamaoto.2015.2338 [DOI] [PubMed] [Google Scholar]
- 25.Jono H., Xu H., Kai H., Lim D. J., Kim Y. S., Feng X. H., Li J. D. (2003) Transforming growth factor-beta-Smad signaling pathway negatively regulates nontypeable Haemophilus influenzae-induced MUC5AC mucin transcription via mitogen-activated protein kinase (MAPK) phosphatase-1-dependent inhibition of p38 MAPK. J. Biol. Chem. 278, 27811–27819 10.1074/jbc.M301773200 [DOI] [PubMed] [Google Scholar]
- 26.Preciado D., Poley M., Tsai S., Tomney A., Brown K., Val S. (2016) A proteomic characterization of NTHi lysates. Int. J. Pediatr. Otorhinolaryngol. 80, 8–16 10.1016/j.ijporl.2015.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chun Y. M., Moon S. K., Lee H. Y., Webster P., Brackmann D. E., Rhim J. S., Lim D. J. (2002) Immortalization of normal adult human middle ear epithelial cells using a retrovirus containing the E6/E7 genes of human papillomavirus type 16. Ann. Otol. Rhinol. Laryngol. 111, 507–517 10.1177/000348940211100606 [DOI] [PubMed] [Google Scholar]
- 28.Val S., Burgett K., Brown K. J., Preciado D. (2016) SuperSILAC quantitative proteome profiling of murine middle ear epithelial cell remodeling with NTHi. PLoS One 11, e0148612. 10.1371/journal.pone.0148612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown K. J., Seol H., Pillai D. K., Sankoorikal B. J., Formolo C. A., Mac J., Edwards N. J., Rose M. C., Hathout Y. (2013) The human secretome atlas initiative: implications in health and disease conditions. Biochim. Biophys. Acta 1834, 2454–2461 10.1016/j.bbapap.2013.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu X., Peters-Hall J. R., Ghimbovschi S., Mimms R., Rose M. C., Peña M. T. (2011) Glandular gene expression of sinus mucosa in chronic rhinosinusitis with and without cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 45, 525–533 10.1165/rcmb.2010-0133OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villarroya-Beltri C., Gutiérrez-Vázquez C., Sánchez-Cabo F., Pérez-Hernández D., Vázquez J., Martin-Cofreces N., Martinez-Herrera D. J., Pascual-Montano A., Mittelbrunn M., Sánchez-Madrid F. (2013) Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 4, 2980. 10.1038/ncomms3980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santangelo L., Giurato G., Cicchini C., Montaldo C., Mancone C., Tarallo R., Battistelli C., Alonzi T., Weisz A., Tripodi M. (2016) The RNA-binding protein SYNCRIP is a component of the hepatocyte exosomal machinery controlling microRNA sorting. Cell Rep. 17, 799–808 10.1016/j.celrep.2016.09.031 [DOI] [PubMed] [Google Scholar]
- 33.Wu Y., Deng W., Klinke D. J., II (2015) Exosomes: improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Analyst (Lond.) 140, 6631–6642 10.1039/C5AN00688K [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin J., Tsuboi Y., Rimell F., Liu G., Toyama K., Kawano H., Paparella M. M., Ho S. B. (2003) Expression of mucins in mucoid otitis media. J. Assoc. Res. Otolaryngol. 4, 384–393 10.1007/s10162-002-3023-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim D. J., Birck H. (1971) Ultrastructural pathology of the middle ear mucosa in serous otitis media. Ann. Otol. Rhinol. Laryngol. 80, 838–853 10.1177/000348947108000611 [DOI] [PubMed] [Google Scholar]
- 36.Ryan A. F., Jung T. T., Juhn S. K., Li J. D., Andalibi A., Lin J., Bakaletz L. O., Post C. J., Ehrlich G. D. (2005) Recent advances in otitis media. 4A. Molecular biology. Ann. Otol. Rhinol. Laryngol. Suppl. 194, 42–49 10.1177/00034894051140S106 [DOI] [PubMed] [Google Scholar]
- 37.Dreyfuss G., Matunis M. J., Piñol-Roma S., Burd C. G. (1993) hnRNP proteins and the biogenesis of mRNA. Annu. Rev. Biochem. 62, 289–321 10.1146/annurev.bi.62.070193.001445 [DOI] [PubMed] [Google Scholar]
- 38.Lässer C. (2012) Exosomal RNA as biomarkers and the therapeutic potential of exosome vectors. Expert Opin. Biol. Ther. 12 (Suppl 1), S189–S197 10.1517/14712598.2012.680018 [DOI] [PubMed] [Google Scholar]
- 39.Keller S., Ridinger J., Rupp A. K., Janssen J. W., Altevogt P. (2011) Body fluid derived exosomes as a novel template for clinical diagnostics. J. Transl. Med. 9, 86. 10.1186/1479-5876-9-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jørgensen M., Bæk R., Pedersen S., Søndergaard E. K., Kristensen S. R., Varming K. (2013) Extracellular vesicle (EV) array: microarray capturing of exosomes and other extracellular vesicles for multiplexed phenotyping. J. Extracell. Vesicles 2, 20920. 10.3402/jev.v2i0.20920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kesimer M., Gupta R. (2015) Physical characterization and profiling of airway epithelial derived exosomes using light scattering. Methods 87, 59–63 10.1016/j.ymeth.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J., Drescher K. M., Chen X. M. (2009) MicroRNAs and epithelial immunity. Int. Rev. Immunol. 28, 139–154 10.1080/08830180902943058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H. G., Zhao L. H., Bao X. B., Sun P. C., Zhai B. P. (2014) Meta-analysis of the differentially expressed colorectal cancer-related microRNA expression profiles. Eur. Rev. Med. Pharmacol. Sci. 18, 2048–2057 [PubMed] [Google Scholar]
- 44.Krishn S. R., Batra S. K., Kaur S. (2015) Advances in miRNA-mediated mucin regulation. Curr. Pharmacol. Rep. 1, 355–364 10.1007/s40495-014-0010-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pellatt D. F., Stevens J. R., Wolff R. K., Mullany L. E., Herrick J. S., Samowitz W., Slattery M. L. (2016) Expression profiles of miRNA subsets distinguish human colorectal carcinoma and normal colonic mucosa. Clin. Transl. Gastroenterol. 7, e152. 10.1038/ctg.2016.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin J., Wang Y., Zou Y. Q., Chen X., Huang B., Liu J., Xu Y. M., Li J., Zhang J., Yang W. M., Min Q. H., Sun F., Li S. Q., Gao Q. F., Wang X. Z. (2016) Differential miRNA expression in pleural effusions derived from extracellular vesicles of patients with lung cancer, pulmonary tuberculosis, or pneumonia. Tumour Biol. 37, 15835–15845 [DOI] [PubMed] [Google Scholar]
- 47.Bracken C. P., Li X., Wright J. A., Lawrence D. M., Pillman K. A., Salmanidis M., Anderson M. A., Dredge B. K., Gregory P. A., Tsykin A., Neilsen C., Thomson D. W., Bert A. G., Leerberg J. M., Yap A. S., Jensen K. B., Khew-Goodall Y., Goodall G. J. (2014) Genome-wide identification of miR-200 targets reveals a regulatory network controlling cell invasion. EMBO J. 33, 2040–2056 https://doi.org/10.15252/embj.201488641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inamura K., Ishikawa Y. (2016) MicroRNA in lung cancer: novel biomarkers and potential tools for treatment. J. Clin. Med. 5, 36. 10.3390/jcm5030036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samuels T. L., Yan J., Khampang P., MacKinnon A., Hong W., Johnston N., Kerschner J. E. (2016) Association of microRNA 146 with middle ear hyperplasia in pediatric otitis media. Int. J. Pediatr. Otorhinolaryngol. 88, 104–108 10.1016/j.ijporl.2016.06.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song J. J., Kwon S. K., Cho C. G., Park S. W., Chae S. W. (2011) Microarray analysis of microRNA expression in LPS induced inflammation of human middle ear epithelial cells (HMEECs). Int. J. Pediatr. Otorhinolaryngol. 75, 648–651 10.1016/j.ijporl.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 51.Staedel C., Darfeuille F. (2013) MicroRNAs and bacterial infection. Cell. Microbiol. 15, 1496–1507 10.1111/cmi.12159 [DOI] [PubMed] [Google Scholar]
- 52.Mobergslien A., Sioud M. (2014) Exosome-derived miRNAs and cellular miRNAs activate innate immunity. J. Innate Immun. 6, 105–110 10.1159/000351460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kesimer M., Scull M., Brighton B., DeMaria G., Burns K., O’Neal W., Pickles R. J., Sheehan J. K. (2009) Characterization of exosome-like vesicles released from human tracheobronchial ciliated epithelium: a possible role in innate defense. FASEB J. 23, 1858–1868 10.1096/fj.08-119131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whitby P. W., VanWagoner T. M., Seale T. W., Morton D. J., Stull T. L. (2013) Comparison of transcription of the Haemophilus influenzae iron/heme modulon genes in vitro and in vivo in the chinchilla middle ear. BMC Genomics 14, 925. 10.1186/1471-2164-14-925 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.