Abstract
The investigation of orphan GPCRs (GPRs) has the potential to uncover novel insights into whole animal physiology. In this study, our goal was to determine the renal localization of Gprc5c, a receptor that we previously reported to be highly expressed in murine whole kidney, and to examine physiologic parameters in Gprc5c knockout (KO) mice to gain insight into function. Gprc5c localized to the apical membrane of renal proximal tubules (PTs) in mice, rats, and humans. With the comparison of Gprc5c wild-type (WT) and KO mice, we found that Gprc5c KO mice have altered acid-base homeostasis. Specifically, Gprc5c KO mice have lower blood pH and higher urine pH compared with WT mice, with a reduced level of titratable acids in their urine. In an in vitro GPCR internalization assay, we observed that Gprc5c internalization (an index of activation) was triggered by alkaline extracellular pH. Furthermore, with the use of an in vitro BCECF assay, we observed that Gprc5c increases Na+/H+ exchanger 3 (NHE3) activity at alkaline pH. We also find that the NHE3 activity is reduced in Gprc5c KO mice by 2 photon imaging in seminaphthorhodafluors (SNARF)-4F-loaded kidney sections. NHE3 is a primary contributor to apical transport of H+ in the renal PT. Together, these data imply that Gprc5c modulates the renal contribution to systemic pH homeostasis, at least in part, by taking part in the regulation of NHE3.—Rajkumar, P., Cha, B., Yin, J., Arend, L. J., Păunescu, T. G., Hirabayashi, Y., Donowitz, M., Pluznick, J. L. Identifying the localization and exploring a functional role for Gprc5c in the kidney.
Keywords: pH regulation, proximal tubule, NHE3
Kidneys play a vital role in maintaining physiologic homeostasis by properly balancing reabsorption and secretion of a varied array of substances. Recent studies have shown that novel GPCRs are used as specialized chemosensors in various organs, including the kidneys. Renal GPCRs aid in the kidney’s battle to maintain homeostasis by helping to assess the levels of physiologic substances and metabolites in the blood or urine and/or by triggering downstream signaling pathways to regulate physiologic homeostasis (1–13). For example, it has been well established that orphan GPCR (Gpr)91 in the kidneys detects succinate, a metabolic intermediate of citric acid cycle, and triggers renin secretion to modulate systemic blood pressure (14–16). Furthermore, the recent deorphanization of several GPRs [apelin receptor (17, 18), Gpr4 (19–21), Gpr30 (22–24), Gpr41 and Gpr43 (9, 25, 26), Gpr48 (27–31), and Gpr99 (16, 32, 33)] and investigation of their renal-specific function have established that novel GPCRs and metabolic intermediates of known pathways are involved in important regulatory functions.
We previously used a TaqMan Mouse GPCR Array and identified that Gprc5c, a novel renal GPR, is highly expressed in the kidney. Among ∼380 GPCRs targeted in this screen, the average ΔCt value of Gprc5c obtained from the whole mouse kidney was comparable with several well-studied renal receptors, including angiotensin II 1a receptor, arginine vasopressin 2 receptor, and parathyroid hormone 1 receptor (34). Gprc5c is an orphan receptor that belongs to the 4-member Gprc5 family (Gprc5a, Gprc5b, Gprc5c, and Gprc5d) (35–38). The Gprc5 receptors belong to the larger class of family C metabotropic GPCRs, which include the calcium-sensing receptor, taste receptors, vasopressin receptor 2, metabotropic glutamate receptors, and GABA receptors. All four Gprc5 receptors are orphan receptors with no known ligand; however, they have distinct tissue localization profiles. Gprc5a is expressed specifically in the lung (37–39); Gprc5b in the brain and placenta (38, 40, 41); Gprc5c in the brain, liver, and kidneys (37, 38, 42); and Gprc5d in the skin (36, 43). Although the physiologic functions of these receptors are not yet extensively understood, it is known that the Gprc5a receptor can act as a tumor suppressor (44, 45) and that the Gprc5b receptor regulates the progenitor cell fate decision during neurogenesis (46). Gprc5c knockout (KO) mice have been previously generated (42) and have been reported to show relatively mild abnormalities in the hematopoietic system, including elevated numbers of reticulocytes, mean corpuscular volume, basophil percentage, and reduced mean corpuscular hemoglobin concentration. However, despite Gprc5c expression in the brain, Gprc5c KO mice were not found to manifest defects in their cognitive capabilities. The renal-specific function of Gprc5c KO mice has not been previously examined.
In this study, our objective was to identify the localization of Gprc5c in the kidney and to examine the physiologic parameters of Gprc5c wild-type (WT) and KO mice to gain insight into Gprc5c function. We find that Gprc5c localizes to the apical membrane of renal proximal tubules (PTs) in mice, rats, and humans and that Gprc5c KO mice have defects in acid-base homeostasis. Furthermore, we find that Gprc5c is responsive to changes in extracellular pH in an in vitro assay. On the apical membrane of the PT, the Na+/H+ exchanger 3 (NHE3) plays a key role in modulating renal pH handling; in an in vitro assay, we show that Gprc5c increases NHE3 activity, and in an ex vivo assay, we find that the NHE3 activity is reduced in the renal PTs of Gprc5c KO mice. Thus, we show here, for the first time, that Gprc5c is involved in regulation of renal acid-base homeostasis.
MATERIALS AND METHODS
Animals
Mice were housed and treated in accordance with institutional guidelines. All experimental protocols were approved by the Johns Hopkins University Institutional Animal Care and Use Committee. Gprc5c heterozygote mice, backcrossed at least 10 generations with C57BL/6 mice [previously generated as described in Sano et al. (42)], were obtained and bred in house to obtain Gprc5c WT and KO littermates. Genotyping of mice was performed by PCR using tail DNA. PCR reactions were performed using Hotstar Plus PCR Master Mix (Qiagen, Germantown, MD, USA) by following standard cycling conditions [95°C, 5 min (94°C, 30 s; 60°C, 30 s; 72°C, 30 s) × 30 cycles, 72°C, 5 min, 4°C, hold] and these primers (WT forward: GCCAATGCCTGGACCTTTGT, WT reverse: ATACCTATGATCCCAGCAACTAGGAGAAGG, 411 bp; KO forward: ATCCTCTGCATGGTCAGGTC, KO reverse: CGTGGCCTGATTCATTCC, 315 bp). Mice were given unrestricted access to food (Teklad 2018SX, 18% protein diet; sodium content: 0.2%) and water throughout the duration of the experiments. Experiments were performed on age-matched WT or KO mice that included both males and females. Data were analyzed both between genotypes and between sexes; no genotypic sex-dependent differences were seen, and thus, all data are presented with males and females grouped together. Kidneys were collected for cryosectioning from adult male Sprague-Dawley rats that were housed and treated in compliance with all experimental protocols approved by the Massachusetts General Hospital Subcommittee on Research Animal Care.
Human kidney biopsy
This study was approved by the Johns Hopkins Institutional Review Board (Protocol 00090103, which includes a waiver of consent for subjects whose tissue was used for studies covered by this protocol). Kidney biopsies were processed for immunofluorescence microscopy using standard technique.
Antibody validation
To confirm the specificity of the Gprc5c antibody [as previously generated and described in Sano et al. (42)], we cloned the full-length coding sequence of Gprc5c (identical to the coding sequence of NM_001110338.1) from mouse kidney cDNA by PCR into the mammalian expression vector, pME18S. We expressed this construct in human embryonic kidney 293T (HEK293T) cells by transient transfection (Lipofectamine 2000; Thermo Fisher Scientific, Waltham, MA, USA). Transfected cells were assayed using an immunocytochemistry staining procedure, in which cells were fixed with 4% paraformaldehyde, permeabilized using 0.3% Triton X-100, and then exposed to rabbit anti-Gprc5c antibody (1:5000). Fluorescent anti-rabbit secondary antibodies (AlexaFluor; Thermo Fisher Scientific) were used to identify Gprc5c expression in HEK293T cells. HEK293T cells transiently transfected with an empty pME18S vector construct were used as a negative control. Gprc5c has been reported to contain a 22-aa-long signal peptide at the N terminus (38). To generate an N-terminally tagged Gprc5c construct, we inserted the flag-tag sequence between A22 and Q23. Furthermore, we used overlap extension PCR with site-directed mutagenesis primers to create a humanized Gprc5c construct in which a single amino acid in the C-terminal of the N-terminally tagged mice Gprc5c construct was mutated to match the human Gprc5c epitope sequence (humanized Gprc5c: CDGKNSQVFRNPYVWD; mouse: CDGKISQVFRNPYVWD; underline indicates the single amino acid that was mutated).
Immunofluorescence
Kidneys collected from mice and rats were perfusion fixed in periodate-lysine-paraformaldehyde (4%) solution, followed by immersion in 30% sucrose solution and mounting in optimum cutting temperature compound (Tissue-Tek). Cryosections (mice: ∼10–16 μm; rats: 5 μm) were washed 3 times in Tris-buffered saline (TBS), incubated for 5 min in TBS with 1% SDS, and washed in TBS again. After blocking [TBS supplemented with 1% bovine serum albumin (BSA) + 0.2% milk], sections were incubated with primary antibody [anti-Gprc5c at 1:1000 or anti-NHE3 at 1:100 (Novus Biologicals, Littleton, CO, USA)]. Sections were then washed in TBS-high salt buffer (TBS + 2.5% NaCl and 0.1% BSA) and incubated with appropriate secondary fluorescent antibodies (AlexaFluor; Thermo Fisher Scientific). Sections were then washed with TBS and mounted in Vectashield (Vector Labs, Burlingame, CA, USA). To confirm PT staining of Gprc5c, kidney sections were costained with either anti-Megalin at 1:500 (a kind gift from Dr. Peter S. Aronson, Yale School of Medicine, New Haven, CT, USA) or lotus tetragonolobus lectin (LTL), along with secondary antibody (Vector Labs) for 1 h and postfixed with 4% paraformaldehyde. Sections incubated without any primary antibody were used as a negative control. Human kidney cryosections were washed 3 times in TBS, incubated for 5 min in TBS with 1% SDS, and washed in TBS again. After blocking (TBS supplemented with 1% BSA + 0.2% milk), sections were incubated with primary antibody (anti-Gprc5c at 1:100). Sections were then washed in TBS and incubated with appropriate secondary fluorescent antibodies (AlexaFluor; Thermo Fisher Scientific).
Trafficking of GPCRs to the cell membrane surface was assayed in HEK293T cells, as previously described (47). In brief, nonpermeabilized live cells, transiently transfected with the GPCR, were labeled with the rabbit polyclonal anti-flag antibody at 4°C. After 1 h, cells were washed, fixed with 4% paraformaldehyde, permeabilized with 0.3% Triton X-100, and labeled with the mouse (M2) anti-flag mAb. Secondary antibodies with fluorescent labels were used to visualize the localization of flag-tagged receptors in HEK293T cells.
Internalization assay
HEK293T cells were grown on poly-L-lysine-coated coverslips and were transiently transfected with the postsignal peptide flag-tagged Gprc5c construct [or flag-Olfr78 (9) as a control]. To detect internalization of Gprc5c, live, nonpermeabilized HEK293T cells were first surface labeled with a rabbit polyclonal anti-flag antibody (Sigma-Aldrich, St. Louis, MO, USA) at 4°C for 1 h. This labels only the pool of receptors at the plasma membrane surface. Subsequently, the plate was sealed and moved back to 37°C for 20 min with medium adjusted to a pH of 6.8, 7.4, or 8.0. In addition, we used media (non-pH adjusted), with or without addition of various physiologic compounds. After 20 min of treatment, cells were fixed in 4% paraformaldehyde and permeabilized with 0.3% Triton X-100. Fluorescent anti-rabbit secondary antibody (AlexaFluor; Thermo Fisher Scientific) was used to identify flag-tagged Gprc5c localization.
In vitro Na+/H+ exchange activity assay
Na+/H+ exchange activity was measured in HEK293T cells that were acid loaded for at least 3 passages and transiently transfected with either flag-tagged Gprc5c (postsignal peptide) or empty vector. Acid loading was performed to increase and maintain high Na+/H+ exchange activity in cells. The procedure has been described previously in detail (48), but in brief, cells were exposed to a high acid load by the NH4Cl prepulse method (40 mM NH4Cl/saline solution) for 1 h, followed by incubation in an isotonic 3 mM Na+ solution for 1 h. Cells that survived this acid load were then placed in normal culture medium and allowed to reach at least 50% confluence. The acidification process was repeated initially every 2–3 d until >50% of cells survived the procedure and was then repeated once every week. Cells grown on glass coverslips were loaded with the intracellular pH (pHi)-sensitive dye 2′,7′-bis(carboxyethyl)5-6-carboxyfluorescein (BCECF)-acetoxymethyl ester (5 μM) for 15 min and mounted onto a Ratio-Master fluorescence system (Photon Technology International, Lawrenceville, NJ, USA) for imaging. After dye loading, the cells were sequentially subjected to NH4Cl solution [40 mM NH4Cl, 130 mM tetramethylammonium (TMA) chloride, 5 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 1 mM NaH2PO4, 25 mM glucose, and 20 mM HEPES], TMA solution (130 mM TMA chloride, 5 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 1 mM NaH2PO4, 25 mM glucose, and 20 mM HEPES), and Na+ solution (138 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 1 mM NaH2PO4, 25 mM glucose, and 20 mM HEPES). The pH of the buffers was adjusted to either 7.4 or 8.0, as described in Results. Subsequently, K+/nigericin (10 μm) was used to calibrate the pHi for each coverslip. K+ clamp buffer contained 20 mM HEPES, 20 mM 2-(N-morpholino)ethanesulfonic acid, 75 mM KCl, 35 mM potassium gluconate, 14 mm sodium gluconate, 1 mM CaCl2, 1 mM MgSO4, 2 mM TMA-Cl, adjusted to pH 6.0, 6.8, or 7.4. The fluorescence measurements were captured using the Photon Technology International spectrophotometer and converted to pHi using the values obtained from the high potassium/nigericin K+ clamp method.
Ex vivo NHE3 activity assay
We measured NHE3 activity in the Gprc5c WT and KO mice (8–12 wk old), as previously described (49). Male littermate mice were anesthetized with isoflurane, and the abdomen was immediately opened by midline incision. Kidneys were dissected and placed immediately in cold Na+ buffer (138 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgSO4, 1 mm NaH2PO4, 25 mm glucose, and 20 mm HEPES, pH 7.4). Following kidney isolation, the mice were euthanized by cervical dislocation under deep isoflurane. The kidney capsule was removed, and an ∼1–2 mm-thick section in the cortex was cut using a scalpel blade and mounted on a glass coverslip with Krazy Glue (Elmer’s Products, Columbus, OH, USA). This entire procedure was performed on ice. The glue used for mounting had no autofluorescent signal and did not affect cell viability.
The kidney slices on mounted coverslips were loaded with 20 μM seminaphthorhodafluors (SNARF)-4F in Na+ buffer at 37°C for 45 min with 5% CO2. The coverslip was then placed in a perfusion chamber (RC-21BDW; Warner Instruments, Hamden, CT, USA), mounted on the microscope stage, and perfused using a peristaltic pump (Reglo; Imatec, Wertheim, Germany) at 1 ml/min with NH4Cl buffer (40 mM NH4Cl, 130 mM TMA chloride, 5 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 1 mM NaH2PO4, 25 mM glucose, and 20 mM HEPES, pH 7.4) for 15 min to acidify the tissue by the prepulse method. Subsequently, the cells were sequentially perfused with TMA solution (130 mM TMA chloride, 5 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 1 mM NaH2PO4, 25 mM glucose, and 20 mM HEPES, pH 7.4) for 15 min, immediately switched to Na+ solution (138 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 1 mM NaH2PO4, 25 mM glucose, and 20 mM HEPES, pH 7.4), and imaged every 20 s. K+/nigericin clamp buffer, containing 10 µm nigericin, along with 20 mM HEPES, 20 mM 2-(N-morpholino)ethanesulfonic acid, 75 mM KCl, 35 mM potassium gluconate, 14 mm sodium gluconate, 1 mM CaCl2, 1 mM MgSO4, 2 mM TMA-Cl, and pH, adjusted to 6.0, 6.7, or 7.4, was used to calibrate the pHi for each coverslip. Probenecid (1 mM) and 50 μM HOE694 were added in the buffer solutions to prevent SNARF-4F leakage and eliminate the contributions of NHE1 and NHE2, respectively.
For each sample, 5 optical sections at 10 μm each were captured using a multiphoton laser-scanning microscope (FV1000-MPE; ×25 objective; Olympus, Waltham, MA, USA) at 780 nm excitation and 580/640 nm emission at 580 and 640 nm, and the images were stored. Several regions of interest (ROIs) were chosen on multiple PTs in each kidney section, and the fluorescence intensity in gray levels that corresponds to relative amounts of SNARF-4F for each ROI was calculated using MetaMorph. The 640/580 ratio for each ROI was calculated, and the averages were determined for each time point. The 640/580 ratios over time were converted to pH values by using the internal K clamp pH values as standards using Microsoft Excel. The Na+/H+ exchange activity of NHE3 was determined by calculating the initial pHi slope change for 2 min after the addition of Na+ buffer and measured as ΔpH/min. For a single trial, NHE3 activity was measured for a Gprc5c WT and KO mice on the same day, the relative activity of Gprc5c KO mice was calculated by normalizing the data to Gprc5c WT mice, and this was repeated in 4 independent experiments. One sample Student’s t test was used to analyze results using GraphPad Software (La Jolla, CA, USA).
Urine analysis
Mice in metabolic cages were allowed to acclimate for 1 d, and then their 24 h urine was collected the following day under mineral oil. Urine pH was measured using a pH electrode (Orion Ross; Thermo Fisher Scientific). Mice urine was diluted 1:20, and urinary ammonia was quantified using an A7553 kit (Pointe Scientific, Canton, MI, USA). Urinary titratable acid (TA) was measured by following previously published protocol (50), modified for small urine volume. In brief, the urine sample was acidified by addition of an equal volume of 0.1 M HCl, boiling for 2 min, and then cooling to 37°C. The amount of 0.4 M NaOH required to titrate the sample to pH 7.4 was measured. A deionized water sample was analyzed in parallel, and results for water were subtracted from urine samples to yield net TA as the following: (volume of NaOHsample − volume of NaOHwater)/ml × 0.4 NaOH M. Urinary glucose and creatinine values were measured from spot urine collections by using the VetACE Clinical Chemistry system (Alfa Wassermann, West Caldwell, NJ, USA).
Blood analysis
For blood electrolyte measurements, ∼100 μl of sample was collected from the facial vein of ∼6- to 10-wk-old Gprc5c mice and analyzed using the iStat Chem8+ cartridge with a hand-held iStat system. With the use of blood electrolyte values obtained from the iStat Chem8+ cartridge, anion gap was calculated using a modified version of the equation used by Abbott Point of Care: {Na+ − [Cl− + (TCO2 − 1)]}. To measure pH and blood gases, blood collected from either the facial vein or tail artery of unanesthetized mice or from the abdominal aorta of mice under isoflurane anesthesia was analyzed using the iStat CG4+ cartridge. Blood from the abdominal aorta was drawn directly into a sterile heparin-coated syringe (Monovette; Sarstedt, North Rhine-Westphalia, Germany) to minimize exposure to air.
Blood pressure measurement
Blood pressure was measured by tail-cuff plethysmography in conscious mice with the BP-2000 Tail Cuff Analysis System (Visitech, Apex, NC, USA). Blood pressure was recorded daily for 5 consecutive days following 1 wk of acclimation to the setup.
RESULTS
Gprc5c localizes apically in the renal PTs
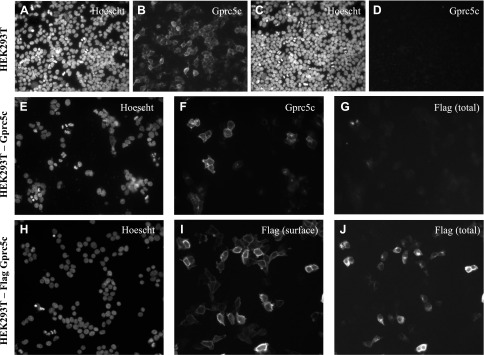
To validate the specificity of Gprc5c antibody, we first tested it on HEK293T cells transiently transfected with either a flag-tagged Gprc5c construct or empty vector (Fig. 1A–D). We found that the antibody specifically reacted with cells on Gprc5c-transfected coverslips. Gprc5c has a predicted N-terminal signal peptide sequence (38); indeed, when we added a flag tag to the extreme N terminus of Gprc5c, we detected a signal with the Gprc5c antibody but not with the flag antibody (Fig. 1E–G), presumably because both the signal sequence and the flag tag were cleaved. Therefore, we generated a flag-tagged construct in which the flag tag was inserted directly after the N-terminal signal peptide sequence between A22 and Q23 (postsignal peptide flag Gprc5c). By performing a surface labeling for the flag tag in HEK293T cells overexpressing this construct, we observed that this Gprc5c construct traffics to the cell surface (Fig. 1H–J).
Figure 1.
Confirming the specificity of the Gprc5c antibody and showing that Gprc5c contains a 22-aa long cleavable signal peptide in its N terminus in HEK293T cells. Either the Gprc5c construct (A, B) or the empty vector construct (C, D) was transiently transfected in HEK293T cells, which were then fixed and permeabilized. Cells were stained for nuclei with Hoechst, and Gprc5c expression with the anti-Gprc5c antibody (1:2500). E–G) HEK293T cells were transiently transfected with a Gprc5c construct containing a flag tag on its extreme N terminus and then fixed, permeabilized, and immunostained for both Gprc5c and flag. These cells stained for Gprc5c (F) but not for flag (G); and Hoechst is shown (E). H–J) HEK293T cells were transiently transfected with a Gprc5c construct containing a flag tag between A22 and Q23. Staining for (poly) flag in live, nonpermeabilized cells shows that the flag tag is now present on the cell surface (I). Cells were also stained with a different (mono) flag antibody following fixative and permeabilization, revealing addition flag staining intracellularly (J); and nuclear labeling is shown (H). These data confirm that Gprc5c contains a 22-aa-long cleavable signal peptide on its extreme N terminus. Original magnification, ×20.
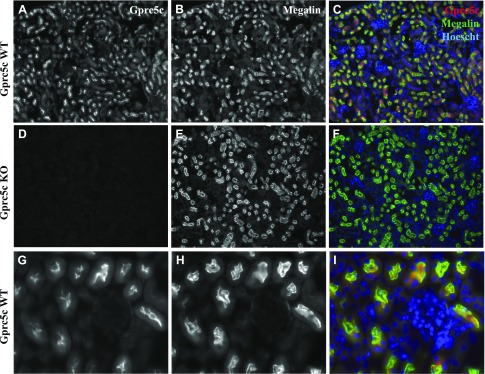
In murine kidney, the Gprc5c antibody stained the renal cortex of WT but not Gprc5c KO mice (Fig. 2A–F), further demonstrating the specificity of Gprc5c antibody. Double staining confirmed that Gprc5c localizes apically in the renal PT; Gprc5c signal colocalizes with 2 PT markers: megalin (Fig. 2) and LTL (Supplemental Fig. 1). Additionally, as the Gprc5c KO mice were generated by replacing the gene encoding Gprc5c with β-galactosidase (42), we performed β-galactosidase staining in the Gprc5c KO mice kidneys and found robust β-galactosidase staining (a surrogate for Gprc5c expression) in the renal PTs of Gprc5c KO mice (Supplemental Fig. 2). Together, these data indicate that Gprc5c is expressed primarily in the apical renal PTs of mice.
Figure 2.
Gprc5c localizes apically in the renal PTs of mice. Perfusion-fixed kidneys collected from either Gprc5c WT (A–C, G–I) or KO mice (D–F) were cryosectioned at ∼10 μm and stained with anti-Gprc5c (A, D) and megalin (B, E). Colocalization of Gprc5c expression (red) with megalin (green) is shown for Gprc5c WT and KO, indicating that the Gprc5c antibody is specific (signal is absent in Gprc5c KO), and it colocalizes with megalin, a marker for PTs. A–F) Original magnification, ×10. Hoechst (nuclear stain) is shown in blue. G–I) Original magnification, ×40; Gprc5c WT shows that Gprc5c localizes apically in the renal PTs of mice.
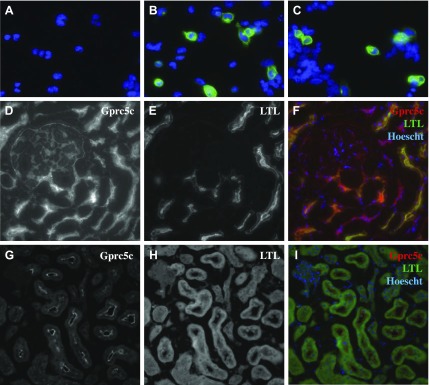
The epitope sequence of the Gprc5c antibody is conserved in rats and differs by only 1 aa in humans. To determine whether the Gprc5c antibody would recognize the human epitope, we modified our murine Gprc5c construct by mutating the single amino acid in the epitope sequence to match the human (humanized Gprc5c construct). With the use of this construct, we confirmed that the antibody recognizes the human epitope with no discernable decrease in signal (Fig. 3A–C). Subsequently, with the use of immunohistochemistry, we confirmed that Gprc5c localizes apically in the PTs of both humans and rats (Fig. 3D–I). In human kidneys, we also noted Gprc5c expression in LTL-negative tubule segments. Together, these data demonstrate that Gprc5c localizes to the apical membrane of PTs in both rodents and humans, implying that its role is conserved between these species.
Figure 3.
Gprc5c localization in the human and rat kidneys. As the Gprc5c antibody epitope sequence in human Gprc5c differs by 1 aa compared with mice, we generated a humanized Gprc5c construct in which this single amino acid was mutated to match the human sequence (humanized Gprc5c: CDGKNSQVFRNPYVWD; mouse: CDGKISQVFRNPYVWD; underline indicates the single amino acid that was mutated). A–C) HEK293T cells transiently transfected with empty vector (A), mouse Gprc5c (B), or humanized Gprc5c (C) were permeabilized and stained with anti-Gprc5c to confirm that the antibody can recognize the human Gprc5c epitope. D–F) Human kidney cryosections were stained with anti-Gprc5c (D) and LTL (E). F) Merged image shows Gprc5c staining in red, LTL in green, and Hoechst in blue and indicates that Gprc5c localization is primarily apical in the renal PTs. G–I) Gprc5c localization in the rat kidneys. The antibody epitope sequence in rat Gprc5c is identical to mice; thus, rat kidney cryosections were stained with the Gprc5c antibody (G) and LTL (H). I) Merged image of Gprc5c staining in red, LTL in green, and Hoechst in blue shows that Gprc5c localizes apically in the rat kidney PTs. Rat renal PTs were identified by LTA stain and morphology.
Gprc5c KO mice exhibit an altered pH phenotype
We examined a cohort of Gprc5c WT and KO mice (6–10 wk old) and found no overt differences between KO and WT littermates with regards to kidney weight/body weight ratio. We also did not find any statistical difference in blood pressure (by tail cuff), blood electrolytes, hematocrit, or nonfasting blood glucose, measured from conscious Gprc5c WT and KO mice (Table 1).
TABLE 1.
Physiological parameters in Gprc5c WT and KO mice with corresponding significance values
| Gprc5c | |||
|---|---|---|---|
| Parameter | WT | KO | P |
| Kidney weight/body weight | 0.0126 ± 0.0005 (n = 6) | 0.0125 ± 0.0003 (n = 7) | 0.82 |
| Blood pressure [tail cuff, systolic/diastolic (mmHg)] | 129 ± 5.4/104 ± 2.8 (n = 4) | 128 ± 2.1/105 ± 1.8 (n = 4) | 0.91, 0.89 |
| Blood Na+ (mM) | 147 ± 1.2 (n = 4) | 147 ± 1.7 (n = 4) | 0.73 |
| Blood Cl− (mM) | 122 ± 1.9 (n = 4) | 122 ± 2.5 (n = 4) | 1.00 |
| Blood iCa mM/L | 1.1 ± 0.06 (n = 4) | 1.1 ± 0.03 (n = 4) | 0.67 |
| Nonfasting blood glucose (mg/dl) | 226 ± 23 (n = 4) | 227 ± 18 (n = 4) | 0.96 |
| Blood urea nitrogen (mg/dl) | 28.5 ± 6.29 (n = 4) | 37.3 ± 6.09 (n = 4) | 0.36 |
| Hematocrit [packed cell volume (%)] | 40.3 ± 1.3 (n = 4) | 43.7 ± 2.1 (n = 4) | 0.20 |
| Hemoglobin (g/dl) | 13.7 ± 0.43 (n = 4) | 14.9 ± 0.71 (n = 4) | 0.22 |
| Blood pH (cheek pouch) | 7.45 ± 0.09 (n = 3) | 7.17 ± 0.09 (n = 5) | 0.077 |
| Blood pH (tail artery) | 7.44 ± 0.04 (n = 4) | 7.32 ± 0.02 (n = 4) | 0.04 |
| Urine creatinine (µM/L) | 1614.05 ± 30.61 (n = 4) | 1644.24 ± 177.09 (n = 4) | 0.87 |
| Urine glucose/creatinine (mM/µM/L) | 0.0022 ± 0.0002 (n = 4) | 0.0021 ± 0.0002 (n = 4) | 0.69 |
| Urinary ammonia (µM/d) | 80.25 ± 7.4 (n = 6) | 66.22 ± 4.02 (n = 6) | 0.13 |
| Urinary TAs (μM/d) | 125.24 ± 4.24 (n = 6) | 79.02 ± 1.51 (n = 6) | 1.24e−06 |
Underline indicates that P value reached statistical significance.
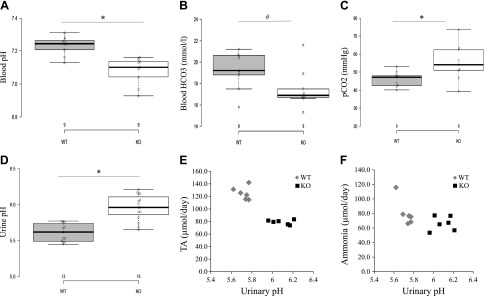
However, we did note differences in blood pH between genotypes (Table 1). In blood collected from the cheek pouch and tail artery of conscious animals, the blood pH of Gprc5c KO mice is lower than that of Gprc5c WT mice (Table 1). Although we took care to collect and measure blood as swiftly as possible, these measurements (from cheek pouch or tail artery of conscious animals) are complicated by the exposure of blood to air, which altered blood gas values. Therefore, we collected blood from the abdominal aorta under isoflurane anesthesia using a needle/syringe to minimize air exposure and found that the blood pH of Gprc5c KO mice is again lower than that of WT mice (Fig. 4A; WT: 7.23 ± 0.02, n = 9; KO: 7.08 ± 0.03, n = 9, P < 0.05). WT pH values of ∼7.2 are consistent with what is reported in the literature for mice under isoflurane (51). When we examined the blood pCO2 and HCO3 values, we found that HCO3 is lower in Gprc5c KOs compared with WT littermates (Fig. 4B: WT: 19.24 ± 0.57, n = 9; KO: 17.48 ± 0.68, n = 9, P = 0.051), but pCO2 is higher (Fig. 4C; WT: 46.16 ± 1.57, n = 9; KO: 55.43 ± 3.61, n = 9, P < 0.05). We did not find a significant difference in the blood anion gap of Gprc5c WT and KO mice (WT: 11 ± 1.2, n = 4; KO: 9 ± 1.8, n = 4, P = 0.52), suggesting that the Gprc5c KO mice exhibit normal anion gap acidosis.
Figure 4.
Physiologic parameters of Gprc5c WT and KO mice; data in are displayed as a box plot (A–C). A) The pH of whole blood, obtained from the abdominal aorta under isoflurane anesthesia, is lower in Gprc5c KO compared with WT mice (WT: n = 9; KO: n = 9). B-C) Blood pCO2 is increased, and HCO3− is decreased in whole blood obtained from the abdominal aorta under isoflurane anesthesia (WT: n = 9; KO: n = 9). D) The pH of urine (24 h urine collections) of Gprc5c KO mice is less acidic than that of WT mice (WT: n = 13; KO: n = 15). E–F) Urinary pH, TA, and ammonia were measured on the same urine samples; each point represents the values for a single animal (WT: n = 6; KO: n = 6). Urine pH in the KOs correlates with lower levels of TAs but does not correlate with any change in ammonia. This indicates that Gprc5c KO mice excrete less TA in their urine, resulting in less acidic urine pH levels compared with WT mice. *P < 0.05, #P = 0.051 (by Student’s t test).
In examining 24 h urine samples collected using metabolic cages, we observed that the urine pH of Gprc5c KO mice is higher compared with WT mice (Fig. 4D; WT: 5.61 ± 0.03, n = 13; KO: 5.96 ± 0.05, n = 15, P < 0.05). The finding that Gprc5c KO mice excrete higher urine pH, despite exhibiting systemic acidosis, indicates that Gprc5c KO mice have a renal acid excretion disorder. For a subset of Gprc5c WT and KO mice (n = 6), we collected 24 h urine samples and measured urinary pH, TAs, and ammonium from each sample. We observed that the urinary TAs are significantly lower in Gprc5c KOs compared with WT mice (Fig. 4D, E and Table 1; WT: 125.24 ± 4.24 µmol/d, n = 6; KO: 79.02 ± 1.51 µmol/d, n = 6, P < 0.05). However, we did not detect a significant difference in urinary ammonium levels between Gprc5c WT and KO mice (Fig. 4F and Table 1). Together, these results indicate that Gprc5c KO mice excrete reduced amounts of TAs in their urine, indicating that Gprc5c KO mice have a renal acid excretion disorder.
Gprc5c increases NHE3 activity in response to alkaline pH
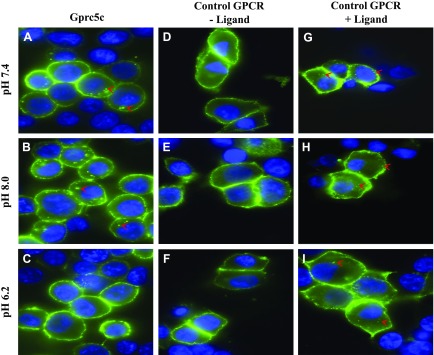
NHE3 is a sodium proton exchanger that is responsible for acid secretion in the apical membrane of PTs. Thus, we hypothesized that Gprc5c may regulate NHE3 function. To investigate if Gprc5c can affect the exchanger activity of NHE3, we first wanted to identify activators of Gprc5c. Because the downstream second-messenger signaling pathway of Gprc5c has not been previously identified, we used an internalization assay that works independently of any G-protein activation. This assay labels surface Gprc5c (using live-cell staining of transfected HEK293T cells), and the cells are subsequently exposed to potential activators. Because GPCRs commonly internalize postactivation, receptor internalization postexposure is taken as an index of activation. Thus, this assay allows us to test potential ligands without knowing the downstream signaling pathway, albeit in a low-throughput manner. With the use of this assay, we tested various compounds (glucose, lactate, malonate, propionate, oxalate, succinate, and chemical mixes: Thi-Di, MA, Oxlk, BzB, BzC, cycone) (34) but did not see changes in internalization. However, we noted that even in basal media, we always observed some internalization for Gprc5c but not for the control GPCR (Olfr78); therefore, we hypothesized that something already present in the media may be activating Gprc5c. Subsequently, we found that internalization of Gprc5c increases when the pH of the media is adjusted to alkaline values and decreases when the pH of the extracellular media is acidic (Fig. 5). We observed the same results when we used other pH-adjusted buffers (PBS and TBS) and when we adjusted the pH using different buffer solutions (HCl/NaOH and NaH2PO4/Na2HPO4). In cells transfected with a different GPCR (Olfr78), which has a known ligand (propionate), we noted internalization only in the presence of ligand and regardless of changes in pH (Fig. 5G–I). These results indicate that alkaline extracellular pH activates Gprc5c, which fits well with previously known reports about other family C GPCRs, such as calcium-sensing receptor and GABA receptors, whose activity is also modulated by extracellular pH conditions.
Figure 5.
Extracellular pH influences internalization of Gprc5c in HEK293T cells. Flag-tagged Gprc5c (postsignal peptide) or control GPCR (Olfr78) was transiently transfected in HEK293T cells. The surface pool of receptors in live cells was labeled at time 0 with a flag antibody, and live cells were then incubated for 20 min in a solution of the indicated pH. Subsequently, the cells were fixed, permeabilized, and stained with a secondary antibody to visualize the flag-tag location at ×100. Internalized receptors as punctate structures are labeled with red arrowheads. Gprc5c (A-C) internalized when the extracellular media was alkaline (pH 8.0 or 7.4) but not when it was acidic (pH 6.2). In contrast, a control GPCR was on the cell surface in the absence of ligand and internalized in the presence of ligand, regardless of pH (D–I).
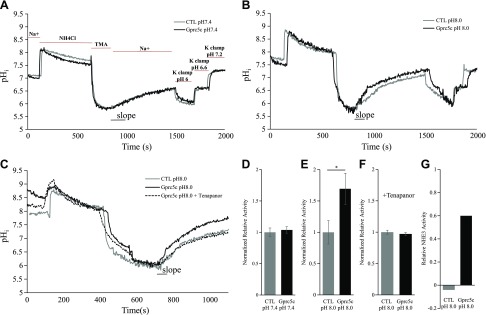
To determine whether Gprc5c may modulate NHE3 activity in vitro, we used BCECF in acid-loaded HEK293T cells to evaluate sodium proton exchanger activity with and without the presence of Gprc5c (Fig. 6). With the use of BCECF, we determined that sodium-dependent proton exchanger activity increases by 0.6-fold in cells transfected with Gprc5c compared with empty vector at pH 8 (Fig. 6B, E). However, no difference was found between Gprc5c and empty vector when the assay was performed at pH 7.4 (Fig. 6A, D). This increase in exchanger activity at pH 8 in Gprc5c-transfected cells is tenapanor sensitive (tenapanor is a specific NHE3 inhibitor), demonstrating that Gprc5c increases NHE3 activity at pH 8 (Fig. 6C, F, G). Previous studies have demonstrated that NHE3 is inactive above pH 7.4 (52–54); our results indicate that Gprc5c activation modulates this set point of NHE3 activity.
Figure 6.
Gprc5c increases NHE3 exchanger activity at alkaline extracellular pH. Acid-loaded HEK293T cells were transfected with either empty vector [control (ctl)] or Gprc5c (postsignal peptide) and subsequently loaded with BCECF. The initial rate of sodium-dependent alkalization with which the cells recover from acidification (slope) is a measure of sodium-dependent proton exchanger activity. A) Representative pHi tracing of CTL and Gprc5c-transfected cells at pH 7.4. Bars on the graph indicate when the appropriate solutions were applied to the cells. K+ clamp buffer containing 10 μm nigericin was adjusted to pH 6.0, 6.8, or 7.4 and applied to each coverslip at the end of recording to calibrate the pHi. When the pH of the extracellular solution was pH 7.4, we did not observe any difference in exchanger activity between ctl and Gprc5c-transfected cells. B) When the pH of extracellular solution is 8.0, we find that the sodium proton exchanger activity is higher in Gprc5c-transfected (vs. empty vector-transfected) cells. C) Addition of 100 nM tenapanor (NHE3-specific inhibitor) abolished the increase in sodium proton activity seen in Gprc5c-transfected cells at pH 8.0. D–F) Summary of the sodium proton exchanger activity of cells calculated from the slope and normalized to the averaged activity of empty vector-transfected cells at pH 7.4 (D), pH 8 (E), and pH 8 with 100 nM tenapanor (F). The sodium proton exchanger activity of Gprc5c-transfected cells at pH 8.0 is significantly increased compared with empty vector-transfected cells. G) An estimation of the tenapanor-sensitive NHE3 activity at pH 8 between empty vector and Gprc5c-transfected cells. For this graph, the tenapanor-sensitive activity at pH 8 for both empty vector and Gprc5c is plotted; all values were normalized to empty vector pH 8 (without tenapanor). Each measurement was performed on separate coverslips with independent transfections (CTL pH 7.4: n = 4; Gprc5c pH 7.4: n = 4; ctl pH 8.0: n = 7; Gprc5c pH 8.0: n = 7; ctl pH 8.0 + tenapanor: n = 3; Gprc5c pH 8.0 + tenapanor: n = 3). *P < 0.05 (Student’s t test).
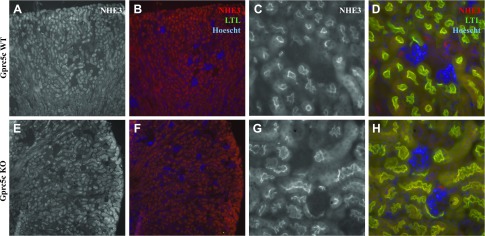
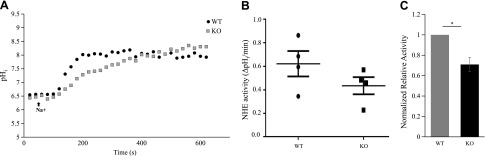
We next examined the expression level and localization of NHE3 in Gprc5c WT and KO mice. In our hands, the anti-NHE3 antibody did not identify a specific NHE3 band by Western blot, either in kidney lysates or in homogenates from NHE3-overexpressed cell lysates; however, we did examine NHE3 expression in Gprc5c WT and KO by immunofluorescence staining (Fig. 7). We did not note any differences in expression levels or in apical membrane localization of NHE3 in Gprc5c KO. We then investigated whether Gprc5c has any effect on the NHE3 activity level in the PTs of Gprc5c WT and KO mice. With the use of SNARF-4F-loaded ex vivo renal cortex preparations from Gprc5c WT and KO male mice, we measured pHi levels by 2 photon imaging to determine basal NHE3 activity (Fig. 8A). Gprc5c KO animals exhibit a significant decrease in NHE3 activity compared with WT littermates (Fig. 8B, C), suggesting that Gprc5c plays an important role in the renal regulation of NHE3 activity in the PTs.
Figure 7.
No difference in the localization of NHE3 was observed in the kidneys of Gprc5c WT and KO mice. A–D) Gprc5c WT and Gprc5c KO (E, F) kidney sections were stained with anti-NHE3. No overt differences were noticed in merged images with NHE3 (red), LTL (green), and Hoechst (blue). Original magnification, ×10 (A, B, E, F); ×40 (C, D, G, H).
Figure 8.
Gprc5c KO mice exhibit reduced NHE3 activity in the renal PT. A) Basal NHE3 activity was studied fluorometrically by 2 photon imaging using the pH-sensitive dye SNARF-4F. A representative experiment is shown, demonstrating that the NHE3 activity is reduced in Gprc5c KO mice compared with WT. The black arrow indicates the start of Na+ buffer perfusion to initiate sodium-dependent proton exchange to restore pHi. B) NHE3 activity in Gprc5c WT and KO mice (n = 4), measured as the initial rate of change in pHi, measured as ΔpH/min. C) To best control for day-to-day variability in measurements, NHE3 activity was measured from 1 WT and 1 KO mouse on each experimental day, and the data were normalized to the WT mouse from that day’s experiment. Results are means ± sem of 4 independent experiments. *P < 0.05 (1-sample Student’s t test).
DISCUSSION
Gprc5c is a GPR that we reported to be highly expressed in the kidneys but whose renal functional role has not previously been examined. Here, we show for the first time that Gprc5c localizes to the apical membrane of the renal PT in mice, rats, and humans, implying that its functional role is highly conserved. Our results show that Gprc5c modulates systemic pH homeostasis, potentially by regulating urinary acid excretion. Our in vitro studies indicate that Gprc5c activity increases when the extracellular pH is alkaline and that Gprc5c activation increases NHE3 activity.
Although GPCRs in the kidney have been studied at length, most of these studies have focused on a few GPCRs that already have known functional roles. In contrast, many orphan receptors, including Gprc5c, have never been examined in the kidney, despite the fact that Gprc5c expression levels are comparable with both the angiotensin II 1a receptor and the arginine vasopressin 2 receptor (34). In contrast to these well-studied receptors, however, neither the ligand nor the downstream second messenger for Gprc5c has been previously identified. Indeed, to a large extent, many orphan receptors are understudied precisely because little is known about them, making experimental design quite difficult. To explore a renal role for Gprc5c, we identified the localization of Gprc5c in the kidney and examined the physiologic parameters of Gprc5c WT and KO mice. We found that Gprc5c localizes apically in the renal PTs and is involved in regulation of systemic pH homeostasis. To preserve life, arterial blood pH is tightly maintained at 7.35–7.45, and pH abnormalities have been linked to inflammation, abnormal immune responses, cardiac arrhythmias, bone disorders, and increased mortality in patients with and without chronic kidney disease (55–58).
Physiologically, the HCO3−/CO2 system primarily regulates arterial blood pH as a result of its high buffering capacity. HCO3− and CO2 levels are regulated independently by the kidneys and lungs, respectively. In the kidneys, the PT nephron segment, where Gprc5c is localized, plays a pivotal role in pH regulation. The PT is responsible for reabsorption of 70–90% of ∼4300 mEq/d of HCO3− from the urinary filtrate and excretion of H+ in the final urine via TAs and NH4+ (59–61). The ability of PTs to secrete H+ into the lumen plays a vital part in both HCO3− reabsorption and H+ excretion in the final urine. Filtered HCO3− in the renal tubule cannot pass through the apical membrane of the PT cells, but instead, it is reclaimed by combining with the secreted H+ (mainly via NHE3) to form H2CO3. In the PT lumen, H2CO3 is broken down to H2O and CO2, which passes though the apical membrane and subsequently, gets converted back into HCO3− and H+ inside of the PT cells. The resulting, newly generated HCO3− in the PT is reclaimed back into the blood across the basolateral membrane. Therefore, for every H+ secreted into the lumen, the PT has the ability to reclaim HCO3− back into the blood. Furthermore, secreted H+ in the PT lumen also combines with TAs (primarily phosphate) or ammonium and gets excreted in the urine. Therefore, any disruption in the ability of PTs to secrete H+ in the lumen, as we hypothesize in Gprc5c KO mice with a reduced NHE3 activity in the PTs, will affect HCO3− reclamation in the blood and TA excretion in the urine. If not properly compensated, this will alter the pH of blood and urine, just as we observe in Gprc5c KO mice.
As systemic pH homeostasis can be regulated by both the kidney and lung, even if one organ fails to function optimally, the other organ can often compensate to maintain homeostasis at the expense of changes in HCO3− and CO2 levels. Of note, we were unable to detect expression of Gprc5c mRNA in the lung by RT-PCR (34) or Gprc5c protein in the lung by immunostaining or by a lacZ stain; therefore, the acidosis in the Gprc5c KO mice is likely to be primarily metabolic. However, in uncomplicated primary metabolic acidosis, one would expect a decrease in pCO2 (as a result of respiratory compensation). In contrast, we find that pCO2 increases, whereas HCO3− decreases in Gprc5c KO mice compared with WT animals. This implies that metabolic and respiratory acidosis coexist and explains why the acidosis in the Gprc5c KO is uncompensated. Although we were unable to detect Gprc5c in the lung, Gprc5c is expressed in the brain (42), and therefore, we cannot preclude that Gprc5c maybe involved in the control of breathing or CO2 levels. Future work is required to examine respiratory parameters, such as respiratory rate and tidal volume in Gprc5c WT and KO mice by plethysmography during basal and challenged conditions.
To identify an activator of Gprc5c, we adopted the receptor-internalization assay, which has been used previously to deorphanize other GPRs (62–64). In this in vitro assay, we find that internalization of Gprc5c (a surrogate of activation) is influenced by media pH and that it is maximal at alkaline conditions. There are a number of possibilities for how extracellular pH activates Gprc5c. First, it is possible that changes in extracellular pH directly affect the structural conformation of Gprc5c, thereby modulating its activity; likewise, protons may act as inhibitors of Gprc5c, and thus, at an alkaline pH, the activity of Gprc5c may be increased. Alternatively, pH may affect the protonation state of a potential ligand, such that it can only bind to and activate Gprc5c under alkaline conditions. However, as we find that Gprc5c internalization at alkaline pH occurs both in cell culture media and in pH-adjusted saline buffers, this last possibility is somewhat less likely. We cannot rule out the possibility that Gprc5c might be activated by other metabolites or compounds. In addition, although we feel that this assay is an excellent surrogate for receptor activation, when the G-protein signaling pathway is unknown, we acknowledge that we are not truly measuring activation per se. Future studies that focus on screening physiologic compounds as potential agonists and antagonists for Gprc5c and on identifying the downstream G-protein will help us to understand better novel mechanisms underlying renal acid-base homeostasis.
In our in vitro assay, Gprc5c activation by alkaline extracellular pH increased the activity of NHE3. Thus, we hypothesized that Gprc5c deletion would reduce NHE3 activity in the kidneys of KO mice. When we measured NHE3 activity in the renal PTs of Gprc5c WT and KO mice, we found that the NHE3 activity was indeed reduced in the Gprc5c KOs compared with the WT animals. Such a decrease in NHE3 activity would reduce the amount of H+ secreted in the PT lumen and alter HCO3− reabsorption, causing acidosis, and would also decrease the amount of H+ excreted into the urine as TAs, rendering the urine more alkaline. Our blood and urine pH measurements in Gprc5c KO mice are in agreement with this hypothesis. However, it is puzzling that in our in vitro assay, Gprc5c does not increase NHE3 activity unless the extracellular environment is alkaline; one would imagine that when the early tubular fluid (and thus, the plasma) is alkaline, one would want to reabsorb less bicarbonate and excrete less acid, not more (that is, one would want to inhibit NHE3 rather than activate it). However, the ex vivo studies were performed using an extracellular buffer with pH 7.4, so it may be simply that the HEK cells cannot fully recapitulate the phenotype. Clearly, it will be informative to perform future studies on WT and KO mice under conditions of acidosis and alkalosis, screen for other potential agonists for Gprc5c, and examine any potential adaptations in the localization and activity of acid-base exchangers in other parts of the nephron to understand this pathway better.
We did not find a difference in blood pressure values between Gprc5c WT and KO animals measured by tail cuff. This is surprising given the role of NHE3 in systemic salt homeostasis, and therefore, future work would benefit from using a more reliable telemetric blood pressure monitoring system and characterizing any differences in these animals in both baseline and salt-challenged conditions. However, it should also be noted that PT-specific NHE3 KO mice did not exhibit a blood pressure phenotype (65). Furthermore, as our current study focused our efforts on examining NHE3, in the future, we aim to investigate potential interactions of Gprc5c with other well-characterized H+ and HCO3 transporters, such as NBCe2 and PAT1 in the kidney. In addition, Gprc5c, in the pancreas, has been implicated in regulation of glucose-induced insulin secretion, along with survival and proliferation of β cells (66). It will be interesting to investigate the renal role of Gprc5c-mediated glucose handling as well, as it could lead to important therapeutic implications.
In summary, we have examined the function of the GPR, Gprc5c, in the kidney for the first time. We find that Gprc5c localizes to the renal PT and modulates systemic acid-base homeostasis. Our findings underline the importance of exploring the roles of orphaned GPCRs in the kidney and highlight the work that is left to be done. Indeed, previous studies have shown that numerous GPRs are expressed in the kidney (34). Future studies should specifically aim to unravel the function of these receptors to reveal novel mechanisms of renal regulation.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Gregory Blass and Alexander Staruschenko (Medical College of Wisconsin, Milwaukee, WI, USA) and P. Richard Grimm and Paul Welling (University of Maryland, College Park, MD, USA) for sharing their protocols for urine ammonia measurement and blood collection from the abdominal aorta, respectively. The authors are grateful to Peter S. Aronson (Yale University, New Haven, CT, USA) for sharing the Megalin antibody. The authors are also grateful to the members of the J.L.P. and M.D. laboratories for their helpful discussions. This work was supported by U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grant R00-DK081610 (to J.L.P.), and a National Kidney Foundation of Maryland Mini-Grant (to P.R.). The authors declare no conflicts of interest.
Glossary
- BCECF
2′,7′-bis(carboxyethyl)-5 (or 6)-carboxyfluorescein
- BSA
bovine serum albumin
- GPR
orphan GPCR
- HEK293T
human embryonic kidney 293T
- KO
knockout
- LTL
lotus tetragonolobus lectin
- NHE
Na+/H+ exchanger
- pHi
intracellular pH
- PT
proximal tubule
- ROI
region of interest
- SNARF
seminaphthorhodafluors
- TA
titratable acid
- TBS
Tris-buffered saline
- TMA
tetramethylammonium
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
P. Rajkumar performed the immunofluorescence in vitro and in vivo experiments; P. Rajkumar and B. Cha performed the in vitro NHE3 activity assay; P. Rajkumar and J. Yin performed the ex vivo NHE3 activity assay; P. Rajkumar, B. Cha, L. J. Arend, T. G. Păunescu, M. Donowitz, Y. Hirabayashi, and J. L. Pluznick performed data interpretation; P. Rajkumar, M. Donowitz, and J. L. Pluznick developed the study concept and design; and P. Rajkumar and J. L. Pluznick prepared the manuscript.
REFERENCES
- 1.Busse D., Kudella P., Grüning N. M., Gisselmann G., Ständer S., Luger T., Jacobsen F., Steinsträßer L., Paus R., Gkogkolou P., Böhm M., Hatt H., Benecke H. (2014) A synthetic sandalwood odorant induces wound-healing processes in human keratinocytes via the olfactory receptor OR2AT4. J. Invest. Dermatol. 134, 2823–2832 10.1038/jid.2014.273 [DOI] [PubMed] [Google Scholar]
- 2.Feldmesser E., Olender T., Khen M., Yanai I., Ophir R., Lancet D. (2006) Widespread ectopic expression of olfactory receptor genes. BMC Genomics 7, 121. 10.1186/1471-2164-7-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang A. L., Chen X., Hoon M. A., Chandrashekar J., Guo W., Tränkner D., Ryba N. J., Zuker C. S. (2006) The cells and logic for mammalian sour taste detection. Nature 442, 934–938 10.1038/nature05084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang N., Koo J. (2012) Olfactory receptors in non-chemosensory tissues. BMB Rep. 45, 612–622 10.5483/BMBRep.2012.45.11.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang N., Bahk Y. Y., Lee N., Jae Y., Cho Y. H., Ku C. R., Byun Y., Lee E. J., Kim M. S., Koo J. (2015) Olfactory receptor Olfr544 responding to azelaic acid regulates glucagon secretion in α-cells of mouse pancreatic islets. Biochem. Biophys. Res. Commun. 460, 616–621 10.1016/j.bbrc.2015.03.078 [DOI] [PubMed] [Google Scholar]
- 6.Kim S. H., Yoon Y. C., Lee A. S., Kang N., Koo J., Rhyu M. R., Park J. H. (2015) Expression of human olfactory receptor 10J5 in heart aorta, coronary artery, and endothelial cells and its functional role in angiogenesis. Biochem. Biophys. Res. Commun. 460, 404–408 10.1016/j.bbrc.2015.03.046 [DOI] [PubMed] [Google Scholar]
- 7.Pluznick J. L., Zou D. J., Zhang X., Yan Q., Rodriguez-Gil D. J., Eisner C., Wells E., Greer C. A., Wang T., Firestein S., Schnermann J., Caplan M. J. (2009) Functional expression of the olfactory signaling system in the kidney. Proc. Natl. Acad. Sci. USA 106, 2059–2064 10.1073/pnas.0812859106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pluznick J. L., Caplan M. J. (2012) Novel sensory signaling systems in the kidney. Curr. Opin. Nephrol. Hypertens. 21, 404–409 10.1097/MNH.0b013e328354a6bd [DOI] [PubMed] [Google Scholar]
- 9.Pluznick J. L., Protzko R. J., Gevorgyan H., Peterlin Z., Sipos A., Han J., Brunet I., Wan L. X., Rey F., Wang T., Firestein S. J., Yanagisawa M., Gordon J. I., Eichmann A., Peti-Peterdi J., Caplan M. J. (2013) Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA 110, 4410–4415 10.1073/pnas.1215927110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pluznick J. L. (2013) Renal and cardiovascular sensory receptors and blood pressure regulation. Am. J. Physiol. Renal Physiol. 305, F439–F444 10.1152/ajprenal.00252.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah A. S., Ben-Shahar Y., Moninger T. O., Kline J. N., Welsh M. J. (2009) Motile cilia of human airway epithelia are chemosensory. Science 325, 1131–1134 10.1126/science.1173869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spehr M., Gisselmann G., Poplawski A., Riffell J. A., Wetzel C. H., Zimmer R. K., Hatt H. (2003) Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science 299, 2054–2058 10.1126/science.1080376 [DOI] [PubMed] [Google Scholar]
- 13.Wu C., Jia Y., Lee J. H., Kim Y., Sekharan S., Batista V. S., Lee S. J. (2015) Activation of OR1A1 suppresses PPAR-γ expression by inducing HES-1 in cultured hepatocytes. Int. J. Biochem. Cell Biol. 64, 75–80 10.1016/j.biocel.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 14.Toma I., Kang J. J., Sipos A., Vargas S., Bansal E., Hanner F., Meer E., Peti-Peterdi J. (2008) Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J. Clin. Invest. 118, 2526–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vargas S. L., Toma I., Kang J. J., Meer E. J., Peti-Peterdi J. (2009) Activation of the succinate receptor GPR91 in macula densa cells causes renin release. J. Am. Soc. Nephrol. 20, 1002–1011 10.1681/ASN.2008070740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He W., Miao F. J., Lin D. C., Schwandner R. T., Wang Z., Gao J., Chen J. L., Tian H., Ling L. (2004) Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature 429, 188–193 10.1038/nature02488 [DOI] [PubMed] [Google Scholar]
- 17.Kleinz M. J., Davenport A. P. (2005) Emerging roles of apelin in biology and medicine. Pharmacol. Ther. 107, 198–211 10.1016/j.pharmthera.2005.04.001 [DOI] [PubMed] [Google Scholar]
- 18.Roberts E. M., Newson M. J., Pope G. R., Landgraf R., Lolait S. J., O’Carroll A. M. (2009) Abnormal fluid homeostasis in apelin receptor knockout mice. J. Endocrinol. 202, 453–462 10.1677/JOE-09-0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludwig M. G., Vanek M., Guerini D., Gasser J. A., Jones C. E., Junker U., Hofstetter H., Wolf R. M., Seuwen K. (2003) Proton-sensing G-protein-coupled receptors. Nature 425, 93–98 10.1038/nature01905 [DOI] [PubMed] [Google Scholar]
- 20.Sun X., Yang L. V., Tiegs B. C., Arend L. J., McGraw D. W., Penn R. B., Petrovic S. (2010) Deletion of the pH sensor GPR4 decreases renal acid excretion. J. Am. Soc. Nephrol. 21, 1745–1755 10.1681/ASN.2009050477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun X., Stephens L., DuBose T. D. Jr., Petrovic S. (2015) Adaptation by the collecting duct to an exogenous acid load is blunted by deletion of the proton-sensing receptor GPR4. Am. J. Physiol. Renal Physiol. 309, F120–F136 10.1152/ajprenal.00507.2014 [DOI] [PubMed] [Google Scholar]
- 22.Hofmeister M. V., Damkier H. H., Christensen B. M., Olde B., Fredrik Leeb-Lundberg L. M., Fenton R. A., Praetorius H. A., Praetorius J. (2012) 17β-Estradiol induces nongenomic effects in renal intercalated cells through G protein-coupled estrogen receptor 1. Am. J. Physiol. Renal Physiol. 302, F358–F368 10.1152/ajprenal.00343.2011 [DOI] [PubMed] [Google Scholar]
- 23.Thomas P., Pang Y., Filardo E. J., Dong J. (2005) Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146, 624–632 10.1210/en.2004-1064 [DOI] [PubMed] [Google Scholar]
- 24.Revankar C. M., Cimino D. F., Sklar L. A., Arterburn J. B., Prossnitz E. R. (2005) A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307, 1625–1630 10.1126/science.1106943 [DOI] [PubMed] [Google Scholar]
- 25.Le Poul E., Loison C., Struyf S., Springael J. Y., Lannoy V., Decobecq M. E., Brezillon S., Dupriez V., Vassart G., Van Damme J., Parmentier M., Detheux M. (2003) Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 278, 25481–25489 10.1074/jbc.M301403200 [DOI] [PubMed] [Google Scholar]
- 26.Natarajan N., Hori D., Flavahan S., Steppan J., Flavahan N. A., Berkowitz D. E., Pluznick J. L. (2015) Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol Genomics 48, 826–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carmon K. S., Gong X., Lin Q., Thomas A., Liu Q. (2011) R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/β-catenin signaling. Proc. Natl. Acad. Sci. USA 108, 11452–11457 10.1073/pnas.1106083108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dang Y., Liu B., Xu P., Zhu P., Zhai Y., Liu M., Ye X. (2014) Gpr48 deficiency induces polycystic kidney lesions and renal fibrosis in mice by activating Wnt signal pathway. PLoS One 9, e89835. 10.1371/journal.pone.0089835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Lau W., Barker N., Low T. Y., Koo B. K., Li V. S., Teunissen H., Kujala P., Haegebarth A., Peters P. J., van de Wetering M., Stange D. E., van Es J. E., Guardavaccaro D., Schasfoort R. B., Mohri Y., Nishimori K., Mohammed S., Heck A. J., Clevers H. (2011) Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476, 293–297 10.1038/nature10337 [DOI] [PubMed] [Google Scholar]
- 30.Glinka A., Dolde C., Kirsch N., Huang Y. L., Kazanskaya O., Ingelfinger D., Boutros M., Cruciat C. M., Niehrs C. (2011) LGR4 and LGR5 are R-spondin receptors mediating Wnt/β-catenin and Wnt/PCP signalling. EMBO Rep. 12, 1055–1061 10.1038/embor.2011.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohri Y., Oyama K., Akamatsu A., Kato S., Nishimori K. (2011) Lgr4-deficient mice showed premature differentiation of ureteric bud with reduced expression of Wnt effector Lef1 and Gata3. Dev. Dyn. 240, 1626–1634 10.1002/dvdy.22651 [DOI] [PubMed] [Google Scholar]
- 32.Tokonami N., Morla L., Centeno G., Mordasini D., Ramakrishnan S. K., Nikolaeva S., Wagner C. A., Bonny O., Houillier P., Doucet A., Firsov D. (2013) α-Ketoglutarate regulates acid-base balance through an intrarenal paracrine mechanism. J. Clin. Invest. 123, 3166–3171 10.1172/JCI67562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grimm P. R., Lazo-Fernandez Y., Delpire E., Wall S. M., Dorsey S. G., Weinman E. J., Coleman R., Wade J. B., Welling P. A. (2015) Integrated compensatory network is activated in the absence of NCC phosphorylation. J. Clin. Invest. 125, 2136–2150 10.1172/JCI78558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajkumar P., Aisenberg W. H., Acres O. W., Protzko R. J., Pluznick J. L. (2014) Identification and characterization of novel renal sensory receptors. PLoS One 9, e111053. 10.1371/journal.pone.0111053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bräuner-Osborne H., Jensen A. A., Sheppard P. O., Brodin B., Krogsgaard-Larsen P., O’Hara P. (2001) Cloning and characterization of a human orphan family C G-protein coupled receptor GPRC5D. Biochim. Biophys. Acta 1518, 237–248 10.1016/S0167-4781(01)00197-X [DOI] [PubMed] [Google Scholar]
- 36.Bräuner-Osborne H., Krogsgaard-Larsen P. (2000) Sequence and expression pattern of a novel human orphan G-protein-coupled receptor, GPRC5B, a family C receptor with a short amino-terminal domain. Genomics 65, 121–128 10.1006/geno.2000.6164 [DOI] [PubMed] [Google Scholar]
- 37.Cheng Y., Lotan R. (1998) Molecular cloning and characterization of a novel retinoic acid-inducible gene that encodes a putative G protein-coupled receptor. J. Biol. Chem. 273, 35008–35015 10.1074/jbc.273.52.35008 [DOI] [PubMed] [Google Scholar]
- 38.Robbins M. J., Michalovich D., Hill J., Calver A. R., Medhurst A. D., Gloger I., Sims M., Middlemiss D. N., Pangalos M. N. (2000) Molecular cloning and characterization of two novel retinoic acid-inducible orphan G-protein-coupled receptors (GPRC5B and GPRC5C). Genomics 67, 8–18 10.1006/geno.2000.6226 [DOI] [PubMed] [Google Scholar]
- 39.Xu J., Tian J., Shapiro S. D. (2005) Normal lung development in RAIG1-deficient mice despite unique lung epithelium-specific expression. Am. J. Respir. Cell Mol. Biol. 32, 381–387 10.1165/rcmb.2004-0343OC [DOI] [PubMed] [Google Scholar]
- 40.Robbins M. J., Charles K. J., Harrison D. C., Pangalos M. N. (2002) Localisation of the GPRC5B receptor in the rat brain and spinal cord. Brain Res. Mol. Brain Res. 106, 136–144 10.1016/S0169-328X(02)00420-5 [DOI] [PubMed] [Google Scholar]
- 41.Imanishi S., Sugimoto M., Morita M., Kume S., Manabe N. (2007) Changes in expression and localization of GPRC5B and RARalpha in the placenta and yolk sac during middle to late gestation in mice. J. Reprod. Dev. 53, 1131–1136 10.1262/jrd.18102 [DOI] [PubMed] [Google Scholar]
- 42.Sano T., Kim Y. J., Oshima E., Shimizu C., Kiyonari H., Abe T., Higashi H., Yamada K., Hirabayashi Y. (2011) Comparative characterization of GPRC5B and GPRC5CLacZ knockin mice; behavioral abnormalities in GPRC5B-deficient mice. Biochem. Biophys. Res. Commun. 412, 460–465 10.1016/j.bbrc.2011.07.118 [DOI] [PubMed] [Google Scholar]
- 43.Inoue S., Nambu T., Shimomura T. (2004) The RAIG family member, GPRC5D, is associated with hard-keratinized structures. J. Invest. Dermatol. 122, 565–573 10.1046/j.0022-202X.2004.12628.x [DOI] [PubMed] [Google Scholar]
- 44.Deng J., Fujimoto J., Ye X. F., Men T. Y., Van Pelt C. S., Chen Y. L., Lin X. F., Kadara H., Tao Q., Lotan D., Lotan R. (2010) Knockout of the tumor suppressor gene Gprc5a in mice leads to NF-kappaB activation in airway epithelium and promotes lung inflammation and tumorigenesis. Cancer Prev. Res. (Phila.) 3, 424–437 10.1158/1940-6207.CAPR-10-0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tao Q., Fujimoto J., Men T., Ye X., Deng J., Lacroix L., Clifford J. L., Mao L., Van Pelt C. S., Lee J. J., Lotan D., Lotan R. (2007) Identification of the retinoic acid-inducible Gprc5a as a new lung tumor suppressor gene. J. Natl. Cancer Inst. 99, 1668–1682 10.1093/jnci/djm208 [DOI] [PubMed] [Google Scholar]
- 46.Kurabayashi N., Nguyen M. D., Sanada K. (2013) The G protein-coupled receptor GPRC5B contributes to neurogenesis in the developing mouse neocortex. Development 140, 4335–4346 10.1242/dev.099754 [DOI] [PubMed] [Google Scholar]
- 47.Shepard B. D., Natarajan N., Protzko R. J., Acres O. W., Pluznick J. L. (2013) A cleavable N-terminal signal peptide promotes widespread olfactory receptor surface expression in HEK293T cells. PLoS One 8, e68758. 10.1371/journal.pone.0068758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levine S. A., Nath S. K., Yun C. H., Yip J. W., Montrose M., Donowitz M., Tse C. M. (1995) Separate C-terminal domains of the epithelial specific brush border Na+/H+ exchanger isoform NHE3 are involved in stimulation and inhibition by protein kinases/growth factors. J. Biol. Chem. 270, 13716–13725 10.1074/jbc.270.23.13716 [DOI] [PubMed] [Google Scholar]
- 49.Murtazina R., Kovbasnjuk O., Zachos N. C., Li X., Chen Y., Hubbard A., Hogema B. M., Steplock D., Seidler U., Hoque K. M., Tse C. M., De Jonge H. R., Weinman E. J., Donowitz M. (2007) Tissue-specific regulation of sodium/proton exchanger isoform 3 activity in Na(+)/H(+) exchanger regulatory factor 1 (NHERF1) null mice. cAMP inhibition is differentially dependent on NHERF1 and exchange protein directly activated by cAMP in ileum versus proximal tubule. J. Biol. Chem. 282, 25141–25151 10.1074/jbc.M701910200 [DOI] [PubMed] [Google Scholar]
- 50.Lee H. W., Verlander J. W., Bishop J. M., Igarashi P., Handlogten M. E., Weiner I. D. (2009) Collecting duct-specific Rh C glycoprotein deletion alters basal and acidosis-stimulated renal ammonia excretion. Am. J. Physiol. Renal Physiol. 296, F1364–F1375 10.1152/ajprenal.90667.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iversen N. K., Malte H., Baatrup E., Wang T. (2012) The normal acid-base status of mice. Respir. Physiol. Neurobiol. 180, 252–257 10.1016/j.resp.2011.11.015 [DOI] [PubMed] [Google Scholar]
- 52.Donowitz M., Mohan S., Zhu C. X., Chen T. E., Lin R., Cha B., Zachos N. C., Murtazina R., Sarker R., Li X. (2009) NHE3 regulatory complexes. J. Exp. Biol. 212, 1638–1646 10.1242/jeb.028605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kinsella J. L., Aronson P. S. (1980) Properties of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am. J. Physiol. 238, F461–F469 [DOI] [PubMed] [Google Scholar]
- 54.Brown S. E., Heming T. A., Benedict C. R., Bidani A. (1991) ATP-sensitive Na(+)-H+ antiport in type II alveolar epithelial cells. Am. J. Physiol. 261, C954–C963 [DOI] [PubMed] [Google Scholar]
- 55.Mitch W. E. (2006) Metabolic and clinical consequences of metabolic acidosis. J. Nephrol. 19 (Suppl 9), S70–S75 [PubMed] [Google Scholar]
- 56.Kovesdy C. P., Anderson J. E., Kalantar-Zadeh K. (2009) Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol. Dial. Transplant. 24, 1232–1237 10.1093/ndt/gfn633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Navaneethan S. D., Schold J. D., Arrigain S., Jolly S. E., Wehbe E., Raina R., Simon J. F., Srinivas T. R., Jain A., Schreiber M. J. Jr., Nally J. V. Jr (2011) Serum bicarbonate and mortality in stage 3 and stage 4 chronic kidney disease. Clin. J. Am. Soc. Nephrol. 6, 2395–2402 10.2215/CJN.03730411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raphael K. L., Murphy R. A., Shlipak M. G., Satterfield S., Huston H. K., Sebastian A., Sellmeyer D. E., Patel K. V., Newman A. B., Sarnak M. J., Ix J. H., Fried L. F.; Health ABC Study (2016) Bicarbonate concentration, acid-base status, and mortality in the health, aging, and body composition study. Clin. J. Am. Soc. Nephrol. 11, 308–316 10.2215/CJN.06200615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skelton L. A., Boron W. F., Zhou Y. (2010) Acid-base transport by the renal proximal tubule. J. Nephrol. 23 (Suppl 16), S4–S18 [PMC free article] [PubMed] [Google Scholar]
- 60.Curthoys N. P., Moe O. W. (2014) Proximal tubule function and response to acidosis. Clin. J. Am. Soc. Nephrol. 9, 1627–1638 10.2215/CJN.10391012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weiner I. D., Verlander J. W. (2017) Ammonia transporters and their role in acid-base balance. Physiol. Rev. 97, 465–494 10.1152/physrev.00011.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meyer R. C., Giddens M. M., Schaefer S. A., Hall R. A. (2013) GPR37 and GPR37L1 are receptors for the neuroprotective and glioprotective factors prosaptide and prosaposin. Proc. Natl. Acad. Sci. USA 110, 9529–9534 10.1073/pnas.1219004110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scarselli M., Donaldson J. G. (2009) Constitutive internalization of G protein-coupled receptors and G proteins via clathrin-independent endocytosis. J. Biol. Chem. 284, 3577–3585 10.1074/jbc.M806819200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walther C., Nagel S., Gimenez L. E., Mörl K., Gurevich V. V., Beck-Sickinger A. G. (2010) Ligand-induced internalization and recycling of the human neuropeptide Y2 receptor is regulated by its carboxyl-terminal tail. J. Biol. Chem. 285, 41578–41590 10.1074/jbc.M110.162156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li H. C., Du Z., Barone S., Rubera I., McDonough A. A., Tauc M., Zahedi K., Wang T., Soleimani M. (2013) Proximal tubule specific knockout of the Na+/H+ exchanger NHE3: effects on bicarbonate absorption and ammonium excretion. J. Mol. Med. (Berl.) 91, 951–963 10.1007/s00109-013-1015-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Amisten S., Mohammad Al-Amily I., Soni A., Hawkes R., Atanes P., Persaud S. J., Rorsman P., Salehi A. (2017) Anti-diabetic action of all-trans retinoic acid and the orphan G protein coupled receptor GPRC5C in pancreatic β-cells. Endocr. J. 64, 325–338 10.1507/endocrj.EJ16-0338 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.