Abstract
The mechanisms by which lung structural cells survive toxic exposures to cigarette smoke (CS) are not well defined but may involve proper disposal of damaged mitochondria by macro-autophagy (mitophagy), processes that may be influenced by pro-apoptotic ceramide (Cer) or its precursor dihydroceramide (DHC). Human lung epithelial and endothelial cells exposed to CS exhibited mitochondrial damage, signaled by phosphatase and tensin homolog-induced putative kinase 1 (PINK1) phosphorylation, autophagy, and necroptosis. Although cells responded to CS by rapid inhibition of DHC desaturase, which elevated DHC levels, palmitoyl (C16)-Cer also increased in CS-exposed cells. Whereas DHC augmentation triggered autophagy without cell death, the exogenous administration of C16-Cer was sufficient to trigger necroptosis. Inhibition of Cer-generating acid sphingomyelinase reduced both CS-induced PINK1 phosphorylation and necroptosis. When exposed to CS, Pink1-deficient (Pink1−/−) mice, which are protected from airspace enlargement compared with wild-type littermates, had blunted C16-Cer elevations and less lung necroptosis. CS-exposed Pink1−/− mice also exhibited significantly increased levels of lignoceroyl (C24)-DHC, along with increased expression of Cer synthase 2 (CerS2), the enzyme responsible for its production. This suggested that a combination of high C24-DHC and low C16-Cer levels might protect against CS-induced necroptosis. Indeed, CerS2−/− mice, which lack C24-DHC at the expense of increased C16-Cer, were more susceptible to CS, developing airspace enlargement following only 1 month of exposure. These results implicate DHCs, in particular, C24-DHC, as protective against CS toxicity by enhancing autophagy, whereas C16-Cer accumulation contributes to mitochondrial damage and PINK1-mediated necroptosis, which may be amplified by the inhibition of C24-DHC-producing CerS2.—Mizumura, K., Justice, M. J., Schweitzer, K. S., Krishnan, S., Bronova, I., Berdyshev, E. V., Hubbard, W. C., Pewzner-Jung, Y., Futerman, A. H., Choi, A. M. K., Petrache, I. Sphingolipid regulation of lung epithelial cell mitophagy and necroptosis during cigarette smoke exposure.
Keywords: ceramide, sphingosine, cell death, cell survival, inflammation
Caused primarily by cigarette smoke (CS) exposure, chronic obstructive pulmonary disease (COPD) is a major health problem. Increased death of structural cells that comprise the alveolar wall has been implicated in the pathogenesis of COPD, in particular, its emphysema phenotype (1). The mechanisms of lung epithelial and endothelial cell death induced by CS are incompletely elucidated. These cells may die by apoptosis or by necroptosis, a culmination of cell stress that features induction of mitophagy, a form of macro-autophagy that targets damaged mitochondria for disposal. We set out to investigate the role of ceramide (Cer) in CS-induced lung cell mitophagy and cell death, given that CS exposure increases Cer, which in turn triggers lung cell mitochondrial damage, autophagy, and apoptosis (2–4).
As the intracellular abundance of Cer is determined by sphingolipid metabolism, we interrogated two main pathways of Cer synthesis: the de novo and the sphingomyelinase pathways. In the de novo synthesis pathway, serine palmitoyl (C16) transferase produces 3-ketodihydrosphingosine, which is immediately converted to sphinganine. Cer synthases (CerS) generate dihydroceramide (DHC) from sphinganine, followed by desaturation of DHC by DHC desaturase (DEGS) to form Cer. In the sphingomyelinase pathway, the production of Cer from sphingomyelin is catalyzed by neutral sphingomyelinase (NSM) or acid sphingomyelinase (ASM). It is increasingly evident that distinct Cer species, defined by the type of fatty acid linked to the sphingoid base of the molecule, are synthesized by specific CerS and play distinct roles in cell biology. For example, C16-Cer, synthesized primarily by CerS5, is involved in cell death (3, 4). In contrast, lignoceroyl (C24)-Cer, produced mostly by CerS2, may be lung protective, as mice deficient in CerS2 (CerS2−/−) have increased airspace enlargement and inflammation, associated with marked increases in C16-Cer (5). Although the role of Cer as inducer and mediator of apoptosis has been studied extensively, multiple reports have suggested that Cer can also trigger autophagy with opposing outcomes of cell survival or cell death, a paradox recently reviewed (6).
Autophagy is an evolutionarily conserved process of cytoplasmic content engulfment by complex reorganization of subcellular membranes (7), followed by fusion with the lysosome, where this content is degraded and reused to generate energy for homeostasis and repair (7). During stress, such as nutrient deprivation, autophagy is increased as a necessary prosurvival response, but unremitted or defective autophagy culminates in cell death. We have previously implicated autophagy in COPD models, identifying an important role of autophagy initiation molecules in maintaining homeostatic airspace size and an essential role of autophagy completion (via fusion with the lysosome) in mucociliary clearance (8, 9). More recently, we determined that mitophagy is also increased in COPD models and may be linked to lung epithelial cell death induced by CS exposure (10).
Mitophagy is independently regulated by Parkin or the phosphatase and tensin homolog-induced kinase 1 (PINK1) (11). Parkin’s involvement in CS-induced airspace enlargement has been investigated (12), but the role of PINK1 and the mechanisms of mitophagy in CS-induced lung injury are not fully elucidated. In addition, although mitophagy generally functions as a protective program for mitochondrial homeostasis (13), lethal mitophagy has been described in the context of either lack of lysosomal fusion and completion of mitophagy or that of excess Cer that anchors autophagolysosomes to (undamaged) mitochondrial membranes, inappropriately targeting them for lysosomal degradation (14). Recently, we have found that CS-induced mitophagy can culminate in necroptosis, a form of programmed necrosis (15), which together with apoptosis, may contribute to the pathogenesis of COPD (10). The kinases receptor-interacting protein (RIP)-1, RIP-3, and mixed-lineage kinase domain-like protein (MLKL) form multiprotein complexes, termed the necrosome and the ripoptosome, which are key regulators of necroptosis (16–19). Unlike apoptosis, which is considered a weak inducer of inflammation with little release of damage-associated molecular patterns from dying cells, necroptosis causes a massive release of damage-associated molecular patterns and is believed to be a strong inducer of inflammation (20). We hypothesized that sphingolipids, such as Cer, are important mediators of mitophagy and necroptosis during CS exposure.
In this study, with the use of human pulmonary epithelial and endothelial cells and mice, we found that CS exposure triggers necroptosis through a mechanism that involves ASM activation and excessive accumulation of C16-Cer. CS-induced lung injury and necroptosis required PINK1 stabilization with mitophagy, as CS-exposed Pink1−/− mice were protected against airspace enlargement. Investigation of the sphingolipid metabolism in these models revealed that whereas C16-Cer can induce PINK1 phosphorylation and necroptosis, C24-Cer synthesis via CerS2 and not C16-Cer accumulation was downstream of PINK1 activation, suggesting important crosstalk between sphingolipid synthesis and mitophagy during CS exposure.
MATERIALS AND METHODS
Reagents
Unless otherwise stated, all chemicals and reagents were purchased from MilliporeSigma (St. Louis, MO, USA). The following antibodies were used: rabbit antibody to human PINK1 (BC100-494; Novus Biologicals, Littleton, CO, USA), rabbit antibody to mouse RIP3 (AHP1797; AbD Serotec, Hercules, CA, USA), mouse antibody to human and mouse β-actin (A2228; MilliporeSigma), rabbit antibody to human phospho-dynamin-related protein 1 (Drp1) (3455; Cell Signaling Technology, Danvers, MA, USA), rabbit antibody to human phospho-MLKL (ABC234; EMD Millipore, Billerica, MA, USA), and rabbit antibody to human MLKL (M6697; MilliporeSigma). Necrostatin-1 (Nec1) and necrox-5 (Nex5) were from Enzo Life Sciences (Farmingdale, NY, USA). Polyethylene glycol C16-Cer, sphingosine, sphingosine-1-phosphate, N-[(1R,2S)-2-hydroxy-1-hydroxymethyl-2-(2-tridecyl-1-cyclopropenyl)ethyl]octanamide (GT11), and sphingomyelin were purchased from Avanti Polar Lipids (Alabaster, AL, USA). D-Erythro-2-N-[12′-(1-pyridinium)-dodecanoyl]-4,5-dihydrosphingosine bromide (dhCCPS) was from Dr. A. Bjelawska (Medical University of South Carolina, Charleston, SC, USA).
Cells
Human primary lung pulmonary artery endothelial cells and their culture medium 200, containing a low serum growth supplement, were from Cascade Biologics (Portland, OR, USA). Upon attaining 70–90% confluence, cells were washed with PBS, and fresh medium containing low serum growth supplement was added for 2 h before treatments. Human primary lung microvascular endothelial cells and small airway epithelial cells (SAECs) were from Lonza (Walkersville, MD, USA). Human immortalized bronchial epithelial airway cells (Beas-2B) were from American Type Culture Collection (Manassas, VA, USA), cultured in DMEM from Thermo Fisher Scientific (Waltham, MA, USA) containing 15% fetal bovine serum (FBS) and penicillin/streptomycin or gentamicin (100 μg/ml), and maintained at 37°C with 5% CO2. When cells were 50–70% confluent, medium was aspirated, and they were washed with PBS before DMEM containing 2% fetal bovine serum, 2 h before treatments.
C16-Cer and DHC treatments
Cer and DHC treatments were performed by use of C16-Cer conjugated with polyethylene glycol (Avanti Polar Lipids), which was then solubilized in water. DHC was solubilized in ethanol.
CS exposures
Filtered research-grade cigarettes (2R4F or 3R4F) from the Kentucky Tobacco Research and Development Center (University of Kentucky, Lexington, KY, USA) were used for preparing aqueous CS extract (100%) by bubbling smoke from 2 cigarettes into 20 ml PBS at a rate of 1 cigarette/min to 0.5 cm above the filter, followed by pH adjustment to 7.4- and 0.2-μm filtration. A similar procedure was followed for air-control extract preparation, bubbling ambient air instead of CS. Treatments were performed with CS extract at the indicated concentrations (v/v).
Cer determination
Following treatments, culture medium was washed with PBS, and cells were collected in methanol, followed by lipid extraction, using a modified Bligh and Dyer method. Total lipid phosphorus (Pi) content of each lipid extract was measured by Pi labeling with NH4-molybdate. Sphingolipid analyses were performed via combined liquid chromatography-tandem mass spectrometry (LC-MS/MS) using an API4000 triple-quadrupole mass spectrometer (AB Sciex, Foster City, CA, USA) interfaced with an Agilent 1100 series liquid chromatograph (Agilent Technologies, Wilmington, DE, USA), as previously described. Analytes were ionized via positive ion electrospray ionization. Elution of the Cer and DHC was detected by multiple reaction-monitoring characteristics for 14:0-, 16:0-, 18:0-, 18:1-, 20:0-, 24:0-, and 24:1-Cer and -DHC. C17:0-Cer was used as an internal standard. All Cer measurements were normalized by Pi.
Sphingolipid inhibitory studies
The following inhibitors were used by treating cells with the indicated concentration and amount of time before CS: CerS inhibitor, fumonisin 1 (FB1; 10 μM, 2 h; Cayman Chemical, Ann Arbor, MI, USA); serine C16 transferase inhibitor, myriocin (Myr; 50 nM; 2 h; Biomol International, Plymouth Meeting, PA, USA); NSM inhibitor (GW4869; 20 µM; 2 h); and ASM inhibitor imipramine (Imi; 50 μM; 2 h; MilliporeSigma).
Immunoblotting
Cells were lysed in 1% v/v Triton X-100, 150 mM NaCl, 50 mM Tris buffer with protease, and phosphatase inhibitors (Complete and Phostop, respectively; Roche, Indianapolis, IN, USA). Lysates were boiled for 5 or 10 min in NuPAGE or Laemmli 4x sample-loading buffer (Thermo Fisher Scientific or Boston BioProducts; Ashland, MA, respectively). Samples with equal protein amount, determined by bicinchoninic acid protein analysis (Pierce, Rockford, IL, USA), were resolved using SDS-PAGE: NuPage 4–12% Bis-Tris gels (Thermo Fisher Scientific); transferred onto PVDF or nitrocellulose membranes using semidry transfer (Bio-Rad Laboratories, Hercules, CA, USA), following the manufacturer’s protocol; and probed with primary antibodies against Parkin (1:1000 dilution; Cell Signaling Technology) or LC3B (1:100,000 dilution; MilliporeSigma). Protein expression was detected using ECL, ECL Plus (GE Healthcare, Piscataway, NJ, USA), and Luminata Forte (MilliporeSigma), quantified by densitometry and normalized by a housekeeping protein, using specific β-actin antibody (1:50,000 dilution; MilliporeSigma), vinculin (1:5,000 dilution; MilliporeSigma), or GAPDH (1:1000 dilution; Abcam, Cambridge, United Kingdom).
Immunocytochemistry and immunohistochemistry
Cells were grown on gelatin-coated coverslips overnight after being transfected with a plasmid expressing N-terminally tagged enhanced green fluorescent protein (eGFP)-LC3B under the control of a cytomegalovirus promoter (a kind gift from William Jackson, Stanford University, Stanford, CA, USA) via Amaxa Nucleofector (Gaithersburg, MA, USA), according to the manufacturer’s recommendations, followed by indicated treatments. After fixation in 4% paraformaldehyde, nuclei were visualized with DAPI. Epifluorescent images were collected using a Nikon Eclipse 80i microscope and Nikon Plan Apo 100 times oil-immersion objective, NA 1.7. Formalin-fixed, paraffin-embedded, 5 μm-thick lung sections were used for immunohistochemistry, following deparafinization in xylene, rehydration, and antigen retrieval with the Immunosaver Antigen Retriever (64142; Electron Microscopy Sciences, Hatfield, PA, USA). The lung tissue was immunostained with rabbit anti-mouse RIP3 antibody (AHP1797; AbD Serotec), using the Vectastain Elite ABC Kit (PK-6101; Vector Laboratories, Burlingame, CA, USA).
Electron microscopy
Cells were fixed using 2%/2% glutaraldehyde/paraformaldehyde in a 0.1 M phosphate buffer. Images were taken with a Tecnai G2 12 Bio Twin (FEI, Hillsboro, OR, USA), equipped with an Advanced Microscopy Techniques charge-coupled device (Advanced Microscopy Techniques, Danvers, MA, USA).
Cell death determination
To discriminate live from dead cells, cells were simultaneously stained with green fluorescent calcein-acetoxymethyl (AM) to indicate intracellular esterase activity and red fluorescent ethidium homodimer-1 to indicate loss of plasma membrane integrity using the Live/Dead Viability/Cytotoxicity Kit (Thermo Fisher Scientific). Data were acquired with a FACSCanto II (BD Biosciences, San Jose, CA, USA) and were analyzed with FlowJo analytical software (Tree Star, Ashland, OR, USA).
Animal experiments
All animal studies were conducted in compliance with the Institutional Animal Care and Use Committee guidelines of Indiana University and the Harvard Standing Committee for Animal Welfare. DBA2/J mice (females, 3 mo; The Jackson Laboratory, Bar Harbor, ME, USA) were exposed to 3R4F cigarettes for 5 h/d, 5 d/wk, for 4 mo, using a Teague 10E apparatus (21). Lungs were harvested 24 h following the last scheduled day of CS exposure. Pink1−/− mice were from Dr. Jie Shen (Center for Neurologic Diseases, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA) and were generated as previously described (22). Experiments involving Pink1−/− mice used age-matched control mice (6–12 wk old) and exposure to room air or CS in whole-body exposure chambers, as previously described (8, 23), for 2 h/d (150 mg/m3), 5 d/wk, for 6 mo. Similar exposures were used for 1 mo in CerS2−/− mice and age-matched control mice (6–12 wk old, male and female), which were generated as previously described (24).
Statistical analysis
SigmaStat 3.5 or Prism 6 was used for comparisons among groups by ANOVA, followed by a Tukey’s post hoc test. For experiments in which 2 conditions were being compared, a 2-tailed Student’s t test was used. All data are expressed as means ± sem, and differences were considered statistically significant if P < 0.05.
RESULTS
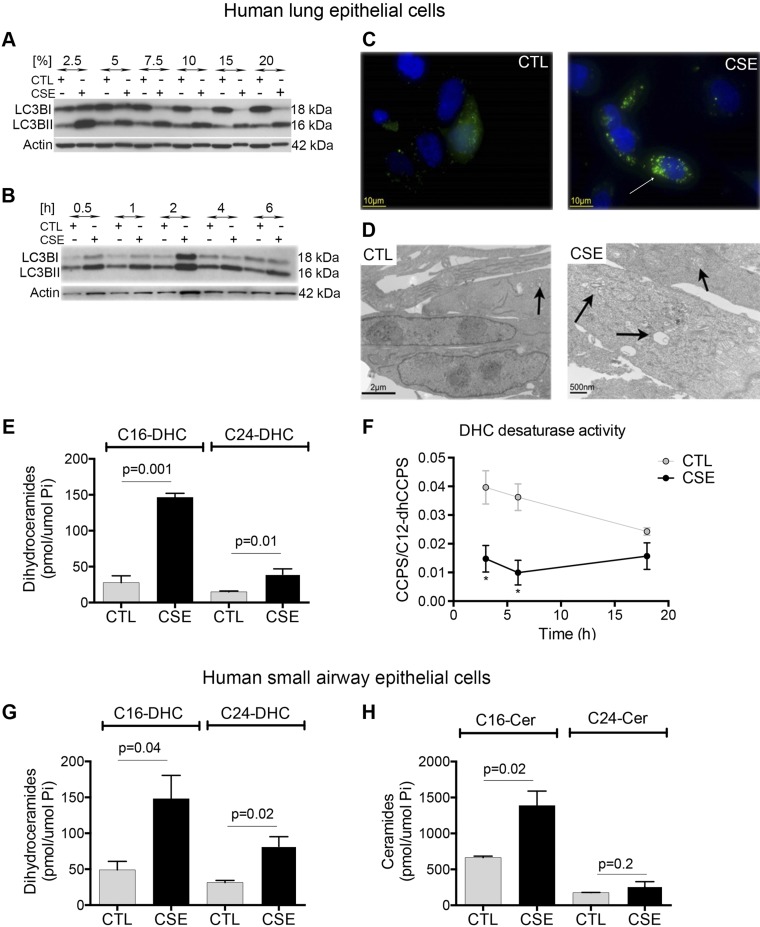
Exposure of cultured lung cells to CS extract is a widely used model to determine cell-specific mechanisms of injury or adaptation to components of CS that are absorbed across the epithelial lining fluid and lung cellular barriers. Lung epithelial and endothelial cell responses to CS may culminate in cell death by apoptosis or necroptosis (10, 25). Previous reports indicate that lung epithelial cells can also respond to CS with autophagy, identified by lipidation of LC3BI, a modification detected as a lower MW band (LC3BII) when immunoblotted with LC3B-specific antibodies. With the use of CS from reference cigarettes, we recapitulated these findings, noting that epithelial bronchial cell line Beas2B cells responded to CS with increased LC3BII, in a dose-dependent manner, as early as 30 min after exposure (Fig. 1A, B). Increased amounts of LC3BII were present in all CS conditions, whereas higher concentrations of CS decreased the amount of LC3BI, suggesting either cell toxicity (death) or an increased rate of conversion and use of LC3B that was not accompanied by further LC3B production (Fig. 1A). As a complementary approach, we transfected cells with eGFP-LC3B, followed by exposure to CS extract. Autophagy was identified by the appearance of green florescent punctae in the cytoplasm following CS exposure, indicating aggregation of LC3B in autophagosomes (Fig. 1C). Furthermore, electron microscopy of epithelial cells showed the presence of multiple autophagosomes in cells exposed to CS, whereas few similar structures were present in control cells (Fig. 1D). Overall, these results indicate that our model of CS-induced autophagy in lung cells is congruent with that reported (23). We used this model to determine the role of sphingolipids in determination of cell fate in response to CS.
Figure 1.
Effect of CS exposure on autophagy and Cer in lung epithelial cells. A, B) Immunoblot analysis of LC3BII, with β-actin as a loading control in Beas-2B cells exposed to ambient air control (ctl) or CS extract (CSE) at the indicated concentrations (5%; A), or for the indicated time (6 h; B). C) Representative immunofluorescence images (n = 3) of Beas-2B cells transiently transfected with eGFP-LC3B plasmid and then exposed to ctl or CSE (5% for 6 h). DAPI staining (blue) indicates nuclei. Note LC3B staining (green) in a cytosolic-diffuse pattern in ctl cells compared with bright-green puncta (arrow) in CSE-exposed cells. D) Representative electron microscopy images (n = 2) of Beas-2B cells exposed to CSE (5% for 6 h). Scant autolysosomes (arrow) were noted in ctl, whereas multiple structures with double-limiting membranes (autophagosomes and autolysosomes) can be seen CSE-exposed cells (arrows). E) C16- and C24-DHC species abundance in Beas-2B cells exposed ctl or CSE (5% for 6 h). F) DEGS activity in Beas-2B cells exposed to ctl or CSE (5%, indicated time), measured by the rate of conversion of C12-dhCCPS to C12-CCPS; *P < 0.05. G, H) C16- and C24-DHC (G) and C16- and C24-Cer (H) abundance in primary human SAECs exposed to ctl or CSE (5% for 6 h; n = 3). All graphs are means ± sem; 2-way ANOVA with Holm-Sidak post hoc analysis.
With the use of LC-MS/MS, we noted that DHC was significantly increased by the CS exposure, affecting mainly the most common DHC species, i.e., C16:0 and C24:0 and C24:1 (Fig. 1E), which we abbreviate herein as C16-DHC and C24-DHC. To determine whether DHC accumulation is a result of decreased conversion of DHC to Cer, we interrogated the DHC desaturase activity by using exogenous, desaturated dhCCPS (26). CS significantly inhibited DEGS activity to <50% of control, following 3 h of exposure and for at least 16 h (Fig. 1F). However, as we noted that the activity of the DEGS also decreased in control cells following 16 h of culture conditions, our subsequent studies were conducted before this time point. We next determined whether primary human lung cells, such as SAECs, respond to CS exposure in a similar manner as the exposed cell line. In SAEC, CS significantly increased C16-DHC and C24-DHC, as well as C16-Cer, but not C24-Cer (Fig. 1G, H).
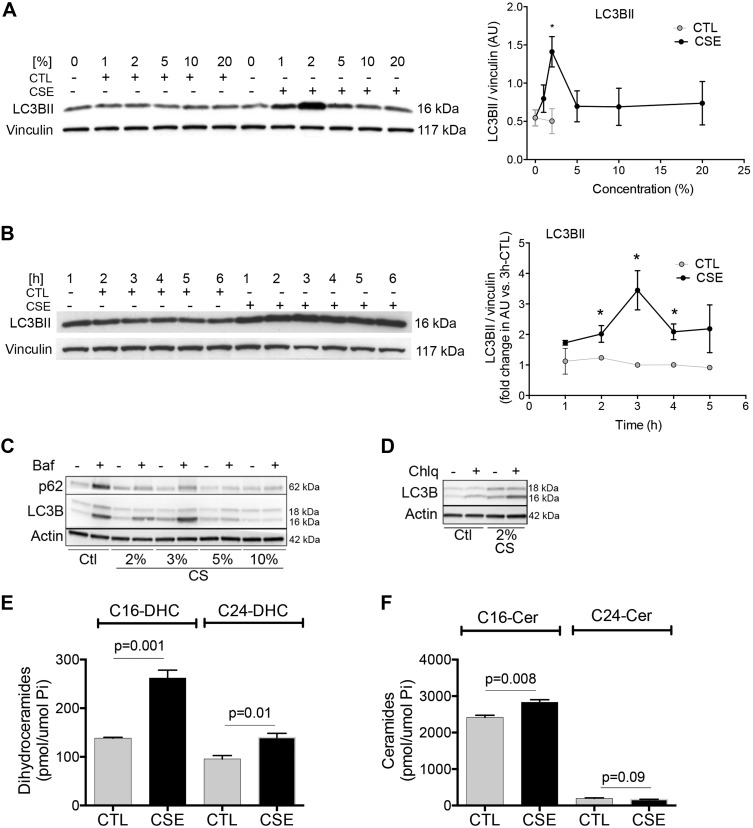
Injury of lung endothelial cells is also critical to the development of CS-induced emphysema, and to our knowledge, CS has not been reported yet to induce autophagy in these cells. Like epithelial cells, primary human lung endothelial cells of either large pulmonary artery or pulmonary microvasculature origin increased LC3BII when exposed to CS extract (Fig. 2A–D). We noted that CS-induced increases in LC3BII in lung endothelial cells required relatively low concentrations (<5%) of CS extract (Fig. 2A, C, D) and at least 2 h of exposure (Fig. 2B). CS decreased p62, suggesting at least a partial preservation of lysosomal degradation during these conditions (Fig. 2D). This was further supported by the fact that treatment with lysosomal inhibitors bafilomycin or chloroquine further increased LC3BII and p62 levels during CS exposure (Fig. 2C, D). Of note, similar to lung epithelial cells, CS-induced endothelial cell autophagy was associated with significant increases of C16- and C24-DHC (Fig. 2E) and C16-Cer but not C24-Cer (Fig. 2F). This suggested inhibition of DEGS with excessive production of C16-Cer through a different pathway than de novo synthesis.
Figure 2.
Effect of CS exposure on LC3BII and Cer in primary human lung endothelial cells. A–D) Immunoblot analysis of autophagy markers LC3B I or -II and p62 and loading controls (vinculin or actin) in human primary lung endothelial cells from the pulmonary artery (A, B) or the lung microvasculature (C, D) exposed to CTL or CSE for the indicated concentrations (%; A, 6 h; C, D, 24 h) or time (hours; B, 2%). Bar graphs depict LC3BII levels relative to vinculin, quantified by densitometry; *P < 0.05 vs. CTL. C, D) Cells were incubated in the presence of lysosomal inhibitors bafilomycin (Baf; 5 nM) or chloroquine (Chlq; 20 μM). E, F) C16- and C24-DHC (E) and C16- and C24-Cer (F) species abundance measured by MS in SAECs exposed to ctl or CSE (5%; 6 h; n = 3). All graphs are means ± sem; 2-way ANOVA with Holm-Sidak post hoc analysis.
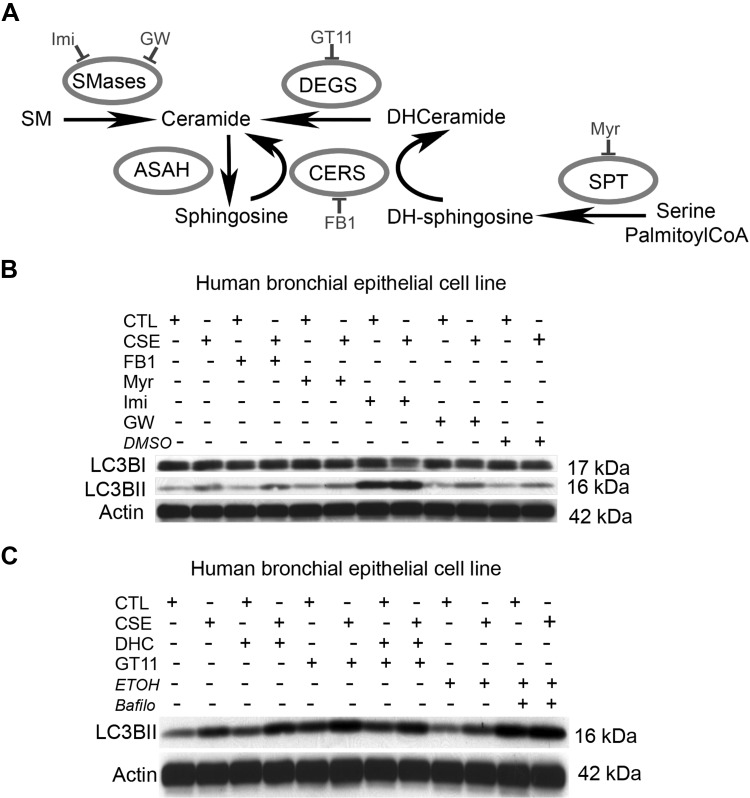
To determine if DHC or Cer production was involved in LC3BII increases during CS exposure, we treated cells with pharmacological inhibitors of specific sphingolipid metabolism pathways, including FB1, inhibitor for CerS in both de novo and recycling pathways of Cer production; Myr, which targets the first step of de novo synthesis; Imi, which inhibits ASM; and GW4869, which targets NSM (Fig. 3A). Of these, only Myr, which inhibits both DHC and Cer production, partially inhibited LC3BII increase (Fig. 3B). In turn, inhibition of ASM augmented LC3BII, which suggested that Cer production by this pathway might inhibit autophagy (Fig. 3C). To determine if DHC is sufficient to increase LC3BII, we exposed cells to exogenous DHC, or we elevated endogenous DHC by treatment with a DEGS inhibitor (GT11). Bafilomycin, a V-ATPase inhibitor that increases lysosomal pH, inhibiting the degradation of autolysosome contents and the turnover of LC3BII, was used as a positive control. Treatment with either DHC or GT11 increased LC3BII in both control conditions and in cells exposed to CS (Fig. 3C).
Figure 3.
Effect of Cer synthesis inhibition on LC3BII abundance during CS exposure. A) Schematic of Cer synthesis via sphingomyelin (SM) hydrolysis catalyzed by sphingomyelinases (SMases); de novo synthesis from serine and C16 coenzyme A (CoA), catalyzed by the serial action of serine C16 transferase (SPT), CerS, and DEGS; and via the recycling pathway, which uses sphingosine produced from Cer hydrolysis by ceramidases (ASAH) to resynthesize Cer via CERS. Noted are pharmacological inhibitors used to target enzymes involved in Cer production. DH, Dihydro; GW, GW4869; Imi, Imipramine. B, C) Representative immunoblots (n = 3) of LC3B and the loading control actin in cells exposed to ctl or CSE (2% CSE for 3 h; n = 3), while treated with inhibitors of Cer synthesis, DHC, or vehicle controls [DMSO and ethanol (ETOH)] or the lysosomal inhibitor bafilomycin (Bafilo) as a positive control for LC3BII accumulation. Note that increased LC3BII in response to CSE is enhanced by Imi (ASM inhibitor) and modestly decreased by Myr (SPT inhibitor); also, note that either exogenous DHC exposure or inhibition of endogenous DHC metabolism (GT11) increased LC3BII, even in ctl cells.
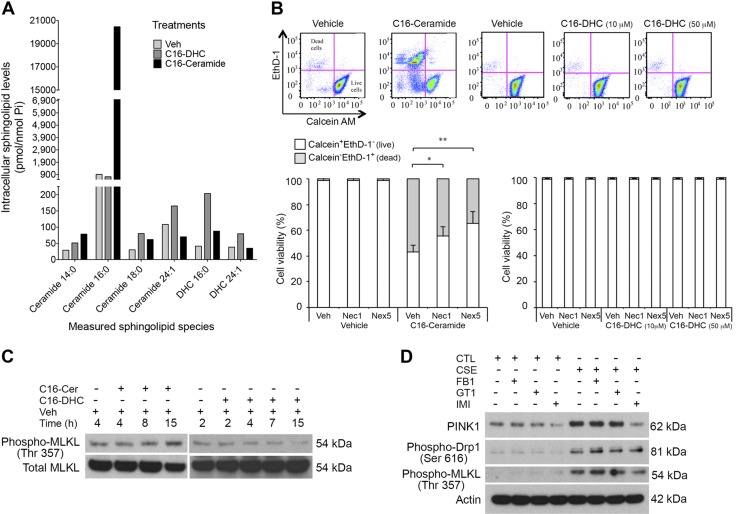
As autophagy is typically a prosurvival stress response, we further distinguished between the biologic effects of DHC and Cer by assessing cellular viability following their exogenous addition to cultured cells. By measuring with MS increases in respective intracellular species (Fig. 4A), we confirmed that long-chain C16-Cer and DHC are cell permeable. With the use of a live/dead cell assay, we noted that C16-Cer but not C16-DHC induced cell death (Fig. 4B).
Figure 4.
Effect of Cer and DHC on lung cell necroptosis and mitophagy. A) Intracellular sphingolipid levels in primary human lung epithelial cells following addition of C16-Cer or -DHC (10 nM), measured by LC-MS/MS and normalized by lipid phosphorus. B) Cell viability and cell death were determined with calcein-AM/ethidium homodimer-1 (EthD-1) staining and flow cytometry of Beas-2B cells exposed to C16-Cer (10 μM; 15 h) or C16-DHC (indicated concentrations; 15 h) and treated with Nec1 (50 μM, 1 h), Nex5 (30 μM, 1 h), or vehicle (DMSO, 1 h). A, B) Bar graphs show means ± sem. *P < 0.05, **P < 0.01; n = 3; representative panels are shown above, where x-axes indicate calcein-AM staining of live cells, and y-axes indicate EthD-1 staining of dead cells. C) Immunoblot of phospho-MLKL (Thr357) and total MLKL in Beas-2B cells treated with C16-Cer (10 μM) or C16-DHC (50 μM) for the indicated time. D) Immunoblot of PINK1, phospho-Drp1 (Ser616), phospho-MLKL (Thr357), and β-actin (as loading control) in Beas-2B cells exposed to CTL or CSE (20%, 6 h) and treated with sphingolipid enzyme inhibitors FB1 (5 μM, 1 h), GT11 (12 μM, 1 h), or Imi (50 μM, 1 h).
We have previously shown that C16-Cer increases induce lung endothelial apoptosis. However, as CS can induce necroptosis in lung epithelial cells through mitophagy (10), and Cer has been reported to trigger lethal mitophagy in other cell types (14), we tested the contribution of necroptosis to C16-Cer-induced epithelial cell death. Treatments with Nec1, a necroptosis inhibitor, or with Nex5, a necrosis inhibitor with antioxidant activity that primarily targets mitochondria, significantly inhibited C16-Cer-induced lung epithelial cell death (Fig. 4B). To determine the signaling events that linked the sphingolipid pathway to mitophagy, we focused on PINK1, a master mitophagy regulator. We also investigated the involvement of MLKL, a critical necroptosis mediator (19), which is activated via Thr-357 and Ser-358 phosphorylation by the RIP3 kinase. Exposure of Beas-2B cells to C16-Cer but not C16-DHC increased MLKL phosphorylation (Fig. 4C), suggesting that C16-Cer but not DHC may be associated with CS-induced necroptosis. To determine which sphingolipid metabolic pathway is involved in CS-induced necroptosis, we first demonstrated that CS exposure of these cells increases PINK1 expression, MLKL Thr-357 phosphorylation, and mitochondrial fission regulator Drp1 phosphorylation (Fig. 4D). Of the various disruptors of sphingolipid metabolism, only the ASM inhibitor Imi, but not inhibitors of de novo and recycling pathway (FB1) or of DEGS (GT11) attenuated the CS effect on mitophagy and necroptosis effectors (Fig. 4D). These data implicate C16-Cer produced via ASM in CS-induced mitophagy and necroptosis in lung epithelial cells.
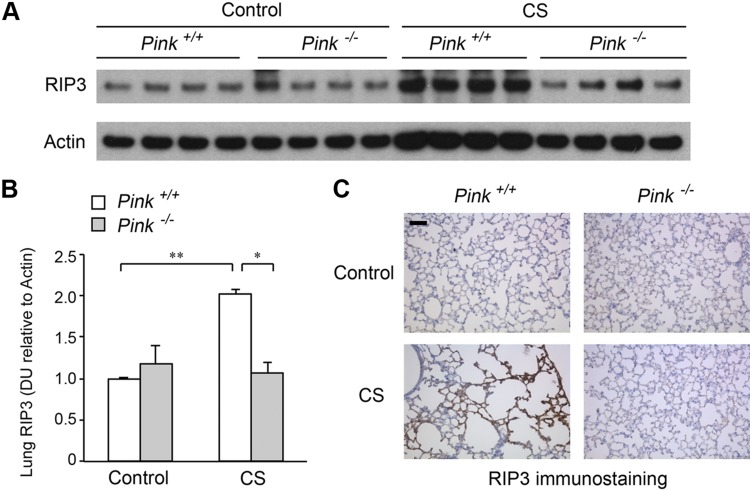
To explore the relationship between CS-induced mitophagy and necroptosis in vivo, we exposed Pink1−/− mice and corresponding wild-type mice to CS for 6 mo and measured lung levels of the necroptosis marker RIP3 by Western blotting (Fig. 5A, B) and immunohistochemistry (Fig. 5C). CS markedly increased RIP3 in the lung of wild-type mice relative to air-treated controls (Fig. 5A–C). In contrast CS-exposed Pink1−/− mouse lungs had reduced lung RIP3 abundance (Fig. 5A–C). These results suggest that Pink1 is at least partially required for chronic CS exposure-induced lung cell necroptosis.
Figure 5.
Effect of PINK1 on lung necroptosis marker RIP3 during chronic CS exposure. A) Immunoblot of RIP3 and β-actin (as loading control) in wild-type (Pink1+/+) and Pink1−/− mouse lung homogenates following exposure to ambient air control or CS (6 mo). B) Bar graphs of RIP3 protein abundance in mouse lungs measured by densitometry; means ± sem. DU, densitometry units. *P < 0.05, **P < 0.01 (n = 4). C) Representative immunohistochemistry image of RIP3 in lung parenchyma of Pink1+/+ and Pink1−/− mice chronically exposed to CS (6 mo). Scale bar, 50 μm. Image is representative of 5 images/mouse lung; n = 3 mice/group.
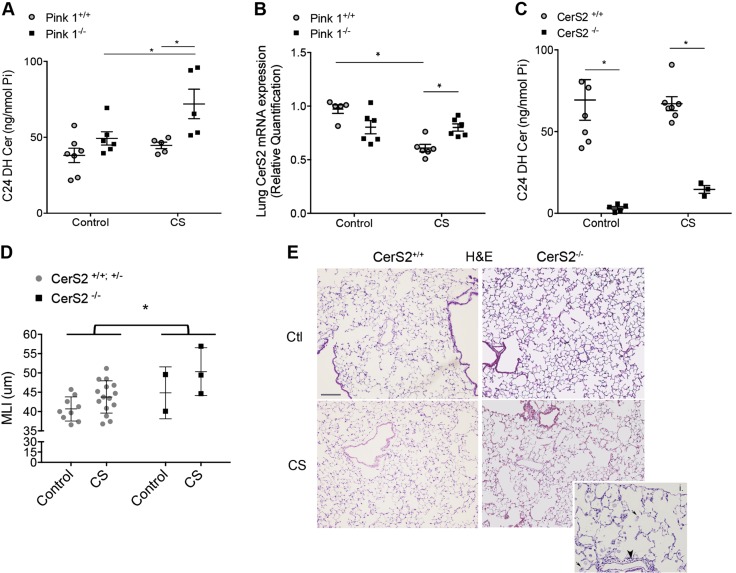
Having shown that both C16-Cer and Pink1 are involved in necroptosis, we next studied their relationship by using lungs of CS-exposed Pink1−/− mice. C16-Cer levels were not decreased in Pink1−/− mice, either at baseline or following chronic CS exposure (data not shown). However, there was a significant 2-fold increase in C24-DHC levels in Pink1−/− mice vs. wild-type mice following CS exposure (Fig. 6A), with a trend for C24-Cer increases (of only 20%; data not shown). As the enzyme CerS2 preferentially produces C24-DHC, we measured CerS2 mRNA expression in these lungs. Unlike the lungs of wild-type animals, in which CerS2 mRNA significantly decreased following CS exposure, CerS2 expression was significantly increased in Pink1−/− lungs exposed to CS (Fig. 6B).
Figure 6.
Effect of PINK1 on CerS2 during CS exposure. A) C24-DHC abundance in Pink1−/− mice compared with Pink1+/+ mice after CS exposure (6 mo). B) Expression levels of CerS2 mRNA in whole lungs of Pink1−/− and Pink1+/+ mice after CS exposure (6 mo). C) C24-DHC Cer levels in lungs of CerS2−/− and CerS2+/+ mice following chronic CS exposure (6 mo). D, E) Mean linear intercepts (MLI; D) and representative hematoxylin and eosin (H&E) staining images of lung sections from CerS2+/+ and CerS2−/− mice after CS exposure (1 mo; E). Graphs show individual data points identifying distinct mice; mean and sem. *P < 0.05, 2-way ANOVA with Holm-Sidak post hoc analysis. Inset shows select areas of marked airspace enlargement, foamy macrophages (arrows), and inflammatory cells (arrowhead) in CerS2−/− mice exposed to CS.
These results implicated for the first time Pink1 in the regulation of CerS2. We previously reported that Pink1−/− mice are protected from developing airspace enlargement from CS exposure (10). We now show that decreased Pink1 is associated with increased CerS2 and increased C24-DHC. This may suggest that CerS2 and its product C24-DHC is lung protective, and as a corollary, decreases in CerS2 or C24-DHC may increase the susceptibility of lungs to CS-induced injury. We have previously characterized CerS2−/− mice, which as expected, lack C24 sphingolipids, including C24-DHC (Fig. 6C), at the expense of increased C16 sphingolipids (5). To test whether the lack of CerS2 increases the susceptibility to airspace enlargement following CS exposure, we exposed CerS2−/− to CS for 1 mo. As determined by stereological-automated morphometry and calculation of mean linear intercepts, in control wild-type mice, CS exposure during this time frame did not significantly change lung morphology (Fig. 6D, E). However, CS exposure for only 1 mo significantly increased the airspace size of CerS2−/− mice (Fig. 6D, E).
DISCUSSION
Our findings indicate that C16-Cer induces mitophagy-mediated necroptosis in lung structural cells. Furthermore, CerS2 and its immediate product C24-DHC may have a role in the maintenance of lung structural cells, but they are vulnerable to inhibition by chronic CS. We also demonstrated that similar to hypoxia (26), CS inhibits DEGS activity, which may represent an adaptive response to limit the production of proapoptotic Cer, while engaging prosurvival autophagy via DHC accumulation.
These findings are important, as the underlying mechanisms of CS-induced autophagy and mitophagy, although increasingly appreciated to contribute to the pathogenesis of COPD, have not been fully elucidated (8, 10, 27). The involvement of C16-Cer in this process is not surprising, as several sphingolipids, including Cer and DHC, have been previously involved in cell death and in mito/autophagy in other models (14, 25).
In this study, we found that the increases in the two main DHC species, C16 and C24, during CS exposure were associated with inhibition of DEGS activity. The inhibition of DEGS would be expected to increase DHC and to limit the production of Cer in the de novo pathway. However, we measured an increase in C16-Cer levels in pulmonary epithelial and endothelial cells exposed to CS, likely the result of the ASM pathway. Our data indicate that whereas C16-Cer triggers epithelial cell necroptosis, DHC induces prosurvival autophagy. Of note, sphingolipid species with 16- and 24-carbon fatty acid chains, including Cer and DHC, are the most prevalent in the lung (28). These findings corroborate our previous reports that CS exposure increases Cer in lung structural cells, such as endothelial and epithelial cells (29). We also previously demonstrated that Cer activates mitochondrial membrane potential and triggers autophagy and eventually death by apoptosis in lung endothelial cells isolated from nonsmokers (4). This body of work, together with the current findings that ASM inhibition reduced CS-induced mitophagy and necroptosis, leads us to conclude that during CS, ASM-generated C16-Cer may damage mitochondria, triggering mitophagy, which when unmitigated by prosurvival cellular adaptations, culminates in cell death via necroptosis. Our results implicate ASM as a potential mediator of the previously reported lethal mitophagy induced by CS exposure in pulmonary epithelial cells (10). As inhibition of Cer production did not affect CS-induced phosphorylation of Drp1, a mitochondrial fission regulator, our data suggest that C16-Cer, synthesized via ASM, is an upstream modulator of CS-induced mitophagy and necroptosis but not of mitochondrial fission.
Mitophagy is a homeostatic program that maintains a healthy mitochondrial population with a cytoprotective role in the context of disease pathogenesis (11). However, similar to other examples of macroautophagy, in certain conditions, mitophagy is a pathway to cell death (14, 30) and therefore, may be implicated in conditions of unwanted cell demise. It remains unclear how C16-Cer accumulation bridges CS-induced mitophagy and necroptosis. Such effects may be related to oxidative stress, as excessive C16-Cer and sphinganine, resulting from adaptations to CerS2 inhibition, increased mitochondria damage in hepatocytes, affecting the mitochondrial complex IV that led to chronic oxidative stress and progressive hepatopathy (31). A different mechanism may operate in cancer cells, where similar conditions were associated with activation of the unfolded protein response and nonlethal autophagy (32). Although our data suggest that DHC may promote prosurvival autophagy during CS exposure, the pathophysiological differences among C16-Cer-induced lethal, necroptotic mitophagy, and DHC-regulated autophagy remain unclear and deserve further studies. We tested directly the differential effect of C16-Cer vs. DHC, either by the direct addition of the respective sphingolipids to cell cultures or by the increase of endogenous DHC via pharmacological GT11. We could not directly dissect a functional difference between C16- and C24-DHC, as both were increased by CS, likely via desaturase inhibition. However, the abundance of de novo synthesized C16 and C24 sphingolipid species appears to be inversely linked. Likewise, our data suggest that during CS exposure, there is an overall decrease in C24 species accompanied by increases in C16 species. This suggests a crosstalk between CerS2 and CerS5 regulation. We took advantage of the transgenic CerS2−/− mouse, which exhibits concomitant increases of C16 and decreases of C24 sphingolipids to garner insight into the functional meaning of the changes of the two sphingolipids in an opposite direction. Data in this mouse are highly supportive of the notion of a coupled and differential effect of these two most abundant sphingolipid species in the lung, whereby C16-Cer accumulation and C24-DHC loss are detrimental to lung health. Whereas we cannot rule out that other C16 or C24 sphingolipid species may be implicated, our evidence supports that at least these particular molecules have an important role.
In the current report, with the use of Pink1−/− mice and with the evaluation of lung RIP3 expression, we determined, for the first time, the direct contribution of mitophagy to CS-induced necroptosis in vivo. In addition, our sphingolipid measurements in CS-exposed Pink1−/− mice corroborated our data obtained in cell cultures, which suggested that C16-Cer is upstream of PINK1 activation by CS. Furthermore, we found that CS decreases CerS2 mRNA expression and that this suppressive effect is absent in Pink1−/− mice, which have significantly elevated lung C24-DHC levels. These data suggest that when activated, PINK1 has regulatory effects on Cer synthesis via the de novo pathway by inhibiting CerS2 expression in the lungs. It is unclear whether this effect is directly linked to C16-Cer upregulation of PINK1. However, the association of elevated CerS2 and its products of C24-DHC, which was the most upregulated species in Pink1−/− mice, protected from CS, suggests a potential role of CerS2 and possibly C24-DHC in the lung defense against CS. Whether this is a result of a proautophagic effect of C24-DHC remains to be demonstrated. Nevertheless, the notion that CerS2 is lung protective and that PINK1-mediated CerS2 inhibition during CS is detrimental was further supported by the increased susceptibility to CS noted in CerS2−/− mice. With the use of our CS exposure protocol, 4–6 mo of exposure are typically required to measure significant airspace enlargement differences in the wild-type mouse strain used to generate the CerS2 transgenic deletion. In contrast, CerS2−/− mice manifested increased airspace enlargement after only 1 mo of CS exposure.
Several limitations of our study deserve mentioning. Although the role of PINK1 in mitophagy is essential, superseding that of Parkin (11), PINK1 may interact with other signaling platforms unrelated to mitophagy (33), which were not addressed in our study. In addition, although we focused on the Cer and DHC species role in the models studied, other sphingolipid metabolites, including sphinganine, known to be elevated in CerS2−/− mice, may be responsible for some of the biologic effects noted. We also did not elucidate either the cell-specific contribution of the signaling pathways investigated to the CS-induced, emphysema-like phenotype or the relative contribution of necroptosis vs. apoptosis to airspace enlargement. Finally, as our cell culture data relied on pharmacological inhibitors with potential off-target effects, we performed our in vivo studies using transgenic animals to target more specifically the enzymes of interest.
In summary, we demonstrated that C16-Cer produced via ASM is an upstream initiator of PINK1-regulated lethal mitophagy by necroptosis in CS-exposed lung structural cells. In turn, during chronic CS exposure, PINK1 triggered lung necroptosis while inhibiting a potentially protective CerS2 expression in the lung. Our study highlights the importance of lethal mitophagy in CS-induced chronic lung injury, provides a mechanistic explanation for how CS induces mitophagy-driven necroptosis, and further supports the fact that the dysregulated sphingolipid metabolism is implicated in the pathogenesis of structural cell injury in emphysema.
ACKNOWLEDGMENTS
The authors acknowledge the support from U.S. National Institute of Health, National Heart, Lung, and Blood Institute Grants RO1HL077328 (to I.P.), F31HL126459 (to M.J.J.), and P01HL114501 and R01HL132198 (to A.M.K.C.); and support from the Binational Science Foundation (to I.P. and A.H.F.). K.M. and M.J.J. are cofirst authors on this manuscript. The authors declare no conflicts of interest.
Glossary
- AM
acetoxymethyl
- ASM
acid sphingomyelinase
- Beas2B
human immortalized bronchial epithelial airway cell
- C16
palmitoyl
- C24
lignoceroyl
- Cer
ceramide
- CerS
ceramide synthases
- COPD
chronic obstructive pulmonary disease
- CS
cigarette smoke
- DEGS
dihydroceramide desaturase
- DHC
dihydroceramide
- dhCCPS
D-erythro-2-N-[12′-(1-pyridinium)-dodecanoyl]-4,5-dihydrosphingosine bromide
- Drp1
dynamin-related protein 1
- eGFP
enhanced green fluorescent protein
- FB1
fumonisin 1
- GT11
N-[(1R,2S)-2-hydroxy-1-hydroxymethyl-2-(2-tridecyl-1-cyclopropenyl)ethyl]octanamide (dihydroceramide desaturase inhibitor)
- Imi
imipramine
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- MLKL
mixed-lineage kinase domain-like protein
- Myr
myriocin
- Nec
necrostatin
- Nex5
necrox-5
- NSM
neutral sphingomyelinase
- Pi
total lipid phosphorus
- PINK1
phosphatase and tensin homolog-induced putative kinase 1
- RIP
receptor-interacting protein
- SAEC
small airway epithelial cell
AUTHOR CONTRIBUTIONS
K. Mizumura, M. J. Justice, and E. V. Berdyshev designed and performed experiments, analyzed data, and wrote the manuscript; K. S. Schweitzer and W. C. Hubbard performed experiments and analyzed data; S. Krishnan and I. Bronova performed experiments; Y. Pewzner-Jung designed and performed experiments and analyzed data; A. H. Futerman and A. M. K. Choi designed experiments and analyzed data; and I. Petrache designed experiments, analyzed data, and wrote manuscript.
REFERENCES
- 1.Tuder R. M., Petrache I. (2012) Pathogenesis of chronic obstructive pulmonary disease. J. Clin. Invest. 122, 2749–2755 10.1172/JCI60324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medler T. R., Petrusca D. N., Lee P. J., Hubbard W. C., Berdyshev E. V., Skirball J., Kamocki K., Schuchman E., Tuder R. M., Petrache I. (2008) Apoptotic sphingolipid signaling by ceramides in lung endothelial cells. Am. J. Respir. Cell Mol. Biol. 38, 639–646 10.1165/rcmb.2007-0274OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamocki K., Van Demark M., Fisher A., Rush N. I., Presson R. G., Jr., Hubbard W., Berdyshev E. V., Adamsky S., Feinstein E., Gandjeva A., Tuder R. M., Petrache I. (2013) RTP801 is required for ceramide-induced cell-specific death in the murine lung. Am. J. Respir. Cell Mol. Biol. 48, 87–93 10.1165/rcmb.2012-0254OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrusca D. N., Van Demark M., Gu Y., Justice M. J., Rogozea A., Hubbard W. C., Petrache I. (2014) Smoking exposure induces human lung endothelial cell adaptation to apoptotic stress. Am. J. Respir. Cell Mol. Biol. 50, 513–525 10.1165/rcmb.2013-0023OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrache I., Kamocki K., Poirier C., Pewzner-Jung Y., Laviad E. L., Schweitzer K. S., Van Demark M., Justice M. J., Hubbard W. C., Futerman A. H. (2013) Ceramide synthases expression and role of ceramide synthase-2 in the lung: insight from human lung cells and mouse models. PLoS One 8, e62968. 10.1371/journal.pone.0062968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang W., Ogretmen B. (2014) Autophagy paradox and ceramide. Biochim. Biophys. Acta 1841, 783–792 10.1016/j.bbalip.2013.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizushima N., Komatsu M. (2011) Autophagy: renovation of cells and tissues. Cell 147, 728–741 10.1016/j.cell.2011.10.026 [DOI] [PubMed] [Google Scholar]
- 8.Chen Z. H., Lam H. C., Jin Y., Kim H. P., Cao J., Lee S. J., Ifedigbo E., Parameswaran H., Ryter S. W., Choi A. M. (2010) Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc. Natl. Acad. Sci. USA 107, 18880–18885 10.1073/pnas.1005574107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam H. C., Cloonan S. M., Bhashyam A. R., Haspel J. A., Singh A., Sathirapongsasuti J. F., Cervo M., Yao H., Chung A. L., Mizumura K., An C. H., Shan B., Franks J. M., Haley K. J., Owen C. A., Tesfaigzi Y., Washko G. R., Quackenbush J., Silverman E. K., Rahman I., Kim H. P., Mahmood A., Biswal S. S., Ryter S. W., Choi A. M. (2013) Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction. J. Clin. Invest. 123, 5212–5230 10.1172/JCI69636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizumura K., Cloonan S. M., Nakahira K., Bhashyam A. R., Cervo M., Kitada T., Glass K., Owen C. A., Mahmood A., Washko G. R., Hashimoto S., Ryter S. W., Choi A. M. (2014) Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J. Clin. Invest. 124, 3987–4003 10.1172/JCI74985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazarou M., Sliter D. A., Kane L. A., Sarraf S. A., Wang C., Burman J. L., Sideris D. P., Fogel A. I., Youle R. J. (2015) The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524, 309–314 10.1038/nature14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmad T., Sundar I. K., Lerner C. A., Gerloff J., Tormos A. M., Yao H., Rahman I. (2015) Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: implications for chronic obstructive pulmonary disease. FASEB J. 29, 2912–2929 10.1096/fj.14-268276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Youle R. J., Narendra D. P. (2011) Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 12, 9–14 10.1038/nrm3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sentelle R. D., Senkal C. E., Jiang W., Ponnusamy S., Gencer S., Selvam S. P., Ramshesh V. K., Peterson Y. K., Lemasters J. J., Szulc Z. M., Bielawski J., Ogretmen B. (2012) Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat. Chem. Biol. 8, 831–838 10.1038/nchembio.1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linkermann A., Green D. R. (2014) Necroptosis. N. Engl. J. Med. 370, 455–465 10.1056/NEJMra1310050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandenabeele P., Galluzzi L., Vanden Berghe T., Kroemer G. (2010) Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 11, 700–714 10.1038/nrm2970 [DOI] [PubMed] [Google Scholar]
- 17.Zhang D. W., Shao J., Lin J., Zhang N., Lu B. J., Lin S. C., Dong M. Q., Han J. (2009) RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325, 332–336 10.1126/science.1172308 [DOI] [PubMed] [Google Scholar]
- 18.He S., Wang L., Miao L., Wang T., Du F., Zhao L., Wang X. (2009) Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137, 1100–1111 10.1016/j.cell.2009.05.021 [DOI] [PubMed] [Google Scholar]
- 19.Sun L., Wang H., Wang Z., He S., Chen S., Liao D., Wang L., Yan J., Liu W., Lei X., Wang X. (2012) Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 10.1016/j.cell.2011.11.031 [DOI] [PubMed] [Google Scholar]
- 20.Pasparakis M., Vandenabeele P. (2015) Necroptosis and its role in inflammation. Nature 517, 311–320 10.1038/nature14191 [DOI] [PubMed] [Google Scholar]
- 21.Clauss M., Voswinckel R., Rajashekhar G., Sigua N. L., Fehrenbach H., Rush N. I., Schweitzer K. S., Yildirim A. O., Kamocki K., Fisher A. J., Gu Y., Safadi B., Nikam S., Hubbard W. C., Tuder R. M., Twigg H. L., III, Presson R. G., Sethi S., Petrache I. (2011) Lung endothelial monocyte-activating protein 2 is a mediator of cigarette smoke-induced emphysema in mice. J. Clin. Invest. 121, 2470–2479 10.1172/JCI43881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitada T., Pisani A., Porter D. R., Yamaguchi H., Tscherter A., Martella G., Bonsi P., Zhang C., Pothos E. N., Shen J. (2007) Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc. Natl. Acad. Sci. USA 104, 11441–11446 10.1073/pnas.0702717104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Z. H., Kim H. P., Sciurba F. C., Lee S. J., Feghali-Bostwick C., Stolz D. B., Dhir R., Landreneau R. J., Schuchert M. J., Yousem S. A., Nakahira K., Pilewski J. M., Lee J. S., Zhang Y., Ryter S. W., Choi A. M. (2008) Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. PLoS One 3, e3316. 10.1371/journal.pone.0003316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pewzner-Jung Y., Park H., Laviad E. L., Silva L. C., Lahiri S., Stiban J., Erez-Roman R., Brügger B., Sachsenheimer T., Wieland F., Prieto M., Merrill A. H., Jr., Futerman A. H. (2010) A critical role for ceramide synthase 2 in liver homeostasis: I. alterations in lipid metabolic pathways. J. Biol. Chem. 285, 10902–10910 10.1074/jbc.M109.077594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrache I., Natarajan V., Zhen L., Medler T. R., Richter A. T., Cho C., Hubbard W. C., Berdyshev E. V., Tuder R. M. (2005) Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat. Med. 11, 491–498 10.1038/nm1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devlin C. M., Lahm T., Hubbard W. C., Van Demark M., Wang K. C., Wu X., Bielawska A., Obeid L. M., Ivan M., Petrache I. (2011) Dihydroceramide-based response to hypoxia. J. Biol. Chem. 286, 38069–38078 10.1074/jbc.M111.297994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizumura K., Choi A. M., Ryter S. W. (2014) Emerging role of selective autophagy in human diseases. Front. Pharmacol. 5, 244. 10.3389/fphar.2014.00244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrache I., Berdyshev E. V. (2016) Ceramide signaling and metabolism in pathophysiological states of the lung. Annu. Rev. Physiol. 78, 463–480 10.1146/annurev-physiol-021115-105221 [DOI] [PubMed] [Google Scholar]
- 29.Elias J. A., Lee C. G. (2005) Lipid let loose in pulmonary emphysema. Nat. Med. 11, 471–472 10.1038/nm0505-471 [DOI] [PubMed] [Google Scholar]
- 30.Dorn G. W., II, Kitsis R. N. (2015) The mitochondrial dynamism-mitophagy-cell death interactome: multiple roles performed by members of a mitochondrial molecular ensemble. Circ. Res. 116, 167–182 10.1161/CIRCRESAHA.116.303554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zigdon H., Kogot-Levin A., Park J. W., Goldschmidt R., Kelly S., Merrill A. H., Jr., Scherz A., Pewzner-Jung Y., Saada A., Futerman A. H. (2013) Ablation of ceramide synthase 2 causes chronic oxidative stress due to disruption of the mitochondrial respiratory chain. J. Biol. Chem. 288, 4947–4956 10.1074/jbc.M112.402719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spassieva S. D., Mullen T. D., Townsend D. M., Obeid L. M. (2009) Disruption of ceramide synthesis by CerS2 down-regulation leads to autophagy and the unfolded protein response. Biochem. J. 424, 273–283 10.1042/BJ20090699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arena G., Valente E. M. (2017) PINK1 in the limelight: multiple functions of an eclectic protein in human health and disease. J. Pathol. 241, 251–263 10.1002/path.4815 [DOI] [PubMed] [Google Scholar]