ABSTRACT
Vaccination status is more often evaluated by up-to-date vaccination coverage rather than timeliness of immunization. Delaying vaccination may be dangerous during infancy. The aim of this study was to identify the importance of potentially dangerous vaccination delay (previously defined) and determinants of these delays. We conducted a national, prospective, vaccination survey in June 2014, with primary care pediatricians. Children, 2 to 24 months of age, were included. Data about vaccination were extracted from their health books. Additional data were collected through a standardized questionnaire. Vaccine coverage rate and timeliness were calculated. Variables associated with a potentially dangerous vaccination delay as previously defined were determined by a multivariable analysis. Among the 443 included children (mean age 10.8 months, 49% males), 13% to 58% of vaccine doses according to vaccine type were done with a potentially dangerous delay. Globally, 47% of children had at least one potentially dangerous immunization delay. We identified two risk factors of potentially dangerous delayed immunization globally: an increasing age of the child (adjusted odds ratio: 1.2, 95% confidence interval [CI]: 1.1–1.3, p < 10−3), and a working mother (adjusted OR: 2.4, 95% CI: 1.2–4.7, p = 0.01). Despite a good vaccine coverage rate, a large number of children had a potentially dangerous vaccination delay. A high level of vigilance regarding these immunization delays, and particularly to the patients sharing the risk factors of immunization delay identified here, can increase quality and effectiveness of the vaccine protection.
KEYWORDS: children, immunization delay, pediatricians, timeliness, vaccine
Introduction
Immunization is one of the most important achievements in the 20th century. Objective of the World Health Organization (WHO) Expanded Program on Immunization is to increase rates of childhood immunization to over 90 percent worldwide in order to reduce global childhood morbidity and mortality due to vaccine-preventable diseases (VPD).1
Vaccination coverage was the main indicator of immunization, but several studies showed that up-to-date vaccination does not mean age-appropriate vaccination.2-6 Delayed immunization recently appeared as a key element in the risk reduction of VPD, especially when epidemic peak is close to the recommended age of vaccination.2,3 A delayed immunization is defined by a real age of vaccination over the recommended age of vaccination. This period matches with maximal vulnerability time in which children are exposed to VPD4 and implies two risks: not to be protected at maximal vulnerability ages and not to complete immunization schedule by domino effect.5,6 For example, a pertussis outbreak in 2010 showed that 140/275 infants (51%) with pertussis could have had one dose of the vaccine and 22% at least 2 doses, with 33% intensive care unit admissions and one death.7 A cost-analysis model to explore the factors associated with vaccination delay could prevent annually approximately 278 pertussis cases, 103 hospitalizations and one death in infants aged less than one year in the US, saving approximately $1.03 million in healthcare costs.8
The results of a Delphi study which defined what a potentially dangerous vaccination delay is, were recently published.9 This process included French experts in vaccination and pediatric infectious diseases specialists. They determined limits of the potentially dangerous vaccination delay of the 10 vaccine-doses recommended for French children aged less than 2 years. The purpose of our study was to quantify the frequency of potentially dangerous vaccination delay for children 2 to 24 months followed by primary-care pediatricians.
Results
Populations' characteristics
A total of 473 responses for children 2–24 months of age were obtained from 105 participating pediatricians (4.5 patients/physician) and 443 patients were included (Fig. 1). The mean age of included children was 10.8 months, with 49% of males and 49% attending day-care centers. Included children had a past-history of prematurity in 9%, severe infection in 4%, chronic disease in 7%, and multidisciplinary approach in 4%. Mothers had a high school education level for 66%, were not working for 14% and were isolated parent for 3%. One of the parents had a chronic disease in 21%. A family history of vaccine preventable disease was reported by 12% of the parents; a history of vaccine adverse event by 3%.
Figure 1.
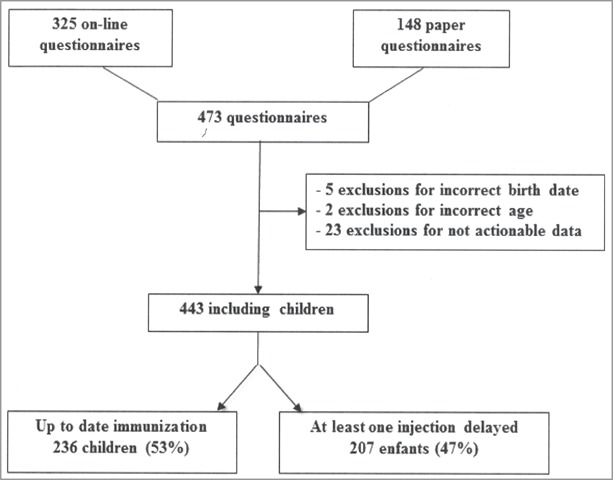
Flow chart of screened children 2–24 months of age, followed by primary-care pediatricians for the presence or not of a potentially dangerous vaccination delay.
Among the 70 pediatricians that responded to their own questionnaire, 81% were women and 24% had a subspecialty. They were 50 years of age or older for 64% and 50% had a work experience in primary care for more than 20 years. They had a median number of 83 visits per week.
Immunization coverage and potentially dangerous immunization delays
Immunization coverage rates for each dose of each vaccine are presented in Fig. 2. More than 90% of children had PCV doses and diphtheria-tetanus-acellular pertussis-poliomyelitis-Haemophilus influenza b ± Hepatitis B (DTaP-IPV-Hib ± VHB) vaccine doses. Only 77 to 81% had had Measles-Mumps-Rubella (MMR) vaccine doses, and 66% the meningococcal C (MenC) vaccine.
Figure 2.
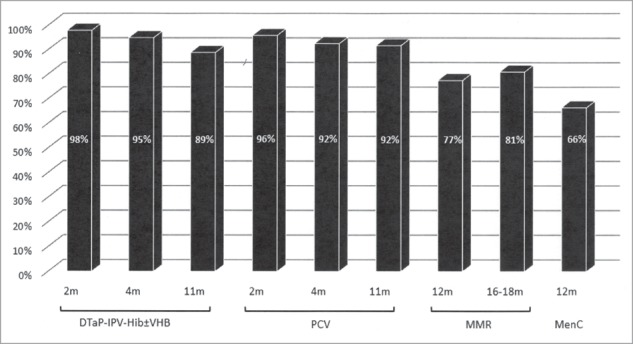
Immunization coverage of children 2–24 months of age, followed by primary-care pediatricians DTaP-IPV-Hib ± VHB: Diphtheria-Tetanus-acellular Pertussis-Inactivated Polio Vaccine-Haemophilus influenzae b ± Hepatitis B Vaccine; PCV: Pneumococcal Conjugate Vaccine; MMR: Measles-Mumps-Rubella vaccine; MenC: Meningococcal C vaccine.
For DTaP-IPV-Hib ± VHB, at least one potentially dangerous immunization delay was identified for 36% of children. Details per vaccine dose are provided in Table 2. For the PCV, at least one potentially dangerous immunization delay was identified in 34% of children. For MMR and the MenC vaccine at 12 months, 31% and 57% of children respectively presented potentially dangerous immunization delay. In all, 47% of children had at least one potentially dangerous immunization delay, 41% at least two and 15% at least four potentially dangerous immunization delays.
Table 2.
Frequency and importance of delayed immunizations of children 2–24 months followed by primary-care pediatricians.
| Potentially dangerous delayed immunization* |
Potentially dangerous delays (days) |
||||||
|---|---|---|---|---|---|---|---|
| Vaccination dose | n/N vaccinated | % | 95%CI | Means | Median | Min | Max |
| 2 m DTaP-IPV-Hib ± VHB | 65/441 | 14.7% | 11.7–18.4 | 30 | 14 | 1 | 347 |
| 4 m DTaP-IPV-Hib ± VHB | 98/386 | 25.4% | 21.3–30.0 | 29 | 16 | 1 | 413 |
| 11m DTaP-IPV-Hib ± VHB | 58/209 | 27.8% | 22.1–34.2 | 98 | 91 | 8 | 350 |
| 2 m PCV | 81/440 | 18.4% | 15.1–22.3 | 44 | 16 | 1 | 537 |
| 4 m PCV | 105/384 | 27.3% | 23.1–32.0 | 40 | 20 | 1 | 477 |
| 11 m PCV | 27/209 | 12.9% | 9.0–18.1 | 83 | 62 | 6 | 350 |
| 12 m MMR** | 54/172 | 31.4% | 24.9–38.7 | 42 | 24 | 1 | 216 |
| 12 m MenC | 97/170 | 57.1% | 50.0–64.3 | 90 | 60 | 1 | 350 |
DTaP-IPV-Hib ± VHB: diphtheria-tetanus-acellular pertussis-inactivated polio vaccine-Haemophilus influenzae b ± hepatitis B vaccine; PCV: pneumococcal conjugate vaccine; MMR: Measles-Mumps-Rubella vaccine; MenC: meningococcal C vaccine.
As defined in Table 1.
The potential damaging delay of the second dose of MMR could not be estimated because only children less than 2 years of age have been included.
Reasons and risk factors for delayed immunization
Reasons for potentially dangerous immunization delay could be identified for 66% of cases. It was due to personal organizational issues in 24%, related to the child in 14% (acute illness or chronic disease decompensating, prematurity), related to the parents in 11% and related to recent modifications of immunization schedule in 6%. Other reasons were described in 10%: forgetting, domino effect, initial vaccination following foreign vaccination schedule, follow-up by another physician, breast-feeding.
The univariate analysis of risk factors according to the age of vaccination is presented in the Table 3. Globally, several factors were associated with a potentially dangerous immunization delay for any dose of vaccine: the child's increasing age, as continuous variable (8.2 ± 4.8 months vs. 13.8 ± 6.4 months, p < 10−6); for dichotomous variables, a child medical history of severe infection (OR = 3.9; 95% CI, 1.2–14.5; p = 0.01) and a child attending day-care center (OR = 1.5; 95% CI, 1.0–2.2; p = 0.03), were associated with a risk of a potentially dangerous immunization delay. A working mother was a variable associated with a higher risk of potentially dangerous immunization delay (OR = 2.0; 95% CI, 1.1–3.3; p = 0.01). Seven variables were introduced in the multivariable analysis. Two of them (i.e., the child's increasing age and a working mother) were significantly and independently associated with the risk of potentially damaging immunization delay (Table 4). None of the pediatrician-related factors analyzed were significantly associated with an immunization delay.
Table 3.
Univariate analysis of risk factors of potentially damaging immunization delay of children 2–24 months followed by primary-care pediatricians.
| 2 m vaccines (n = 440) | 4m vaccines (n = 384) | 11 m vaccines (n = 209) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (PCV + DTaP-IPV Hib ± VHB) |
(PCV + DTaP-IPV-Hib ± VHB) |
(PCV + DTaP-IPV-Hib ± VHB) |
12 m vaccines (n = 170)(1st MMR + MenC) |
Global delay |
|||||||||||
| Variables | OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p |
| Children's characteristics | |||||||||||||||
| Male | 1.0 | 0.6–1.6 | 0.83 | 1.1 | 0.7–1.7 | 0.84 | 0.9 | 0.5–1.7 | 0.70 | 0.9 | 0.5–1.7 | 0.72 | 0.8 | 0.5–1.1 | 0.16 |
| Rank in the sibling ≥3 | 1.7 | 0.9–3.3 | 0.09 | 1.3 | 0.7–2.6 | 0.34 | 1.3 | 0.5–3.2 | 0.54 | 0.9 | 0.3–2.4 | 0.87 | 1.4 | 0.8–2.2 | 0.20 |
| Child attending day-care center | 0.7 | 0.4–1.2 | 0.14 | 0.7 | 0.4–1.1 | 0.10 | 1.2 | 0.6–2.5 | 0.55 | 1.6 | 0.8–3.2 | 0.16 | 1.5 | 1.0–2.2 | 0.03 |
| Prematurity < 37SA | 1.0 | 0.4–2.6 | 0.95 | 1.3 | 0.6–3.0 | 0.49 | 1.5 | 0.4–5.4 | 0.34 | 1.7 | 0.4–6.9 | 0.40 | 1.3 | 0.6–2.7 | 0.44 |
| PMH of severe infection | 1.9 | 0.6–6.1 | 0.23 | 2.1 | 0.7–6.3 | 0.15 | 5.1 | 1.1–26.9 | 0.02 | 4.6 | 0.5–104 | 0.13 | 3.9 | 1.2–14.5 | 0.01 |
| Chronic disease | 1.3 | 0.5–3.2 | 0.61 | 1.4 | 0.6–3.2 | 0.42 | 1.4 | 0.5–4.1 | 0.50 | 0.8 | 0.2–3.0 | 0.75 | 1.3 | 0.6–2.9 | 0.45 |
| Multidisciplinary management | 1.2 | 0.3–4.8 | 0.50 | 2.3 | 0.7–8.0 | 0.13 | 4.1 | 0.7–25.0 | 0.07 | 5.1 | 0.6–118 | 0.10 | 1.6 | 0.5–5.4 | 0.40 |
| Familiy's characteristics | |||||||||||||||
| Study of mother: higher education | 1.0 | 0.6–1.7 | 0.85 | 0.8 | 0.5–1.3 | 0.28 | 1.3 | 0.6–2.7 | 0.42 | 1.0 | 0.5–2.1 | 0.94 | 1.0 | 0.7–1.5 | 0.99 |
| Working mother | 1.5 | 0.8–3.0 | 0.19 | 2.2 | 1.2–4.2 | 0.008 | 1.7 | 0.6–4.8 | 0.24 | 2.0 | 0.7–10.0 | 0.14 | 2.0 | 1.1–3.3 | 0.01 |
| Isolated parents | 2.9 | 0.8–10.1 | 0.06 | 2.6 | 0.6–10.8 | 0.12 | 1.2 | 0.0–17.1 | 0.65 | 0.7 | 0.0–27.3 | 0.66 | 1.9 | 0.5–6.7 | 0.28 |
| Chronic disease of one parent | 0.6 | 0.3–1.2 | 0.12 | 0.5 | 0.3–1.0 | 0.03 | 0.9 | 0.4–2.0 | 0.73 | 0.7 | 0.3–1.5 | 0.32 | 0.7 | 0.4–1.2 | 0.13 |
| Vaccine related adverse event* | 0.7 | 0.1–3.6 | 0.50 | 0.6 | 0.1–2.9 | 0.36 | 7.3 | 0.7–189 | 0.08 | / | / | 0.19 | 0.9 | 0.3–2.8 | 0.77 |
| PMH of VPD* | 1.0 | 0.4–2.1 | 0.93 | 1.1 | 0.5–2.2 | 0.81 | 1.3 | 0.5–3.4 | 0.56 | 3.8 | 1.0–17.7 | 0.06 | 1.0 | 0.6–1.9 | 0.93 |
DTaP-IPV-Hib ± VHB-PCV: diphtheria-tetanus-acellular pertussis-inactivated polio vaccine-Haemophilus influenzae b ± hepatitis B vaccine-pneumococcal conjugate vaccine; MMR: Measles-Mumps-Rubella vaccine; MenC: meningococcal C vaccine; PMH: past medical history; VPD: vaccine preventable disease;
related to siblings, father, mother and great-parents.
Table 4.
Multivariable analysis of risk factors of potentially damaging immunization delay of children 2–24 months followed by primary-care pediatricians.
| Variables | Adjusted OR | 95%CI | P |
|---|---|---|---|
| Male sex | 0.8 | 0.5–1.3 | 0.39 |
| Rank in the sibling ≥3 | 1.2 | 0.9–1.5 | 0.26 |
| Child attending day-care center | 1.0 | 0.6–1.7 | 0.96 |
| PMH of severe infection | 2.6 | 0.8–9.3 | 0.13 |
| Working mother | 2.4 | 1.2–4.7 | 0.01 |
| Chronic disease of one parent | 0.6 | 0.3–1.0 | 0.05 |
| Child age | 1.2 | 1.1–1.3 | <10−3 |
Discussion
This national survey performed with the national-AFPA has shown that immunization coverage of children 2–24 months of age followed by primary care pediatricians was satisfactory. Immunization coverage rates estimated in our study at the time of the visit (and not at 2 years of age) matched with published national data.10-12 It showed still insufficient coverage rates for MMR and MenC vaccines regarding the WHO objectives.1 Despite a satisfactory immunization coverage rates at 2, 4 or 11 months of age (> 89%), a potentially dangerous immunization delay as previously defined was observed in an important number of cases: 13 to 58% of vaccine doses according to vaccine type. Overall, 47% of children presented at least one potentially dangerous immunization delay before the age of 24 months, with longer delay for the vaccines most recently introduced such as the MenC.
The importance of immunization delay in childhood has been highlighted in the literature worldwide in the past. In a wide study performed in the United States, 80% of children aged 25 to 72 months were up-to-date for the studied vaccines, but only 33% had no delay.13 In the study of Luman et al, only 9% of children received all recommended vaccines at the recommended ages.14 Other studies showed also significant immunization delays in Iran (42% to 67.6% of infants received vaccine with delay),15 in Greece (administration of booster doses delayed in 33.7–97.4% of children at 60 months of age),16 in 45 low-income and middle-income countries17 and in Salvador.18 Importantly, time limit of immunization delay was not defined in most of these studies19,20 or defined by a similar time-limit for all vaccine doses3,21-24 without considering the epidemiology of the diseases. In our study, immunization delays were based on the definition of a potentially dangerous immunization delay obtained by a Delphi method taking into account epidemiologic data for each disease and age for each injection.9 Therefore, our study provides more precise data compared to previous studies.
At 2 and 4 months of age, the mean period of delay was 30 to 44 days after the defined deadline. Thus, first doses were done between 3 months and 3.5 months. Consequently, these children were exposed to vaccine preventable diseases like pertussis (peak of incidence at 2 months of age) or pneumococcal related diseases (peak of pneumococcal meningitis incidence at 4 months of age). The timeliness was even much longer at 11 and 12 months (i.e., between 1.5 and 3 months), leading to a prolonged exposure to concerned diseases. Direct consequences have been highlighted still recently.7,8,25
Identification of risk factors for immunization delays is necessary to guide policies of decision makers. First, regarding all vaccine doses, two risk factors were identified. The strong association between child's age and vaccination delay observed in this study, with all vaccines except MMR vaccine, is consistent with previous findings.16 The more the child is old, the more he is at risk of delay probably due to less watchfulness. Contrary to other reports, having a working mother was significantly associated with more delay in vaccines administration.20,26 In another study, maternal work was also associated with a significantly lower likelihood of on-time vaccination; this likelihood was significantly higher when families were not receiving welfare.27 In our study it was not possible to associate economic status and work. It was also not possible to distinguish true immunization delays from vaccination refusal, even if refusal is rare. However vaccine hesitancy may be associated with immunization delays, which could not be evaluated here. Second, we identified risk factors at 2, 4, 11 and 12 months of age (vaccine appointment ages). None of the pediatrician-related factors analyzed were significantly associated with an immunization delay.
The present study may have some limitations. We would have expected a greater number of participants. But the investment required has probably limited the participation. A selection bias was possible by the participation of pediatricians with greater awareness about immunization that may lead to underestimation of the immunization delay. It was conducted among primary-care pediatricians and was, therefore, not representative of the whole country and particularly of the general practitioners practice. Missing data and non-participation were below 0.5%, which induced a limited bias. We could not evaluate delayed immunization for second injection of MMR vaccine, which delays concerned children older than 24 months. Reasons for the vaccine delays were available for only 66% of cases, which is good for this memorization work. Based on the ability of parents and pediatricians to remember the cause of the delay, a memorization bias is however possible. Finally in case of non-vaccination, immunization delay was calculated on the basis of the day of the study and we did not distinguish vaccine refusal and immunization delay. Strengths of this study should also be noted. This was the first national study in France, which evaluated immunization delays in children 2 to 24 months followed by primary-care pediatricians. To improve accuracy of information and validity of findings, healthcare provider records were used to control the vaccination histories.
The influence of immunization delay appears to play an increasingly important role in persistence of vaccine preventable diseases. Spreading the concept of immunization delay should be a major focus of vaccine preventable diseases reduction policy. All infants are concerned but particularly those with risk of severe vaccine preventable diseases such as very high premature babies20 or children with chronic diseases.28 A high level of vigilance should be given to those patients but also to patients sharing the risk factors of immunization delay identified here. Finally, in case of lawsuits related to vaccine preventable diseases, the existence of potentially damaging immunization delay could become one day a crucial issue.
In conclusion, the results of this study indicate that although vaccination coverage of the infancy period was nearly complete, considerable number of infants received vaccines with potentially dangerous delays. Longer delays were identified for the most recent vaccines such as the MenC.
Methods
Study design and inclusion criteria
This was a prospective national survey conducted in June 2014 in children aged 2 to 24 months followed by primary-care pediatricians, members of the Association Française de Pédiatrie Ambulatoire (AFPA, association of the French primary care pediatricians). This was a one-day survey chosen by each pediatrician within a predefined week. Each pediatrician had to include five consecutive male or female children, 2 to 24 months of age, seen at the outpatient visit. Patients were excluded from the study if health-book was absent or in case of parental refusal. Approval by the ethics committees was not needed for such anonymous observational survey. Only an information sheet was provided and non-opposition was obtained from the parents of each included infant as recommended at that time.
Objectives and definitions
The primary endpoint of this study was to quantify the frequency of potentially dangerous vaccination delay overall and for each vaccine dose recommended before the age of 2 years. The secondary endpoints were: (i) to assess vaccination coverage at the time of the visit for each dose of each vaccine, (ii) to identify reasons for immunization delay related to the patient or to the vaccinator and (iii) to identify variables associated with immunization delay. The National French Immunization Schedule, published in May 2014, was used as the reference. A potentially dangerous immunization delay was defined by a real age of vaccination over the age at risk as identified in a previous Delphi study (Table 1).9 Vaccination delays, presented Table 2, were calculated in days between the age at risk9 and the real age of vaccination.
Table 1.
Recommended age for immunization and definitions of potentially dangerous vaccination delays according to expert advices obtained through a Delphi process.9
| Vaccination dose | Recommended age for routine immunization | Potentially dangerous vaccination delay |
|---|---|---|
| DTaP-IPV-Hib ± VHB | 2 months | > 15 days |
| 4 months | > 15 days | |
| 11 months | > 2 months | |
| PCV | 2 months | > 15 days |
| 4 months | > 15 days | |
| 11 months | > 2 months | |
| MMR | 12 months | > 1 month |
| 18 months | > 6 months | |
| MenC | 12 months | > 1 month |
DTaP-IPV-Hib ± VHB: diphtheria-tetanus-acellular pertussis-inactivated polio vaccine-Haemophilus influenzae b ± hepatitis B vaccine; PCV: pneumococcal conjugate vaccine; MMR: Measles-Mumps-Rubella vaccine; MenC: meningococcal C vaccine.
Data collection
Each pediatrician had to include five children to avoid an overrepresentation of some pediatricians with a greater activity. For each child, a standardized on-line questionnaire was filled at convenience either on “SurveyMonkey” website or on a paper version available on the AFPA website. The first part of the questionnaire was about vaccination data: specific dates of vaccine administrations (based on written record in the health-book) were collected and potentially dangerous vaccination delays were identified, if applicable. An easy-to-use excel program was available on the AFPA website to calculate, for each child, vaccination delays according to his birth date. In case of potentially dangerous vaccination delay, pediatricians had to look for the reasons of the delay through a pre-established questionnaire, with yes/no answers and could add an extra-reason. In a second part, data on demographic (sex, siblings, mother's age, maternal education level) and medical characteristics (underlying conditions and treatments) were collected through interviews. At the end of the day, each pediatrician completed an on-line questionnaire about his own characteristics (gender, age, pediatric subspecialty, source of information about vaccines, number of years of private practice, and average number of visiting patients per week).
Expected number of patients
At the time of study design, AFPA was counting 1500 members. Because of the type of research (survey) and the need for an active participation with several files to complete, between 5 and 10% of participants were expected. With five children that should be included by each pediatrician, 375 to 750 responses were expected.
Statistical methods
Estimates of coverage rates, potentially dangerous immunization delay rates, odds ratios (ORs), and 95% confidence intervals (CIs) were calculated using the Epi-Info 6.04fr statistical software (Centers for Disease Control and Prevention, Atlanta, Ga). Duration of vaccination delays during the period of infancy was assessed using Kaplan-Meier hazard function; means, medians and standard deviations were evaluated for each vaccine. In order to examine associations of potential determinants with a complete and age-appropriate vaccination we applied Mann-Whitney test for continuous variables and Chi-2 or Fisher-exact tests where appropriate for dichotomous variables. A stepwise approach was used for the multivariable logistic regression with the inclusion of variables with a p value ≤ 0.20 in this univariate analysis.
Disclosure of potential conflicts of interest
ACB has had her “advanced pediatric life support” courses paid by Guigoz; PG and JFL have no conflict of interest; JCR has been invited to the 2017 ESPID meeting by Sanofi-Pasteur; FVlS has been investigator for phase III or IV studies for vaccine companies and has been invited for congresses, symposium, workshops, lecture and consultancy/advice (GSK, Sanofi-Pasteur MSD, MSD, Pfizer); AM has had appointments for lecture and consultancy/advice (GSK vaccines, Pfizer), and invitations to ESPID meetings (GSK vaccines, Pfizer); FD has been invited to the 2014 ESPID meeting and for a lecture without fees at the national pediatric primary-care meeting (AFPA) 2017 by GSK vaccines. He has received fees from Biocodex for a lecture at the 2017 pediatric gastroenterology national meeting (GFHGPN).
References
- 1.Bustreo F, Okwo-Bele JM, Kamara L. World Health Organization perspectives on the contribution of the global alliance for vaccines and immunization on reducing child mortality. Arch Dis Child. 2015;100(Suppl 1):S34–7. doi: 10.1136/archdischild-2013-305693. PMID:25613965. [DOI] [PubMed] [Google Scholar]
- 2.Buttery JP, Graham SM. Immunisation timing: the protective layer in vaccine coverage. Lancet. 2009;373(9674):1499–500. doi: 10.1016/S0140-6736(09)60340-8. PMID:19303632. [DOI] [PubMed] [Google Scholar]
- 3.Grant CC, Roberts M, Scragg R, Stewart J, Lennon D, Kivell D, Ford R, Menzies R. Delayed immunisation and risk of pertussis in infants: unmatched case-control study. BMJ. 2003;326(7394):852–3. doi: 10.1136/bmj.326.7394.852. PMID:12702617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akmatov MK, Mikolajczyk RT. Timeliness of childhood vaccinations in 31 low and middle-income countries. J Epidemiol Community Health. 2012;66(7):e14. doi: 10.1136/jech.2010.124651. PMID:21551179. [DOI] [PubMed] [Google Scholar]
- 5.Guerra FA. Delays in immunization have potentially serious health consequences. Paediatr Drugs. 2007;9(3):143–8. doi: 10.2165/00148581-200709030-00002. PMID:17523694. [DOI] [PubMed] [Google Scholar]
- 6.Strine TW, Luman ET, Okoro CA, McCauley MM, Barker LE. Predictors of age-appropriate receipt of DTaP dose 4. Am J Prev Med. 2003;25(1):45–9. doi: 10.1016/S0749-3797(03)00093-X. PMID:12818309. [DOI] [PubMed] [Google Scholar]
- 7.Winter K, Glaser C, Watt J, Harriman K. Centers for Disease Control and Prevention (CDC). Pertussis epidemic–California, 2014. MMWR Morb Mortal Wkly Rep. 2014;63(48):1129–32. PMID:25474033. [PMC free article] [PubMed] [Google Scholar]
- 8.Curran D, Terlinden A, Poirrier JE, Masseria C, Krishnarajah G. Vaccine timeliness: a cost analysis of the potential implications of delayed pertussis vaccination in the US. Pediatr Infect Dis J. 2016;35(5):542–7. doi: 10.1097/INF.0000000000001071. PMID:26835971. [DOI] [PubMed] [Google Scholar]
- 9.Gras P, Bailly AC, Lagree M, Dervaux B, Martinot A, Dubos F. What timing of vaccination is potentially dangerous for children younger than 2 years? Hum Vaccin Immunother. 2016;12(8):2046–52. doi: 10.1080/21645515.2016.1157239. PMID:27215704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Institut de veille sanitaire (France ). Vaccinal data by age group, diphteria-tetanus, poliomyelitis, pertussis. Available at: http://invs.santepubliquefrance.fr/Dossiers-thematiques/Maladies-infectieuses/Maladies-a-prevention-vaccinale/Couverture-vaccinale/Donnees/Diphterie-tetanos-poliomyelite-coqueluche [Accessed 2017August10]
- 11.Institut de veille sanitaire (France ). Vaccinal data by age group, pneumococcus. Available at:http://invs.santepubliquefrance.fr/Dossiers-thematiques/Maladies-infectieuses/Maladies-a-prevention-vaccinale/Couverture-vaccinale/Donnees/Pneumocoque [Accessed 2017August10]
- 12.Institut de veille sanitaire (France ). Vaccinal data by age group, meningococcus C. Available at:http://www.invs.sante.fr/Dossiers-thematiques/Maladies-infectieuses/Maladies-a-prevention-vaccinale/Couverture-vaccinale/Donnees/Meningocoque-C [Accessed 2017August10]
- 13.Dombkowski KJ, Lantz PM, Freed GL. The need for surveillance of delay in age-appropriate immunization. Am J Prev Med. 2002;23(1):36–42. doi: 10.1016/S0749-3797(02)00442-7. PMID:12093421. [DOI] [PubMed] [Google Scholar]
- 14.Luman ET, McCauley MM, Stokley S, Chu SY, Pickering LK. Timeliness of childhood immunizations. Pediatrics. 2002;110(5):935–9. doi: 10.1542/peds.110.5.935. PMID:12415033. [DOI] [PubMed] [Google Scholar]
- 15.Poorolajal J, Khazaei S, Kousehlou Z, Bathaei S, Zahiri A. Delayed Vaccination and Related Predictors among Infants. Iran J Public Health. 2012;41(10):65–71. PMID:23304664. [PMC free article] [PubMed] [Google Scholar]
- 16.Pavlopoulou ID, Michail KA, Samoli E, Tsiftis G, Tsoumakas K. Immunization coverage and predictive factors for complete and age-appropriate vaccination among preschoolers in Athens, Geece: a cross–sectional study. BMC Public Health. 2013;13:908. doi: 10.1186/1471-2458-13-908. PMID:24083352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark A, Sanderson C. Timing of children's vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet. 2009;373(9674):1543–9. doi: 10.1016/S0140-6736(09)60317-2. PMID:19303633. [DOI] [PubMed] [Google Scholar]
- 18.Suárez-Castaneda E, Pezzoli L, Elas M, Baltrons R, Crespin-Elías EO, Pleitez OA, de Campos MI, Danovaro-Holliday MC. Routine childhood vaccination programme coverage, El Salvador, 2011-In search of timeliness. Vaccine. 2014;32(4):437–44. doi: 10.1016/j.vaccine.2013.11.072. PMID:24315884. [DOI] [PubMed] [Google Scholar]
- 19.Lernout T, Theeten H, Hens N, Braeckman T, Roelants M, Hoppenbrouwers K, Van Damme P. Timeliness of infant vaccination and factors related with delay in Flanders, Belgium. Vaccine. 2014;32(2):284–9. doi: 10.1016/j.vaccine.2013.10.084. PMID:24252698. [DOI] [PubMed] [Google Scholar]
- 20.Tozzi AE, Piga S, Corchia C, Di Lallo D, Carnielli V, Chiandotto V, Fertz MC, Miniaci S, Rusconi F, Cuttini M. Timeliness of routine immunization in a population-based Italian cohort of very preterm infants: results of the action follow-up project. Vaccine. 2014;32(7):793–9. doi: 10.1016/j.vaccine.2013.12.044. PMID:24397902. [DOI] [PubMed] [Google Scholar]
- 21.Luman ET, Chu SY. When and why children fall behind with vaccinations: missed visits and missed opportunities at milestone ages. Am J Prev Med. 2009;36(2):105–11. doi: 10.1016/j.amepre.2008.09.035. PMID:19062241. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka M, Vitek CR, Pascual FB, Bisgard KM, Tate JE, Murphy TV. Trends in pertussis among infants in the United States, 1980-1999. JAMA. 2003;290(22):2968–75. doi: 10.1001/jama.290.22.2968. PMID:14665658. [DOI] [PubMed] [Google Scholar]
- 23.Akmatov MK, Kretzschmar M, Krämer A, Mikolajczyk RT. Timeliness of vaccination and its effects on fraction of vaccinated population. Vaccine. 2008;26(31):3805–11. doi: 10.1016/j.vaccine.2008.05.031. PMID:18565626. [DOI] [PubMed] [Google Scholar]
- 24.Yadav K, Srivastava R, Kumar R, Chinnakal P, Rai SK, Krishnan A. Significant vaccination delay can occur even in a community with very high vaccination coverage: evidence from Ballabgarh, India. J Trop Pediatr. 2012;58(2):133–8. doi: 10.1093/tropej/fmr059. PMID:21742766. [DOI] [PubMed] [Google Scholar]
- 25.Robison SG, Liko J. The timing of pertussis cases in unvaccinated children in an outbreak year: Oregon 2012. J Pediatr. 2017;183:159–63 doi: 10.1016/j.jpeds.2016.12.047. PMID:28088399. [DOI] [PubMed] [Google Scholar]
- 26.Odutola A, Afolabi MO, Ogundare EO, Lowe-Jallow YN, Worwui A, Okebe J, Ota MO. Risk factors for delay in age-appropriate vaccinations among Gambian children. BMC Health Serv Res. 2015;15:346. doi: 10.1186/s12913-015-1015-9. PMID:26315547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sohn MW, Yoo J, Oh EH, Amsden LB, Holl JL. Welfare, maternal work, and on-time childhood vaccination rates. Pediatrics. 2011;128(6):1109–16 doi: 10.1542/peds.2011-0931. PMID:22123896. [DOI] [PubMed] [Google Scholar]
- 28.Pandolfi E, Carloni E, Marino MG, Ciofi degli Atti ML, Gesualdo F, Romano M, Giannattasio A, Guarino A, Carloni R, Borgia P, et al.. Immunization coverage and timeliness of vaccination in Italian children with chronic diseases. Vaccine. 2012;30(34):5172–8. doi: 10.1016/j.vaccine.2011.02.099. PMID:21414380. [DOI] [PubMed] [Google Scholar]


