ABSTRACT
The present study is to measure the expression of programmed death (PD)-1 / programmed death ligand-1 (PD-L1) negative costimulatory molecules, soluble format sPD-1 in patients with immune thrombocytopenia (ITP), and to investigate their correlation with the secretion of cytokines. A total of 35 patients with ITP were included in the present study. Twenty healthy subjects who received physical examination at our hospital were included as control group. Peripheral blood was collected from all ITP patients and healthy subjects. Flow cytometry was performed to determine the percentages of PD-1+CD4+T cells and PD-L1+DCs in ITP patients and healthy subjects. Enzyme-linked immunosorbent assay was performed to measure the concentrations of interferon (IFN)-γ, interleukin (IL)-17 and sPD-1 in peripheral blood from ITP patients and healthy subjects. Percentages of PD-1+CD4+T cells and PD-L1+DCs in peripheral blood from ITP patients before treatment were significantly higher than that from healthy subjects, but were not different from those after treatment. Serum concentrations of IFN-γ, IL-17 and sPD-1 in ITP patients before treatment were significantly higher than those in healthy subjects, and these concentrations were significantly reduced after treatment. The concentration of sPD-1 was positively correlated with the concentration of IFN-γ, and negatively correlated with platelet count. Percentages of PD-1+CD4+T cells and PD-L1+DCs in ITP patients are higher than those in healthy subjects, but elevated sPD-1 concentration in the blood blocks PD-1/PD-L1 signaling pathway, leading to unaffected Th cell function. Elevated concentrations of IFN-γ and IL-17 in the blood may participate in the occurrence and development of ITP.
KEYWORDS: cytokine, primary immune thrombocytopenia, programmed death-1, programmed death-ligand 1, soluble programmed death-1
Introduction
Primary immune thrombocytopenia (ITP) is a kind of disease that is mainly characterized by humoral and cellular immune-mediated platelet destruction, and patients with ITP lose immune tolerance to platelet antigens.1 Overactivation of Th1 and Th17 cells is observed in ITP patients, and cytokines secretion by Th1 or Th17 cells are also increased.2,3 Th1 cells can secrete interferon (IFN)-γ, which mediates cytotoxicity responses by promoting the activation and proliferation of cytotoxic lymphocytes (CTL), natural killer (NK) cells or macrophages. Th17 cells mainly secrete interleukin (IL)-17, which stimulates the production of inflammatory mediators and chemokines by various types of cells, accumulates neutrophils at inflammatory sites, and mediates inflammatory reactions. Th17 cells also participate in the occurrence and development of autoimmune diseases, such as psoriasis, rheumatoid arthritis and multiple system sclerosis.4-6
Programmed death (PD)-1 belongs to CD28/B7 family and is an important negative regulator that is mainly expressed on the surface of activated T cells, B cells, monocytes, NK cells, dendritic cells (DCs) and Treg cells. PD-1 has 2 ligands, programmed death ligand-1 (PD-L1) and programmed death ligand-2 (PD-L2). PD-L1 is widely expressed on activated DCs, Langerhans cells, islet cells and endothelial cells and plays important roles in inhibiting inflammation and preventing autoimmune diseases, while PD-L2 is mainly on macrophages, DCs or some tumor cells.7-10 After PD-1 binds with PD-L1, tyrosine in immunoreceptor tyrosine-based switch motif region is phosphorylated, and protein tyrosine phosphatase is recruited to phosphorylate downstream effector cells, playing negative regulation effect. It is reported that PD-1/PD-L1 signaling pathway plays negative regulation roles in autoimmune diseases such as glomerular nephritis, autoimmune encephalomyelitis, psoriatic arthritis, and Sjogren's syndrome.11-15 In addition, activation of PD-1/PD-L1 signaling pathway in rheumatoid arthritis patients effectively inhibits the proliferation of Th1 and Th17 cells and the secretion of IFN-γ and IL-17 cytokines.16
sPD-1 is the soluble form of PD-1 that is obtained by phosphoric acid hydrolysis of membrane type PD-1. Studies show that sPD-1 level is elevated in scleroderma and rheumatoid arthritis patients.16,17 Furthermore, in vitro experiments show that PD-1Fc promotes the proliferation of Th1 and Th17 cells, which is related with joint damage of mice with collagen-induced arthritis. Therefore, sPD-1 is believed to block PD-1/PD-L1 signaling pathway. However, there are few studies on the role of PD-1/PD-L1 negative costimulatory molecules and soluble sPD-1 in the pathogenesis of ITP by now. In the present study, we determine the expression of PD-1/PD-L1 negative costimulatory molecules and sPD-1 in ITP patients before and after treatments, and investigate how their expression is related with the secretion of cytokines by Th1 and Th17 cells.
Materials and methods
Patients
A total of 35 patients with newly diagnosed ITP who received treatments at Department of Hematology at The First Affiliated Hospital of Xinjiang Medical University between January 2015 and June 2016 were included in the present study. All patients were diagnosed to have ITP according to the widely used diagnostic standards in China.18 The white blood cell counts of 33 patients were 4 – 10 × 109/L, that of one patient was lower than 4 × 109/L, and that of one patients was higher than 10 × 109/L (average white blood cell count for all patients was 6.2 ± 1.9 × 109/L). The lymphocyte counts of 5 patients were lower than 1 × 109/L, that of 20 patients were 1 – 2 × 109/L, that of 9 patients were 2 – 3 × 109/L, and that of 1 patient was 3 – 4 × 109/L (average white lymphocyte count for all patients was 1.6 ± 0.7 × 109/L). None of the patients received platelet transfusion and splenectomy, or treatment with corticosteroids and gamma globulin within one month before the study. Twenty healthy subjects who received physical examination at our hospital were included as control group (Table 1). Healthy control subjects received no treatment, while patients with platelet count < 30 × 109/L were given intravenous infusion with 40 mg dexamethasone for 4 d after blood collection. On the first day after treatment, blood routine examination was performed, and hormone therapy was considered effective only if platelet count was equal to or greater than 30 × 109/L and at least 2 times higher than baseline platelet count.19 Among the 35 ITP patients, 29 patients (platelet count < 30 × 109/L) were given dexamethasone shock therapy, and all treatments were effective. After treatment, the platelet count in 15 patients was in the range of 30 – 100 × 109/L, with a mean value of 70.2 ± 17 × 109/L. In addition, the platelet count in the remaining 14 patients was higher than 100 × 109/L, with a mean value of 168 ± 71 × 109/L. Before treatment and on the day after treatment, peripheral blood (5 ml) was collected from all ITP patients. Peripheral blood (5 ml) was also collected from all healthy control subjects. All procedures were approved by the Ethics Committee of Xinjiang Medical University. Written informed consents were obtained from all patients or their families.
Table 1.
General information of healthy subjects and patients.
| Healthy subjects | ITP patients | P | |||
|---|---|---|---|---|---|
| Total No. | 20 | 35 | |||
| Median age (year) | 41 | 42 | P > 0.05 | ||
| Gender | P > 0.05 | ||||
| Male | 8 | 14 | |||
| Female | 12 | 21 | |||
| Platelet count (1 × 109/L) |
120 ± 20* |
Before treatment |
After treatment |
|
|
| 17.6 ± 12.2* | 105 ± 69* | ||||
| 0–10 | 0 | 12 | 0 | ||
| 10–20 | 0 | 10 | 0 | ||
| 20–30 | 0 | 7 | 0 | ||
| 30–100 | 0 | 6 | 21 | ||
| > 100 | 20 | 0 | 14 | ||
Note:
, data are expressed as means ± standard deviations.
Flow cytometry
Into a flow cytometric tube (labeled as T tube), 5 μl CD4-PERCP, PD-1-PE, and CD3-APC mouse anti-human monoclonal antibodies (BD Biosciences, Franklin Lakes, NY, USA) were added. Into another flow cytometric tube (labeled as DC tube), 5 μl lin1-FITC, HLA-DR-PE-cy7, CD11c-PERC, and PD-L1-PE mouse anti-human monoclonal antibodies (BD Biosciences, Franklin Lakes, NY, USA) were added. Anticoagulant blood (100 μl) was added into each of the 2 tubes before thorough mixing, and the 2 tubes were kept in dark at room temperature for 20 min. Then, 1,000 μl red blood cell lysis buffer (BD Biosciences, Franklin Lakes, NY, USA) was added into each tube. After thorough mixing, the tubes were placed in dark at room temperature for 10 min for lysis of red blood cells. After centrifugation at 1,000 rpm for 5 min, the supernatant was discarded. After resuspension in 2 ml phosphate-buffered saline (PBS), the samples were centrifuged again at 1,000 rpm for 5 min before discarding supernatant. Then, the cells were resuspended in 500 μl PBS before flow cytometry (AriaII; BD Biosciences, Franklin Lakes, NY, USA). SSC-FSC-CD3+-CD4+ was defined as CD4+T lymphocytes, and the percentage of PD-1+CD4+T lymphocytes in all CD4+T lymphocytes was calculated. SSC-FSC-lin1−HLA-DR+-CD11c+ was defined as CD11c+DCs, and the percentage of PD-L1+CD11c+DCs in all CD11c+DCs was determined.
Enzyme-linked immunosorbent assay (ELISA)
Peripheral blood was centrifuged at 2,500 rpm for 10 min before separating serum. Serum was subjected to ELISA using sPD-1, IFN-γ and IL-17 detection kits (eBioscience, San Diego, CA, USA) according to the manufacturer's manuals. First, 50 μl serum and standards were added into 96-well plates, and then 50 μl biotinylated antibodies were added into each well before incubation at 37°C for 1 h. After washing the ELISA plate for 3 times, 80 μl streptavidin-horseradish peroxidase were added into each well before incubation at 37°C for 30 min. After washing for 3 times, 50 μl substrate A and 50 μl substrate B were added before incubation at 37°C in dark for 10 min. Finally, 50 μl stop solution was added and absorbance was measured at 450 nm. Then, serum concentrations of sPD-1, IFN-γ and IL-17 were calculated. Negative and positive controls were also set in the 96-well plate to ensure the quality of the tests.
Statistical analysis
The results were analyzed using SPSS17.0 statistical software (IBM, Armonk, NY, USA). All data were expressed as means ± standard deviations. Differences between groups of measurement data were analyzed using independent t-test. Correlation test was performed using Pearson correlation analysis. Differences with P < 0.05 were considered statistically significant.
Results
Treatment against ITP cannot alter the percentage of PD-1+CD4+T cells or PD-L1+DCs in ITP patients
To examine the percentages of PD-1+CD4+T cells and PD-L1+DCs, flow cytometry was performed. The data showed that the percentage of PD-1+CD4+T cells in peripheral blood from ITP patients was significantly higher than that from healthy subjects (P < 0.05) (Fig. 1A and B). In addition, the percentage of PD-L1+DCs in peripheral blood from ITP patients was also significantly higher than that from healthy subjects (P < 0.05) (Fig. 1C and D). Of note, the percentages of PD-1+CD4+T cells and PD-L1+DCs after treatment were not significantly different from those before treatment, respectively (Fig. 1A–D). The results suggest that treatment against ITP cannot alter the percentage of PD-1+CD4+T cells or PD-L1+DCs in ITP patients.
Figure 1.
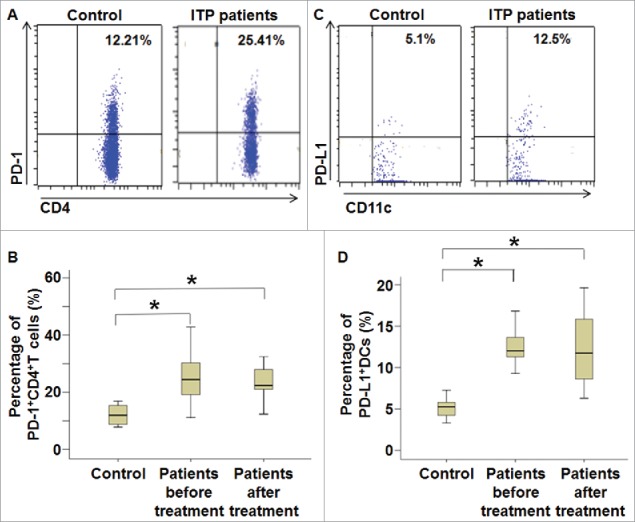
Percentages of PD-1+CD4+T cells and PD-L1+DCs in peripheral blood from ITP patients before and after treatment. Fresh anticoagulant blood was collected from ITP patients and healthy subjects, and subjected to flow cytometry. (A) Flow cytometry of CD4+T lymphocytes and (B) percentage of PD-1+CD4+T cells in all CD4+T cells. *, P < 0.05. (C) Flow cytometry of CD11c+DCs and (D) percentage of PD-L1+DCs in all CD11c+DCs. For T cell tests, 5 μl CD4-PERCP, CD3-APC and 5 μl IgG-PE were added, and coordinates of isotype control tubes were used to determine cutoff. When examining DC cells, 5 μl lin1-FITC, HLA-DR-PE-cy7, CD11c-PERC, and IgG-PE were added, and coordinates of isotype control tubes were used to determine cutoff. *, P < 0.05.
Percentage of PD-1+CD4+T cells in ITP patients is correlated with platelet count, and treatment of ITP decreases IFN-γ and IL-17 concentrations in the blood
To test how the secretion of cytokines, ELISA was used to determine the serum levels of IFN-γ and IL-17. The data showed that serum concentrations of IFN-γ and IL-17 in ITP patients before treatment were significantly higher than those in healthy subjects, respectively (P < 0.05) (Fig. 2A and B). Moreover, serum concentrations of IFN-γ and IL-17 in ITP patients after treatment were significantly lower than those in ITP patients before treatment, respectively (P < 0.05), but were not different from those in healthy subjects (Fig. 2A and B). Correlation analysis showed that the percentage of PD-1+CD4+T cells was not correlated with serum concentrations of IFN-γ or IL-17 (Fig. 2C and D), but was negatively correlated with platelet count in ITP patients before treatment (r = −0.508, P < 0.05) (Fig. 2E). The results indicate that the percentage of PD-1+CD4+T cells in ITP patients is correlated with platelet count, and treatment of ITP decreases IFN-γ and IL-17 concentrations in the blood.
Figure 2.
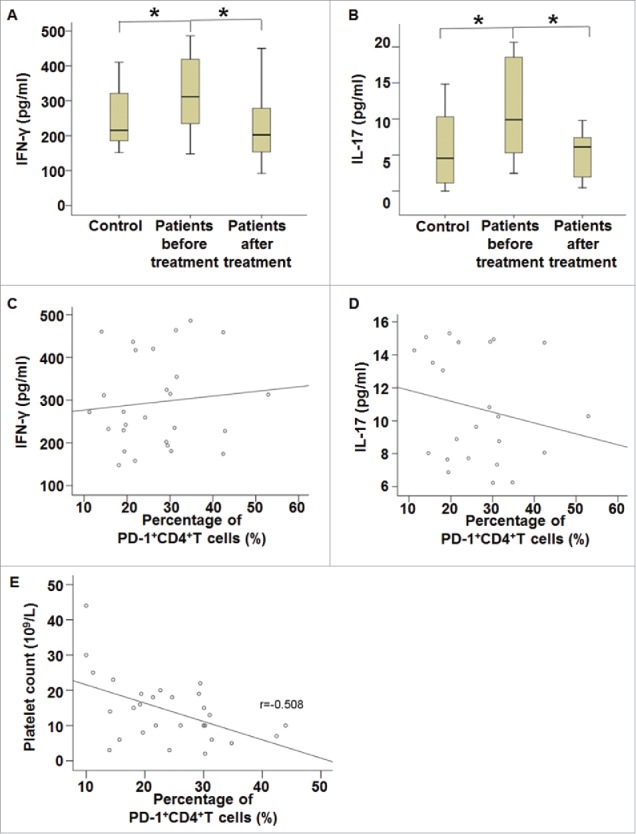
Serum concentrations of IFN-γ and IL-17 and their correlation with the percentage of PD-1+CD4+(T)cells. (A-B) Serum concentrations of (A) IFN-γ and (B) IL-17 in healthy subjects and ITP patients before and after treatment. Peripheral blood was collected from all patients and healthy subjects and subjected to ELISA assay. *, P < 0.05. (C-E) Correlation analysis of (C) IFN-γ concentration, (D) IL-17 concentration and (E) platelet count with the percentage of PD-1+CD4+T cells in ITP patients before treatment. Correlation was analyzed using Pearson correlation analysis.
Serum concentration of sPD-1 in ITP patients is correlated with IFN-γ concentration and platelet count, and treatment of ITP reduces sPD-1 concentration in the blood
To measure the secretion of sPD-1 by ITP patients, ELISA was performed. The data showed that serum concentration of sPD-1 in ITP patients before treatment was significantly higher than that in healthy subjects (P < 0.05). After treatment, serum concentration of sPD-1 in ITP patients was significantly reduced compared with that before treatment (P < 0.05), but was not different from that in healthy subjects (Fig. 3A). Correlation analysis showed that serum concentration of sPD-1 before treatment was positively correlated with serum concentration of IFN-γ (r = 0.516, P < 0.05) (Fig. 3B), but was negatively correlated with platelet count (r = −0.562, P < 0.05) (Fig. 3C). The results suggest that serum concentration of sPD-1 in ITP patients is correlated with IFN-γ concentration and platelet count, and treatment of ITP reduces sPD-1 concentration in the blood.
Figure 3.
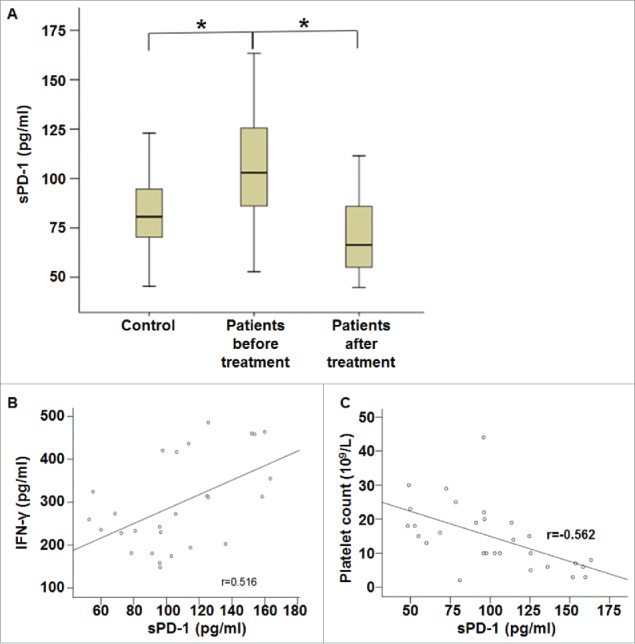
Serum concentration of sPD-1 and its correlation with IFN-γ concentration and platelet count. (A) Serum concentrations of sPD-1 in healthy subjects and ITP patients before and after treatment. Peripheral blood was collected from all patients and healthy subjects and subjected to ELISA assay. *, P < 0.05. (B-C) Correlation analysis of (B) IFN-γ concentration or (C) platelet count with serum concentration of sPD-1 in ITP patients before treatment. Correlation was analyzed using Pearson correlation analysis.
Discussion
ITP is an autoimmune disease caused by immune imbalance of the body, and its immune mechanisms are diverse and complex. Abnormal proportion of Th cell subsets and imbalance of cytokines are involved in the pathogenesis ITP. PD-1/PD-L1 signaling pathway plays an important role in the regulation of immune balance and the maintenance of peripheral immune tolerance. Activation of PD-1/PD-L1 signaling pathway inhibits the proliferation of T cells and the production of cytokines. For example, knockout of PD-1 gene aggravates the symptoms of lupus nephritis in C57BL/6 mice.20 In addition, PD-1−/− mice develop more severe collagen-induced arthritis than normal mice.21 The present study shows that the percentages of PD-1+CD4+T cells and PD-L1+DCs are enhanced in ITP patients, being consistent with previous reports.21,22
Our data also show that serum concentrations of IFN-γ and IL-17 in ITP patients are higher than healthy subjects, being consistent with previous findings.2,3 These data suggest that the secretion of cytokines that are related with Th1 and Th17 cells is increased. Similarly, Dolff et al. report that the percentage of PD-1+CD4+T cells and the level of serum IFN-γ in patients with systemic lupus erythematosus are both enhanced and positively correlated with each other, suggesting that elevated IFN-γ level may lead to the upregulation of PD-1 expression.22 Amalia et al. find that increased percentage of PD-1+CD4+T cells is positively correlated with DAS-28 disease activity score in rheumatoid arthritis patients, suggesting that sustained antigen stimulation may lead to increased expression of PD-1.21 Our data in the present study show that the percentage of PD-1+CD4+T cells is not correlated with serum levels of IFN-γ or IL-17, but further analysis show that the percentage of PD-1+CD4+T cells is negatively correlated with platelet count, suggesting that the amount of activated CD4 T cells (given that PD-1 is a marker of activation) inversely correlates with platelet counts.
The present study shows that increased percentages of PD-1+CD4+T cells and PD-L1+DCs fail to inhibit the activation and proliferation of T cells. However, the only piece of data looking at this subset of DCs looks at their percentages in healthy as well as ITP patients. There are no functional assays such as inhibition or proliferation assays that interrogate this matter directly. It is also reported that sPD-1 blocks the regulation of T cells by PD-1/PD-L1 signaling pathway. For example, the concentrations of sPD-1 in serum and synovial fluid from patients with rheumatoid arthritis are significantly higher than those in normal subjects, and are positively correlated with rheumatoid factors.17 Animal experiments show that IFN-γ mRNA and IL-17 mRNA expression in mice treated with PD-1Fc is enhanced than that before treatment, suggesting that sPD-1 blocks PD-1/PD-L1 pathway.17 Similarly, another study shows that high expression of PD-1 and PD-L1 coexists with sustained activation of autoreactive T cells, suggesting that sPD-1 suppresses functions of PD-1/PD-L1 signaling pathway.23 The present study demonstrates that serum concentration of sPD-1 in ITP patients before treatment is higher than that in healthy subjects, and positively correlated with IFN-γ level but negatively correlated with platelet count. After treatment, serum concentrations of sPD-1, IFN-γ and IL-17 are reduced compared with those before treatment, suggesting that sPD-1 is correlated with ITP disease activity. Therefore, we believe that elevated sPD-1 level in patients blocks PD-1/PD-L1 signaling pathway, leading to imbalance of immune regulation. After treatment, sPD-1 level is decreased and the regulation by PD-1/PD-L1 signaling pathway is restored, leading to regulated immune imbalance, reduced concentrations of IFN-γ and IL-17, and decreased damage of platelets. Birtas Atesoglu et al. report that serum sPD-1 levels of patients with newly diagnosed ITP or recurrent ITP are reduced than healthy subjects, and these levels are not altered before and after immunotherapy, before and after splenectomy, or before and after the disease is alleviated.24 These researchers believe that sPD-1 levels in ITP patients can be used as a predictor for the recurrence of ITP. In the meantime, sPD-L1 levels in newly diagnosed ITP patients are lower than those in patients with recurrent ITP, suggesting that reduced sPD-L1 level may affect the function of PD-1/PD-L1 signaling pathway and cause T cell apoptosis, finally leading to ITP.24 However, these authors have only studied the serum levels of PD-1/PD-L1 but not the expression of PD-1/PD-L1 on cell membrane in the patients. This may be the reason why the observations made in the present study are contrasting to the mentioned report by Birtas Atesoglu et al. Therefore, their results cannot show the function of PD-1/PD-L1 signaling pathway, or explain the abnormal immune regulation in ITP patients. In conclusion, the present study demonstrates that the percentages of PD-1+CD4+T cells and PD-L1+DCs are increased in ITP patients, but their serum concentration of sPD-1 is also elevated. Increased sPD-1 level may block PD-1/PD-L1 signaling pathway, leading to uninhibited Th cell function. As a result, elevated serum concentrations of IFN-γ and IL-17 may participate in the occurrence and development of ITP. Serum level of sPD-1 is reduced in patients who respond well to treatments, and negative regulation of PD-1/PD-L1 signaling pathway is improved.
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 81360086).
Disclosure of potential conflicts of interest
All authors declare no non-financial or financial competing interests.
References
- [1].Nomura S. Advances in Diagnosis and Treatments for Immune Thrombocytopenia. Clin Med Insights Blood Disord 2016; 9:15-22; PMID:27441004; https://doi.org/ 10.4137/CMBD.S39643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhou L, Xu F, Chang C, Tao Y, Song L, Li X. Interleukin-17-producing CD4+ T lymphocytes are increased in patients with primary immune thrombocytopenia. Blood Coagul Fibrinolysis. 2016; 27:301-7; PMID:26484642; https://doi.org/ 10.1097/MBC.0000000000000423 [DOI] [PubMed] [Google Scholar]
- [3].Guo XH, Zhao F, Shi W, Ma XM, Xu Q, Patiguli AB, Halida YS. Detection and clinical significance of Th1/Th2 cytokines in patients with idiopathic thrombocytopenic purpura. Chin J Cell Mol Immunol 2012; 28:1185-7. [PubMed] [Google Scholar]
- [4].AbuHilal M, Walsh S, Shear N. The Role of IL-17 in the Pathogenesis of Psoriasis and Update on IL-17 Inhibitors for the Treatment of Plaque Psoriasis. J Cutan Med Surg 2016; 20:509-516; PMID:27207350; https://doi.org/ 10.1177/1203475416651605 [DOI] [PubMed] [Google Scholar]
- [5].Kaneko S, Kondo Y, Yokosawa M, Sumida T. Rheumatoid arthritis and cytokines. Nihon Rinsho 2016; 74:913-8; PMID:27311178 [PubMed] [Google Scholar]
- [6].Yan JW, Wang YJ, Peng WJ, Tao JH, Wan YN, Li BZ, Mei B, Chen B, Yao H, Yang GJ, Li XP, Ye DQ, Wang J. Therapeutic potential of interleukin-17 in inflammation and autoimmune diseases. Expert Opin Ther Targets 2014; 18:29-41; PMID:24147601; https://doi.org/ 10.1517/14728222.2013.843669 [DOI] [PubMed] [Google Scholar]
- [7].Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26:677-704; PMID:18173375; https://doi.org/ 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009; 206:3015-29; PMID:20008522; https://doi.org/ 10.1084/jem.20090847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Luo Q, Huang Z, Ye J, Deng Y, Fang L, Li X, Guo Y, Jiang H, Ju B, Huang Q, Li J. PD-L1-expressing neutrophils as a novel indicator to assess disease activity and severity of systemic lupus erythematosus. Arthritis Res Ther 2016; 18:47; PMID:26867643; https://doi.org/ 10.1186/s13075-016-0942-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Araki K, Youngblood B, Ahmed R. Programmed Cell Death 1-Directed Immunotherapy for Enhancing T-Cell Function. Cold Spring Harb Symp Quant Biol 2013; 78:239-47; PMID:25028401; https://doi.org/ 10.1101/sqb.78.019869 [DOI] [PubMed] [Google Scholar]
- [11].Jiao Q, Liu C, Yang Z, Ding Q, Wang M, Li M, Zhu T, Qian H, Li W, Tu N, et al.. Upregulated PD-1 Expression Is Associated with the Development of Systemic Lupus Erythematosus, but Not the PD-1.1 Allele of the PDCD1 Gene. Int J Genomics 2014; 2014:950903; PMID:24860805; https://doi.org/ 10.1155/2014/950903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Reynolds J, Sando GS, Marsh OB, Salama AD, Evans DJ, Cook HT, Pusey CD. Stimulation of the PD-1/PDL-1 T-cell co-inhibitory pathway is effective in treatment of experimental autoimmune glomerulonephritis. Nephrol Dial Transplant 2012; 27:1343-50; PMID:21965585; https://doi.org/ 10.1093/ndt/gfr529 [DOI] [PubMed] [Google Scholar]
- [13].Salama AD, Chitnis T, Imitola J, Ansari MJ, Akiba H, Tushima F, Azuma M, Yagita H, Sayegh MH, Khoury SJ. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med 2003; 198:71-8; PMID:12847138; https://doi.org/ 10.1084/jem.20022119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Peled M, Strazza M, Azoulay-Alfaguter I, Silverman GJ, Scher JU, Mor A. Analysis of Programmed Death-1 in Patients with Psoriatic Arthritis. Inflammation 2015; 38:1580; PMID:25847627; https://doi.org/ 10.1007/s10753-015-0165-6 [DOI] [PubMed] [Google Scholar]
- [15].Xu T, Wu M, Cai MY. The expression and clinical significance of programmed death-1/programmed death ligands-1 in peripheral blood in patients with primary sjfigren'S syndrome. Chin j Rheumatol 2013; 17:318-22. [Google Scholar]
- [16].Liu C, Jiang J, Gao L, Wang X, Hu X, Wu M, Xu T, Shi Q, Zhang X. Soluble PD-1 aggravates progression of collagen-induced arthritis through Th1 and Th17 pathways. Arthritis Res Ther 2015; 17:340; PMID:26608464; https://doi.org/ 10.1186/s13075-015-0859-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yanaba K, Hayashi M, Yoshihara Y, Nakagawa H. Serum levels of soluble programmed death-1 and programmed death ligand-1 in systemic sclerosis: Association with extent of skin sclerosis. J Dermatol 2016; 43:954-7; PMID:26945563; https://doi.org/ 10.1111/1346-8138.13339 [DOI] [PubMed] [Google Scholar]
- [18].Zhang Z, Shen T. Diagnostic and therapeutic criteria of hematologic diseases (3rd edition). Science Press, 2008; 08:172-6. [Google Scholar]
- [19].2016 edition of expert consdensus of ault ITP diagnosis and treatment. [Google Scholar]
- [20].Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motifcarrying immunoreceptor. Immunity 1999; 11:141-51; https://doi.org/ 10.1016/S1074-7613(00)80089-8 [DOI] [PubMed] [Google Scholar]
- [21].Raptopoulou AP, Bertsias G, Makrygiannakis D, Verginis P, Kritikos I, Tzardi M, Klareskog L, Catrina AI, Sidiropoulos P, Boumpas DT. The programmed death 1/programmed death ligand 1 inhibitory pathway is up-regulated in rheumatoid synovium and regulates peripheral T cell responses in human and murine arthritis. Arthritis Rheum 2010; 62:1870-80; PMID:20506224 [DOI] [PubMed] [Google Scholar]
- [22].Dolff S, Quandt D, Feldkamp T, Jun C, Mitchell A, Hua F, Specker C, Kribben A, Witzke O, Wilde B. Increased percentages of PD-1 on CD4+ T cells is associated with higher INF-γ production and altered IL-17 production in patients with systemic lupus erythematosus. Scand J Rheumatol 2014; 43:307-13; PMID:25088926; https://doi.org/ 10.3109/03009742.2013.869830 [DOI] [PubMed] [Google Scholar]
- [23].Wan B, Nie H, Liu A, Feng G, He D, Xu R, Zhang Q, Dong C, Zhang JZ. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J Immunol 2006; 177:8844-50. [DOI] [PubMed] [Google Scholar]
- [24].Birtas Atesoglu E, Tarkun P, Demirsoy ET, Geduk A, Mehtap O, Batman A, Kaya F, Cekmen MB, Gulbas Z, Hacıhanefioglu A. Soluble Programmed Death 1 (PD-1) Is Decreased in Patients With Immune Thrombocytopenia (ITP): Potential Involvement of PD-1 Pathway in ITP Immunopathogenesis. Clin Appl Thromb Hemost 2016; 22:248-51; PMID:25510412; https://doi.org/ 10.1177/1076029614562952 [DOI] [PubMed] [Google Scholar]


