ABSTRACT
Cross-talk by pattern recognition receptors may facilitate the maturation of dendritic cells and fine tune the immune response. Thus, the inclusion of ligands agonistic to multiple receptors in a vaccine formula may be an effective strategy to elicit robust antitumor cellular immunity. We tested the adjuvant effects and possible synergy of CpG (CpG oligodeoxynucleotide), Poly I:C (polyinosinic-polycytidylic acid) and the cationic peptide Cramp (cathelicidin-related antimicrobial peptide) formulated in a DOTAP (1,2-dioleoyl-3-trimethylammonium-propane) liposomal HPV E7 epitope vaccine on a TC-1 grafted mouse model. The vaccine formulations were administered both preventively and therapeutically. Based on our results, both CpG and Poly I:C-adjuvanted vaccines abolished tumor development in a preventive trial and significantly suppressed tumor growth in a therapeutic trial. Increased interferon (IFN)-γ expression and potent memory T cells in splenocytes as well as elevated CD8+IFN-γ+ cells in both spleen and tumor tissue indicated an elevated E744-62-specific cellular immune response. Although synergistic effects were detected between CpG and Poly I:C, their adjuvant effects were not enhanced further when combined with Cramp. Because the enhancement of tumor antigen-specific cellular immune responses is vital for the clearance of infected and cancerous cells, our results contribute a potential adjuvant combination for cancer vaccines.
KEYWORDS: CpG, Poly I:C, adjuvant, cramp, tumor, therapeutic vaccine
Introduction
Invasive cervical cancer caused by high-risk human papillomavirus (HPV) infection leads to approximately 0.25 million deaths worldwide according to 2011 statistics.1 Although two preventive vaccines have been available since 2006 and 2008, they are not effective for women with persistent infections.2,3 Since viruses are naturally eliminated during most cases of infection, active immune intervention during infection may significantly lower the risk of cancer development.4 During infection with high-risk HPVs, E6 and E7 are expressed to induce tumorigenesis and are presented by infected cells in a sustained manner. Targeting these neoantigens with therapeutic vaccines may help eliminate infected and cancerous cells.5,6 Several vaccines design strategies involving antigen targeting have previously been reported, including DNA vaccines, vector vaccines, a recombinant subunit protein, and synthetic long peptides.7-10 A robust CD8+ T cell response is critical for cancer elimination.11-14
Poly I:C (polyinosinic-polycytidylic acid) is a synthetic analog of double-stranded RNA that mimics viral nucleic acids recognized by the antiviral pattern receptors TLR-3 (Toll-like receptor 3) and RIG-1 (retinoic acid-inducible gene 1)/MDA5 (melanoma differentiation-associated protein 5). The activation of these receptor-mediated signal pathways boosts downstream acquired immunity, particularly T cell responses aimed at eliminating virus-infected cells.15-17 Experimental and clinical trials using Poly I:C as an adjuvant have elicited good effects for the treatment of several cancers, including thymomas and melanomas.18,19 Notably, some studies showed only good effects in combination with PD-1 (programmed cell death protein 1) blockade.20,21 Furthermore, CpG (CpG oligodeoxynucleotide), an unmethylated CpG nucleic acid, has demonstrated significant adjuvant effects when used in vaccines by down-regulating PD-1 expression in effector CD8+ cells via the IL-12 pathway.22-24 Several studies indicate that Poly I:C and CpG exert synergistic effects for antiviral protection and tumor vaccination, but little has been reported for mouse model for HPV-associated tumors or liposome-based vaccines.18,25-27 Though the combination of CpG and PolyI:C as tumor vaccine adjuvant has been reported in an ovalbumin-expressing thymoma cells grafted tumor model, reports concerning TC-1 model either used carcinogenic whole HPV E7 protein as antigens, or used peptide antigens with CAF09 adjuvant formulations.18,28-30
Cationic peptides enhance the adjuvant effects of oligonucleotides by forming nanostructures and stimulating their uptake and internalization by DCs (dendritic cells).31-34 Cathelicidin-related antimicrobial peptide (Cramp) is a mouse-derived cationic peptide that has been reported to recruit monocytes, neutrophils and DCs via CXCLs (chemokine (C-X-C) motif ligands) and FPRs (formyl peptide receptors) and promote the rapid sensing of oligodeoxynucleotides, thus playing different roles in immunity against pathogen infections, autoimmune diseases, and cancers.35-37 In this study, we tested the adjuvant effects and possible synergy of Poly I:C, CpG and a cationic peptide on a TC-1 grafted mouse model to develop a DOTAP (1,2-dioleoyl-3-trimethylammonium-propane) liposomal peptide vaccine.
Results
CpG and Poly I:C-adjuvanted vaccines effectively suppressed the development of grafted TC-1 tumors following preventive immunization
TC-1 cells were grafted three weeks after the final immunization (Fig. 1A), which eliminated any possible innate immune effects exerted by vaccine components on vaccine-elicited adaptive immunity. The PBS and DOTAP-CpG-Poly I:C-Cramp groups showed similar tumor growth, and no significant differences in tumor size were detected (Fig. 1B). Mice immunized with un-adjuvanted E744-62 peptides developed smaller tumors than the control mice in the PBS group (P<0.05); furthermore, tumors in the mice immunized with DOTAP-Cramp-Ag (P<0.01) were much smaller than tumors in the mice immunized with un-adjuvanted E744-62 peptides or PBS, indicating that the combination of DOTAP and Cramp resulted in significant adjuvant effects. Tumor development was barely detectable in the groups immunized with DOTAP and nucleic acid adjuvants. The experiment ended on day 70, when the tumor diameters of the PBS group became greater than 10 mm.
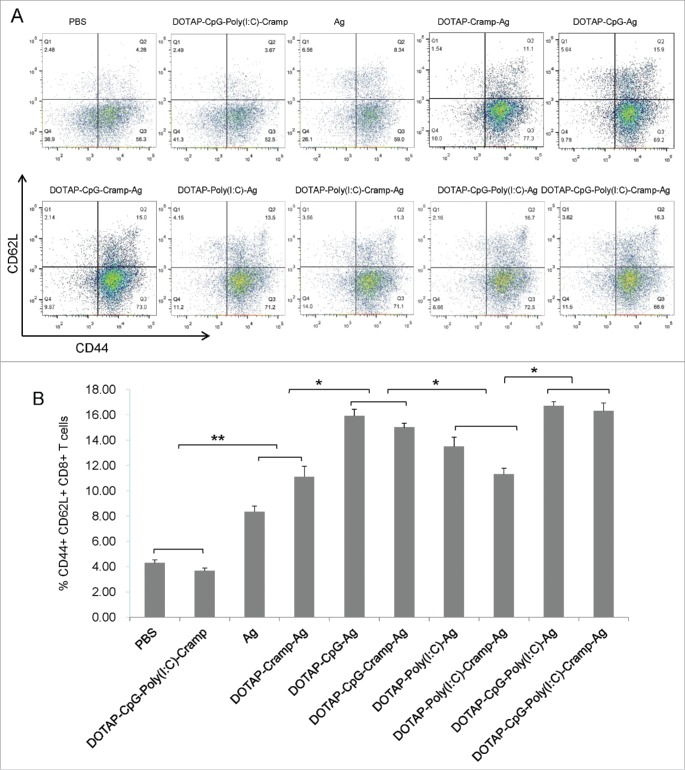
Figure 1.
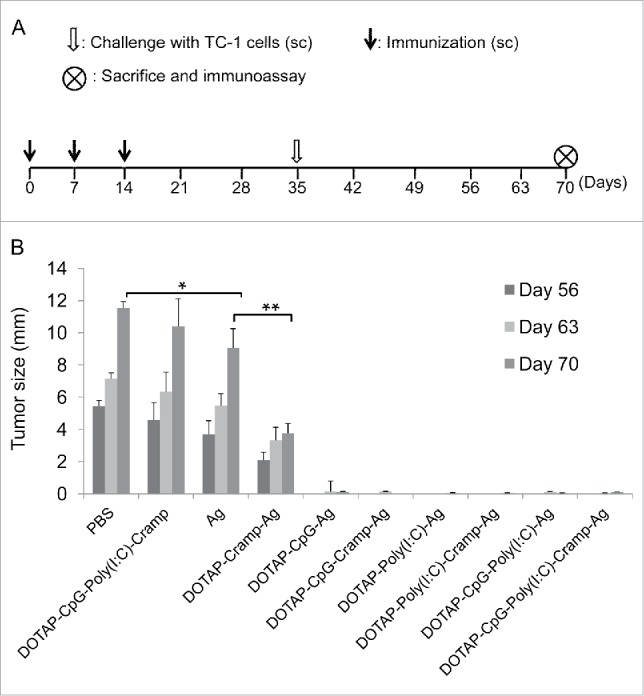
Preventive immunization with CpG and Poly I:C-adjuvanted vaccines significantly prevented the development of grafted TC-1 tumors in mice. (A) Protocol – Mice were immunized sc with vaccines three times at one-week intervals and challenged sc in the right flank with 1 × 105 TC-1 cells. The mice were euthanized humanely, and immunoassays were performed on day 70. (B) Changes in tumor size. One-way ANOVA followed by Tukey's multiple comparisons test, *P<0.05, **P<0.01, n = 5 per group. sc, subcutaneous; PBS, phosphate buffered saline; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane; CpG, CpG oligodeoxynucleotide; Poly I:C, polyinosinic-polycytidylic acid; Cramp, cathelicidin-related antimicrobial peptide; Ag, antigen E744-62; ANOVA, analysis of variance.
CpG and Poly I:C-adjuvanted vaccines induced potent memory T cell responses
Because tumor development was limited in the CpG and Poly I:C preventively immunized mice, only splenocytes were analyzed. Potent proliferative and lymph node-homing CD8+ T cells (CD44+CD62 L+) were more numerous in Ag-immunized mice (Fig. 2, PBS and DOTAP-CpG-Poly I:C-Cramp groups vs Ag and DOTAP-Cramp-Ag groups, P<0.01).38,39 These memory cells were also more numerous in the Poly I:C-adjuvanted groups (although no significant differences were detected between the Ag and DOTAP-Cramp-Ag groups vs the DOTAP-Poly I:C-Ag and DOTAP-Poly I:C-Cramp-Ag groups, P>0.05) and peaked in the CpG-adjuvanted groups (compared to groups without CpG, P<0.05) (Fig. 2).
Figure 2.
Preventive immunization with CpG and Poly I:C-adjuvanted vaccines induced memory T cell responses. (A) Representative picture of flow cytometry analysis. (B) CD44+CD62 L+ CD8+ T cells detected by flow cytometry. One-way ANOVA followed by Tukey's multiple comparisons test, *P<0.05, **P<0.01, n = 5 per group.
T cell responses were further analyzed by performing ELISPOT assays (Fig. 3). After stimulation with E744-62 peptides, splenocytes were activated and produced IFN-γ. Consistent with memory cell analysis, nucleic acid-adjuvanted mice contained greater numbers of IFN-γ-producing splenocytes (Ag and DOTAP-Cramp-Ag vs DOTAP-Poly I:C-Ag and DOTAP-Poly I:C-Cramp-Ag, P<0.01), and synergistic effects were observed between the Poly I:C and CpG-adjuvanted groups (P<0.05).
Figure 3.
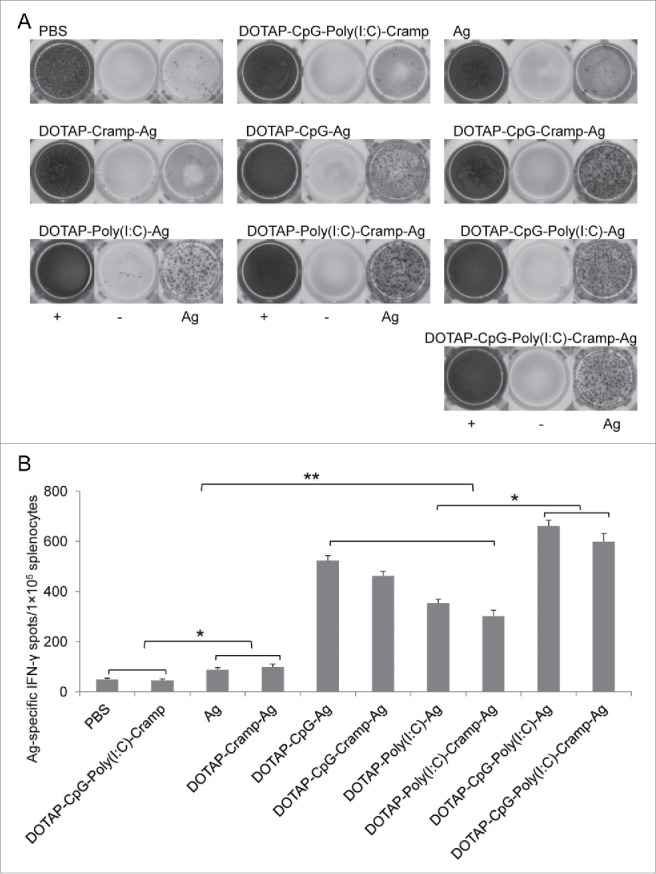
Preventive immunization with CpG and Poly I:C-adjuvanted vaccines induced potent tumor-specific IFN-γ-expressing lymphocytes. (A) Representative ELISPOT picture. (B) E744-62-specific IFN-γ-expressing lymphocytes detected by ELISPOT. One-way ANOVA followed by Tukey's multiple comparisons test, *P<0.05, **P<0.01, n = 5 per group. IFN-γ, interferon-γ; ELISPOT, enzyme-linked immunospot assay.
CpG and Poly I:C-adjuvanted vaccines significantly suppressed tumor development via therapeutic immunization in TC-1 grafted mice
At the end of the therapeutic experiment on day 56 the average tumor diameter was 20 mm in the PBS and DOTAP-CpG-Poly I:C-Cramp groups (Fig. 4A and B). The Ag and DOTAP-Cramp-Ag groups developed smaller tumors than the PBS and DOTAP-CpG-Poly I:C-Cramp groups (P<0.01), but these tumors were still much larger than those in the nucleic acid-adjuvanted groups (P<0.01). Consistent with the T cell responses observed in the preventive immunization experiment, mice in the Poly I:C-adjuvanted groups (including DOTAP-Poly I:C-Ag and DOTAP-Poly I:C-Cramp-Ag) developed larger tumors than those in the CpG-adjuvanted groups (including DOTAP-CpG-Ag and DOTAP-CpG-Cramp-Ag) (p<0.05), and synergistic effects (P<0.01) were detected in the groups adjuvanted with a combination of Poly I:C and CpG. Similar results were obtained when tumor weight was analyzed (Fig. 4C).
Figure 4.
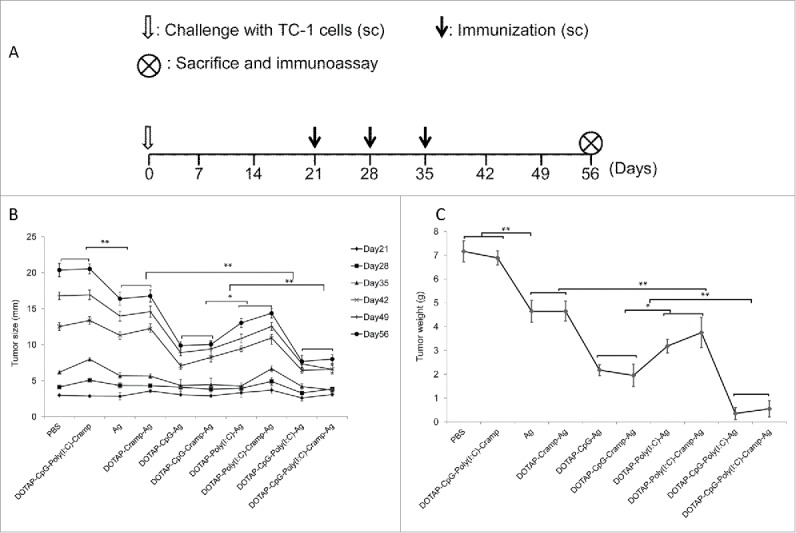
Therapeutic immunization with CpG and Poly I:C-adjuvanted vaccines significantly prevented the development of grafted TC-1 tumors in mice. (A) Protocol – Mice were challenged sc in the right flank with 1 × 105 TC-1 cells. Three weeks later, when the tumors had diameters of approximately 2.5 mm, vaccines were administered sc three times at one-week intervals. The mice were euthanized humanely, and immunoassays were performed on day 56. (B) Changes in tumor size. (C) Changes in tumor weight. One-way ANOVA followed by Tukey's multiple comparisons test, *P<0.05, **P<0.01, n = 5 per group.
CpG and Poly I:C-adjuvanted vaccines induced and activated greater numbers of CD8+ cells
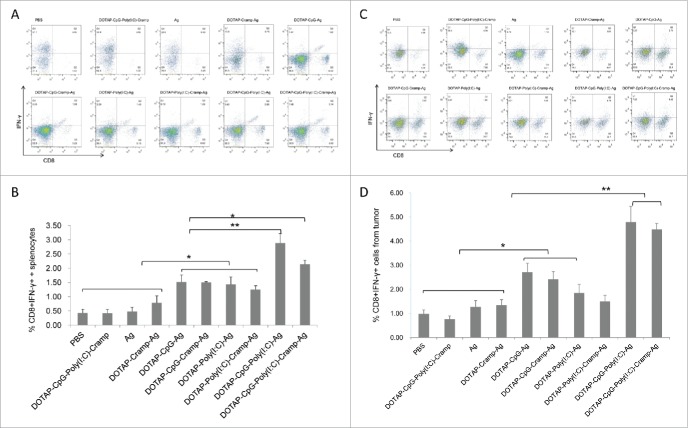
Activated CD8+ cells produce tremendous amounts of IFN-γ and are effective killers of cancerous and infected cells. Nucleic acid-adjuvanted vaccines effectively induced E44-62-specific IFN-γ-producing CD8+ cells in the spleen (P<0.05), and these effects were prominently enhanced when CpG and Poly I:C were used together (Fig. 5A and B). T cell responses were further confirmed by ELISPOT analysis (Fig. 6). Ag-specific IFN-γ spots induced by nucleic acid-adjuvanted vaccines were higher than in the other groups (P<0.01), and synergistic effects between CpG and Poly I:C were detected (P<0.05) (Fig. 6). Furthermore, similar results were obtained with flow cytometry analysis of tumor cells (Fig. 5C and D), indicating that tumor-specific CTLs were not only induced but also successfully penetrated into tumor tissue and contributed to tumor cell clearance.40
Figure 5.
Therapeutic immunization with CpG and Poly I:C-adjuvanted vaccines induced potent tumor-specific IFN-γ-expressing CD8+ T cells in spleens and tumors. (A) Representative picture of flow cytometry analysis of splenocytes. (B) CD8+ IFN-γ+ splenocytes detected by flow cytometry. (C) Representative picture of flow cytometry analysis of tumor cells. (D) CD8+ IFN-γ+ tumor cells detected by flow cytometry. One-way ANOVA followed by Tukey's multiple comparisons test, *P<0.05, **P<0.01, n = 5 per group.
Figure 6.
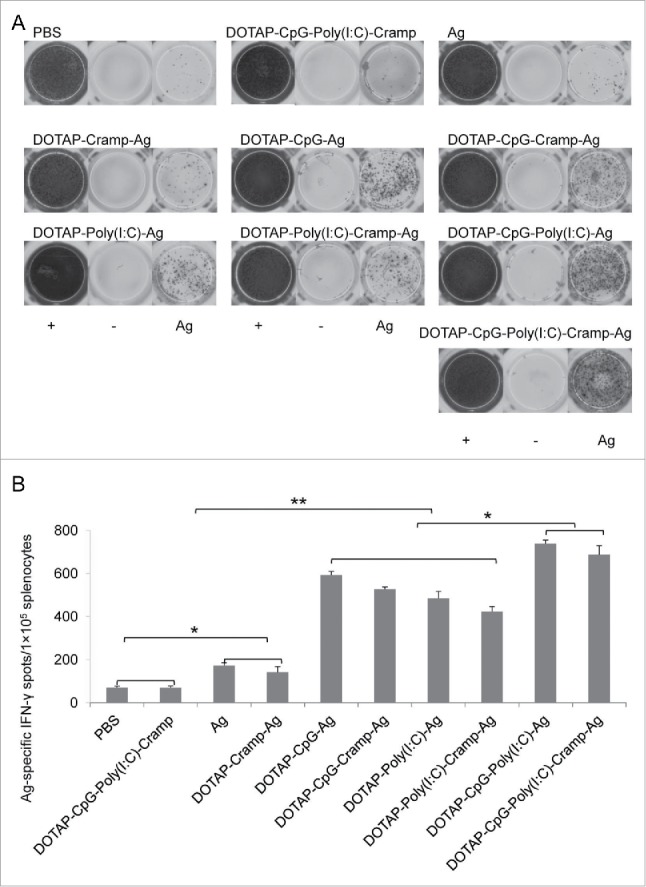
Therapeutic immunization with CpG and Poly I:C-adjuvanted vaccines induced potent tumor-specific IFN-γ expressing lymphocytes. (A) Representative ELISPOT picture. (B) E744-62-specific IFN-γ-expressing lymphocytes detected by ELISPOT. One-way ANOVA followed by Tukey's multiple comparisons test, *P<0.05, **P<0.01, n = 5 per group.
Discussion
We previously reported that the formation of chimeric HBcAg VLPs (virus-like particles) is important to induce vigorous antitumor immunity; however, the nucleic acids entrapped in VLPs may serve as adjuvants and facilitate the elicitation of tumor-specific cellular immunity.41,42 The inclusion of nucleic acid residues is nearly unavoidable during the preparation of VLP-based vaccines such as HPV L1 VLPs, influenza VLPs and HBsAg VLPs, and nucleic acids enhance the immunogenicity of these vaccines.43,44 CpG and Poly I:C are potent adjuvants for eliciting cellular immunity, but we were concerned with the associated risks of producing or enhancing autoimmunity. The use of liposomes to restrict the distribution of CpG and Poly I:C and avoid systemic diffusion may increase the safety of CpG and Poly I:C-adjuvanted vaccines. Therefore, we sought to investigate the adjuvant effects of nucleic acid immune stimulators in a DOTAP liposomal vaccine, which may mimic VLP vaccines and simultaneously deliver both antigen and nuclear acid contents to antigen-presenting cells.
CpG showed excellent adjuvant effects for the elicitation of tumor antigen-specific cellular responses via preventive or therapeutic immunization in a TC-1 grafted tumor model. Adjuvant effects manifested as the suppression of tumor growth during development (Fig. 1B and Fig. 4B) and reduced tumor weight at the end of the experiment (Fig. 4C). Additionally, the use of CpG elevated tumor-specific CTL numbers both in the spleen and in the tumor, as indicated by flow cytometry analysis (Fig. 5B and D) and ELISPOT analysis (Fig. 3 and Fig. 6). In a preventive TC-1 grafted model, CpG also elicited effective memory T cells (Fig. 2), which may play an important role in controlling the recurrence of cancer cells after surgical excision or radiation therapy.45,46
Cross-talk between pattern recognition receptors may facilitate the maturation of dendritic cells and fine tune the immune response against tumor development.18,47 Both TLR3 and TLR9 activate IFN-dependent immune responses that trigger acquired and long-lasting antitumor immunity that is dependent on CD8+ T cells and IFN-γ.21 Activation of the IFN system with immunostimulatory RNA combined with immune-inhibitory receptor blockade is a rational strategy for cancer vaccine design, and CpG itself down-regulates the expression of PD-120,24; therefore, we tested the synergistic adjuvant effects of CpG and Poly I:C. A combination of ODN 1826 (class B) and ODN 2395 (class C) (5 μg each) induced higher numbers of memory T cells in DOTAP liposomal peptide vaccine-immunized mice and suppressed tumor development to a greater degree than 25 μg of Poly I:C in therapeutically immunized mice (Fig. 2 and 4). As expected, the synergistic effects of CpG and Poly I:C were evidenced by higher numbers of activated CTLs in the preventive study (Fig. 3) and the suppression of tumor development (Fig. 4) and higher numbers of activated CTLs in the therapeutic study (Fig 5 and 6).
Although the DOTAP-Cramp-Ag group developed much smaller tumors than the un-adjuvanted E744-62 group (P<0.01) in the preventive experiment (Fig. 1B), no significant CTL elevation was detected (Fig. 3B), possibly because cationic lipids delay the release of Cramp and Ag,17 resulting in the ability of Cramp remnants to kill tumor cells by enhancing the proliferation and activation of natural killer cells but not CD4+ or CD8+ T cells.35 Delayed antigen release is one mechanism utilized by adjuvants.48 We believe the latter explanation is more likely because the DOTAP-CpG-Poly I:C-Cramp group without Ag demonstrated tumors comparable in size to those of the PBS group at different time points, and there was no significant difference (Fig. 1B).
Surprisingly, Cramp did not enhance the adjuvant effects of either CpG or Poly I:C, as indicated by tumor size and immune cell activation in both the preventive and therapeutic experiments. Conversely, Cramp appeared to dampen the adjuvant effects of CpG and Poly I:C. A dose of 200 μg of Cramp (equal to 10 mg of Cramp/kg body weight) is considered a high dose; therefore, factors other than dosage may be responsible for this result. Although reported cationic peptides exert adjuvant effects in cancer vaccines, these peptides are short (i.e., less than 15 aa) and form 200-300-nm-sized uniform nanorings with CpG. In contrast, larger peptides (e.g., Cramp, which has a length of 39 aa) may form aggregates with nucleic acids and thus dampen the adjuvant effects of both CpG and Poly I:C,31,32,34 as indicated by the presence of a visible precipitate when we mixed Cramp/CpG/Poly I:C with a pipettor.
It seems that the increases in immunogenicity and tumor protection using the vaccine formulated in this current study are not notably superior to those using other vaccine strategies as documented by other groups.29 Actually, the vaccine efficacies are hard to be simply compared between studies from different publications, due to the specific experimental designs and conditions. For example, the intervening time of vaccination (concerning tumor size at the beginning) and vaccination frequencies may affect the results greatly. In addition, other factors, including the status of grafted cells line, animal conditions and time points for sampling and analysis, etc. may also have important impacts on the presented data. According to the previous experiences, our vaccine generally begins to induce significant antitumor immune responses on the second vaccination. It should be mentioned that, at that time tumors have reached the diameters of nearly 5mm in the current study, which is definitely a great challenge to therapeutic vaccines since the tumor immune-suppressive microenvironment has been fully established. In this study, a subcutaneous tumor cells-grafted model was used, which may have limitations as compared to an orthotopic model for fully evaluating the potentials of a candidate vaccine, taking the possible impacts of tumor microenvironment into account. HPV-associated orthotopic tumor models have been reported previously, including a successful head-neck tumor model and a genital model which however has some drawbacks, for example it did not represent a proper orthotopic transplantation.49 To our knowledge, no successful orthotopic mouse model for cervical cancer has been established yet. Our interests are to develop a vaccine against genital or cervical tumors, for which a reliable orthotopic model is not available yet. Though TC-1 grafted model employed in this study is not perfect, it is useful and widely accepted for evaluating the efficacy of HPV-specific immune responses caused by therapeutic vaccines. Our results did testify that CpG and PolyI:C showed synergistic adjuvant effects in the vaccine recipe and tumor model we employed, which is our main purpose of this study.
In conclusion, the combination of Poly I:C with the TLR9 ligand CpG, an IFN-dependent immune response activator, exerts prominent synergistic effects in a DOTAP liposomal vaccine and is sufficient to trigger acquired and long-lasting antitumor immunity. It may be useful as a powerful complex adjuvant in therapeutic vaccines against cancer and persistent intracellular infections.
Materials and methods
Mice, cell line and vaccines
Female C57 BL/6 mice (6–8 weeks; 16–18 g) were purchased from Vital River Laboratory Animal Technology Ltd. (Beijing, People's Republic of China). All mice were maintained under specific pathogen-free conditions in the Central Animal Care Services of the Institute of Medical Biology of the Chinese Academy of Medical Sciences, Peking Union Medical College, People's Republic of China. All animal experiments were approved by the Animal Ethics Committee of the Institute of Medical Biology.
The TC-1 tumor cell line, which was derived from the primary lung epithelial cells of a C57 BL/6 mouse co-transformed with the HPV-16 oncoproteins E6 and E7 as well as the c-Ha-ras oncogene, was purchased from the Tumor Center of the Chinese Academy of Medical Sciences. The cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 with 10% fetal bovine serum.
A previously reported HPV16 segment, E744-62 (44QAEPDRAHYNIVTFCCKCD62), which contains both cytotoxic T lymphocyte (CTL) and T helper (Th) cell epitopes, and mouse Cramp (GLLRKGGEKIGEKLKKIGQKIKNFFQKLVPQPEQ) were synthesized by GL Biochem Ltd. (Shanghai, People's Republic of China).50 ODN 1826 (class B) and ODN 2395 (class C) with DNase-protected phosphorothioate bonds and low-molecular-weight (LMW) Poly I:C were supplied by InvivoGen (San Diego, USA). Cationic lipid DOTAP was purchased from Roche Diagnostic Corp. (Basel, Switzerland). Vaccines were formulated by mixing the following amounts of E744-62/Cramp/CpG/Poly I:C/DOTAP with a pipettor: 100 μg of Ag (E744-62) peptide, 20 μg of DOTAP, 5 μg of CpG ODN 1826 and 5 μg of CpG ODN 2395, 25 μg of Poly I:C, and 200 μg of Cramp.26,27
Tumor challenge and mouse immunization
To establish a grafted tumor model, 1 × 105 TC-1 cells mixed with Basement Membrane Matrix (BD Bioscience, San Jose, CA, USA) were injected subcutaneously (sc) into the right flank of each mouse. The mice were randomly divided into ten groups (n = 5). In both the preventive and therapeutic experiments, the following groups of mice were immunized: PBS (phosphate buffered saline), DOTAP-CpG-Poly(I:C)-Cramp, Ag (E744-62 peptide), DOTAP-Cramp-Ag, DOTAP-CpG-Ag, DOTAP-CpG-Cramp-Ag, DOTAP-Poly(I:C)-Ag, DOTAP-Poly(I:C)-Cramp-Ag, DOTAP-CpG-Poly(I:C)-Ag, and DOTAP-CpG-Poly(I:C)-Cramp-Ag. For the preventive experiment, the mice were immunized sc three times at one-week intervals and then challenged with TC-1 cells three weeks after the last immunization to avoid any innate immune effects of the vaccine components. For the therapeutic experiment, the mice were first challenged with TC-1 cells and then immunized three times at one-week intervals when the tumor diameters reached approximately 2–3 mm. The mice were monitored once per week for tumor growth using a slide caliper as described previously.41 The animal study was performed twice and one of the studies is shown in the figures.
Enzyme-linked immunospot assay (ELISPOT)
Spleens were removed from the mice and dispersed using a 70-μm cell strainer (BD Bioscience, San Jose, CA, USA). Antigen-specific IFN-γ-producing lymphocytes were determined using an ELISPOT assay kit (DAKEWE Biotechnology Co., Ltd., Shenzhen, People's republic of China) according to the manufacturer's protocol. Briefly, 1 × 105 splenic lymphocytes were plated per well into a 96-microwell plate and incubated with 5 μg/ml E744-62 peptide or no peptide, followed by incubation for 16–20h at 37°C and 5% CO2. After immuno-imaging, spots were counted using an ELISPOT reader system (AID Diagnostika GmbH, Straβberg, Germany). The results were expressed as the mean number of IFN-γ-positive cells per 1 × 105 lymphocytes ± standard error of the mean (SEM).
Flow cytometry
Cells isolated from the spleens or tumors of immunized mice were plated into 96-microwell plates at 1 × 106 cells per well. For an antigen-specific CTL assay, lymphocytes were stimulated for 7 h with 5 μg/ml E744-62 peptide, and cytokine release was blocked by adding brefeldin A during the final 4 h of culture. Intracellular staining was performed using fixation/permeabilization buffer according to the manufacturer's protocol. Cell surface staining was performed by incubating cells with corresponding antibodies for 20 min on ice. All reagents were purchased from BioLegend, San Diego, CA, USA. Cells were analyzed using a BD Accuri C6 (BD Bioscience), and the data were analyzed using FlowJo software (FlowJo LLC, Ashland, Oregon, USA).
Statistical analysis
Significant differences among experimental groups were analyzed by performing an ANOVA (one-way analysis of variance) followed by Tukey's multiple comparisons test (GraphPad Prism 5.0, GraphPad Software, Inc., La Jolla, CA, USA). The values are reported as the mean ± SEM.
Funding Statement
This work was financially supported by the CAMS Initiative for Innovative Medicine (grant number 2017-I2M-3-022), Fundamental Research Funds for the Central Universities of China (grant number 2016ZX350073, 2017310059), the Science and Technology Project of Yunnan Province (2016FA049), and the National Natural Science Foundation of China (grant number 81503117, 81573206, 81773270).
Disclosure of potential conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. PMID:21296855 [DOI] [PubMed] [Google Scholar]
- 2.Govan VA. A novel vaccine for cervical cancer: quadrivalent human papillomavirus (types 6, 11, 16 and 18) recombinant vaccine (Gardasil). Ther Clin Risk Manag. 2008;4:65–70. doi: 10.2147/TCRM.S856. PMID:18728721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keam SJ, Harper DM. Human papillomavirus types 16 and 18 vaccine (recombinant, AS04 adjuvanted, adsorbed) [Cervarix]. Drugs. 2008;68:359–72. doi: 10.2165/00003495-200868030-00007. PMID:18257611 [DOI] [PubMed] [Google Scholar]
- 4.Farhat S, Nakagawa M, Moscicki AB. Cell-mediated immune responses to human papillomavirus 16 E6 and E7 antigens as measured by interferon gamma enzyme-linked immunospot in women with cleared or persistent human papillomavirus infection. Int J Gynecol Cancer. 2009;19:508–12. doi: 10.1111/IGC.0b013e3181a388c4. PMID:19509544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Jong A, van Poelgeest MI, van der Hulst JM, Drijfhout JW, Fleuren GJ, Melief CJ, Kenter G, Offringa R, van der Burg SH. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Cancer Res. 2004;64:5449–55. doi: 10.1158/0008-5472.CAN-04-0831. PMID:15289354 [DOI] [PubMed] [Google Scholar]
- 6.de Vos van Steenwijk PJ, Piersma SJ, Welters MJ, van der Hulst JM, Fleuren G, Hellebrekers BW, Kenter GG, van der Burg SH. Surgery followed by persistence of high-grade squamous intraepithelial lesions is associated with the induction of a dysfunctional HPV16-specific T-cell response. Clin Cancer Res. 2008;14:7188–95. doi: 10.1158/1078-0432.CCR-08-0994. PMID:19010835 [DOI] [PubMed] [Google Scholar]
- 7.Khan S, Oosterhuis K, Wunderlich K, Bunnik EM, Bhaggoe M, Boedhoe S, Karia S, Steenbergen RDM, Bosch L, Serroyen J, et al.. Development of a replication-deficient adenoviral vector-based vaccine candidate for the interception of HPV16- and HPV18-induced infections and disease. Int J Cancer. 2017;141:393–404. doi: 10.1002/ijc.30679. PMID:28263390 [DOI] [PubMed] [Google Scholar]
- 8.Trimble CL, Morrow MP, Kraynyak KA, Shen X, Dallas M, Yan J, Edwards L, Parker RL, Denny L, Giffear M, et al.. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386:2078–88. doi: 10.1016/S0140-6736(15)00239-1. PMID:26386540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu CC, Wu FC, Hsu YT, Hsiao YC, Yang YC, Chang CA, Chang CL. Enhanced anti-tumor therapeutic efficacy of DNA vaccine by fusing the E7 gene to BAFF in treating human papillomavirus-associated cancer. Oncotarget. 2017;8:33024–36. PMID:28423693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang A, Farmer E, Wu TC, Hung CF. Perspectives for therapeutic HPV vaccine development. J Biomed Sci. 2016;23:75. doi: 10.1186/s12929-016-0293-9. PMID:27809842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim TJ, Jin HT, Hur SY, Yang HG, Seo YB, Hong SR, Lee CW, Kim S, Woo JW, Park KS, et al.. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat Commun. 2014;5:5317. doi: 10.1038/ncomms6317. PMID:25354725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansilla C, Berraondo P, Durantez M, Martínez M, Casares N, Arribillaga L, Rudilla F, Fioravanti J, Lozano T, Villanueva L, et al.. Eradication of large tumors expressing human papillomavirus E7 protein by therapeutic vaccination with E7 fused to the extra domain a from fibronectin. Int J Cancer. 2012;131:641–51. doi: 10.1002/ijc.26412. PMID:21898393 [DOI] [PubMed] [Google Scholar]
- 13.Okoye IS, Houghton M, Tyrrell L, Barakat K, Elahi S. Coinhibitory receptor expression and immune checkpoint blockade: Maintaining a balance in CD8+ T cell responses to chronic viral infections and cancer. Front Immunol. 2017;8:1215. doi: 10.3389/fimmu.2017.01215. PMID:29033936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostroumov D, Fekete-Drimusz N, Saborowski M, Kuhnel F, Woller N. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell Mol Life Sci. 2017. doi: 10.1007/s00018-017-2686-7. PMID:29032503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T, Akira S, et al.. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med 2008;205:1601–10. doi: 10.1084/jem.20080091. PMID:18591409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulz O, Diebold SS, Chen M, Näslund T, Nolte MA, Alexopoulou L, Azuma YT, Flavell RA, Liljeström P, Reis e Sousa C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–92. doi: 10.1038/nature03326. PMID:15711573 [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Zhang S, Li W, Hu Y, Zhao J, Liu F, Lin H, Liu Y, Wang L, Xu S, et al.. A novel rabies vaccine based-on toll-like receptor 3 (TLR3) agonist PIKA adjuvant exhibiting excellent safety and efficacy in animal studies. Virology. 2016;489:165–72. doi: 10.1016/j.virol.2015.10.029. PMID:26765968 [DOI] [PubMed] [Google Scholar]
- 18.Bayyurt B, Tincer G, Almacioglu K, Alpdundar E, Gursel M, Gursel I. Encapsulation of two different TLR ligands into liposomes confer protective immunity and prevent tumor development. J Control Release. 2017;247:134–44. doi: 10.1016/j.jconrel.2017.01.004. PMID:28069554 [DOI] [PubMed] [Google Scholar]
- 19.Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, et al.. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–21. doi: 10.1038/nature22991. PMID:28678778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bald T, Landsberg J, Lopez-Ramos D, Renn M, Glodde N, Jansen P, Gaffal E, Steitz J, Tolba R, Kalinke U, et al.. Immune cell-poor melanomas benefit from PD-1 blockade after targeted type I IFN activation. Cancer Discov. 2014;4:674–87. doi: 10.1158/2159-8290.CD-13-0458. PMID:24589924 [DOI] [PubMed] [Google Scholar]
- 21.Charlebois R, Allard B, Allard D, Buisseret L, Turcotte M, Pommey S, Chrobak P, Stagg J. PolyI:C and CpG synergize with Anti-ErbB2 mAb for treatment of breast tumors resistant to immune checkpoint inhibitors. Cancer Res. 2017;77:312–9. doi: 10.1158/0008-5472.CAN-16-1873. PMID:27872096 [DOI] [PubMed] [Google Scholar]
- 22.Hanagata N. CpG oligodeoxynucleotide nanomedicines for the prophylaxis or treatment of cancers, infectious diseases, and allergies. Int J Nanomedicine. 2017;12:515–31. doi: 10.2147/IJN.S114477. PMID:28144136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S, Campos J, Gallotta M, Gong M, Crain C, Naik E, Coffman RL, Guiducci C. Intratumoral injection of a CpG oligonucleotide reverts resistance to PD-1 blockade by expanding multifunctional CD8+ T cells. Proc Natl Acad Sci U S A. 2016;113:E7240–E9. doi: 10.1073/pnas.1608555113. PMID:27799536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin P, Liu X, Mansfield AS, Harrington SM, Li Y, Yan Y, Dong H. CpG-induced antitumor immunity requires IL-12 in expansion of effector cells and down-regulation of PD-1. Oncotarget. 2016;7:70223–31. PMID:27602959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitmore MM, DeVeer MJ, Edling A, Oates RK, Simons B, Lindner D, Williams BR. Synergistic activation of innate immunity by double-stranded RNA and CpG DNA promotes enhanced antitumor activity. Cancer Res. 2004;64:5850–60. doi: 10.1158/0008-5472.CAN-04-0063. PMID:15313929 [DOI] [PubMed] [Google Scholar]
- 26.Zhu Q, Egelston C, Gagnon S, Sui Y, Belyakov IM, Klinman DM, Berzofsky JA. Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. J Clin Invest. 2010;120:607–16. doi: 10.1172/JCI39293. PMID:20101095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Q, Egelston C, Vivekanandhan A, Uematsu S, Akira S, Klinman DM, Belyakov IM, Berzofsky JA. Toll-like receptor ligands synergize through distinct dendritic cell pathways to induce T cell responses: implications for vaccines. Proc Natl Acad Sci U S A. 2008;105:16260–5. doi: 10.1073/pnas.0805325105. PMID:18845682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L, Xu C, Long J, Shen D, Zhou W, Zhou Q, Yang J, Jiang M. E6 and E7 gene silencing results in decreased methylation of tumor suppressor genes and induces phenotype transformation of human cervical carcinoma cell lines. Oncotarget. 2015;6:23930–43. doi: 10.18632/oncotarget.4525. PMID:26329329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korsholm KS, Hansen J, Karlsen K, Filskov J, Mikkelsen M, Lindenstrøm T, Schmidt ST, Andersen P, Christensen D. Induction of CD8+ T-cell responses against subunit antigens by the novel cationic liposomal CAF09 adjuvant. Vaccine. 2014;32:3927–35. doi: 10.1016/j.vaccine.2014.05.050. PMID:24877765 [DOI] [PubMed] [Google Scholar]
- 30.Domingos-Pereira S, Decrausaz L, Derre L, Bobst M, Romero P, Schiller JT, Jichlinski P, Nardelli-Haefliger D. Intravaginal TLR agonists increase local vaccine-specific CD8 T cells and human papillomavirus-associated genital-tumor regression in mice. Mucosal Immunol. 2013;6:393–404. doi: 10.1038/mi.2012.83. PMID:22968420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aichinger MC, Ginzler M, Weghuber J, Zimmermann L, Riedl K, Schütz G, Nagy E, von Gabain A, Schweyen R, Henics T. Adjuvating the adjuvant: facilitated delivery of an immunomodulatory oligonucleotide to TLR9 by a cationic antimicrobial peptide in dendritic cells. Vaccine. 2011;29:426–36. doi: 10.1016/j.vaccine.2010.11.003. PMID:21093498 [DOI] [PubMed] [Google Scholar]
- 32.Fritz JH, Brunner S, Birnstiel ML, Buschle M, Av Gabain, Mattner F, Zauner W. The artificial antimicrobial peptide KLKLLLLLKLK induces predominantly a TH2-type immune response to co-injected antigens. Vaccine. 2004;22:3274–84. doi: 10.1016/j.vaccine.2004.03.007. PMID:15308350 [DOI] [PubMed] [Google Scholar]
- 33.Gungor B, Yagci FC, Gursel I, Gursel M. Forging a potent vaccine adjuvant: CpG ODN/cationic peptide nanorings. Oncoimmunology. 2014;3:e950166. doi: 10.4161/21624011.2014.950166. PMID:25610738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gungor B, Yagci FC, Tincer G, Bayyurt B, Alpdundar E, Yildiz S, Ozcan M, Gursel I, Gursel M. CpG ODN nanorings induce IFNalpha from plasmacytoid dendritic cells and demonstrate potent vaccine adjuvant activity. Sci Transl Med. 2014;6:235ra61. doi: 10.1126/scitranslmed.3007909. PMID:24807558 [DOI] [PubMed] [Google Scholar]
- 35.Chuang CM, Monie A, Wu A, Mao CP, Hung CF. Treatment with LL-37 peptide enhances antitumor effects induced by CpG oligodeoxynucleotides against ovarian cancer. Hum Gene Ther. 2009;20:303–13. doi: 10.1089/hum.2008.124. PMID:19272013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurtado P, Peh CA. LL-37 promotes rapid sensing of CpG oligodeoxynucleotides by B lymphocytes and plasmacytoid dendritic cells. J Immunol. 2010;184:1425–35. doi: 10.4049/jimmunol.0902305. PMID:20042575 [DOI] [PubMed] [Google Scholar]
- 37.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, Cao W, Wang YH, Su B, Nestle FO, et al.. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–9. doi: 10.1038/nature06116. PMID:17873860 [DOI] [PubMed] [Google Scholar]
- 38.Esser MT, Marchese RD, Kierstead LS, Tussey LG, Wang F, Chirmule N, Washabaugh MW. Memory T cells and vaccines. Vaccine. 2003;21:419–30. doi: 10.1016/S0264-410X(02)00407-3. PMID:12531640 [DOI] [PubMed] [Google Scholar]
- 39.Liu L, Cao F, Liu X, Wang H, Zhang C, Sun H, Wang C, Leng X, Song C, Kong D, et al.. Hyaluronic Acid-Modified Cationic Lipid-PLGA Hybrid Nanoparticles as a Nanovaccine Induce Robust Humoral and Cellular Immune Responses. ACS Appl Mater Interfaces. 2016;8:11969–79. doi: 10.1021/acsami.6b01135. PMID:27088457 [DOI] [PubMed] [Google Scholar]
- 40.Calcinotto A, Grioni M, Jachetti E, Curnis F, Mondino A, Parmiani G, Corti A, Bellone M. Targeting TNF-alpha to neoangiogenic vessels enhances lymphocyte infiltration in tumors and increases the therapeutic potential of immunotherapy. J Immunol. 2012;188:2687–94. doi: 10.4049/jimmunol.1101877. PMID:22323546 [DOI] [PubMed] [Google Scholar]
- 41.Chu X, Li Y, Long Q, Xia Y, Yao Y, Sun W, Huang W, Yang X, Liu C, Ma Y. Chimeric HBcAg virus-like particles presenting a HPV 16 E7 epitope significantly suppressed tumor progression through preventive or therapeutic immunization in a TC-1-grafted mouse model. Int J Nanomedicine. 2016;11:2417–29. PMID:27313455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song S, Wang Y, Zhang Y, Wang F, He Y, Ren D, Guo Y, Sun S. Augmented induction of CD8+ cytotoxic T-cell response and antitumor effect by DCs pulsed with virus-like particles packaging with CpG. Cancer Lett. 2007;256:90–100. doi: 10.1016/j.canlet.2007.06.004. PMID:17656012 [DOI] [PubMed] [Google Scholar]
- 43.Lee SH. Melting profiles may affect detection of residual HPV L1 gene DNA fragments in Gardasil(R). Curr Med Chem. 2014;21:932–40. doi: 10.2174/0929867321999140102110933. PMID:24083601 [DOI] [PubMed] [Google Scholar]
- 44.Margine I, Martinez-Gil L, Chou YY, Krammer F. Residual baculovirus in insect cell-derived influenza virus-like particle preparations enhances immunogenicity. PLoS One. 2012;7:e51559. doi: 10.1371/journal.pone.0051559. PMID:23236516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Enomoto K, Sho M, Wakatsuki K, Takayama T, Matsumoto S, Nakamura S, Akahori T, Tanaka T, Migita K, Ito M, et al.. Prognostic importance of tumour-infiltrating memory T cells in oesophageal squamous cell carcinoma. Clin Exp Immunol. 2012;168:186–91. doi: 10.1111/j.1365-2249.2012.04565.x. PMID:22471279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wakatsuki K, Sho M, Yamato I, Takayama T, Matsumoto S, Tanaka T, Migita K, Ito M, Hotta K, Nakajima Y. Clinical impact of tumor-infiltrating CD45RO(+) memory T cells on human gastric cancer. Oncol Rep. 2013;29:1756–62. PMID:23440298 [DOI] [PubMed] [Google Scholar]
- 47.Benko S, Magyarics Z, Szabo A, Rajnavolgyi E. Dendritic cell subtypes as primary targets of vaccines: the emerging role and cross-talk of pattern recognition receptors. Biol Chem. 2008;389:469–85. doi: 10.1515/BC.2008.054. PMID:18953714 [DOI] [PubMed] [Google Scholar]
- 48.Brito LA, O'Hagan DT. Designing and building the next generation of improved vaccine adjuvants. J Control Release. 2014;190:563–79. doi: 10.1016/j.jconrel.2014.06.027. PMID:24998942 [DOI] [PubMed] [Google Scholar]
- 49.Venuti A, Curzio G, Mariani L, Paolini F. Immunotherapy of HPV-associated cancer: DNA/plant-derived vaccines and new orthotopic mouse models. Cancer Immunol Immunother. 2015;64:1329–38. doi: 10.1007/s00262-015-1734-0. PMID:26138695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reinis M, Stepanek I, Simova J, Bieblova J, Pribylova H, Indrova M, Bubenik J. Induction of protective immunity against MHC class I-deficient, HPV16-associated tumours with peptide and dendritic cell-based vaccines. Int J Oncol. 2010;36:545–51. doi: 10.3892/ijo_00000528. PMID:20126973 [DOI] [PubMed] [Google Scholar]