ABSTRACT
The present incidence of leptospirosis in China is significantly lower than past rates, although small localized outbreaks continue to occur in epidemic regions. Improvements in sanitation, as well as vaccination of high-risk populations, have played crucial roles in reducing the disease burden. Several types of human leptospirosis vaccines have been developed, including inactivated whole-cell, outer-envelope, and recombinant vaccines. Of these, only a multivalent inactivated leptospirosis vaccine is available in China, which was added to the Chinese Expanded Program on Immunization in 2007. However, this vaccine elicits serogroup-specific immunity, and serogroup epidemiology should continue to be monitored to enhance vaccine coverage and distribution. On the other hand, the efficiency of the inactivated vaccine should be further improved by optimizing the formulation, and by expanding the target population. More importantly, additional investments should be made to develop universal recombinant vaccines.
KEYWORDS: leptospirosis, human vaccines, inactivated whole-cell vaccine, recombinant vaccine
Introduction
Leptospirosis is a serious worldwide zoonotic disease caused by pathogenic species of the genus Leptospira. Leptospires are maintained in an array of wild and domestic animal hosts, and survive for extended periods after being shed in the urine. Consequently, humans are infected through contact with carrier animals or with contaminated soil, sewage, or water.1 Pathogenic leptospires then disseminate via the bloodstream, and cause a range of clinical symptoms, including high fever, headache, acute renal failure, and pulmonary hemorrhage.2 Although most patients recover well after treatment, some rapidly develop severe disease with fatality rates exceeding 30%.3 On the other hand, infection may present no other symptoms except bacteriuria, and may then persist as a chronic condition.2
Approximately 1.03 million human leptospirosis cases and 58,900 deaths are reported worldwide each year, suggesting that the disease has become a leading zoonotic cause of morbidity and mortality.4 Most cases occur in developing and underdeveloped countries, but international travel and global warming have led to an apparent surge in its incidence in industrialized countries.1,4 Furthermore, expanding urban slum settlements have also created environmental hotspots for Leptospira transmission.5 Therefore, leptospirosis may become even more prevalent, and it has been recently recognized as a re-emerging infectious disease.4,6 In China, leptospirosis is common and widespread, with more than 2.4 million cases and over 20,000 deaths occuring from 1955 to 2016 (Fig. 1).7–9 In this review, we briefly describe the epidemiological characteristics of leptospirosis and Leptospira spp., and summarize the development of various human leptospirosis vaccines in China. Quality control assessments of inactivated whole-cell vaccines currently used in the country are also reviewed.
Figure 1.
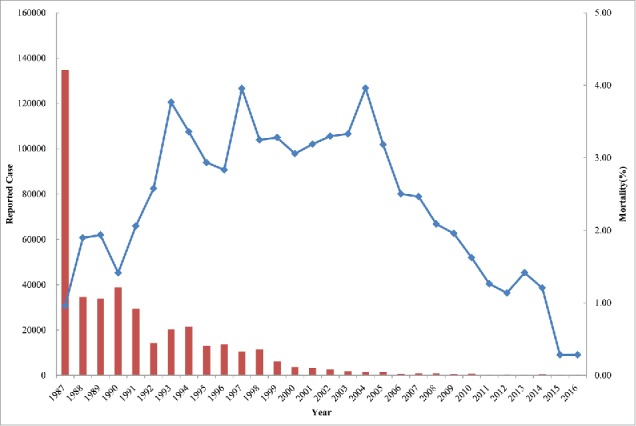
Reported leptospirosis cases and mortality in China in 1987–2016. The red columns and blue line represent the annual reported cases and disease mortality, respectively.
Leptospirosis and Leptospira spp.
The first documented case of human leptospirosis in mainland China can be traced to the 1930s.10 Leptospirosis became a reportable disease in the country after 1955,7 although the reported annual incidence in the 1950s ranged only from 0.029 to 2.21 cases per 100,000 in 10 provinces.10 However, it is likely that some cases were overlooked by physicians who were unfamiliar with the disease at the time. A severe epidemic broke out in the 1960–1970s, with the average annual incidence spiking to more than 10 cases per 100,000. During this period, more than 10 large outbreaks also occurred following severe flooding events. Such outbreaks seriously endangered public health, and affected agricultural production and disaster relief. The epidemic area also expanded during this period, with cases spreading to 26 provinces.10 In 1987, another large outbreak was reported in Sichuan province in southwest China, with 102,872 cases and 419 fatalities (Fig. 1).7,11 To prevent and control leptospiral infections, several measures were implemented in the past decades, including improvements in sanitation, water conservancy, reduction of leptospiral infection in animal hosts, and vaccination of high-risk populations. As a result, the incidence of leptospirosis has dropped to less than 1 per 100,000 since 1997, with only 355 and 354 cases reported in 2015 and 2016, respectively (Fig. 1).
In mainland China, the National Centers for Disease Control and Prevention and its provincial branches are legally required to formally report human leptospirosis cases to the Ministry of Health monthly. The provincial branches are also responsible for testing suspected human patient and animal sera, collecting infected animals, and identifying infectious isolates by culture and microscopic agglutination test according to the Diagnostic Criteria for Leptospirosis from Chinese Centers for Disease Control and Prevention.12 Results are then verified by the national agency, and finally reported to health authorities. However, the disease prevalence in humans is likely to be significantly underestimated, owing to the wide spectrum of clinical symptoms and rare use of diagnostic methods in hospitals such as the microscopic agglutination test, enzyme-linked immunosorbent assay (ELISA), and polymerase chain reaction.9,10 Indeed, leptospirosis remains endemic in China with local outbreaks still occurring in the southern provinces.9,13,14
Human leptospirosis has been reported in all provinces except Gansu, Qinghai, Ningxia, Xinjiang, and Xizang (Fig. 2).7,9 For purposes of disease control, China has been subdivided into 4 regions, designated A, B, C, and D based on incidence and geographical location. The B region, in which the incidence is highest, encompasses 13 provinces such as Sichuan, Yunan, Zhejiang, Jiangxi, and Anhui, and it is located in the middle and lower Yangtze River region (Fig. 2).10,15 Although leptospiral infections occur throughout the year, most are reported in July-October,9 presumably because the temperatures in these months, typically 25°C to 30°C, are suitable for the survival and transmission of leptospires.15 Furthermore, heavy rain and flooding are frequent during this period, inevitably increasing the risk of human infection.16 Notably, the concentration of cases has shifted from 15–35-year-olds to > 35-year-olds in 2006–2010, with the gap between these age groups continuing to increase gradually.9 In addition, 13.20% (13/98) and 13.51% (10/74) of patients more than 60 years old resided in Sichuan in 2005–2011 and in Guizhou in 2010–2014, respectively.17,18 These epidemiological changes imply that the elderly in rural areas continue to be more active in field work than in other areas, and are therefore at a higher risk of infection.
Figure 2.
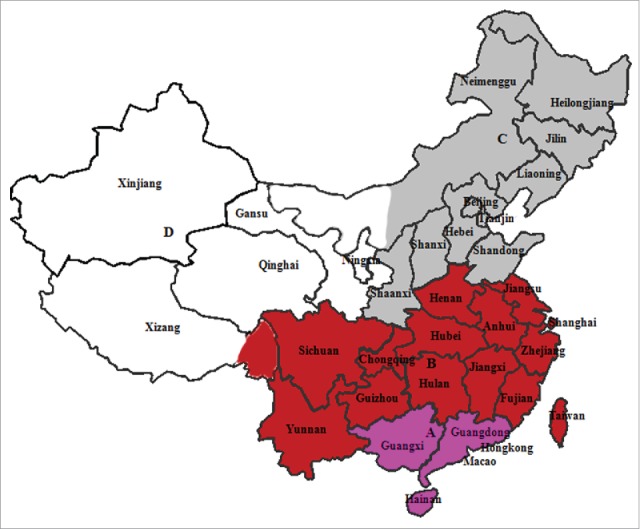
Four regions with different leptospirosis incidence and geographical locations in China.10,15 The A region (pink) is located in tropical or subtropical areas, where typhoons commonly occur, and it has moderate incidence. The B region (red), in which incidence is the highest, encompasses 13 provinces such as Sichuan, Yunan, Zhejiang, Jiangxi, Fujian and Anhui, and is located in the middle and lower Yangtze River regions. The C region (gray) comprises the temperate region located in the middle and lower areas of the Yellow River, with lower leptospirosis incidence. Meanwhile, no human leptospirosis has been reported in the D region (white), which has a dry climate.
Based on antigenic similarity, leptospires have been divided into numerous servovars. Two strains are considered to belong to different serovars if more than 10% of the homologous titer remains in at least 1 of the 2 antisera in repeated tests following the cross-absorption with adequate amounts of heterologous antigen. Serovars that are antigenically related have traditionally been grouped into serogroups.2,10,19 Thus far,, more than 300 pathogenic serovars have been clustered into 24 serogroups throughout the world.10 In China, existing and prevailing serovars are far more diverse than in other countries due to the variety of geographic and climatic conditions. More than 70 serovars in 18 serogroups of pathogenic Leptospira have been isolated from and detected in different hosts, of which some serogroups, e.g., Manhao, are only found in China.19,20 Furthermore, 38 new serovars, e.g., serogroup Icterohaemorrhagiae serovar lai, serogroup Canicola serovar dokou, and serogroup Grippotyphosa serovar liangguang, have been reported in the past fifty years.10,20 Serogroup Icterohaemorrhagiae is the most predominant epidemic strain throughout China, and other prevailing serogroups, e.g., Pomona, Canicola, Autumnalis, Grippotyphosa, and Hebdomadis, are often found to be epidemic in some regions.10,15,21 The predominant serogroups have also shifted in some areas. For example, serogroup Hebdomadis was predominant in 1988–1989 in Wuping county in Fujian province, but it was replaced by serogroup Bataviae in 1990–1991.21 The shift was believed to be closely linked to several local outbreaks,10,15 highlighting the need to monitor serogroup epidemiology in humans and host animals.
Pathogenic Leptospira have been isolated from 67 species of wild and domestic animals in China.10 In earlier years, pigs were reported to be the major animal host, although continuous improvements in large-scale pig farming over the past 20 years has greatly reduced the risk of pig exposure to contaminated water, and has decreased the infection rate accordingly.10,15 At present, Apodemus agrarius, is the most important carrier. For example, all 56 strains of spirochetes were isolated from Apodemus agrarius in the epidemic area of Guizhou province in 2011–2014.18,22
Inactivated whole-cell vaccines
Vaccination of at-risk populations remains the most viable strategy to control leptospirosis. An inactivated whole-cell vaccine was first used in the 1920s,23 and it continues to be used in humans and some animals in certain countries.24-28 Available literatures have shown that current human leptospirosis vaccines contain mono- or polyvalent inactivated whole-cell leptospires (Table 1). For example, in France, the monovalent vaccine contained the serogroup Icterohaemorrhagiae, have been demonstrated to be well-tolerated and having a high seroconversion rate (95–100%).29 The Cuban vax-SPIRAL vaccine containing serovars canicola, copenhageni and mozdok was developed in 1998 and included within the Leptospirosis Prevention and Control Program.26 It is estimated that as a result of the administration of more than 8 million doses, the morbility due to leptospirosis has greatly diminished in Cuba.30
Table 1.
Current licensed human leptospirosis vaccines.
| Products | Manufacturer/country | Vaccine components | Description of technology | Immunization program | Results from clinical trials |
|---|---|---|---|---|---|
| Trivalent inactivated vaccine26,30 | Cuba's Finlay Institute, Cuba | Containing 5–8 × 107 organisms of serogroup canicola serovar canicola, serogroup icterohemorragiae serovar copenhageni and serogroup pomona serovar mozdok in single-use package with 0.5 mL dose of vaccine | Inactivated with formaldehyde, adsorbed onto aluminum hydroxide, with 0.01% thimerosal as preservative | Intramuscular; two 0.5 mL doses administered in a 6-week interval | No serious adverse events and mild spontaneous pain at the injection site was the most frequent local effect |
| Monovalent inactivated vaccine29 | Sanofi-Pasteur, France | Containing 2 × 108 organisms of serogroup icterohemorragiae in single-use package with 0.1 mL dose of vaccine | Inactivated with formaldehyde and purified, together with sodium mercurothiolate (0.008 mg/ syringe) as a preservative | Subcutaneous or Intramuscular; 2 doses of 1 mL each at a 15-d interval, with the first and second boost dose 6 and 30 month after the first dose | Systemic reactions were rare and local reactions about within 3 h were more frequent after the booster injection. IgG seroconversion rates after the first booster were 96% (95% CI 80–100%) and reached 100% for IgG after the second booster. |
| Polyvalent inactivated vaccine25,28 | Japan | Consists of 2 × 108 organisms/mL each of leptospiral serovar australis, autumnalis, and hebdomadis and 5 × 108 organisms/mL of serovar copenhageni. | The leptospires are grown in media containing rabbit serum and/or bovine serum albumin, and inactivated with formalin. | Subcutaneous; two 1.0 mL doses given at a 7-day interval with the booster injection, and given within 5 years after the second initial dose | Unavailable* |
| Polyvalent inactivated vaccine34 | Wuhan Institue of Biological Products Co., Ltd, PR.China | Serovar lai, linhai, autumnalis, canicola, pomona, australis, hebdomadis and australis are used as leptospirosis vaccine strain.The vaccine of ≤ 5 or ≥ 6 strains should contain no less than 1.5 × 108 organisms/mL and 1.0 ´ 108 organisms/mL, respectively, but no more than 1.25 ´ 109 organisms/mL. | The leptospires are grown in protein-free synthetic media and concentrated using ultrafiltration method. Inactivated with phenol. | Subcutaneous; 0.5 mL doses administered in a 7–14 days interval with 1.0 mL dose booster injection | No serious adverse events. 7.46 %, 10.49 % and 24.63 % of subjects appeared local reactions following the first and booster at 7 days and one year after the first injection. Seroconversion rates were 95 -100 % after the first and booster, respectively. |
| Bivalent outer envelope vaccine47 | Shanghai Institue of Biological Products Co., Ltd, PR.China | Consists of 200 μg/mL each outer envelope antigens from Serogroups Icterohaemorrhagiae and Hebdomadis | The leptospires are grown in protein-free synthetic media.After the ultrafiltration concentration of the bacteria, outer envelope is extracted using SDS method. Inactivated with phenol. | Subcutaneous; single dose with 1.0 mL vaccine | No severe side-effect and abnormal reaction was found, only 2 case suffered from slight fever and local edema within 48 h after injection. Protection from outer-envelope vaccines was 95.57 % and 100 % against serogroups Icterohaemorrhagiae and Hebdomadis within one year |
*limited literature was available because the clinical studies were performed more than 40 years ago in Japan,
In China, a leptospirosis vaccine was successfully developed in 1958, which has been used for immunization of risk populations of epidemic regions till now.8,10,31 With advancements in bio-technology, the vaccine production has gradually improved.10 In 1958–1962, a trivalent inactivated vaccine containing serogroups Icterohaemorrhagiae, Autumnalis, and Pyrogenes was used for large-scale vaccination in epidemic areas, and it greatly diminished morbidity. However, approximately 15% of vaccinees developed allergies, since 3% rabbit serum was used to produce the vaccine.15 To address this issue, a serum-free medium containing human placenta extract was developed in 1963, and extensively used to produce an equally effective, but nearly nonallergenic vaccine in 1965–1975.10 However, with the limited availability of human placenta extract and the rapid development of chemically defined media,32,33 the medium was again gradually replaced with protein-free synthetic media in the 1970s. Other processes were also improved; for example, large, deeply ventilated culture flasks and hollow fiber ultrafiltration have been used to concentrate bacterial cells.10 In 2004, a concentrated pentavalent vaccine was tested for safety and immunogenicity in Fujian province, and was found to elicit no systemic reactions after immunization. However, 7.46% (15/201), 10.49% (17/162) and 24.63% (17/69) of subjects developed local reactions following the first and second booster at 7 days and 1 year after the first injection, respectively,34 suggesting that local reactions may slowly intensify after booster doses. Nevertheless, these results were consistent with other studies demonstrating good tolerance of and only low-grade local reactions from inactivated leptospirosis vaccine produced in other countries.27,29 Seroconversion rates were also as high as 95% and 100% after the first and second booster, respectively.34 Furthermore, a field trial in epidemic areas in Hubei province in 1999 demonstrated that tested vaccines were 85.34% effective by the end of surveillance,35 confirming the efficacy of leptospirosis vaccines.27,29
However, immunity from inactivated leptospirosis vaccines is serovar-specific, although potential cross-protection might be acquired from some serogroups or serovars.24,36 Hence, a universal inactivated vaccine is challenging to develop owing to the large variability in local leptospiral strains in different countries or regions.1,2,37-39 Furthermore, protection from inactivated vaccines lasts no longer than about 1 year23,24,34 when administered in 2 doses 7 days apart,34 although strong and specific antibody responses persisted for 2 years after primary vaccination25,29 with 2 doses 14 days apart and a booster after 6 months.29
Inactivated whole-cell leptospiral vaccines have been produced for many years in China at 5 mL per ampoule. However, single-dose formulations have become standard for human vaccines to minimize waste and potential pollution. Thus, the formulation of inactivated leptospiral vaccines remains to be improved. On the other hand, clinical trials for these vaccines were completed in the 1980–1990s.34,35 As the average life expectancy was 67.77 years in 1981 in China, individuals older than 60 years old were not considered at-risk, and the vaccine was indicated only for individuals aged 7–60 years. However, the average life expectancy has risen to 76 years in 2013,40 perhaps coinciding with the gradual increase in incidence in people over 60 years old, as noted earlier.17,18 Hence, the target population for vaccination should be expanded to enhance prevention.
Outer-envelope vaccines
Due to inherent side-reactions and short-lived protection from inactivated vaccines, other potential vaccines have been developed in some countries.10,23,25 For example, many studies demonstrated that the leptospiral outer envelope is immunogenic and protective, even in animal hosts such as dogs and cattle.41-43 The outer envelope surrounds the slender, helically coiled leptospires; is approximately 11 nm in width; and consists of 3–5 electron-dense layers44 with a gross chemical composition of 23% lipid, 47% protein, and 23% carbohydrate.45
The development of human vaccines based on the outer envelope can be traced to the 1970s in China.46-48 Of note, comparative clinical trials in 1993 showed that bivalent outer-envelope vaccines from the serogroups Icterohaemorrhagiae and Hebdomadis elicited fewer side-effects than whole-cell vaccines. Importantly, immunity acquired from a single dose of 100 μg outer envelope was comparable to that acquired from 2 doses of whole-cell vaccines.49 Large phase III clinical trials further demonstrated that protection from outer-envelope vaccines was 95.57% and 100% against the serogroups Icterohaemorrhagiae and Hebdomadis within 1 year, respectively. Accordingly, such vaccines were licensed in China for immunization of at-risk populations in epidemic regions with circulating serogroups Icterohaemorrhagiae and Hebdomadis.47 Trivalent or pentavalent outer-envelope vaccines, which should protect against more serovars, have also been shown to be as well-tolerated and immunogenic as the corresponding multivalent whole-cell vaccines.50
Considering the chemical composition of the outer envelope and the high titer of agglutinating antibodies induced, the most immunodominant antigen is believed to be lipopolysaccharide.44 However, lipopolysaccharide fractions were found to protect against homologous, but not heterologous serogroups,51,52 implying that immunity from outer-envelope vaccines appears to also be serogroup-specific.53,54
Recombinant vaccines
Advances in molecular techniques have enabled the search for novel antigens, proteins, and genes that may better protect against leptospirosis.23,55,56 Indeed, many studies have demonstrated that recombinant antigens potently elicit protective immunity. Such antigens include multiple leptospiral outer membrane proteins and lipoproteins that play vital roles in pathogenesis, such as OmpL1,57 immunoglobulin-like protein,58-60 LipL21,61 LipL32,62 LipL36,63 LipL 41,57 and LipL 45.64
Researchers in China have also investigated the protective characteristics of recombinant OmpL1, LipL21, LipL32, and LipL41 in animal models, and identified conserved T and B cell epitopes in these proteins.65-67 Recently, Lin et al. expressed and purified a recombinant, chimeric protein containing multiple epitopes from leptospiral OmpL1, LipL32, and LipL21, and found that it induces wide-ranging protection against Leptospira, suggesting that the recombinant antigens might yield a universal cross-reactive vaccine.68
The rapid development of high-throughput sequencing has also enabled reverse vaccinology against many infectious diseases.69-71 In 2005, Gamberini et al. identified potential vaccine candidates from the genome of Leptospira interrogans serovar Copenhageni using reverse vaccinology. Accordingly, 150 coding sequences were cloned and expressed in Escherichia coli, of which 16 reacted with sera from infected patients, indicating that these proteins may be useful as vaccine antigens.72 Similarly, 70% of 238 recombinant proteins identified by reverse vaccinology were found to be immunogenic in hamsters, although none of 49 combinations of up to 5 antigens protected the animals against infection.73 Recently, Zeng and colleagues implemented a pan-genomic screening of surface-exposed proteins from 17 representative L. interrogans strains covering 11 epidemic serovars and 17 multilocus sequence types from around the world. In addition to several known outer membrane proteins and lipoproteins, 118 new candidate antigens were identified.74 To date, more than 300 genomes from leptospiral strains isolated worldwide are available in public databases,75-77 and are anticipated to be a rich resource for identifying potential vaccine antigens by reverse vaccinology. Nevertheless, extensive studies remain necessary to pinpoint potential vaccine components that are actually protective.55
Although recombinant leptospirosis vaccines remain in preclinical development stage, outer membrane proteins and lipoproteins are generally accepted to be among the most potent immunogens that elicit remarkable immune responses during infection,10,23,78 and are thus currently considered leading candidate vaccine antigens.16,47,52,61,64,66,68 In addition, many of these proteins, unlike lipopolysaccharides, are antigenically conserved among many pathogenic Leptospira species regardless of the serovar or serogruop.61,62,78-80 A shortcoming associated with inactivated whole-cell vaccines is that the immune response induced is mainly directed against leptospiral lipopolysaccharide, which protects against infection by closely related serovars.23,25 In contrast, cross-species protection induced recombinant protein vaccines confer protection against many Leptospira species, implying that recombinant vaccines may prove to be universal.65,68 Furthermore, recombinant antigens can be easily combined in various configurations to further improve cross-protective immunity,73,81,82 as is achieved by a recently licensed Group B meningococcal vaccine that contains a mix of the New Zealand vaccine, NadA, and the recombinant proteins NHBA-GNA1030 and fHbp-GNA2091.71,83 Haake et al. reported that a combination of OmpL1 and LipL41 provides significant levels of protection 28 days after challenge in comparison to OmpL1 alone.57 Similarly, recombinant forms of the putative outer membrane proteins Lp1454, rLp1118, and rMceII are also more protective when administered together.84
Importantly, the addition of adjuvants may also improve the efficiency of multicomponent recombinant vaccines and lower vaccination frequency.85,86 Although aluminum hydroxide is the most commonly used adjuvant, Faisal and colleagues reported that leptospiral LigA confers better protection when formulated with liposomes and microspheres instead of aluminum hydroxide, as evidenced by enhanced survival and reduced histopathological lesions in immunized animals.87 Furthermore, a pool of LigA, LIC10009, LIC10301, LIC10507, LIC10704, LIC11030, and LIC11087 adjuvanted with flagellin confers protection and significantly inhibits renal colonization, while adjuvantation with aluminum hydroxide induces protection only, but does not prevent renal colonization.82 In contrast, leptospiral immunoglobulin-like B protein adsorbed on aluminum hydroxide confers sterile immunity against lethal challenge in hamsters.88 Collectively, these observations suggest that potential antigens and adjuvants should be carefully selected in developing recombinant leptospirosis vaccines.
Quality control of leptospirosis vaccines
Only a single multivalent, inactivated leptospirosis vaccine from a domestic manufacturer is currently available in China, which was added to the Chinese Expanded Program on Immunization in 2007. Three highly virulent (serogroups Icterohaemorrhagiae, Grippotyphosa, and Autumnalis) and 4 low-virulence strains (serogroups Canicola, Pomona, Australis, and Hebdomadis) are used as vaccine strains, which are the major L. interrogans serogroups and cover more than 80% serogroup coverage of circulating strains in the country.9,10 The vaccine is recommended for at-risk populations during annual epidemic periods.31
Since the vaccines are used in healthy populations, it is essential to ensure consistent quality in each vaccine lot released to the market. Accordingly, the Chinese government has required since January 1, 2006 that all marketed preventative vaccines be released lot by lot by the national regulatory authority,89,90 following review and independent testing. The review evaluates whether critical raw materials and manufacturing processes are consistent with approved parameters, and whether the vaccine bulk and final product meet the current national pharmaceutical criteria.31,90 Independent tests include tests of identity and sterility.
Naturally, strain quality directly impacts the quality of the final inactivated whole-cell vaccine.91 Unlike other bacterial pathogens, the virulence of pathogenic Leptospira spp. gradually diminishes after 3–6 passages in vitro.92 Hence, strains are passaged in guinea pigs prior to production to preserve virulence, which is closely associated with efficacy. Accordingly, specific physicochemical and microbiological parameters such as morphological and cultural characteristics, virulence, immunogenicity, and antigenicity (Table 2) are prescribed by the Chinese Pharmacopoeia (2015 Edition)31 for strains to be used as vaccines. Molecular tools were also recently used to assess genetic stability and further guarantee quality, in the light of possible bacterial adaptation to the animal host and during in vitro culture.93,94 For example, pulsed-field gel electrophoresis and multilocus sequence typing are reproducible and reliable tests of identity and genetic stability,91 and can be combined with existing tests for a more comprehensive evaluation of biological quality.
Table 2.
Quality control tests for leptospiral vaccine strains used as vaccines in China.
| Test | Method and specification |
|---|---|
| Morphological and cultural characteristics | Leptospiral vaccine strains shall be inoculated into production medium at less than 5 %, and incubated at 28–32°C for 5–10 days. The cultures shall contain more than 100 organisms per high-power field at 400 ×. Leptospires appear as motile rods with regular shape and with both ends curved. |
| Serum agglutination | The microscopic agglutination test using serogroup reference serum shall be performed on leptospiral vaccine strains grown for 3–10 days and adjusted to 50–100 organisms per high-power field at 400 ×. The agglutination titer shall be no less than half of the titer of the reference serum. |
| Virulence | Six guinea pigs (180–220 g), divided into 2 groups, are subcutaneously injected with 2 mL of leptospiral vaccine strains grown for 5–10 days and adjusted to 50–100 organisms per high-power field at 400 ×. In 1 group, the blood shall be drawn from the heart 48 h after injection, and inoculated into the production medium or other appropriate media, and incubated for 14 days. The strain shall be considered low-virulence if growth is observed. In the other group, the guinea pigs are observed for up to 10 days after injection. The strain is considered highly virulent if at least 2 animals die of leptospirosis. |
| Immunogenicity | Three guinea pigs (120–220 g) are subcutaneously immunized with 0.5 mL leptospiral vaccine strains inactivated by heating at 56–58°C for 1 h or by adding 3.0 g/L phenol, adjusted to 70–100 organisms per high-power field at 400 ×, and diluted 3-fold with saline. A boost of 1 mL inactivated culture is administered at 5-day intervals. Three control animals are injected with equal volumes of saline. On days 10–12 after the last injection, the animals shall be challenged subcutaneously with 2 mL of the same strain grown for 5–10 days and adjusted to 50–100 organisms per high-power field at 400 ×. For highly virulent strains, the animals shall be observed for up to 10 days after challenge. All immunized animals shall survive, and have normal appearance, appetite, activity, and weight gain, but have no piloerection or jaundice. At least 2 control animals should die of leptospirosis. For low-virulence strains, blood will be collected from the heart 24 h after challenge, inoculated at about 1 % into Leptospira medium containing 5–8 % rabbit serum, and incubated for 14 days. The test shall be considered passed only if more than 2 cultures from immunized animals are negative, and all cultures from control animals are positive. |
| Antigenicity | Three healthy rabbits (2.0–2.5 kg) are intravenously immunized at 5-day intervals with 1, 2, and 5 mL leptospiral vaccine strains inactivated by heating at 56–58°C for 1 h or by adding 3.0 g/L phenol, and adjusted to 70–100 organisms per high-power field at 400 ×. On days 10–15 after the last injection, rabbit sera are collected, and tested by the microscopic agglutination test against the same strain. The test shall be considered passed if the serum titers of at least 2 rabbits are 1:10,000 or more. |
*Three highly virulent (serogroups Icterohaemorrhagiae serovar lai, serogroup Grippotyphosa serovar linhai, and serogroup Autumnalis serovar autumnalis) and 4 low-virulence strains (serogroup Canicola serovar canicola, serogroup Pomona serovar pomona, serogroup Australis serovar australis, and serogroup Hebdomadis serovar hebdomadis) are currently used.
Bacterial culture bulks are inactivated with phenol or other appropriate bactericides. According to the Chinese pharmacopeia,31 a vaccine of ≤ 5 or ≥ 6 strains should contain no less than 1.5 × 108 organisms/mL and 1.0 × 108 organisms/mL, respectively, but no more than 1.25 × 109 organisms/mL. The quality control tests required by the Chinese pharmacopoeia31 for the final leptospirosis vaccine are listed in Table 3, along with specifications for safety and potency. Of note, potency is tested in European and North American countries by hamster vaccination and challenge,95,96 although both guinea pigs and hamsters were instrumental in understanding the pathophysiology of lethal leptospirosis.97 However, animal challenge method has been heavily criticized on the grounds of animal welfare. Thus, an alternative rabbit serological potency assay, which avoided the use of challenge and decreased the number of animals required, was validated and authorized for canine Leptospira vaccines in the European Union.98 Furthermore, in vitro ELISAs were also developed to test the potency of US vaccines containing L. canicola, L. grippotyphosa, L. icterohaemorrhagiae, and L. pomona.95 Nevertheless, similar or other alternative assays should still be revalidated in China due to differences in vaccine production and desired clinical outcomes. On the other hand, early biomarkers of adaptive and protective immunity would be valuable, and system immunology data from genomic and proteomic studies may facilitate the development of alternative assays.77
Table 3.
Quality control tests for the final leptospirosis vaccines used in China.
| Test | Specification |
|---|---|
| Identity | The microscopic agglutination test shall be performed on the final product using serogroup reference sera, and specific agglutination shall be observed. |
| Physical inspection | The product is a slightly opalescent liquid free of abnormal odor, foreign matter, or clumps not dispersed on shaking. |
| pH | 6.4–7.4 |
| Sodium chloride content | 7.5–9.5 g/L |
| Phenol content | Not more than 3.0 g/L |
| Potency | The final product is diluted with physiological saline to 5 × 107 organisms/mL, and tested by the immunogenicity test described in Table 1. |
| Sterility | Sterile |
| Abnormal toxicity | Immunized animals should gain weight and survive without abnormality. |
Conclusion
Although the incidence of leptospirosis has greatly diminished in the past 20 years, small localized outbreaks continue to occur in some regions in China due to the diversity of pathogenic Leptospira species and animal hosts, as well as frequent flooding and typhoons. This also implies that the burden of leptospirosis likely continues to be substantial, but is unknown because of limited deployment of diagnostic methods. Therefore, reducing infection in animal reservoirs should continue to be a top priority. Similarly, immunization of high-risk populations in epidemic regions, as well as those previously considered low-risk, should also reduce the disease burden, as it has in the past decades. However, serogroup epidemiology should also be monitored to guide the production of appropriate inactivated vaccines, which induce only serogroup-specific immunity. In addition, the quality of inactivated leptospirosis vaccines should be guaranteed by appropriate testing, or improved by optimizing formulations. Suitable tests to replace animal testing are also urgently needed on grounds of animal welfare. Finally, the availability of numerous genome sequences, combined with advances in reverse vaccinology facilitate the high-throughput screening and discovery of potential vaccine candidates, and further investments are required to develop a universal recombinant leptospirosis vaccine, considering the short-lived serogroup-specific immunity acquired from current vaccines.
Disclosure of potential conflicts of interest
The authors declare that they have no competing interests.
Funding
This work was supported by National Major Scientific and Technological Special Project for “Major new drug development” (No. 2013ZX09304101) from the Ministry of Science and Technology, China and The National natural science Funds, China (No. 81471968), and NIFDC Professional Leader Fund (No.2013 × 4).
References
- 1.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, et al.. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–71. doi: 10.1016/S1473-3099(03)00830-2. PMID:14652202. [DOI] [PubMed] [Google Scholar]
- 2.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. PMID:11292640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gouveia EL, Metcalfe J, de Carvalho AL, Aires TS, Villasboas-Bisneto JC, Queirroz A, Santos AC, Salgado K, Reis MG, Ko AI. Leptospirosis-associated severe pulmonary hemorrhagic syndrome, Salvador, Brazil. Emerg Infect Dis. 2008;14:505–8. doi: 10.3201/eid1403.071064. PMID:18325275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, Stein C, Abela-Ridder B, Ko AI. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS NeglTrop Dis. 2015;9:e0003898. doi: 10.1371/journal.pntd.0003898. PMID:26379143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reis RB, Ribeiro GS, Felzemburgh RD, Santana FS, Mohr S, Melendez AX, Queiroz A, Santos AC, Ravines RR, Tassinari WS, et al.. Impact of environment and social gradient on Leptospira infection in urban slums. PLoS NeglTrop Dis. 2008;2:e228. doi: 10.1371/journal.pntd.0000228. PMID:18431445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meites E, Jay MT, Deresinski S, Shieh WJ, Zaki SR, Tompkins L, Smith DS. Reemerging leptospirosis, California. Emerg Infect Dis. 2004;10:406–12. doi: 10.3201/eid1003.030431. PMID:15109405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi M, Shao Z, Chen J, He J, Liu L, Guo S. Analysis of epidemic situation of leptospirosis in China from 1987 to 1997. Dis Suveillance. 1998;13:460–2. [Google Scholar]
- 8.Shi M, Jiang X. Leptospirosis in the past fifty years in China. Chin J Epidemiol. 2000;21:228–30. [Google Scholar]
- 9.Liu B, Ding F, Jiang X, Yin W. Epidemiology of leptospirosis in China, 2006–2010. Dis Surveillance. 2012;27:46–50. [Google Scholar]
- 10.Yan J, Dai B, Yu E. Leptospirosis. 3rd Ed Beijing: People's Medical Publisheing House; 2006. [Google Scholar]
- 11.Wu M. Epidemiological analysis on the outbreak of Leptospira in Sichuan province in 1987. Dis Surveillance. 1987;3:110–2. [Google Scholar]
- 12.National Institute for Communicable Disease Control and Prevention Diagnostic criteria for Leptospirosis (WS290-2008). Beijing: People's Medical Publishing House (PMPH); 2008. [Google Scholar]
- 13.Li Z, Jiang L, Zhang M, Li X, Ying K, Lin G. Surveillance of Leptospira after leptospirosis outbreak in Pan'an county, Zhejiang. Chin J Vector Biol Control. 2013;24:272–4. [Google Scholar]
- 14.Wang Y, Yang X, Yu B, Yang H, Yang F, Zhang Q, Ma H, Li Z, Peng H. Investigation of leptospirosis infection in patients with fever of unknown origin in Yunnan province. China Trop Med. 2013;13:1480–2. [Google Scholar]
- 15.Zhang C, Wang H, Yan J. Leptospirosis prevalence in Chinese populations in the last two decades. Microbes Infect. 2012;14:317–23. doi: 10.1016/j.micinf.2011.11.007. PMID:22155621. [DOI] [PubMed] [Google Scholar]
- 16.Sarkar U, Nascimento SF, Barbosa R, Martins R, Nuevo H, Kalofonos I, Grunstein I, Flannery B, Dias J, Riley LW, et al.. Population-based case-control investigation of risk factors for leptospirosis during an urban epidemic. Am J Trop Med Hyg. 2002;66:605–10. doi: 10.4269/ajtmh.2002.66.605. PMID:12201599. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Tian H. Leptospirosis in Anyue County, 2005–2011. Parasitoses Infect Dis. 2012;10:201–3. [Google Scholar]
- 18.Liu Y, Chen Z, Li S, Yao G, Huang H, Ma Q, Zhou J, Tang G, Wang D. Epidemic characteristics of leptospirosis in Guizhou province from 2010 to 2014. Chin J Zoonose. 2016;32:1034–38. [Google Scholar]
- 19.Kmety E, Dikken H. Classification of the species Leptospira interrogans and history of its serovars. 104 pp. Groningen: University Press; 1993. [Google Scholar]
- 20.Xu Y, Zheng H, Zhang Y, Wang Y, Zhang J, Li Z, Cui S, Xin X, Ye Q, Chang YF, et al.. Genomic Analysis of a New Serovar of Leptospira weilii Serogroup Manhao. Front Microbiol. 2017;8:149. doi: 10.3389/fmicb.2017.00149. PMID:28210253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu A, Qin J, Shi M, Tu Y, Yang W, Li Q, Xu H, Liang Z. Distribution of serogroup of pathogenic Leptospira in China. Chin J Zoonose. 1995;11:38–40. [Google Scholar]
- 22.Li S, Wang D, Zhang C, Wei X, Tian K, Li X, Nie Y, Liu Y, Yao G, Zhou J, et al.. Source tracking of human leptospirosis: serotyping and genotyping of Leptospira isolated from rodents in the epidemic area of Guizhou province, China. BMC Microbiol. 2013;13:75. doi: 10.1186/1471-2180-13-75. PMID:23548108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adler B. Vaccines against leptospirosis. Curr Top Microbiol Immunol. 2015;387:251–72. PMID:25388138. [DOI] [PubMed] [Google Scholar]
- 24.Verma R, Khanna P, Chawla S. Whole-cell inactivated leptospirosis vaccine: future prospects. Hum Vaccin Immunother. 2013;9:763–5. doi: 10.4161/hv.23059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koizumi N, Watanabe H. Leptospirosis vaccines: past, present, and future. J Postgrad Med. 2005;51:210–4. PMID:16333195. [PubMed] [Google Scholar]
- 26.Martinez R, Perez A, Quinones Mdel C, Cruz R, Alvarez A, Armesto M, Fernandez C, Menendez J, Rodriguez I, Baro M, et al.. [Efficacy and safety of a vaccine against human leptospirosis in Cuba]. Rev Panam Salud Publica. 2004;15:249–55. PMID:15193180. [DOI] [PubMed] [Google Scholar]
- 27.Martinez Sanchez R, Perez Sierra A, Baro Suarez M, Alvarez AM, Menendez Hernandez J, Diaz Gonzalez M, Cruz de la Paz R, de los Reyes G, Montoya Batista B, Sierra Gonzalez G, et al.. [Evaluation of the effectiveness of a new vaccine against human leptospirosis in groups at risk]. Rev Panam Salud Publica. 2000;8:385–92. PMID:11209251. [DOI] [PubMed] [Google Scholar]
- 28.Masuzawa T, Suzuki R, Yanagihara Y. Comparison of protective effects with tetra-valent glycolipid antigens and whole cell-inactivated vaccine in experimental infection of Leptospira. Microbiol Immunol. 1991;35:199–208. doi: 10.1111/j.1348-0421.1991.tb01548.x. PMID:1870437. [DOI] [PubMed] [Google Scholar]
- 29.Laurichesse H, Gourdon F, Smits HL, Abdoe TH, Estavoyer JM, Rebika H, Pouliquen P, Catalina P, Dubray C, Beytout J. Safety and immunogenicity of subcutaneous or intramuscular administration of a monovalent inactivated vaccine against Leptospira interrogans serogroup Icterohaemorrhagiae in healthy volunteers. Clin Microbiol Infect. 2007;13:395–403. doi: 10.1111/j.1469-0691.2007.01662.x. PMID:17359323. [DOI] [PubMed] [Google Scholar]
- 30.Martínez R. vax-SPIRAL®: Cuban antileptospirosis vaccines for humans: Clinical and field assays and impact of the vaccine on the disease after 11 years of application in Cuba. Int J Infect Dis. 2010, 14(S1):e448. doi: 10.1016/j.ijid.2010.02.613. PMID:19700358.19700358 [DOI] [Google Scholar]
- 31.National Pharmacopoeia Committee The Chinese Pharmacopoeia (2015 version) 3rd Part. Beijing: Chinese Medical Science and Technology Press; 2015. [Google Scholar]
- 32.Shenberg E. Growth of pathogenic Leptospira in chemically defined media. J Bacteriol. 1967;93:1598–606. PMID:6025446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shenberg E, Torten M. A new leptospiral vaccine for use in man. I. Development of a vaccine from Leptospira grown on a chemically defined medium. J Infect Dis. 1973;128:642–6. doi: 10.1093/infdis/128.5.642. PMID:4768915. [DOI] [PubMed] [Google Scholar]
- 34.Xu G, Pan M, Lin G, Yan Y, Wang L, L P. Observation on the immune efficiency after vaccination with new pentavalent whole cell vaccine for leptospirosis in Fujian province. Chin J Zoonose. 2005;21:1020. [Google Scholar]
- 35.Yan Y. Epidemiological efficiency of large – scale field trial of whole cell Leptospira vaccine. Chin J Biologicals. 2000;13:188–9. [Google Scholar]
- 36.Maneewatch S, Sakolvaree Y, Saengjaruk P, Srimanote P, Tapchaisri P, Tongtawe P, Klaysing B, Wongratanacheewin S, Chongsa-Nguan M, Chaicumpa W. Monoclonal antibodies to LipL32 protect against heterologous Leptospira spp. challenge. Hybridoma. 2008;27:453–65. doi: 10.1089/hyb.2008.0056. PMID:19108618. [DOI] [PubMed] [Google Scholar]
- 37.Dreyfus A, Dyal JW, Pearson R, Kankya C, Kajura C, Alinaitwe L, Kakooza S, Pelican KM, Travis DA, Mahero M, et al.. Leptospira Seroprevalence and Risk Factors in Health Centre Patients in Hoima District, Western Uganda. PLoS NeglTrop Dis. 2016;10:e0004858. doi: 10.1371/journal.pntd.0004858. PMID:27487398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ayral FC, Bicout DJ, Pereira H, Artois M, Kodjo A. Distribution of Leptospira serogroups in cattle herds and dogs in France. Am J Trop Med Hyg. 2014;91:756–9. doi: 10.4269/ajtmh.13-0416. PMID:25092816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Assenga JA, Matemba LE, Muller SK, Mhamphi GG, Kazwala RR. Predominant leptospiral serogroups circulating among humans, livestock and wildlife in Katavi-Rukwa ecosystem, Tanzania. PLoS NeglTrop Dis. 2015;9:e0003607. doi: 10.1371/journal.pntd.0003607. PMID:25806825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization. Geneva: Available at: http://apps.who.int/gho/data/view.main.680?lang=en. [Google Scholar]
- 41.Bey RF, Auran NE, Johnson RC. Immunogenicity of whole cell and outer envelope leptospiral vaccines in hamsters. Infect Immun. 1974;10:1051–6. PMID:16558088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Auran NE, Johnson RC, Ritzi DM. Isolation of the outer sheath of Leptospira and its immunogenic properties in hamsters. Infect Immun. 1972;5:968–75. PMID:4635506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bey RF, Johnson RC. Leptospiral vaccines in dogs: immunogenicity of whole cell and outer envelope vaccines prepared in protein-free medium. Am J Vet Res. 1982;43:831–4. PMID:7091847. [PubMed] [Google Scholar]
- 44.Jost BH, Adler B, Faine S. Reaction of monoclonal antibodies with species specific determinants in Leptospira interrogans outer envelope. J Med Microbiol. 1988;27:51–7. doi: 10.1099/00222615-27-1-51. PMID:2459387. [DOI] [PubMed] [Google Scholar]
- 45.Zeigler JA, VanEseltine WP. Isolation and chemical characterization of outer envelope of Leptospira pomona. Can J Microbiol. 1975;21:1102–12. doi: 10.1139/m75-160. PMID:1148944. [DOI] [PubMed] [Google Scholar]
- 46.Bao XH. [The effect of leptospiral outer envelope vaccine. IV. A study among a small population]. Zhonghua Liu Xing Bing Xue Za Zhi. 1984;5:212–5. PMID:6509528. [PubMed] [Google Scholar]
- 47.Cheng J, Qin J, Xie G. [Epidemiological observation on effect of Leptospiral outer membrane vaccine]. Zhonghua Liu Xing Bing Xue Za Zhi. 2001;22:108–10. PMID:11860856. [PubMed] [Google Scholar]
- 48.Yan Y, Chen Y, Liou W, Ding J, Chen J, Zhang J, Zhang A, Zhou W, Gao Z, Ye X, et al.. An evaluation of the serological and epidemiological effects of the outer envelope vaccine to leptospira. J Chin Med Assoc. 2003;66:224–30. PMID:12854874. [PubMed] [Google Scholar]
- 49.Zhang J, Xie G, Qin J, Yu L, Zhu F, Gao J, Wang Z, Wang Y, Hu M, Hu J. Obsevation on the immunologic response of inequality dose of leptospira envelope vaccine. Chin J Zoonoses. 1996;12:28–31. [Google Scholar]
- 50.Xie G, Zhang J, Qin J, Zhu F, Yu L, Zhang Y, Wang Y, Wu M, Hu J, Wang Z, et al.. Safety and immunogenicity of trivalent and pentavalent leptospira outer membrane vaccine. Chin J Zoonoses. 2000;16:38–40. [Google Scholar]
- 51.Challa S, Nally JE, Jones C, Sheoran AS. Passive immunization with Leptospira LPS-specific agglutinating but not non-agglutinating monoclonal antibodies protect guinea pigs from fatal pulmonary hemorrhages induced by serovar Copenhageni challenge. Vaccine. 2011;29:4431–4. doi: 10.1016/j.vaccine.2011.04.041. PMID:21549788. [DOI] [PubMed] [Google Scholar]
- 52.Sonrier C, Branger C, Michel V, Ruvoen-Clouet N, Ganiere JP, Andre-Fontaine G. Evidence of cross-protection within Leptospira interrogans in an experimental model. Vaccine. 2000;19:86–94. doi: 10.1016/S0264-410X(00)00129-8. PMID:10924790. [DOI] [PubMed] [Google Scholar]
- 53.Bey RF, Johnson RC. Immunogenicity and humoral and cell-mediated immune responses to leptospiral whole cell, outer envelope, and protoplasmic cylinder vaccines in hamsters and dogs. Am J Vet Res. 1982;43:835–40. PMID:7201285. [PubMed] [Google Scholar]
- 54.Bey RF, Johnson RC. Humoral immune response of dogs vaccinated with leptospiral pentavalent outer envelope and whole culture vaccines. Am J Vet Res. 1978;39:831–6. PMID:727583. [PubMed] [Google Scholar]
- 55.Dellagostin OA, Grassmann AA, Rizzi C, Schuch RA, Jorge S, Oliveira TL, McBride AJ, Hartwig DD. Reverse Vaccinology: An Approach for Identifying Leptospiral Vaccine Candidates. Int J Mol Sci. 2017;18:E158. doi: 10.3390/ijms18010158. PMID:28098813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dellagostin OA, Grassmann AA, Hartwig DD, Felix SR, da Silva EF, McBride AJ. Recombinant vaccines against leptospirosis. Hum Vaccin. 2011;7:1215–24. doi: 10.4161/hv.7.11.17944. PMID:22048111. [DOI] [PubMed] [Google Scholar]
- 57.Haake DA, Mazel MK, McCoy AM, Milward F, Chao G, Matsunaga J, Wagar EA. Leptospiral outer membrane proteins OmpL1 and LipL41 exhibit synergistic immunoprotection. Infect Immun. 1999;67:6572–82. PMID:10569777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin YP, Chang YF. A domain of the Leptospira LigB contributes to high affinity binding of fibronectin. Biochem Biophys Res Commun. 2007;362:443–8. doi: 10.1016/j.bbrc.2007.07.196. PMID:17707344. [DOI] [PubMed] [Google Scholar]
- 59.Faisal SM, Yan W, Chen CS, Palaniappan RU, McDonough SP, Chang YF. Evaluation of protective immunity of Leptospira immunoglobulin like protein A (LigA) DNA vaccine against challenge in hamsters. Vaccine. 2008;26:277–87. doi: 10.1016/j.vaccine.2007.10.029. PMID:18055070. [DOI] [PubMed] [Google Scholar]
- 60.Cao Y, Faisal SM, Yan W, Chang YC, McDonough SP, Zhang N, Akey BL, Chang YF. Evaluation of novel fusion proteins derived from extracellular matrix binding domains of LigB as vaccine candidates against leptospirosis in a hamster model. Vaccine. 2011;29:7379–86. doi: 10.1016/j.vaccine.2011.07.070. PMID:21803087. [DOI] [PubMed] [Google Scholar]
- 61.Cullen PA, Haake DA, Bulach DM, Zuerner RL, Adler B. LipL21 is a novel surface-exposed lipoprotein of pathogenic Leptospira species. Infect Immun. 2003;71:2414–21. doi: 10.1128/IAI.71.5.2414-2421.2003. PMID:12704111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haake DA, Chao G, Zuerner RL, Barnett JK, Barnett D, Mazel M, Matsunaga J, Levett PN, Bolin CA. The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection. Infect Immun. 2000;68:2276–85. doi: 10.1128/IAI.68.4.2276-2285.2000. PMID:10722630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haake DA, Martinich C, Summers TA, Shang ES, Pruetz JD, McCoy AM, Mazel MK, Bolin CA. Characterization of leptospiral outer membrane lipoprotein LipL36: downregulation associated with late-log-phase growth and mammalian infection. Infect Immun. 1998;66:1579–87. PMID:9529084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsunaga J, Young TA, Barnett JK, Barnett D, Bolin CA, Haake DA. Novel 45-kilodalton leptospiral protein that is processed to a 31-kilodalton growth-phase-regulated peripheral membrane protein. Infect Immun. 2002;70:323–34. doi: 10.1128/IAI.70.1.323-334.2002. PMID:11748198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin X, Sun A, Ruan P, Zhang Z, Yan J. Characterization of conserved combined T and B cell epitopes in Leptospira interrogans major outer membrane proteins OmpL1 and LipL41. BMC Microbiol. 2011;11:21. doi: 10.1186/1471-2180-11-21. PMID:21269437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dong H, Hu Y, Xue F, Sun D, Ojcius DM, Mao Y, Yan J. Characterization of the ompL1 gene of pathogenic Leptospira species in China and cross-immunogenicity of the OmpL1 protein. BMC Microbiol. 2008;8:223. doi: 10.1186/1471-2180-8-223. PMID:19087358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin X, Zhao J, Qian J, Mao Y, Pan J, Li L, Peng H, Luo Y, Yan J. Identification of immunodominant B- and T-cell combined epitopes in outer membrane lipoproteins LipL32 and LipL21 of Leptospira interrogans. Clin Vaccine Immunol. 2010;17:778–83. doi: 10.1128/CVI.00405-09. PMID:20237196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin X, Xiao G, Luo D, Kong L, Chen X, Sun D, Yan J. Chimeric epitope vaccine against Leptospira interrogans infection and induced specific immunity in guinea pigs. BMC Microbiol. 2016;16:241. doi: 10.1186/s12866-016-0852-y. PMID:27737644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seib KL, Zhao X, Rappuoli R. Developing vaccines in the era of genomics: a decade of reverse vaccinology. Clin Microbiol Infect. 2012;18(Suppl 5):109–16. doi: 10.1111/j.1469-0691.2012.03939.x. PMID:22882709. [DOI] [PubMed] [Google Scholar]
- 70.Bowman BN, McAdam PR, Vivona S, Zhang JX, Luong T, Belew RK, Sahota H, Guiney D, Valafar F, Fierer J, et al.. Improving reverse vaccinology with a machine learning approach. Vaccine. 2011;29:8156–64. doi: 10.1016/j.vaccine.2011.07.142. PMID:21864619. [DOI] [PubMed] [Google Scholar]
- 71.Vesikari T, Esposito S, Prymula R, Ypma E, Kohl I, Toneatto D, Dull P, Kimura A, group EUMBIVS . Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: results of two randomised trials. Lancet. 2013;381:825–35. doi: 10.1016/S0140-6736(12)61961-8. PMID:23324563. [DOI] [PubMed] [Google Scholar]
- 72.Gamberini M, Gomez RM, Atzingen MV, Martins EA, Vasconcellos SA, Romero EC, Leite LC, Ho PL, Nascimento AL. Whole-genome analysis of Leptospira interrogans to identify potential vaccine candidates against leptospirosis. FEMS Microbiol Lett. 2005;244:305–13. doi: 10.1016/j.femsle.2005.02.004. PMID:15766783. [DOI] [PubMed] [Google Scholar]
- 73.Murray GL, Lo M, Bulach DM, Srikram A, Seemann T, Quinsey NS, Sermswan RW, Allen A, Adler B. Evaluation of 238 antigens of Leptospira borgpetersenii serovar Hardjo for protection against kidney colonisation. Vaccine. 2013;31:495–9. doi: 10.1016/j.vaccine.2012.11.028. PMID:23176980. [DOI] [PubMed] [Google Scholar]
- 74.Zeng L, Wang D, Hu N, Zhu Q, Chen K, Dong K, Zhang Y, Yao Y, Guo X, Chang YF, et al.. A Novel Pan-Genome Reverse Vaccinology Approach Employing a Negative-Selection Strategy for Screening Surface-Exposed Antigens against leptospirosis. Front Microbiol. 2017;8:396. doi: 10.3389/fmicb.2017.00396. PMID:28352257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu Y, Zhu Y, Wang Y, Chang YF, Zhang Y, Jiang X, Zhuang X, Zhu Y, Zhang J, Zeng L, et al.. Whole genome sequencing revealed host adaptation-focused genomic plasticity of pathogenic Leptospira. Sci Rep. 2016;6:20020. doi: 10.1038/srep20020. PMID:26833181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fouts DE, Matthias MA, Adhikarla H, Adler B, Amorim-Santos L, Berg DE, Bulach D, Buschiazzo A, Chang YF, Galloway RL, et al.. What Makes a Bacterial Species Pathogenic?:Comparative Genomic Analysis of the Genus Leptospira. PLoS NeglTrop Dis. 2016;10:e0004403. doi: 10.1371/journal.pntd.0004403. PMID:26890609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Website of the European Bioinformatics Institute. 2017. Available from: www.ebi.ac.uk.
- 78.Cullen PA, Haake DA, Adler B. Outer membrane proteins of pathogenic spirochetes. FEMS Microbiol Rev. 2004;28:291–318. doi: 10.1016/j.femsre.2003.10.004. PMID:15449605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haake DA, Zuckert WR. The leptospiral outer membrane. Curr Top Microbiol Immunol. 2015;387:187–221. PMID:25388136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shang ES, Summers TA, Haake DA. Molecular cloning and sequence analysis of the gene encoding LipL41, a surface-exposed lipoprotein of pathogenic Leptospira species. Infect Immun. 1996;64:2322–30. PMID:8675344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yan W, Faisal SM, McDonough SP, Divers TJ, Barr SC, Chang CF, Pan MJ, Chang YF. Immunogenicity and protective efficacy of recombinant Leptospira immunoglobulin-like protein B (rLigB) in a hamster challenge model. Microbes Infect. 2009;11:230–7. doi: 10.1016/j.micinf.2008.11.008. PMID:19070678. [DOI] [PubMed] [Google Scholar]
- 82.Monaris D, Sbrogio-Almeida ME, Dib CC, Canhamero TA, Souza GO, Vasconcellos SA, Ferreira LC, Abreu PA. Protective Immunity and Reduced Renal Colonization Induced by Vaccines Containing Recombinant Leptospira interrogans Outer Membrane Proteins and Flagellin Adjuvant. Clin Vaccine Immunol. 2015;22:965–73. doi: 10.1128/CVI.00285-15. PMID:26108285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Andrews SM, Pollard AJ. A vaccine against serogroup B Neisseria meningitidis: dealing with uncertainty. Lancet Infect Dis. 2014;14:426–34. doi: 10.1016/S1473-3099(13)70341-4. PMID:24679664. [DOI] [PubMed] [Google Scholar]
- 84.Chang YF, Chen CS, Palaniappan RU, He H, McDonough SP, Barr SC, Yan W, Faisal SM, Pan MJ, Chang CF. Immunogenicity of the recombinant leptospiral putative outer membrane proteins as vaccine candidates. Vaccine. 2007;25:8190–7. doi: 10.1016/j.vaccine.2007.09.020. PMID:17936448. [DOI] [PubMed] [Google Scholar]
- 85.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. PMID:21029960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leroux-Roels G. Unmet needs in modern vaccinology: adjuvants to improve the immune response. Vaccine. 2010;28(Suppl 3):C25–36. doi: 10.1016/j.vaccine.2010.07.021. PMID:20713254. [DOI] [PubMed] [Google Scholar]
- 87.Faisal SM, Yan W, McDonough SP, Chang YF. Leptospira immunoglobulin-like protein A variable region (LigAvar) incorporated in liposomes and PLGA microspheres produces a robust immune response correlating to protective immunity. Vaccine. 2009;27:378–87. doi: 10.1016/j.vaccine.2008.10.089. PMID:19022317. [DOI] [PubMed] [Google Scholar]
- 88.Conrad NL, Cruz McBride FW, Souza JD, Silveira MM, Felix S, Mendonca KS, Santos CS, Athanazio DA, Medeiros MA, Reis MG, et al.. LigB subunit vaccine confers sterile immunity against challenge in the hamster model of leptospirosis. PLoS NeglTrop Dis. 2017;11:e0005441. doi: 10.1371/journal.pntd.0005441. PMID:28301479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jia L, Yang R, Dong G. Study on Improvement of National Lot Release Regulations on Biological Products. Chin Pharm Affairs. 2010;24:523–30. [Google Scholar]
- 90.Xu M, Liang Z, Xu Y, Wang J. Chinese vaccine products go global: vaccine development and quality control. Expert Rev Vaccin. 2015;14:763–73. doi: 10.1586/14760584.2015.1012503. PMID:25697690. [DOI] [PubMed] [Google Scholar]
- 91.Xu Y, Zhang J, Cui S, Li M, Zhang Y, Xue H, Xin X, Wang J. Genetic stability of vaccine strains by multilocus sequence typing and pulsed-field gel electrophoresis analysis: Implications for quality control of the leptospiral vaccine. Hum Vaccin Immunother. 2015;11:1272–6. doi: 10.1080/21645515.2015.1020266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Johnson RC, Harris VG. Differentiation of pathogenic and saprophytic letospires. I. Growth at low temperatures. J Bacteriol. 1967;94:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blot M. Transposable elements and adaptation of host bacteria. Genetica. 1994;93:5–12. doi: 10.1007/BF01435235. PMID:7813917. [DOI] [PubMed] [Google Scholar]
- 94.Toft C, Andersson SG. Evolutionary microbial genomics: insights into bacterial host adaptation. Nat Rev Genet. 2010;11:465–75. doi: 10.1038/nrg2798. PMID:20517341. [DOI] [PubMed] [Google Scholar]
- 95.Walker A, Srinivas GB. Opportunities and strategies to further reduce animal use for Leptospira vaccine potency testing. Biologicals. 2013;41:332–7. doi: 10.1016/j.biologicals.2013.06.006. PMID:23891496. [DOI] [PubMed] [Google Scholar]
- 96.Srinivas GB, Walker A, Rippke B. USDA regulatory guidelines and practices for veterinary Leptospira vaccine potency testing. Biologicals. 2013;41:298–302. doi: 10.1016/j.biologicals.2013.06.005. PMID:23838570. [DOI] [PubMed] [Google Scholar]
- 97.Gomes-Solecki M, Santecchia I, Werts C. Animal Models of Leptospirosis: Of Mice and Hamsters. Front Immunol. 2017;8:58. doi: 10.3389/fimmu.2017.00058. PMID:28270811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stirling C, Novokova V. Product-specific validation of a serological potency test for release of Leptospira vaccines in the European Union. Biologicals. 2013;41:330–1. doi: 10.1016/j.biologicals.2013.06.004. PMID:23849308. [DOI] [PubMed] [Google Scholar]


