ABSTRACT
Human papillomavirus (HPV) infection is a frequent cause of malignant and non-malignant disease, in particular among persons with HIV. HPV serotype-specific anti L1 antibodies protect against HPV infection but little is known about prophylactic HPV vaccine-induced cell-mediated immunity against HPV in high-risk individuals. We recently showed that both HPV vaccines (Gardasil® and Cervarix®) induce solid, serological immune responses in HIV-infected persons. This study aimed to characterize HPV-specific CD4 T cells in HIV-infected HPV-vaccine recipients, T cell responses being critical for B cell activation and antibody-isotype switching. Thirty HIV-infected patients on long-term antiretroviral treatment (ART) received 3 doses of either Cervarix (n = 15) or Gardasil (n = 15) vaccine at month 0, 1.5 and 6. Cryopreserved peripheral blood mononuclear cells (PBMC) from baseline, 7 and 12 months were subjected to 24-hour stimulation with specific pools of HPV L1-peptides (HPV6, 11, 16, 18, 31 and 45) and HPV E6/E7-peptide pools (HPV6/11 and HPV16/18). Fluorescence-activated cell sorting with intracellular staining (IC-FACS) against CD4, CD154, IL-2, and IFNγ was performed. Frequencies (%) of HPV-antigen specific CD4+ T cells (CD154+/IL-2+ or CD154+/ IFNγ+) were determined. Both HPV-vaccines significantly and comparably enhanced cell-mediated vaccine L1 antigen-specific immunity in HIV-positive adults receiving ART therapy at month 7 and 12 after first vaccine dose. This suggests that the vaccines induce CD4 T cellular memory despite HIV-induced immune compromisation.
KEYWORDS: cell mediated immunity, human papillomavirus vaccines, hiv, vaccinology, T helper cell response
Introduction
Human papillomavirus (HPV) infection is the most prevalent sexually transmitted infection world-wide. HIV-infected women have high rates of HPV infection and they are more likely to have multiple HPV types and persistent infections compared to HIV-negative women.1 HIV-infected men who have sex with men (MSM) are also at increased risk for HPV-associated anal cancer2 and the incidence continues to rise despite wide-spread use of antiretroviral treatment.3 Furthermore, HIV-infected adults have high rates of genital warts, which are often recalcitrant to conventional therapies.4
Both HPV vaccines have been reported to be safe and immunogenic in HIV-infected individuals with high seroconversion rates, but lower antibody titers than in age-matched HIV-negative persons.5–9 To our knowledge, no other studies have reported comparative CD4 T cellular responses following HPV immunization in HIV-infected adults. Such T cell responses demonstrate the capacity for cellular immune memory in HIV-compromised, potentially CD4-depleted, patients. CD4 helper responses are also important for antibody isotype switching during the development of the B cell mediated humoral response initiating and contributing to serologic responses. We have previously reported serologic responses from HPV-vaccination in a head-to-head trial comparing immunization with Gardasil and Cervarix in individuals with HIV infection.10 We showed induction of robust antibody responses including isotype switching to IgG.
This sub-study was conducted to investigate the induction of cellular immunity following HPV-vaccination by a characterization of the HPV-specific T helper cells (CD4) in ART-treated, HIV-infected men and women.
Materials and methods
Study design
This was a blinded study conducted as a cooperation between the Department of Infectious Diseases, Aarhus Universitetshospital, Aarhus, Denmark, and the Laboratory of Gynecologic Tumor Immunology, Charité Universitätsmedizin, Berlin, Germany, as a continuation of the randomized, double-blind, head-to-head trial randomizing persons with HIV to receive vaccination with either Gardasil and Cervarix at three time-points (0, 1.5 and 6 months).10 The study protocol was approved by the Danish Medicines Agency, the Regional Ethical Committee and the Danish Data Protection Agency.
Study population
As described in Toft L. et al.,10 HIV-infected patients seen at the outpatient clinic during 2011 at the Department of Infectious Diseases, Aarhus University Hospital, Aarhus, Denmark, were invited by letter to participate. Consenting HIV-seropositive volunteers were eligible for enrollment when i) 18 years or older, ii) non-vaccinated to HPV, iii) with no malignancy, allergy, or receiving immunosuppressive treatment, iv) non-pregnant or breastfeeding, v) having a HIV RNA level <200 copies/ml. Excluded were individuals who i) had previously been vaccinated against HPV, ii) had a history of malignancy or autoimmune disease, iii) had received systemic immunosuppressive treatment, iv) had previously had an allergic reaction to vaccination, v) were pregnant, breastfeeding or unwilling to use reliable contraception methods for the duration of the trial and/or vi) had an HIV RNA level >200 copies/ml if receiving antiretroviral treatment.
Eighty ART-treated, HIV-positive patients were randomized to receive vaccination with either Gardasil (n = 40) and Cervarix (n = 40) and all patients were evaluated for vaccine safety and antibody production.10 A sub-cohort of 30 patients (Gardasil n = 15 and Cervarix n = 15; matched by gender) with sufficient numbers (total of >107 PBMC per time point) of cryopreserved cells were included in the present study.
Preparation of samples
During the initial trial, PBMC were cryopreserved in a freezing medium consisting of medium (RPMI 1640 w/L-Glutamine, Biowest) supplemented with 20% Fetal Calf Serum (FCS), 10% dimethylsulfoxid (DMSO) and 1% Penicillin/Streptomycin (P/S) and stored below 150°C.
For the T cell analyses the lab personal was blinded to the vaccination type, gender, and dose. PBMC samples were thawed out in a 37°C water bath. Samples from baseline, 7 and 12 months were transferred to the pre-heated medium (RPMI-medium 1640 (1X) + GlutaMAX™ –I, Thermo Fisher) supplemented with 10% FCS and 1% P/S. Between 10 and 17 million cryopreserved PBMCs per patient per time-point were thawed. Cells were washed with medium twice by centrifugation at 1300 rpm for 15 minutes.
The cells equilibrated over night in the incubator in a 24 well culture plate in 2 ml medium. After resting, viable cells were manually counted in a Neubauer chamber and cell number adjusted according to viable cell numbers.
Stimulation of samples
In order to activate the vaccine-specific memory T cell responses 5 × 105 PBMC were stimulated with 1 μl/ml anti-CD28 (1 mg/ml BD Pharmingen™) and 10 μl/ml HPV-peptide pool consisting of synthetically produced, overlapping (12 amino acids) 30 mer peptides representing the whole L1 sequence in a concentration of 1 μg/μl (Peptides and Elephants, Nuthetal, Germany).11 Individual pools from L1 of HPV type 6, 11, 16, 18, 31, 45 and E6/E7 of HPV type 6/11 and 16/18 were used, respectively. The full list of peptide sequences for the 14 HPV antigens used in this study is available from the corresponding author upon request. One sample per time-point was stimulated with 10 μl/ml staphylococcal enterotoxin B (SEB) as a positive control and one sample per time-point was left without any stimulation as a negative T cell background control (TC). The cells were incubated for 90–120 minutes before adding Brefeldin A (10 μg/ml) to block cytokine secretion and CD154 transport and then incubated overnight (16–20 hours, 37℃). Finally, the cells were fixed (Formalin, 2%, in PBS) and permeabilized (FACS™ 10x Permeabilizing Solution 2, BD Biosciences, San Jose, CA, USA) according to the suppliers protocol.
Assessment of T cell response
The PBMC were stained using the following antibodies for IC-FACS analysis: anti-CD154-APC (conc. 1:10, reference number: 130–092-290), anti-CD4-PerCP (conc. 1:5, reference number: 345770), anti-IFNγ-FITC (conc. 1:100, reference number: 554551), and anti-IL-2-PE (conc. 1:100, reference number: 554566). Beriglobin (conc. 1:50, reference number: 176a/92) was added to perform Fc-receptor blocking.
The acquisition of flow cytometric data was performed using a FACSCalibur (BD Biosciences, Franklin Lakes, NJ, USA) flow cytometer. The number of CD4+ cells acquired from the flow cytometry varied from 10,000 to 160,000 due to individual variations, number of cells frozen initially, number of cells lost or recollected after thawing and other protocol procedures.
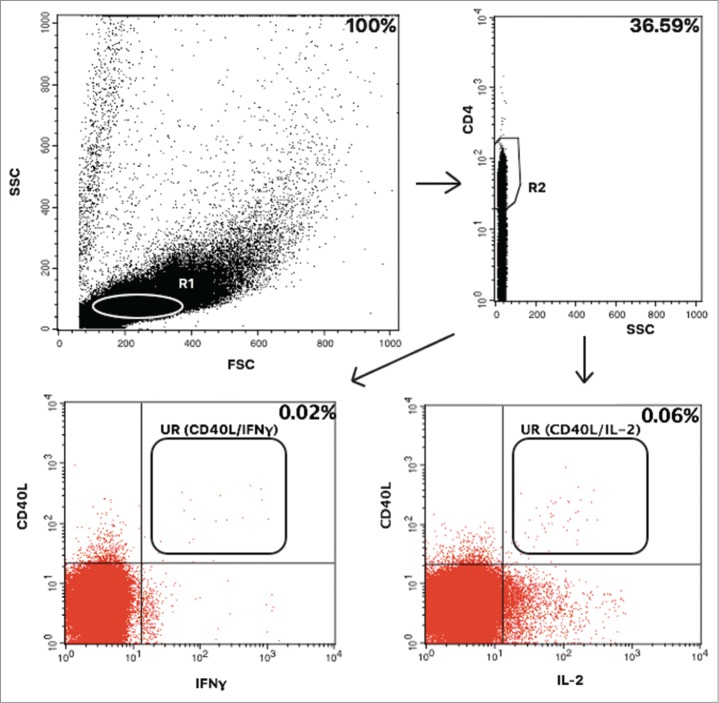
Gating strategy
The forward (FSC) and side-scatter (SSC) were gating the lymphocytes into region 1 (R1) (Fig. 1). Subsequently, the CD4+ T cells were gated into region 2 (R2). Data were analysed by CellQuest PRO software (BD Biosciences) and CD154 plotted against IFNγ or IL-2, respectively. Each dot plot was adjusted to the TC sample by gating and positive cells in the TC sample were substracted from each value in a HPV antigen-stimulated sample. SEB-stimulated samples were viability indicators. The reactogenicity varied widely between individuals and time points but did not correlate with the L1 antigen-specific CD4 T cells and vice versa. Per cent of antigen-specific CD4 positive T cells expressing the activation marker CD154 (CD40L) and a cytokine (either IFNγ or IL-2) were evaluated. The lowest accepted SEB-value was a value of minimum 1.5% IL-2/IFNγ positive CD4 T cells due to potential immune compromization in the HIV infected participants. This limit value was determined in a pre-study on young, healthy quadrivalent vaccine receiving women. All 10 samples at each time point were analysed with the exact same settings for the gates and quadrant positions aiming to get as standardized a procedure as possible per patient. As the rate of positive events varied a lot between subjects and timepoints it would not have improved the objectivity of the results to apply one standardized gate to all IC-FACS patient samples.
Figure 1.
Gating strategy. The T cell life gate (R1) was gated into a CD4/SCC plot and CD4 positive cells in region 2 (R2) were analysed for positivity of CD40L (CD154) and IFNγ or IL-2 positivity, respectively. Events in the upper right (UR) quadrant falling into the analysis region were counted as antigen specific cells. Per cent of positively gated cells are given in this representative patient.
Statistical analysis
Data were analysed using SPSS (IBM SPSS Statistics 23). Non-parametric data were summerized using median and IQR and analysed using Wilcoxon signed rank test with a p-value <0.05 considered statistically significant. A comparison of vaccine groups was performed using Analysis of Variance (ANOVA) in a general linear model. This analysis required data to be stated in mean values, which was not fullfilled. For this reason a more strict p-value of <0.01 was considered statistically significant.
HPV16 and HPV18 antibody titers evaluated in the main trial10 and corresponding T cell frequencies at baseline, 7 and 12 months were correlated using Kendall´s Tau ß.
Correlations between HPV DNA anal and vaginal swab results from baseline and month 710 and the corresponding T cell frequencies were performed in order to investigate any influence on T cell responses from prevalent infection. Correlations between HPV E6/E7 positive/negative baseline-status and T cell results were performed to see if HPVE6/E7-status at baseline, as a marker of natural virus infection, influenced the ex vivo cellular responses to HPVL1 stimulation.
Results
Study population
Thirty patients from the main trial were investigated, fifteen of the Gardasil- and Cervarix vaccine recipients, respectively. The participants in the two vaccine groups were similar in immune status and all other baseline characteristics (Table 1).
Table 1.
Baseline characteristics.
| Gardasil vaccinated | Cervarix vaccinated | |
|---|---|---|
| (n = 15) | (n = 15) | |
| Age (years), median (IQR) | 45 (38.0–53.0) | 46 (34.0–60.0) |
| Sex (%) | ||
| Male | 11 (73.3) | 10 (66.7) |
| Female | 4 (26.7) | 5 (33.3) |
| Undergoing ART | 15 | 15 |
| BMI, kg/m2, median (IQR) | 25.4 (21.8–31.6) | 21.6 (20.5–27.4) |
| Current smoker (%) | 5 (33.3) | 3 (20) |
| CD4+ cell count, cells/μl, median (IQR) | 620 (475–925) | 610 (580–745) |
| HIV RNA level, log10 copies/mL, median (IQR) | 1.28 (1.28–1.31) | 1.28 (1.28) |
Immunogenicity
The primary objective was to determine the frequency of reactive, HPV L1-specific CD4 T cells before and after vaccination.
Immunogenicity endpoints for Gardasil and Cervarix, separately, are shown in Table 2 for IL-2+ cells. The Gardasil vaccine recipients showed to significantly increase T cell frequencies specific for HPV6, 11, 16, 18, 31 and 45 L1 from baseline to 7 and 12 months for IL-2+ cells. Percentages of HPV16 L1 specific IL-2+ T cells increased from 0.005 percent at baseline to 0.040 and 0.050 percent at 7 and 12 months, respectively (p = 0.004 and 0.018, respectively). The Cervarix vaccine recipients also showed significant increases in T cell frequencies, except for HPV18 L1 (at 12 months) and HPV45 L1. Percentages of HPV16 L1-specific IL-2+ T cells increased from 0.010 at baseline to 0.050 and 0.030 percent at 7 and 12 months, respectively (p = 0.003 and 0.008, respectively). Results for INFγ+ cells were similar to the results of IL-2+ cells, though generally the INFγ+ results were of lower magnitude and HPV45 L1 at 7 months did not show a significant increase for Gardasil (Table 3).
Table 2.
IL-2 T cell responses.
| Antigen | Gardasil vaccinated CD4/CD154/IL-2 positive T cells Median frequency (IQR) | Number of subjects | p-value | Cervarix vaccinated CD4/CD154/IL-2 positive T cells Median frequency (IQR) | Number of subjects | p-value |
|---|---|---|---|---|---|---|
| HPV6 L1 | ||||||
| Baseline | 0.000 (0.000–0.003) | 14 | 0.000 (0.000–0.015) | 13 | ||
| 7 months | 0.080 (0.045–0.130) | 13 | 0.003 | 0.030 (0.010–0.090) | 15 | 0.028 |
| 12 months | 0.060 (0.040–0.090) | 15 | 0.001 | 0.040 (0.010–0.070) | 15 | 0.021 |
| HPV11 L1 | ||||||
| Baseline | 0.000 (0.000–0.010) | 14 | 0.000 (0.000–0.005) | 13 | ||
| 7 months | 0.060 (0.030–0.120) | 15 | 0.001 | 0.020 (0.010–0.110) | 15 | 0.009 |
| 12 months | 0.050 (0.010–0.090) | 15 | 0.004 | 0.020 (0.008–0.070) | 14 | 0.007 |
| HPV16 L1 | ||||||
| Baseline | 0.005 (0.000–0.030) | 14 | 0.010 (0.000–0.020) | 15 | ||
| 7 months | 0.040 (0.020–0.100) | 15 | 0.004 | 0.050 (0.010–0.113) | 14 | 0.003 |
| 12 months | 0.050 (0.020–0.070) | 15 | 0.018 | 0.030 (0.010–0.050) | 15 | 0.008 |
| HPV18 L1 | ||||||
| Baseline | 0.000 (0.000–0.003) | 0.000 (0.000–0.005) | 14 | |||
| 7 months | 0.030 (0.010–0.040) | 15 | 0.016 | 0.040 (0.010–0.070) | 15 | 0.003 |
| 12 months | 0.020 (0.010–0.050) | 15 | 0.016 | 0.020 (0.000–0.060) | 15 | 0.108 |
| HPV31 L1 | ||||||
| Baseline | 0.000 (0.000–0.010) | 14 | 0.000 (0.000–0.020) | 15 | ||
| 7 months | 0.020 (0.010–0.080) | 15 | 0.013 | 0.030 (0.010–0.100) | 15 | 0.006 |
| 12 months | 0.030 (0.000–0.050) | 15 | 0.028 | 0.030 (0.015–0.075) | 13 | 0.018 |
| HPV45 L1 | ||||||
| Baseline | 0.000 (0.000–0.020) | 14 | 0.000 (0.000–0.033) | 14 | ||
| 7 months | 0.020 (0.015–0.035) | 13 | 0.026 | 0.030 (0.000–0.053) | 14 | 0.325 |
| 12 months | 0.020 (0.010–0.050) | 15 | 0.011 | 0.010 (0.000–0.030) | 13 | 0.929 |
Table 3.
IFNγ T cell responses.
| Antigen | Gardasil vaccinated CD4/CD154/IFNγ positive T cells Median frequency (IQR) | Number of subjects | p-value | Cervarix vaccinated CD4/CD154/IFNγ positive T cells Median frequency (IQR) | Number of subjects | p-value |
|---|---|---|---|---|---|---|
| HPV6 L1 | ||||||
| Baseline | 0.000 (0.000–0.010) | 14 | 0.000 (0.000–0.010) | 13 | ||
| 7 months | 0.020 (0.015–0.065) | 13 | 0.006 | 0.020 (0.000–0.050) | 15 | 0.017 |
| 12 months | 0.030 (0.020–0.060) | 15 | 0.001 | 0.010 (0.000–0.060) | 15 | 0.18 |
| HPV11 L1 | ||||||
| Baseline | 0.000 (0.000–0.010) | 14 | 0.000 (0.000–0.005) | 13 | ||
| 7 months | 0.030 (0.010–0.050) | 15 | 0.015 | 0.010 (0.000–0.030) | 15 | 0.024 |
| 12 months | 0.020 (0.010–0.030) | 15 | 0.022 | 0.010 (0.000–0.020) | 14 | 0.023 |
| HPV16 L1 | ||||||
| Baseline | 0.000 (0.000–0.010) | 14 | 0.000 (0.000–0.000) | 15 | ||
| 7 months | 0.030 (0.010–0.050) | 15 | 0.011 | 0.015 (0.000–0.040) | 14 | 0.007 |
| 12 months | 0.030 (0.010–0.050) | 15 | 0.047 | 0.010 (0.000–0.030) | 15 | 0.007 |
| HPV18 L1 | ||||||
| Baseline | 0.000 (0.000–0.000) | 14 | 0.000 (0.000–0.020) | 14 | ||
| 7 months | 0.010 (0.000–0.020) | 15 | 0.022 | 0.010 (0.000–0.030) | 15 | 0.039 |
| 12 months | 0.010 (0.000–0.020) | 15 | 0.014 | 0.000 (0.000–0.020) | 15 | 0.719 |
| HPV31 L1 | ||||||
| Baseline | 0.000 (0.000–0.003) | 14 | 0.000 (0.000–0.010) | 15 | ||
| 7 months | 0.010 (0.000–0.040) | 15 | 0.016 | 0.010 (0.000–0.040) | 15 | 0.011 |
| 12 months | 0.010 (0.000–0.030) | 15 | 0.014 | 0.020 (0.010–0.040) | 15 | 0.009 |
| HPV45 L1 | ||||||
| Baseline | 0.000 (0.000–0.010) | 14 | 0.000 (0.000–0.010) | 14 | ||
| 7 months | 0.010 (0.000–0.020) | 13 | 0.19 | 0.010 (0.000–0.010) | 14 | 0.558 |
| 12 months | 0.010 (0.010–0.030) | 15 | 0.015 | 0.010 (0.000–0.015) | 13 | 0.715 |
Gardasil was found to induce higher T cell frequencies against HPV6 and -11 L1 compared to Cervarix, which does not contain these antigens. However, significant reactive T cell responses could still be identified after vaccination in recipients of the Cervarix vaccine. A general linear model was used to investigate any significant differences in the increase in T cell frequencies for the vaccine types and cross-reactive types over time between the two vaccines. No significant differences over time were detected (p significance = 0.01, values: 0.031 – 0.931, data not shown) using this model. This was the case for all HPV types investigated in the trial.
Antibody titers, HPV E6/E7 baseline-status and HPV-DNA-status
The antibody titers for HPV 16 and 18 L1 of the individuals included in this substudy were reevaluated according to their baseline conditions for serological and HPV DNA positivity (Table 4). Generally, a strong induction of antibody responses at month 7 was found that dropped towards month 12. There was a significant difference between Gardasil and Cervarix for HPV18L1 specific responses in patients who were serologically and DNA negative at time of initial vaccination. No patients serologically negative and HPV16 or 18 positive at baseline were identified. Also for HPV18 no patient was serologically positive and DNA positive.
Table 4.
GMT of individuals included in the subgroup analysis for CD4 T cells.
| Gardasil vaccinated | Cervarix vaccinated | |||||||
|---|---|---|---|---|---|---|---|---|
| HPV type | Baseline HPV serostatus and DNA status | Month | No. | GMT mean values | 95% CI | No. | GMT mean values | 95% CI |
| HPV16 | ||||||||
| Sero- and HPV DNA- | (1) | |||||||
| 0 | 6 | 40 | / | 5 | 40 | / | ||
| 7 | 6 | 91750 | (42 323 – 141 177) | 5 | 186024 | (27 960 – 344 088) | ||
| 12 | 6 | 21074 | (-2 955 – 45 103) | 5 | 55998 | (9 292 – 102 704) | ||
| Sero+ and HPV DNA- | (1) | |||||||
| 0 | 7 | 907 | (138 – 1676) | 7 | 1225 | (406 – 2 044) | ||
| 7 | 7 | 98232 | (51 403 – 145 061) | 7 | 136280 | (80 057 – 192 503) | ||
| 12 | 7 | 47294 | (19 193 – 75 995) | 7 | 59797 | (24 458 – 95136) | ||
| Sero+ and HPV DNA+ | ||||||||
| 0 | 1 | 5179 | / | 2 | 2776 | / | ||
| 7 | 1 | 101426 | / | 2 | 33187 | / | ||
| 12 | 1 | 70668 | / | 2 | 14397 | / | ||
| HPV18 | ||||||||
| Sero- and HPV DNA- | (2) | |||||||
| 0 | 6 | 40 | / | 6 | 40 | / | ||
| 7 | 6 | 5581 | (759 – 10 403) | 6 | 103150 | (29 071 – 177 229) | ||
| 12 | 6 | 733 | (175 – 1 291) | 6 | 30159 | (9 329 – 50 988) | ||
| Sero+ and HPV DNA- | (1) | |||||||
| 0 | 8 | 723 | (-39 – 1 485) | 7 | 448 | (-18 – 014) | ||
| 7 | 8 | 24083 | (5 529 – 42 637) | 7 | 39439 | (4 360 – 74 518) | ||
| 12 | 8 | 9065 | (1 579 – 16 551) | 7 | 13125 | (3 933 – 22 317) |
For HPV16 no Sero- and HPV+ data was found.
For HPV18 no Sero- and HPV+ as well as Sero+ and HPV+ data was found.
/ = no result.
() = did not have a swab done.
Results with negative CI = non-significant passing 0.
When evaluating anti-HPV antibody titers to HPV16 and 18 L1 and corresponding T cell frequencies, we found no or only few weak correlations calculated by Kendall's Tau ß test (data not shown). We observed a positive correlation between anti-HPV16 antibody titers at baseline and HPV16 L1 T cell frequencies (IFNγ+) at month 12 (tau β = 0.291; p = 0.040) and anti-HPV18 antibody titers at month 7 and HPV18 T cell frequencies (INFγ+) at baseline (tau β = 0.340; p = 0.028).
We found no significant correlations between the vaginal or anal swab results for HPV DNA-status of the participants at baseline and the T cell frequencies after vaccination.
The reactivity to E6/E7 antigens of low and high risk HPV types was measured to evaluate former or current infection by vaccine HPV types. The proportion of patients reacting by INFγ or IL-2 secretion was comparable between both vaccine groups and time points (supplementary Table 1). For correlations between HPV E6/E7 baseline-status ”positive” or ”negative” as a marker of natural infection and HPV L1-specific T cell results, there was a correlation between a positive baseline HPV6/11 E6/E7 status and the T cell response for HPV11 L1 at baseline and at month 7 (tau β = 0.68; p = 0.001 and 0.303; p = 0.038, respectively). Also, it showed a correlation between a positive HPV16/18 E6/E7 baseline-status and the T cell response to HPV18 L1 at baseline (tau β = 0.378; p = 0.040).
Discussion
The purpose of this study was to determine the cellular immunity to HPV vaccines in HIV-infected, ART-treated men and women by measuring frequencies of HPV vaccine-specific T cells before and after HPV vaccination. The results of our study suggest that irrespective of the type of HPV vaccine used, T cell frequencies increase significantly after vaccination in HIV infected adults of both sexes.
It is a well-known fact that the two vaccines induce a vaccine-specific antibody response in healthy individuals with Cervarix inducing higher antibody titers than Gardasil.12 For the HIV positive participants in the current trial the results on the antibody titers10 also showed that Cervarix induces higher anti-HPV18 L1-specific antibody titers compared to Gardasil, but for HPV16 L1 there was no significant difference in the antibody response between the two vaccines.
Increases in T helper cell type 1 (Th1) and type 2 (Th2) cytokines have been reported to occur following injection with L1 virus-like-particles (VLP) vaccines in healthy individuals looking at investigations from before the approval of either of the vaccines.13,14,15,16 To our knowledge, only limited data have been published on T cell frequencies in healthy HPV vaccinees upon vaccination with either vaccine.17 We have found CD4 T cell frequencies after HPV vaccination in healthy individuals at 6 months and 5 years after the last vaccine dose, respectively, showing similar magnitude to those found in this trial. In this healthy study population the CD4 T cell response was sustainable 5 years after vaccination. Furthermore, these results on the healthy study population found a tendency towards Cervarix inducing a stronger cellular immune response against the high-risk HPV types than Gardasil (unpublished data).
When comparing the results on cellular immunity in each vaccine arm in this HIV positive study population, none of the vaccines seemed superior. Surprisingly, both vaccines resulted in an induction of a significant T cell response to HPV6 and 11 L1. A greater increase in T cell frequencies to HPV6 and 11 L1 was seen in the Gardasil recipients compared to the Cervarix recipients. However, as these two low risk HPV types are not included in Cervarix, it was surprising to find that this significant increase was observed. It raises the question if there are cross-reacting T cells between high-risk and low-risk HPV types? Interestingly, we have found that clonal CD4+ HPV16-specific T cells do cross-react with naturally processed and structually conserved L1-derived peptides of HPV6, 11, 18, 31 and 45 L1 in healthy Cervarix and Gardasil recipients (manuscript in preparation). Future clinical studies evaluating endpoints on incidences of genital warts in Cervarix compared to Gardasil recipients would be interesting in order to evaluate the clinical value of these findings.
A decline in genital warts has been found in genitourinary medicine clinics in England after vaccination with Cervarix.18,19 The decrease is directly correlated with the younger age group, which might be explained by the higher vaccine coverage in this group. Interestingly, a parallel decrease is observed among heterosexual young men of the same age-range, but not in MSM.
Cross-protective efficacy of the two vaccines is a familiar observation with a tendency towards Cervarix being more efficacious against non-vaccine HPV types than Gardasil, when investigating HPV31, 33 and 45 L1.20 Looking at the anti-HPV31 and −45 antibody titers from the HIV patients in our former study, both vaccines induced cross-neutralizing antibodies against HPV31 and 45 L1, also with a trend towards more seroconverters in the Cervarix recipients compared to the Gardasil recipients.21 However, for HPV31 and 45 L1 specific CD4 T cell frequencies this difference was not seen between both vaccines. Here we found that HPV31 and 45 L1 specific CD4 T cells increased after vaccination with either of the vaccines and the increase was not statistically significant for HPV45 L1 in the Cervarix recipients.
We investigated if there were any correlations between the antibody response and the CD4 T cell response of the HIV infected individuals. From comparisons of antibody titers presented in Table 4 and T cell frequencies in Tables 2 and 3 it can be appreciated that there is no strong direct correlation as the antibodies decrease towards month 12 while the T cells remain stable. We found only two weak, positive correlations between anti-HPV16 L1 antibody titers at baseline and HPV16 L1-specific CD4 T cell frequencies (IFNγ+) at month 12 and a weak positive correlation between anti-HPV18 L1 antibody titers at 7 months and HPV18 L1-specific CD4+ T cell frequencies (INFγ+) at baseline. This suggests that the antibody titers are independent of the magnitude of the increase in L1-specific CD4 T cells. Also, it might suggest that underlying cellular immune responses vary considerably between individuals or that the peripheral circulating CD4 cell pool may not represent the frequency in lymphoid organs. Furthermore, we investigated whether a former natural infection with HPV would influence the vaccine-specific response. The antigens E6 and E7 are not present in the vaccine and observing a specific response to E6/E7 should thus be generated by a natural infection. For this reason we investigated if a positive HPV E6/E7 baseline-status would influence the T cell responses after vaccination compared to those HPV E6/E7 baseline negative. With only a few correlations found this did not seem convincing.
We do find Cervarix to be comparable to Gardasil in CD4 T cell responses in our study, despite reports of stronger cellular immunogenicity on Cervarix in healthy women potentially due to the AS04 adjuvant system.12 An explaination could be that the amount of lipopolysaccharides (LPS) is increased in HIV positive individuals, which is related to a reduced response via TLR-4 receptors in vaccination.22
Limitations of the study
The power of this study is limited by the restricted sample number (n = 15 per vaccine), which influences the reliability of the results. This was a follow up study with only limited residual material available. Samples with sufficient numbers of PBMC (appx. 1 × 107) were selected and included, balanced for gender and vaccine type and presence of sufficient material at three time points. This was possible for only 15 of the 40 patients per vaccine group initially recruited. Hence the statistical analyses have more an explorative character.
The limited amount of PBMC also led to the decision to use IC-FACS instead of the more sensitive ELISPOT as a read out. IC-FACS can measure multiple parameters at a time within the cell, allows to identify CD4 and activation markers (CD154) simultaneously with cytokines, it has an acceptable throughput, and the cells were swiftly fixed and any potentially infectious HIV inactivated.
Natural infection and high antibody titers were expected to influence the results in the direction of higher T cell frequencies. The small study population available may partly explain why no relations are found between natural infection (HPV E6/E7-status), antibody titers, HPV DNA-status and CD4 T cells. Furthermore, the study did not have a long-term follow-up and for this reason it cannot predict potential decreases in immunological responses over time. Neither can the study predict the clinical importance of the findings, as it does not evaluate any clinical end-points. Since the initial serological study by Toft et al.10,21 did not include a healthy control population no cellular material from a comparably treated healthy group was available in this follow up study. Not having included an HIV-negative group of vaccine recipients limits the comparison to findings from healthy individuals. Nevertheless, a longitudinal comparison of CD4 T cell responses in HIV+ vaccinated patients, in a head to head comparison of the vaccines was investigated.
The baseline mean CD4 frequency in all study participants was 28.8% ± 9.6 (data not shown). This is somewhat lower than expected in a healthy population and could be due to HIV-induced immunocompromised individuals, and cryopreservation of the PBMC. However significant vaccine-induced increase of HPV L1-specific CD4 responses could be demonstrated from this material. The comparability of the blood drawings from the different time points was enhanced by processing all samples of an individual at once.
The results in this study support the serology data from the main trials.10,21 Immunologically, the HIV-positive adults benefit from HPV vaccination with either of the vaccines included in this study as evidenced by increases in vaccine-specific T cells after vaccination, albeit with less difference in response between the two vaccines compared to what has been found in the healthy population.
Supplementary Material
Funding Statement
MZMM was supported by internal funds of Charite-Universitätsmedizin Berlin, Clinic for Gynecology Laboratory.
Abbreviations
- ART
antiretroviral therapy
- BMI
body mass index
- HIV
human immunodeficiency virus
- HPV
human papilloma virus
- IQR
interquartile range
Disclosure of potential conflicts of interest
AMK received speakers honoraria and travel support from GSK and SPMSD.
Acknowledgments
Det Frie Forskningsråd – The Danish Council For Independent Research. The contribution of serological test data by Martin Müller and Peter Sehr, Heidelberg, Germany, and the HPV genotyping results by Jesper Bonde, Copenhagen, Denmark, is gratefully acknowledged.
References
- 1.Palefsky JM, Minkoff H, Kalish LA, Levine A, Sacks HS, Garcia P, Young M, Melnick S, Miotti P, Burk R. Cervicovaginal human papillomavirus infection in human immunodeficiency virus-1 (HIV)-positive and high-risk HIV-negative women. J Natl Cancer Inst. 1999;91:226–236. doi: 10.1093/jnci/91.3.226. PMID:10037100. [DOI] [PubMed] [Google Scholar]
- 2.Goedert JJ, Cote TR, Virgo P, Scoppa SM, Kingma DW, Gail MH, Jaffe ES, Biggar RJ. Spectrum of AIDS-associated malignant disorders. Lancet. 1998;351(9119):1833–1839. doi: 10.1016/S0140-6736(97)09028-4. PMID:9652666. [DOI] [PubMed] [Google Scholar]
- 3.Piketty C, Selinger-Leneman H, Grabar S, Duvivier C, Bonmarchand M, Abramowitz L, Costagliola D, Mary-Krause M. Marked increase in the incidence of invasive anal cancer among HIV-infected patients despite treatment with combination antiretroviral therapy. AIDS. 2008;22(10):1203–1211. doi: 10.1097/QAD.0b013e3283023f78. PMID:18525266. [DOI] [PubMed] [Google Scholar]
- 4.De Panfilis G, Melzani G, Mori G, Ghidini A, Greifemberghi S. Relapses after treatment of external genital warts are more frequent in HIV-positive patients than in HIV-negative controls. Sex Transm Dis. 2002;29:121–5. doi: 10.1097/00007435-200203000-00001. PMID:11875372. [DOI] [PubMed] [Google Scholar]
- 5.Denny L, Hendricks B, Gordon C, Thomas F, Hezareh M, Dobbelaere K, Durand C, Hervé C, Descamps D. Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in HIV-positive women in South Africa: a partially-blind randomised placebo-controlled study. Vaccine. 2013;31(48):5745–53. doi: 10.1016/j.vaccine.2013.09.032. PMID:24091311. [DOI] [PubMed] [Google Scholar]
- 6.Kahn JA, Burk RD, Squires KE, Kapogiannis BG, Rudy B, Xu J, Gonin R, Liu N, Worrell C, Wilson CM. Prevalence and risk factors for HPV in HIV-positive young women receiving their first HPV vaccination. J Acquir Immune Defic Syndr. 2012;61(3):390–9. doi: 10.1097/QAI.0b013e3182676fe3. PMID:22820809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinberg A, Song LY, Saah A, Brown M, Moscicki AB, 3rd Meyer WA, Bryan J, Levin MJ. Humoral, mucosal, and cell-mediated immunity against vaccine and nonvaccine genotypes after administration of quadrivalent human papillomavirus vaccine to HIV-infected children. J Infect Dis. 2012;206(8):1309–18. doi: 10.1093/infdis/jis489. PMID:22859825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levin MJ, Moscicki AB, Song LY, Fenton T, Meyer WA 3rd, Read JS, Handelsman EL, Nowak B, Sattler CA, Saah A, et al.. Safety and immunogenicity of a quadrivalent human papillomavirus (Types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. J Acquir Immune Defic Syndr. 2010;55(2):197–204. doi: 10.1097/QAI.0b013e3181de8d26. PMID:20574412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkin T, Lee JY, Lensing SY, Stier EA, Goldstone SE, Berry JM, Jay N, Aboulafia D, Cohn DL, Einstein MH, et al.. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis. 2010;202(8):1246–53. doi: 10.1086/656320. PMID:20812850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toft L, Storgaard M, Müller M, Sehr P, Bonde J, Tolstrup M, Østergaard L, Søgaard OS. Comparison of the immunogenicity and reactogenicity of Cervarix and Gardasil human papillomavirus vaccines in HIV-infected adults: a randomized, double-blind, clinical trial. J Infect Dis. 2014;209(8):1165–73. doi: 10.1093/infdis/jit657. PMID:24273179. [DOI] [PubMed] [Google Scholar]
- 11.Gaiser MR, Textor S, Senger T, Schädlich L, Waterboer T, Kaufmann AM, Süsal C, Pawlita M, Enk AH, Gissmann L, et al.. Evaluation of specific humoral and cellular immune responses against the major capsid L1 protein of cutaneous wart-associated alpha-Papillomaviruses in solid organ transplant recipients. J Dermatol Sci. 2015. January;77(1):37–45. doi: 10.1016/j.jdermsci.2014.11.002 Epub 2014November18. doi: 10.1016/j.jdermsci.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, Carletti I, Dessy FJ, Trofa AF, Schuind A, et al.. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccin. 2009;5(10):705–19. doi: 10.4161/hv.5.10.9518. PMID:19684472. [DOI] [PubMed] [Google Scholar]
- 13.Evans TG, Bonnez W, Rose RC, Koenig S, Demeter L, Suzich JA, O´Brien D, Campbell M, White WI, Balsley J, et al.. A phase 1 study of a recombinant viruslike particle vaccine against human papillomavirus type 11 in healthy adult volunteers. J Infect Dis. 2001;183(10):1485–93. doi: 10.1086/320190. PMID:11319684. [DOI] [PubMed] [Google Scholar]
- 14.Emeny RT, Wheeler CM, Jansen KU, Hunt WC, Fu TM, Smith JF, MacMullen S, Esser MT, Paliard X. Priming of human papillomavirus type 11-specific humoral and cellular immune responses in college-aged women with a virus-like particle vaccine. J Virol. 2002;76(15):7832–42. doi: 10.1128/JVI.76.15.7832-7842.2002. PMID:12097595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinto LA, Edwards J, Castle PE, Harro CD, Lowy DR, Schiller JT, Wallace D, Kopp W, Adelsberger JW, Baseler MW, et al.. Cellular immune responses to human papillomavirus (HPV)-16 L1 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. J Infect Dis. 2003;188(2):327–38. doi: 10.1086/376505. PMID:12854090. [DOI] [PubMed] [Google Scholar]
- 16.Pinto LA, Viscidi R, Harro CD, Kemp TJ, García-Pineres AJ, Trivett M, Demuth F, Lowy DR, Schiller JT, Berzofsky JA, et al.. Cellular immune responses to HPV-18, −31 and −53 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. Virology. 2006;353(2):451–62. doi: 10.1016/j.virol.2006.06.021. PMID:16863657. [DOI] [PubMed] [Google Scholar]
- 17.Einstein MH, Levin MJ, Chatteree A, Chakhtoura N, Takacs P, Catteau G, Dessy FJ, Moris P, Lin L, Struyf F, et al.. Comparative humoral and cellular immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18–45 years: Follow-up through month 48 in a Phase III randomized study. Hum Vaccin Immunother. 2014;10(12):3455–3465. doi: 10.4161/hv.36117. PMID:25483700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canvin M, Sinka K, Hughes G, Mesher D. Decline in genital warts diagnoses among young women and young men since the introduction of the bivalent HPV (16/18) vaccination programme in England: an ecological analysis. Sex Transm Infect. 2017;93(2):125–128. doi: 10.1136/sextrans-2016-052626. [DOI] [PubMed] [Google Scholar]
- 19.Szarewski A, Skinner SR, Garland SM, Romanowski B, Schwarz TF, Apter D, Chow SN, Paavonen J, Del Rosario-Raymundo MR, Teixeira JC, et al.. Efficacy of the HPV-16/18 AS04-adjuvanted vaccine against low-risk HPV types (PATRICIA randomized trial): an unexpected observation. J Infect Dis. 2013;208(9):1391–6. doi: 10.1093/infdis/jit360. PMID:24092907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malagón T, Drolet M, Boily MC, Franco EL, Jit M, Brisson J, Brisson M. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(10):781–9. doi: 10.1016/S1473-3099(12)70187-1. PMID:22920953. [DOI] [PubMed] [Google Scholar]
- 21.Toft L, Tolstrup M, Müller M, Sehr P, Bonde J, Storgaard M, Østergaard L, Søgaard OS. Comparison of the immunogenicity of Cervarix® and Gardasil® human papillomavirus vaccines for oncogenic non-vaccine serotypes HPV-31, HPV-33, and HPV-45 in HIV-infected adults. Hum Vaccin Immunother. 2014;10(5):1147–54. doi: 10.4161/hv.27925. PMID:24553190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvesteri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al.. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–71. doi: 10.1038/nm1511. PMID:17115046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.