ABSTRACT
Objectives: To evaluate the initiation and completion and timeliness of inactivated hepatitis A vaccine (HAV-I) series, to identify the missed opportunities for HAV-I series, and to examine determinants associated with the completion of HAV-I.
Methods: Children born from 1 January 2005 to 31 December 2014 and registered in Zhejiang provincial immunization information system (ZJIIS), were enrolled in this study. Descriptive statistics were used to describe the initiation and completion, the timeliness and the missed opportunities for HAV-I. Logistic regression analysis was applied to explore the determinants of the completeness of HAV-I.
Results: The initiation rate of HAV-I increased from 8.1% for the 2005 birth cohort to 13.2% for the 2014 birth cohort. The completion rate of HAV-I increased from 4.2% for the 2005 birth cohort to 7.8% for the 2014 birth cohort. The timeliness rate of the 1st dose of HAV-I increased from 38.2% for the 2005 birth cohort to 57.9% for the 2014 birth cohort. The timeliness rate the 2nd dose of HAV-I increased from 17.3% for the 2005 birth cohort to 35.3% for the 2014 birth cohort. 78.3% of the children who did not initiated any hepatitis A vaccine, had at least one immunization clinic visit after their 18th month of age. 36.0% of the children who received the 1st dose of HAV-I but did not receive the 2nd dose, had at least one immunization clinic visit after 6 months from the date of receiving the 1st dose of HAV-I. The determinants including year of birth, socio-economic development level of municipals, place of delivery, receipt of MMR/VarV were associated with the completion of HAV-I series.
Conclusion: Although the initiation and completion of HAV-I series had improved in recent years, these indicators were still lower than those for other vaccines scheduled at the similar age. It is important for providers to identify every opportunity for HAV-I vaccination and to assure that children get protection from this vaccine-preventable disease.
KEYWORDS: completion, children, inactivated hepatitis A vaccine, initiation, vaccination
Introduction
Hepatitis A infection is an acute illness, typically caused by the hepatitis A virus, which is transmitted through the contaminated water or food or from person-to-person by the fecal-oral route. Children are high risk group of infection, and they are often asymptomatic while still play an important role in transmission.1 Infection in adolescent or adult can lead to a symptomatic illness characterized by an abrupt onset of fever, malaise, nausea, abnormal discomfort and jaundice.2
Hepatitis A can be prevented through vaccination. There are two types of hepatitis A vaccine available in Zhejiang province. One is live attenuated hepatitis A vaccine (HAV-L), which has been licensed in 1990s and has been included in Chinese expanded program on immunization (CEPI) as a category I vaccine since 2008, with the schedule of one dose administrated for free at 18 months of age. The other is inactivated hepatitis A vaccine (HAV-I), which has been licensed in 2002 as a category II (parent-pay) vaccine. HAV-I is voluntary and considered as an alternative of HAV-L. Since 2004, Chinese advisory committee of immunization practice (CACIP) has recommended a 2-doses HAV-I schedule with the 1st dose administrated at 18 months of age and the 2nd dose at 24 months of age. CACIP recommends that the two doses of HAV-I should be given with a minimal interval of 6 months. Furthermore, CACIP stipulates that children need to be vaccinated with two doses of HAV-I to replace the HAV-L. If parents can't afford the 2nd dose of HAV-I, an additional dose of the HAV-L would be given instead. Actually, CACIP suggests vaccination providers should clearly inform the total price of the full series of HAV-I before they make decisions to choose HAV-I to avoid that situation.
Zhejiang is a province located in east China, with 70 million inhabitants living in 11 municipals. Zhejiang has an annual birth cohort of approximately 700,000 newborns in recent years. The incidence of hepatitis A infection of Zhejiang province was estimated to be 0.6 cases per 100000 in 2016, with a total of 465 cases reported. In general, the risk of hepatitis A infection has become lower due to the improvement on sanitation and hygiene, especially in elder susceptible adults. For example, the incidence of hepatitis A infection among adults ≥ 20 years has decreased from 120.7 cases per 100000 in 1988 to 0.2 cases per 100000 in 2016 in Zhejiang province. However, the hepatitis A outbreaks still occurred and the control measures were difficult, which required the rigorous public health efforts with the significant direct or indirect costs to society.3
In Zhejiang province, two types of hepatitis A vaccine were available since the time points of their license. Although, almost 20% of the age-appropriate children chose the self-paid HAV-I to replace HAV-L in Zhejiang province in recent years, the completion of the 2-doses series of HAV-I among children aged ≥ 2 years was lower than other childhood vaccines that needed multiple doses. Low completion rate of HAV-I could be partially due to the relatively recent introduction as well as it needed the out of pocket fee. Similarly, high levels of missed opportunities for immunization including HAV-I had been reported in other settings, where the missed opportunities of vaccination might contributed to the under immunization.4,5 However, little is known specifically on the missed opportunities for the completion of HAV-I in Zhejiang province by now.
As such, we used the vaccination records from Zhejiang provincial immunization information system (ZJIIS) to evaluated the rates of the HAV-I series initiation and completion among children aged ≥ 2 years who chose the HAV-I. Furthermore, we also explored the determinants of the completion of HAV-I from the demographic and socio-economic variables as well as the vaccination status of the other vaccine doses scheduled at the similar age points.
Results
A total of 5722735 children born from 1 January 2005 to 31 December 2014 were enrolled in this study. Of the enrolled children, 51.1% were male, 35.9% were migrant, and 35.2% were from the high socio-economic development municipals. The majority of those children were Han race and were delivered in hospital. Among the enrolled children, the coverage rates of MMR and VarV were 96.1% and 75.3%, respectively (Table 1).
Table 1.
Characteristics of the study subjects.
| Variable | Value | % |
|---|---|---|
| Year of Birtha | 2005(443523) | 7.8 |
| 2006(456209) | 8.0 | |
| 2007(501126) | 8.8 | |
| 2008(519361) | 9.1 | |
| 2009(532126) | 9.3 | |
| 2010(543753) | 9.5 | |
| 2011(619650) | 10.8 | |
| 2012(684232) | 12.0 | |
| 2013(705528) | 12.3 | |
| 2014(717227) | 12.5 | |
| Gender | Male | 51.1 |
| Female | 48.9 | |
| Socio-economic stratab | Low (5 municipals) | 29.9 |
| Middle (3 municipals) | 34.9 | |
| High (3 municipals) | 35.2 | |
| Immigration status of children | Resident | 64.1 |
| Migrant | 35.9 | |
| Race | Han | 93.7 |
| Other minorities | 6.3 | |
| Place of delivery | Home | 4.2 |
| Hospital | 95.8 | |
| Receipt of MMR | No | 3.9 |
| Yes | 96.1 | |
| Receipt of VarV | No | 24.7 |
| Yes | 75.3 |
Note:
: the number of children for each birth cohort was listed.
: All of the 11 municipals were divided into 3 socio-economic strata (high, middle, and low) by Gross Domestic Product (GDP) per capita level according to the 2016 statistics of Zhejiang province. Hangzhou, Ningbo and Zhoushan were classified as high socio-economic development areas for GDP per capita >15000 USD. Shaoxing, Jiaxing and Huzhou were classified as middle socio-economic development areas for GDP per capita between 10000 and 15000 USD. Wenzhou, Taizhou, Jinhua, Quzhou and lishui were classified as low socio-economic development areas for GDP per capita < 10000 USD
The initiation rate of hepatitis A vaccine series was 64.0% for birth cohorts from 2005 to 2014, with 52.5% for HAV-L and 11.5% for HAV-I, respectively. The initiation rate of HAV-I increased from 8.1% for the 2005 birth cohort to 13.2% for the 2014 birth cohort. The completion rate of HAV-I series was 6.4% for birth cohorts from 2005 to 2014, with an increase from 4.2% for the 2005 birth cohort to 7.8% for the 2014 birth cohort (Fig. 1).
Figure 1.
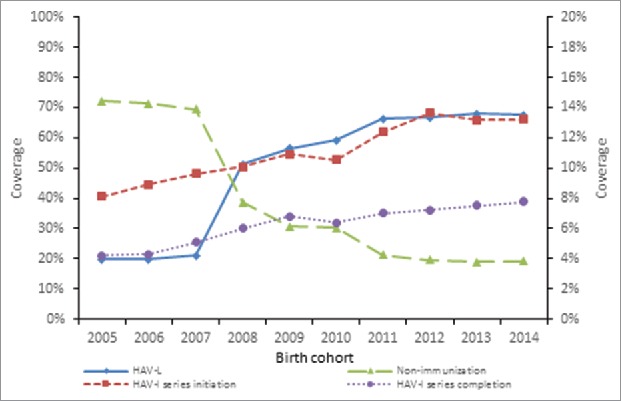
The coverage of HAV-L and the coverage of initiation and completeness of HAV-I for birth cohorts from 2005 to 2014 in Zhejiang province Note: The left Y axis was for the HAV-L coverage and the proportion of non-immunization and the right Y axis was for the HAV-I coverage.
There were 660576 children received at least one dose of HAV-I, 51.7% of which were vaccinated the 1st dose of HAV-I in a timely manner. The coverage of timeliness increased from 38.2% for the 2005 birth cohort to 57.9% for the 2014 birth cohort. There were 368050 children received two doses of HAV-I, 30.8% of which were vaccinated the 2nd dose of HAV-I in a timely manner. The coverage of timeliness increased from 17.3% for the 2005 birth cohort to 35.3% for the 2014 birth cohort (Fig. 2).
Figure 2.
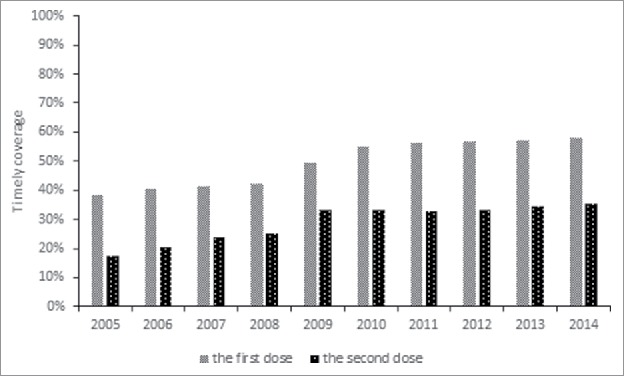
The timeliness of the 1st and 2nd dose of HAV-I for birth cohorts from 2005 to 2014 in Zhejiang province Note: the timeliness of the 1st dose of HAV-I was obtained from 660576 children who received at least one dose of HAV-I, while the timeliness of the 2nd dose of HAV-I was obtained from368050 children who completed the HAV-I series.
There were 2059667(36.0%) enrolled children who did not initiate any vaccination of hepatitis A vaccine. Of these children, 78.3% had at least one immunization clinic visit after the age 18 months. There were 660576 (36.0%) enrolled children who had received the 1st dose of HAV-I but had not received the 2nd dose. Of these children, 61.5% had at least one immunization clinic visit after 6 months from the date of receiving the 1st dose of HAV-I (Table 2).
Table 2.
Missed opportunity of the 1st dose of HAV and the 2nd dose of HAV-I.
| Number of immunization clinic visits |
||||
|---|---|---|---|---|
| Number of target children | 0 | 1 | ≥2 | |
| % of missed opportunity for the 1st dose of HAV (among children never vaccinated with HAV-L or HAV-I) | 2059667 | 21.7 | 52.6 | 25.7 |
| % of missed opportunity for the 2nd dose of HAV-I (among children only vaccinated the 1st dose of HAV-I) | 292526 | 38.5 | 48.9 | 12.6 |
Children born from 2011 to 2014 were likely to complete the 2-doses schedule of HAV-I. Children from high socio-economic municipals, born in hospital were significantly associated with the completion of HAV-I series. Receipt of MMR/VarV were independent determinants positively associated with the completion of HAV-I series (Table 3).
Table 3.
Determinants associated with the completion of HAV-I series.
| Determinants associated with the completion of HAV-I series |
|||
|---|---|---|---|
| Variable | Value | COR(95% CI) | AOR(95% CI) |
| Year of Birth | 2005-2007 | Ref | Ref |
| 2008–2010 | 0.7(0.5–1.1) | 0.8(0.7–1.0) | |
| 2011–2014 | 1.2(1.0–1.3)# | 1.5(1.3–1.7)* | |
| Gender | Male | Ref | — |
| Female | 1.1(0.8–1.3) | — | |
| Socio-economic strata | Low (5 municipals) | Ref | Ref |
| Middle (3 municipals) | 1.2(1.1–1.4)# | 1.0(0.8–1.1) | |
| High (3 municipals) | 1.6(1.3–2.0)# | 1.4(1.2–1.7)* | |
| Immigration status of children | Resident | Ref | Ref |
| Migrant | 0.8(0.7–0.9)# | 0.9(0.8–1.2) | |
| Race | Han | Ref | — |
| Other minorities | 0.8(0.7–1.3) | — | |
| Place of delivery | Home | Ref | Ref |
| Hospital | 2.2(1.9–2.4)# | 2.6(2.3–3.0)* | |
| Receipt of MMR | No | Ref | Ref |
| Yes | 2.7(2.3–3.3)# | 2.9(2.4–3.5)* | |
| Receipt of VarV | No | Ref | Ref |
| Yes | 3.8(3.2–4.1)# | 4.1(3.9–4.3)* | |
Note: COR = crude OR, AOR = Adjusted OR;
: P < 0.10,
: P < 0.05.
Discussion
In this study, we found that the young birth cohorts were more likely to initiate and complete the HAV-I series. This increased trend might be a result of the better acceptance of the recommendation of HAV-I vaccination. However, the coverage of HAV-I still lagged behind the CEPI vaccines and some other non-CEPI vaccines, such as MMR or VarV. This finding was similar to our previous report from Yiwu city,6 where the coverage for CEPI vaccines ranged between 87.9% and 100.0% but the coverage for non-CEPI vaccines ranged between 0% and 74.8%. The main reason for lower initiation and completion rates among non-CEPI vaccines is its out-of pocket fee of vaccination. As we known, the fee of non-CEPI vaccine is sometimes expensive, which may impede the utilization of non-CEPI vaccinations among children from households with low incomes. The difference in coverage among non-CEPI vaccines may be as a result of the difference in the year of its license or introduction. Our previous study indicated that the public perception and training of physician required additional time since new vaccines were licensed or introduced in the market.6 For non-CEPI vaccines, this issue may be more prominent, because the existing public health resources was limited and the majority of these resources were restricted into the CEPI vaccines.
A poor adherence of HAV-I schedule was observed in this study. As we known, the significant implication of untimely vaccination is that a pool of children with delayed vaccinations is at an unnecessary risk of infection. With the presence of such a pool of susceptible children, outbreaks can probably occur when the epidemic threshold is exceeded and it may occur much faster when the delayed immunization is coupled with the low coverage rate.7–9 Although it was difficult to identify the risk factors of untimely vaccination of both two HAV-I doses as there was a proportion of children administrated with HAV-L, we strongly suggested that the timeliness or up-to-date coverage be considered as another important indicator of the performance of vaccination service.
Our results indicated that there were numerous opportunities to administer either HAV-L or HAV-I when children were age-appropriate, but they were not being capitalized upon. Missed opportunities for the 1st dose of HAV or for the 2nd dose of HAV-I highlighted the gaps in the ability to timely and fully protect these children against the hepatitis A infection. The CACIP has set a target coverage of 90% for HAV-L, or full series of HAV-I as an alternative, since the HAV-L included in CEPI. However, the aggregate coverage of both HAV-L and HAV-I was around 80% in 2014. If the providers are unable to decrease the number of missed opportunities for the vaccination of hepatitis A vaccine, the CEPI's goal will be unattainable. Furthermore, many children in our study were observed with the opportunities for immunization clinic visits, but they did not receive any hepatitis A vaccines.
Results of the multivariate analysis found some determinants of the completion of HAV-I, from the utilization side of the vaccination. First, children from low socio-economic municipals were likely to have incompleteness of HAV-I series, which showed a relation between poverty and hindered utilization of HAV-I vaccinations. It was probably due to the poor accessibility caused by the indirect costs (like transport cost or medication for non-serious adverse events) or the deducting wages for work leave for children's vaccination.10 Second, children delivered at hospitals were more likely to complete the HAV-I series. It was possible that mothers who delivered their babies at health facilities would use the health services more frequently,11 including the childhood vaccination service. The administration of the 1st dose of hepatitis B vaccine at birth in the obstetric hospitals might partly account for a better timeliness of the subsequent vaccinations. Third, a high completion rate was observed among children if they had received either MMR or VarV. It suggested that the recommendations, reservation and administration of those vaccines were successful in inducing the broad uptake of HAV-I. A previous study had reported that one of the primary reasons of missed opportunities for immunization was fail to review children's vaccination records completely and carefully, and could not recognize the opportunities for immunization.12 As such, it is important for vaccination providers to verify whether children are current on all recommended vaccinations when they are brought for an immunization clinic visit. Future studies on developing methods applied by providers are necessary to make sure that children's vaccinations are up to date. Another effective intervention to improve the initiation and completeness of HAV-I is to implement the vaccination requirements on the daycare and school entry, which have been demonstrated as an effective method to improve the coverage and reduce heterogenicity within the different sub-populations.13-15
There was a limitation for this study. We evaluated the association between HAV-I completeness and the variables only obtained from ZJIIS. There might be other risk factors from providers or parents that would confound the associations observed. For example, parental refusal might be a variable of interest as the levels of vaccine hesitancy had increased in recent years.16 Future research is needed to focus on this aspect, especially with regard to its association with the missed opportunities for HAV-I vaccination.
Conclusion
Although the initiation and completion of HAV-I series had improved in recent years, these indicators were still lower than those for other vaccines scheduled at the similar age. Reducing missed opportunities can leverage to increase both initiation and completion of HAV-I. It is important for providers to identify every opportunity for HAV-I vaccination and to assure that children get protection from this vaccine-preventable disease.
Methods
Data sources
The database of this study was obtained from ZJIIS, which was established in 2004 by Zhejiang provincial center for disease control. The main functions of ZJIIS can be found elsewhere.17 ZJIIS covers all immunization clinics in Zhejiang province and it includes the demographic information and vaccination records of children born from 1999 to present, with approximately 12 million individual records collected.
Study subjects
Children born from 1 January 2005 to 31 December 2014 and registered in ZJIIS, were enrolled in this study. Appropriately anonymized individual records of the target children with their information on hepatitis A vaccine vaccination (including HAV-L and HAV-I) were extracted from ZJIIS on 31 December 2016. Children who were designated in ZJIIS as permanently inactive (i.e., deceased) or “moved or gone elsewhere” were excluded. The range of birth date was decided based on the schedule of HAV-I as a follow-up period of 2 years would captured this full range.
Measurements
HAV-I series initiation rate was defined as the proportion of eligible children who received at least 1 dose of HAV-I. HAV-I series completion rate was defined as the proportion of children who received at least 2 doses of HAV-I, among the eligible children who received the 1st dose of HAV-I before. The HAV-I series initiation rate and the HAV-I series completion rate were stratified by the demographic variables, the socio-economic variables, and the vaccination status of measles-mumps-rubella combined vaccine (MMR, CEPI vaccine) and varicella vaccine (VarV, category II vaccine), as these two vaccines were scheduled within the similar age range as HAV-I.
For children who received at least 1 dose of HAV-I, the timeliness of the 1st dose of HAV-I was evaluated and the administration within the 18th month of age was defined as timely. For children who received 2 doses of HAV-I, the timeliness of the 2nd dose of HAV-I was evaluated and the administration within the 24th month of age was defined as timely.
Among children who did not initiate any hepatitis A vaccine, we checked the missed opportunities by measuring the number of immunization clinic visit after 18 months of age. For children who received the 1st dose but did not receive the 2nd dose, we examined missed opportunities by measuring number of immunization clinic visit after 6 months from the date of receiving the 1st dose of HAV-I. Immunization clinic visit was defined as a visit at which children received vaccinations rather than HAV-I or HAV-L. Missed opportunities of vaccinations were tallied, and categorized into 3 groups: 0, 1, and ≥2.
Data analysis
Descriptive statistics were used to describe the characteristics and study measurements. Both univariate analysis and multivariate analysis were applied to explore the determinants of the completion of HAV-I series among children who received the 1st dose of HAV-I before. In the univariate analysis, we cross-tabulated each explanatory variables with the completion of HAV-I series. χ2 test was used to obtain P values and crude odds ratio (COR) with 95% confidence interval (CI) was calculated. In the multivariate analysis, variables associated with the completion of HAV-I with P-value < 0.10 were entered into the multivariate model. Logistic regression analysis was adopted to obtain adjusted odds ratio (AOR) with 95% CI. All statistical analyses were performed using statistics package for socio science software, version 13.0(SPSS Inc, Chicago, IL, USA). Statistical significance was defined as a two-tailed P-value of 0.05.
Ethical considerations
This study was approved by the ethical review board of Zhejiang provincial center for disease control and prevention. All the data were anonymous when we extracted them from ZJIIS and kept confidential without individual identifiers.
Funding Statement
This study was supported by the General Medical Research Program of Zhejiang province in 2015 (program number: 2015KYB081).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Rui Wu from Suzhou Shensu Automatic Technology Co., Ltd. for providing assistance with the ZJIIS data.
Author Contributions
Yu Hu and Yaping Chen conceived and designed the experiments; Yu Hu and Yaping Chen performed the experiments; Yu Hu and Hui Liang analyzed the data; Ying Wang contributed reagents/materials/analysis tools; Yu Hu wrote the paper.
References
- 1.Rizzo C, Alfonsi V, Bruni R, Busani L, Ciccaglione A, De Medici D, Di Pasquale S, Equestre M, Escher M, Montano-Remacha M, et al.. Ongoing outbreak of hepatitis A in Italy: preliminary report as of 31 May 2013. Euro Surveill. 2013;18:20518. doi: 10.2807/1560-7917.ES2013.18.7.20518. PMID:23870075. [DOI] [PubMed] [Google Scholar]
- 2.Donnan EJ, Fielding JE, Gregory JE, Lalor K, Rowe S, Goldsmith P, Antoniou M, Fullerton KE, Knope K, Copland JG, et al.. A multistate outbreak of hepatitis A associated with semidried tomatoes in Australia, 2009. Clin Infect Dis. 2012;54:775–81. doi: 10.1093/cid/cir949. PMID:22238166. [DOI] [PubMed] [Google Scholar]
- 3.Wasley A, Grytdal S, Gallagher K, Centers for Disease C, Prevention . Surveillance for acute viral hepatitis–United States, 2006. MMWR Surveill Summ. 2008;57:1–24. PMID:18354374. [PubMed] [Google Scholar]
- 4.Fu LY, Zook K, Gingold J, Gillespie CW, Briccetti C, Cora-Bramble D, Joseph JG, Moon RY. Frequent vaccination missed opportunities at primary care encounters contribute to underimmunization. J Pediatrics. 2015;166:412–7. doi: 10.1016/j.jpeds.2014.10.066. [DOI] [PubMed] [Google Scholar]
- 5.Sun M, Ma R, Zeng Y, Luo F, Zhang J, Hou W. Immunization status and risk factors of migrant children in densely populated areas of Beijing, China. Vaccine. 2010;28:1264–74. doi: 10.1016/j.vaccine.2009.11.015. PMID:19941996. [DOI] [PubMed] [Google Scholar]
- 6.Hu Y, Chen Y, Guo J, Tang X, Shen L. Completeness and timeliness of vaccination and determinants for low and late uptake among young children in eastern China. Hum Vaccines Immunotherapeutics. 2014;10:1408–15. doi: 10.4161/hv.28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith PJ, Humiston SG, Parnell T, Vannice KS, Salmon DA. The association between intentional delay of vaccine administration and timely childhood vaccination coverage. Public Health Reports. 2010;125:534–41. doi: 10.1177/003335491012500408. PMID:20597453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yadav K, Srivastava R, Kumar R, Chinnakal P, Rai SK, Krishnan A. Significant vaccination delay can occur even in a community with very high vaccination coverage: evidence from Ballabgarh, India. J Tropical Pediatrics. 2012;58:133–8. doi: 10.1093/tropej/fmr059. [DOI] [PubMed] [Google Scholar]
- 9.Lernout T, Theeten H, Hens N, Braeckman T, Roelants M, Hoppenbrouwers K, Van Damme P. Timeliness of infant vaccination and factors related with delay in Flanders, Belgium. Vaccine. 2014;32:284–9. doi: 10.1016/j.vaccine.2013.10.084. PMID:24252698. [DOI] [PubMed] [Google Scholar]
- 10.Kitamura T, Komada K, Xeuatvongsa A, Hachiya M. Factors affecting childhood immunization in Lao People's Democratic Republic: a cross-sectional study from nationwide, population-based, multistage cluster sampling. Bioscience Trends. 2013;7:178–85. PMID:24056168. [PubMed] [Google Scholar]
- 11.Frew PM, Chung Y, Fisher AK, Schamel J, Basket MM. Parental experiences with vaccine information statements: Implications for timing, delivery, and parent-provider immunization communication. Vaccine. 2016;34:5840–4. doi: 10.1016/j.vaccine.2016.10.026. PMID:27789148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease C, Prevention Hepatitis A vaccination coverage among U.S. children aged 12–23 months – immunization information system sentinel sites, 2006–2009. MMWR. 2010;59:776–9. PMID:20592688. [PubMed] [Google Scholar]
- 13.Cawkwell PB, Oshinsky D. Childhood vaccination requirements: Lessons from history, Mississippi, and a path forward. Vaccine. 2015;33:5884–7. doi: 10.1016/j.vaccine.2015.08.087. PMID:26409142. [DOI] [PubMed] [Google Scholar]
- 14.Bugenske E, Stokley S, Kennedy A, Dorell C. Middle school vaccination requirements and adolescent vaccination coverage. Pediatrics. 2012;129:1056–63. doi: 10.1542/peds.2011-2641. PMID:22566425. [DOI] [PubMed] [Google Scholar]
- 15.Lindley MC, Lorick SA, Spinner JR, Krull AR, Mootrey GT, Ahmed F, Myers R, Bednash GP, Cymet TC, Maeshiro R, et al.. Student vaccination requirements of U.S. health professional schools: a survey. Ann Inter Med. 2011;154:391–400. doi: 10.7326/0003-4819-154-6-201103150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Stahl JP, Cohen R, Denis F, Gaudelus J, Martinot A, Lery T, Lepetit H. The impact of the web and social networks on vaccination. New challenges and opportunities offered to fight against vaccine hesitancy. Medecine Et Maladies Infect. 2016;46:117–22. doi: 10.1016/j.medmal.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Li Q, Hu Y, Zhong Y, Chen Y, Tang X, Guo J, Shen L. Using the Immunization Information System to determine vaccination coverage rates among children aged 1–7 years: a report from Zhejiang Province, China. Int J Environmental Res Public Health. 2014;11:2713–28. doi: 10.3390/ijerph110302713. [DOI] [PMC free article] [PubMed] [Google Scholar]


