ABSTRACT
The objective of the study was to evaluate the role of age and sex and their combined effect in the development of post-herpetic neuralgia (PHN) in a large population-based study, in order to confirm the results published previously by Amicizia et al. Data were extracted from population and healthcare databases from the Valencia Region (2009–2014). Logistic regressions were implemented to estimate the effect of increasing age on the probability of developing PHN stratified by sex. From a cohort of 2,289,485 subjects ≥ 50 years, 87,086 cases of HZ were registered and 13,658 (15.7%) of them developed PHN. In our population, PHN cases were more common in women and rose with increasing age independently of the sex.
KEYWORDS: age-sex combined effect, healthcare databases, Herpes zoster, post-herpetic neuralgia, population-based study, risk factor
To the editor
We are writing in regards to the article “The role of age-sex interaction in the development of post-herpetic neuralgia” published by Daniela Amicizia and colleagues in volume 13, number 2 of Human Vaccines and Immunotherapeutics. These authors studied the role of age and sex and their combined effect in the development of post-herpetic neuralgia (PHN)1 and pointed out the small sample size as one of the limitations of the study and the need for larger studies to confirm their results.
In order to corroborate or contrast these results in a larger population, we have developed a population-based study with a total cohort of 2,289,485 subjects ≥ 50 years. Data were extracted from population and healthcare databases from the Valencia Region of Spain (2009–2014), which has a population of approximately 5 million inhabitants.2 Over 98% of them are insured by the Regional Health System (RHS),3 which consists of 24 Health Departments.4 Each of them includes at least one hospital, one specialties center and a number of ambulatory centers. All primary care visits and hospitalizations are recorded in clinical databases.
About 37% of the Valencian population is 50 years of age or older. The study cohort included all individuals aged 50 years or older, living in the Valencia Region and insured by the RHS between the 1st of January 2009 and the 31st of December 2014. Subjects were included in the cohort if they were aged 50 years or older on 1st January 2009, or on the day of entry into RHS, or on their fiftieth birthday, whichever occurred last. Follow-up ended when they left Valencia (data of deletion from RHS), on their death or at the end of the study (31 December 2014), whichever occurred first. Patients with immunosuppressive conditions were excluded.5
Herpes zoster (HZ) and PHN were defined using ICD-9 codes and reported prescription of specific HZ and PHN medications.5 For descriptive purposes PHN was defined as pain persisting beyond the acute phase of an HZ considering periods from 30 (PHN1) and 90 (PHN3) days post HZ diagnosis, however only pain persisting beyond 90 days after rash onset is considered true PHN.
The percentage of HZ cases that developed PHN by age groups and by sex was calculated. Logistic regressions were implemented to estimate the effect of increasing age on the probability of developing PHN stratified by sex and by PHN definition.
From this cohort, 87,086 cases of HZ were registered and 13,658 (15.7%) of them developed PHN3 [19,101 (21.9%) developed PHN1]. In a first descriptive analysis, PHN was differently distributed among males (19% for PHN1 and 12% for PHN3) and females (24% for PHN1 and 18% for PHN3) in contrast with Amicizias et al. observations who reported equal distribution among both sexes.
Also contrary to what they reported, we observed a significant raise of the number of PHN cases with the increasing age for both males and females (Fig. 1 and Table 1) and for both PHN definitions. Table 2 shows the age odds ratio for the sex-stratified analysis and for both PHN definitions. Males aged between 60–69 years were 50% [OR: 1.50; 95% CI: (1.35–1.66)] more likely to develop PHN3 than aged between 50 and 59, and it was 2.31 and 2.67 times more probable for 70–79 and ≥ 80 HZ patients respectively. A similar increase was observed in females and for both PHN definitions (Table 2). According to our results, significant age-associated pattern was observed in both men and women.
Figure 1.
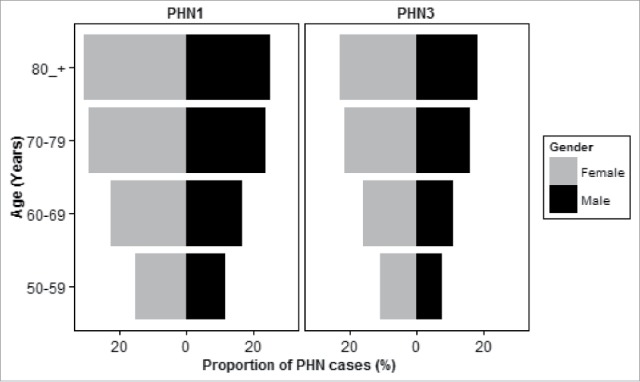
Proportion of PHN cases by sex and age for both PHN definitions.
Table 1.
Number of PHN cases and proportion (%) of HZ cases developing PHN by age and sex for both PHN definitions.
| PHN1 cases (%) |
PHN3 cases (%) |
|||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Age | ||||
| 50–59 | 938 | 1974 | 608 | 1409 |
| (11.7) | (15.1) | (7.6) | (10.8) | |
| 60–69 | 1741 | 3462 | 1155 | 2503 |
| (16.5) | (22.2) | (11) | (16.1) | |
| 70–79 | 2153 | 4265 | 1461 | 3119 |
| (23.5) | (28.9) | (15.9) | (21.1) | |
| ≥ 80 | 1250 | 3318 | 904 | 2499 |
| (24.9) | (30.3) | (18) | (22.8) | |
Table 2.
Logistic regressions implemented to estimate the effect of the age on the probability of developing of PHN stratified by sex and by PHN definition.
| OR (95% CI)* |
||||
|---|---|---|---|---|
| PHN1 |
PHN3 |
|||
| Male | Female | Male | Female | |
| Age | ||||
| 50–59 | 1 | 1 | 1 | 1 |
| 60–69 | 1.49 (1.37–1.63) | 1.60 (1.50–1.70) | 1.50 (1.35–1.66) | 1.58 (1.47–1.69) |
| 70–79 | 2.31 (2.13–2.52) | 2.28 (2.14–2.42) | 2.31 (2.09–2.55) | 2.21 (2.07–2.37) |
| ≥ 80 | 2.50 (2.27–2.74) | 2.43 (2.28–2.59) | 2.67 (2.39–2.98) | 2.44 (2.27–2.62) |
OR: Odds ratio; 95% CI: 95% confidence interval
Although both are retrospective studies, there are some differences that could explain the discrepancies in the results. There is a lack of a standard definition for PHN among GPs in the Amicizias' study, such as mentioned by the authors in the limitations section. Concerning to the population of both studies, ours is a population-based study were data and results correspond to the entire population while conclusions from the other study represent a small sample size. Moreover, we analyzed data from people ≥ 50 years, when incidence rate for HZ rises sharply,6,7 while Amicizia et al. enrolled adult subjects ≥ 18 years. Opstelten, for example, reported previously that female sex was an independent risk factor for HZ in the 25- to 64-year-old age groups while sex effect was inverse in young adults (age, 15 to 24 years).8
Some previous studies have also reported inconsistencies with respect to whether there are sex differences in the risk of PHN.9–18 Methodological differences such as design of the study (retrospective or prospective), case definitions, study population (a sample of the total population or population-based studies), age of the study population (all ages, ≥18, ≥50, ≥60), data sources (primary care, hospitalizations, specialties centers, all) or statistical analysis might be responsible for these discrepancies. Among these studies, the most similar to ours regarding the design and their large populations, reported similar results, with more than 60% of the PHN cases documented in female patients in United Kingdom and in Italy.11,18
Pathophysiological causes of these sex differences are unknown. It has been hypothesized that females might have a different response to latent viral infections, which is strongly supported by the sex difference also reported in the incidence of herpes simplex.19 However, further research is needed to elucidate the difference in the immune response between sexes.
In conclusion, our results point to a higher probability of developing PHN in females and a similar increase of PHN probability with increasing age independently of the sex. These findings respond to the need mentioned by Amicizias et al. for a more robust and large scale study to ascertain their results.
Disclosure of potential conflicts of interest
This study was not funded but FISABIO had a contract with Sanofi Pasteur MSD (SPMSD) to study the epidemiology of HZ. SPMSD had no role in the analysis or discussion of the results.JDD and his institution received research grants from SPMSD related to HZ vaccine. He also acted as advisor for these vaccines to GSK and SPMSD. CMQ and AOS have attended several congresses and their registration, travel and accommodation costs have been covered by SPMSD. MLL has reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Acknowledgments
The authors thank Topi Turunen for manuscript review.
Author contributions
JDD is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article.
CMQ contributed to study conception and design; data acquisition, analysis, and interpretation; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. CMQ takes responsibility for the integrity of the data and the accuracy of the data analysis and serves as principal author.
MLL contributed to data acquisition, data cleaning, analysis and interpretation; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published.
JDD and AOS contributed to study design; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published.
References
- 1.Amicizia D, Domnich A, Arata L, Zoli D, Zotti CM, Cacello E, Gualano MR, Gasparini R, Panatto D. The role of age-sex interaction in the development of post-herpetic neuralgia. Hum Vaccin Immunother. 2017;13(2):376–378. doi: 10.1080/21645515.2017.1264799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Instituto nacional de estadística: Cifras oficiales de población resultantes de la revisión del padrón municipal a 1 de enero de 2014. 2014. [accessed] http://www.ine.es/dynt3/inebase/es/index.html?padre=517&dh=1.
- 3.Indra sistemas sa: Proyecto abucasis conselleria sanitat valencia. 2012. [accessed] http://www.indracompany.com/sectores/sanidad/proyectos/179/proyecto-abucasis-conselleria-de-sanitat-valencia.
- 4.Munoz-Quiles C, Lopez-Lacort M, Ubeda-Sansano I, Aleman-Sanchez S, Perez-Vilar S, Puig-Barbera J, Diez-Domingo J. Population-based analysis of bronchiolitis epidemiology in valencia, spain. Pediatric Infect Dis J. 2016;35(3):275–280. doi: 10.1097/INF.0000000000000993. [DOI] [PubMed] [Google Scholar]
- 5.Muñoz-Quiles C, López-Lacort M, Ampudia-Blasco FJ, Díez-Domingo J. Risk and impact of herpes zoster on patients with diabetes: A population-based study, 2009–2014. Hum Vaccin Immunother. 2017;2017 Sep 21:0. doi: 10.1080/21645515.2017.1368600 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmader K. Herpes zoster in older adults. Clinical Infectious Diseases. 2001;32(10):1481–1486. doi: 10.1086/320169. [DOI] [PubMed] [Google Scholar]
- 7.Morant-Talamante N, Diez-Domingo J, Martinez-Ubeda S, Puig-Barbera J, Aleman-Sanchez S, Perez-Breva L. Herpes zoster surveillance using electronic databases in the valencian community (spain). BMC Infect Dis. 2013;13:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Opstelten W, Van Essen GA, Schellevis F, Verheij TJ, Moons KG. Gender as an independent risk factor for herpes zoster: A population-based prospective study. Ann Epidemiol. 2006;16(9):692–695. doi: 10.1016/j.annepidem.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Forbes HJ, Thomas SL, Smeeth L, Clayton T, Farmer R, Bhaskaran K, Langan SM. A systematic review and meta-analysis of risk factors for postherpetic neuralgia. Pain. 2016;157(1):30–54. doi: 10.1097/j.pain.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung BF, Johnson RW, Griffin DRJ, Dworkin RH. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology. 2004;62(9):1545–1551. doi: 10.1212/01.WNL.0000123261.00004.29. [DOI] [PubMed] [Google Scholar]
- 11.Gauthier A, Breuer J, Carrington D, Martin M, Remy V. Epidemiology and cost of herpes zoster and post-herpetic neuralgia in the united kingdom. Epidemiol Infect. 2009;137(1):38–47. doi: 10.1017/S0950268808000678. [DOI] [PubMed] [Google Scholar]
- 12.Jih J-S, Chen Y-J, Lin M-W, Chen Y-C, Chen T-J, Huang Y-L, Chen C-C, Lee D-D, Chang Y-T, Wang W-J, et al.. Epidemiological features and costs of herpes zoster in taiwan: A national study 2000 to 2006. Acta Derm-Venereol. 2009;89(6):612–616. doi: 10.2340/00015555-0729. [DOI] [PubMed] [Google Scholar]
- 13.Scott FT, Johnson RW, Leedham-Green M, Davies E, Edmunds WJ, Breuer J. The burden of herpes zoster: A prospective population based study. Vaccine. 2006;24(9):1308–1314. doi: 10.1016/j.vaccine.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 14.Dieleman JP, Kerklaan J, Huygen FJPM, Bouma PAD, Sturkenboom MCJM. Incidence rates and treatment of neuropathic pain conditions in the general population. Pain. 2008;137(3):681–688. doi: 10.1016/j.pain.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Bouhassira D, Chassany O, Gaillat J, Hanslik T, Launay O, Mann C, Rabaud C, Rogeaux O, Strady C. Patient perspective on herpes zoster and its complications: An observational prospective study in patients aged over 50 years in general practice. Pain. 2012;153(2):342–349. doi: 10.1016/j.pain.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Opstelten W, Mauritz JW, de Wit NJ, van Wijck AJM, Stalman WAB, van Essen GA. Herpes zoster and postherpetic neuralgia: Incidence and risk indicators using a general practice research database. Family Practice. 2002;19(5):471–475. doi: 10.1093/fampra/19.5.471. [DOI] [PubMed] [Google Scholar]
- 17.Parruti G, Tontodonati M, Rebuzzi C, Polilli E, Sozio F, Consorte A, Agostinone A, Di Masi F, Congedo G, D'Antonio D, et al.. Predictors of pain intensity and persistence in a prospective italian cohort of patients with herpes zoster: Relevance of smoking, trauma and antiviral therapy. Bmc Medicine. 2010;8:58. doi: 10.1186/1741-7015-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gialloreti LE, Merito M, Pezzotti P, Naldi L, Gatti A, Beillat M, Serradell L, di Marzo R, Volpi A. Epidemiology and economic burden of herpes zoster and post-herpetic neuralgia in italy: A retrospective, population-based study. Bmc Infect Dis. 2010;10 230 Published online 2010 Aug 3. doi: 10.1186/1471-2334-10-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleming DM, Cross KW, Cobb WA, Chapman RS. Gender difference in the incidence of shingles. Epidemiol Infect. 2004;132(1):1–5. doi: 10.1017/S0950268803001523. [DOI] [PMC free article] [PubMed] [Google Scholar]


