ABSTRACT
Objective: In China, Hib vaccine is a private-sector vaccine that is an option for parents to select to give to their children; it must be paid for out-of-pocket because it is not included in the government's Expanded Program on Immunization (EPI). We evaluated utilization patterns of Hib vaccine to provide evidence in support of development of a national Hib vaccination strategy. Methods: We obtained lists of children from immunization information systems (IIS) of counties or districts in 8 provinces of China. Using these lists, we selected 10 children at random from each birth cohort from 2008 through 2012. We obtained Hib vaccination dates from official vaccination certificates. The target sample size was 1,000 children. Results: We were able to obtain records for 978 subjects of the selected subjects; of these, 44.79% had received at least 1 dose of Hib vaccine, and 15.54%, 5.83%, 12.27%, and 11.15% had received one, two, three, and four doses, respectively. Per capita GDP was positively correlated with receipt of at least one dose of Hib vaccine. Among the 438 subjects who received Hib vaccine, 27% received 1 dose after 12 months of age; 15%, 7%, and 23% received one of three other patterns of Hib vaccination recommended by the World Health Organization (WHO) [a 3-dose primary series; 2 primary series doses and 1 booster; or 3 primary series doses and 1 booster]. The other 28% of subjects received patterns of Hib vaccination not recommended by WHO. Considering protection from Hib disease as receipt of a WHO-recommended Hib vaccine schedule, 29% of subjects could be considered protected after 12 months of age, 52% could be considered protected during infancy and beyond, and 19% could be considered to not have been protected adequately, despite being vaccinated. Conclusions: Coverage with Hib vaccine was low. There were significant differences between WHO recommendations and actual patterns of use of Hib vaccine, with half of vaccine recipients receiving no protection during infancy and one fifth receiving non-protective Hib vaccination patterns. Inclusion of Hib vaccine into China's EPI system, which provides vaccine at no charge to parents and makes specific vaccination schedule standards, has potential to make more effective use of Hib vaccine.
KEYWORDS: Haemophilus influenzae type B vaccine, vaccination rate, vaccination age, interval between doses, concomitant vaccination
Introduction
Haemophilus influenzae has caused significant morbidity and mortality worldwide. In the pre-vaccine era, 95% of Haemophilus influenzae infections were caused by serotype b (Hib).1 Hib meningitis and pneumonia can be fatal in 3% to 20% of patients and can cause serious sequelae among the 30% to 40% of survivors. Invasive Hib disease generally occurs during the first 5 years of life, and is most common among children less than 2 years of age. The most serious cases of invasive Hib disease are among infants 4 to 8 months old.
Immunizing infants with Hib vaccine is the most effective and cost-effective means to prevent invasive Hib disease; the World Health Organization (WHO) recommends inclusion of Hib vaccine in all national immunization programs. As of December 2013, 192 countries and regions include Hib vaccine in their immunization programs – all WHO Member States except China, and Thailand.2 In China, Hib vaccine is a widely-used private-sector vaccine that is voluntarily administered and paid for out-of-pocket by parents or caregivers. At the national level, neither a Hib vaccine schedule nor Hib vaccine administration guidelines have been developed and promoted. Vaccination staff recommend and administer Hib vaccine based on manufacturers' package inserts or promotional materials rather than on program guidance.
Even though Hib vaccine is one of the most widely used private-sector vaccines in China,3 there are no published studies on Hib vaccine utilization patterns. We report a study to evaluate patterns of Hib vaccination in China to provide evidence for the development of national Hib vaccination guidelines.
Materials and methods
We conducted a cross-sectional survey of Hib vaccination status; all vaccination data came from official records. The survey was conducted between January and July of 2014.
Subjects and settings
Subjects were children born between January 1, 2008 and December 31, 2012. We selected 8 provinces for study – at least 2 provinces from each region of China – eastern, middle and western. We selected at least 2 counties or districts from these provinces. County/district selection was based on local health authorities' opinions that the selected settings had high-quality official certificate records of private-sector vaccines and had functioning immunization information systems (IIS) which contained the most complete lists of children born from 2008 to 2012.
Figure 1 shows the selected provinces. We selected 20 counties/districts – in eastern China, we selected 2 districts from Shanghai, 2 counties/districts from Jiangsu, and 3 districts from Guangdong; in middle China, we selected 2 counties from Hebei, 4 counties from Heilongjiang, and 2 counties from Jiangxi; and from western China, we selected 2 counties from Gansu, and 3 counties from Chongqing.
Figure 1.
Provinces selected for study.
Sampling methods and sample size
Our desired sample size was based on the calculation:
where n was the desired sample size and P was the coverage rate reported in the literature for receiving 1 or more doses of Hib vaccine.3 A minimum of 470 subjects was needed. To account for the cluster sampling, we used a design effect multiplier of 2. We allowed for a 5%, lost-to-follow-up rate. The final target sample size was 1000.
We obtained lists of children born between 2008 and 2012 from the 20 countries or districts IISs, and we selected a simple random sample of 10 children from each of the 5 birth cohorts, for a total sample of 20 countries/districts * 5 birth cohorts * 10 subjects = 1,000 subjects.
We obtained our random sample from IIS lists of children, ranked by their unique codes in IIS. We then used a sequence of computer-generated random numbers, matched them by rank with the IIS list and then selected the ten children with the lowest computer-generated random numbers as subjects.
Per capita gross domestic product (GDP)
GDP and population data were obtained from the “Statistical bulletin on per capita national economic and social development in 2012,” as reported on county/district Bureau of Statistics websites. If the GDP at county/district level was not available, we used prefecture-level per capita GDP data.
We calculated the average value of per capita GDP of the selected counties/districts in each province as the sum of selected counties (or districts) GDPs from the same province divided by the total number of individuals registered in these selected counties/districts at the end of 2012.
Vaccination status and rates
The names of selected children were provided to the corresponding county or district CDC. The CDCs were responsible for identifying the clinic at which children were vaccinated and for obtaining Hib-containing vaccination dates from official vaccination certificates.
We categorized each subject by the number of Hib-containing vaccine doses they received: 1 dose, 2 doses, 3 doses, 4 or more doses, and by whether they received any dose of Hib vaccine. For vaccination rate calculations, numerators were the sum of children meeting the appropriate vaccination status, and denominators were the number of children in the specific grouping. For example, the overall Hib vaccination rate was the number of children receiving any dose of Hib vaccine divided by the number of subjects, and the rate of 4-or-more dose recipients for a given province was the number of 4-or-more-dose recipients divided by the number of subjects sampled from that province.
Statistical methods
We used Epidata 3.1 to establish a vaccination record database and double-entered data to ensure transcription accuracy. We compared the utilization pattern with WHO recommendations. We used chi square test or Fisher exact probability methods for categorical data analyses; we used Student's t tests, analysis of variance, and rank sum testing for continuous variable analyses. We considered P-values less than 0.05 to be statistically significant.
WHO recommendations
The WHO position paper on Hib vaccine lists three acceptable Hib vaccination schedules: 3 primary-series doses (3P); 3 primary doses plus 1 booster dose (3P+1); and 2 primary doses plus 1 booster dose (2P+1). The WHO-recommended interval between doses should be at least 4 weeks if 3 primary-series doses are given, and at least 8 weeks if 2 primary-series doses are given. Booster doses should be administered at least 6 months after completion of a primary series. When a first dose is given to a child >12 months of age, only one dose is recommended. Hib vaccine is not recommended for healthy children more than 5 years of age.
Ethics approval and consent to participate
The Chinese Center for Disease Control and Prevention's Ethical Review Committee (ERC) considers vaccination record checks to be routine work, without the need for additional, specific ERC approval. Personally-identifiable data were not retained in the analytic data sets.
Results
We obtained vaccination data on 978 subjects (97.8% overall response rate), including 543 male and 435 female subjects – a male to female ratio of 1.25:1. Among the subjects, 197, 197, 195, 195, and 194 children were born in 2008, 2009, 2010, 2011 and 2012, respectively; and 143, 127, 106, 200, 100, 102, 100, and 100 children were located in Gansu, Guangdong, Hebei, Heilongjiang, Jiangsu, Jiangxi, Shanghai, and Chongqing, accounting for 14.62%, 12.99%, 10.84%, 20.45%, 10.22%, 10.43%, 10.22%, and 10.22% of the subjects, respectively.
Uptake of Hib vaccine
Of the 978 subjects, 438 received at least 1 dose of Hib vaccine, for a vaccination rate of 44.79% (95% CI:43.20%-46.38%). By birth cohort, vaccination rates were 47.69% (95% CI: 44.13%-51.25%), 44.67% (95% CI: 41.13%-48.21%), 47.72% (95% CI: 44.14%-51.30%), 43.59% (95% CI: 40.04%-47.14%), and 40.21% (95% CI: 36.69%-43.73%) for the 2008 – 2012 cohorts, respectively (P = 0.54). By sex, 42.91%(95% CI:40.79%-45.03%) of males and 47.13%(95% CI: 44.74%-49.52%) of females received at least one dose (P = 0.19). There was a significant difference in coverage by province (P = 0.00).
Among all subjects, 152 (15.54%, 95% CI: 14.38%-16.70%) received 1 dose of Hib vaccine, 57 (5.83%, 95% CI:5.10%-6.58%) received 2 doses, 120 (12.27%, 95% CI: 11.22%-13.32%) received 3 doses, and 109 (11.15%, 95% CI: 10.14%-12.16%) received 4 or more doses. Dose-specific vaccination rates were different among different birth cohorts and provinces. As the birth year increased, the vaccination rate with 3 or more doses increased. Most children in Gansu, Hebei, Heilongjiang, and Jiangsu provinces received 1 dose of Hib vaccine, while most children in Jiangxi, Shanghai, Chongqing, and Guangdong received 3 or more doses (Fig. 2).
Figure 2.
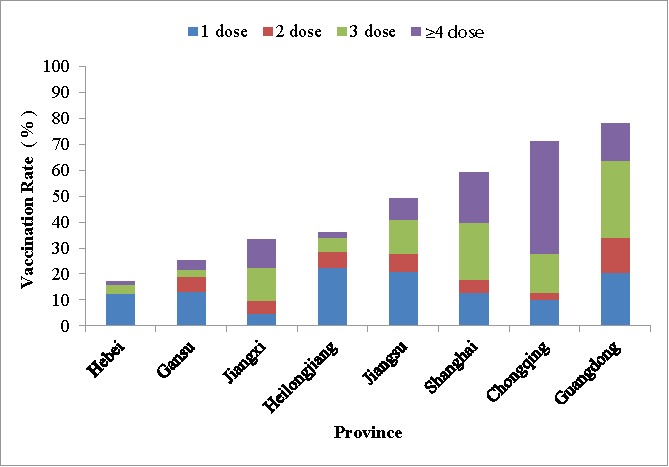
Hib vaccination rate by province.
We conducted a simple linear correlation analysis between vaccination rate and per capita GDP without stratification or considering other confounders. The correlation analysis showed that there was a statistically significant linear relation between per capita GDP and vaccination rate (r = 0.88, P = 0.0038). As per capita GDP increased, vaccination rate increased (Fig. 3).
Figure 3.
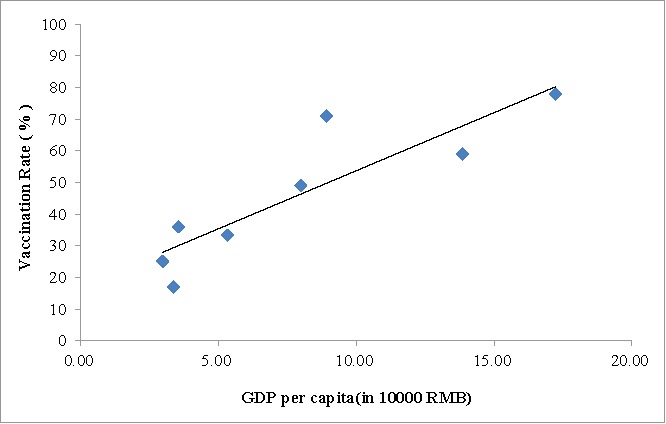
Hib vaccination rate and per capita GDP.
Type of Hib-containing vaccine
Among the 438 children who had received Hib-containing vaccine, 422 (96.35%) received monovalent Hib vaccine; 16 (3.65%) received DTP-Hib-IPV pentavalent vaccine; and 2 (0.46%) received DTP-Hib quadrivalent vaccine.
Concomitant use of Hib vaccine and other vaccines
Among the 438 children who had received Hib vaccine, 159 (36.30%) received Hib vaccine and at least 1 other vaccine at the same visit. Concomitant use rates by birth cohort were 32.26%, 37.50%, 38.30%, 42.35%, and 30.77% for each of the 2008 through 2012 birth cohorts, respectively (no statistically significantly differences). The proportion of concomitant use by province was 16.67% for Gansu, 9.09% for Guangdong, 27.78% for Hebei, 22.22% for Heilongjiang, 30.61% for Jiangsu, 85.29% for Jiangxi, 32.20% for Shanghai, and 84.51% for Chongqing. There were significant differences in concomitant use by province – for example 85% of children in Jiangxi and Chongqing received Hib vaccine with other vaccines, while only 9.09% of children in Guangdong received Hib vaccine with other vaccines.
Vaccines given simultaneously with Hib vaccine included OPV, IPV, DTaP, DTwP, measles, rotavirus, influenza, Hepatitis B, varicella, Meningococcal serogroup A, measles-rubella, attenuated Japanese Encephalitis, Meningococcal serogroup A+C, MMR, attenuated Hepatitis A, inactivated Hepatitis A, 7-valent Pneumococcal Conjugate, and BCG vaccines. The respective percentages of concomitant use were 70.44%, 1.89%, 51.57%, 10.06%, 1.26%, 8.18%, 3.14%, 3.77%, 0.63%, 10.06%, 2.52%, 3.77%, 0.63%, 6.29%, 1.26%, 2.52%, 0.63%, 0.63%.
Hib vaccination status and protection provided by Hib vaccination
The age distribution of Hib vaccination were as follows: Among the children receiving only one dose of Hib vaccine, the majority (66.45%; 101/152) received the 1st dose at 1 year old; among the children receiving ≥2 doses of Hib vaccine, the majority received the first dose at 2 months, accounting for 19.30% (11/57), 39.17% (47/120) and 63.30% (69/109) for children receiving only 2 doses, only 3 doses, or no less than 4 doses respectively. Among the children receiving only 2 doses, the majority received the 2nd dose at 12–23 months old and at 11 months old, accounting for 45.61% and 21.05% respectively. Among the children receiving ≥3 doses of Hib vaccine, the majority received the 2nd dose at 3, 4 and 5 months old. Among the children receiving 3 doses of Hib vaccine, the majority received the 3rd dose at 12–23 months old. Among the children receiving ≥4 doses of Hib vaccine, the majority received the 3rd dose at 4 months old, followed by 5 and 7 months old, and the majority received the 4th dose at 1 year old, accounting for 86.2% (94/109). The 5th doses were all administered after 12 months old.
We analyzed patterns of Hib vaccine use in China with respect to the WHO recommendation (Fig. 4). The non-recommended patterns included 33 subjects who received only 1 dose of Hib vaccine before 12 months of age; 57 subjects who received 2 doses (49 starting before 12 months and 8 starting after 12 months); and 3 subjects who received 5 doses, 25 subjects who received 2P+1 schedule and 4 subjects who received a 3P+1 schedule with improper intervals between doses.
Figure 4.
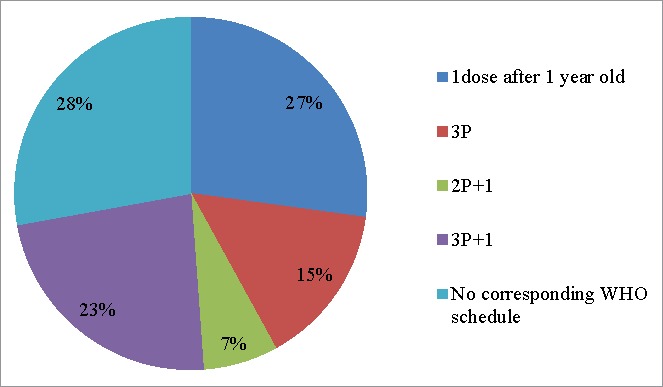
Hib vaccination patterns in China among children receiving at least 1 dose of Hib vaccine, with respect schedules stated as acceptable in the WHO Hib Vaccine position paper.
Receipt of ≥1 dose of Hib vaccine after 12 months of age can provide protection after vaccination, and completion of the 3P, 2P+1, and 3P+1 patterns and receiving more than 4 doses can provide protection in infancy and beyond. The other patterns are improper and cannot be considered to provide adequate protection from invasive Hib disease. With these considerations, Fig. 5 shows patterns of protection against invasive Hib disease.
Figure 5.
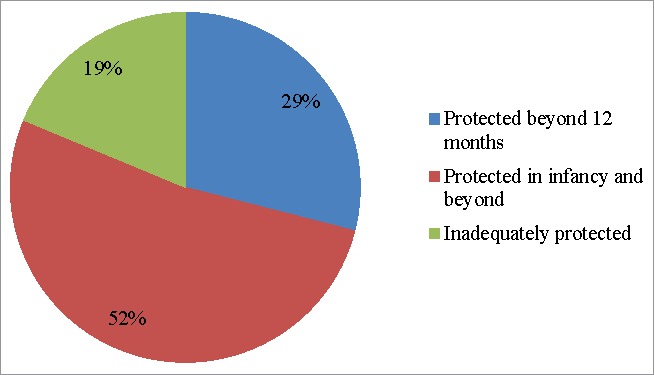
Degree and age of protection provided by Hib vaccination in China among children receiving at least 1 dose of Hib vaccine.
Discussion
We have shown that use of Hib vaccine as a private-sector vaccine in China has resulted in low and stagnate coverage, with only 45% of children receiving one or more doses of Hib vaccine in a non-increasing trend of coverage from between 2008 and 2012. Coverage varied significantly by province and showed a positive linear relation between per-capita gross domestic product and coverage – wealthier areas had a higher percent of children vaccinated against invasive Hib disease. We further showed that common patterns of Hib vaccine use in China were incompletely protective. Only half of the children who received any Hib vaccine were protected during infancy – the age at which the most serious invasive Hib disease occurs. One fifth of Hib-vaccinees received patterns of Hib vaccine that could not be considered protective – for example, receipt of a single dose of Hib vaccine during infancy with no other doses being provided.
Study in relation to other literature
Our study of utilization patterns was not nationally-representative, but our coverage findings are consistent with other studies. The coverage rate for at least one dose of Hib vaccine that we found in our study (44.79%) is similar to a 2009 survey-based national study by Zheng and colleagues in 2009 (45.31%).3 The coverage of Hib vaccine in 2 districts in Chongqing city we found (71%) is consistent with a study by Tang and colleagues (69.7%).4 Our coverage finding in Guangdong (77.95%) is consistent with a 2013 study in Futian District in Shenzhen (77.11%),5 and is slightly higher than a 2008 result in Panyu District in Guangdong (72.4%),6 suggesting a possible increase in Hib vaccine coverage in Guangdong Province.
We found that although there was no temporal trend in the percent of children receiving 1 or more doses of Hib vaccine, the pattern of use did change over time. In 2008, most children receiving Hib vaccine received 1 dose, but in 2012, most children receiving Hib vaccine received 3 or 4 doses. The number of doses received per Hib-vaccine-recipient varied by province, with a 1-dose pattern most prominent in Gansu, Hebei, and Heilongjiang, and a 3- or 4-dose pattern most prominent in Chongqing, Guangdong, Shanghai, and Jiangxi.
Hib vaccine coverage varied widely by province, and coverage was highest in Guangdong, Chongqing, and Shanghai and lowest in Hebei, Heilongjiang and Gansu. There was a linear correlation between the coverage and per capita GDP, but coverage was not higher in Shanghai than in Chongqing, indicating that Hib vaccination rate is not only related with the per capita GDP, but also with other unmeasured factors. Further research is warranted to identify such factors.
Actual versus recommended schedules
Although WHO supports any of 3 Hib schedules: 2P+1, 3P, or 3P+1, a systematic review found that 2 and 3 primary doses induce similar short-term protection, while the 2P+1 schedule can induce longer-term protection than 3 primary doses without a booster dose.7-10The immune response induced by a 3P+1 schedule is similar to that of 2P+1 schedule.11 The immune response of 3P+1 schedule is more robust than that of 3 primary doses.12 To produce a better, longer-lasting immune response, the 2P+1 or 3P+1 schedules are preferable. In our study, only 30% subjects received 2P+1 or 3P+1 schedules, which suggest that optimal effectiveness and efficacy is not being achieved for 70% of the Hib vaccine recipients.
At present, there is no consensus whether there is difference in immune response between monovalent Hib vaccine and DTaP-Hib combined vaccine.13 The advantages of a combined vaccine include fewer injections, fewer clinic visits, and higher vaccination compliance.14 Since Hib vaccine is well-suited for combination vaccines, we believe that Hib-containing combination vaccines should be promoted. Co-administration of vaccines can also save time and improve compliance. We found use of combination vaccines (4.11%) and co-administration with other vaccines (36.3%) were both low, demonstrating a need for improvement.
Program implications
In China, all vaccines are classified into two categories: Category I vaccines (EPI vaccines) and Category II (non-EPI, private-sector vaccines). Category I vaccines are provided by the government at no charge, while Category II vaccines must be paid for by parents or guardians. At present, there are 14 Category I vaccines in China's National Immunization Program (11 for universal use among children and 3 for emergency use). Hib vaccine was licensed and first used in 1997 in China; in 2004, a domestic-manufactured Hib vaccine was licensed. Although Hib vaccine is still a Category II vaccine, a very large amount of Hib vaccine is used each year in China – 30–40 million doses annually from 2012 through 2014.15 This amount is equal to half of the number of Hib vaccine doses (82.7million) reported by 161 countries in 2014.16 China's use of Hib vaccine is thus important globally.
There are significant gaps between the WHO recommendations for Hib vaccine and actual patterns of use of Hib vaccine in China. Coverage is low, and even among children vaccinated, half are not protected during infancy and one fifth receive inadequately-protective schedules. Although China is correctly listed by WHO as not having Hib vaccine in the routine program, paradoxically, China uses more Hib vaccine than almost any other country, but its use not part of the national schedule for all infants. If Hib vaccine is to be used, we believe that it should be used properly and for greatest impact.
Limitations
Our study has strengths and limitations that should be considered during its interpretation. Strengths include random sampling of children from lists and use of only official vaccination records for vaccination status ascertainment. Limitations include non-random sampling of provinces and counties, making our study unable to be considered as nationally representative. However, where coverage studies were conducted in the same areas, we found that our results were similar, giving us confidence in the ascertainment of Hib vaccine use. We selected provinces to be exemplars of eastern, middle, and western China – a stratification by socioeconomic development of China.
Scientific contribution
Although there are studies estimating Hib vaccine coverage in China as a Category II vaccine, our study contributes to the scientific literature with an analysis of utilization patterns of Hib vaccine in China showing which vaccinated children receive adequate protection from invasive Hib disease by age group. We also compare China's Hib vaccine utilization patterns with WHO-recommended technical recommendations. We believe that the analyses of utilization patterns will provide evidence for developing a national Hib vaccination strategy for infants and children in China.
Recommendations
We have 2 recommendations based on our study. First, national technical guidelines should be developed that recommend an effective Hib vaccination schedule for use in China. Second, China should consider including Hib vaccine into the EPI system, which would provide the vaccine at no cost to parents or guardians. Because overall coverage is currently low, stagnant, and varies markedly by socioeconomic development, and because China's EPI system consistently attains high coverage with program (Category I) vaccines among all sociodemographic groups, inclusion of Hib vaccine into the program would almost certainly lead to higher and equitable coverage among China's annual birth cohort of over 16 million.
Funding Statement
This study was performed with financial support from UNICEF China.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed. The authors have no financial relationships relevant to this article to disclose.
Acknowledgments
We gratefully appreciate the efforts of the health workers from Gansu CDC, Guangdong CDC, Hebei CDC, Heilongjiang CDC, Jiangsu CDC, Jiangxi CDC, Shanghai CDC, Chongqing CDC and their associated affiliated county CDC, community health service centers or township hospitals who have participated in the field investigation. We thank Dr. Lance Rodewald for his critical review and suggestions for the manuscript.
References
- 1.World Health Organization Haemophilus influenzae type b (Hib) Vaccination Position Paper– July 2013. Wkly Epidemiol Rec. 2013;88(39):413–426. [PubMed] [Google Scholar]
- 2. http://www.who.int/immunization/monitoring_surveillance/data/en/
- 3.Zheng JS, Cao L, Guo SC, et al.. Investigation and analysis of the current situation of the type 2 vaccines in children aged 1–2 years in China [J]. Chinese Journal of Vaccine and Immunization. 2012;18(3):233–237. [Google Scholar]
- 4.Tang L. Analysis of the coverage rate of type 2 vaccines in children aged between 0 and 2 [J]. 43–45. Chinese Clinical Dortor. 2013;41(5):43–45. [Google Scholar]
- 5.Fang Q, Wang YG, Cai L, A KZ, Wang L, Yu WZ, et al.. Investigation and analysis of the status of type 1 and type 2 vaccines among local and migrant children in Futian District, Shenzhen. 2013, Practical Preventive Medicine. 2015,22(3):322–323. [Google Scholar]
- 6.Xu XY, Li GL, Li WQ, Chen GY, et al.. Investigation and analysis of the situation of Haemophilus influenzae type b (Hib) vaccination and influencing factors in Panyu District [J]. Journal of Tropical Medicine. 2008,8(12):1282–1284. [Google Scholar]
- 7.Watt JP, Chen S, Santosham M. Haemophilus influenzae type b conjugate vaccine:review of observational data on long term vaccine impact to inform recommendations for vaccine schedules, Geneva, World Health Organization, 2012. Available From http://www.who.int/immunization/sage/meetings/2012/november/5_Review_observational_data_long_term_vaccineimpactrecommendations_vaccine_schedules_Watt_J_et_al_2012.pdf; accessed September2013.
- 8.Jackson C, Mann A, Mangtani P, Fine P. Effectiveness of Haemophilus influenzae Type b Vaccines Administered According to Various Schedules: Systematic Review and Meta-Analysis of Observational Data, Pediatric Infectious Disease Journal, post-acceptance, July 2013, online PDF version only: http://journals.lww.com/pidj/Abstract/publishahead/Effectiveness_of_Haemophilus_influenza_Type_b.98281.aspx [DOI] [PubMed]
- 9.Low N, Redmond SM, Rutjes AW, Martínez-González NA, Egger M, di Nisio M, Scott P. Comparing Haemophilus influenzae type b Conjugate Vaccine Schedules: A Systematic Review and Meta-Analysis of Vaccine Trials, Pediatric InfectiousDisease Journal: post-acceptance, June 2013, online PDF version only:http://journals.lww.com/pidj/Abstract/publishahead/Comparing_Haemophilus_influenzae_type_b_Conjugate.98295.aspx [DOI] [PubMed]
- 10.Grading of scientific evidence –Table 2: Hib vaccination schedules: 3 primary doses versus 2 primary doses plus one booster dose. Available at http://www.who.int/immunization/position_papers/Hib_schedule_3p0_vs_2p1.pdf
- 11.Grading of scientific evidence –Table 3: Hib vaccination schedules: 3 primary doses plus one booster dose versus 2 primary doses plus one booster dose. Available at http://www.who.int/immunization/position_papers/Hib_schedule_3p1_vs_2p1.pdf
- 12.Grading of scientific evidence –Table 4: Hib vaccination schedules: 3 primary doses plus one booster versus 3 primary doses only. Available at http://www.who.int/immunization/position_papers/Hib_schedule_3p1_vs_3p0.pdf
- 13.Bar-On ES, Goldberg E, Hellmann S, Leibovici L. Combined DTP-HBV-HIB vaccine versus separately administered DTP-HBV and HIB vaccines for primary prevention of diphtheria, tetanus, pertussis,hepatitis B and Haemophilus influenzae b (Hib) (Review). Cochrane Database systematic reviews. 2012;18(4):CD005530. doi: 10.1002/14651858.cd005530.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurosky Samantha K., Davis Keith L. & Krishnarajah Girishanthy. Effect of combination vaccines on completion and compliance of childhood vaccinations in the United States. Human Vaccines & Immunotherapeutics, 2017;13(11):2494–2502. doi: 10.1080/21645515.2017.1362515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institution for Food and Drug Control http://www.nicpbp.org.cn/ClL0621CL0428CL0737. Download in 2015.
- 16.World Health Organization http://www.who.int/immunization/monitoring_surveillance/data/en/. Download in 2017.


