ABSTRACT
Basophils are circulating cells that are associated quite exclusively with allergy response and hypersensitivity reactions but their role in the immune network might be much more intriguing and complex than previously expected. The feasibility of testing their biology in vitro for allergy research and diagnosis, due fundamentally to their quite easy availability in the peripheral blood, made them the major source for assessing allergy in the laboratory assay, when yet many further cells such as mast cells and eosinophils are much more involved as effector cells in allergy than circulating basophils. Interestingly, basophil numbers change rarely in peripheral blood during an atopic response, while we might yet observe an increase in eosinophils and modification in the biology of mast cells in the tissue during an hypersensitivity response. Furthermore, the fact that basophils are very scanty in numbers suggests that they should mainly serve as regulatory cells in immunity, rather than effector leukocytes, as still believed by the majority of physicians. In this review we will try to describe and elucidate the possible role of these cells, known as “innate IL4-producing cells” in the immune regulation of allergy and their function in allergen immunotherapy.
KEYWORDS: basophils, immunotherapy, T cell, immunomodulation, T reg, allergy, immune modulators, vaccinology
Introduction
Overview on the origin and main function of basophils
Basophils are polymorhonucleate leukocytes considered, with eosinophils, the major circulating immune population of effector granulocytes in allergy.1-3 Their haematopoietic origin is still controversial. As recently reported in mouse models, both basophils and mast cells, which share functional similarities such as the expression of the high affinity IgE receptor (FcεRI) on their membrane and the involvement in allergy and hypersensitivity reactions,4,5 derive from a common haematopoietic precursor, while their separated differentiation occurs via the differential expression of specific transcription factors.6-8 According to recent investigations in murine models, STAT5, P1-RUNX, GATA1, GATA2 and C/EBPα are involved in basophil differentiation while STAT5, GATA1, GATA2, FOG-1 and MITF are fundamental for the mast cell commitment.7 According to Arinobu et al., the different interplay in both the dosage and time of expression of the transcription factors C/EBPα and GATA-2 regulates the fates of mast cells, basophils and eosinophils from their myeloid precursors. The up-regulation of GATA-2 rules the differentiation towards eosinophils in the GMP, while the concurrent down-regulation of C/EBPα and the up-regulation of GATA-2 lead to the basophil/mast cell precursor (BMCP) from GMPs.8 C/EBPα reactivation is fundamental to the basophil commitment, while the continuous suppression of C/EBPα leads to the mast cell differentiation.8 Other studies revealed that the balance between GATA-2 and STAT5 is fundamental for basophil and mast cell differentiation and that GATA2 should act downstream of the interferon regulatory factor-8 (IRF8), which modulates several myeloid lineages, at least in mice.9,10 The mouse model offers the possibility to investigate also the origin of basophils in humans, where the mechanism should appear much more complex and most probably the STAT5/GATA2 axis induces the up-regulation of C/EBPα for mast cell and MITF for basophil commitment.6,11
Actually, for a long time, basophils were functionally mismatched with mast cells, because of their shared activity in allergy, and because, likewise mast cells, they release histamine, prostaglandins and leukotrienes, are involved in innate immunity, release cytokines such as IL4 and IL13, and express FcεRI; yet, due to the complex cross talk between basophils and other immune cells, mast cells are quite far from being compared with basophils in allergy respect to other granulocytes such as eosinophils, which are more similar to basophils during an allergy response.12
Once released from bone marrow, basophils are usually present in the peripheral blood and in the spleen but can enter also the lymph nodes and tissue inflammatory sites, where activated cells release serine proteases (mMCP-8 and mMCP-11) and elicit an enhancement in the local micro-vascular permeability to allow T-cells and innate immune cells reaching the inflammatory site.13-15 Circulating basophils account for the smallest proportion of leukocytes present in the whole blood, reaching a value that is ≤ 1.0% of the whole white blood cell count (WBC).16 Their relatively easy withdrawal from venous peripheral whole blood by venipuncture allows the setting of successful assay tests for allergy diagnosis and immunotherapy, usually much cheaper and much less time-consuming than a possible assay set with tissue mast cells. Technical approaches, such as flow cytometry (FC), are able to thoroughly investigate basophil biology in vitro, giving the opportunity to build up a cell test (basophil activation test or BAT) considered of major importance because of the lack of specific cell lines and knock out animals available to invesigate basophil biology.2 Basophils may be activated either by triggering the FcεRI-dependent signaling, via specific IgEs cross-linking (IgE-mediated response) or by non IgE-mediated stimuli, such as bacterial formylated peptides,17 TLR ligands,18 adenosine and extracellular nucleotides,19 several cytokines and immune mediators,3,20 xenobiotics and IgGs.21,22 Activated basophils release a great deal of immune and vasoactive mediators, including histamine,2,4 cysteinyl leukotrienes, which play a major role in allergic rhinitis,23 prostaglandins, with a different COX-1 and COX-2 expression pattern between basophils and mast cells,24 cytokines, chemokines and immune mediators, often quite different from those released by mast cells.3,25 The pattern of released mediators does not necessarily change with the onset of an allergic or an innate immune response but basophil degranulation might be quite different in chronic allergy respect to controls.26 Basophils can migrate in inflamed tissues, where they are recruited via the membrane up-regulation of chemokines receptors, such as CCR1 and CCR2, in response to inflammatory chemokines such as RANTES or MCP-1.27
From an immune point of view, basophils are circulating innate cells that not only elicit micro-vascular permeability to allow leukocytes transmigration in the inflamed tissues but also contribute in the Th2 immune response, as they are the most important CD3neg cells producing IL4.1-3 Basophils participate actively in the eradication of helminths from the infected host via a Th2-mediated response, together with other innate immune cells and dendritic cells, as basophils release IL4 and IL13 and respond to further cytokines involved in the response such as IL9, IL5, IL33 and IL18.28-30 Their involvement in innate immunity has been recently extensively reviewed. For example, murine models showed recently that, besides to IL4, basophils release also IL6, which is able to induce naïve CD4+ T cells to Th17 differentiation and the response of Th17 both in vitro and in vivo.31 Actually, the role of basophils in Th17-mediated immunity has been investigated in lung and bowel inflammatory diseases, where recent reports highlighted the involvement of basophils in the increase of Th17/Th1 cytokine expression in memory CD4+ T cells.32
Basophil paucity in circulating blood deserves a particular attention, because it may suggest for a major regulatory role in innate and acquired immunity rather than the traditional effector function in hypersensitivity and atopic response.33 In this perspective, new insightful data are available in the current literature focusing onto the immune regulatory role of basophils.
Background on the immune regulatory role of basophils
The very recently reported papers on basophils would suggest that these cells have mainly an immune regulatory function, both in innate and acquired immunity, rather than solely a forthright involvement in the simplest histamine releasing activity.2,34-37
For example, recent evidence suggest that basophil activation and development can be promoted by thymic stromal lymphopoietin (TSLP) and that probably two different functional lineages of basophils may exist, i.e. TSLP-elicited and IL3-elicited basophils.38,39 The presumptive existence of two functional kinds of basophils, associated with their relative paucity in the peripheral blood, should lead to the consideration that basophils are much more addressed as immune regulatory cells than effector leukocytes. For certain aspects, research in allergy might be interested in elucidating if these two functional purported subpopulations of basophils could differentially affect any immunotherapeutic approach, i.e. if these cells have a differential immunological profile and/or behaviour in a specific allergen immunotherapy.40 To tentatively reply to this fundamental question, one should turn any effort to focus onto the “immunological” role of basophils, rather than their classical activity in the allergy response. Actually, many interesting suggestions are coming from the very recent literature in the field.
During allergy, basophils are circulating cells that release various mediatior in order to address acquired immunity towards a dampening response to allergy and hypersensitivity. This immune activity occurs in tissues, where recent reports showed that the mucosal barrier, the role of the alarmin IL33 from epithelia and the microbiome play a major role in the immune activity of mast cells, basophils and eosinophils.41 Depending on the immune microenvironment and the immune disorder occurred upon an etiopathological insult, basophils release different mediators and express different membrane markers. This should ensure these leukocytes to address several different modalities of biological response in the immune network. For example, basophils dysregulate the expression of membrane CRTH2 during chronic spontaneous urticaria (CSU), showing CRTH2 as a possible therapic target for CSU.42 During this pathology, basophils upregulate the expression of substance P, which in turn activates degranulation and histamine release (acting as an histamine releasing factor), upregulating basophil NKR1 on the cell membrane and exacerbating CSU and the related pro-inflammatory response.43 A major task of basophils is to orchestrate the immune response in order to dampen the causative ignition of the allergen-mediated inflammation. In doing so, they might even exacerbate the inflammatory response.
Their ability in modulating the organism's immune response has been recently reported also towards M2 macrophages, as basophils appear to regulate their differentiation.44 Basophils activated by TLR4 or TLR2 are causative, with macrophages, of the pathophysiology of type 1 autoimmune pancreatitis44 and interestingly this pathology is characterized by a high serum level and pancreas infiltration of IgG4, which are notoriously induced by basophils to inhibit hypersensitivity reactions.45,46
Besides their immunoregulatory activity, the major effector function of basophils is to activate micro-vascular permeability and promote a Th2 response, addressed to the production of IgE specific antibodies.2,3 During a so called pseudo-allergic response, basophils exert mainly an immunoregulatory activity, for example, towards drugs, xenobiotics and chemicals and particularly for certain non-IgE related immediate drug hypersensitivity reactions (IDHRs) the diagnostic tools may appear quite scanty to address a therapic intervention.47 This is probably because a non IgE-mediated response from basophils cannot be directly evaluated by the classical diagnostic approaches used for allergy and therefore it may emphasize the immune rather than the allergic role of these cells. For example, the ability of basophils to respond to bacterial derived molecules, such as formylated peptides, allows these cells to actively participate to chronic non allergic diseases, such as chronic kidney disease, where membrane markers up-regulated upon an IgE-mediated stimulus, such as CD63, are also up-regulated by fMLP.48 Membrane markers, usually activated upon an IgE-mediated stimulation, such as CD203c, CD63, CD107a, CD193 (CCR3), CD164 and so forth, can be up-regulated also by a non IgE-mediated stimulus.2,3 Furthermore, the marker CD300a is expressed on human basophils and, when rapidly up-regulated upon the allergic cross linking IgEs/FcεRI, inhibits basophil massive degranulaion following anaphylaxis.49 Several markers up-regulated upon activation of basophils, such as CD203c, deserved interest as possible prognostic biomarker in non allergic immunotherapy, such as in carboplatin desensitization.50
During allergen immunotherapy basophils themselves may be able to dampen allergy induced by the therapy, even apparently without an immune T cell-mediated surveillance. A possible mechanism is basophil anergy. In the case of peanut oral immunotherapy (OIT) a mechanism of basophil anergy was recently described.51 In this paper, the authors used an RPMI stimulation buffer containing 2 ng/mL IL-3, they gated basophils as CD123+CD203c+HLA−DR−CD41a− events in a flow cytometry (FC) approach and reported that, following peanut OIT, basophils reduced significantly the in vitro CD63% in FC, a reduction observed indifferently if challenging cells with specific or aspecific allergens, probably indicating an anergic hypo-responsivity.51 According to recent views, basophil anergy should be considered as a mechanism to counteract excessive cell activation, also towards minor antigens, and it often involves the Syk tyrosine kinase down-regulation.52 Anergy is a well known down-regulation mechanism in immune cells and it is used to modulate lymphocyte response during inflammation. The mechanism might be significantly different respect to basophil and mast cell desensitization, which can be elicited by immunotherapy with a sequential allergen challenge and hence an immune desensitization, a process that should rapidly attenuate basophil reactivity in a non-specific way by acting at a level probably upstream of the p38-MAPK signaling.53 Furthermore, the role of IL-3 in the anergy mechanism is particularly intriguing and might even suggest that “anergic” basophils belong to a defined subpopulation of activated cells.38,39 This intriguing aspect on anergy related to basophils does not seem to involve mast cells as well, which are considered a much more heterogeneous population of immune cells.25 Mast cells respond to pro-inflammatory and allergenic stimuli via their heterogeneity while basophils via their ability to adaptation to different immune microenvironments. For example the ability of mast cells to release vasoactive mediators during allergy is higher than basophils, which possess less leukotriene subtypes than mast cells, although both basophils and mast cells actively participate in the innate response.33,34
Some cytokines enable basophils to act as immune regulatory cells. The in vitro incubation of basophils with IL-3 increases the expression of the beta-subunit of the basophil membrane IgE receptor, FcεRI.54,55 The up-regulation of the beta subunit of the FcεRI competes with the subunit alpha of the same high affinity IgE-receptor, FcεRIα, so modulating the allergy response.56 We do not know if basophils by alone might be able to account for the effectiveness of an allergen immunotherapy via the induction of anergy, yet, actually, their complex relationship with the immune system may lead to a more correct and thorough interpretation of their biology.
Is there a difference between basophil anergy and allergen desensitization at the level of membrane FcεRI-IgE-mediated signaling? Recent insights have shown that basophil anergy is a mechanism deputed to dampen unwanted aspecific reactions against minor antigens.52 However we do not know if this is a mechanism generally adopted also during allergen immunotherapy at the level of the FcεRI/IgE membrane signaling system. Any possible response to this interesting conundrum may shed a light on the regulatory role exerted by basophils in the immune system.
At a molecular level, immunotherapy desensitization should possibly involve the Bruton tyrosine kinase (BTK). Recent reports showed an involvement of BTK in IgE-mediated allergy and the ability of BTK inhibitors to switch off the allergic stimulus.57,58 Ibrutinib is a selective BTK inhibitor, which was successfully used for a constitutively activated BTK in B-cell malignancies and which has been recently suggested for the inhibition of the NRLP3-mediated immune response.59 Usually, BTK activation involves the signaling mechanism mediated by Lyn and Syk, i.e. Lyn or Syk phosphorylates BTK at a critical tyrosine (Y551), which in turn activates the catalytic domain resulting in a BTK auto-phosphorylation at a second tyrosine (Y223). This mechanism has suggested some authors the use of Ibrutinib® to attenuate allergen reactivity through a molecular interference with the FcεRI/IgE-mediated signaling in basophils and mast cells.60 Allergen immuno-therapy accounts on the ability of basophils to be down-regulated by a continuously induced allergic stimulus or a constitutive self-stimulation.61,62 This perspective takes into account the fundamental role of basophils in the immune regulation of allergy, a role that somehow may neglect or put in a background the much more important task exerted by lymphocytes in immunity.63 The immune regulatory role of basophils might not be a simple curiosity. Mutual interactions between T cells and basophils have been recently reviewed and further commented,64,65 suggesting that basophils should play a fundamental role in the immune network. This should mean that the role of basophils, either in allergy or in immunotherapy, is fundamentally regulatory, a consideration that it may also suggest a kind of speculative shortcut in describing basophils as “innate T-like cells”.66
In summary, to date we have described basophils as simple allergy effector cells. The increasing bulk of evidence and reports regarding their active participation in the regulation of the immune response, should give us the serious possibility to thoroughly reappraise their role in immunity. Furthermore, as regard immunotherapy, a possible association between basophil anergy (as a regulatory mechanism of allergy) and allergen-mediated desensitization might exist and may serve to suggest new immuno-therapeutic approaches to counteract and prevent chronic allergy manifestations. This relationship might be further orchestrated by the participation of basophils with other immune cells, such as T cells. A possible approach to highlight this issue, therefore, is to deepen the role of basophils in the immune regulation. In the next section we will address the role of basophils as immune regulatory cells and their impact in allergen immunotherapy.
Basophils as regulatory immune cells in allergy
Background
As detailed before, basophils are still considered as circulating land mines able to exacerbate the hypersensitive response to an external (allergenic) stimulus by releasing histamine and leukotrienes. This reductive look, yet currently attributed to these highly complex cells, is loosing some impact on the most recent overviews about the role of these leukocytes in humans.3 In the regulatory immune network, basophils have yet a major role, much more important than previously suspected.2 For example, a mutal interplay between basophils and regulatory T cells (Tregs) in allergen immunotherapy (AIT) or specific immunotherapy (SIT) with pollen allergoids has been recently reported.67 During the first year of SIT basophil may interact with T cells by producing leukotrienes and contributing to the recruitment and/or activation of Tregs, which increased in the second year of SIT concurrently with a reduction in lipid mediators.67 As assessed before, the paucity of basophils within the peripheral circulating blood should suggest for a modulatory rather than an effective role of these cells in hypersensitivity and its relationship with innate immunity, while a more effective action in allergy should be associated with mast cells and eosinophils. If this is true, the role of basophils in SIT may be radically different than expected. Basophils are still used as fundamental models to assess allergen immunization and immunotherapy in vitro.68 The involvement of basophils in immunotherapy has been demonstrated long time ago.69 A renowned study from Kimura et al., showed modifications in the cell function of basophils (morphological changes, reduction in IgE and in histamine production), when challenged with housedust extracts and anti-IgE in patients with a severe form of bronchial asthma sensitive to D. pteronyssinus allergens.69 These changes were further investigated and partly confirmed in the following years.70-72 Insightful evidence suggests that a major change in basophil responsiveness to allergens administered to patients via reiterated challenges, i.e. during immunotherapy, is cell desensitization, caused by a progressive exposition to causative stimuli such as allergens or drugs. Sub-threshold desensitization leads to a loss of two fundamental signaling proteins, Syk and FcεRI, although only signaling systems, which are downstream of Syk activation, are mainly involved and are regulated by IL-3.73 The FcεRI/IgE desensitization mechanism was observed also in mast cells and therefore represents a physiological mechanism of regulation at the IgE receptor level.73,74 Desensitization has been considered as a fundamental mechanism underlying AIT.75-77 The immune model involves also the skewing of an allergen-specific effector T cell to FoxP3+ CD4+CD25+ Treg and inducible type 1 Treg (Tr1) cells.75 According to the most common opinion, allergen desensitization in basophils should occur after years of AIT, although evidence was reported showing that, for Hymenoptera stings or airway allergens, subjects may be desensitized at the earliest stage of the allergen challenge or SIT.78,79 Desensitization involves also a wider mechanism in the regulation of the response to an allergen, such as the involvement of allergen-specific Treg cells, causing a peripheral T cell tolerance to the allergen in AIT.80-82 In this perspective, basophils might act as a fundamental hub in the immune regulation of allergy.
Basophils in the immune regulation and in immune disorders
An orchestrated mechanism of allergy modulation is performed by circulating basophils in association with other immune cells, via the fundamental cross-talk that they have at the crossroad of innate and acquired immunity.83-85 For example, during rush immunotherapy (RIT) a marked reduction in the basophil-dependent release of IL4 and IL13 was recently observed.86 A marked reduction of histamine release and of the membrane activation marker CD203c early in the treatment was also reported, with a recovery within one week following RIT.86 Reduction in the pro-allergenic response mediated by basophils can be considered a feedback-like response of basophils to an allergen challenge, which notoriously involves the production of fundamental cytokines such as IL4 and IL13; yet, it may involve a more complex mechanism of immune regulation upon immunotherapy. During AIT with dust mite allergens, a down-regulation in IL4 genetic expression in CD4+-Th cells occurs even through the IL4 gene promoter DNA methylation.87 Dampening IL4-mediated signaling is fundamental to modulate allergic inflammation. For example, IL4 modulates regulatory T cells (Treg) activity, via the IL4Rα/STAT6 axis, so decreasing the expression of Foxp3 in Tregs and promoting allergy response.88 Basophil modulation of releasibility, either histamine, lipid mediators or cytokines, is a way by which these cells decrease the hypersensitive response, which may lead to allergy-mediated inflammation, although advocating this mechanism as the major regulating pathway in allergy fails in elucidating how basophils directly respond to AIT. The ongoing allergen challenge, either natural or provoked (as in a SIT), should recruit basophils as major cells able to modulate allergy or alternatively dampen the same by innate immunity.
A fundamental role might be exerted by IL4, which in the case of basophils should activate and enhance a Th2-response only during allergy, while in AIT, where allergy desensitization mechanisms prevail, IL4 from basophils induces a Treg response, while at the same time basophils may contribute in inhibiting IL4 production from CD4+T cells. Fig. 1 summarizes this point, trying to deepen the fundamental role of basophil in the immune regulation of allergy.
Figure 1.
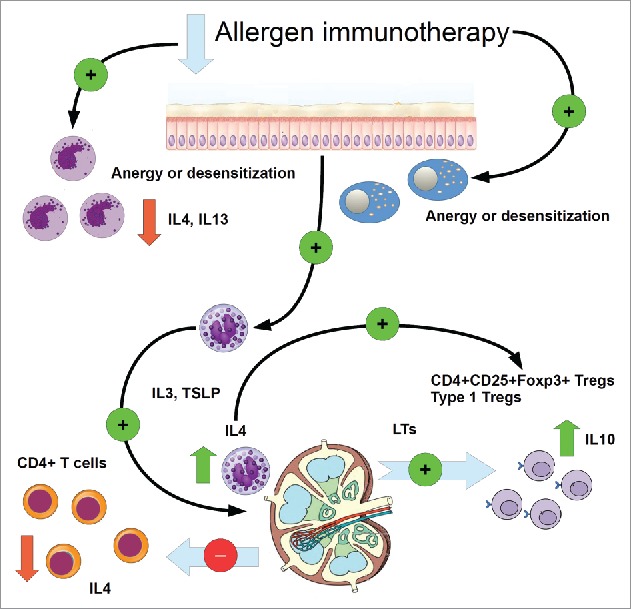
Cartoon showing two major assessed pathways of allergy dampening via allergen specific immunotherapy (AIT), in which basophils may be directly involved. As explained in the text, both anergy and desensitization could be a possible effect of AIT, involving both indifferently mast cells as well as basophils, probably mast cells are an early target of desensitization. Basophils reduce their release of IL4 and IL13. Alternatively, basophils may activate in lymphoid tissues via a basophil IL4-mediated signaling, the differentiation and development of Tregs and IL10-producing Tregs from naïve CD4+ T cells, thus reducing the expression of IL4 in these cells and the inhibition of a Th2-mediated response. Green arrow: increase. Red arrow: decrease. Green circle: activation (+), red circles inhibition (−).
Mediators in such a mechanism include a certain amount of reactive substances, obviously encompassing also histamine.89 It is well known that histamine released from mast cells inhibits Treg suppressor function (essentially by inducing a decrease in the expression of CD25 and Foxp3 in these cells via the H1-receptor-mediated signaling).90
Impairment in the activity of basophils able to induce immune disorders was rarely reported. Major data should come from mastocytosis, where anyway recent reports do not report an increased activity of peripheral basophils, despite any expectation.91 Alterations in the basophil function have been reported in some diseases where basophils play a fundamental immune role. For example in the Kimura disease, where parotid glands are involved and a more systemic disease leading to the formation of painless masses in enlarged lymph nodes occur, basophils greatly enhance the production and release of IL4 and increase their number in the bloodstream.92 One of the major physiological role of basophils is B cell maturation, which should lead to the specific IgE production.93 This evidence may shed a light on why during autoimmune disease the role of basophils is imbalanced. In many sytemic autoimmune rheumatic diseases the ratio basophils/lymphocytes is lowered, respect to other granulocytes/lymphocytes ratios, assessing that basophils are most probably recruited in the lymph nodes and inflammatory sites.94 Production of IgE does not only deal with allergy but recent findings show that autoreactive IgEs, such as dsDNA-specific IgE as occrring in systemic lupus erythematosus (SLE), are able to potentiate plasmacytoid dendritic cells (pDCs) via the FcεRI, followed by the TLR9 activation and to exacerbate SLE-related inflammation.95-97 Basophils are particularly able to cross talk with DCs, besides to partcipate in B cell antibody switching, in a complex interrelationship with mast cells98,99 and therefore their involvement in the etipathogenesis of autoimmune diseases is particularly intriguing if the allergic response is impaired or dysregulated. When dysregulation occurs in the modulation of the Foxo-1 expression in Treg cells, for example, the activated Treg function may lead to autoimmunity.100
In summary, some complex immunological pathologies involve basophils, though no allergy mechanism is directly implicated, showing that these cells are deeply related with immune modulation.
Insights on the relationship with regulatory T cells
Actually, the role of Tregs is fundamental in preventing impairment in the allergy response, even in AIT.101 AIT should induce a specific Treg response with the purpose to abolish the allergen-caused proliferation of Th1 and Th2 cells, by releasing in the immune microenvironment IL10, TGF-β and surface molecules such as CTLA-4, PD-1, mTGF-β, m-IL10, IL10R and TGF-βR.101 Furthermore, Tregs drive the immune response caused by allergy to the suppression of pro-inflammatory cells and via the IL10 and TGF-β skewing an isotype switching from IgE towards IgG4 and IgA.101,102 While mast cells inhibit Treg suppressor function via the histamine-H1 signaling, circulating basophils might have an opposite role. During SIT for grass pollen, some authors reported an increase in the plasma levels of specific IgG and IgG4 during the first year of SIT, while the significance of this increase was reached during and after the second year of SIT, when also a substantial enhancement in the number and activity of Tregs and a decrease in leukotrienes were observed.67 A speculative overview of this evidence should suggest that while an early response to allergy or allergen specific immunotherapy involving mast cells dampened the Treg involvement in the immune response, the second (late) response might involve much more significantly circulating basophils, recruited in the antigen-presenting sites to modulate the hypersensitivity reaction
It is tempting to speculate that, in this circumstance, while vasoactive mediators, such as leukotrienes and histamine, have a role in promoting an immune response from tissue mast cells, the same mediators should mainly have a regulatory activity on the immune pro-inflammatory response via the basophil.103 Within few minutes following allergen challenge, i.e. upon allergen binding on the FcεRI/IgE complex, basophils produce and release leukotriene-B4 (LTB4).104 A past report showed that the release of LTB4 from leukocytes preceded anergy in these cells.105 Furthermore, a more recent paper reported that both LTB4 and prostaglandin E2 (PGE2), the latter produced by basophils only upon a FcεRI, rather than a FcγRI signal,106 are able to independently modulate the differentiation of the immunosuppressive CD4+CD25+Foxp3+ T regulatory cells and Th17. While LTB4 dose-dependently increased RORγt expression and secretion of IL-17 in Th17 cells, PGE2 exerted an opposite effect, while both LTB4 and PGE2 inhibited Treg function and Foxp3 expression.107 This increase occurred when CD4+CD62L+ T cells (central memory T cells) were activated under Th17-promoting conditions in the presence of TGF-β1 and IL-6.107 Interestingly, CD4+CD62L+ T cells can be converted to Foxp3+ T cells by TGF-β.108
All these findings show that the involvement of basophils in the complex milieu of lymphocytes and other leukocytes is particularly dense. The immune “role” is exerted also by basophil released leukotrienes. For example, the LTB4-promoting differentiation of IL17-producing T cells, although apparently observed only in mice, might elucidate the involvement in allergy of the “natural” regulatory T cells respect to the TGF-β “induced” Tregs. The expression of Foxp3 in CD4+CD25+ regulatory T cells confers a set of different properties and markers to these leukocytes, in order to set them as an immuno-regulatory phenotype able to dampen allergy-related inflammation, produce IL10 and TGF-β and induce anergy. These “natural” regulatory T cells, which should account for the maintenance of immune tolerance and anti-inflammatory response, are neither terminally fixed, nor dependent from different functional immune conditions, yet they may change phenotype markers, cytokine profile and function.109 In the absence of a CD8+ signal, both “natural” Tregs (nTregs) and those regulatory T cells induced by other stimuli, such as TGF-β, (“induced” Tregs, or iTregs),110-112 though sharing the Foxp3+ suppressor phenotype, will develop in IL13 and IL17 producing T cells, respectively.109 Initially, the induction of iTregs requires TGF-β and consequently the presence of IL-6, which should induce the development of a Th17 phenotype.113
Under Th17-polarizing conditions, CD4+CD25+Foxp3+ regulatory T cells do not express an immunosuppressive or pro-tolerant phenotype but exhibit a pro-inflammatory, pro-allergic behaviour, for example by promoting and enhancing the expression of IL17A, IL17F and IL22 from CD4+CD44loCD62LhiCD25− naive T cells, which can be differentiated into Th17 cells also by IL6,114,115 which is also produced by basophils.18 The Treg/Th17 balance is a fundamental hallmark of regulation of allergy and it is not surprising that circulating basophils, able to access the lymphatic trafficking, actively participate in modulating this mechanism.116,117 In allergic asthmatic patients the ratio Treg/Th17 is decreased while the expression of Notch1 mRNA increased,118 an evidence that should assess the idea that, at the crossroad of the anti-allergic/pro-inflammatory regulation, a key role is exerted by the Treg/Th17 balance.
Actually, the overall picture is much more complex than expected.
A much more differentiated population of regulatory T cells involved in allergy has been described in recent years. Besides to CD4+CD25+Foxp3+ natural T regs (nTregs), which include also the CD4+CD25+Foxp3+ ICO5+ inducible co-stimulator Tregs, further functional sub-populations were described as involved in allergy, such as the CD4+CD25−Foxp3+inducible/adataive Tregs, the CD4+CD25+Foxp3− IL10 type 1 producing Tregs (Tr1), the CD4+Foxp3+CCR6+RORγt+ IL17 producing Tregs and the CD8+CD25+CD28+Foxp3+ CD8+ Tregs.119 What is interesting for our purposes is that Tregs actively participate in allergen specific immunotherapy and different subpopulations appear to be induced by different routes of immunotherapy, either if epicutaneous (EPIT), oral (OIT) or sublingual (SLIT).120 EPIT induced the greatest increase of Foxp3+ Tregs, while as OIT caused the increase of LAP+ Tregs and only SLIT the induction of IL10+ Tregs (i.e. Foxp3-negative Tregs, which should express GARP/LAP and, when activated, Helios instead of Foxp3).121 In EPIT the suppressive activity depends on the expression of CTLA-4, for OIT on IL10 and CTLA4 and for SLIT only on IL10.120 Although this evidence was reported in laboratory animals, it presumably should fit to AIT in humans. As a matter of fact, recent reports suggest a major role for extra-thymically induced regulatory T cells as fundamental target of allergen immunotherapy.122
Possible specific role for basophils in the immune regulation
In this complex functional network, which is the main regulatory role of basophils?
Recent papers outlined the evidence that basophils are engaged in a complex cross talk with a great deal of immune cells, B-cells, T-cells, dendritic cells, monocytes and so on.123 Besides to pro-Th2 cytokines, such as IL4, IL13, IL25 and TSLP, basophils participate in the modulation of the innate and acquired immunity by producing IL6.123 A fundamental evidence is that basophil-derived IL6 is a major cytokine for Th17 cell differentiation and the immune memory response.3,31 At least in an experimental model of murine colitis, basophil-derived IL6, together with IL4, is able to modulate a T-cell driven autoimmune reaction by down-regulating in these cells the expression of IL2, IFN-γ and TNF-α.124 The strategic role played by basophil, through IL6, in the differentiation of Th17 cells, must be of the utmost interest in the aforementioned Treg/Th17 balance and its role in the immune managing of the allergic response.124
Upon allergen cross-linking of the IgE/FcεRI complex, basophils release histamine, which is notoriously a mediator of inflammation.125 Th17 cells, particularly if polarized by IL1β and IL23, express the histamine H4 receptor, evidence that should assess the relationship between histamine producing basophils and the Th17 function.126 Recent evidence reported that basophils are able to express MHC class II and co-stimulatory molecules, migrate into draining lymph nodes, present antigen to naive CD4+ T cells and promote Th2 cell differentiation.127 Previous reports showed that this recruitment in draining lymph nodes is most probably led by IL3.128 In this micro-environment, basophils should exert their immuno-regulatory function. During a hypersensitivity reaction, a TLR4/MyD88-dependent recruitment of basophils into lymph nodes, (locating close to the CD4+ naïve T cells), concurrently with the expression of the surface FcεRI on dendritic cells, has been observed.129,130 Although IL-3 was reported as a key cytokine able to enhance IL4 production in basophils and to recruit them in lymphoid tissues, basophils are also induced by thymic stromal lymphopoietin (TSLP) to enter lymph nodes.8,131 TSLP, which is expressed and released also by mast cells and basophils, orchestrates the Th17-driven immune response through the activation of dendritic cells (DCs).123,132-135 The TSLP-mediated triggering of basophils should generate a different functional lineage of these cells respect to the IL3-elicited ones, which are able to respond to cytokines IL1, IL33 and IL18 and produce much higher levels of IL6, besides to IL4.8,136 Interestingly, biochemical and hormonal regulators of the blood pressure, which exert a fundamental role also in immunity, can elicit TSLP and contribute substantially to the TSLP-driven Th2 or Th17-mediated responses.135,137 This evidence allows to assoiate the role of tissue mast cells with this basophil-orchestrated signaling network, which are quite far from the mechanisms occurring in lymphoid tissues and in the bloodstream and having a primary role in the early contact with the allergen. Besides to mast cells, also keratinocytes produce large amounts of this cytokine, as reported in atopic dermatitis-affected subjects.138,139
In this context, the role of TSLP in basophil activation, however, has been discussed as a fundamental mechanism of skin allergy and protease-induced asthma or, more generally, in the context of an IgE-independent allergy.3 These basophils, able to respond to IL33 and IL18, may migrate from bloodstream to the skin, but might not have any significant role in specific immunotherapy, except for EPIT.140
Regulation of basphils. Insights on the basophils desensitization/anergy mechanism and relationship with immunity during AIT
Besides to the central role exerted by basophils in the immune regulation of allergy, these cells should play a major role in the control of hypersensitivity reactions, i.e. if they lead to an inflammatory response or should be rapidly dampened. Allergy desensitization must therefore initially occur at the level of basophils and mast cells. In this perspective, the role of circulating IgGs appears also fundamental. At the mast cell levels, for example, IgG-mediated allergy desensitization may occur either through the allergen neutralization or also via the FcγRIIB recruitment of the SHIP-1 phosphatase, which inhibits the FcεRI-mediated signaling. Furthermore, at least in mice, in experimental models with Fel d1 AIT, the receptor FcγRIIB enhances FcεRI internalization and inhibits mast cell activation via the blocking of calcium flux and degranulation.141-142 Interestingly, specific immunotherapy involves the production of specific IgGs,143,144 besides to the production of blocking IgG4.145,146 In EPIT, the production of IgG antibodies mediates the inhibition of basophil function.147 Cat hair allergen specific IgGs desensitize the basophil-mediated allergy response through the involvement of both FcγRIIA and FcγRIIB,147 a mechanism reported also in humans.148-150 However, the mechanism of IgG/FcγR-mediated desensitization of allergy, occurring in immunotherapy, is quite different from other allergy modulating pathways, e.g. those ones involving Treg-mediated immune tolerance or basophil anergy, which, as previously indicated, should act as a barrier from negligible allergens.52 As already indicated, some experimental evidence in SIT, suggested an anergy mechanism for basophils to explain allergy desensitization.51
It is fundamental, therefore, to deepen the bio-molecular mechanism and signaling underlying any event of basophil desensitization occurring following AIT. In the case of basophil anergy, which is supposed to be a physiological mechanism to reduce the impact of a hypersensitive reaction to minor allergens,52 recent reports indicate a loss in the spleen tyrosine kinase (Syk) function, though controversial opinions and evidence were also reported.151 This tyrosine kinase activates phospholipase C (PLC), playing a fundamental role in the FcεRI-mediated signaling. Any intracellular portion of Fc receptors contains an immuno-receptor tyrosine-based activation motif (ITAM) and tyrosine kinases as Syk add a phosphate in a Tyr residue of ITAM, generating a signaling cascade within the cell.52,152-154 The down-regulation of both Syk expression and function has been suggested as the major molecular cause of basophil anergy, which has been described as a possible intermediate state between “naïve”, non responder basophils and activated ones.52 Some case of oral immunotherapy with peanut allergens showed possible basophil anergy lasting several months following OIT.51
To date, we are unable to assess if specific immunotherapy (SIT) preferentially generates anergy in basophils or a Treg-mediated response. A recent evidence showed that specific birch pollen immunotherapy caused a Bet v1-specific Tr1 cells (IL10 producing Tregs), with an enhancement in the production and release of specific IgG antibodies.155 This mechanism should explicate the generation of blocking IgGs, able to dampen the allergic response via the FcγRs. Basophils might have a pivotal role in these events.74,1056-158 The fundamental role in the regulation of allergy-mediated inflammation exerted by basophils was also associated with tissue mast cells, even described as Yin-Yang modulators of allergy.159
The role of mast cells in the finest regulation of allergy may be marginalised, however, because of the functional interaction of basophils with T and B cells in the lymphoid organs, which renders basophils able to orchestrate a complex functional network of immune cells, probably in a greater extent than tissue resident mast cells.2,160 Actually, basophils are able, through a mechanism that is CD28-dependent and apparently IL4-independent, to induce CD8+ T cells (without any Treg phenotype) in the production of IL10.161 This is a fundamental hallmark also for skin and tissue immunology. Tissue resident memory CD8+ T cells are the first line of defence against repeated insults, infections and allergen ongoing challenges.162 The production of IL10 initiates a modulating mechanism that would finally recruit the CD25+ regulatory T cells. The experiments of Kim and colleagues reported that the stimulation of ovalbumin-specific OT-I CD8+ T cells with splenic dendritic cells pulsed with ovalbumin-derived peptides, induces primarily the production of IFN-γ, yet, by adding basophils into the coculture a futher IL-10 production was induced.161
Furthermore, basophils can present antigen to CD8+ T cells and the co-stimulation with CD28, without ICOS or CD86, induced an enhancement in IL10 production.162 IL10 is an inhibitory cytokine for CD4+ T cells and antigen presenting cells (APCs) but it seems that, at least initially, IL10 stimulates CD8+ T cell function, though lately inhibiting OT-1 CD8+ T cell expansion (in a secondary stimulation), therefore most probably it acts as a priming cytokine for CD8+ T cell activity.163 The contribution of basophils in this sense might be to enhance the immune cell response to the allergen (antigen) in the tissues, where mast cells are addressing the allergenic agent. Moreover, some basophil-released chemokines, such as CCL3 and CCL4, are also able to recruit CD8+ T cells in many inflammatory sites, including tumors.164 Desensitized basophils, which reduced their release in IL4 and IL13, are most probably induced to dampen the CD4+ T cell-mediated response and activate the initial tissue CD8+ one, with or without a subsequent CD25+ Treg involvement.
As reported earlier in this review, activated basophils produce mainly IL4 and IL13. Production of IL4 from basophils can be triggered by the histamine releasing factor (HRF), induced from tissue also by monocytes through the chemokines MCP-1.2,3 The complex HRF/TCTP (translationally controlled tumor protein) is a potent segretagogue for histamine and IL4 in basophils.165 Furthermore, HRF/TCTP primes non responder basophils to release histamine and IL4 in the course of an allergic response.166 This mechanism of priming is quite different from the same induced by IL3 and it is still far to be fully elucidated.166 Priming and desensitization are two opposite mechanisms enabling cell to respond promptly to minor stimuli, usually anticipating a major functional event. It is tempting to speculate that both priming and desensitization correspond to two “bifurcation” events suited to drive cells to a defined functional decision. In this sense, they are functional modes of the complex balance involving basophils in the immune network.167,168
Desensitized basophils may contribute to mast cell down-regulation of allergy functional markers. At least in mice, during contact dermatitis a CD8+ T cell population expressing PD-1 and having an effector memory phenotype, is able to produce IL10 in a way comparable to that of effector CD4+ T cells.169 Further studies have highlighted the mechanism underlying contact hypersensitivity and shown that an initial IFN-γ producing CD8+ T cells, infiltrating the skin, characterized the first 48 hours of the hypersensitivity response, just earlier respect to the recruiting of IL4-producing CD4+ T cells.170
Basophils produce a great deal of pro-inflammatory or anti-inflammatory (Th2-type) cytokines, which modulate the immune response to allergen, also in AIT.
Interferon gamma (IFN-γ) is produced upon IgE-mediated activation in basophils.171 This cytokine is also produced by anergic T cells. Past evidence has shown that T cells reactive to the allergen Fel d1 peptide 4 or to the influenza A haemagglutinin peptide, become anergic or regulatory if the agonist peptide dosage is high and in the absence of APCs.172 These cells are induced in producing IFN-γ but not IL2.172 At least in asthmatic models, IFN-γ, produced by Th1 cells, serves as an anti-inflammatory cytokine, likewise IL12, which is produced by macrophages, dendritic cells (DCs) and B cells to regulate Th1 cells differentiation.173 Invariant natural killer T cells (iNKT cells) are particularly sensitive to DC-derived IL12 and, at least in mouse models, they trigger basophil reduction by apoptosis, a mechanism elicited by the ability of Th1-produced IFN-γ to activate iNKT.174 Therefore, it is particularly interesting to speculate that IFN-γ directly produced by basophils upon IgE-mediated stimulation or by the indirect activation of the CD8+ T cells, should finally promote basophil reduction via apoptosis, thus reducing or lowering the allergy response.
We do not know if this mechanism is occurring also in humans, anyway. If yes, reduction of allergy may derive from either anergy or desensitization, or regulatory T cells or basophil apoptosis or any of them, in concurrent events.
Further insights are needed to elucidate this issue.
In summary, the role of basophils in the immune network is highly complex and a great deal of evidence domonstrates that these cells actively participate in modulating both innate and acquited immunity, even in allergen immunotharapy, an issue that will be addressed in the next chapter.
Basophils as modulatory cells in allergen immunotherapy
The role of basophils in immunotherapy should not apparently be so different respect to a common type I hypersensitivity reaction, yet only if we exclude some fundamental issues regarding SIT. The activity of basophils in the mucosal microenvironment, as occurring in OIT and SLIT, should be radically different respect to skin, as occurring in EPIT.
In oral immunotherapy (OIT), a food allergy-preventing approach, the production of immuno-globulins IgGs dampens the IgE-mediated response through the activity of FcγRIIB.175 The low affinity immunoglobulin G receptor FcγRIIB (CD32) is a potent target for immunoglobulin therapy and is expressed also in B- and T cells, where it inhibits the downstream signaling cascade leading to the activation of these cells.176,177 The receptor should be also involved in the mechanism of IgG-mediated anaphylaxis in mouse.178 CD32 was recently indicated as a fundamental cause of inhibition of the basophil activation and degranulation as well as in mast cells biology.179-181 The cluster of FcγRs, in mast cells as well as in basophils, includes two stimulatory receptors, i.e. FcγRI (CD64) and FcγRIII (CD16) and one major inhibitory one, i.e. FcγRIIB (CD32).182-184 FcγRs are necessary for an IL4-mediated immune response, also from basophils, which produce and release IL4 in response to bacterial products such as lipopolysaccharide (LPS or endotoxin), IL18, IL33 and IL3, when a FcγR-Syk axis is activated.185
From the basophil point of view, there are few major mediators that rule the immune balancing during immunotherapy and are represented, presumably, by histamine and IL4. Furthermore, in the allergy mechanism, earliest mediators of IL3-primed basophils are leukotrienes (such as LTC4) and histamine.186 These mediators can be produced by basophils acting as regulatory cells also during the early onset of a Th1-mediated response, when these cells produce and release type I IFNs.187 This might assess the hypothesis that histamine is a basophil-released mediator secreted to regulate the immune response and promote the induction of immune tolerance.188-190 During a pro-inflammatory or allergenic stimulus, basophils can arrange a set of mechanisms that should respond to allergens, then rapidly dampen the same reaction while they are mediating a wider and much more proper immune response, through the involvement of DCs and T cells. Major mediators of such response from basophils are, therefore, most probably histamine and IL4.
In addition, during oral immunotherapy, either oral (OIT) or sublingual (SLIT), the participation of secreted mucosal IgA immuno-globulins should be also considered.191 After peanut-allergens oral immunotherapy, a dramatic increase in peanut-specific IgAs in patients’ saliva, correlating with food challenge outcomes, was recently observed.192 Interestingly, the FcαRI (CD89) is expressed in those cells co-expressing also the FcεRI, and evidence was reported about the co-aggregation of FcαRI and FcεRI in regulating basophils activation and degranulation by an intracellular modulation of signaling (via the phosphorylation of the inositol polyphosphate 5′-phosphatase SHIP1 and FcεRβ.193 At least in mouse models, the production of mucosal IgA antibodies and the presence of lymphoid tissues, such as Peyer patch, represents distinct elements in immune tolerance to food and oral antigens.194 Nevertheless, at least in gut, a fundamental Treg/IgA axis has been reported to support the immune tolerance and homeostasis with microbiota in the intestinal microenvironment.195 Therefore, the role of IgAs also in oral mucosa may be related to the maintenance of an immune tolerance, particularly in OIT or SLIT.
However, many of these experimental or even speculative suggestions about how OIT, SLIT or EPIT (or subcutaneous immunotherapy or SCIT) are really working in the immune system, still need to be further addressed. The many forwarded hypotheses, basophil anergy, basophil and/or mast cell desensitization or cell number reduction, increase in IgG4 and humoral IgA immunoglobulins, Th cell skewing from Th2 to Th1, induction of tolerant regulatory T cells, induction of Tr1 (IL10 producing T regs) and so on, cannot account by alone to explain the great complexity underlying an AIT-dependent outcome.196 Most probably, the continuous allergen challenge induced by AIT should induce a mechanism of rapid desensitization and of immune tolerance, leading to the positive outcome of the therapeutic approach.
The current interesting question is: why this does not happen usually in the natural course of allergy, for which AIT could be a good therapy?
We do not know if a possible reason would be found by investigating the type of allergen, its amount during challenge and its administration routes.197 Immune tolerance was observed in OIT but requires higher doses of allergen than SLIT or EPIT, its efficacy is higher than SLIT but with more systemic adverse effects compared with EPIT or SLIT, suggesting new improving approaches.197-201 A different set of compensatory and modulatory mechanisms are probably induced, depending on the type of AIT.120 The different immune microenvironment in oral, gut and dermal tissues may account for the different outcome of the many kind of AIT.
Possible role of tissue innate lymphoid cells
Mast cells in the mucosal tissue of oral cavity have a pro-inflammatory phenotype.202 Turning from a pro-allergenic to a pro-inflammatory phenotype might be a fundamental mechanism for tuning the activity from “effector” to “modulating”. Basophils are also major actors of allergy-mediated inflammation and allergy-induced exacerbation of the inflammatory response.203 They migrate in lymphoid tissues and orchestrate, with DCs and other immune cells, the Th2 cells differentiation and activation, promoting the production of Th2-type cytokines.203 If this mechanism of allergy response regards skin, such as in atopic dermatitis (AD), basophil infiltrate and accumulate with the group 2 innate lymphoid cells (ILC2s) in the inflamed lesions of skin, via the basophil-produced IL4, which increases ILC2s proliferation and AD pathogenesis.204
These ILC2s have a major role in the regulation of epithelial immunity, as they localize at the functional boundaries represented by the barrier organism/external environment, such as lung, gut, skin or mucosal tissues and seem to play a Th2-like role, producing also IL13 and IL5 (sometimes also IL4 and IL9) and responding to tissue-damage signals such as IL33, TSLP, IL25.205,206 In airway tissues, such as lung or nasal mucosa, ILC2s are able to migrate into tissues, usually responding to cytokines (IL25) or prostaglandins (PGD2), inducing tissue eosinophilia ad mast cell response.207 Interestingly, NK cells can inhibit the type 2-immune response of ILC2s, in a IFN-γ mediated way.208 The modulation of peripheral allergy, occurring in tissues facing at the external environment, should be orchestrated by basophils and ILC2s, rather than single mast cells.
In lung and mucosal tissues of the upper airway tracts, ILC2s might have a strategic role for the ability of basophils to modulate allergy and participate in AIT.209-211 This may represent an interesting issue for immunotherapy of airway allergens.212 AIT is able to immuno-modulate ILC2s. Innate lymphoid cells can be grouped in at least three subgroups.213 ILC2s are induced to produce type 2 cytokines, such as IL4, IL5, IL13 and IL9 by IL33, IL25 or TSLP.214,215 In humans, the Lin− CD25+ ST2+ c-Kit+ CD127+ ICOS+ subpopulation of ILC2s, non expressing ILC3, CD4, NKp46, and RORγt, has been recently described.215 The involvement of ILC2s in skin and airway allergy, such as allergic rhinitis, has been extensively studied in recent years.216 A subcutaneous immunotherapy (SCIT) with airway allergens, such as grass pollen, reduced drastically the increase in ILC2s observed during seasonal allergy and identified as CD117+ILC2s and IL-13+ILC2s, though a short four-months SLIT did not get the same efficacy.217 Actually, levels of circulating ILC2s appeared comparable in atopic and non atopic (non allergic) subjects, despite the differential regulation of these cells during allergy.212 218
Actually, basophil are able to regulate, via the IL4/IL4R-mediated signaling, many innate and type 2 immune cells, including ILC2s.219 In this perspective, it is tempting to speculate that dermal ILC2s might even have a role in EPIT via either an IL25 or a TSLP-mediated pathway, with the major contribution of basophils. The role of IL25 and IL33 in basophil activation has been deepened quite recently in a human model of asthma. Following allergen inhalation challenge in asthmatic patients, an increase in circulating basophils expressing IL17RB, intracellular IL25 and the membrane marker ST2 was observed, and it was associated with a increase in type 2 cytokine-producing activated basophils when these cells were triggered with IL25 or IL33 in vitro.220 This perspective assesses the “alarmin” role exerted by IL25 and IL33, also in basophils, not only in IL2Cs. Therefore, basophils actively participate in promoting an early pro-inflammatory and type 2 immune response in tissues triggered with allergens, particularly because IL25 and IL33 increase the migration potential of basophils towards damaged or inflamed tissues.220
Moreover, most probably basophils also orchestrate the modulation of allergy via their complex cross-talk with anti-inflammatory cells, including DCs, Tregs, Tr1, Th1 cells, IL10-producing CD8+ T cells and others. This modulatory role might not be fully accomplished by IL33-triggered IL2cs, as their IL4 production promotes food allergy by blocking Treg function.221 IL33 induce the production of IL4 also in activated basophils,220 yet IL4 from basophils induces naïve CD4+ T cells to differentiate in iTregs.222 In this context, the production of IL9 from basophils should prevent the massive release of IL4 from Th2 cells, which could block the differentiation of iTregs by inhibiting the TGF-β mediated expression of Foxp3.223
Furthermore, IL33 induces the production of IL9 in basophils, besides CD4+ T cells.224 IL9 is predominantly produced by Th17 cells; basophils therefore collaborate with Th17 cells, which are induced by basophils themselves, in the release of IL9. This cytokine is fundamental to synergize with TGF-β in the differentiation of Th17 cells from naïve CD4+ T cells and also to enhance the suppressive activity of CD4+CD25+Foxp3+ T regs, via a STAT3 and STAT5 signaling.225
Figure 2 summarizes this point.
Figure 2.
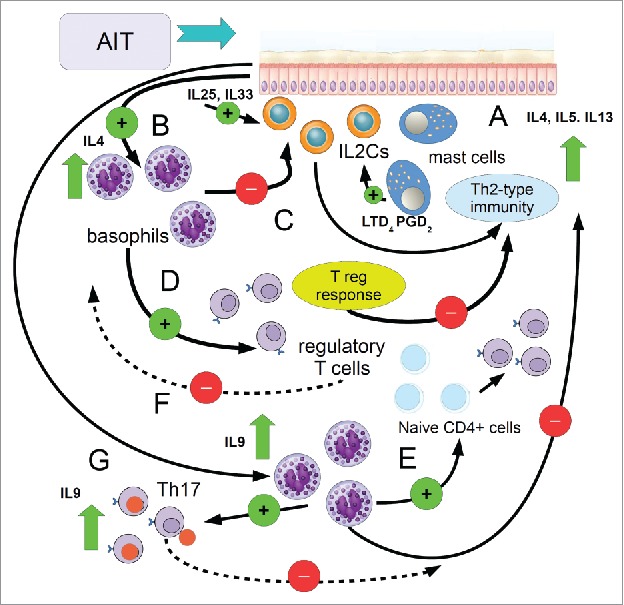
Cartoon showing the complex regulatory activity of basophils following AIT. A) Initially, allergen challenge elicits a mast cell response, which triggers the type2 innate lymphoid cells (IL2Cs), via mediators such as LTD4 and PGD2, to support a type 2 immune response, with the production of IL4, IL5, IL13. B) Tissue allergen challenge induces the production of alarmins and cytokines, such as IL25 and IL33, which induce IL2Cs to the Th2 response and activate basophils to produce IL4, which in turn inhibit the activity of IL2Cs, to reduce (C) the onset of inflammation; D) In these circumstances, IL4 from basophils induces the differentiation and activation of Tregs, which in turn dampen allergy reactions (E) through cytokines (IL10, TGF-β) and IgG4; F) here basophils produce also IL9, which helps the differentiation of Tregs from naiveCD4+ T cells; G) the promotion of the development of IL17-producing T regs (Th17) from basophils contributes both in increasing IL9 and inhibiting CD4+CD25+Foxp3+ T regs differentiation, in order to switch off the Treg-mediated signaling. Green circle: activation (+), red circles inhibition (−).
IL2cs have been recently described as fundamental cells in allergy.226,227 While IL9 is able to promote the production of TNF-α and IL6, in mast cells and basophils,226 basophils should also be able to directly inhibit IL2Cs, most probably via an IL6-mediated signaling. This suggestion arises from the evidence that dendritic cells (DCs)-mediate innate and adaptive responses, during oral immunotherapy, either SLIT or OIT, and are modulated by a TLR-dependent mechanism and IL6. During SLIT, but not OIT, the TLR-induced production of IL6 from myeloid DCs (mDCs) is inhibited and upon OIT it affects also the production of IL10 in mDCs.228 Moreover, OIT decreases IL6 production in plasmacytoid DCs (pDCs), while increases IFN-α expression,228 which consequently inhibits the induction of IL13 release from pDCs; furthermore, both SLIT and OIT decreased the TLR-mediated Th2-type cytokine production from pDCs.228
In this context, basophils should act primarily as immuno-modulatory cells, rather than effector pro-allergic leukocytes. They can either promote or dampen a pro-inflammatory response from an allergen challenge. As a matter of fact, basophils express FcδRs for IgD, which might represent an ancient form of immune surveillance.229-231 Most probably, as emerging from some recent evidence, the interaction FcδR/IgD, which activates signal transduction in T cells,232 in basophils may enhance the production and release of IL6.233 IgD signal for basophils might be specific to drive these cells to a pro-inflammatory response. When serum IgDs level is elevated, as occurring in many autoimmune diseases, these immunoglobulins are potent inducers of the proliferation of peripheral blood mononuclear cells (PMNCs) and enhancers of the production of pro-inflammatory cytokines from these leukocytes, such as IL-1α, IL-1β, TNF-α, IL-6.234 IgDs in serum induce also the proliferation of activated B cell subsets (i.e. CD19+CD23+, CD19+CD21+, CD19−CD138+ and CD19+IgD+) and also T cell subsets (CD4+CD154+ and CD4+CD69+).234
Interestingly, past evidence suggested a role for IgDs in blocking B-cell apoptosis (so interfering with immune tolerance) following the antigen-mediated cross-linking of membrane-bound IgM (sIgM).235 Whether a role for IgDs in tolerance induction and immuno-suppression should exist and if this role may affect the modulatory role of basophils in the relationship with Tregs, is still a mater of debate. IgDs elicit the production of IL10 in PBMCs.234 Immunoglobulin D should be a key regulator in the humoral immune response, particularly important in the function of the B cell receptor (BCR).236,237 In this perspective, IL6 produced by IgD-activated basophils, might help B cells in producing antibodies and CD4+ T helper cells in their activity by increasing IL21 production.238
Conclusions
The activity of basophils in the complex network of the immune system has gaining major attention in recent years, due to the many fundamental insightful results about their modulatory rather than effector actions in allergy and immunotherapy.
Recently, new advances have been proposed in targeting basophils or mast cells, via their membrane receptors or released mediators, such as serine proteinases, histamine 4-receptor, 5-lipoxygenase-activating protein, 15-lipoxygenase-1, prostaglandin D2, and proinflammatory cytokines, giving the opportunity to plan possible promising therapeutic suggestion to dampem allergy inflammtion and the role of these cells in exacerbating immune disorders.239
The many relationships of basophils with both innate ad acquired immunity suggest for the existence of a tuning role in balancing host's response to external stimuli or allergens, which basophils should exert by interacting with immune cells in lymphoid tissues and in epithelia and sensing soluble mediators, such as immuno-globulins, cytokines, vaso-active amines, leukotrienes and prostaglandins, via membrane receptors, which could be used also to gate basophils in cellular diagnostic assays. The central regulatory role in the immune system, which these leukocytes are endowed with, makes basophil a fundamental target for immunotherapy and a fascinating topic for further research in medical immunology.
Acronyms
- C/EBPα
CCAAT-enhancer binding protein alpha
- CCR1
C-C chemokine receptor 1
- COX
cyclooxygenase
- CTLA-4
Cytotoxic T-Lymphocyte Antigen 4
- FOG-1
friend of GATA 1
- GATA
(consenss seqence guanine-adenine-thymine-adenine)
- GARP
glycoprotein A repetitions predominant
- ICOS
Inducible T-cell costimulator
- IFN
interferon
- IL
interleukin
- IRF-8
interferon regulatory factor-8
- LAP
latency-associated peptide
- LTB4
leukotriene B4
- mMCP-8
mouse mast cell protease 8
- MCP-1
monocyte chemotactic protein-1
- MITF
microphtalmia-associated transcription factor
- OT
obese transgenic
- PD-1
programmed cell death 1
- PGE2
prostaglandin E2
- P1RUNX
Promoter 1 Runt-related transcription factor 1
- RANTES
regulated on activation, normal T cell expressed and secreted
- RORγr
RAR-related orphan recetor gamma t
- SHIP-1
SH-2 containing inositol 5′ polyphosphatase 1
- STAT5
signal transducer and activator of transcripton 5
- TGF
tumor growth factor
- TLR
toll-like receptor
- TSLP
thymic stromal lymphopoietin
- TNF-α
tumor necrosis factor alpha
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
A special thank to Dr. James Walmadge, Senjor English Lectuter, University of Padua, for his precious help in the manuscript revision for English grammar and syntax.
References
- 1.Yamanishi Y, Miyake K, Iki M, Tsutsui H, Karasuyama H. Recent advances in understanding basophil-mediated Th2 immune responses. Immunol Rev. 2017;278(1):237–245. [DOI] [PubMed] [Google Scholar]
- 2.Chirumbolo S. State-of-the-art review about basophil research in immunology and allergy: is the time right to treat these cells with the respect they deserve? Blood Transfus. 2012;10(2):148–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyake K, Karasuyama H. Emerging roles of basophils in allergic inflammation Allergol Int. 2017;66(3):382–391. [DOI] [PubMed] [Google Scholar]
- 4.Knol EF, Olszewski M. Basophils and mast cells: Underdog in immune regulation? Immunol Lett. 2011;138(1):28–31. [DOI] [PubMed] [Google Scholar]
- 5.Kubo M. Basophil and mast cells. Their similarity and functional difference Arerugi. 2016;65(7):926–31. [DOI] [PubMed] [Google Scholar]
- 6.Huang H, Li Y. Mechanisms controlling mast cell and basophil lineage decisions. Curr Allergy Asthma Rep. 2014;14(9):457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang H, Li Y, Liu B. Transcriptional regulation of mast cell and basophil lineage commitment. Semin Immunopathol. 2016;38(5):539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arinobu Y, Iwasaki H, Akashi K. Origin of basophils and mast cells. Allergol Int. 2009. March;58(1):21–8. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Qi X, Liu B, Huang H. The STAT5-GATA2 pathway is critical in basophil and mast cell differentiation and maintenance. J Immunol. 2015;194(9):4328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasaki H, Kurotaki D, Osato N, Sato H, Sasaki I, Koizumi S, Wang H, Kaneda C, Nishiyama A, Kaisho T, Aburatani H, Morse HC 3rd, Ozato K, Tamura T. Transcription factor IRF8 plays a critical role in the development of murine basophils and mast cells. Blood. 2015. January 8;125(2):358–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasaki H, Kurotaki D, Tamura T. Regulation of basophil and mast cell development by transcription factors. Allergol Int. 2016;65(2):127–134. [DOI] [PubMed] [Google Scholar]
- 12.Dwyer DF, Barrett NA, Austen KF. Immunological Genome Project Consortium Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat Immunol. 2016;17(7):878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyake K, Karasuyama H. Emerging roles of basophils in allergic inflammation. Allergol Int. 2017;66(3):382–391. [DOI] [PubMed] [Google Scholar]
- 14.Yamanishi Y, Miyake K, Iki M, Tsutsui H, Karasuyama H. Recent advances in understanding basophil-mediated Th2 immune responses. Immunol Rev. 2017;278(1):237–245. [DOI] [PubMed] [Google Scholar]
- 15.Yamanishi Y, Miyake K, Iki M, Tsutsui H, Karasuyama H. Recent advances in understanding basophil-mediated Th2 immune responses. Immunol Rev. 2017;278(1):237–245. [DOI] [PubMed] [Google Scholar]
- 16.Borzova E, Dahinden CA. The absolute basophil count. Methods Mol Biol. 2014;1192:87–100. [DOI] [PubMed] [Google Scholar]
- 17.MacGlashan D., Jr. Expression profiling of human basophils: modulation by cytokines and secretagogues. PLoS One. 2015;10(5):e0126435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeon JH, Ahn KB, Kim SK, Im J, Yun CH, Han SH. Bacterial flagellin induces IL-6 expression in human basophils. Mol Immunol. 2015;65(1):168–76. [DOI] [PubMed] [Google Scholar]
- 19.Nakano M, Ito K, Yuno T, Soma N, Aburakawa S, Kasai K, Nakamura T, Takami H. UDP/P2Y6 receptor signaling regulates IgE-dependent degranulation in human basophils. Allergol Int. 2017. October;66(4):574–580. [DOI] [PubMed] [Google Scholar]
- 20.Voehringer D. Recent advances in understanding basophil functions in vivo. F1000Res. 2017;6:1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borrelli I, Loffredo S, Staiano RI, Frattini A, Marone G, Triggiani M. Benzene metabolites inhibit the release of proinflammatory mediators and cytokines from human basophils. Int J Immunopathol Pharmacol. 2010;23(3):737–44. Erratum in: Int J Immunopathol Pharmacol. 2011 Jan-Mar;24(1):2 p following 274. [DOI] [PubMed] [Google Scholar]
- 22.Malbec O, Cassard L, Albanesi M, Jönsson F, Mancardi D, Chicanne G, Payrastre B, Dubreuil P, Vivier E, Daëron M. Trans-inhibition of activation and proliferation signals by Fc receptors in mast cells and basophils. Sci Signal. 2016. December 20;9(459):ra126. [DOI] [PubMed] [Google Scholar]
- 23.Peters-Golden M, Gleason MM, Togias A. Cysteinyl leukotrienes:multi-functional mediators in allergic rhinitis. Clin Exp Allergy. 2006;36(6):689–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bando T, Fujita S, Nagano N, Yoshikawa S, Yamanishi Y, Minami M, Karasuyama H. Differential usage of COX-1 and COX-2 in prostaglandin production by mast cells and basophils. Biochem Biophys Rep. 2017. March 15;10:82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chhiba KD, Hsu CL, Berdnikovs S, Bryce PJ. Transcriptional Heterogeneity of Mast Cells and Basophils upon Activation. J Immunol. 2017;198(12):4868–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilmartin L, Tarleton CA, Schuyler M, Wilson BS, Oliver JM. A comparison of inflammatory mediators released by basophils of asthmatic and control subjects in response to high-affinity IgE receptor aggregation. Int Arch Allergy Immunol. 2008;145(3):182–92. [DOI] [PubMed] [Google Scholar]
- 27.Pan Q, Feng Y, Peng Y, Zhou H, Deng Z, Li L, Han H, Lin J, Shi L, Wang S, An N, Yang C, Liu HF. Basophil Recruitment to Skin Lesions of Patients with Systemic Lupus Erythematosus Mediated by CCR1 and CCR2. Cell Physiol Biochem. 2017;43(2):832–839. [DOI] [PubMed] [Google Scholar]
- 28.Webb LM, Tait Wojno ED. The role of rare innate immune cells in Type 2 immune activation against parasitic helminths. Parasitology. 2017;144(10):1288–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuzuki H, Arinobu Y, Miyawaki K, Takaki A, Ota SI, Ota Y, Mitoma H, Akahoshi M, Mori Y, Iwasaki H, Niiro H, Tsukamoto H, Akashi K. Functional interleukin-33 receptors are expressed in early progenitor stages of allergy-related granulocytes. Immunology. 2017 Jan;150(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders NL, Mishra A. Role of interleukin-18 in the pathophysiology of allergic diseases. Cytokine Growth Factor Rev. 2016;32:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuk CM, Park HJ, Kwon BI, Lah SJ, Chang J, Kim JY, Lee KM, Park SH, Hong S, Lee SH. Basophil-derived IL-6 regulates T(H)17 cell differentiation and CD4 T cell immunity. Sci Rep. 2017. January 30;7:41744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wakahara K, Baba N, Van VQ, Bégin P, Rubio M, Ferraro P, Panzini B, Wassef R, Lahaie R, Caussignac Y, Tamaz R, Richard C, Soucy G, Delespesse G, Sarfati M. Human basophils interact with memory T cells to augment Th17 responses. Blood. 2012;120(24):4761–71. [DOI] [PubMed] [Google Scholar]
- 33.Merluzzi S, Betto E, Ceccaroni AA, Magris R, Giunta M, Mion F. Mast cells, basophils and B cell connection network. Mol Immunol. 2015. January;63(1):94–103. [DOI] [PubMed] [Google Scholar]
- 34.Marone G, Varricchi G, Loffredo S, Galdiero MR, Rivellese F, de Paulis A. Are Basophils and Mast Cells Masters in HIV Infection? Int Arch Allergy Immunol. 2016;171(3–4):158–165. [DOI] [PubMed] [Google Scholar]
- 35.Jiang AP, Jiang JF, Guo MG, Jin YM, Li YY, Wang JH. Human Blood-Circulating Basophils Capture HIV-1 and Mediate Viral trans-Infection of CD4+ T Cells. J Virol. 2015;89(15):8050–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steiner M, Huber S, Harrer A, Himly M. The Evolution of Human Basophil Biology from Neglect towards Understanding of Their Immune Functions. Biomed Res Int. 2016;2016:8232830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voehringer D. Regulation of type 2 immunity by basophils. Adv Exp Med Biol. 2013;785:37–41. [DOI] [PubMed] [Google Scholar]
- 38.Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, Wherry EJ, Jessup HK, Siegel LA, Kambayashi T, Dudek EC, Kubo M, Cianferoni A, Spergel JM, Ziegler SF, Comeau MR, Artis D. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477(7363):229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siracusa MC, Wojno ED, Artis D. Functional heterogeneity in the basophil cell lineage. Adv Immunol. 2012;115:141–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Till SJ, Francis JN, Nouri-Aria K, Durham SR. Mechanisms of immunotherapy. J Allergy Clin Immunol. 2004;113(6):1025–34. [DOI] [PubMed] [Google Scholar]
- 41.Rothenberg ME, Saito H, Peebles RS Jr.. Advances in mechanisms of allergic disease in 2016. J Allergy Clin Immunol. 2017;140(6):1622–1631. pii: S0091-6749(17)31576-2. doi: 10.1016/j.jaci.2017.08.029. PMID:29038009. [DOI] [PubMed] [Google Scholar]
- 42.Oliver ET, Sterba PM, Devine K, Vonakis BM, Saini SS. Altered expression of chemoattractant receptor-homologous molecule expressed on T(H)2 cells on blood basophils and eosinophils in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. 2016. January;137(1):304–306. [DOI] [PubMed] [Google Scholar]
- 43.Zheng W, Wang J, Zhu W, Xu C, He S. Upregulated expression of substance P in basophils of the patients with chronic spontaneous urticaria: induction of histamine release and basophil accumulation by substance P. Cell Biol Toxicol. 2016;32(3):217–28. [DOI] [PubMed] [Google Scholar]
- 44.Yanagawa M, Uchida K, Ando Y, Tomiyama T, Yamaguchi T, Ikeura T, Fukui T, Nishio A, Uemura Y, Miyara T, et al.. Basophils activated via TLR signaling may contribute to pathophysiology of type 1 autoimmune pancreatitis. J Gastroenterol. 2017. doi: 10.1007/s00535-017-1390-6. PMID:28921377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukui Y, Uchida K, Sumimoto K, Kusuda T, Miyoshi H, Koyabu M, Ikeura T, Sakaguchi Y, Shimatani M, Fukui T, Matsushita M, Takaoka M, Nishio A, Shikata N, Sakaida N, Uemura Y, Satoi S, Kwon AH, Okazaki K. The similarity of Type 1 autoimmune pancreatitis to pancreatic ductal adenocarcinoma with significant IgG4-positive plasma cell infiltration. J Gastroenterol. 2013;48(6):751–61. [DOI] [PubMed] [Google Scholar]
- 46.James LK, Till SJ. Potential Mechanisms for IgG4 Inhibition of Immediate Hypersensitivity Reactions. Curr Allergy Asthma Rep. 2016. March;16(3):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Decuyper II, Mangodt EA, Van Gasse AL, Claesen K, Uyttebroek A, Faber M, Sabato V, Bridts CH, Mertens C, Hagendorens MM, De Clerck LS, Ebo DG. In Vitro Diagnosis of Immediate Drug Hypersensitivity Anno 2017: Potentials and Limitations. Drugs R D. 2017;17(2):265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aljadi Z, Nopp A, Winqvist O, Russom A, Hylander B, Jacobson SH, Lundahl J. Altered basophil function in patients with chronic kidney disease onhemodialysis. Clin Nephrol. 2017;88(8):86–96. [DOI] [PubMed] [Google Scholar]
- 49.Sabato V, Verweij MM, Bridts CH, Levi-Schaffer F, Gibbs BF, De Clerck LS, Schiavino D, Ebo DG. CD300a is expressed on human basophils and seems to inhibit IgE/FcεRI-dependent anaphylactic degranulation. Cytometry B Clin Cytom. 2012;82(3):132–8. [DOI] [PubMed] [Google Scholar]
- 50.Iwamoto T, Sugimoto H, Tabata T, Okuda M. Clinical Utility of Basophil CD203c as a Biomarker for Predicting the Timing of Hypersensitivity Reaction in Carboplatin Rechallenge: Three Case Reports. Clin Ther. 2016;38(6):1537–1541. [DOI] [PubMed] [Google Scholar]
- 51.Thyagarajan A, Jones SM, Calatroni A, Pons L, Kulis M, Woo CS, Kamalakannan M, Vickery BP, Scurlock AM, Wesley Burks A, Shreffler WG. Evidence of pathway-specific basophil anergy induced by peanut oral immunotherapy in peanut-allergic children. Clin Exp Allergy. 2012;42(8):1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Puan KJ, Andiappan AK, Lee B, Kumar D, Lai TS, Yeo G, Bercin D, Starke M, Haase D, Lum J, Chew FT, Connolly J, Wong SC, Zolezzi F, Poidinger M, Wang Y, Rötzschke O. Systematic characterization of basophil anergy. Allergy. 2017. March;72(3):373–384. [DOI] [PubMed] [Google Scholar]
- 53.Witting Christensen SK, Kortekaas Krohn I, Thuraiaiyah J, Skjold T, Schmid JM, Hoffmann HJ. Sequential allergen desensitization of basophils is non-specific and may involve p38 MAPK. Allergy. 2014. October;69(10):1343–9. [DOI] [PubMed] [Google Scholar]
- 54.Saini S, Richardson JJ, Wofsy C, Lavens-Phillips Bochner B, MacGlashan DW Jr.. Expression and modulation of FceRIa and FceRIb in human blood basophils. J. All. Clin. Immunol. 2001;107:832–41. [DOI] [PubMed] [Google Scholar]
- 55.Miura K, Saini SS, Gauvreau G, MacGlashan DW Jr.. Differences in functional consequences and signal transduction induced by IL-3, IL-5 and NGF in human basophils. J. Immunol. 2001;167:2282–91. [DOI] [PubMed] [Google Scholar]
- 56.Chirumbolo S. Immunotherapy in allergy and cellular tests: state of art. Hum Vaccin Immunother. 2014;10(6):1595–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dispenza MC, Regan JA, Bochner B. Potential applications of Bruton's tyrosine kinase inhibitors for the prevention of allergic reactions. Expert Rev Clin Immunol. 2017;13(10):921–923. doi: 10.1080/1744666X.2017.1370374. PMID:28829205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smiljkovic D, Blatt K, Stefanzl G, Dorofeeva Y, Skrabs C, Focke-Tejkl M, Sperr WR, Jaeger U, Valenta R, Valent P. BTK inhibition is a potent approach to block IgE-mediated histamine release in human basophils. Allergy. 2017;72(11):1666–1676. doi: 10.10.1111/all.13166. PMID:28328081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Banoth B, Cassel SL. Bruton tyrosine kinase inhibition: Clinical relevance beyond B cells. J Allergy Clin Immunol. 2017;140(4):985–987. pii: S0091-6749(17)30679-6. doi: 10.1016/j.jaci.2017.03.041. PMID:28456622. [DOI] [PubMed] [Google Scholar]
- 60.Regan JA, Cao Y, Dispenza MC, Ma S, Gordon LI, Petrich AM, Bochner BS. Ibrutinib, a Bruton's tyrosine kinase inhibitor used for treatment of lymphoproliferative disorders, eliminates both aeroallergen skin test and basophil activation test reactivity. J Allergy Clin Immunol. 2017;140(3):875–879. pii: S0091-6749(17)30512-2. PMID:28389390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Čelesnik Smodiš N, Šilar M, Eržen R, Rijavec M, Košnik M, Korošec P. Down-regulation of FcεRI-mediated CD63 basophil response during short-term VIT determined venom-nonspecific desensitization. PLoS One. 2014;9(4):e94762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Čelesnik N, Vesel T, Rijavec M, Šilar M, Eržen R, Košnik M, Kloft Žitnik SE, Avčin T, Korošec P. Short-term venom immunotherapy induces desensitization of FcεRI-mediated basophil response. Allergy. 2012;67(12):1594–600. [DOI] [PubMed] [Google Scholar]
- 63.Mortuaire G, Michel J, Papon JF, Malard O, Ebbo D, Crampette L, Jankowski R, Coste A, Serrano E. Specific immunotherapy in allergic rhinitis. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;134(4):253–258. [DOI] [PubMed] [Google Scholar]
- 64.Sarfati M, Wakahara K, Chapuy L, Delespesse G. Mutual Interaction of Basophils and T Cells in Chronic Inflammatory Diseases. Front Immunol. 2015;6:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chirumbolo S. Commentary: Mutual interaction of basophils and T cells in chronic inflammatory diseases. Front Immunol. 2016;7:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karasuyama H, Yamanishi Y. Basophils have emerged as a key player in immunity. Curr Opin Immunol. 2014. December;31:1–7. [DOI] [PubMed] [Google Scholar]
- 67.Rosewich M, Schulze J, Eickmeier O, Telles T, Rose MA, Schubert R, Zielen S. Tolerance induction after specific immunotherapy with pollen allergoids adjuvanted by monophosphoryl lipid A in children. Clin Exp Immunol. 2010;160(3):403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun N, Zhou C, Zhou X, Sun L, Che H. Use of a rat basophil leukemia (RBL) cell-based immunological assay for allergen identification, clinical diagnosis of allergy, and identification of anti-allergy agents for use in immunotherapy. J Immunotoxicol. 2015;12(2):199–205. [DOI] [PubMed] [Google Scholar]
- 69.Kimura I, Tanizaki Y, Goda Y, Komagoe H, Kitani H. Decrease in reactivity of basophils by immunotherapy with housedust extract. Clin Allergy. 1985. January;15(1):1–7. [DOI] [PubMed] [Google Scholar]
- 70.Shim JY, Kim BS, Cho SH, Min KU, Hong SJ. Allergen-specific conventional immunotherapy decreases immunoglobulin E-mediated basophil histamine releasability. Clin Exp Allergy. 2003. January;33(1):52–7. [DOI] [PubMed] [Google Scholar]
- 71.Yuta A, Nishimura S, Sakakura Y, Majima Y. [Changes of histamine release rate from basophils measured by HRT Shionogi kit in immunotherapy for cedar pollinosis]. Arerugi. 2002;51(8):634–8. [PubMed] [Google Scholar]
- 72.Nagao M, Hiraguchi Y, Hosoki K, Tokuda R, Usui T, Masuda S, Yamaguchi M, Fujisawa T. Allergen-induced basophil CD203c expression as a biomarker for rush immunotherapy in patients with Japanese cedar pollinosis. Int Arch Allergy Immunol. 2008;146 Suppl 1:47–53. [DOI] [PubMed] [Google Scholar]
- 73.MacGlashan D., Jr. Subthreshold desensitization of human basophils re-capitulates the loss of Syk and FcεRI expression characterized by other methods of desensitization. Clin Exp Allergy. 2012;42(7):1060–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abramson J, Barbu EA, Pecht I. Regulation of mast cell secretory response to the type I Fcepsilon receptor: inhibitory elements and desensitization. Novartis Found Symp. 2005;271:78–89; discussion 89–99. [DOI] [PubMed] [Google Scholar]
- 75.Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ J. 2015;8(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2011. January;127(1):18–27; quiz 28–9. [DOI] [PubMed] [Google Scholar]
- 77.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol. 2014. March;133(3):621–31. [DOI] [PubMed] [Google Scholar]
- 78.Tarzi M, Klunker S, Texier C, Verhoef A, Stapel SO, Akdis CA, Maillere B, Kay AB, Larché M. Induction of interleukin-10 and suppressor of cytokine signalling-3 gene expression following peptide immunotherapy. Clin Exp Allergy. 2006;36(4):465–74. [DOI] [PubMed] [Google Scholar]
- 79.Alexander C, Ying S, B Kay A, Larché M. Fel d 1-derived T cell peptide therapy induces recruitment of CD4+ CD25+; CD4+ interferon-gamma+ T helper type 1 cells to sites of allergen-induced late-phase skin reactions in cat-allergic subjects. Clin Exp Allergy. 2005. January;35(1):52–8. [DOI] [PubMed] [Google Scholar]
- 80.Akdis CA, Akdis M, Blesken T, Wymann D, Alkan SS, Müller U, Blaser K. Epitope-specific T cell tolerance to phospholipase A2 in bee venom immunotherapy and recovery by IL-2 and IL-15 in vitro. J Clin Invest. 1996. October 1;98(7):1676–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Akdis CA, Blesken T, Akdis M, Wüthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998;102(1):98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jutel M, Akdis M, Budak F, Aebischer-Casaulta C, Wrzyszcz M, Blaser K, Akdis CA. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003;33(5):1205–14. [DOI] [PubMed] [Google Scholar]
- 83.Gibbs BF. Human basophils as effectors and immunomodulators of allergic inflammation and innate immunity. Clin Exp Med. 2005;5(2):43–9. [DOI] [PubMed] [Google Scholar]
- 84.Min B, Le Gros G, Paul WE. Basophils: a potential liaison between innate and adaptive immunity. Allergol Int. 2006;55(2):99–104. [DOI] [PubMed] [Google Scholar]
- 85.Sokol CL, Medzhitov R. Emerging functions of basophils in protective and allergic immune responses. Mucosal Immunol. 2010. March;3(2):129–37. [DOI] [PubMed] [Google Scholar]
- 86.Plewako H, Wosińska K, Arvidsson M, Bjorkander J, Skov PS, Håkansson L, Rak S. Basophil interleukin 4 and interleukin 13 production is suppressed during the early phase of rush immunotherapy. Int Arch Allergy Immunol. 2006;141(4):346–53. [DOI] [PubMed] [Google Scholar]
- 87.Wang CM, Chang CB, Chan MW, Wen ZH, Wu SF. Dust mite allergen-specific immunotherapy increases IL4 DNA methylation and induces Der p-specific T cell tolerance in children with allergic asthma. Cell Mol Immunol. 2017. doi: 10.1038/cmi.2017.26. PMID:28603280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y, Souabni A, Flavell RA, Wan YY. An intrinsic mechanism predisposes Foxp3-expressing regulatory T cells to Th2 conversion in vivo. J Immunol. 2010;185(10):5983–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jutel M, Blaser K, Akdis CA. The role of histamine in regulation of immune responses. Chem Immunol Allergy. 2006;91:174–87. [DOI] [PubMed] [Google Scholar]
- 90.Forward NA, Furlong SJ, Yang Y, Lin TJ, Hoskin DW. Mast cells down-regulate CD4+CD25+ T regulatory cell suppressor function via histamine H1 receptor interaction. J Immunol. 2009;183(5):3014–22. [DOI] [PubMed] [Google Scholar]
- 91.Gülen T, Möller Westerberg C, Lyberg K, Ekoff M, Kolmert J, Bood J, Öhd J, James A, Dahlén SE, Nilsson G, Dahlén B. Assessment of in vivo mast cell reactivity in patients with systemic mastocytosis. Clin Exp Allergy. 2017;47(7):909–917. [DOI] [PubMed] [Google Scholar]
- 92.Nonaka M, Sakitani E, Ono E, Yamamura Y, Seo Y, Shibata N, Pawankar R, Yoshihara T. Basophils are increased and express increased levels of interleukin-4 in the parotid lesions of Kimura disease. Asia Pac Allergy. 2017. October;7(4):221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dijkstra D, Meyer-Bahlburg A. Human Basophils Modulate Plasma Cell Differentiation and Maturation. J Immunol. 2017;198(1):229–238. [DOI] [PubMed] [Google Scholar]
- 94.Yang Z, Zhang Z, Lin F, Ren Y, Liu D, Zhong R, Liang Y. Comparisons of neutrophil-, monocyte-, eosinophil-, and basophil- lymphocyte ratios among various systemic autoimmune rheumatic diseases. APMIS. 2017;125(10):863–871. [DOI] [PubMed] [Google Scholar]
- 95.Henault J, Riggs JM, Karnell JL, Liarski VM, Li J, Shirinian L, Xu L, Casey KA, Smith MA, Khatry DB, Izhak L, Clarke L, Herbst R, Ettinger R, Petri M, Clark MR, Mustelin T, Kolbeck R, Sanjuan MA. Self-reactive IgE exacerbates interferon responses associated with autoimmunity. Nat Immunol. 2016;17(2):196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sanjuan MA, Sagar D, Kolbeck R. Role of IgE in autoimmunity. J Allergy Clin Immunol. 2016;137(6):1651–1661. [DOI] [PubMed] [Google Scholar]
- 97.Ettinger R, Karnell JL, Henault J, Panda SK, Riggs JM, Kolbeck R, Sanjuan MA. Pathogenic mechanisms of IgE-mediated inflammation in self-destructive autoimmune responses. Autoimmunity. 2017;50(1):25–36. [DOI] [PubMed] [Google Scholar]
- 98.Merluzzi S, Betto E, Ceccaroni AA, Magris R, Giunta M, Mion F. Mast cells, basophils and B cell connection network. Mol Immunol. 2015. January;63(1):94–103. [DOI] [PubMed] [Google Scholar]
- 99.Breedveld A, Groot Kormelink T, van Egmond M, de Jong EC. Granulocytes as modulators of dendritic cell function. J Leukoc Biol. 2017. October;102(4):1003–1016. [DOI] [PubMed] [Google Scholar]
- 100. [Google Scholar]
- 101.Taylor A, Verhagen J, Akdis CA, Akdis M. T regulatory cells in allergy and health: a question of allergen specificity and balance. Int Arch Allergy Immunol. 2004;135(1):73–82. [DOI] [PubMed] [Google Scholar]
- 102.Akdis M, Blaser K, Akdis CA. T regulatory cells in allergy. Chem Immunol Allergy. 2006;91:159–73. [DOI] [PubMed] [Google Scholar]
- 103.Gibbs BF. Basophils as Key Regulators of Allergic Inflammation and Th2-type Immunity. World Allergy Organ J. 2008;1(7):123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Metcalfe DD, Pawankar R, Ackerman SJ, Akin C, Clayton F, Falcone FH, Gleich GJ, Irani AM, Johansson MW, Klion AD, Leiferman KM, Levi-Schaffer F, Nilsson G, Okayama Y, Prussin C, Schroeder JT, Schwartz LB, Simon HU, Walls AF, Triggiani M. Biomarkers of the involvement of mast cells, basophils and eosinophils in asthma and allergic diseases. World Allergy Organ J. 2016;9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Braquet M, Ducousso R, Garay R, Guilbaud J, Carsin H, Braquet P. Leucocyte leukotriene B4 secretion precedes anergy in burn-injured patients. Lancet. 1984. October 27;2(8409):976–7. [DOI] [PubMed] [Google Scholar]
- 106.Ugajin T, Satoh T, Kanamori T, Aritake K, Urade Y, Yokozeki H. FcεRI, but not FcγR, signals induce prostaglandin D2 and E2 production from basophils. Am J Pathol. 2011;179(2):775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen H, Qin J, Wei P, Zhang J, Li Q, Fu L, Li S, Ma C, Cong B. Effects of leukotriene B4 and prostaglandin E2 on the differentiation of murine Foxp3+ T regulatory cells and Th17 cells. Prostaglandins Leukot Essent Fatty Acids. 2009;80(4):195–200. [DOI] [PubMed] [Google Scholar]
- 108.Zhang X, Chang Li X, Xiao X, Sun R, Tian Z, Wei H. CD4(+)CD62L(+) central memory T cells can be converted to Foxp3(+) T cells. PLoS One. 2013. October 14;8(10):e77322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Joetham A, Schedel M, O'Connor BP, Kim S, Takeda K, Abbott J, Gelfand EW. Inducible and naturally occurring regulatory T cells enhance lung allergic responses through divergent transcriptional pathways. J Allergy Clin Immunol. 2017;139(4):1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003. December 15;198(12):1875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen JF, Gao J, Zhang D, Wang ZH, Zhu JY. CD4+Foxp3+ regulatory T cells converted by rapamycin from peripheral CD4+CD25(−) naive T cells display more potent regulatory ability in vitro. Chin Med J (Engl). 2010;123(7):942–8. [PubMed] [Google Scholar]
- 112.Selvaraj RK, Geiger TL. A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-beta. J Immunol. 2007;178(12):7667–77. Erratum in: J Immunol. 2007 Jul 15;179(2):1390. Corrected and republished in: J Immunol. 2007 Jul 15;179(2):11 p following 1390. [DOI] [PubMed] [Google Scholar]
- 113.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40(7):1830–5. [DOI] [PubMed] [Google Scholar]
- 114.Pandiyan P, Conti HR, Zheng L, Peterson AC, Mathern DR, Hernández-Santos N, Edgerton M, Gaffen SL, Lenardo MJ. CD4(+)CD25(+)Foxp3(+) regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 cell infection model. Immunity. 2011. March 25;34(3):422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ziegler SF, Buckner JH. FOXP3 and the regulation of Treg/Th17 differentiation Microbes Infect. 2009;11(5):594–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martín-Orozco E, Norte-Muñoz M, Martínez-García J. Regulatory T Cells in Allergy and Asthma. Front Pediatr. 2017;5:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stelmaszczyk-Emmel A. Regulatory T cells in children with allergy and asthma: it is time to act. Respir Physiol Neurobiol. 2015;209:59–63. [DOI] [PubMed] [Google Scholar]
- 118.Li C, Sheng A, Jia X, Zeng Z, Zhang X, Zhao W, Zhang W. Th17/Treg dysregulation in allergic asthmatic children is associated with elevated notch expression. J Asthma. 2017;2:1–7. PMID:28463581. [DOI] [PubMed] [Google Scholar]
- 119.Zhang H, Kong H, Zeng X, Guo L, Sun X, He S. Subsets of regulatory T cells and their roles in allergy. J Transl Med. 2014;12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dioszeghy V, Mondoulet L, Puteaux E, Dhelft V, Ligouis M, Plaquet C, Dupont C, Benhamou PH. Differences in phenotype, homing properties and suppressive activities of regulatory T cells induced by epicutaneous, oral or sublingual immunotherapy in mice sensitized to peanut. Cell Mol Immunol. 2017;14(9):770–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Elkord E, Abd Al Samid M, Chaudhary B. Helios, and not FoxP3, is the marker of activated Tregs expressing GARP/LAP. Oncotarget. 2015;6(24):20026–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Verhagen J, Wegner A, Wraith DC. Extra-thymically induced T regulatory cell subsets: the optimal target for antigen-specific immunotherapy. Immunology. 2015;145(2):171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schneider E, Thieblemont N, De Moraes ML, Dy M. Basophils: new players in the cytokine network. Eur Cytokine Netw. 2010;21(3):142–53. [DOI] [PubMed] [Google Scholar]
- 124.Gomez MR, Talke Y, Hofmann C, Ketelsen I, Hermann F, Reich B, Goebel N, Schmidbauer K, Dunger N, Brühl H, Renner K, Syed SN, Mack M. Basophils control T-cell responses and limit disease activity in experimental murine colitis. Mucosal Immunol. 2014. January;7(1):188–99. [DOI] [PubMed] [Google Scholar]
- 125.MacGlashan D., Jr. Histamine: A mediator of inflammation. J Allergy Clin Immunol. 2003. October;112(4 Suppl):S53−9. [DOI] [PubMed] [Google Scholar]
- 126.Mommert S, Gschwandtner M, Koether B, Gutzmer R, Werfel T. Human memory Th17 cells express a functional histamine H4 receptor. Am J Pathol. 2012. January;180(1):177–85. [DOI] [PubMed] [Google Scholar]
- 127.Siracusa MC, Perrigoue JG, Comeau MR, Artis D. New paradigms in basophil development, regulation and function. Immunol Cell Biol. 2010. Mar-Apr;88(3):275–84. [DOI] [PubMed] [Google Scholar]
- 128.Kim S, Prout M, Ramshaw H, Lopez AF, LeGros G, Min B. Cutting edge: basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J Immunol. 2010;184(3):1143–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, Muskens F, Lambrecht BN. Inflammatory dendritic cells–not basophils–are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207(10):2097–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008. March;9(3):310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Min B, Brown MA, Legros G. Understanding the roles of basophils: breaking dawn. Immunology. 2012. March;135(3):192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ziegler SF. The role of thymic stromal lymphopoietin (TSLP) in allergic disorders. Curr Opin Immunol. 2010. December;22(6):795–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ziegler SF, Roan F, Bell BD, Stoklasek TA, Kitajima M, Han H. The biology of thymic stromal lymphopoietin (TSLP). Adv Pharmacol. 2013;66:129–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ziegler SF. Thymic stromal lymphopoietin and allergic disease. J Allergy Clin Immunol. 2012. October;130(4):845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao H, Li M, Wang L, Su Y, Fang H, Lin J, Mohabeer N, Li D. Angiotensin II induces TSLP via an AT1 receptor/NF-KappaB pathway, promoting Th17 differentiation. Cell Physiol Biochem. 2012;30(6):1383–97. [DOI] [PubMed] [Google Scholar]
- 136.Giacomin PR, Siracusa MC, Walsh KP, Grencis RK, Kubo M, Comeau MR, Artis D. Thymic stromal lymphopoietin-dependent basophils promote Th2 cytokine responses following intestinal helminth infection. J Immunol. 2012;189(9):4371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Crowley SD, Rudemiller NP. Immunologic Effects of the Renin-Angiotensin System. J Am Soc Nephrol. 2017;28(5):1350–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Landheer J, Giovannone B, Sadekova S, Tjabringa S, Hofstra C, Dechering K, Bruijnzeel-Koomen C, Chang C, Ying Y, de Waal Malefyt R, Hijnen D, Knol E. TSLP is differentially regulated by vitamin D3 and cytokines in human skin. Immun Inflamm Dis. 2015. March;3(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, de Waal-Malefyt Rd R, Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3(7):673–80. [DOI] [PubMed] [Google Scholar]
- 140.Muto T, Fukuoka A, Kabashima K, Ziegler SF, Nakanishi K, Matsushita K, Yoshimoto T. The role of basophils and proallergic cytokines, TSLP and IL-33, in cutaneously sensitized food allergy. Int Immunol. 2014. October;26(10):539–49. [DOI] [PubMed] [Google Scholar]
- 141.Uermösi C, Zabel F, Manolova V, Bauer M, Beerli RR, Senti G, Kündig TM, Saudan P, Bachmann MF. IgG-mediated down-regulation of IgE bound to mast cells: a potential novel mechanism of allergen-specific desensitization. Allergy. 2014. March;69(3):338–47. [DOI] [PubMed] [Google Scholar]
- 142.Cemerski S, Chu SY, Moore GL, Muchhal US, Desjarlais JR, Szymkowski DE. Suppression of mast cell degranulation through a dual-targeting tandem IgE-IgG Fc domain biologic engineered to bind with high affinity to FcγRIIb. Immunol Lett. 2012. March 30;143(1):34–43. [DOI] [PubMed] [Google Scholar]
- 143.Mortuaire G, Michel J, Papon JF, Malard O, Ebbo D, Crampette L, Jankowski R, Coste A, Serrano E. Specific immunotherapy in allergic rhinitis. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;134(4):253–258. [DOI] [PubMed] [Google Scholar]
- 144.Wachholz PA, Soni NK, Till SJ, Durham SR. Inhibition of allergen-IgE binding to B cells by IgG antibodies after grass pollen immunotherapy. J Allergy Clin Immunol. 2003;112(5):915–22. [DOI] [PubMed] [Google Scholar]
- 145.Fujita H, Soyka MB, Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. Clin Transl Allergy. 2012. January 5;2(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aalberse R. The role of IgG antibodies in allergy and immunotherapy. Allergy. 2011;66 Suppl 95:28–30. [DOI] [PubMed] [Google Scholar]
- 147.Cady CT, Powell MS, Harbeck RJ, Giclas PC, Murphy JR, Katial RK, Weber RW, Hogarth PM, Johnson S, Bonvini E, Koenig S, Cambier JC. IgG antibodies produced during subcutaneous allergen immunotherapy mediate inhibition of basophil activation via a mechanism involving both FcgammaRIIA and FcgammaRIIB. Immunol Lett. 2010;130(1–2):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cassard L, Jönsson F, Arnaud S, Daëron M. Fcγ receptors inhibit mouse and human basophil activation. J Immunol. 2012;189(6):2995–3006. [DOI] [PubMed] [Google Scholar]
- 149.Bruhns P, Frémont S, Daëron M. Regulation of allergy by Fc receptors. Curr Opin Immunol. 2005. December;17(6):662–9. [DOI] [PubMed] [Google Scholar]
- 150.Zhang M, Murphy RF, Agrawal DK. Decoding IgE Fc receptors. Immunol Res 2007;37(1):1–16. [DOI] [PubMed] [Google Scholar]
- 151.MacGlashan D Jr, Undem BJ. Inducing an anergic state in mast cells and basophils without secretion. J Allergy Clin Immunol. 2008;121(6):1500–6. [DOI] [PubMed] [Google Scholar]
- 152.Wilson BS, Pfeiffer JR, Oliver JM. FcepsilonRI signaling observed from the inside of the mast cell membrane. Mol Immunol. 2002;38(16–18):1259–68. [DOI] [PubMed] [Google Scholar]
- 153.Goldstein B, Faeder JR, Hlavacek WS, Blinov ML, Redondo A, Wofsy C. Modeling the early signaling events mediated by FcepsilonRI. Mol Immunol. 2002;38(16–18):1213–9. [DOI] [PubMed] [Google Scholar]
- 154.MacGlashan D Jr, Lavens-Phillips S, Katsushi M. IgE-mediated desensitization in human basophils and mast cells. Front Biosci. 1998;3:d746−56. [DOI] [PubMed] [Google Scholar]
- 155.Möbs C, Slotosch C, Löffler H, Jakob T, Hertl M, Pfützner W. Birch pollen immunotherapy leads to differential induction of regulatory T cells and delayed helper T cell immune deviation. J Immunol. 2010;184(4):2194–203. [DOI] [PubMed] [Google Scholar]
- 156.Rust B, Wambre E. Human Immune Monitoring Techniques during Food Allergen Immunotherapy. Curr Allergy Asthma Rep. 2017;17(4):22. [DOI] [PubMed] [Google Scholar]
- 157.Rachid R, Umetsu DT. Immunological mechanisms for desensitization and tolerance in food allergy. Semin Immunopathol. 2012;34(5):689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Maggi E. T-cell responses induced by allergen-specific immunotherapy. Clin Exp Immunol. 2010;161(1):10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kraneveld AD, Sagar S, Garssen J, Folkerts G. The two faces of mast cells in food allergy and allergic asthma: the possible concept of Yin Yang. Biochim Biophys Acta. 2012. January;1822(1):93–9. [DOI] [PubMed] [Google Scholar]
- 160.Jutel M, Van de Veen W, Agache I, Azkur KA, Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy and novel ways for vaccine development. Allergol Int. 2013;62(4):425–33. [DOI] [PubMed] [Google Scholar]
- 161.Kim S, Shen T, Min B. Basophils can directly present or cross-present antigen to CD8 lymphocytes and alter CD8 T cell differentiation into IL-10-producing phenotypes. J Immunol. 2009;183(5):3033–9. [DOI] [PubMed] [Google Scholar]
- 162.Nomura T, Kabashima K, Miyachi Y. The panoply of αβT cells in the skin. J Dermatol Sci. 2014. October;76(1):3–9. [DOI] [PubMed] [Google Scholar]
- 163.Kang SS, Allen PM. Priming in the presence of IL-10 results in direct enhancement of CD8+ T cell primary responses and inhibition of secondary responses. J Immunol. 2005;174(9):5382–9. [DOI] [PubMed] [Google Scholar]
- 164.Sektioglu IM, Carretero R, Bulbuc N, Bald T, Tüting T, Rudensky AY, Hämmerling GJ. Basophils Promote Tumor Rejection via Chemotaxis and Infiltration of CD8+ T Cells. Cancer Res. 2017. January 15;77(2):291–302. [DOI] [PubMed] [Google Scholar]
- 165.Schroeder JT, Lichtenstein LM, MacDonald SM. An immunoglobulin E-dependent recombinant histamine-releasing factor induces interleukin-4 secretion from human basophils. J Exp Med. 1996. March 1;183(3):1265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vonakis BM, Macglashan DW Jr, Vilariño N, Langdon JM, Scott RS, MacDonald SM. Distinct characteristics of signal transduction events by histamine-releasing factor/translationally controlled tumor protein (HRF/TCTP)-induced priming and activation of human basophils. Blood. 2008;111(4):1789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bernardes AT, dos Santos RM. Immune network at the edge of chaos. J Theor Biol. 1997;186(2):173–87. [DOI] [PubMed] [Google Scholar]
- 168.Li R, Yang M, Chu T. Controllability and observability of Boolean networks arising from biology. Chaos. 2015;25(2):023104. [DOI] [PubMed] [Google Scholar]
- 169.Dolch A, Kunz S, Dorn B, Roers A, Martin SF, Jakob T. Contact allergens induce CD8(+) T cell-derived interleukin 10 that appears dispensable for regulation of contact hypersensitivity. Exp Dermatol. 2017;26(5):449–451. [DOI] [PubMed] [Google Scholar]
- 170.Okazaki F, Kanzaki H, Fujii K, Arata J, Akiba H, Tsujii K, Iwatsuki K. Initial recruitment of interferon-gamma-producing CD8+ effector cells, followed by infiltration of CD4+ cells in 2,4,6-trinitro-1-chlorobenzene (TNCB)-induced murine contact hypersensitivity reactions. J Dermatol. 2002;29(11):699–708. [DOI] [PubMed] [Google Scholar]
- 171.Brunner T, Heusser CH, Dahinden CA. Human peripheral blood basophils primed by interleukin 3 (IL-3) produce IL-4 in response to immunoglobulin E receptor stimulation. J Exp Med. 1993;177(3):605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Carlin LM, Yanagi K, Verhoef A, Nolte-’t Hoen EN, Yates J, Gardner L, Lamb J, Lombardi G, Dallman MJ, Davis DM. Secretion of IFN-gamma and not IL-2 by anergic human T cells correlates with assembly of an immature immune synapse. Blood. 2005;106(12):3874–9. [DOI] [PubMed] [Google Scholar]
- 173.Chung F. Anti-inflammatory cytokines in asthma and allergy: interleukin-10, interleukin-12, interferon-gamma. Mediators Inflamm. 2001;10(2):51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Park HJ, Lee SW, Park SH, Hong S. iNKT Cells Are Responsible for the Apoptotic Reduction of Basophils That Mediate Th2 Immune Responses Elicited by Papain in Mice Following γPGA Stimulation. PLoS One. 2016;11(4):e0152189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Burton OT, Logsdon SL, Zhou JS, Medina-Tamayo J, Abdel-Gadir A, Noval Rivas M, Koleoglou KJ, Chatila TA, Schneider LC, Rachid R, Umetsu DT, Oettgen HC. Oral immunotherapy induces IgG antibodies that act through FcγRIIb to suppress IgE-mediated hypersensitivity. J Allergy Clin Immunol. 2014. December;134(6):1310–1317.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Brauweiler AM, Cambier JC. Fc gamma RIIB activation leads to inhibition of signalling by independently ligated receptors. Biochem Soc Trans. 2003;31(Pt 1):281–5. [DOI] [PubMed] [Google Scholar]
- 177.Jensen WA, Marschner S, Ott VL, Cambier JC. FcgammaRIIB-mediated inhibition of T-cell receptor signal transduction involves the phosphorylation of SH2-containing inositol 5-phosphatase (SHIP), dephosphorylation of the linker of activated T-cells (LAT) and inhibition of calcium mobilization. Biochem Soc Trans. 2001;29(Pt 6):840–6. [DOI] [PubMed] [Google Scholar]
- 178.Clay CD, Strait RT, Mahler A, Khodoun MV, Finkelman FD. Anti-FcγRIIB mAb suppresses murine IgG-dependent anaphylaxis by Fc domain targeting of FcγRIII. J Allergy Clin Immunol. 2017. pii: S0091-6749(17)30934-X. doi: 10.1016/j.jaci.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chirumbolo S, Olivieri M. Increase in human basophils IgE-mediated stimulation by omalizumab: a role for membrane FcγRs? J Allergy Clin Immunol. 2014;133(5):1493–4. [DOI] [PubMed] [Google Scholar]
- 180.Ekoff M, Möller C, Xiang Z, Nilsson G. Coaggregation of FcepsilonRI with FcgammaRIIB Inhibits Degranulation but Not Induction of Bcl-2 Family Members A1 and Bim in Mast Cells. Allergy Asthma Clin Immunol. 2006;2(3):87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Malbec O, Attal JP, Fridman WH, Daëron M. Negative regulation of mast cell proliferation by FcgammaRIIB. Mol Immunol. 2002;38(16–18):1295–9. [DOI] [PubMed] [Google Scholar]
- 182.Hulett MD, Hogarth PM. Molecular basis of Fc receptor function. Adv Immunol. 1994;57:1–127. [DOI] [PubMed] [Google Scholar]
- 183.Getahun A, Cambier JC. Of ITIMs, ITAMs, and ITAMis: revisiting immunoglobulin Fc receptor signaling. Immunol Rev. 2015;268(1):66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bolland S, Ravetch JV. Inhibitory pathways triggered by ITIM-containing receptors. Adv Immunol. 1999;72:149–77. [DOI] [PubMed] [Google Scholar]
- 185.Kamijo S, Nunomura S, Ra C, Kanaguchi Y, Suzuki Y, Ogawa H, Okumura K, Takai T. Innate basophil IL-4 responses against allergens, endotoxin, and cytokines require the Fc receptor γ-chain. J Allergy Clin Immunol. 2016;137(5):1613–1615.e2. [DOI] [PubMed] [Google Scholar]
- 186.Singh RK, Gupta S, Dastidar S, Ray A. Cysteinyl leukotrienes and their receptors: molecular and functional characteristics. Pharmacology. 2010;85(6):336–49. [DOI] [PubMed] [Google Scholar]
- 187.Chen YH, Bieneman AP, Creticos PS, Chichester KL, Schroeder JT. IFN-alpha inhibits IL-3 priming of human basophil cytokine secretion but not leukotriene C4 and histamine release. J Allergy Clin Immunol. 2003;112(5):944–50. [DOI] [PubMed] [Google Scholar]
- 188.Smaldini PL, Orsini Delgado ML, Fossati CA, Docena GH. Orally-Induced Intestinal CD4+ CD25+ FoxP3+ Treg Controlled Undesired Responses towards Oral Antigens and Effectively Dampened Food Allergic Reactions. PLoS One. 2015. October 30;10(10):e0141116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Novak N, Mete N, Bussmann C, Maintz L, Bieber T, Akdis M, Zumkehr J, Jutel M, Akdis C. Early suppression of basophil activation during allergen-specific immunotherapy by histamine receptor 2. J Allergy Clin Immunol. 2012;130(5):1153–1158. [DOI] [PubMed] [Google Scholar]
- 190.Kmiecik T, Otocka-Kmiecik A, Górska-Ciebiada M, Ciebiada M. T lymphocytes as a target of histamine action. Arch Med Sci. 2012;8(1):154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Monteiro RC, Van De Winkel JG. IgA Fc receptors. Annu Rev Immunol. 2003;21:177–204. [DOI] [PubMed] [Google Scholar]
- 192.Kulis M, Saba K, Kim EH, Bird JA, Kamilaris N, Vickery BP, Staats H, Burks AW. Increased peanut-specific IgA levels in saliva correlate with food challenge outcomes after peanut sublingual immunotherapy. J Allergy Clin Immunol. 2012;129(4):1159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Matsui T, Nunomura S, Shimokawa T, Yoshimaru T, Ra C. Functionality of the IgA Fc receptor (FcalphaR, CD89) is down-regulated by extensive engagement of FcepsilonRI. Clin Immunol. 2008. October;129(1):155–62. [DOI] [PubMed] [Google Scholar]
- 194.Fujihashi K, Kato H, van Ginkel FW, Koga T, Boyaka PN, Jackson RJ, Kato R, Hagiwara Y, Etani Y, Goma I, Fujihashi K, Kiyono H, McGhee JR. A revisit of mucosal IgA immunity and oral tolerance. Acta Odontol Scand. 2001. October;59(5):301–8. [DOI] [PubMed] [Google Scholar]
- 195.Feng T, Elson CO, Cong Y. Treg cell-IgA axis in maintenance of host immune homeostasis with microbiota. Int Immunopharmacol. 2011;11(5):589–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.van der Valk JP, de Jong NW, Gerth van Wijk R. Review on immunotherapy in airway allergen sensitised patients. Neth J Med. 2015;73(6):263–9. [PubMed] [Google Scholar]
- 197.Gernez Y, Nowak-Węgrzyn A. Immunotherapy for Food Allergy: Are We There Yet? J Allergy Clin Immunol Pract. 2017;5(2):250–272. [DOI] [PubMed] [Google Scholar]
- 198.Sato S, Yanagida N, Ogura K, Asaumi T, Okada Y, Koike Y, Iikura K, Syukuya A, Ebisawa M. Immunotherapy in food allergy: towards new strategies. Asian Pac J Allergy Immunol. 2014;32(3):195–202. [PubMed] [Google Scholar]
- 199.Pesek RD, Jones SM. Current and Emerging Therapies for IgE-Mediated Food Allergy. Curr Allergy Asthma Rep. 2016;16(4):28. [DOI] [PubMed] [Google Scholar]
- 200.Jones SM, Burks AW, Dupont C. State of the art on food allergen immunotherapy: oral, sublingual, and epicutaneous. J Allergy Clin Immunol. 2014;133(2):318–23. [DOI] [PubMed] [Google Scholar]
- 201.Sindher S, Fleischer DM, Spergel JM. Advances in the Treatment of Food Allergy: Sublingual and Epicutaneous Immunotherapy. Immunol Allergy Clin North Am. 2016;36(1):39–54. [DOI] [PubMed] [Google Scholar]
- 202.Gaje PN, Amalia Ceausu R, Jitariu A, Stratul SI, Rusu LC, Popovici RA, Raica M. Mast Cells: Key Players in the Shadow in Oral Inflammation and in Squamous Cell Carcinoma of the Oral Cavity. Biomed Res Int. 2016;2016:9235080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Siracusa MC, Kim BS, Spergel JM, Artis D. Basophils and allergic inflammation. J Allergy Clin Immunol. 2013;132(4):789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kim BS, Wang K, Siracusa MC, Saenz SA, Brestoff JR, Monticelli LA, Noti M, Tait Wojno ED, Fung TC, Kubo M, Artis D. Basophils promote innate lymphoid cell responses in inflamed skin. J Immunol. 2014;193(7):3717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Roediger B, Weninger W. Group 2 innate lymphoid cells in the regulation of immune responses. Adv Immunol. 2015;125:111–54. [DOI] [PubMed] [Google Scholar]
- 206.Li BW, Hendriks RW. Group 2 innate lymphoid cells in lung inflammation. Immunology. 2013;140(3):281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Eastman JJ, Cavagnero KJ, Deconde AS, Kim AS, Karta MR, Broide DH, Zuraw BL, White AA, Christiansen SC, Doherty TA. Group 2 innate lymphoid cells are recruited to the nasal mucosa in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2017;140(1):101–108.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bi J, Cui L, Yu G, Yang X, Chen Y, Wan X. NK Cells Alleviate Lung Inflammation by Negatively Regulating Group 2 Innate Lymphoid Cells. J Immunol. 2017;198(8):3336–3344. [DOI] [PubMed] [Google Scholar]
- 209.Halim TYF, McKenzie ANJ. New kids on the block: group 2 innate lymphoid cells and type 2 inflammation in the lung. Chest. 2013;144(5):1681–1686. [DOI] [PubMed] [Google Scholar]
- 210.Gold MJ, Antignano F, Halim TY, Hirota JA, Blanchet MR, Zaph C, Takei F, McNagny KM. Group 2 innate lymphoid cells facilitate sensitization to local, but not systemic, TH2-inducing allergen exposures. J Allergy Clin Immunol. 2014;133(4):1142–8. [DOI] [PubMed] [Google Scholar]
- 211.Kubo M. Innate and adaptive type 2 immunity in lung allergic inflammation. Immunol Rev. 2017;278(1):162–172. [DOI] [PubMed] [Google Scholar]
- 212.Kouser L, Kappen J, Walton RP, Shamji MH. Update on Biomarkers to Monitor Clinical Efficacy Response During and Post Treatment in Allergen Immunotherapy. Curr Treat Options Allergy. 2017;4(1):43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015. January 15;517(7534):293–301. [DOI] [PubMed] [Google Scholar]
- 214.Imai Y, Yasuda K, Sakaguchi Y, Haneda T, Mizutani H, Yoshimoto T, Nakanishi K, Yamanishi K. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci U S A. 2013;110(34):13921–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, Hepworth MR, Van Voorhees AS, Comeau MR, Artis D. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5(170):170ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Doherty TA, Scott D, Walford HH, Khorram N, Lund S, Baum R, Chang J, Rosenthal P, Beppu A, Miller M, Broide DH. Allergen challenge in allergic rhinitis rapidly induces increased peripheral blood type 2 innate lymphoid cells that express CD84. J Allergy Clin Immunol. 2014;133(4):1203–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lao-Araya M, Steveling E, Scadding GW, Durham SR, Shamji MH. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2014;134(5):1193–5.e4. [DOI] [PubMed] [Google Scholar]
- 218.Lombardi V, Beuraud C, Neukirch C, Moussu H, Morizur L, Horiot S, Luce S, Wambre E, Linsley P, Chollet-Martin S, Baron-Bodo V, Aubier M, Moingeon P. Circulating innate lymphoid cells are differentially regulated in allergic and nonallergic subjects. J Allergy Clin Immunol. 2016;138(1):305–8. [DOI] [PubMed] [Google Scholar]
- 219.Yamanishi Y, Karasuyama H. Basophil-derived IL-4 plays versatile roles in immunity. Semin Immunopathol. 2016;38(5):615–22. [DOI] [PubMed] [Google Scholar]
- 220.Salter BM, Oliveria JP, Nusca G, Smith SG, Tworek D, Mitchell PD, Watson RM, Sehmi R, Gauvreau GM. IL-25 and IL-33 induce Type 2 inflammation in basophils from subjects with allergic asthma. Respir Res. 2016. January 14;17:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Noval Rivas M, Burton OT, Oettgen HC, Chatila T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J Allergy Clin Immunol. 2016;138(3):801–811.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Skapenko A, Kalden JR, Lipsky PE, Schulze-Koops H. The IL-4 receptor alpha-chain-binding cytokines, IL-4 and IL-13, induce forkhead box P3-expressing CD25+CD4+ regulatory T cells from CD25-CD4+ precursors. J Immunol. 2005;175(9):6107–16. [DOI] [PubMed] [Google Scholar]
- 223.Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2007;104(46):18169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Blom L, Poulsen BC, Jensen BM, Hansen A, Poulsen LK. IL-33 induces IL-9 production in human CD4+ T cells and basophils. PLoS One. 2011;6(7):e21695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, Bettelli E, Oukka M, van Snick J, Renauld JC, Kuchroo VK, Khoury SJ. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A. 2009;106(31):12885–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lund S, Walford HH, Doherty TA. Type 2 Innate Lymphoid Cells in Allergic Disease. Curr Immunol Rev. 2013;9(4):214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Barlow JL, McKenzie AN. Type-2 innate lymphoid cells in human allergic disease. Curr Opin Allergy Clin Immunol. 2014. October;14(5):397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Frischmeyer-Guerrerio PA, Keet CA, Guerrerio AL, Chichester KL, Bieneman AP, Hamilton RG, Wood RA, Schroeder JT. Modulation of dendritic cell innate and adaptive immune functions by oral and sublingual immunotherapy. Clin Immunol. 2014;155(1):47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chen K, Xu W, Wilson M, He B, Miller NW, Bengtén E, Edholm ES, Santini PA, Rath P, Chiu A, Cattalini M, Litzman J, B Bussel J, Huang B, Meini A, Riesbeck K, Cunningham-Rundles C, Plebani A, Cerutti A. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol. 2009;10(8):889–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Chen K, Cerutti A. The function and regulation of immunoglobulin D. Curr Opin Immunol. 2011;23(3):345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Chen K, Cerutti A. New insights into the enigma of immunoglobulin D. Immunol Rev. 2010;237(1):160–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lakshmi Tamma SM, Wu Y, Toporovsky I, Lima V, Coico RF. IgD receptor-mediated signal transduction in T cells. Cell Immunol. 2001;207(2):110–7. [DOI] [PubMed] [Google Scholar]
- 233.Sechet B, Meseri-Delwail A, Arock M, Wijdenes J, Lecron JC, Sarrouilhe D. Immunoglobulin D enhances interleukin-6 release from the KU812 human prebasophil cell line. Gen Physiol Biophys. 2003;22(2):255–63. [PubMed] [Google Scholar]
- 234.Wu Y, Chen W, Chen H, Zhang L, Chang Y, Yan S, Dai X, Ma Y, Huang Q, Wei W. The Elevated Secreted Immunoglobulin D Enhanced the Activation of Peripheral Blood Mononuclear Cells in Rheumatoid Arthritis. PLoS One. 2016;11(1):e0147788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Carsetti R, Köhler G, Lamers MC. Transitional B cells are the target of negative selection in the B cell compartment. J Exp Med. 1995;181(6):2129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Geisberger R, Lamers M, Achatz G. The riddle of the dual expression of IgM and IgD. Immunology. 2006;118(4):429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Soulas P, Koenig-Marrony S, Julien S, Knapp AM, Garaud JC, Pasquali JL, Martin T. A role for membrane IgD in the tolerance of pathological human rheumatoid factor B cells. Eur J Immunol. 2002;32(9):2623–34. [DOI] [PubMed] [Google Scholar]
- 238.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–88. [DOI] [PubMed] [Google Scholar]
- 239.Toft-Petersen M, Stidsholt Roug A, Plesner T, Ebbesen L, Brown GD, Nederby L. The CLEC12A receptor marks human basophils: Potential implications for minimal residual disease detection in acute myeloid leukemia. Cytometry B Clin Cytom. 2017. doi: 10.1002/cyto.b.21540. PMID:28718199. [DOI] [PubMed] [Google Scholar]


