Abstract
Red seaweed Gracilaria, one of the largest genus in Division Rhodophyta inhabits Sarawak coastal water. This study was designed to identify the species of Gracilaria using morphological approach and to assess selected water quality parameters in Gracilaria habitats. Three field samplings were carried out in Santubong and Asajaya, Sarawak from November 2013 to December 2014. Overall, three species were identified namely Gracilaria changii, G. blodgettii and G. coronopifolia, attached to net of cage culture in Santubong and root of mangrove trees in Asajaya. In addition, three different taxa of aquatic macroinvertebrates (polychaete, small crab, bivalve) and single species of red seaweed (Acanthophora sp.) were observed in Gracilaria assemblages. An estimate of 37% to 40% of the upper part of the cage net in Santubong was covered by seaweeds and only 16% to 20% in Asajaya’s mangrove. The study had provided better information on identification of Gracilaria and their habitat in Sarawak. Future work involving DNA barcoding of each species is in progress.
Keywords: Sarawak, Gracilaria, Morphological Approach, Water Quality, Habitat
Abstract
Rumput laut merah Gracilaria, salah satu genus terbesar bagi spesies Rhodophyta yang mendiami pesisir pantai Sarawak. Kajian ini dijalankan untuk mengenal pasti spesies Gracilaria menggunakan pendekatan morfologi dan untuk menilai parameter kualiti air terpilih dalam habitat Gracilaria. Tiga pensampelan lapangan dijalankan di Santubong dan Asajaya, Sarawak dari November 2013 hingga Disember 2014. Secara keseluruhannya, tiga spesies telah dikenal pasti iaitu Gracilaria changii, G. blodgettii dan G. coronopifolia, yang tersangkut pada jaringan sangkar di Santubong dan akar pokok bakau di Asajaya. Di samping itu, tiga jenis makroinvertebrata akuatik (polychaete, ketam kecil, dwicangkerang) dan spesies tunggal rumput laut merah (Acanthophora sp.) diperhatikan dalam himpunan Gracilaria. Anggaran 37% hingga 40% daripada bahagian atas sangkar di Santubong dilindungi oleh rumpai laut dan hanya 16% hingga 20% di bakau Asajaya. Kajian ini telah memberikan maklumat yang lebih baik mengenai pengenalan Gracilaria dan habitat mereka di Sarawak. Kajian masa depan yang membabitkan pengkodan DNA bagi setiap spesies juga sedang dijalankan.
INTRODUCTION
Gracilaria is one of the Genus in Family Gracilariaceae with more than 100 species worldwide, inhabiting temperate and tropical seawaters, covering from intertidal to subtidal areas (Food and Agriculture Organisation (FAO) (1990); Gulbransen et al. (2012)). Gracilaria is important as source of income in country such as Chile, where they have been cultured commercially with total landings of 120,000 wet metric tons (Buschmann et al. 2001). In Sarawak, Malaysia, wild Gracilaria are collected by the local people as source of food and generate income by selling them in market (Othman et al. 2015). From ecological aspect, Gracilaria act as natural habitat for aquatic organisms and protect them from predators, waves and tides (Nyberg et al. 2009) and certain fishes, crabs and isopods prefer Gracilaria as food (Veeragurunathan et al. 2014).
In Sarawak, effort has been done to document the seaweed resources from coastal waters (Ariffin 2006; Esa 2014; Jusoh 2012; Nurridan 2007; 2013; Wong et al. 2012; Zakaria et al. 2006; Zawawi et al. 2015). According to Nurridan (2007), there are about 87 species of marine seaweeds found along the coastal water of Sarawak, belongs to 27 species of Division Chlorophyta, 21 species of Division Phaeophyta and 39 species of Division Rhodophyta. Up to now, there are 10 species of Gracilaria recorded in Sarawak namely Gracilaria arcuata, G. articulata, G. changii, G. coronopifolia, G. blodgetti, G. salicornia, G. edulis, G. textorii and two unidentified species (Nurridan 2013).
Gracilaria is an ambiguous species where misidentification often occur due simple morphology and high plasticity problems (Phang et al. 2001; Saunders 2009). Besides, the different features of male and female in same species as well as variation in morphologies of their life cycle also could cause difficulty during identification (Baghel et al. 2011; Robba et al. 2006). In Sarawak, the identification of Gracilaria was done by Nurridan (2007; 2013) and the study should be continued to resolve the taxonomy and create clear understanding on their morphology. Besides, the available studies of seaweed in Sarawak focusing on the species checklist and the habitat information especially Gracilaria is limited. Therefore, the objectives of this study were to: (i) describe the morphological characteristics of Gracilaria species found in Santubong and Asajaya, Sarawak and (ii) assess selected water quality parameters in Gracilaria habitats as well as the aquatic organisms associated with it.
MATERIALS AND METHODS
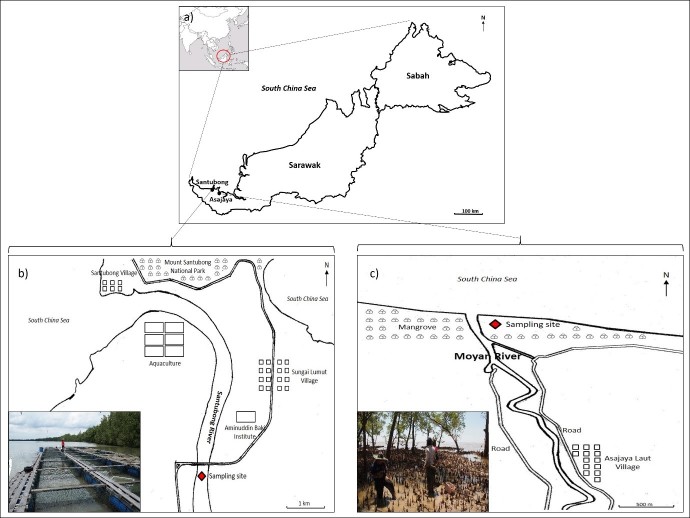
Three field samplings were carried out in cage culture area, Santubong, Sarawak (N 01° 40′42.1″ E 110° 20′2.4″) and mangrove area, Asajaya, Sarawak (N 01° 35′57.8″ E 110° 36′15.9″) (Fig. 1). For Santubong, the samplings were done during flooding tide while for Asajaya, the samplings were done during ebbing tide. All the samplings were done at daylight. The descriptions of the sampling sites are shown in Table 1.
Figure 1.
Field samplings area in Santubong and Asajaya, Sarawak.
Table 1.
Coordinate of each sampling sites
| Sampling Sites | Sampling times | Brief descriptions |
|---|---|---|
| Santubong | November 2013, March 2014, October 2014 | Located in Santubong River, near to the Santubong bridge. The cage culture is run by three local residents (En. Poli B. Pa’ee, En. Joni B. Pa’ee, En. Sudiman B. Bujang) and received guidance from Department of Fisheries Sarawak. There are 30 cages with length of 2.5 metres (m), width of 2.0 m and approximate depth of 3 m. The mesh size of the nets used is 3.5 centimetres (cm). Examples of fish culture here are grouper, red fish, yellow fish, sea bass and mud crab. |
| Asajaya | April 2013, March 2014, December 2014 | Located in the mangrove area of Asajaya Laut village. Examples of mangrove trees observed in the area are Rhizophora, Sonneratia and Avicennia. |
Selected water quality parameters namely dissolved oxygen (DO), temperature, turbidity, pH, conductivity and salinity were measured in-situ. DO and temperature were measured using Hanna instrument (model 9142). Turbidity was taken using Eutech instrument (model TN-100). Milwaukee instrument was used to record salinity while Hanna instrument (model HI 8424) was used to take pH reading. Other parameters such as water transparency was recorded using secchi disk while the depth of water was measured using depth finder (Speedtech instrument, model 65054). Triplicate readings of all the water quality parameters were recorded. Four litres of water samples were collected using acid wash polyethylene bottles for nutrient (nitrite, orthophosphate, silicate), chlorophyll a (chl a) and total suspended solid (TSS) analyses. In Santubong, the water samples were collected at the water surface during flooding tide while in Asajaya, the water samples were collected at water puddle during ebbing tide. The samples were kept in cooler box with ice and brought back to laboratory in Universiti Malaysia Sarawak (UNIMAS) for further analysis. Chl a analysis followed the method proposed by Aminot and Rey (2000), TSS analysis followed the method proposed by Jacobs Engineering Group (2010) and nutrients analysis followed the standard protocol Hach (2007) using spectrophotometer (Hach, DR 2010). The values obtained were compared with Malaysian Marine Water Quality Criteria and Standard (MMWQCS) (Department of Environment (DOE) 2010). In Asajaya, 30 g of subsurface sediment was collected using scope for particle size analysis. The samples were collected in triplicates, stored in plastic bag with appropriate labels and brought back to laboratory for further analysis. Sediment particle size was analysed using method proposed by Buchanan (1984). One-way analysis of variance (ANOVA) was performed to test the significant difference of water parameters among field samplings in each location. The test was significant at p < 0.05.
The whole thallus of 15 Gracilaria individuals were collected at cage culture in Santubong and mangrove area in Asajaya consists of holdfast, blade and stipe. The samples were washed, stored in plastic bag with zipper and brought back to laboratory for further analysis. Other organisms associated with Gracilaria were also observed, collected, stored in plastic bag and brought back to laboratory for identification. At the lab, the Gracilaria samples were preserved using wet preservation and dry preservation, methods suggested by Dhargalkar and Kavlekar (2004). The other organisms were preserved in 10% formalin. Identification of Gracilaria species and other seaweed were based on the identification keys from Nurridan (2007; 2013), Dhargalkar and Kavlekar (2004), Ismail (1995) and Lin (2009).
Percentage coverage of seaweeds were observed in four cages of Santubong cage culture using modified grid transparency quadrate (0.058 m2). In December 2014, a 100 m transect line was set up parallel to the seashore in Asajaya and a quadrate (0.25 m2) was randomly throw for every 25 m to determine the percentage coverage of seaweeds.
RESULTS AND DISCUSSION
A total of three Gracilaria species were found in Santubong and Asajaya, Sarawak namely G. changii, G. blodgettii and G. coronopifolia. Table 2 showed the voucher list of the seaweed specimens that were deposited in the Department of Aquatic Science Museum (Botanical Section), UNIMAS. Summary of their morphological characteristics shown in Table 3.
Table 2.
Voucher lists for seaweed preservation.
| Division | Species | Voucher number | Habitat | Location | Collection date |
|---|---|---|---|---|---|
| Rhodophyta | Order Gracilariales | ||||
| Family Gracilariaceae | |||||
| G. coronopifolia | SW 001-SW005 | CG | Santubong | 18/10/2014 | |
| G. blodgettii | SW 006-SW010 | E, M | Asajaya | 27/11/2014 | |
| G. changii | SW 011-SW015 | CG | Santubong | 18/10/2014 |
Note: CG = cage net; E = epiphyte; M = mud
Table 3.
Summary of morphological characteristics of all Gracilaria found in this study.
| G. blodgettii | G. changii | G. coronopifolia | |
|---|---|---|---|
| Locality | Asajaya, Sarawak | Santubong, Sarawak | Santubong, Sarawak |
| Type of habitat | Mangrove | Cage culture | Cage culture |
| Type of substrate | Mangrove root | Cage net | Cage net |
| Height of thallus (mm) | 200 | 180–220 | - |
| Colour | Dark red | Dark red | Purplish red |
| Type of Holdfast | Discoid | Discoid | Discoid |
| Constriction at base | Present | Present | Absent |
| Branching pattern | Secund or irregular, frequent branching | Irregular, branching occasionally | Irregular, branching shorter than previous branches |
| Shape of tip | Pointed | Pointed | Pointed |
| Diameter (mm) | 1–2 | 1–2 | 1–2 |
| No of parenchymatous layers (medulla) | 3–4 | 3–4 | 3–4 |
| No of cortical layers (cortex) | 2–3 | 2–3 | 1–2 |
G. blodgettii Harvey, 1853
Synonyms
None
Taxonomy Remarks
G. blodgettii had a dark red colour and the thallus could grow up to 200 mm tall (Fig. 2a). The holdfast shape was discoid while the branch shape was cylindrical with diameter between 1 to 2 mm. Primary branches were longer compare to secondary branches and could reach up between 10 mm to 90 mm long while secondary branches could reach up to 5 mm to 85 mm. The branches either secund or irregular, slightly constrict at the base, enlarged at the middle and become attenuate at the tip. Frequent formation of branches was observed at secondary branches which make this species look compact. New formation of short branches with pointed tip were observed at tertiary branches. The cross section of stipe show that the medulla was composed of 3–4 layers of parenchymatous cells and surround by 2–3 layer of small rounded cortical cells at the cortex.
Figure 2.
(a) G. blodgettii, (b) G. changii, (c) G. coronopifolia.
Location
Asajaya, Sarawak
Ecology and Distribution
The specimen was found attached to root of mangrove trees in Asajaya, Sarawak. This finding was similar to report by Nurridan (2007). Other than Asajaya, G. blodgettii was also found in Muara Mengkuang, Miang Kecil, Sungai Sibu, Salak, Kuching Division.
G. changii (Xia & Abbott) Zhang & Xia, 1991
Synonyms
Polycavernosa changii (Xia & Abbott), Hydropuntia changii (Xia & Abbott) (Wynne, 1989)
Taxonomy Remarks
the colour of G. changii was dark red while the thallus could grow between 180 mm to 220 mm tall (Fig. 2b). Primary branches were shorter compare to secondary branches and could reach up between 25 mm to 40 mm long while secondary branches could reach up to 40 mm to 170 mm. The species had discoidal holdfast and the branches were irregular with diameter between 1 to 2 mm. Constriction occur at base of branches, swelling at middle and tapering toward the end. The formation of branches occurs occasionally. The tip of secondary branches either pointed or divide into two short branchlets. Formation of new branches with pointed tip were observed along tertiary branches. The cross section of stipe show that the medulla was composed of 3–4 layers of parenchymatous cells and surround by 2–3 layer of small rounded cortical cells at the cortex.
Location
Santubong, Sarawak
Ecology and Distribution
The specimen was found attach to net of cage culture in Santubong, Sarawak. Nurridan (2007) reported that G. changii attach to net, buoys and floating net cages. G. changii could be found in other areas of Sarawak namely Pulau Salak, Kuching Division. In Peninsular Malaysia, G. changii was found in Morib, Selangor (Chan et al. 2002). G. changii can also be found in Malacca, Penang, Selangor, Negeri Sembilan, Johor, Kedah, and Sabah.
G. coronopifolia J. Agardh, 1852
Synonyms
G. lichenoides f. coronopifolia (J. Agardh) (May, 1948)
Taxonomy Remarks
G. coronopifolia had a purplish-red colour (Fig. 2c). The species had discoid holdfast, cylindrical and irregular branches with diameter between 1 to 2 mm. shorted pointed tip, frequent formation of branches were observed at the end of thallus where each subsequent branching was shorter than previous branches. Upper part of thallus more densely branches and form small bush. The branches had no constrict base and tapering toward the end. The last branches were bifurcate. The cross section of stipe show that the medulla was composed of 3–4 layers of parenchymatous cells and surround by 1–2 layer of small rounded cortical cells at the cortex.
Location
Santubong, Sarawak
Ecology and Distribution
The specimen was found attach to net of cage culture in Santubong, Sarawak. Nurridan (2007) reported that G. coronopifolia attach to net and buoy at floating net cage system. G. coronopifolia could be found in other areas of Sarawak namely Pulau Salak, Kuching Division.
Based on Table 4, the surface water temperature in Santubong had range of 28.80°C–29.90°C [F (2,6) = 273.00; p = 0.000] while Asajaya had range of 29.30°C–31.37°C [F (2,6) = 70.26; p = 0.000]. Santubong had recorded pH with range of 6.60–7.82 [F (2,6) = 16.12; p = 0.004] while Asajaya had recorded pH with range 7.41–7.80 [F (2,6) = 83.27; p = 0.000]. In Santubong, the pH probably affected by the decomposition of foods and waste products that come from culturing activities (Suratman et al. 2014). For Asajaya, the water pH possibly affected by photosynthesis rate where the presence of Gracilaria used the CO2 for photosynthesis process thus increasing the pH (Tucker & D’Abramo 2008).
Table 4.
Selected water quality parameters of cage culture site measured in-situ in Santubong and Asajaya Sarawak.
| Temperature (°C) | pH | DO (mg/L) | Salinity (PSU) | Turbidity (NTU) | Depth (m) | Transparency (m) | ||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Mean ± SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||
| Santubong | 13 Nov | 29.30±0.00 | 6.60±0.00 | 5.44±0.46 | 28.33±0.58 | 17.16±1.10 | N/A | N/A |
| 14 Mar | 29.90±0.00 | 7.82±0.06 | 5.29±0.07 | 26.00±0.00 | 35.10±1.85 | N/A | N/A | |
| 14 Oct | 28.80±0.10 | 7.18±0.45 | 6.69±0.26 | 20.33±0.58 | 10.17±1.05 | 7.80±0.00 | 0.73±0.05 | |
| Asajaya | May 13 | 29.30±0.00 | 7.41±0.02 | 5.27±0.01 | 18.33±1.53 | 253.33±14.29 | N/A | N/A |
| Mar 14 | 29.37±0.06 | 7.80±0.01 | 4.05±0.03 | 21.00±1.73 | 37.63±6.60 | N/A | N/A | |
| Dec 14 | 31.37±0.42 | 7.41±0.07 | 5.68±1.01 | 23.44±0.77 | 115.22±64.00 | N/A | N/A | |
N/A: not available
Santubong had recorded DO with range of 5.29 mg/L – 6.69 mg/L [F (2,6) = 19.00; p = 0.003] while Asajaya had recorded DO with range of 4.05 mg/L – 5.68 mg/L [F (2,6) = 6.36; p = 0.033]. Noraini et al. (2010). reported that abundance of aquatic plants could increase the water DO where the presence of Gracilaria and other type of seaweeds could be observed in Santubong and Asajaya. Santubong had recorded salinity with range of 20.33 PSU – 28.33 PSU) [F (2,6) = 228.50; p = 0.000] while Asajaya had recorded salinity with range of 18.33 PSU – 23.44 PSU [F (2,6) = 9.93; p = 0.013]. Water salinity in Asajaya and Santubong could be affected by tidal change, evaporation and mixing of freshwater and seawater (Wong et al. 2012; Coelho et al. 2007).
The turbidity in Santubong had range of 10.17 NTU – 35.10 NTU [F (2,6) = 259.74; p = 0.000] while Asajaya had range of 36.63 NTU – 253.33 NTU [F (2,6) = 24.73; p = 0.001]. In Santubong, the water turbidity could be affected by water discharge from human settlement areas, aquaculture activities along the Santubong river and soil erosion due to construction at the upper part of the river. The turbidity in Asajaya was high with range of 37.63 NTU – 253.33 NTU because the readings were taken during low tide where the suspended solid had been brought by the turbid river and deposited at the mangrove area thus increasing the turbidity reading. Due to technical problems, the depth and transparency readings in Santubong were only taken during the third sampling (October 2014). The depth and transparency readings for Santubong were 7.80 m and 0.73 m respectively.
Based on Table 5, Santubong had recorded TSS with range of 27.33 mg/L – 63.33 mg/L [F (2,6) = 2.04; p = 0.211] while Asajaya had recorded TSS with range of 161.10 mg/L – 655.57 mg/L [F (2,6) = 9.24; p = 0.015]. The TSS of Santubong river is possibly influenced by water discharge from residential areas, aquaculture and industrial activities. TSS in Asajaya was high with range of 161.10 mg/L – 655.57 mg/L because the readings were taken during low tide where the turbid river brings the particles such as silt, clay, sand, organic and inorganic matter from the upstream to the mangrove areas, trapped by the mangrove roots and deposited in the mangrove areas.
Table 5.
Selected water quality parameters of cage culture site measured ex-situ in Santubong and Asajaya, Sarawak.
| TSS (mg/L) | NO2 (mg/L) | PO43 (mg/L) | SiO2 (mg/L) | Chlorophyll a (mg/m3) | ||
|---|---|---|---|---|---|---|
|
|
||||||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Santubong | 13 Mar | 63.33±20.42 | 0.012±0.001 | 0.053±0.02 | 1.070±0.286 | 3.037±0.254 |
| 14 Mar | 52.00±32.74 | 0.034±0.009 | 0.050±0.01 | 3.509±0.275 | 2.556±1.805 | |
| 14 Oct | 27.33±2.31 | 0.046±0.002 | 0.063±0.06 | 0.579±0.354 | 7.663±0.129 | |
| Asajaya | 13 May | 655.57±258.91 | 0.015±0.00 | 0.160±0.03 | 2.740±0.090 | 5.937±0.663 |
| 14 Mar | 161.10±29.30 | 0.005±0.004 | 0.057±0.01 | 1.560±0.056 | 5.089±2.402 | |
| 14 Dec | 234.67±38.02 | 0.027±0.001 | 0.173±0.03 | 1.796±0.072 | 3.236±0.336 | |
Santubong had recorded nitrite (NO2) with range of 0.012 mg/L – 0.046 mg/L C [F (2,6) = 27.00; p = 0.001], orthophosphate (PO43−) with range of 0.050 mg/L – 0.063 mg/L [F (2,6) = 0.12; p = 0.888] and silicate (SiO2) with range of 0.579 mg/L – 3.509 mg/L [F (2,6) = 78.36; p = 0.000]. Asajaya had recorded NO2 with range of 0.005 mg/L – 0.015 mg/L [F (2,6) = 78.00; p = 0.000], PO43− with range of 0.057 mg/L – 0.173 mg/L [F (2,6) = 20.77; p = 0.002] and SiO2 with range of 1.560 mg/L – 2.740 mg/L [F (2,6) = 214.06; p = 0.000]. The runoff of sewage from residential areas, waste products from industrial activities with addition of aquaculture activities could influenced the nitrite and orthophosphate concentration in Santubong river. Leach of fertilizers and pesticides from the farms own by Asajaya’s local people may indirectly affected the nutrients. Santubong had recorded mean chl a reading with range of 2.556 mg/m3 – 7.663 mg/m3 [F (2,6) = 21.44; p = 0.002] while Asajaya had recorded chl a with range of 3.236 mg/m3 – 5.937 mg/m3[F (2,6) = 2.72; p = 0.145].
Table 6 showed the comparison of water quality results in Santubong, Sarawak with Malaysian Marine Water Quality Criteria and Standard (MMWQCS) values. The range of DO for all field samplings in Santubong was higher compared to the standard value set for Marine life, fisheries, coral reefs, recreational and mariculture (Class 2). The mean TSS in October 2014 was lower than Class 2 of MWQCS whereas the mean TSS in November 2013 and March 2014 were higher than standard value. the range of NO2 and PO43 for all field samplings were lower compared to the standard value of Class 2.
Table 6.
Comparison of water quality results in Santubong with Malaysian Marine Water Quality Criteria and Standard (MMWQCS) values.
| DO | TSS | NO2 | PO43 | |
|---|---|---|---|---|
| Reference value (Class 2) (mg/L) | 5.00 | 50 | 0.055 | 0.075 |
| Observed value (mg/L) | 5.29–6.69 | 27.33–63.33 | 0.012–0.046 | 0.050–0.063 |
Table 7 showed the comparison of water quality results in Asajaya with Malaysian Marine Water Quality Criteria and Standard (MMWQCS) values. The range of DO and TSS for all field samplings were higher compared to the standard value set for mangrove estuarine and river mouth water (Class E). In Comparison, the range of NO2 for all field samplings were lower compared to the standard value set for mangrove estuarine and river mouth water (Class E). the mean PO43 in March 2014 was lower than Class E of MWQCS whereas the mean PO43 in May 2014 and December 2014 were higher than standard value.
Table 7.
Comparison of water quality results in Asajaya with Malaysian Marine Water Quality Criteria and Standard (MMWQCS) values.
| DO | TSS | NO2 | PO43 | |
|---|---|---|---|---|
| Reference value (Class E) (mg/L) | 4.00 | 100 | 0.055 | 0.075 |
| Observed value (mg/L) | 4.05–5.68 | 161.10–655.57 | 0.005–0.027 | 0.057–0.173 |
Based on the observation at the field, Gracilaria in Asajaya prefer root of mangrove trees compare to muddy sediment as their substrate. In contrast, Gracilaria found in Santubong river grow well by attaching themselves to the cage culture net which is a man-made structure. In Asajaya, the sediment comprised of high silt and clay contents (58.5%–65.8%) compare to sand (34.2%–41.5%) which make the substrate loosely arranged (Table 8). This probably the reason for Gracilaria to grow on more stable and strong mangrove roots that can protect them from incoming waves and prevent from swept away by the water current during flooding and ebbing tides. Based on studies done by Molloy and Bolton (1995), the type of sediment did influence the distribution of Gracilaria.
Table 8.
Percentage of sand, silt and clay content in Asajaya based on sampling times.
| Sampling | Sediment particle size (%) | Sand (%) | Silt and clay (%) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| >1000 μm | >500 μm | >250 μm | >125 μm | >63 μm | <63 μm | |||
| May 13 | 4.5 | 6.0 | 5.8 | 7.6 | 10.3 | 65.8 | 34.2 | 65.8 |
| Mar 14 | 5.4 | 4.5 | 4.5 | 5.8 | 21.3 | 58.5 | 41.5 | 58.5 |
| Dec 14 | 0.7 | 5.2 | 8.5 | 12.5 | 13.4 | 59.8 | 40.3 | 59.8 |
In Santubong, the estimated percentage cover of seaweeds on the cage net in October 2014 is 37% to 40% meanwhile the estimated percentage cover of seaweeds in Asajaya mangrove (December 2014) is 16% to 20%. No information on seaweeds percentage cover collected on first and second samplings due to technical problem. Gracilaria in Santubong cage culture were found living in patchy throughout the three samplings and similar case also was observed in Asajaya mangrove area. The actual percentage cover of Gracilaria in both sampling sites could not be obtained because the Gracilaria existed there probably had been collected regularly by the local people for personal consumption and selling for extra income.
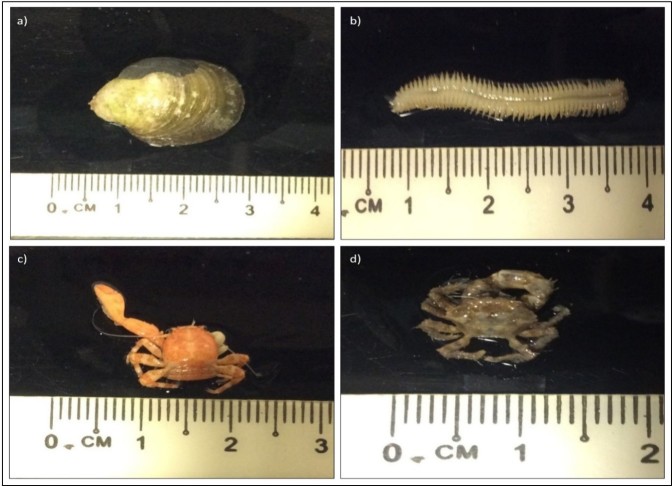
Based on Fig. 3, three different taxa of aquatic macroinvertebrates (polychaete, small crab, bivalve) and single species of red seaweed (Acanthophora sp.) found living together with Gracilaria assemblages in Santubong (Fig. 3). According to Veeragurunathan et al. (2014), various organisms such as green seaweed, brown seaweed, red seaweed, blue green algae, decapod, gastropod, bivalvia, polychaete, ophiuroidea, isopod, ectoprocta, maxillopod, ascidiacea and anthozoa were found together with G. dura. Thirteen species of green seaweeds, 8 species of brown seaweeds, 14 species of red seaweeds and single species of blue-green alga were found grow together with G. edulis cultured in Gulf of Mannar and Palk Bay, India (Ganesan et al. 2011; Kaliaperumal et al. 1993). This suggests that Gracilaria assemblages as one of the important habitat that supports wide range of living organisms including flora and fauna where it provides protection against tides, predators, waves and also as food sources.
Figure 3.
Aquatic macroinvertebrates (polychaete, small crab, bivalve) found living together with Gracilaria assemblages in Santubong.
CONCLUSION
Based on morphological characteristics, three species namely G. changii, G. coronopifolia and G. blodgettii were found attach to net of cage culture in Santubong river and root of mangrove trees in Asajaya. In this study, it is found that Gracilaria able to grow both on man-made structure and natural habitat. Several aquatic organisms were found associated with Gracilaria assemblages such as Acanthophora, small crab, polychaete and bivalve. The actual percentage cover of seaweeds in Santubong and Asajaya could not be determine due to frequent collection of Gracilaria by the local people. Overall, the water quality in Santubong river are suitable for fisheries and mariculture while Asajaya is within normal range for mangrove in Malaysia.
ACKNOWLEDGEMENTS
This work is supported by Ministry of Education Malaysia through Research Acculturation Collaborative Effort (RACE) Grant Scheme RACE/g(2)891/2012(09). Authors would like to thank local people of Santubong and Asajaya for their kind assistance during sampling trips. Thank you to UNIMAS for laboratory facilities and transportation. The first author is the recipient of MyBrain 15 scholarship and Zamalah Siswazah UNIMAS (ZSU) scholarship.
REFERENCES
- Aminot A, Rey F. Standard procedure for the determination of chlorophyll a by spectroscopic methods. ICES Techniques in Marine Environmental Sciences. 2000:1–17. [Google Scholar]
- Ariffin MKF. Unpublished Bachelor thesis. Universiti Malaysia Sarawak; Sarawak, Malaysia: 2006. Seaweed community in Asajaya mangrove area. [Google Scholar]
- Baghel RS, Kumari P, Bijo AJ, Gupta V, Reddy CRK, Jha B. Genetic analysis and marker assisted identification of life phases of red alga Gracilaria corticata (J. Agardh) Molecular Biology Reports. 2011;38:4211–4218. doi: 10.1007/s11033-010-0543-y. https://doi.org/10.1007/s11033-010-0543-y. [DOI] [PubMed] [Google Scholar]
- Buchanan JB. Methods for the study of marine benthos. Victoria, Australia: Blackwell Scientific Publications; 1984. [Google Scholar]
- Buschmann AH, Correa JA, Westermeier R, Hernandez-Gonzalez C, Norambuena R. Red algal farming in Chile: a review. Aquaculture. 2001;194:203–220. https://doi.org/10.1016/S0044-8486(00)00518-4. [Google Scholar]
- Chan CX, Ho CL, Othman RY, Phang SM. Optimisation of RNA extraction for Gracilaria changii (Gracilariales, Rhodophyta) In: Phang SM, Chong VC, Mokhtar N, Ooi JLS, editors. Marine science into the new millenium: New perspectives & challenges. Kuala Lumpur, Malaysia: Maritime Research Centre; 2002. [Google Scholar]
- Coelho S, Gamito S, Perez-Ruzafa A. Trophic state of Foz de Almargem coastal lagoon (Algarve, South Portugal) based on the water quality and the phytoplankton community. Estuarine, Coastal and Shelf Science. 2007;71:218–231. https://doi.org/10.1016/j.ecss.2006.07.017. [Google Scholar]
- Department of Environment (DOE) Malaysia marine water quality criteria and standard. 2010. [Accessed on 11 March 2016]. http://www.doe.gov.my/portalv1/en/info-umum/piawaian-dan-kriteriakualiti-air-marin-malaysia/301.
- Dhargalkar VK, Kavlekar D. Seaweeds: A field manual. Goa, India: National Institute of Oceanography; 2004. [Google Scholar]
- Esa FA. Unpublished Master thesis. Universiti Malaysia Sarawak; Sarawak, Malaysia: 2014. Taxanomy, species composition and ecology of seaweed plus preliminary molecular assessment of Caulerpa spp. in Satang and Sampadi Island, Sarawak. [Google Scholar]
- Food and Agriculture Organisation (FAO) Training manual of Gracilaria culture and seaweed processing in China. 1990. [Accessed on 20 December 2015]. http://www.fao.org/docrep/field/003/ab730e/AB730E00.htm#TOC.
- Ganesan M, Sahu N, Eswaran K. Raft culture of Gracilaria edulis in open sea along the south-eastern coast of India. Aquaculture. 2011;321:141–151. https://doi.org/10.1016/j.aquaculture.2011.08.040. [Google Scholar]
- Gulbransen DJ, McGlathery KJ, Marklund M, Norris JN, Gurgel CFD. Gracilaria vermiculophylla (Rhodophyta, Gracilariales) in the Virginia coastal bays, USA: cox1 analysis reveals high genetic richness of an introduced macroalga. Phycological Society of America. 2012;48:1278–1282. doi: 10.1111/j.1529-8817.2012.01218.x. [DOI] [PubMed] [Google Scholar]
- Hach DR 2800 Spectrophotometer procedures manual. 2007. [Accessed on 10 November 2014]. http://www.hach.com/asset-get.download.jsa?id=7639982436.
- Ismail A. Rumpai laut Malaysia. Kuala Lumpur, Malaysia: Dewan Bahasa dan Pustaka; 1995. [Google Scholar]
- Jacobs Engineering Group. [Accessed on 30 January 2015];Standard operating procedure: Total suspended solids determination using vacuum filtration and oven drying. 2010 http://waterfacts.net/Treatment/Activated_Sludge/Wastewater_Tests/TSS/TVA-KIF-SOP49_Total_Suspended_Solids__Apr_10_.pdf. [Google Scholar]
- Jusoh NI. Unpublished Master thesis. Universiti Malaysia Sarawak; Sarawak, Malaysia: 2012. Seaweed community from intertidal areas of Satang Besar Island, Sarawak. [Google Scholar]
- Kaliaperumal N, Chennubhotla VSK, Kalimuthu S, Ramalingam JR, Muniyandi K. Growth of Gracilaria edulis in relation to environmental factors in field cultivation. Seaweed Research and Utilization. 1993;6:167–176. [Google Scholar]
- Lin SM. Marine benthic macroalgal flora of Taiwan. Taiwan: National Taiwan Ocean University Publication; 2009. [Google Scholar]
- Molloy FJ, Bolton JJ. Distribution, biomass and production of Gracilaria in Luderitz Bay, Namibia. Journal of Applied Phycology. 1995;7:381–392. https://doi.org/10.1007/BF00003795. [Google Scholar]
- Noraini R, Gandaseca S, Johan I, Jailan MI. Comparative study of water quality at different peat swamp forest of Batang Igan, Sibu Sarawak. American Journal of Environmental Sciences. 2010;6:416–421. [Google Scholar]
- Nurridan AH. Seaweeds of Sarawak Malaysia Borneo. Sarawak, Malaysia: Fisheries Research Institute; 2007. [Google Scholar]
- Nurridan AH. Diversity and new record of seaweed in Sarawak. In: Nyanti L, Norhadi I, Samsur M, Hassan R, Rahim SAKA, editors. Aquatic Science Colloqium 2012: Experience Sharing in Aquatic Science Research II, Talang-Satang National Park to Santubong. Sarawak, Malaysia: Department of Aquatic Science; 2013. [Google Scholar]
- Nyberg CD, Thomsen MS, Wallentinus I. Flora and fauna associated with the introduced red alga Gracilaria vermiculophylla. Europe Journal Phycology. 2009;44:395–403. https://doi.org/10.1080/09670260802592808. [Google Scholar]
- Othman MNA, Hassan R, Harith MN, Sah ASRM. Red seaweed Gracilaria arcuata in cage culture area of Lawas, Sarawak. Borneo Journal of Resource Science and Technology. 2015;5:53–61. [Google Scholar]
- Phang SM, Lim PE, Thong KW. Molecular differentiation of two morphological variants of Gracilaria salicornia. Journal of Applied Phycology. 2001;13:335–342. https://doi.org/10.1023/A:1017532624398. [Google Scholar]
- Robba L, Russell SJ, Barker G, Brodie J. Assessing the use of the mitochondrial cox1 marker for use in DNA barcoding of red algae (Rhodophyta) American Journal of Botany. 2006;93:1101–1108. doi: 10.3732/ajb.93.8.1101. https://doi.org/10.3732/ajb.93.8.1101. [DOI] [PubMed] [Google Scholar]
- Saunders GW. Routine DNA barcoding of Canadian Gracilariales (Rhodophyta) reveals the invasive species Gracilaria vermiculophylla in British Columbia. Molecular Ecology Resources. 2009;9:140–150. doi: 10.1111/j.1755-0998.2009.02639.x. https://doi.org/10.1111/j.1755-0998.2009.02639.x. [DOI] [PubMed] [Google Scholar]
- Suratman S, Hussein ANAR, Latif MT, Weston K. Reassessment of physicochemical water quality in Setiu Wetland, Malaysia. Sains Malaysiana. 2014;43:1127–1131. [Google Scholar]
- Tucker CS, D’Abramo LR. Managing high pH in freshwater ponds. 2008. [Accessed on 20 January 2016]. http://www.ca.uky.edu/wkrec/High-pH-Ponds.pdf.
- Veeragurunathan V, Eswaran K, Malarvizhi J, Gobalakrishnan M. Cultivation of Gracilaria dura in the open sea along the southeast coast of India. Journal of Applied Phycology. 2014;26:1–13. [Google Scholar]
- Wong SC, Harah ZM, Sidik BJ, Arshad A. Comparison of seaweed communities of the two rocky shores in Sarawak, Malaysia. Coastal Marine Science. 2012;35:78–84. [Google Scholar]
- Zakaria MH, Bujang JS, Amit R, Awing SA, Ogawa H. Marine macrophytes: Macroalgae species and life forms from Golden Beach, Similanjau National Park, Bintulu, Sarawak, Malaysia. Coastal Marine Science. 2006;30:243–246. [Google Scholar]
- Zawawi MH, Idris MH, Kamal AH, Wong SK. Taxanomic assessment of seaweed community from the coastal areas of Bintulu, Sarawak, Malaysia. Songklanakarin Journal of Science & Technology. 2015;37:147–153. [Google Scholar]