Abstract
Rationale
Effective pharmacological treatments to prevent cocaine relapse remain elusive. In male rats, ceftriaxone attenuates the reinstatement of cocaine-seeking while increasing glutamate transporter-1 (GLT-1) and xCT expression in the nucleus accumbens core (NAc). Despite reported sex differences in cocaine relapse, these effects have not yet been confirmed in female rats.
Objective
We investigated the effects of ceftriaxone on cue-primed reinstatement and cocaine-induced alterations in glutamatergic proteins in the NAc of female rats. Potential interactions between estrous phase and treatment were also assessed.
Method
Male and female rats self-administered cocaine in the presence of discrete cues for 12 days, followed by 2–3 weeks of extinction. Ceftriaxone or vehicle was administered daily for a minimum of 6 days immediately preceding a cue-primed reinstatement test.
Results
Total cocaine intake was greater in females than in males, but reinstatement behavior was similar. Ceftriaxone attenuated reinstatement in both sexes and was accompanied by increased expression of GLT-1a and xCT in the NAc. However, ceftriaxone attenuated reinstatement only when females were tested during met-, di-, and proestrus phases and not during estrus. A significant increase in AMPA receptor subunit GluA1 surface expression was also observed during estrus, potentially influencing reinstatement.
Conclusion
These findings extend the beneficial effects of ceftriaxone on persistent cocaine-seeking from males to females, increasing its potential as a pharmacological treatment for preventing relapse. The effects of estrus on GluA1 expression and reinstatement observed here indicate that females may need additional interventions during some phases of the menstrual cycle.
Keywords: GLT-1, xCT, mGlu5, GluA1, GluA2
Introduction
The high rate of relapse associated with cocaine addiction remains a significant treatment challenge. To date, there are no pharmacological interventions to reduce the probability of relapse. The development of a pharmacological intervention to reduce drug-seeking during abstinence would significantly benefit millions of people suffering from addiction.
Persistent drug-seeking behavior is observed in rodents, and the operant self-administration extinction-reinstatement paradigm is often used to identify neurobiological changes associated with cocaine intake. Animals are trained to self-administer drug by performing an operant response that is reinforced by intravenous drug infusion. After days to weeks of self-administration, the drug-seeking response is extinguished and subsequently reinstated by one of the stimuli known to cause relapse in humans, such as stress, contextual and/or discrete drug-associated cues, or the drug itself (for review, see Epstein et al. 2006). Synaptic release of glutamate in the nucleus accumbens core (NAc) mediates the reinstatement of cocaine-seeking primed by drug-associated cues, context, and cocaine itself (LaCrosse et al. 2016; McFarland et al. 2003; Smith et al. 2017). Accordingly, antagonism of NAc glutamate receptors, such as AMPA (Cornish and Kalivas 2000; Xie et al. 2012) and mGlu5 (Wang et al. 2013) receptors, attenuates the reinstatement of cocaine-seeking.
Within the NAc, decreased basal levels of extrasynaptic glutamate occur 2–3 weeks following cessation of cocaine self-administration (Baker et al. 2003; Knackstedt et al. 2010b). Glutamate levels are regulated by the cystine-glutamate exchanger (system xC-), which exports intracellular glutamate in exchange for extracellular cystine in a 1:1 ratio. The catalytic subunit of system xC-, xCT, is downregulated after repeated cocaine use (Knackstedt et al. 2010a). Downregulation of the Na+ -dependent glial glutamate transporter (GLT-1) also occurs after repeated use of cocaine (Knackstedt et al. 2010a). GLT-1 is the main mechanism for the removal of synaptically released glutamate and is responsible for more than 90% of glutamate reuptake (Danbolt 2001). The cocaine-induced alterations in glutamate homeostasis in the NAc are restored to pre-cocaine levels by chronic (5–7 days) treatment with ceftriaxone, an FDA approved betalactam antibiotic (Knackstedt et al. 2010a). Ceftriaxone (100 or 200 mg/kg/day) attenuates cue- and cocaine-primed reinstatement of cocaine-seeking without affecting sucrose or chow consumption or locomotion (Fischer et al. 2013; Knackstedt et al. 2010a; Sari et al. 2009; Ward et al. 2011; Weiland et al. 2015).
Mechanistically, ceftriaxone has been shown to increase the expression and function of system xC- and GLT-1, resulting in normalized basal extrasynaptic glutamate levels and reduced glutamate overflow during the reinstatement of cocaine-seeking (Knackstedt et al. 2010b; Trantham-Davidson et al. 2012). Ceftriaxone has no effect on GLT-1 and xCT expression in drug-naïve rats (Knackstedt et al. 2010a). Ceftriaxone normalizes the increased total protein expression of the AMPA receptor subunit GluA1 that results from cocaine self-administration followed by abstinence without extinction training (LaCrosse et al. 2016), as well as the increased surface expression of GluA1 present 2–3 weeks after extinguished cocaine-seeking (Lacrosse et al. 2017). The ability of ceftriaxone to normalize GluA1 expression is dependent on the upregulation of xCT (Lacrosse et al. 2017).
Ceftriaxone’s ability to attenuate cocaine-seeking has only been tested in male rodents. While both men and women are vulnerable to cocaine addiction, there are sex differences in rate of progression and relapse and subjective response to cocaine (reviewed by Becker 2016). Although more cocaine addicts are male, females have greater cocaine cravings (Back et al. 2005) and show a faster progression from casual use to addiction and treatment seeking (McCance-Katz et al. 1999). Women also have shorter cocaine-free periods (Griffin et al. 1989) and an increased susceptibility to relapse following events of stress or depression (Back et al. 2005; Elman et al. 2001; Fattore et al. 2008). Thus, the neurobiology underlying cocaine relapse may differ in males and females. Female rats in estrus show greater responding during cocaine-primed reinstatement compared to non-estrus females (Kippin et al. 2005; Feltenstein et al. 2009).
In the absence of any literature indicating sex differences in glutamate adaptations following cocaine, we hypothesized that ceftriaxone would attenuate the reinstatement of cocaine-seeking in female rats. However, we monitored the estrous cycle to assess any interacting effects with ceftriaxone treatment. The current study is the first to assess cocaine-induced changes in NAc glutamatergic proteins, which have previously been demonstrated to regulate the reinstatement of cocaine-seeking, in female rats. Furthermore, we assess the ability of ceftriaxone to normalize the expression of these proteins in different phases of the estrous cycle.
Methods and materials
Animals
Male and female Sprague-Dawley rats (325–375 and 225–275 g at surgery, respectively, Charles River, Laboratories, Raleigh, NC, USA) were used in this study. Animals were individually housed in ventilated cages in a temperature- and humidity-controlled vivarium and maintained on a 12:12-h light/dark cycle (lights on at 7am). Animals were allowed 1 week of habituation to the vivarium with ad libitum access to standard lab chow and water. Following this week, animals underwent surgery for the implantation of jugular catheters and were subsequently fed a restricted diet of 15–20 g and weighed daily to ensure the maintenance of body weight.
Surgery
Rats were anesthetized with ketamine HCl (males 87.5 mg/kg, IP; females 60 mg/kg, IP) and xylazine (males 5 mg/kg, IP; females 5 mg/kg, IP) in preparation for surgical implantation of jugular catheters (SILASTIC tubing, 0.51-mm inner diameter, 0.94 outer diameter, Dow Corning, Midland, MI, USA). The catheter exited the skin between the shoulder blades and attached to a stainless-steel cannula, which was held in place by a harness (Instech, Plymouth Meeting, PA, USA). Ketorolac (3 mg/kg, IP) was administered prior to surgery to provide analgesia, and for 2 days post-surgery, along with the antibiotic cefazolin (100 mg/mL, IV). Rats were allowed to recover for 5 days before the start of the experiment. During this time, and throughout the self-administration period, their catheters were flushed daily with 0.2 mL of heparinized saline (100 U/mL, Elkins-Sinn, Cherry Hill, NJ, USA). Catheter patency was verified periodically by intravenous administration of methohexital sodium (10 mg/mL, IV), which produces a temporary loss of muscle tone.
Food-training, cocaine self-administration, extinction, and cue-primed reinstatement procedures
To assist in the acquisition of cocaine self-administration, food-deprived rats were subjected to a 15 h food-training paradigm. Animals were placed in an operant conditioning chamber (30 × 24 × 30 cm; Med Associates, St. Albans, VT, USA) equipped with two retractable levers. Responses on the active lever delivered a food pellet (0.45 g; LabDiet, St. Louis, MO, USA) into a trough. Responses on the inactive lever were recorded, but yielded no reinforcement. The schedule of reinforcement was a fixed ratio-1 (FR-1). Food training prior to cocaine self-administration does not alter reinstatement levels relative to rats not trained to press the lever with food (Bongiovanni and See 2008). Cocaine self-administration commenced 24 h after the conclusion of food training and occurred daily between 10am and 4pm thereafter. Upon pressing the active lever, rats received an infusion (approximately 0.5 mg/kg/infusion in 0.05 uL) of cocaine-HCL (NIDA Controlled Substances Program, Research Triangle Institute, NC, USA) at a concentration adjusted for males (0.177 mg/infusion) and females (0.141 mg/infusion). Cocaine infusions were paired with presentation of a stimulus light over the active lever and tone (2900 Hz). Rats self-administered on an FR-1 schedule with a 20-s time out following each infusion during which responses were recorded but not reinforced. Daily sessions were 2 h in duration and continued until rats met the criteria of 10 or more infusions for 12 days. Subsequently, animals entered the extinction phase of the experiment, conducted in the same chamber as drug-taking occurred, in which responses were not reinforced with drug or cues. Extinction training lasted 2 h daily for 2–3 weeks and continued until responses on the active lever were less than 20 for at least 2 days in a row. During the final week of extinction, animals were treated with a daily post-session injection of ceftriaxone (200 mg/kg, IP) or vehicle (0.9% physiological saline, 0.3 mL, IP). Rats received a minimum of 6 days of injections. After meeting extinction criterion, animals were returned to the chamber for a 2-h test of cue-primed reinstatement. During the cue test, presses on the active lever once again resulted in the presentation of the previously associated discrete drug-cues (light, tone, and infusion pump), although no infusion was delivered. The number of presses on the active lever was used as the dependent measure of cocaine-seeking. Ceftriaxone and vehicle treatment continued until animals were sacrificed 24–72 h after the reinstatement test. Rats were rapidly decapitated and the NAc harvested for biotinylation and subsequent protein analyses as described below.
Estrous cycle monitoring
During the final week of extinction and prior to any pharmacological treatment, we began the daily monitoring of estrous cycle. This procedure and subsequent assessment of vaginal cytology have been carefully described previously (e.g., Marcondes et al. 2002; Lebron-Milad and Milad 2012; McLean et al. 2012). Briefly, using a vaginal lavage technique, we gently administered a saline (0.9%) flush with a disposable pipette positioned at the opening of the vagina and then expelled the vaginal lumen sample onto a microscope slide. The estimation of estrous phase was conducted immediately after sampling by visual observation of the proportion of cell type within the sample using a light microscope at ×10 magnification. Proestrus was indicated by a majority of nucleated epithelial cells, estrus was indicated by a majority of cornified squamous epithelial cells, diestrus was indicated by a majority of leukocytes, and metestrus by an equal proportion of each of these cell types. Phases were later confirmed following the staining of samples with cresyl violet. Sampling occurred approximately 20–30 min prior to the test session, and no signs of stress or changes in female behavior were observed. However, to control for unobservable effects of handling, males were restrained at a similar position and frequency. Only one female was tested for reinstatement during diestrus and since hormone levels between diestrus and metestrus are similar (Lebron-Milad and Milad 2012; McLean et al. 2012), for analysis, we combined this one female’s data with those in the metestrus group.
Surface protein biotinylation and Western blotting
We conducted biotinylation of surface proteins as reported previously (Knackstedt et al. 2010b). Rats were sacrificed via rapid decapitation and the core of the NA was dissected on ice from 2-mm coronal sections using a 2-mm micropuncher. Tissue was sliced into 200-μM prism-shaped slices with a McIllwain tissue chopper (Ted Pella), incubated in Sulfo-NHS-SS-Biotin (1 mg/mL; Pierce), and the reaction quenched by glycine. A portion of the sample lysate was incubated with streptavidin agarose beads (Thermo Fisher Scientific). The remainder of the sample was stored as the total protein fraction. Biotinylated proteins were captured by streptavidin-coated beads, separated by centrifugation, and subsequently eluted with Laemmli buffer. The amount of GluA1 and GluA2 protein in the total and biotinylation fractions was analyzed by quantitative Western blotting as described below. GLT-1a, xCT and mGlu5 were blotted only in the total protein fraction due to limited amount of the biotinylated fraction permitting only two blots to be conducted on this tissue. We utilized an antibody that recognizes the GLT-1a splice variant, as this is the predominant isoform expressed in the rodent forebrain (see Rimmele and Rosenberg 2016) and was previously shown to be upregulated by ceftriaxone and decreased by cocaine (Rothstein et al. 2005; LaCrosse et al. 2016, 2017; Rothstein et al. 2005).
Proteins were separated using 10% SDS-PAGE and transferred to PVDF membrane. The membranes were probed overnight at 4 °C with primary antibodies diluted in 5% milk/Tris-buffered saline with 0.1% Tween-20. After incubation with HRP-conjugated secondary antiserum (Jackson Immuno; 1:10,000–1:50,000), immunoreactive bands on the membranes were detected by enhanced chemiluminescence (ECL Plus; GE Healthcare Bio-Sciences). Band density was measured using NIH ImageJ software. For total protein expression, blots were re-probed with calnexin as a loading control (Johnson 2012). Antibodies and dilutions are presented in Table 1.
Table 1.
Antibodies and dilutions used in Western blotting
| Antibody | Company | Catalog number | Lot number | Concentration |
|---|---|---|---|---|
| Rabbit anti-Calnexin | Millipore | AB2301 | 2587261 | 1:40,000 |
| Rabbit anti-xCT | Novus | NB300-318 | I-01 | 1:5000 |
| Mouse anti-GluA1 | Millipore | MAB2263 | 2428724 | 1:2000 |
| Rabbit anti-GluA2 | Abcam | ab20673 | GR117410-1 | 1:2000 |
| Rabbit anti-GLT1a | Paul Rosenberg, Harvard | 1:80,000 | ||
| Rabbit anti beta-tubulin | Abcam | ab6046 | GR251141-1 | 1:80,000 |
| Rabbit anti-mGlu5 | Millipore | AB5675 | 2279534 | 1:5000 |
Statistical analyses
SPSS (IBM, Amorak, NY) was used to analyze behavioral data. Comparisons of the dependent measures of cocaine intake (infusion number, mg/kg) or lever pressing (active, inactive) were conducted using Repeated Measures (RM) ANOVAs, with Day as the within-subjects factor, and Treatment (ceftriaxone or vehicle) and Sex as between-subjects factors. Reinstatement of cocaine-seeking induced by the drug-associated cues (i.e., cue-primed reinstatement test) was defined as significantly greater active lever presses compared to the final day of extinction, and so a RM ANOVA with Test day (extinction vs. cue test) was used for all reinstatement analyses. In order to explore potential influences of estrous cycle on the effectiveness of ceftriaxone treatment, this analysis was also done exclusively on female reinstatement data with Test as a within-subjects variable, and Phase and Treatment as between-subject variables. For all RM analyses, Mauchly’s test of sphericity was used to assess homogeneity of the data, and when violated, the Greenhouse-Geisser corrected degrees of freedom are provided. The effects of cocaine use and ceftriaxone on regularity of the female estrous cycle were assessed separately using a one-sided Chi-square test. For all analyses, statistical significance was set at p = 0.05 and data presented are mean ± SEM.
Protein data were analyzed using GraphPad Prism (GraphPad Software, Inc., San Diego, CA, USA). Immunoblotting data from the cocaine groups, represented by integrated density of individual protein bands, were normalized to samples from cocaine-naïve “Control” rats that were run on the same gel in order to control for differences in transfer and antibody coverage between gels. Independent samples t tests were used to test for estrous cycle effects on protein expression in female Control rats. Upon finding no such effect for all proteins, estrus and non-estrus Control rat data were pooled into a single Control group. Immunoblotting data were analyzed using one-way ANOVAs to compare each cocaine group to this cocaine-naïve Control group. For female rats, the same values (percent control) were also analyzed (in the absence of direct comparisons to the cocaine-naïve control group) using 2-way Treatment × Phase ANOVAs to test for main effects of estrous cycle phase or ceftriaxone/vehicle treatment. Controls were not included in these 2-way ANOVAs because the lack of estrous cycle effect on protein expression would obscure any effects detected in cocaine rats. Furthermore, we have previously reported that ceftriaxone does not change either xCT or GLT-1 protein expression in cocaine-naive rats (Knackstedt et al. 2010a) and thus did not include such groups here. We utilized Tukey’s multiple comparisons test for post-hoc analyses. For both sexes, Pearson’s correlations were used to assess the relationship between total cocaine intake (mg/kg) and raw xCT and GLT-1a expression. As ceftriaxone increases the expression of these proteins following cocaine (Knackstedt et al. 2010a), correlation analyses excluded rats treated with ceftriaxone.
Results
Ceftriaxone attenuated cue-primed reinstatement in males and females, dependent on estrous phase
Twenty-eight male and 40 female rats were implanted with jugular catheters and trained using the methods and timeline shown in Fig. 1a. Throughout self-administration, rats were eliminated for catheter failures (n = 4 male and 5 female), failing to meet self-administration criterion (n = 2 male and 2 female), and pressing the inactive lever continuously throughout the experiment, demonstrating a failure to appropriately learn the task (n = 2 male and 1 female). In the end, n = 20 male (9 Coc-Cef, 11 Coc-Veh) and n = 32 female (17 Coc-Cef, 15 Coc-Veh) rats were used in the analyses depicted in Figs. 1 and 2. While no Sex × Day interaction was detected for number of cocaine infusions (Fig. 1b), a significant main effect of Day was found, as both sexes escalated cocaine intake [F(5.258) = 14.9, p < 0.001]. There was again no Sex × Day interaction when intake was calculated in mg/kg (Fig. 1c), although significant main effects of Sex [F(1,50) = 6.5, p = 0.013] and Day [F(11.550) = 16.1, p < 0.001] were detected. Whereas, no effects of Sex or Day on active lever pressing were observed (Fig. 1d), the number of presses on the inactive lever decreased across days [Day: F(3.128) = 2.8, p = 0.047; Fig. 1e] indicating rats learned to discriminate between the two levers.
Fig. 1.
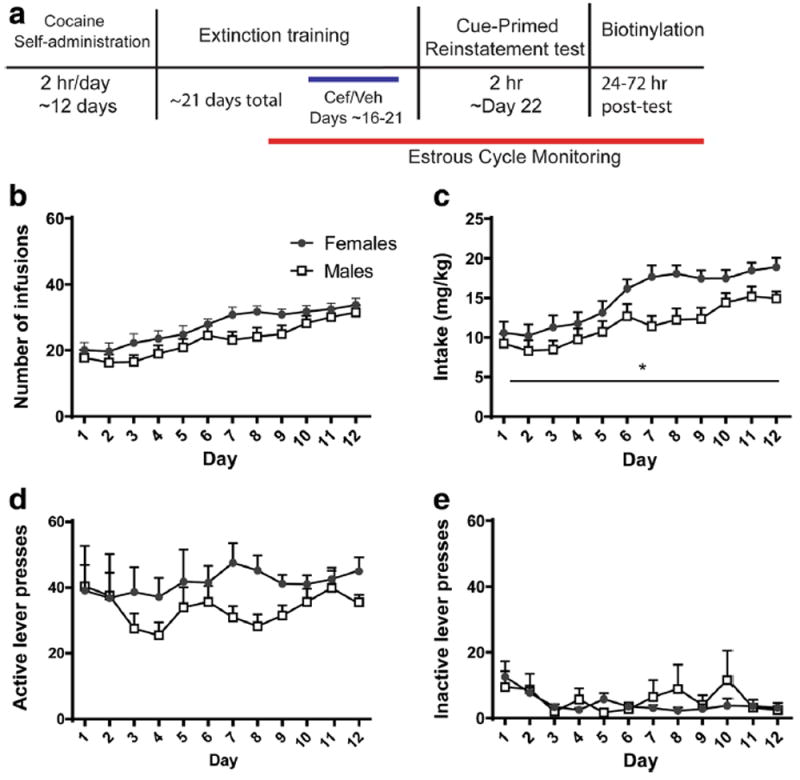
a Shows general experimental methods and timeline. The behavior of males and females during the 12 days of self-administration of cocaine showed b a significant escalation in the number of infusions occurred across days. c The mg/kg intake of cocaine escalated across days and revealed that female intake was significantly greater than males. d There were no differences in the number of active lever presses. e A significant decrease in inactive lever presses occurred across the days. Closed circles denote females and open squares denote males. *p < 0.05 for males compared to females
Fig. 2.
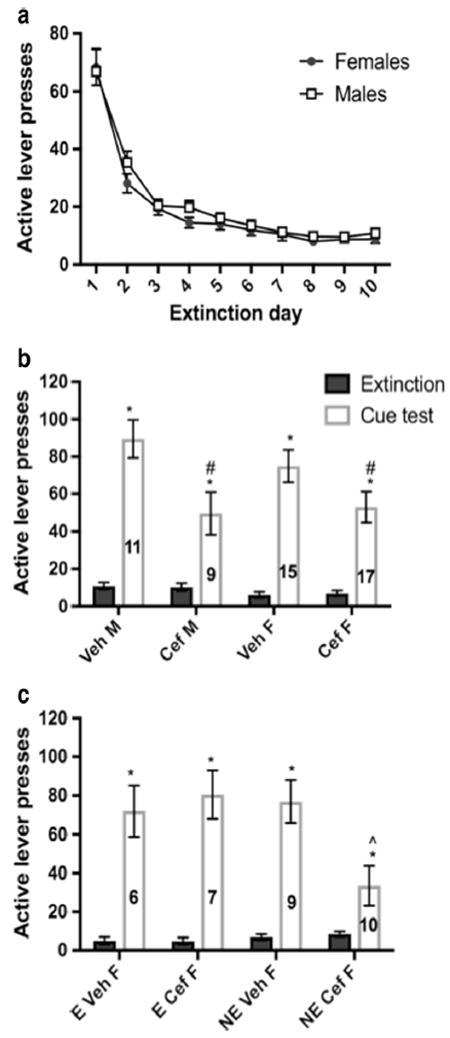
Extinction training and cue-primed reinstatement of cocaine-seeking. a There was a significant decrease in the number of active lever presses across the first 10 days of extinction training. b All groups significantly reinstated; however, compared to vehicle-treated animals, ceftriaxone significantly attenuated lever pressing in both males and females. c All females significantly reinstated cocaine-seeking, although a benefit of ceftriaxone was found only for females tested in non-estrus (NE), and not females in estrus (E), compared to Veh. *p < 0.001 comparing Extinction to Cue test. In panel a, closed circles denote females and open squares denote males. In panels b and c, closed bars denote the last day of extinction training and open bars denote the cue-primed reinstatement test. # = p < 0.01 comparing Cef to Veh. ˆ = p<0.01 comparing non-estrus Cef to Veh
After meeting self-administration criterion of 10 or more infusions for 12 days, rats entered a 2–3 week period of extinction training. The extinction of active lever pressing across the first 10 days was affected only by Day [F(2.99) = 97.7, p < 0.001, Fig. 2a] and not Sex. Males and females were then split into treatment groups (ceftriaxone or vehicle) and matched for number of infusions and active lever presses during self-administration and extinction periods. During the final week of extinction training, ceftriaxone or vehicle injections were administered and estrous cyclicity was monitored.
After 6–10 days of treatment and monitoring, animals were assessed for cocaine-seeking during a 2h cue-primed reinstatement test. The range of treatment days was to service the targeting of estrous phases to ensure there were enough female subjects in each phase and treatment group on reinstatement test day. Reinstatement to cocaine-seeking was defined as a significant increase in active lever pressing compared to the final day of extinction and thus was analyzed using RM ANOVAs. Although all rats reinstated cocaine-seeking during the cue test [Test: F(1.48) = 135.1, p < 0.001], active lever pressing following treatment with ceftriaxone was significantly attenuated compared to vehicle for both males and females, evidenced by a significant main effect of Treatment [F(1.48) = 10.1, p = 0.003; Fig. 2b]. No effect of Sex on reinstatement was detected [F(1.48) = 0.9, p = 0.32]. Due to a previous literature showing cocaine-seeking differences during estrus compared to other phases (e.g., Kippin et al. 2005; Feltenstein et al. 2011), we next considered all phases of the estrous cycle in the analyses. Whereas no effect of Phase was found for reinstatement of females treated with vehicle (p > 0.05; Estrus n = 6, mean = 72 ± 22; Metestrus n = 4, mean = 47 ± 8; Proestrus n = 5, mean = 84 ± 13), there was a main effect of Phase [F(1.14) = 8.3, p = 0.004] in ceftriaxone females due to estrus females (n = 7; mean = 80 ± 12) differing from metestrus (p = 0.008; n = 7, mean = 35 ± 5) and proestrus (p = 0.021, n = 3, mean = 29 ± 8) females, which did not differ from each other (p = 0.92). Because we detected no difference in responding between metestrus and proestrus, we combined these conditions into a “non-estrus” condition. Conducting a Treatment × Phase ANOVA on these data (Fig. 2c), we found that regardless of estrous phase, females reinstated during the cue test compared to the final day of extinction [main effect of Test: F(1.28) = 103.5, p < 0.001], but a significant Treatment × Phase interaction was detected [F(1.28) = 4.2, p = 0.049; Fig. 2c]. Post-hoc analyses revealed that ceftriaxone significantly attenuated lever pressing for females tested in nonestrus relative to their vehicle-treated counterparts [F(1.17) = 17.2, p = 0.001]. Rats in estrus showed no such attenuation (p > 0.05).
Glutamatergic alterations in the NAc in males and females and effects of estrous cycle and ceftriaxone
As we detected an effect of estrous cycle on the ability of ceftriaxone to attenuate cue-primed reinstatement, we next assessed the role of glutamatergic protein alterations in the NAc in mediating such behavioral effects. Two cohorts of female rats were utilized to generate the data in Figs. 1 and 2; in the first cohort, we had not yet detected an effect of estrous cycle and thus cytology was not assessed on the day of biotinylation. Thus, in order to explore the neurobiology underlying the behavioral interaction of estrus and ceftriaxone, we trained an additional 15 females in the self-administration and extinction procedures described above (see Fig. 1a) and targeted their estrous phases on the day of sacrifice to meet sufficient group sizes for protein analyses (as opposed to testing rats for reinstatement once they met extinction criteria). During self-administration, two rats were eliminated due to failure to meet cocaine intake criterion. The remaining 13 females did not undergo reinstatement testing so as to make the targeting of es-trous phase at the time of sacrifice feasible. As they were not tested for reinstatement, we did not include their data in the above behavioral analyses (Figs. 1 and 2). However, to ensure our treatment groups were behaviorally matched before we compared protein levels, we confirmed that rats in the conditions compared with Western blotting (Cef, Veh, Estrus, Non-estrus) did not differ in cocaine self-administration (p’s > 0.05). We also utilized independent samples t tests to ensure that within each treatment/estrous group, the conduction of a reinstatement test did not alter protein expression. In no such case did we observe an effect (all p > 0.05), in line with our previous report in a larger cohort of rats (n = 18– 19) that had received ceftriaxone/vehicle and were or were not tested for cue-primed reinstatement (Knackstedt et al. 2010a). However, we acknowledge that it is possible that the reinstatement test affected expression in a manner that was unable to be detected here due to low power for such comparisons. In addition to cocaine rats, we also used 7 male and 14 female Control rats for Western blotting. Controls were similarly food deprived, injected with ketamine and xylazine, handled, and weighed daily, and females were monitored for estrous cyclicity. The final number of rats used for protein analyses was the following: n = 7 Control males, n = 16 Coc males (7 Coc-Cef, 9 Coc-Veh), n = 14 Control females (7 estrus, 7 non-estrus), n = 15 Coc-Cef females (8 Coc-Estrus-Cef, 7 Coc-Nonestrus-Cef), and n = 16 Coc-Veh females (8 Coc-Estrus-Veh, 8 Coc-Nonestrus-Veh).
Total protein expression of xCT, GLT-1a, and mGlu5 in males and females
A main effect of Group was detected for xCT expression in male rats (F(2.12) = 3.6, p = 0.05; Fig. 3a), and Coc-Veh displayed significantly reduced xCT relative to Coc-Cef rats. In females, significant main effects of Phase (F(1.27) = 6.0, p = 0.020) and Treatment (F(1.27) = 35.4, p < 0.0001) were detected for expression of xCT (Fig. 3b). A one-way ANOVA revealed that total protein expression of xCT differed by Group (F(4.40) = 14.8, p < 0.0001). Post-hoc tests revealed that both Coc-Estrus-Veh and Coc-Nonestrus-Veh groups displayed significantly reduced xCT compared to Controls. The Coc-Estrus-Cef females had significantly greater levels than both vehicle groups, and the Coc-Nonestrus-Cef females had more xCT than Cocaine-Nonestrus-Veh females.
Fig. 3.
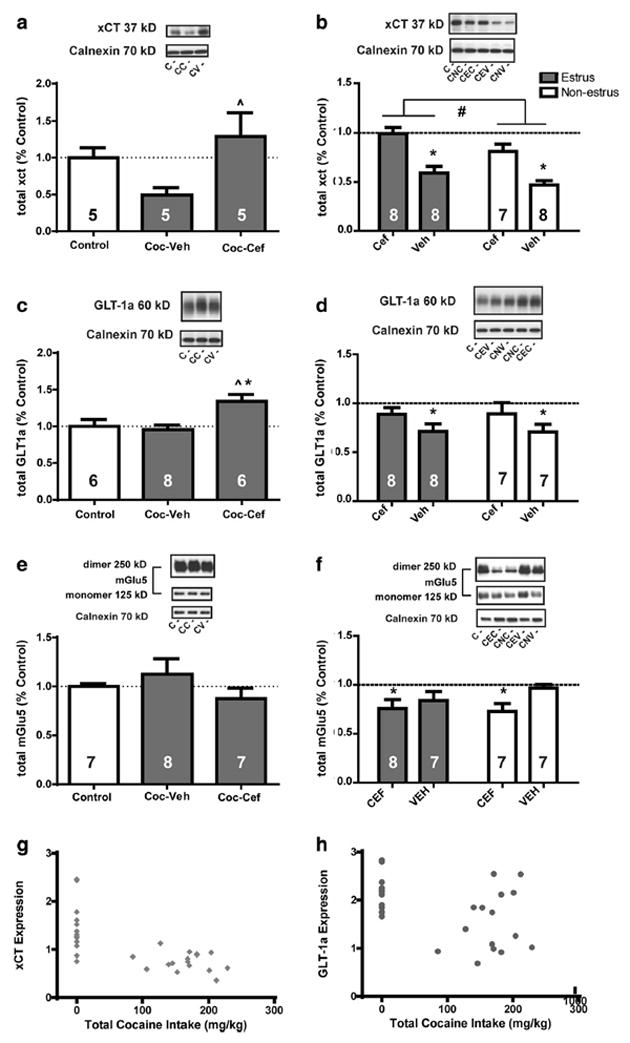
Total protein expression levels of xCT, GLT-1a, and mGlu5 in the NAc of males (left side panels: a, c, e) and females (right side panels: b, d, f) expressed as percent of the Control group. a In males, total protein expression of xCT was decreased by cocaine (Coc) and restored by cef-triaxone (Cef). b In females, total protein expression of xCT was significantly affected by both Phase and Treatment. Total levels of xCT were reduced by Coc and restored by Cef, and females in estrus had more xCT than females in non-estrus. c Total protein expression of GLT-1a is increased in males by treatment with ceftriaxone relative to both controls and vehicle-treated males. d A significant reduction of GLT-1a was found for Coc-Veh females, independent of estrous phase. e There was no difference in male total mGlu5 expression due to ceftriaxone. f In females, a significant reduction of total mGlu5 expression was found due to treatment with ceftriaxone. A significant negative relationship was found in females for total amount of cocaine intake (mg/kg) and total expression levels of g xCT and h GLT-1a. In panels a, c, and e, the open bars denote the control group levels and the closed bars denote cocaine groups. In panels b, d, and f, the open bars denote non-estrus and the closed bars denote estrus. Panel b Controls n = 14, mean = 1.0 ± 0.05; Panel d Controls n = 13, mean = 1.0 ± 0.03; Panel f Controls n = 13, mean = 10.0 ± 0.05. ˆ = p < 0.05 compared to Coc-Veh; # = p < 0.05 for estrus compared to non-estrus; * = p < 0.05 compared to Controls
A significant main effect of Group was detected for GLT-1a expression in male rats (F(2.17) = 6.4, p = 0.008, Fig. 3c). Post-hoc tests revealed that the Coc-Cef group displayed significantly increased expression relative to both Control and Coc-Veh groups. In females, a significant main effect of Treatment was detected for GLT-1a expression (F(1.26) = 4.34, p = 0.047; Fig. 3d). A one-way ANOVA detected Group differences (F(4.38) = 3.64, p = 0.013) and post-hocs revealed that both Coc-Estrus-Veh and Coc-Nonestrus-Veh groups displayed significantly reduced GLT-1a compared to Controls. Pearson correlations revealed a significant negative relationship between amount of cocaine intake (mg/kg) and total expression of xCT [r(28) = − 0.655, p < 0.0001; Fig. 3g] and GLT-1a [r(26) = − 0.437, p = 0.02; Fig. 3h] for females, but no such relationship was detected for either protein in male rats.
Expression of mGlu5 in male rats was unaffected by ceftriaxone and cocaine (Fig. 3e). A significant main effect of Treatment was found for total protein expression of mGlu5 in females (F(1.26) = 3.9, p = 0.05; Fig. 3f). There was no effect of Phase and no Treatment × Phase interaction. When comparing expression to Controls, a one-way ANOVA found a significant main effect (F(4,37) = 3.2, p = 0.022) but post-hoc tests revealed no further significant differences between groups.
Surface expression of GluA1 and GluA2 in female rats
There was a main effect of Phase on surface GluA1 (F(1.22) = 6.7, p = 0.016; Fig. 4a). There was no effect of Treatment and no Treatment × Phase interaction. A one-way ANOVA found that surface GluA1 differed by Group (F(4.30) = 2.7, p = 0.048) and only the Coc-Estrus-Cef group differed from Controls (p < 0.05). There were no main effects of Phase or Treatment on total protein expression of GluA1 (not shown). There were no main effects of Treatment or Phase on surface GluA2 expression (Fig. 4b). Similarly, there were no effects of Treatment or Phase on total expression of GluA2 (not shown).
Fig. 4.
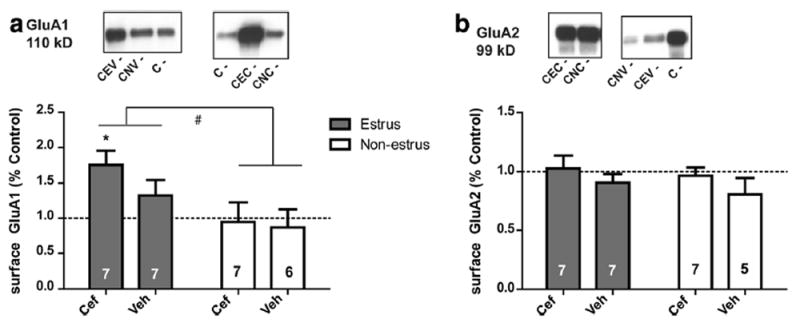
Surface protein expression levels of GluA1 and GluA2 in the NAc of females. Protein expression was expressed as percent of the Control group. a Surface expression levels of GluA1 were significantly increased for females in estrus. b Surface expression levels of GluA2 did not differ due to Phase or Treatment. Closed bars denote females in estrus and open bars denote females in non-estrus phases. Panel a Controls n = 9, mean = 1.0 ± 0.15; Panel b Controls n = 13, mean = 1.0 ± 0.07. # = p < 0.05 for estrus compared to non-estrus
The effects of cocaine and ceftriaxone on estrous cyclicity of female rats
The mean duration for the rat estrous cycle is 4 days, during which normally cycling females will experience each of the four phases in the sequential order of proestrus, estrus, metestrus, and diestrus (Marcondes et al. 2002). Vaginal cytology as described in the methods section above was used to identify the phase of estrous immediately prior to testing, and samples were stained with cresyl violet for confirmation of phase. Using at least one continuous 5-day monitoring period (i.e., Mon–Fri), regularity of the estrous cycle was defined as in (Marcondes et al. 2002) and identified as irregular if one phase lasted longer than 48 h or if the female did not cycle sequentially through each phase. All females that had a complete 5-day period of monitoring were used to assess the effects of cocaine use (n = 43, 31 Coc and 12 Control) and ceftriaxone (n = 20 Coc-Veh vs. n = 11 Coc-Cef) on the regularity of the female estrous cycle. For analysis of ceftriaxone effects on regularity, we compared only females that administered cocaine. Females were abstinent for approximately 2 weeks, when we began to monitor estrous cycles. Treatment with ceftriaxone began at least 1 day after the start of estrous monitoring, and continued for a minimum of 6 days, such that it was given at least once across all estrous phases. We found 0 of the 12 Control and 8 (5 veh, 3 cef) of the 31 Coc (25%) rats cycled irregularly. A one-sided Chi-square test revealed an effect of cocaine use (z = 1.9, p = 0.025) but not ceftriaxone (z = 0.13, p = 0.44) on estrous cycle regularity.
Discussion
Here, we are first to report that ceftriaxone attenuates cue-primed reinstatement of cocaine-seeking in females as it does in males. Compared to males, females self-administered more cocaine (mg/kg), an effect that was not unexpected based on the human and animal literature. Female cocaine addicts escalate their cocaine use faster than men (McCance-Katz et al. 1999), and female rats perform more active lever presses during self-administration indicating increased motivation for cocaine-seeking (e.g., Fuchs et al. 2005; Kippin et al. 2005; Kerstetter et al. 2008). Despite greater cocaine intake, vehicle-treated females reinstated cocaine-seeking at a similar degree as vehicle-treated males. This finding is supported by previous work utilizing the same dose of self-administered cocaine (0.5 mg/kg/infusion; Kerstetter et al. 2008; Feltenstein et al. 2011; Doyle et al. 2014). However, when estrous cycle was considered, ceftriaxone only attenuated reinstatement in female rats that were not in estrus at the time of testing.
To understand the inability of ceftriaxone to attenuate reinstatement during estrus, we assessed glutamatergic protein expression. Similar to previous studies using males (e.g., Knackstedt et al. 2010a; LaCrosse et al. 2016; Sari et al. 2009), we found that ceftriaxone restored cocaine-induced decreases in total expression of xCT and upregulated GLT-1a in males. In females, cocaine produced decreases in both xCT and GLT-1a expression that were restored by ceftriaxone. However, expression of xCT was affected by estrous phase, such that it was increased during estrus. Thus, estrus-induced changes in xCT expression do not underlie the inability of ceftriaxone to attenuate reinstatement during this phase. Although estrous phase was not a factor in total GLT-1a expression, the pattern of GLT-1a expression differed for males and females in response to ceftriaxone. Treatment with ceftriaxone in males increased levels of GLT-1a compared to controls while vehicle-treated cocaine males remained similar to controls. In females, ceftriaxone and control groups displayed the same level of GLT-1a expression, and the vehicle-treated cocaine females showed decreased levels (Fig. 3 b, c). While we have consistently found cocaine-induced decreases in GLT-1 and xCT expression in male rats when blotting in a membrane fraction (Knackstedt et al. 2010a; LaCrosse et al. 2016; Sondheimer and Knackstedt 2011), we recently reported that in the total protein fraction, xCT and GLT-1a are only modestly decreased (Lacrosse et al. 2017). While consistent with this previous report, we acknowledge that the lack of a significant reduction in xCT expression in males in the present work may also stem from insufficient sample size. Thus, membrane trafficking of both xCT and GLT-1a are likely affected by cocaine. A second contributing factor to the absence of decreased GLT-1a and xCT expression here may be the lower dose of cocaine self-administered relative to the higher dose used in our previous work (0.5 mg/kg/infusion vs. 1.0 mg/kg/infusion). Further supporting the role of dose of cocaine in mediating changes in GLT-1a, total cocaine intake (mg/kg) in females was negatively correlated with the expression of GLT-1a and xCT. No such relationship was found in males, possibly due to the smaller sample size and/or range of total cocaine intake.
Total mGlu5 expression did not differ across male groups. We have previously demonstrated that cocaine self-administration followed by extinction training resulted in decreased surface expression of mGlu5 but no change in total protein expression, consistent with the present results (Knackstedt et al. 2010b). Here, we were interested in investigating mGlu5 expression in female rats not only because of our previous work, but also because mGlu5 in the NAc has been shown to be regulated by estradiol. Activation of mGlu5 has been implicated in estradiol-mediated facilitation of cocaine self-administration in ovariectomized females, an effect prevented by an mGlu5 antagonist (MPEP) (Martinez et al. 2016). However, without estradiol treatment, mGlu5 potentiation using a positive allosteric modulator produces no effects (Martinez et al. 2016). Ceftriaxone reduced levels of total mGlu5 expression in females, independent of estrous phase. While surface expression of mGlu5 remains to be evaluated, this reduction is likely functionally relevant because when mGlu5 is trafficked out of the membrane, it is most often reinserted into the membrane and not degraded (Trivedi and Bhattacharyya 2012). Interestingly, mGlu5 was reduced by ceftriaxone even during estrus, indicating that this reduction is not responsible for ceftriaxone’s ability to attenuate reinstatement of cocaine-seeking in female rats.
We have previously demonstrated that following extinguished cocaine-seeking, male rats display increased surface GluA1 in the NAc in the absence of changes in surface GluA2 expression or total protein expression of either protein (Lacrosse et al. 2017). This pattern of expression is characteristic of calcium-permeable (CP)-AMPA receptors (Liu and Zukin 2007), which have a higher conductance and are proposed to partially underlie the increased reactivity of NAc neurons to cocaine-associated cues (Conrad et al. 2008). These receptors have been mechanistically linked to the reinstatement of cocaine-seeking as the CP-AMPA receptor antagonist Naspm attenuates relapse following both abstinence and extinction from cocaine self-administration (White et al. 2016; Conrad et al. 2008). We have also previously demonstrated that ceftriaxone normalizes surface GluA1 expression following extinguished cocaine-seeking in male rats and genetic manipulations which prevent ceftriaxone from normalizing GluA1 expression result in a loss of the ability of ceftri-axone to attenuate reinstatement (Lacrosse et al. 2017). Surface expression of GluA1 increased during estrus compared to non-estrus, and ceftriaxone-treated rats in estrus at the time of biotinylation displayed increased surface GluA1, potentially explaining the failure of ceftriaxone to attenuate reinstatement. We are not the first to report dynamic hormone-induced changes in the NAc in female rats; estradiol decreases dendritic spine density in the NAc (Peterson et al. 2015). Thus, in rats with a history of cocaine self-administration, estrus influences both total xCT expression as well as the trafficking of the GluA1 subunit to the surface, but has no effect on total GLT-1a, GluA1, GluA2, or mGlu5 expression.
The translation of the rodent estrous cycle to female menstrual cycles is somewhat challenging due to differences in length (4 vs. 28 days) and hormonal flux across phase. In general, the beginning of rodent metestrus (low estrogen and progesterone) corresponds to the beginning of the post-ovulation luteal phase in women (Lebron-Milad and Milad 2012; McLean et al. 2012). Decreases of peak estrogen levels during the transition from pre-ovulation follicular phase to post-ovulation luteal phase in women are similar to those of female rats transitioning from the proestrus to post-ovulation estrus phase. However, whereas the progesterone surge in women occurs post-ovulation, in female rats, it closely follows the estrogen surge that precedes ovulation (Lebron-Milad and Milad 2012; McLean et al. 2012). Thus, the approximately 12–18-h estrus phase in rats is marked by decreasing levels of both estradiol and progesterone. While more work is needed to mechanistically link hormone levels to the results observed here, the present results indicate that the decrease in estradiol and progesterone during estrus may trigger increases in GluA1 surface expression, which then enhance cocaine-seeking. Interestingly, effects of estrus phase were not observed in Control rats that were not treated with ceftriaxone or cocaine, indicating an interaction between these manipulations that alter glutamate homeostasis and estrus. While a higher dose of ceftriaxone could also be tested, it should be noted that the dose utilized here is the highest dose that has been tested in rats and increasing the dose may not be translatable to the clinic.
Finally, we found that 2 weeks after cessation of drug use, a history of cocaine intake affected estrous cycle regularity. We did not find an effect of ceftriaxone on estrous cyclicity. Although anecdotal human evidence is common, only one clinical study reported irregular cycling due to chronic cocaine use, and this was comorbid with heroin use (Schmittner et al. 2005). Several laboratory studies found irregularity in cycling due to cocaine administration and in a similar proportion to the data we present here [e.g., monkeys: (Potter et al. 1998, 1999); rats: (Fuchs et al. 2005; Kippin et al. 2005)].
In conclusion, we have shown that ceftriaxone attenuates cue-primed cocaine reinstatement in males and females, an effect associated with increased expression of xCT and GLT-1a in the NAc. In addition, we demonstrated that surface GluA1 expression is upregulated during estrus, providing a mechanism by which ceftriaxone’s ability to attenuate cocaine relapse is prevented during this phase. The current findings uphold previous work and extend the beneficial effects of ceftriaxone on persistent cocaine-seeking from males to females, increasing its potential as a pharmacological treatment for preventing relapse. However, females may need additional prophylactic treatment during certain phases of the menstrual cycle.
Acknowledgments
The authors would like to thank Lizhen Wu for her excellent technical assistance on this project.
Funding information This work was funded by National Institutes of Health grants DA033436 and DA037270 awarded to L.A.K.
Footnotes
Compliance with ethical standards All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Florida and performed in accordance with the Guide for the Care and Use of Laboratory Animals.
Conflict of interest The authors declare that they have no competing interests.
References
- Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology. 2005;180(1):169–176. doi: 10.1007/s00213-004-2129-7. https://doi.org/10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6(7):743–749. doi: 10.1038/nn1069. https://doi.org/10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Becker JB. Sex differences in addiction. Dialogues Clin Neurosci. 2016;18(4):395–402. doi: 10.31887/DCNS.2016.18.4/jbecker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongiovanni M, See RE. A comparison of the effects of different operant training experiences and dietary restriction on the reinstatement of cocaine-seeking in rats. Pharmacol Biochem Behav. 2008;89(2):227–233. doi: 10.1016/j.pbb.2007.12.019. https://doi.org/10.1016/j.pbb.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454(7200):118–121. doi: 10.1038/nature06995. https://doi.org/10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20(15):RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. https://doi.org/10.1016/S0301-0082(00)00067-8) [DOI] [PubMed] [Google Scholar]
- Doyle SE, Ramôa C, Garber G, Newman J, Toor Z, Lynch WJ. A shift in the role of glutamatergic signaling in the nucleus accumbens core with the development of an addicted phenotype. Biol Psychiatry. 2014;76(10):810–815. doi: 10.1016/j.biopsych.2014.02.005. https://doi.org/10.1016/j.biopsych.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Karlsgodt KH, Gastfriend DR. Gender differences in cocaine craving among non-treatment-seeking individuals with cocaine dependence. Am J Drug Alcohol Abuse. 2001;27(2):193–202. doi: 10.1081/ada-100103705. https://doi.org/10.1081/ADA-100103705. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189(1):1–16. doi: 10.1007/s00213-006-0529-6. https://doi.org/10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Altea S, Fratta W. Sex differences in drug addiction: a review of animal and human studies. Women’s Health (Lond Engl) 2008;4(1):51–65. doi: 10.2217/17455057.4.1.51. https://doi.org/10.2217/17455057.4.1.51. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Byrd EA, Henderson AR, See RE. Attenuation of cocaine-seeking by progesterone treatment in female rats. Psychoneuroendocrinology. 2009;34(3):343–352. doi: 10.1016/j.psyneuen.2008.09.014. https://doi.org/10.1016/j.psyneuen.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, See RE. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology. 2011;216(1):53–62. doi: 10.1007/s00213-011-2187-6. https://doi.org/10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer KD, Houston ACW, Rebec GV. Role of the major glutamate transporter GLT1 in nucleus accumbens core versus shell in cue-induced cocaine-seeking behavior. J Neurosci. 2013;33(22):9319–9327. doi: 10.1523/JNEUROSCI.3278-12.2013. https://doi.org/10.1523/JNEUROSCI.3278-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology. 2005;179(3):662–672. doi: 10.1007/s00213-004-2080-7. https://doi.org/10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U. A comparison of male and female cocaine abusers. Arch Gen Psychiatry. 1989;46(2):122–126. doi: 10.1001/archpsyc.1989.01810020024005. https://doi.org/10.1001/archpsyc.1989.01810020024005. [DOI] [PubMed] [Google Scholar]
- Johnson M. Loading controls for Western blots. Mater Methods. 2012;2:114. doi: 10.13070/mm.en.2.114. [DOI] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine with drawal in female relativetoma lerats. Psychopharmacology. 2008;198(1):63–75. doi: 10.1007/s00213-008-1089-8. https://doi.org/10.1007/s00213-008-1089-8. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE. Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology. 2005;182(2):245–252. doi: 10.1007/s00213-005-0071-y. https://doi.org/10.1007/s00213-005-0071-y. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010a;67(1):81–84. doi: 10.1016/j.biopsych.2009.07.018. https://doi.org/10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010b;30(23):7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. https://doi.org/10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCrosse AL, Hill K, Knackstedt LA. Ceftriaxone attenuates cocaine relapse after abstinence through modulation of nucleus accumbens AMPA subunit expression. Eur Neuropsychopharmacol. 2016;26(2):186–194. doi: 10.1016/j.euroneuro.2015.12.022. https://doi.org/10.1016/j.euroneuro.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacrosse AL, O’Donovan SM, Sepulveda-Orengo MT, et al. Contrasting the role of xCT and GLT-1 upregulation in the ability of ceftriaxone to attenuate the cue-induced reinstatement of cocaine-seeking and normalize AMPA receptor subunit expression. J Neurosci. 2017;37(24):5809–5821. doi: 10.1523/JNEUROSCI.3717-16.2017. https://doi.org/10.1523/JNEUROSCI.3717-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebron-Milad K, Milad MR. Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biol Mood Anxiety Disord. 2012;2(1):3. doi: 10.1186/2045-5380-2-3. https://doi.org/10.1186/2045-5380-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30(3):126–134. doi: 10.1016/j.tins.2007.01.006. https://doi.org/10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62(4a):609–614. doi: 10.1590/s1519-69842002000400008. https://doi.org/10.1590/S1519–69842002000400008. [DOI] [PubMed] [Google Scholar]
- Martinez LA, Gross KS, Himmler BT, Emmitt NL, Peterson BM, Zlebnik NE, Foster Olive M, Carroll ME, Meisel RL, Mermelstein PG. Estradiol facilitation of cocaine self-administration in female rats requires activation of mGluR5. Eneuro. 2016;3(5) doi: 10.1523/ENEURO.0140-16.2016. https://doi.org/10.1523/ENEURO.0140-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance-Katz EF, Carroll KM, Rounsaville BJ. Gender differences in treatment-seeking cocaine abusers–implications for treatment and prognosis. Am J Addict. 1999;8(4):300–311. doi: 10.1080/105504999305703. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23(8):3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean AC, Valenzuela N, Fai S, Bennett SAL. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp. 2012:e4389. doi: 10.3791/4389. https://doi.org/10.3791/4389. [DOI] [PMC free article] [PubMed]
- Peterson BM, Mermelstein PG, Meisel RL. Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct Funct. 2015;220(4):2415–2422. doi: 10.1007/s00429-014-0794-9. https://doi.org/10.1007/s00429-014-0794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter DA, Moreno A, Luther MF, et al. Effects of follicular-phase cocaine administration on menstrual and ovarian cyclicity in rhesus monkeys. Am J Obstet Gynecol. 1998;178(1):118–125. doi: 10.1016/s0002-9378(98)70637-4. https://doi.org/10.1016/S0002-9378(98)70637-4. [DOI] [PubMed] [Google Scholar]
- Potter DA, Luther MF, Eddy CA, et al. Low-dose follicular-phase cocaine administration disrupts menstrual and ovarian cyclicity in rhesus monkeys. J Soc Gynecol Investig. 1999;6(2):88–94. doi: 10.1016/s1071-5576(98)00054-9. https://doi.org/10.1177/107155769900600207. [DOI] [PubMed] [Google Scholar]
- Rimmele TS, Rosenberg PA. GLT-1: the elusive presynaptic glutamate transporter. Neurochem Int. 2016;98:19–28. doi: 10.1016/j.neuint.2016.04.010. https://doi.org/10.1016/j.neuint.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su Z, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433(7021):73–77. doi: 10.1038/nature03180. https://doi.org/10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29(29):9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. https://doi.org/10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittner J, Schroeder JR, Epstein DH, Preston KL. Menstrual cycle length during methadone maintenance. Addiction. 2005;100(6):829–836. doi: 10.1111/j.1360-0443.2005.01091.x. https://doi.org/10.1111/j.1360-0443.2005.01091.x. [DOI] [PubMed] [Google Scholar]
- Smith ACW, Scofield MD, Heinsbroek JA, Gipson CD, Neuhofer D, Roberts-Wolfe DJ, Spencer S, Garcia-Keller C, Stankeviciute NM, Smith RJ, Allen NP, Lorang MR, Griffin WC, III, Boger HA, Kalivas PW. Accumbens nNOS interneurons regulate cocaine relapse. J Neurosci. 2017;37(4):742–756. doi: 10.1523/JNEUROSCI.2673-16.2016. https://doi.org/10.1523/JNEUROSCI.2673-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondheimer I, Knackstedt LA. Ceftriaxone prevents the induction of cocaine sensitization and produces enduring attenuation of cue-and cocaine-primed reinstatement of cocaine-seeking. Behav Brain Res. 2011;225(1):252–258. doi: 10.1016/j.bbr.2011.07.041. https://doi.org/10.1016/j.bbr.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham-Davidson H, LaLumiere RT, Reissner KJ, Kalivas PW, Knackstedt LA. Ceftriaxone normalizes nucleus accumbens synaptic transmission, glutamate transport, and export following cocaine self-administration and extinction training. J Neurosci. 2012;32(36):12406–12410. doi: 10.1523/JNEUROSCI.1976-12.2012. https://doi.org/10.1523/JNEUROSCI.1976-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi RR, Bhattacharyya S. Constitutive internalization and recycling of metabotropic glutamate receptor 5 (mGluR5) Biochem Biophys Res Commun. 2012;427(1):185–190. doi: 10.1016/j.bbrc.2012.09.040. https://doi.org/10.1016/j.bbrc.2012.09.040. [DOI] [PubMed] [Google Scholar]
- Wang X, Moussawi K, Knackstedt L, Shen H, Kalivas PW. Role of mGluR5 neurotransmission in reinstated cocaine-seeking. Addict Biol. 2013;18(1):40–49. doi: 10.1111/j.1369-1600.2011.00432.x. https://doi.org/10.1111/j.1369-1600.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, Rasmussen BA, Corley G, Henry C, Kim JK, Walker EA, Rawls SM. Beta-lactam antibiotic decreases acquisition of and motivation to respond for cocaine, but not sweet food, in C57Bl/6 mice. Behav Pharmacol. 2011;22(4):370–373. doi: 10.1097/FBP.0b013e3283473c10. https://doi.org/10.1097/FBP.0b013e3283473c10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland A, Garcia S, Knackstedt LA. Ceftriaxone and cefazolin attenuate the cue-primed reinstatement of alcohol-seeking. Front Pharmacol. 2015;6:44. doi: 10.3389/fphar.2015.00044. https://doi.org/10.3389/fphar.2015.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SL, Vassoler FM, Schmidt HD, Pierce RC, Wimmer ME. Enhanced anxiety in the male offspring of sires that self-administered cocaine. Addict Biol. 2016;21(4):802–810. doi: 10.1111/adb.12258. https://doi.org/10.1111/adb.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Lasseter HC, Ramirez DR, Ponds KCL, Wells AM, Fuchs RA. Subregion-specific role of glutamate receptors in the nucleus accumbens on drug context-induced reinstatement of cocaine-seeking behavior in rats. Addict Biol. 2012;17(2):287–299. doi: 10.1111/j.1369-1600.2011.00325.x. https://doi.org/10.1111/j.1369-1600.2011.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


