Abstract
Objective:
The regenerative potential of bone marrow-derived mononuclear cells (MNCs) and CD133+ stem cells in the heart varies in terms of their pro-angiogenic effects. This phase II/III, multicenter and double-blind trial is designed to compare the functional effects of intramyocardial autologous transplantation of both cell types and placebo in patients with recent myocardial infarction (RMI) post-coronary artery bypass graft.
Materials and Methods:
This was a phase II/III, randomized, double-blind, placebo-controlled trial COMPARE CPM-RMI (CD133, Placebo, MNCs - recent myocardial infarction) conducted in accordance with the Declaration of Helsinki that assessed the safety and efficacy of CD133 and MNCs compared to placebo in patients with RMI. We randomly assigned 77 eligible RMI patients selected from 5 hospitals to receive CD133+ cells, MNC, or a placebo. Patients underwent gated single photon emission computed tomography assessments at 6 and 18 months post-intramyocardial transplantation. We tested the normally distributed efficacy outcomes with a mixed analysis of variance model that used the entire data set of baseline and between-group comparisons as well as within subject (time) and group×time interaction terms.
Results:
There were no related serious adverse events reported. The intramyocardial transplantation of both cell types increased left ventricular ejection fraction by 9% [95% confidence intervals (CI): 2.14% to 15.78%, P=0.01] and improved decreased systolic wall thickening by -3.7 (95% CI: -7.07 to -0.42, P=0.03). The CD133 group showed significantly decreased non-viable segments by 75% (P=0.001) compared to the placebo and 60% (P=0.01) compared to the MNC group. We observed this improvement at both the 6- and 18-month time points.
Conclusion:
Intramyocardial injections of CD133+ cells or MNCs appeared to be safe and efficient with superiority of CD133+ cells for patients with RMI. Although the sample size precluded a definitive statement about clinical outcomes, these results have provided the basis for larger studies to confirm definitive evidence about the efficacy of these cell types (Registration Number: NCT01167751).
Keywords: Autologous Transplantation, Bone Marrow-Cells, Cell Therapy, Mononuclear Cells, Myocardial Infarction
Introduction
Autologous bone marrow-derived cell therapy is under current investigation as a potentially promising therapy to treat patients with ischemic heart disease and potential candidates for revascularization with coronary artery bypass grafts (CABG) (1). The goal of this treatment is to improve myocardial regeneration and angiogenesis through administration of therapeutic cells into the periinfarct areas of the ischemic myocardium. Mononuclear cells (MNCs) (2-6) and CD133+ cells (7-18) are two major bone marrow-derived cells used as potential treatments for ischemic heart diseases. However, some studies report favorable outcomes whereas others indicate no benefits. These discrepancies may be related to factors such as the numbers of injected cells, administration route, time interval from myocardial infarction (MI), type of injected cells, cell isolation and preparation methods, and assessment techniques that include echocardiography, single photon emission computed tomography (SPECT), and magnetic resonance imaging (MRI). Nevertheless, these types of cells are easy to harvest, simple to administer, ethically acceptable, and do not require immunosuppression (19).
CD133+ bone marrow hematopoietic stem cells possess the characteristics of endothelial progenitor cells. These cells have the capability to differentiate into endothelial cells in vitro and play a role in neoangiogenesis processes in vivo (20, 21). Compared to non-selected bone marrow mononuclear cells, CD133+ cells have greater proangiogenic effects due to secretion of related cytokines, graft-host cell interactions (22-24), and resistance to apoptosis (25). The efficacy of intramyocardial injection of bone marrow-derived CD133+ cells versus MNCs in restoring function to an injured myocardium within an established infarct, however, has not been explored.
We sought to determine the functional consequences and clinical events that followed direct intramyocardial delivery of autologous bone marrow-derived MNCs and CD133+ cells in MI patients in this phase II/III multicenter, randomized, double-blind, placebo-controlled study. Findings from a comparison of CD133+ cells or MNCs versus placebo in the COMPARE CPM-RMI (CD133, Placebo, MNCs)-(recent myocardial infarction) trial have implications for the development of cell-based therapies for ischemic heart failure.
Materials and Methods
Study design, enrollment and patient population
We conducted the COMPARE CPM-RMI phase II/III, randomized, double-blind, placebo-controlled trial of the safety and efficacy of the cell procedure in accordance with the Declaration of Helsinki. This study was performed in 5 Tehran, Iran hospitals (Baqiyatallah, Shahid Dr. Lavasani, Tehran Heart Center, Rajaie Cardiovascular Medical and Research Center, and Masih Daneshvari). The patients’ documentations were collected from Royan Institute and the appropriate, related hospital. This study received approval from the Ethical Committee of Royan Institute (reference number: p-85-106). This trial was registered at http://www.Clinicaltrials.gov (identifier: NCT01167751). All patients gave written informed consent.
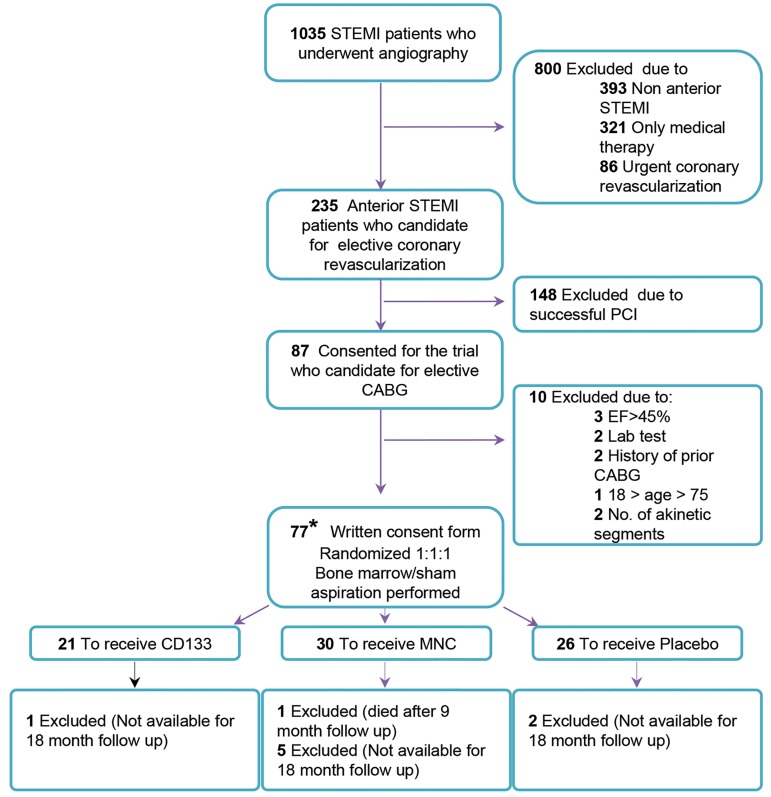
Patients were randomized at Royan Institute beginning in January 2008 with follow-up visits completed in July 2012. The flow chart shows patient eligibility (Fig .1). We selected 1035 patients recently diagnosed with first ST-elevation myocardial infarction (STEMI). The inclusion and exclusion criteria is listed in detail (Table 1). Patients aged 18 to 75 years received standard therapy and were chosen according to a major two-step selection process. Initially, each patient underwent an angiography evaluation that determined their eligibility for elective CABG. For the second step of the selection process, each patient underwent an eligible criteria assessment comprised of laboratory tests, dobutamine stress echocardiography, and SPECT analysis for baseline characteristics. Finally, we randomized 77 eligible patients via a computer-generated randomization sequence in a 1:1:1 ratio between the CD133+, the MNC, and placebo groups. Patients underwent bone marrow or sham aspirations. Patients and investigators not affiliated with the cell-processing laboratory were blinded to preparation and administration of the study product. Each patient had follow up visits at 6 and 18 months after transplantation.
Fig.1.
Flow chart for patient eligibility. We selected patients with STEMI at defined intervals from 10 days to 3 months. Ineligible patients were excluded by angiography results among other criteria as mentioned in the diagram. We divided eligible patients into three groups: placebo, MNC, and CD133+. All patients were intramyocardly injected 2 cc of suspension in 10 points of the marginal zone of the infarcted muscle during open heart surgery and were followed by SPECT scan at 6 and 18 months. From these patients, 8 were not available for follow up and one patient from the MNC group died. STEMI; ST elevation myocardial infarction, CABG; Coronary artery bypass graft, PCI; Percutaneous coronary intervention, LVEF; Left ventricular ejection fraction, MNC; Mononuclear cells, SPECT; Single photon emission computed tomography, and *; According to sample size calculation, we considered 90 patients for enrollment, however financial limitations forced us to stop patient recruitment. This study enrolled and randomly assigned 77 patients to three groups: MNC, CD133+, and placebo.
Table 1.
Patient inclusion and exclusion criteria
| Inclusion criteria | |
| Age: 18-75 Y | |
| First ST elevation myocardial infarction (STEMI) 10 days -3 months | |
| Post-acute myocardial infarction (AMI) left ventricular ejection fraction (LVEF) as assessed by echocardiography: 20-45% | |
| Target lesion must be located in the left anterior descending (LAD) section | |
| Coronary artery bypass graft (CABG) candidate | |
| At least 4 akinetic segments (assessed by stress echocardiography) | |
| Exclusion criteria | |
| History of prior AMI | |
| Active infection, history of recurrent infection, positive test for syphilis (RPR), hepatitis B and C (HBsAg, anti- HBc, anti-HCV), HIV or HTLV-1 | |
| Documented terminal illness or malignancy | |
| Documented autoimmune disease | |
| Pulmonary edema | |
| Urgent or emergency CABG | |
| Systolic blood pressure<80 mmHg | |
| 4000>leukocytes (µL)>15000 | |
| AST>100 IU/L or ALT>100 IU/L | |
| Hemoglobin<10 g/dL | |
| Platelets<100,000/µL | |
| INR>1.8 | |
HBsAg; Hepatitis B surface antigen, HBc; Hepatitis B core, HCV; Hepatitis C virus, HIV; Human immunodeficiency virus, HTLV-1; Human T-cell lymphotropic virus type 1, AST; Aspartate aminotransferase, ALT; Alanine aminotransferase, and INR; International normalized ratio.
Study procedures and timeline
Baseline information included results of chemistry, hematology and virology laboratory tests, in addition to SPECT and stress echocardiography. We conducted patient interviews to determine demographic and clinical variables.
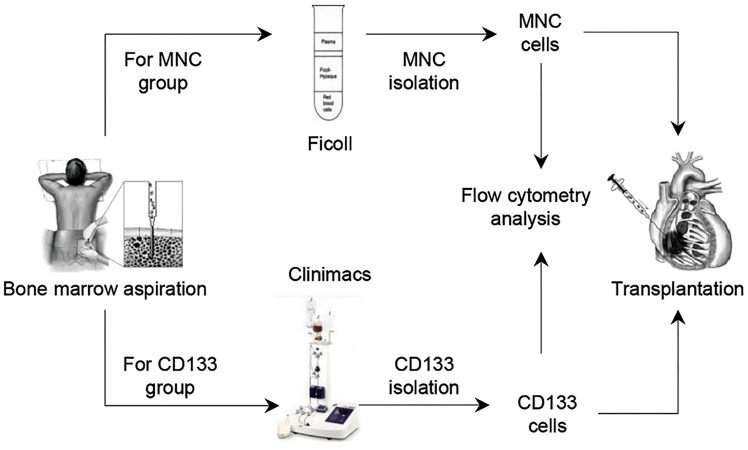
One day prior to surgery, patients in the CD133+ and MNC groups underwent bone marrow aspiration (150 ml) from their iliac crests. The isolation of CD133+ cells and MNCs will be described in a later section. The cells were suspended in 2 ml normal saline supplemented with 2% autologous serum. Placebo group patients underwent a sham aspiration procedure to ensure proper blinding of the treatment assignment. The sham procedure consisted of creating a skin incision under local anesthesia at the iliac crest surface. In the placebo group, patients received vehicle (2 ml) comprised of normal saline supplemented with 2% autologous serum. The MNC and CD133+ cell isolation and transplantation protocol is summarized for the two cell therapy groups (Fig .2).
Fig.2.
MNCs and CD133+ cells isolation and transplantation protocols for CABG candidates. Patients underwent bone marrow aspiration 24 hours before CABG. MNCs and CD133+ cells were isolated by Ficoll density gradient and a CliniMACS machine. Isolated cells were analyzed by flow cytometry before intramyocardial injection. Patients in the placebo group underwent a sham aspiration procedure.
CABG; Coronary artery bypass grafts and MNC; Mononuclear cells.
Patients from all study groups received the injections at the end of the cardiopulmonary bypass and cold blood cardioplegic arrest, and just prior to removal of the aortic cross clamp. Patients received either cells or vehicle placebo as intramyocardial injections to 10 sites (0.2 ml per site) at the marginal zone of the subepicardial infarcted area using a 22-gauge needle. We performed primary echocardiography and SPECT, as the paraclinical imagings, to locate the infarcted area. During the procedure, surgeons visually estimated the border zone of the infarct area and performed the injections. Lists The mean numbers of injected CD133+ and MNC cells were shown for these groups (Table 2). The average number of injected cells in the 2 ml cell suspension was 564.63×106 (± 69.35) MNC cells and 8.19×106 (± 4.26) CD133+ cells per patient. Each patient received 1/10 of each cell suspension injected per site. After the myocardial cell injections, the area was gently massaged, followed by reperfusion. After the CABG, all patients remained hospitalized for a minimum of 4 days. Patients were evaluated at 1 week after CABG, then monthly for 3 months, and at 6 and 18 months. At the long-term (6 and 18 months) follow- ups, we assessed for safety and efficacy that included a clinical interview of the New York Heart Association (NYHA) Classification, physical examination, and cardiac SPECT.
Table 2.
Average number of cells and immunophenotyping of the CD133+ and MNC groups
| Characteristics/patient | MNC groupn=30Mean (SD) | CD133+ groupn=21Mean (SD) |
|---|---|---|
| Bone marrow cell number | 2.11×109(1.62) | 2.39×109(1.42) |
| Total injected cell number | 564.63×106 (69.35) | 8.19×106 (4.26) |
| Injected cell number/site | 564.63×105 (69.35) | 8.19×105 (4.26) |
| Viability (%) | 98.53 (1.67) | 92.91 (7.66) |
| CD133+ (%) | 1.19 (1.21) | 63.69 (17.84) |
| CD34+(%) | 0.20 (0.24) | n.d. |
| CD44+(%) | 88.16 (17.57) | n.d. |
| CD31+ (%) | 42.77 (18.98) | n.d. |
| VEGFR (%) | 38.60 (26.18) | n.d. |
CD; Cluster of differentiation, MNC; Mononuclear cells, n.d.: Not determined, and VEGFR; Vascular endothelial growth factor receptor.
Objectives and endpoints
This study focused on improvement of myocardial perfusion and left ventricular (LV) function in STEMI patients after injections of bone marrow derived cells into the infarcted myocardium compared to a placebo group. Additionally, we evaluated different effects of CD133+ and MNC cells at determined endpoints. The primary endpoint was assessed by changes in global LV ejection fraction (LVEF) at rest by gated SPECT.
Secondary endpoints were: i. Adverse cardiac events that included death, reinfarction, implantable cardioverter defibrillator (ICD) placement, infection, and arrhythmia, ii. Changes in wall motion score (WMS), decreased systolic wall thickening (Dec. Thickening) of the myocardium, non-viable (NV) segments, and perfusion defect score (PDS) assessed by gated SPECT, and iii. NYHA classification. We compared and analyzed endpoint data amongst the three groups during the 18 months of follow up.
Cell separation and flow cytometry
We isolated (150 ml) bone marrow MNCs under Good Manufacturing Practice (GMP) conditions. Bone marrow MNCs were counted by a NucleoCounter instrument (ChemoMetec A/S, Denmark). We isolated the MNCs by centrifuging the resultant bone marrow through a low-density gradient using Ficoll-Paque PREMIUM (Lymphodex, Inno-train, 002041600) according to the manufacturer’s protocol. Briefly, bone marrow samples [diluted 1:1 in phosphate- buffered saline (PBS)] were subsequently loaded over a Ficoll-Paque at a 3:1 ratio and centrifuged for 30 minutes at 400 g with no brake. Cells were collected at the interface and washed in PBS buffer (1:1; Milteny Biotech GmbH, 700-25). Cell viability and counts were determined by trypan blue exclusion staining and a NucleoCounter.
CD133+ cells were isolated by a CliniMACS automated machine. The bone marrow cell suspension was filtered through a 200 µm filter (Baxter S.A, RMC 5849). We used a 10% Gamunex reagent as the blocking antibody (100 mg/ml, Talecris Biotherapeutics, Inc.). A monoclonal magnetic micro-bead antibody that had the capability to detect CD133+ cells (Milteny Biotech GmbH, 172-01) was applied. The magnetically labeled cell bags were subsequently attached to a CliniMACS tubing set LS (Milltenyi Biotec GmbH) and sorted by a CliniMACS instrument. We assessed cell count, viability, and sterility, after which the positive fractions were centrifuged for 5 minutes at 350 g.
We assessed for cell specific marker expressions in the cell populations by flow cytometry. Cells were stained with phycoerythrin (PE) or fluorescein isothiocyanate (FITC) conjugated anti- human CD133 (293C3, Milteny Biotech GmbH, 130090- 853), hVEGFR2/KDR (R&D Systems, FAB357P), CD31 (BD Biosciences, 555445), CD45/34 (BD Biosciences, 341071), and CD44 (BD Biosciences, 555479) antibodies, IgG2b/RPE (BD Biosciences, 556656), and IgG1FITC/IgG1RPE (Dako, X0932). Next, they were analyzed with a BD FACSCalibur flow cytometry system (BD Biosciences, San Jose, CA, USA) and software version 2.5.1 (BD Biosciences) by gating at 2% of the isotype control. A total of 100,000 events were acquired for each marker and isotype.
Single photon emission computed tomography and stress echocardiography imaging
We performed SPECT analysis at baseline, then at 6 and 18 months after transplantation. SPECT images were obtained approximately one hour after an intravenous injection of 740-925 MBq of 99mTc-sestamibi by a single-head camera with the following parameters: low- energy all-purpose (LEAP) collimator, 20% symmetric window at 140 keV, and a 64×64 matrix. Step and shoot acquisition and projection images were reconstructed. We used AutoQUANT software version 4.3.1 (AutoQUANT, ADAC Laboratories, Milpitas, CA) to evaluate PDS, LVEF, WMS, NV, and Dec. Thickening according to a scoring method outlined in Figure S1 (See Supplementary Online Information at www.celljournal.org) (26) that follows a model of 17 cardiac segments. We have assessed the PDS variable according to 4 scores: mild hypoperfusion (1), moderate hypoperfusion (2), severe hypoperfusion (3), and absence of perfusion (4). The NV score (2) segments were important for viability assessment. The 3 scores for abnormal movement included hypokinesia (1), akinesia (2), and dyskinesia (3). We assessed Dec. Thickening as mild (1), moderate (2), or severe (3).
Patients received intravenous injections of low-dose dobutamine for stress echocardiography. The stress echocardiography images were obtained from the parasternal (long and short axis) in the left lateral decubitus and apical (2 and 4 chamber) windows. Five variables that included the LVEF, left ventricular end diastolic diameter (LVEDD), LVE systolic diameter (LVESD), the number of NV segments, and WMS index were evaluated using a 17-segment model from standard parasternal and apical 2D echocardiography recommended by the American Society for Echocardiography. A total of 5 expert echocardiographers blinded to the patient group assignments performed the measurements with the same machine type (GE Vivid 7 with a 3 MHz probe) in each of the 5 centers.
Statistical analysis
We extracted the confidence intervals (CIs) of outcomes to evaluate improvements in myocardial perfusion and LV function in study patients treated with CD133+ cells or MNC versus placebo and in patients that received CD133+ cells versus MNC. All statistical analyses were performed by taking into consideration the intention to treat (ITT) point of view. We used analysis of variance (ANOVA) for quantitative outcomes and the χ2 test for qualitative baseline variables to analyze for differences between the study groups. Normal distributed efficacy outcomes were tested with a mixed analysis of variance model that used the entire data set of baseline, between- group comparisons, within subject (time), and group×time interactions (27, 28).
The NYHA class change scores (baseline vs. 18 months) were calculated to ascertain treatment efficacy by the χ2 test. The sample size was estimated from a study by Zhao et al. (29) and conservative assumptions. At least 27 patients were considered adequate for each group; according to the potential for patient loss during the trial, we determined the total sample size as 90 with a power of >80%. Data analyses were performed by the repeated measures method, standard deviation to 9 units, and a minimum clinically important difference to 6 units in the LVEF scale as a primary end-point, with a correlation between 6 and 18 months to 0.6. We assumed 6 unit differences only for the cell therapy groups against the placebo. Analyses were conducted using SPSS software version 16 (SPSS, Chicago, IL, USA). The data have been presented as mean ± SD. A P<0.05 was considered statistically significant.
Results
Patients
We considered an enrollment of 90 patients according to the sample size calculation. However financial limitations forced us to stop patient recruitment. Therefore, this study enrolled and randomly assigned 77 patients to three groups: MNC, CD133+ and placebo through anterior STEMI at a defined time interval. After opening the study code, we determined that the numbers of patients per group were not similar: MNC (n=30), CD133+ (n=21) and placebo (n=26). There were no significant differences in baseline characteristics detected between groups except for coronary artery disease, LVESD, and LVEDD. The study population was predominantly male (Table 3).
Table 3.
Patient baseline characteristics
| Parameter | Placebo group n=26 | CD133+ group n=21 | MNC group n=30 | P value |
|---|---|---|---|---|
| Age (Y), mean (SD) | 55.50 (8.54) | 53.14 (8.56) | 51.45 (7.49) | NS |
| Females, n (%) | 3 (11.5) | 2 (9.5) | 3 (10) | NS |
| Body mass index, mean (SD) | 26.7 (4.2) | 27.9 (2.6) | 26.0 (2.7) | NS |
| Duration*, (median days) | 32.5 | 30 | 26.5 | NS |
| Min-Max (day) | 10-79 | 10-90 | 10-76 | |
| CAD, n (%) | 0.039 | |||
| LAD only | 2 (7.6) | 0.0 | 5 (16.6) | |
| LAD+RCA/LCX | 4 (15.3) | 6 (28.5) | 11 (36.6) | |
| LAD+RCA+LCX | 20 (76.9) | 15 (71.4) | 14 (46.6) | |
| Diabetes mellitus, n (%) | 10 (38.4) | 6 (28.5) | 11 (36.6) | NS |
| Hypertension, n (%) | 6 (23.0) | 7 (33.3) | 6 (20.0) | NS |
| Hyperlipidemia, n (%) | 14 (53.8) | 9 (42.8) | 15 (50.0) | NS |
| Smoking, n (%) | 12 (46.1) | 7 (33.3) | 15 (50.0) | NS |
| Preoperative medication, n | NS | |||
| Aspirin | 22 | 16 | 24 | |
| Clopidogrel | 13 | 11 | 16 | |
| Βeta-blockers | 24 | 16 | 23 | |
| ACE inhibitors | 13 | 12 | 20 | |
| Statins | 22 | 18 | 21 | |
| Nitrates | 20 | 14 | 8 | |
| Diuretic | 10 | 6 | 8 | |
| Digoxin | 9 | 8 | 7 | |
| Thrombolytic | 12 | 10 | 14 | |
| Lab test (U/L) [mean (SD)] | NS | |||
| Peak CK-total | 774 (541) | 924 (301) | 646 (310) | |
| Peak CK-MB | 52 (37) | 54 (24) | 76 (74) | |
| SPECT [mean (SD)] | NS | |||
| LVEF (%) | 40.47 (15.12) | 39.80 (10.63) | 37.06 (9.01) | |
| PDS | 10.82 (9.24) | 17.10 (10.25) | 16.38 (9.98) | |
| NV | 2.05 (3.12) | 4.14 (3.65) | 3.00 (3.31) | |
| WMS | 5.11 (3.62) | 5.50 (3.74) | 6.93 (5.00) | |
| Dec. Thickening | 8.64 (6.86) | 11.10 (4.25) | 14.46 (8.55) | |
| S. echo, mean (SD) | ||||
| LVEF (%) | 32.90 (8.40) | 31.13 (5.37) | 32.34 (5.86) | NS |
| NV | 5.83 (0.77) | 5.96 (0.75) | 6.32 (0.68) | NS |
| WMSI | 2.00 (0.09) | 2.14 (0.11) | 1.96 (0.07) | NS |
| LVEDD (mm) | 55.60 (6.37) | 59.19 (7.03) | 53.08 (5.36) | 0.012 |
| LVESD (mm) | 43.98 (8.50) | 46.65 (7.78) | 40.68 (6.36) | 0.050 |
| Operative details | NS | |||
| Number of grafts, mean (SD) | 3.6 (0.7) | 3.5 (0.8) | 3.2 (0.8) | |
| Lima grafts, n | 26 | 20 | 30 | |
| X-clamp time (minute) | 39 (10) | 46 (16) | 45 (13) | |
| Perfusion time (minute) | 68 (18) | 74 (27) | 73 (21) | |
MNC; Mononuclear cells, CAD; Coronary artery disease, LAD; Left anterior descending, RCA; Right coronary artery, LCX; Left circumflex artery, SPECT; Single photon emission computed tomography, LVEF; Left ventricular ejection fraction, PDS; Perfusion defect score, NV; Non-viable segments, WMS; Wall motion score, Dec. Thickening; Decreased systolic wall thickening, S. echo; Stress echocardiography, WMSI; Wall motion score index, LVEDD; Left ventricular end diastolic diameter, LVESD; Left ventricular end systolic diameter, NS; Not significant, *; Days from myocardial infarction (MI) to coronary artery bypass graft (CABG).
There were 9 patients who received the study intervention but did not complete the 18 months of follow-up. One patient in the CD133+ and one in the MNC group were lost to follow up due to relocation out of the province. Four patients in MNC group and two in the placebo group were lost to follow up due to lack of telephone access. One patient from the MNC group died in the ninth month after surgery (Fig .1).
The average number of bone marrow MNC cells obtained was 2.11×109 (± 1.62) in the MNC group and 2.39×109 (± 1.42) in the CD133+ group (Table 2). The average number of injected cells prepared in a 2 ml cell suspension of normal saline with 2% autologous serum was 564.63×106 (± 69.35) MNC cells and 8.19×106 (± 4.26) CD133+ cells per recipient.
Safety and adverse events during follow-up
There were no reported study related serious adverse events during the initial hospitalization. However, 2 patients in the placebo group experienced reinfarction at 8 and 11 months after surgery. Additionally, 2 patients who had an LVEF <30% and mild-to-moderate symptoms of heart failure in the MNC group agreed to undergo ICD implantation as a primary prevention at the 6- and 18-month post-surgery follow up visits as recommended by the ACC/AHA Guidelines (30). One patient in MNC group died from cardiac arrest during the 9th month after surgery. There was no autopsy performed for this patient. Additionally, we observed no infections or arrhythmias among patients from all groups. The number of total adverse events per group were: placebo [2], CD133+ [0], and MNC [3].
Physical functional
The NYHA class improved after 18 months compared with baseline in 17 (85.0%) CD133+ patients, 18 (75.0%) MNC patients, and 13 (54.2%) placebo patients. There was no change in 3 (15.0%) CD133+ patients, 6 (25.0%) MNC patients, and 10 (41.7%) placebo patients. There was no worsening in NYHA classification among the CD133+ and MNC patients after 18 months. However, one patient from the placebo group had an increase in the NYHA classification (P=0.178, Table S1) (See Supplementary Online Information at www.celljournal.org).
Global function
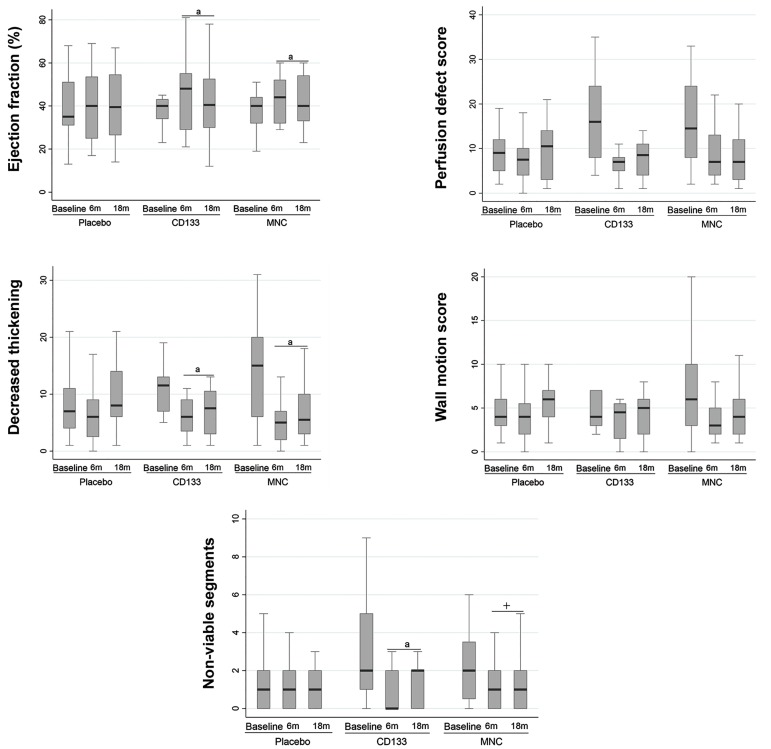
We used cardiac SPECT to assess LVEF, NV segments, Dec. Thickening, WMS, and PDS at baseline, and 6 and 18 months after transplantation (Fig .3, Table S2) (See Supplementary Online Information at www.celljournal. org). Analysis of the parameters showed consistent results at both time points. LVEF increased by 9% in CD133+ group (95% CI: 2.14% to 15.78%, P=0.011) and 7% in the MNC group (95% CI: 1.05% to 12.79%, P=0.022) relative to the placebo group.
Fig.3.
Comparing end-point analyses of different variables between 6 and 18 months by SPECT. Significant differences existed between the cell therapy and placebogroups in ejection fraction, non-viable segments and decreased systolic wall thickening variables as measured by SPECT. There was no time effect observed. Therefore, we considered statistical significance for both the 6- and 18-month time periods. Significant difference between the cell and placebo groups. a; P<0.03, CD133+ or MNC groups versus placebo, +; P<0.01, significant difference between CD133+ and MNC groups. Data markers represent means + SD, and 95% CIs. ANOVA was conducted with repeated measures. CI; Confidence interval, MNC; Mononuclear cells, and SPECT; Single photon emission computed tomography.
NV segments decreased by 75% equals to -1.5 (95% CI:-2.33 to-0.62, P=0.001) after administration of CD133+ cells, yet remained unchanged with MNC -0.30 (95% CI:-1.09 to 0.48, P=0.443) compared to the placebo. Additionally, the NV segments in the CD133+ group significantly decreased 60% equals to -1.2 (95% CI:-2.06 to-0.29, P=0.010) compared to MNC group.
During the 18 months follow up, we observed significantly improved Dec. Thickening in both the CD133+ and MNC groups with a mean change of -3.75 (95% CI:-7.07 to-0.42, P=0.028) for the CD133+ group and -3.55 (95% CI:-6.61 to-0.49, P=0.024) for the MNC group. These improvements remained consistent up to the 18-month follow up (P<0.05). Significant differences existed between the MNC and placebo groups in LVEF and Dec. thickening. We observed comparable results in the CD133+ versus placebo groups. Additionally, there were no significant differences between the CD133+ and MNC groups except in NV segments in CD133+ group.
We assessed the correlations of baseline factors (smoking, diabetes mellitus, hyperlipidemia, and hypertension) with treatment response in all three groups. Patients who smoked in all three groups showed a 7% decrease in LVEF (P=0.046). We observed no correlation in the other variables.
Discussion
Clinical trials of intramyocardial bone marrow-derived cell injections have not reported any related serious adverse events, which this offers promise for MI (218). However, the efficacies of both MNCs and CD133+ cell transplantations remain uncertain. We designed the COMPARE CPM-RMI study with the intent to provide a placebo-controlled, double-blinded, safety and efficacy assessments of two leading candidates for cardiac cell therapy include bone marrow-derived non-selected MNCs and selected CD133+ cells.
The MIXED model of the COMPARE CPM-RMI data suggested that CD133+ cells had slightly greater efficacy compared to MNCs. However, these findings were limited by the small sample size which resulted in preliminary conclusions. The variance-covariance matrix repeated measure analysis was assumed. Due to the type I error rate of 0.05% and observed differences in the treatment groups, 17 samples per arm were considered adequate to achieve an 80% power. Hence, the sample size and power of study were considered reliable despite the study limitations.
CD133+ cells decreased NV segments within the myocardium, which suggested a possible efficacy. The function of CD133+ cells in decreasing of NV segments and improving LVEF and systolic wall thickening remains to be determined. Results from animal studies show that transplanted cells integrate into the new environment, after which they form new vasculature and myocardium (11, 19). We hypothesize that this effect may be due to cytokine release, graft-host interactions (22-24), anti-apoptotic factors (25) and stimulation of neovascularization (20, 21) with cardiomyocyte regeneration primarily and by promotion of endogenous stem cell proliferation.
We did not observe any related serious adverse events from the intramyocardial cell injections. Since the first report on bone marrow-derived cell therapy for the treatment of heart failure in 2001 by Orlic et al. (31) numerous studies have used intracoronary, transendocardial, and/or intramyocardial injections. These trials indicated that the intramyocardial injection approach was the most efficient method due to direct visualization and delivery to the target area.
In this approach, the cells localize more reliably in the heart (32, 33). However, with intracoronary injections many of the injected cells eventually localize in the lungs or liver.
MNCs had a mean number of 565×106 injected cells per recipient compared to 8×106 CD133+ cells per recipient. Although the number of injected CD133+ cells was comparable in both groups, where 6.72×106 (1.19%) of the injected MNCs were CD133+ cells, we observed greater effectiveness with the enriched CD133+ cells. The heterogeneous population of the MNCs could affect homing of the desired cells, which was likely due to competition of multiple progenitors for engraftment and might reduce cell homing with well-known effects (34, 35). Previous human studies have shown that intracoronary transplantation of low numbers of enriched bone marrow progenitor cells have a 7-fold higher homing capacity compared to larger numbers of bone marrow-derived non- selected MNCs (36, 37).
On the other hand, the numbers of cells used for intracoronary cell therapy varied widely, even without taking into consideration trialsthat assessed the importance of cell number on clinical or functional outcome. We selected the number of CD133+ cells based on the positive effects of these cells that have been discussed by previous reports (8, 10, 11, 18) and our phase 1 clinical trial (16, 38). Although the therapeutic effects of at least 50 × 106 bone marrow-MNCs has been noted in previous meta-analyses (3, 39), the debate over the efficacy of the numbers of cells continues. Some meta-analyses have found no association between the number of cells delivered and the outcome (5). Therefore, the effective number of cells has yet to be determined (40, 41).
Appropriate timing of cell delivery following an MI on the course of heart function has yet to be determined. Previous research on the appropriate timing of MNCs administered by percutaneous coronary intervention to patients after an acute MI (AMI) showed no detectable effects on recovery of regional LV function (42-44). Our analysis also indicated no significant time-related improvements in the outcomes.
We observed between-group efficacy improvements after analyses of several outcome measures for the CD133+ cells and MNCs versus placebo. However, comparison between the two cell types showed no significant differences in most cases, with the exception of the N.V segment variable. This finding indicated that the study was not powered to show definitive efficacy comparisons between cell types. Another limitation, due to ethical considerations, was the sham aspiration through a superficial skin incision instead of a bone marrow harvest for placebo treated patients. We attempted to mimic a bone marrow aspiration procedure by placing patients in the prone position where they experienced the sensation of needle pressure during administration of a local anesthetic in the hip area, other related issues, and the intramyocardial injection of a vehicle. MRI is the gold standard for cardiac function and morphology. However, due to limited access, our patients did not undergo MRI imaging. The data quality of an echocardiography is objective but may vary between individuals and centers (45). We took advantage of SPECT modality where the results were reported by one nuclear medicine specialist and double-checked by a second nuclear medicine specialist. Therefore, in order to strengthen our findings, studies with more participants in conjunction with powerful assessments such as MRI imaging is necessary.
Conclusion
This preliminary COMPARE CPM-RMI study showed the safety and possible efficacy of delivering cell-therapy by intramyocardial injections in patients with RMI who underwent CABG. These results provided the basis for larger studies to perform definitive assessments of the efficacy of this approach.
Supplementary Figure
Supplementary PDF
Acknowledgments
This project was financially supported in part by a grant from Royan Institute, the Iran Industrial Development and Renovation Organization (IDRO), and the Small Business Development Center (SBDC). There is no conflict of interest in this study. We would like to express our appreciation to the members of the Department of Regenerative Medicine for their expertise and feedback. We would particularly like to thank the patients and their families for participating in this study.
Author’s Contributions
N.A., M.H.N., S.H.A.T., R.F., A.K., H.M., F.S., N.S., H.B., Ab.S., A.V.D., H.G., M.M.F., H.H., D.K.S., Sa.H., Se.H., Z.H.A., M.D., Al.S., S.M., S.E.H., M.E., H.S., G.B., A.B., Ah.A., Y.S., L.A., M.M., S.D., N.R., A.H., M.N., F.K.; Participated in study concept and design, data collection and evaluation, drafting and statistical analysis. Al.A., H.M., N.A., H.B.; Contributed extensively in interpretation of the data and the conclusion. All authors performed editing and approving the final version and agreeing to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1.Tian T, Chen B, Xiao Y, Yang K, Zhou X. Intramyocardial autologous bone marrow cell transplantation for ischemic heart disease: a systematic review and meta-analysis of randomized controlled trials. Atherosclerosis. 2014;233(2):485–492. doi: 10.1016/j.atherosclerosis.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Pätilä T, Lehtinen M, Vento A, Schildt J, Sinisalo J, Laine M, et al. Autologous bone marrow mononuclear cell transplantation in ischemic heart failure: A prospective, controlled, randomized, doubleblind study of cell transplantation combined with coronary bypass. J Heart Lung Transplant. 2014;33(6):567–574. doi: 10.1016/j.healun.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Afzal MR, Samanta A, Shah ZI, Jeevanantham V, Abdel-Latif A, Zuba-Surma EK, et al. Adult bone marrow cell therapy for ischemic heart disease: evidence and insights from randomized controlled trials. Circ Res. 2015;117(6):558–575. doi: 10.1161/CIRCRESAHA.114.304792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assmus B, Leistner DM, Schächinger V, Erbs S, Elsässer A, Haberbosch W, et al. Long-term clinical outcome after intracoronary application of bone marrow-derived mononuclear cells for acute myocardial infarction: migratory capacity of administered cells determines event-free survival. Eur Heart J. 2014;35(19):1275–1283. doi: 10.1093/eurheartj/ehu062. [DOI] [PubMed] [Google Scholar]
- 5.Gyöngyösi M, Wojakowski W, Lemarchand P, Lunde K, Tendera M, Bartunek J, et al. Meta-Analysis of Cell-based CaRdiac stUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ Res. 2015;116(8):1346–1360. doi: 10.1161/CIRCRESAHA.116.304346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cong XQ, Li Y, Zhao X, Dai YJ, Liu Y. Short-term effect of autologous bone marrow stem cells to treat acute myocardial infarction: a meta-analysis of randomized controlled clinical trials. J Cardiovasc Transl Res. 2015;8(4):221–231. doi: 10.1007/s12265-015-9621-9. [DOI] [PubMed] [Google Scholar]
- 7.Donndorf P, Kaminski A, Tiedemann G, Kundt G, Steinhoff G. Validating intramyocardial bone marrow stem cell therapy in combination with coronary artery bypass grafting, the PERFECT Phase III randomized multicenter trial: study protocol for a randomized controlled trial. Trials. 2012;13:99–99. doi: 10.1186/1745-6215-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stamm C, Kleine HD, Choi YH, Dunkelmann S, Lauffs JA, Lorenzen B, et al. Intramyocardial delivery of CD133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: safety and efficacy studies. J Thorac Cardiovasc Surg. 2007;133(3):717–725. doi: 10.1016/j.jtcvs.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 9.Forcillo J, Stevens LM, Mansour S, Prieto I, Salem R, Baron C, et al. Implantation of CD133+ stem cells in patients undergoing coronary bypass surgery: IMPACT-CABG pilot trial. Can J Cardiol. 2013;29(4):441–447. doi: 10.1016/j.cjca.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Nasseri BA, Ebell W, Dandel M, Kukucka M, Gebker R, Doltra A, et al. Autologous CD133+ bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: the Cardio133 trial. Eur Heart J. 2014;35(19):1263–1274. doi: 10.1093/eurheartj/ehu007. [DOI] [PubMed] [Google Scholar]
- 11.Yerebakan C, Kaminski A, Westphal B, Donndorf P, Glass A, Liebold A, et al. Impact of preoperative left ventricular function and time from infarction on the long-term benefits after intramyocardial CD133(+) bone marrow stem cell transplant. J Thorac Cardiovasc Surg. 2011;142(6):1530–1539. doi: 10.1016/j.jtcvs.2011.05.002. e3. [DOI] [PubMed] [Google Scholar]
- 12.Colombo A, Castellani M, Piccaluga E, Pusineri E, Palatresi S, Longari V, et al. Myocardial blood flow and infarct size after CD133+ cell injection in large myocardial infarction with good recanalization and poor reperfusion: results from a randomized controlled trial. J Cardiovasc Med (Hagerstown) 2011;12(4):239–248. doi: 10.2459/JCM.0b013e328343d708. [DOI] [PubMed] [Google Scholar]
- 13.Mansour S, Roy DC, Bouchard V, Nguyen BK, Stevens LM, Gobeil F, et al. COMPARE-AMI trial: comparison of intracoronary injection of CD133+ bone marrow stem cells to placebo in patients after acute myocardial infarction and left ventricular dysfunction: study rationale and design. J Cardiovasc Transl Res. 2010;3(2):153–159. doi: 10.1007/s12265-009-9145-2. [DOI] [PubMed] [Google Scholar]
- 14.Flores-Ramírez R, Uribe-Longoria A, Rangel-Fuentes MM, Gutiérrez- Fajardo P, Salazar-Riojas R, Cervantes-García D, et al. Intracoronary infusion of CD133+ endothelial progenitor cells improves heart function and quality of life in patients with chronic post-infarct heart insufficiency. Cardiovasc Revasc Med. 2010;11(2):72–78. doi: 10.1016/j.carrev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Kovacic JC, Macdonald P, Feneley MP, Muller DW, Freund J, Dodds A, et al. Safety and efficacy of consecutive cycles of granulocyte- colony stimulating factor, and an intracoronary CD133+ cell infusion in patients with chronic refractory ischemic heart disease: the G-CSF in angina patients with IHD to stimulate neovascularization (GAIN I) trial. Am Heart J. 2008;156(5):954–963. doi: 10.1016/j.ahj.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 16.Ahmadi H, Baharvand H, Ashtiani SK, Soleimani M, Sadeghian H, Ardekani JM, et al. Safety analysis and improved cardiac function following local autologous transplantation of CD133+ enriched bone marrow cells after myocardial infarction. Curr Neurovasc Res. 2007;4(3):153–160. doi: 10.2174/156720207781387141. [DOI] [PubMed] [Google Scholar]
- 17.Klein HM, Ghodsizad A, Marktanner R, Poll L, Voelkel T, Mohammad Hasani MR, et al. Intramyocardial implantation of CD133+ stem cells improved cardiac function without bypass surgery. Heart Surg Forum. 2007;10(1):E66–E69. doi: 10.1532/HSF98.20061054. [DOI] [PubMed] [Google Scholar]
- 18.Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003;361(9351):45–46. doi: 10.1016/S0140-6736(03)12110-1. [DOI] [PubMed] [Google Scholar]
- 19.Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113(6):810–834. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quirici N, Soligo D, Caneva L, Servida F, Bossolasco P, Deliliers GL. Differentiation and expansion of endothelial cells from human bone marrow CD133+ cells. Br J Haematol. 2001;115(1):186–194. doi: 10.1046/j.1365-2141.2001.03077.x. [DOI] [PubMed] [Google Scholar]
- 21.Gehling UM, Ergün S, Schumacher U, Wagener C, Pantel K, Otte M, et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95(10):3106–3112. [PubMed] [Google Scholar]
- 22.Ratajczak J, Kucia M, Mierzejewska K, Marlicz W, Pietrzkowski Z, Wojakowski W, et al. Paracrine proangiopoietic effects of human umbilical cord blood-derived purified CD133+ cells--implications for stem cell therapies in regenerative medicine. Stem Cells Dev. 2013;22(3):422–430. doi: 10.1089/scd.2012.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaipa G, Tilenni M, Straino S, Burba I, Zaccagnini G, Belotti D, et al. GMP-based CD133(+) cells isolation maintains progenitor angiogenic properties and enhances standardization in cardiovascular cell therapy. J Cell Mol Med. 2010;14(6B):1619–1634. doi: 10.1111/j.1582-4934.2009.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma N, Ladilov Y, Moebius JM, Ong L, Piechaczek C, Dávid Á, et al. Intramyocardial delivery of human CD133+ cells in a SCID mouse cryoinjury model: Bone marrow vs.cord blood-derived cells. Cardiovasc Res. 2006;71(1):158–169. doi: 10.1016/j.cardiores.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Forraz N, Pettengell R, Deglesne PA, McGuckin CP. AC133+ umbilical cord blood progenitors demonstrate rapid self-renewal and low apoptosis. Br J Haematol. 2002;119(2):516–524. doi: 10.1046/j.1365-2141.2002.03828.x. [DOI] [PubMed] [Google Scholar]
- 26.Van Train KF, Areeda J, Garcia EV, Cooke CD, Maddahi J, Kiat H, et al. Quantitative same-day rest-stress technetium-99m-sestamibi SPECT: definition and validation of stress normal limits and criteria for abnormality. J Nucl Med. 1993;34(9):1494–1502. [PubMed] [Google Scholar]
- 27.Zhang S, Paul J, Nantha-Aree M, Buckley N, Shahzad U, Cheng J, et al. Empirical comparison of four baseline covariate adjustment methods in analysis of continuous outcomes in randomized controlled trials. Clin Epidemiol. 2014;6:227–235. doi: 10.2147/CLEP.S56554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med. 2002;21(19):2917–2930. doi: 10.1002/sim.1296. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Q, Sun Y, Xia L, Chen A, Wang Z. Randomized study of mononuclear bone marrow cell transplantation in patients with coronary surgery. Ann Thorac Surg. 2008;86(6):1833–1840. doi: 10.1016/j.athoracsur.2008.08.068. [DOI] [PubMed] [Google Scholar]
- 30.Hunt SA; American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46(6):e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 31.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 32.Mäkelä J, Anttila V, Ylitalo K, Takalo R, Lehtonen S, Mäkikallio T, et al. Acute homing of bone marrow-derived mononuclear cells in intramyocardial vs.intracoronary transplantation. Scand Cardiovasc J. 2009;43(6):366–373. doi: 10.1080/14017430903045350. [DOI] [PubMed] [Google Scholar]
- 33.Li SH, Lai TY, Sun Z, Han M, Moriyama E, Wilson B, et al. Tracking cardiac engraftment and distribution of implanted bone marrow cells: comparing intra-aortic, intravenous, and intramyocardial delivery. J Thorac Cardiovasc Surg. 2009;137(5):1225–1233. doi: 10.1016/j.jtcvs.2008.11.001. e1. [DOI] [PubMed] [Google Scholar]
- 34.Zimmet H, Porapakkham P, Porapakkham P, Sata Y, Haas SJ, Itescu S, et al. Short-and long-term outcomes of intracoronary and endogenously mobilized bone marrow stem cells in the treatment of ST-segment elevation myocardial infarction: a meta-analysis of randomized control trials. Eur J Heart Fail. 2012;14(1):91–105. doi: 10.1093/eurjhf/hfr148. [DOI] [PubMed] [Google Scholar]
- 35.Hibino N, Nalbandian A, Devine L, Martinez RS, McGillicuddy E, Yi T, et al. Comparison of human bone marrow mononuclear cell isolation methods for creating tissue-engineered vascular grafts: novel filter system versus traditional density centrifugation method. Tissue Eng Part C Methods. 2011;17(10):993–998. doi: 10.1089/ten.tec.2011.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111(17):2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 37.Bartunek J, Vanderheyden M, Vandekerckhove B, Mansour S, De Bruyne B, De Bondt P, et al. Intracoronary injection of CD133- positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction feasibility and safety. Circulation. 2005;112(9 Suppl):I178–I183. doi: 10.1161/CIRCULATIONAHA.104.522292. [DOI] [PubMed] [Google Scholar]
- 38.Ahmadi H, Farahani MM, Kouhkan A, Moazzami K, Fazeli R, Sadeghian H, et al. Five-year follow-up of the local autologous transplantation of CD133+ enriched bone marrow cells in patients with myocardial infarction. Arch Iran med. 2012;15(1):32–35. [PubMed] [Google Scholar]
- 39.Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126(5):551–568. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, et al. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114(20):2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 41.Dawn B, Tiwari S, Kucia MJ, Zuba-Surma EK, Guo Y, Sanganalmath SK, et al. Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells. 2008;26(6):1646–1655. doi: 10.1634/stemcells.2007-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the time randomized trial. JAMA. 2012;308(22):2380–2389. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choudry F, Hamshere S, Saunders N, Veerapen J, Bavnbek K, Knight C, et al. A randomized double-blind control study of early intra-coronary autologous bone marrow cell infusion in acute myocardial infarction: the REGENERATE-AMI clinical trial†. Eur Heart J. 2016;37(3):256–263. doi: 10.1093/eurheartj/ehv493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sürder D, Manka R, Moccetti T, Lo Cicero V, Emmert MY, Klersy C, et al. The effect of bone marrow derived mononuclear cell treatment, early or late after acute myocardial infarction: Twelve months CMR and long-term clinical results. Circ Res. 2016;119(3):481–490. doi: 10.1161/CIRCRESAHA.116.308639. [DOI] [PubMed] [Google Scholar]
- 45.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC).Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.