Abstract
Previous work showed that primary root elongation in maize (Zea mays L.) seedlings at low water potentials (ψw) requires the accumulation of abscisic acid (ABA) (R.E. Sharp, Y. Wu, G.S. Voetberg, I.N. Saab, M.E. LeNoble [1994] J Exp Bot 45: 1743–1751). The objective of the present study was to determine whether the inhibition of elongation in ABA-deficient roots is attributable to ethylene. At a ψw of −1.6 MPa, inhibition of root elongation in dark-grown seedlings treated with fluridone to impose ABA deficiency was largely prevented with two inhibitors of ethylene synthesis (aminooxyacetic acid and aminoethoxyvinylglycine) and one inhibitor of ethylene action (silver thiosulfate). The fluridone treatment caused an increase in the rate of ethylene evolution from intact seedlings. This effect was completely prevented with aminooxyacetic acid and also when ABA was supplied at a concentration that restored the ABA content of the root elongation zone and the root elongation rate. Consistent results were obtained when ABA deficiency was imposed using the vp5 mutant. Both fluridone-treated and vp5 roots exhibited additional morphological symptoms of excess ethylene. The results demonstrate that an important role of ABA accumulation in the maintenance of root elongation at low ψw is to restrict ethylene production.
Maintenance of root elongation at low water potentials (ψw) is considered to be an adaptive feature that promotes survival of the plant under water-limited conditions (Sharp and Davies, 1989; Spollen et al., 1993). For example, the primary root of maize (Zea mays L.) maintains substantial elongation at a ψw of −1.6 MPa, whereas shoot development is completely inhibited at around −0.8 MPa (Sharp et al., 1988). This differential response is advantageous for seedling establishment under dry conditions.
The mechanisms that allow roots to grow at low ψw have received little attention and are only beginning to be understood. Although hormones are likely to play an important regulatory role in the adaptation of root growth to water stress, the involvement of most of these compounds has not been elucidated. The exception is the accumulation of abscisic acid (ABA), which was shown to be required for maintenance of primary root elongation at low ψw in maize seedlings (Saab et al., 1990; Sharp et al., 1994). This was demonstrated by decreasing endogenous ABA levels chemically using fluridone, which inhibits carotenoid (and ABA) biosynthesis, or genetically using the vp5 mutant, in which carotenoid (and ABA) biosynthesis is deficient. At low ψw, root elongation rate of ABA-deficient seedlings was severely inhibited compared with untreated or wild-type seedlings, and fully recovered when the ABA content of the elongation zone was restored to normal levels with exogenous ABA. Since the seedlings were grown at near-saturation humidity in the dark, indirect effects of altered ABA levels on growth due to stomatal control of plant water balance or photosynthesis were avoided.
The role of ABA accumulation in the maintenance of root elongation at low ψw is not known. There have been several reports that applied ABA can inhibit ethylene production from various organs in a range of species (e.g. Gertman and Fuchs, 1972; Wright, 1980; Yoshii and Imaseki, 1981; Tan and Thimann, 1989). Furthermore, ABA-deficient mutants have been found to exhibit increased ethylene evolution from shoots (tomato: Tal et al., 1979) and whole plants (Arabidopsis: Rakitina et al., 1994). It was suggested by Wright (1980) that endogenous ABA accumulation may limit ethylene production during water stress, and that this interaction may help to determine many of the effects of water deficit, including the responses of root and leaf growth. These hypotheses have not been tested.
In this study, we examined whether elongation of ABA-deficient (fluridone-treated and vp5) maize primary roots at low ψw can be restored with inhibitors of ethylene synthesis or action, and whether ABA-deficiency causes an increase in the rate of ethylene production from water-stressed seedlings. The results show that an important role of ABA accumulation in the maintenance of root elongation at low ψw is to prevent excess ethylene production.
MATERIALS AND METHODS
Fluridone Experiments
For most experiments with fluridone, seeds of maize (Zea mays L. cv FR27 × FRMo17) were germinated for 32 h in well-moistened vermiculite (grade 3, Strong-Lite, Pine Bluff, AR) at 29°C and near-saturation humidity in the dark. Seedlings with primary roots about 5 mm in length were transplanted into Plexiglas boxes or glass beakers containing vermiculite at a ψw of −1.63 ± 0.18 MPa (mean ± sd of all experiments), which was obtained by thorough mixing with a small amount of water. The seedlings were then grown under the same conditions for up to 48 h (Sharp et al., 1988). Vermiculite ψw was measured for each experiment by isopiestic thermocouple psychrometry (Boyer and Knipling, 1965). When necessary for growth measurements and for harvesting, illumination was provided by a green safelight (Saab et al., 1990).
Fluridone (SePRO, Carmel, IN) was added at a final concentration of 1.5 μm to the water mixed with the vermiculite in which seeds were germinated and into which seedlings were transplanted. Details of fluridone preparation are described in Ober and Sharp (1994). Ethanol and Tween 20 (final concentrations of 0.006% and 0.002%, v/v, respectively) were added to control treatments. In previous work, 10 μm fluridone was used to impose ABA deficiency (e.g. Saab et al., 1990). To minimize potential side effects, the relationship of fluridone concentration to root tip ABA level was refined. It was found that inhibition of ABA accumulation at a ψw of −1.6 MPa was almost as large with 1.5 μm as with 10 μm fluridone (data not shown). Therefore, a fluridone concentration of 1.5 μm was used for all experiments.
Experiments to determine whether exogenous ABA could overcome the effects of fluridone were conducted at a later date. A different culture protocol was used because the properties of the vermiculite had changed (although the same brand was used) such that seedlings became Ca2+-deficient unless supplied with supplemental Ca2+ (M.A. Else and R.E. Sharp, unpublished data). Seeds were imbibed for 23 h in 1 mm CaSO4 and germinated for 29 h in vermiculite well moistened with 1 mm CaSO4 (with or without fluridone). Seedlings were then transplanted into vermiculite at a ψw of −1.6 MPa, which was obtained by mixing with 1 mm CaSO4 (with or without fluridone). Preliminary experiments at a range of Ca concentrations showed that seedlings grown using this protocol exhibited maximum root and shoot elongation rates at high and low ψw. (±)-ABA (Sigma-Aldrich, St. Louis) was added at a final concentration of 0.5 mm together with fluridone to the vermiculite into which the seedlings were transplanted, as described by Sharp et al. (1994). (ABA was not added prior to transplanting because it inhibits germination.) In these experiments, root length at transplanting was approximately 20 mm.
vp5 Experiments
Seeds of the vp5 mutant and wild-type maize were obtained by selfing plants grown from heterozygous seed (Maize Genetics Stock Center, Urbana, IL). Only those mutant kernels (identified by a lack of carotenoid pigmentation) that survived desiccation on the plant were used (Saab et al., 1990). The limited amount of such seed restricted the number of experiments that could be conducted with the mutant. Mutant and wild-type seedlings were grown using the first of the culture protocols described above, except that germination times and root lengths at transplanting, respectively, were 54 h and 5 to 10 mm for the wild type, and 54 to 72 h and 2 to 24 mm for vp5. The limited number of mutant seed required that all were used; analysis of the results showed no relationship between initial root length and root length increase after transplanting.
Inhibitors of Ethylene Synthesis and Action
Aminooxyacetic acid (AOA) and aminoethoxyvinylglycine (AVG) are inhibitors of pyridoxal phosphate-requiring enzymes including 1-aminocyclopropane-1-carboxylic acid (ACC) synthase, a key enzyme of ethylene synthesis, and silver thiosulfate (STS) inhibits ethylene action (Abeles et al., 1992). These inhibitors were used to test for the involvement of ethylene in the effects of fluridone and the vp5 mutation. In separate experiments, the different inhibitors were added to the water mixed with the vermiculite in which seeds were germinated and into which seedlings were transplanted. Solutions of STS were made as described by Cameron et al. (1985).
Root Growth and ABA Quantification
Primary root length increase at various times after transplanting to low ψw was determined either by marking the positions of the root apices on the face against which they were growing or by destructive harvesting. Effects of fluridone and the vp5 mutation on root tip swelling were quantified by measuring the spatial distribution of root diameter in the apical 15 mm at the end of experiments. Root tips were photographed immediately after harvest, and diameter profiles were measured from enlarged prints.
Root tips were harvested for measurement of ABA content by radioimmunoassay (Quarrie et al., 1988). Depending on the experiment, the apical 6 or 10 mm (excluding the root cap) was measured. This encompassed the elongation zone, which extends approximately 6 mm from the apex in untreated seedlings at a ψw of −1.6 MPa and is shortened in fluridone-treated seedlings (Sharp et al., 1988; Saab et al., 1992). Harvesting and extraction procedures and assay validation were described in Saab et al. (1990) and Sharp et al. (1994).
Ethylene Evolution
Ethylene evolution rate was measured from intact seedlings using a continuous flow-through system. After germination, up to 35 seedlings were transplanted into a Plexiglas cylinder (2 L) containing vermiculite at a ψw of −1.6 MPa. The cylinder was fitted with a lid containing a rubber O-ring, an air inlet at the bottom, and an air outlet at the top. Ethylene-free air flowed through the chamber at a rate of 40 mL min−1. To maintain the ψw of the vermiculite, the relative humidity of the air was increased prior to entering the chamber by bubbling through water at 50°C. Measurements showed that the vermiculite ψw decreased by only about 0.1 MPa during the 48-h experiments.
At various times after transplanting, samples (60 or 120 mL) of the exiting air stream were collected with syringes, and the ethylene was trapped by injection into a sample loop containing 100 mg of absorbent (Porapak S, Supelco, Bellefonte, PA) and kept at −95°C with melting acetone (De Greef et al., 1976). The sample loop was then heated with boiling water to release the ethylene into the carrier gas (helium) stream and onto a packed alumina F1 column of a gas chromatograph (model no. 3400cx, Varian, Palo Alto, CA) equipped with a photo-ionization detector (lamp energy 10.6 eV). In preliminary experiments for all treatments, the putative ethylene peak was confirmed to be an olefin by its ability to be trapped in a HgClO4 solution and released with the addition of LiCl (Young et al., 1952). In subsequent experiments, the peak was identified by its retention time only. The rate of ethylene evolution from the chamber was divided by the number of seedlings to obtain the average rate per seedling.
Statistical Analysis
Analyses of variance were performed with means compared using Fisher's lsd test at the P = 0.05 level.
RESULTS
Root Elongation of Fluridone-Treated Seedlings
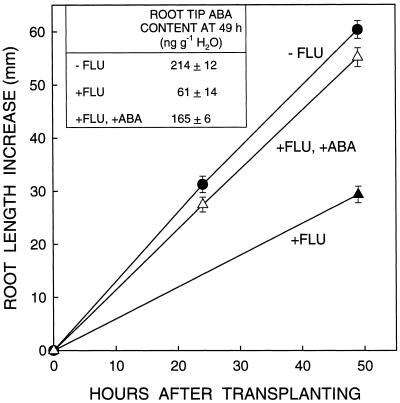
Root elongation of seedlings treated with 1.5 μm fluridone was inhibited by about 45% compared with untreated seedlings after transplanting to a ψw of −1.6 MPa (Figs. 1–3). This was associated with a large decrease in the accumulation of ABA in the apical 6 mm (Fig. 1 inset; the ABA level at high ψw is 10 to 20 ng g−1 H2O in both fluridone-treated and untreated root tips [Saab et al., 1990, 1992]). Preliminary experiments in which a range of ABA concentrations were mixed with the vermiculite into which the seedlings were transplanted determined that an exogenous ABA concentration of 0.5 mm was optimal for restoration of root elongation in seedlings treated with 1.5 μm fluridone. Figure 1 shows that the addition of 0.5 mm ABA almost fully prevented the inhibition of root elongation, in association with substantial restoration of the root tip ABA level (Fig. 1, inset). Root elongation was restored by 83% at 49 h after transplanting. These results show that virtually all of the inhibition of root elongation caused by 1.5 μm fluridone is attributable to ABA deficiency. The requirement for such a high applied ABA concentration to restore the internal ABA content was due to limited uptake from the dry vermiculite (Sharp et al., 1994).
Figure 1.
Effect of treatment with fluridone (FLU) with or without the addition of ABA to the vermiculite on root length increase after transplanting to a ψw of −1.6 MPa. Length increase at 24 h was determined by marking the position of the root apices (in the +FLU treatment, many roots grew into the vermiculite and were thus not visible), and at 49 h by destructive harvesting. Data are means ± se (n = 11–16). The experiment was repeated several times with similar results. Inset, ABA content of the root apical 6 mm at 49 h. Data are means ± se of five samples of three root tips each.
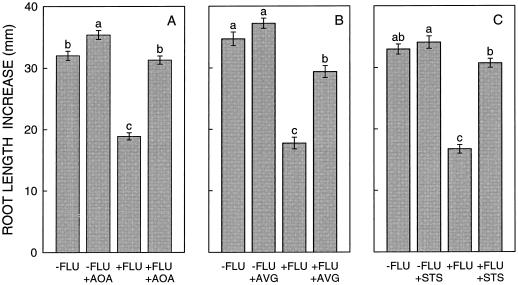
Figure 3.
Effect of treatment with AOA (A), AVG (B), and STS (C) on root length increase of untreated and fluridone (FLU)-treated seedlings 42 h after transplanting to a ψw of −1.6 MPa. Data are means ± se (n = 54–91) combined from three to five experiments. Within each panel, bars with different letters are significantly different at the 0.05 level.
In previous work in which fluridone was applied at 10 μm, root elongation was inhibited about 65%, and a higher applied ABA concentration (0.7 mm) and a longer time after transplanting were required to restore the root elongation rate (Sharp et al., 1994). The faster restoration in the present experiments was probably due at least partly to longer root lengths at transplanting, which increased the root surface area for ABA uptake and may have slowed the rate of stress development (and therefore ABA deficiency) by providing a larger internal source of water.
Inhibitors of Ethylene Synthesis or Action Restore Root Elongation of Fluridone-Treated Seedlings
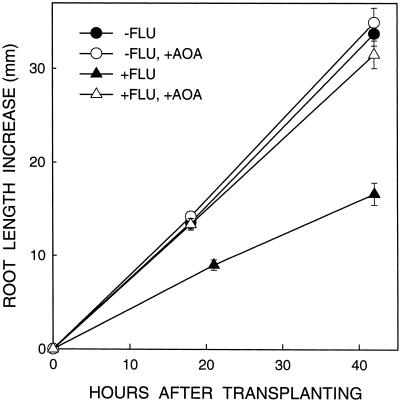
If the inhibition of root elongation in ABA-deficient seedlings at low ψw is caused by ethylene, then elongation should be at least partly restored by inhibitors of ethylene synthesis or action. Therefore, the effects of AOA and AVG, which inhibit ethylene synthesis, and STS, which inhibits ethylene action, were individually examined. The use of three inhibitors was a precaution because of possible side effects of each compound (Abeles et al., 1992). Preliminary experiments determined that the optimum concentrations for restoration of root elongation in fluridone-treated seedlings were 732 μm AOA, 43 μm AVG, and 2.5 mm STS. A typical experiment using AOA is shown in Figure 2, and the mean results from several such experiments using each of the inhibitors are shown in Figure 3. Representative seedlings are illustrated in Figure 4.
Figure 2.
Effect of treatment with AOA on root length increase of untreated and fluridone (FLU)-treated seedlings after transplanting to a ψw of −1.6 MPa. Length increases were determined as described in Figure 1, plus an additional harvest of +FLU seedlings was made at 21 h. Data are means ± se (n = 16–18).
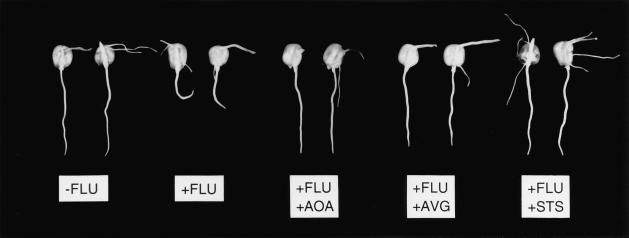
Figure 4.
Representative seedlings treated with or without fluridone (FLU), or with fluridone and AOA, AVG, or STS, 42 h after transplanting to a ψw of −1.6 MPa. Root lengths of selected seedlings were within ±11% of the treatment means in the particular experiments.
Treatment with AOA almost completely prevented the inhibition of root elongation rate in fluridone-treated seedlings in the 42 h after transplanting to a ψw of −1.6 MPa (Fig. 2). The mean restoration of root length increase compared with untreated roots at the end of the experiments was 95% (Figs. 3 and 4). The AOA treatment also caused a small (11%) increase in root elongation of control seedlings. Accordingly, restoration from the effect of fluridone was lessened to 75% compared with the AOA-treated control. However, the absolute promotion of root length by AOA was much greater for fluridone-treated seedlings (12.4 mm) than for the control (3.4 mm).
Results with AVG and STS were similar. Root elongation of fluridone-treated seedlings was restored by 69% and 86%, respectively, compared with untreated seedlings (Figs. 3 and 4). AVG and STS also caused a slight (not significant) increase in root elongation of control seedlings, so that restoration from the effect of fluridone was reduced to 60% and 81%, respectively, compared with the inhibitor-treated controls. As with AOA, the absolute increases in root length caused by AVG and STS were much greater for fluridone-treated seedlings (11.6 and 14.0 mm, respectively) than for the controls (2.5 and 1.1 mm, respectively). Preliminary experiments using seedlings treated with 10 μm fluridone showed that root elongation could also be substantially restored with 2,5-norbornadiene, a competitive inhibitor of ethylene binding, and both α-aminoisobutyric acid and CoCl2, which inhibit ACC oxidase (data not shown).
To ensure that the inhibitors of ethylene synthesis or action did not restore root elongation of fluridone-treated seedlings by restoring root tip ABA levels, the ABA content of the apical 10 mm was measured 20 h after transplanting in all treatments (Table I). Neither AOA, AVG, nor STS had any effect on the ABA content with or without treatment with fluridone. It should be noted that the ABA contents of untreated and fluridone-treated root tips shown in Table I are lower than those shown in Figure 1, because the apical 10 and 6 mm, respectively, were measured. Previous work showed that the ABA content increases steeply in the apical few millimeters of roots at low ψw (Saab et al., 1992).
Table I.
Effect of treatment with AOA, AVG, or STS with or without treatment with fluridone (FLU) on the ABA content of the root apical 10 mm, 20 h after transplanting to a ψw of −1.6 MPa
| Inhibitor | Root Tip ABA Content
|
|
|---|---|---|
| −FLU | +FLU | |
| ng g−1 H2O | ||
| None | 140 ± 8 | 44 ± 4 |
| AOA | 149 ± 6 | 39 ± 2 |
| AVG | 129 ± 10 | 39 ± 2 |
| STS | 146 ± 9 | 36 ± 2 |
Data are means ± se of five samples of three root tips each. The experiment was repeated with similar results.
The results shown in Figures 1 to 4 and Table I indicate that the inhibition of root elongation caused by ABA deficiency in fluridone-treated seedlings at low ψw is largely due to ethylene.
Ethylene Evolution Is Increased in Fluridone-Treated Seedlings
Measurements of ethylene evolution rate were made from whole seedlings to assess whether ABA deficiency at low ψw causes an increase in ethylene production. Shoot development was minimal at the time of transplanting and was completely inhibited thereafter, and seminal root development during the experiments was limited (Fig. 4). Therefore, the measurements reflect rates of ethylene evolution from the primary root plus an unknown contribution from the kernel.
Figure 5 shows that fluridone-treated seedlings exhibited a 5-fold increase in ethylene evolution rate compared with untreated seedlings at 20 h after transplanting to a ψw of −1.6 MPa. This declined to a 3-fold enhancement at 40 h, which is consistent with previous observations that the effects of treatment with fluridone on ABA accumulation and root elongation decrease with time after transplanting (Saab et al., 1990; Sharp et al., 1994). This probably reflects progressive dilution and/or metabolism of the fluridone absorbed during imbibition, combined with limited fluridone uptake from the dry vermiculite.
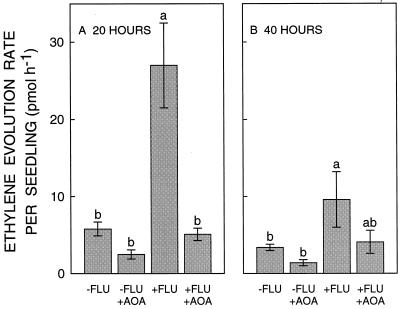
Figure 5.
Ethylene evolution rate from untreated and fluridone (FLU)-treated seedlings with or without treatment with AOA at 20 h (A) and 40 h (B) after transplanting to a ψw of −1.6 MPa. Data are means ± se of four experiments. Within each panel, bars with different letters are significantly different at the 0.05 level. At 40 h, mean seedling fresh weight ranged from 578 to 589 mg in the different treatments.
Treatment with AOA completely prevented the fluridone-induced increase in ethylene evolution at both 20 and 40 h after transplanting (Fig. 5). In these experiments, root elongation was inhibited by 47% in fluridone-treated compared with untreated seedlings, and AOA restored elongation of fluridone-treated roots by 78% and 76%, respectively, compared with untreated and AOA-treated controls. These effects are very similar to those described in Figures 2 and 3, showing that the continuous flow of air that was used to collect evolved ethylene did not affect the results. Treatment with AOA also caused a small decrease in the ethylene evolution rate of control seedlings, although this effect was not significant at either time. This may have caused the slight promotion of root elongation in this treatment (Fig. 3). The differences in ethylene evolution rate among treatments were not attributable to differences in seedling fresh weight, which were similar in all cases (Fig. 5, legend).
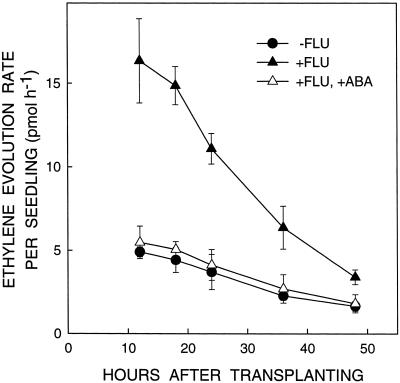
To confirm that the increase in ethylene evolution rate from fluridone-treated seedlings was due to ABA deficiency and not to other effects of fluridone, exogenous ABA was added at a concentration of 0.5 mm, the same concentration used to restore root elongation (Fig. 1). Figure 6 shows that this treatment completely prevented the fluridone-induced increase in ethylene evolution from 12 to 48 h after transplanting to low ψw. The time course of the effect of fluridone on ethylene evolution was similar to that shown in Figure 5, but the increase was less pronounced, perhaps because of the different culture protocol used (see “Materials and Methods”).
Figure 6.
Effect of treatment with fluridone with or without the addition of ABA to the vermiculite on seedling ethylene evolution rate after transplanting to a ψw of −1.6 MPa. Data are means ± se of three experiments. In these experiments, the ABA treatment restored root elongation by 68% at 48 h.
The ability of both AOA and ABA to prevent the increase in ethylene evolution and the inhibition of root elongation in fluridone-treated seedlings indicates that increased ethylene production is an important cause of root growth inhibition in ABA-deficient seedlings at low ψw.
Root Elongation and Ethylene Evolution in vp5 Seedlings
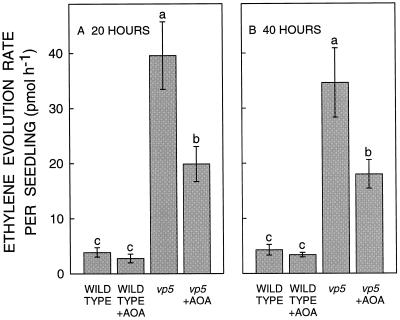
Similar studies were conducted with the vp5 mutant to strengthen the validity of the fluridone experiments. Root elongation of the mutant was severely inhibited (by 67%) compared with the wild type after transplanting to a ψw of −1.6 MPa (Fig. 7). This was associated with a greatly decreased level of ABA in the apical 10 mm (Fig. 7, inset). These effects were more pronounced than in fluridone-treated seedlings (Figs. 1–3; Table I) and were associated with a greater increase in ethylene evolution. The ethylene evolution rate per seedling increased by 10-fold to 40 pmol h−1 in vp5 relative to wild type at 20 h after transplanting (Fig. 8), compared with a 5-fold increase to 27 pmol h−1 observed in fluridone-treated seedlings grown under the same conditions (Fig. 5). Furthermore, in vp5 seedlings the increase was sustained at 40 h, in contrast to the decline observed in the fluridone treatment. Since the mutant (and wild-type) seedlings weighed only about 60% as much as the hybrid seedlings (Figs. 5 and 8, legends), the greater ethylene evolution rate of vp5 compared with fluridone-treated seedlings was even more pronounced on a fresh weight basis.
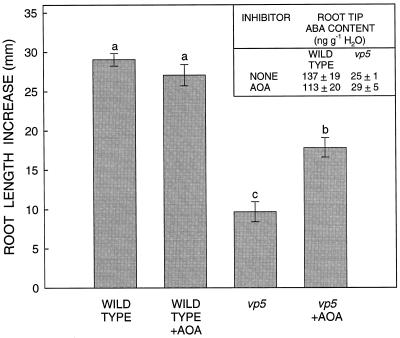
Figure 7.
Root length increase of wild-type and vp5 seedlings with or without treatment with AOA 40 h after transplanting to a ψw of −1.6 MPa. Data are means ± se (n = 12–21). Bars with different letters are significantly different at the 0.05 level. Inset, ABA content of the root apical 10 mm at 40 h. Data are means ± se of three samples of three root tips each. The experiment was repeated with similar results.
Figure 8.
Ethylene evolution rate from wild-type and vp5 seedlings with or without treatment with AOA at 20 h (A) and 40 h (B) after transplanting to a ψw of −1.6 MPa. Data are means ± se of four wild-type and six vp5 experiments. Within each panel, bars with different letters are significantly different at the 0.05 level. At 40 h, mean seedling fresh weight ranged from 323 to 337 mg in the different treatments.
Treatment with AOA (732 μm, as used in the fluridone experiments) caused a significant restoration of root elongation in vp5 seedlings (Fig. 7), but less than the almost complete restoration observed in experiments with fluridone (Fig. 3). Elongation was restored by 42% and 47%, respectively, compared with untreated and AOA-treated wild-type seedlings (Fig. 7). The same restoration was obtained using 900 μm AOA, and higher concentrations were less effective. As in the fluridone experiments, the AOA treatment had no effect on the ABA content of the root tips of vp5 or wild-type seedlings (Fig. 7, inset). Similar results were obtained in one experiment using STS, in which the maximum restoration of elongation was 28% and 32%, respectively, compared with untreated and STS-treated wild-type seedlings (data not shown).
Measurements of ethylene evolution suggest that the incomplete restoration by AOA of root elongation in vp5 seedlings was due to incomplete prevention of the increase in ethylene evolution. At both 20 and 40 h after transplanting, AOA prevented only 55% of the increase (Fig. 8), in contrast to the complete prevention observed in fluridone-treated seedlings (Fig. 5). In these experiments, root elongation was restored by 37% and 32%, respectively, compared with untreated and AOA-treated wild-type seedlings. The difference in effectiveness of AOA between the vp5 and fluridone experiments suggests that the vp5 mutation causes a more pervasive inhibition of ABA content, such that AOA did not penetrate to all the ABA-deficient cells. Consistent with this explanation, in an experiment using seeds from a single ear, AOA restored root elongation in fluridone-treated wild-type seedlings by 76% but in vp5 seedlings by only 34% (data not shown).
The large increase in ethylene evolution rate from vp5 seedlings and the partial recovery of both root elongation and ethylene evolution by treatment with AOA are consistent with the conclusion that increased ethylene production is an important cause of the inhibition of root elongation in ABA-deficient seedlings at low ψw.
Morphology of ABA-Deficient Roots at Low ψw
In addition to the inhibition of elongation, other morphological characteristics of the ABA-deficient roots at low ψw were consistent with an involvement of increased ethylene production. Compared with untreated or wild-type seedlings, root tips of fluridone-treated and vp5 seedlings grown at a ψw of −1.6 MPa were swollen primarily beyond the apical 2 mm (Fig. 9). Exogenous ethylene inhibits elongation and causes a similar pattern of swelling in maize primary roots at high ψw (Moss et al., 1988; Whalen and Feldman, 1988). In both fluridone-treated and vp5 roots, most of the increase in diameter resulted from a greater expansion of the cortex (data not shown). Root tip swelling resulting from treatment with ethylene is also attributable primarily to increase in cortical thickness in roots of maize (Whalen and Feldman, 1988) and barley (Jackson, 1983). In addition, the ABA-deficient roots exhibited a loss of vertical orientation (Fig. 4), which also results when maize roots are treated with ethylene (Curtis, 1968). Both the swelling and the loss of vertical orientation were largely prevented by the addition of 0.5 mm ABA and by treatment with AOA, AVG, and STS (Fig. 4) (determined by visual assessment at the end of the experiments).
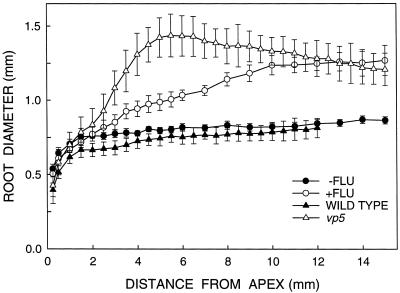
Figure 9.
Diameter as a function of distance from the apex for roots of untreated and fluridone-treated hybrid seedlings (40 h after transplanting, n = 5) and of wild-type and vp5 seedlings (48 h after transplanting, n = 6–8) growing at a ψw of −1.6 MPa. Data are means ± se of roots that elongated within ±10% of the mean rate from at least 20 seedlings per treatment.
In the fluridone-treated roots, swelling became maximal at 10 mm from the apex, where the diameter was 50% greater than that of untreated roots (Fig. 9). In vp5 roots, the increase in diameter was more pronounced and occurred more steeply, reaching a maximum that was 90% greater than that of the wild type at 5.5 mm from the apex. The diameter of the vp5 roots decreased steadily at greater distances from the apex, but was still larger than the wild type at 15 mm. The difference in pattern between the fluridone-treated and vp5 roots is consistent with the decreasing effect of fluridone on ethylene evolution with time after transplanting (Figs. 5 and 6). In contrast, the diameter profile of the vp5 roots indicates that the effect of the mutation increased with time, probably as the tissue water status declined.
DISCUSSION
The combined results of this study provide compelling evidence that an important role of endogenous ABA accumulation in the maintenance of maize primary root elongation at low ψw is to prevent excess ethylene production. When ABA deficiency was imposed at a ψw of −1.6 MPa by treatment with fluridone, root elongation was largely restored by three inhibitors of ethylene synthesis or action, demonstrating that the inhibition of root elongation was primarily attributable to ethylene. The ethylene evolution rate of fluridone-treated seedlings increased several fold, and this effect was prevented when ABA was supplied at a concentration that restored both the ABA content of the root elongation zone and the root elongation rate. The consistent results obtained when ABA deficiency was imposed using the vp5 mutant confirm that the increase in ethylene production was not a side effect of the use of fluridone. It should be noted that our findings do not exclude the possibility that the sensitivity of root elongation to ethylene was also increased by ABA deficiency. This question is under investigation.
In addition, since none of the inhibitors of ethylene synthesis or action substantially increased root elongation when ABA deficiency was not imposed, the study also indicates that ethylene is not an important cause of the inhibition of elongation in water-stressed roots that accumulate normal levels of ABA. (At a ψw of −1.6 MPa, maize primary root elongation is about one-third of the rate at high ψw [Sharp et al., 1988].) The possible involvement of ethylene in the inhibition of growth during water stress is a long-standing question (El-Beltagy and Hall, 1974), but to our knowledge there is no previous information in relation to root growth.
Relationship of the ABA-Ethylene Interaction to Other Processes of Root Elongation
In previous work, ABA accumulation was shown to be required for two other responses thought to contribute to the ability of the maize primary root to elongate at low ψw. These are increases in the activity of the putative wall-loosening enzyme xyloglucan endotransglycosylase (XET) (Wu et al., 1994) and in the concentration of Pro (Ober and Sharp, 1994) within the elongation zone. A preliminary study suggested that the inhibitory effects of ABA deficiency on these responses are not caused by the increase in ethylene production. In fluridone-treated seedlings supplied with AOA, neither the activity of XET nor the concentration of Pro was restored (Sharp et al., 1998). This was probably not due to toxic effects of AOA because there was little effect on XET activity or Pro level in roots of control seedlings. Since AOA almost completely restored root elongation (Fig. 3), the increases in XET activity and Pro concentration (at least to their normal extent) are apparently not essential for root elongation at low ψw (discussed further in Sharp et al., 1998).
At low ψw, the maize primary root becomes thinner, which is believed to be adaptive by increasing the efficiency of resource utilization in the exploration of new soil volume for water (Sharp et al., 1988; Liang et al., 1997). The mechanism of this response is unknown (Baskin et al., 1999). Since ethylene increases lateral expansion of roots (Moss et al., 1988; Whalen and Feldman, 1988), it is tempting to speculate that root thinning at low ψw is related to the restriction of ethylene production by ABA accumulation. However, the pattern of swelling in the ABA-deficient roots suggests that this effect was not a reversal of water-stress-induced thinning. First, the diameter of the apical millimeter was minimally responsive to ABA deficiency (Fig. 9; Saab et al., 1992), whereas this region exhibits the largest decrease in lateral expansion rates at low ψw (Liang et al., 1997). Second, the vp5 roots at low ψw exhibited a maximum diameter that exceeded that of vp5 or wild-type roots at high ψw (data not shown), despite having a higher ABA content (Fig. 7, inset; Saab et al., 1990). These observations make it unlikely that root thinning at low ψw is attributable to the restriction of ethylene production by ABA accumulation.
Generality of the ABA-Ethylene Interaction
Our results confirm that an important role of endogenous ABA accumulation is to limit ethylene production. Moreover, to our knowledge, the study provides the first demonstration that this interaction is involved in the effects of ABA status on plant growth. These ideas were first suggested by Wright (1980) and developed further by Bradford and Hsiao (1982), and were based on the finding that pretreatment with ABA prevented the increase in ethylene production caused by wilting of excised wheat leaves. Results of such experiments have been diverse, however. Although several other studies reported that ABA treatments inhibited ethylene production, there are also many reports of ABA-stimulated ethylene production (Riov et al., 1990 and references therein). Interpretation of these results is further complicated by the uncertainty that effects of applied ABA at high ψw are predictive of the role of endogenous ABA accumulation at low ψw (Trewavas and Jones, 1991; Sharp et al., 1994), and because in most cases excised tissues were employed. Morgan et al. (1990) and Narayana et al. (1991) have shown that the use of excised plant parts can lead to erroneous conclusions in studies of ethylene production, particularly concerning the effects of water stress. Our approach, using chemical and genetic means to manipulate endogenous ABA levels at low ψw, and our use of intact seedlings for ethylene measurements, avoided these concerns.
Consistent with our results, it was reported that ethylene production is enhanced in ABA-deficient mutants of tomato (Tal et al., 1979) and Arabidopsis (Rakitina et al., 1994) grown under well-watered conditions. In the flacca mutant of tomato, it was also shown that ethylene production could be restored to normal levels with exogenous ABA. However, it is uncertain whether the increase in ethylene evolution in those studies was a direct result of ABA deficiency, or if it was an indirect effect of decreased plant water status. Under transpiring conditions, ABA-deficient mutants typically exhibit high stomatal conductance and wilting (Arabidopsis: Koornneef et al., 1982; tomato: Bradford, 1983), and there are many reports that ethylene production can be increased by plant water deficits (but see Morgan et al. [1990]). In fact, Tal et al. (1979) showed that the greater ethylene evolution of flacca was partially to fully prevented (depending on plant age) by growing the plants at high humidity. In the present study, the seedlings were grown under conditions of near-zero transpiration (minimal shoot development, darkness, and near-saturation humidity). Accordingly, the increase in ethylene production resulting from ABA deficiency under water stress was not an indirect effect of differences in stomatal control of plant water balance between treatments.
The possibility that an interaction with ethylene production may be involved in the effects of ABA deficiency on growth has not to our knowledge been assessed in previous studies. Leaf and root growth of ABA-deficient mutants are often substantially inhibited compared with the corresponding wild types (Quarrie, 1987). The mutants of tomato also exhibit morphological symptoms characteristic of excess ethylene, such as leaf epinasty and adventitious rooting (Tal, 1966; Nagel et al., 1994). However, several authors have attributed the inhibition of leaf growth in the tomato mutants to their adverse water relations (Bradford, 1983; Neill et al., 1986; Trewavas and Jones, 1991; Nagel et al., 1994). We have recently shown that when flacca is grown throughout development at the same leaf ψw as well-watered wild-type plants, leaf growth remains greatly inhibited but can be substantially restored by applying ABA or STS (Sharp, et al., 2000). These results indicate that normal ABA levels are required to prevent ethylene-induced inhibition of leaf growth in tomato, at least in well-watered plants, in agreement with the findings of the present study for water-stressed maize roots.
In contrast, our previous work with maize seedlings showed that ABA deficiency at low ψw (either in the vp5 mutant or imposed using fluridone) was associated with increased shoot growth, indicating that ABA accumulation was causally related to shoot growth inhibition (Saab et al., 1990, 1992). This effect of ABA also appears to involve a restriction of ethylene synthesis or sensitivity: preliminary experiments showed that fluridone-induced growth promotion could be prevented by treatment with STS, and that shoot growth could also be increased by applying ACC or ethylene (Feng, 1996). These findings are consistent with reports that ethylene stimulates mesocotyl growth in some species (Suge, 1971; Cornforth and Stevens, 1973).
The commonality of these observations suggests that restriction of ethylene production may be a widespread function of ABA. Depending on the response to ethylene of the organ in question, ABA accumulation may thereby play a role in growth maintenance or inhibition in response to a range of adverse environmental conditions.
Footnotes
This work was supported by award no. 95–37100–1601 from the National Research Initiative Competitive Grants Program, U.S. Department of Agriculture (to R.E.S. and W.G.S.) and by the University of Missouri Food for the 21st Century Program (R.E.S.). This is contribution no. 12,970 from the Missouri Agricultural Experiment Station journal series.
LITERATURE CITED
- Abeles FB, Morgan PW, Saltveit ME., Jr . Ethylene in Plant Biology. Ed 2. San Diego: Academic Press; 1992. [Google Scholar]
- Baskin TI, Meekes HTHM, Liang BM, Sharp RE. Regulation of growth anisotropy in well-watered and water-stressed maize roots. II. Role of cortical microtubules and cellulose microfibrils. Plant Physiol. 1999;119:681–692. doi: 10.1104/pp.119.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JS, Knipling EB. Isopiestic technique for measuring leaf water potentials with a thermocouple psychrometer. Proc Nat Acad Sci USA. 1965;54:1044–1051. [PMC free article] [PubMed] [Google Scholar]
- Bradford KJ. Water relations and growth of the flaccatomato mutant in relation to abscisic acid. Plant Physiol. 1983;72:251–255. doi: 10.1104/pp.72.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford KJ, Hsiao TC. Physiological responses to moderate water stress. In: Lange OL, Nobel PS, Osmond CB, Zeigler H, editors. Encyclopedia of Plant Physiology (New Series) 12B: Physiological Plant Ecology II. Berlin: Springer-Verlag; 1982. pp. 263–324. [Google Scholar]
- Cameron AC, Heins RD, Fonda HN. Influence of storage and mixing factors on the biological activity of silver thiosulfate. Sci Hortic. 1985;26:167–174. [Google Scholar]
- Cornforth IS, Stevens RJ. Ethylene and the germination and early growth of barley. Plant Soil. 1973;38:581–587. [Google Scholar]
- Curtis RW. Mediation of a plant response to malformin by ethylene. Plant Physiol. 1968;43:76–80. doi: 10.1104/pp.43.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Greef J, De Proft M, De Winter F. Gas chromatographic determination of ethylene in large air volumes at the fractional parts-per-billion level. Anal Chem. 1976;48:38–41. [Google Scholar]
- El-Beltagy AS, Hall MA. Effect of water stress upon endogenous ethylene levels in Vicia faba. New Phytol. 1974;73:47–60. [Google Scholar]
- Feng X. Is ABA an inhibitor or promoter of shoot growth in water-stressed plants? MS thesis. Columbia: University of Missouri; 1996. [Google Scholar]
- Gertman E, Fuchs Y. Effect of abscisic acid and its interaction with other plant hormones on ethylene production in two plant systems. Plant Physiol. 1972;50:194–195. doi: 10.1104/pp.50.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB. Regulation of root growth and morphology by ethylene and other externally applied growth substances. In: Jackson MB, Stead AD, editors. Growth Regulators in Root Development, monograph 10. Wantage, UK: British Plant Growth Regulator Group; 1983. pp. 103–116. [Google Scholar]
- Koornneef M, Jorna ML, Brinkhorst-van der Swan DLC, Karssen CM. The isolation of abscisic acid (ABA) deficient mutants by selection of induced revertants in non-germinating gibberellin sensitive lines of Arabidopsis thaliana(L.) Heynh. Theor Appl Genet. 1982;61:385–393. doi: 10.1007/BF00272861. [DOI] [PubMed] [Google Scholar]
- Liang BM, Sharp RE, Baskin TI. Regulation of growth anisotropy in well-watered and water-stressed maize roots. I. Spatial distribution of longitudinal, radial, and tangential expansion rates. Plant Physiol. 1997;115:101–111. doi: 10.1104/pp.115.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PW, He C-J, De Greef JA, De Proft MP. Does water deficit stress promote ethylene synthesis by intact plants? Plant Physiol. 1990;94:1616–1624. doi: 10.1104/pp.94.4.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss GI, Hall KC, Jackson MB. Ethylene and the responses of roots of maize (Zea maysL.) to physical impedance. New Phytol. 1988;109:303–311. [Google Scholar]
- Nagel OW, Konings H, Lambers H. Growth rate, plant development and water relations of the ABA-deficient tomato mutant sitiens. Physiol Plant. 1994;92:102–108. [Google Scholar]
- Narayana I, Lalonde S, Saini HS. Water-stress-induced ethylene production in wheat: a fact or artifact? Plant Physiol. 1991;96:406–410. doi: 10.1104/pp.96.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill SJ, McGaw BA, Horgan R. Ethylene and 1-aminocyclopropane-1-carboxylic acid production in flacca, a wilty mutant of tomato, subjected to water deficiency and pre-treatment with abscisic acid. J Exp Bot. 1986;37:535–541. [Google Scholar]
- Ober ES, Sharp RE. Proline accumulation in maize (Zea maysL.) primary roots at low water potentials. I. Requirement for increased levels of abscisic acid. Plant Physiol. 1994;105:981–987. doi: 10.1104/pp.105.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarrie SA. Use of genotypes differing in endogenous abscisic acid levels in studies of physiology and development. In: Hoad GV, Lenton JR, Jackson MB, Atkin RK, editors. Hormone Action in Plant Development: A Critical Appraisal. London: Butterworths; 1987. pp. 89–105. [Google Scholar]
- Quarrie SA, Whitford PN, Appleford NEJ, Wang TL, Cook SK, Henson IE, Loveys BR. A monoclonal antibody to (S)-abscisic acid: its characterization and use in a radioimmunoassay for measuring abscisic acid in crude extracts of cereal and lupin leaves. Planta. 1988;173:330–339. doi: 10.1007/BF00401020. [DOI] [PubMed] [Google Scholar]
- Rakitina TY, Vlasov PV, Jalilova FK, Kefeli VI. Abscisic acid and ethylene in mutants of Arabidopsis thalianadiffering in their resistance to ultraviolet (UV-B) radiation stress. Russ J Plant Physiol. 1994;41:599–603. [Google Scholar]
- Riov J, Dagan E, Goren R, Yang SF. Characterization of abscisic acid-induced ethylene production in citrus leaf and tomato fruit tissues. Plant Physiol. 1990;92:48–53. doi: 10.1104/pp.92.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab IN, Sharp RE, Pritchard J. Effect of inhibition of ABA accumulation on the spatial distribution of elongation in the primary root and mesocotyl of maize at low water potentials. Plant Physiol. 1992;99:26–33. doi: 10.1104/pp.99.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab IN, Sharp RE, Pritchard J, Voetberg GS. Increased endogenous abscisic acid maintains primary root growth and inhibits shoot growth of maize seedlings at low water potentials. Plant Physiol. 1990;93:1329–1336. doi: 10.1104/pp.93.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp RE, Davies WJ. Regulation of growth and development of plants growing with a restricted supply of water. In: Jones HG, Flowers TL, Jones MB, editors. Plants under Stress. Cambridge, UK: Cambridge University Press; 1989. pp. 71–93. [Google Scholar]
- Sharp RE, LeNoble ME, Else MA, Thorne ET, Gherardi F (2000) Endogenous ABA maintains shoot growth in tomato independently of effects on plant water balance: evidence for the involvement of ethylene. J Exp Bot (in press) [DOI] [PubMed]
- Sharp RE, LeNoble ME, Spollen WG. Regulation of root growth maintenance at low water potentials. In: Flores HE, Lynch JP, Eissenstat D, editors. Radical Biology: Advances and Perspectives on the Function of Plant Roots. Rockville, MD: American Society of Plant Physiologists; 1998. pp. 104–115. [Google Scholar]
- Sharp RE, Silk WK, Hsiao TC. Growth of the maize primary root at low water potentials. I. Spatial distribution of expansive growth. Plant Physiol. 1988;87:50–57. doi: 10.1104/pp.87.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp RE, Wu Y, Voetberg GS, Saab IN, LeNoble ME. Confirmation that abscisic acid accumulation is required for maize primary root elongation at low water potentials. J Exp Bot. 1994;45:1743–1751. [Google Scholar]
- Spollen WG, Sharp RE, Saab IN, Wu Y. Regulation of cell expansion in roots and shoots at low water potentials. In: Smith JAC, Griffiths H, editors. Water Deficits: Plant Responses from Cell to Community. Oxford: BIOS Scientific Publishers; 1993. pp. 37–52. [Google Scholar]
- Suge H. Stimulation of oat and rice mesocotyl growth by ethylene. Plant Cell Physiol. 1971;12:831–837. [Google Scholar]
- Tal M. Abnormal stomatal behavior in wilty mutants of tomato. Plant Physiol. 1966;41:1387–1391. doi: 10.1104/pp.41.8.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M, Imber D, Erez A, Epstein E. Abnormal stomatal behavior and hormonal imbalance in flacca, a wilty mutant of tomato. V. Effect of abscisic acid on indoleacetic acid metabolism and ethylene evolution. Plant Physiol. 1979;63:1044–1048. doi: 10.1104/pp.63.6.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z-Y, Thimann KV. The roles of carbon dioxide and abscisic acid in the production of ethylene. Physiol Plant. 1989;75:13–19. [Google Scholar]
- Trewavas AJ, Jones HG. An assessment of the role of ABA in plant development. In: Davies WJ, Jones HG, editors. Abscisic Acid: Physiology and Biochemistry. Oxford: BIOS Scientific Publishers; 1991. pp. 169–188. [Google Scholar]
- Whalen MC, Feldman LJ. The effect of ethylene on root growth of Zea maysseedlings. Can J Bot. 1988;66:719–723. doi: 10.1139/b88-104. [DOI] [PubMed] [Google Scholar]
- Wright STC. The effect of plant growth regulator treatments on the levels of ethylene emanating from excised turgid and wilted wheat leaves. Planta. 1980;148:381–388. doi: 10.1007/BF00388127. [DOI] [PubMed] [Google Scholar]
- Wu Y, Spollen WG, Sharp RE, Hetherington PR, Fry SC. Root growth maintenance at low water potentials. Increased activity of xyloglucan endotransglycosylase and its possible regulation by abscisic acid. Plant Physiol. 1994;106:607–615. doi: 10.1104/pp.106.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii H, Imaseki H. Biosynthesis of auxin-induced ethylene: effects of indole-3-acetic acid, benzyladenine and abscisic acid on endogenous levels of 1-aminocyclopropane-1-carboxylic acid (ACC) and ACC synthase. Plant Cell Physiol. 1981;22:369–379. [Google Scholar]
- Young RE, Pratt HK, Biale JB. Manometric determination of low concentrations of ethylene with particular reference to plant material. Anal Chem. 1952;24:551–555. [Google Scholar]