Abstract
Objective
Disruption of vitamin D signaling in rodents causes activation of the renin angiotensin system (RAS) and development of hypertension. Observational studies in humans found lower circulating 25-hydroxyvitamin D (25[OH]D) is associated with increased RAS activity and blood pressure (BP). We performed the first randomized control trial to investigate the effects of vitamin D supplementation on the RAS in humans.
Methods
Vitamin D deficient, (25[OH]D ≤20 ng/mL), overweight individuals without hypertension were randomized into a double-blind, placebo-controlled trial of 8 weeks treatment with ergocalciferol or placebo. Kidney-specific RAS activity, measured using renal plasma flow (RPF) response to captopril in high sodium balance, was assessed at baseline and 8 weeks, as was systemic RAS activity and 24-hour ambulatory BP.
Results
84 participants completed the study. Mean 25[OH]D levels increased from 14.7 to 30.3 ng/mL in the ergocalciferol group, p-value < 0.0001, and from 14.3 to 17.4 ng/mL in the placebo group, p-value = 0.3. The RPF response to captopril was 33.9±56.1 mL/min/1.73m2 at baseline and 35.7±47.7 mL/min/1.73m2 at 8 weeks in the ergocalciferol group (p-value=0.83); and was 37.3±46.9 mL/min/1.73m2 at baseline and 35.9±26.2 mL/min/1.73m2 at 8 weeks in the placebo group (p-value=0.78). Ergocalciferol had no effect on PRA, AngII, or 24-hour BP measurements.
Conclusions
This trial found no benefit from correcting vitamin D deficiency on RAS activity or BP after 8 weeks. These findings are not consistent with the hypothesis that vitamin D is a modifiable target for lowering blood pressure in vitamin D deficient individuals.
Keywords: Vitamin D, ambulatory blood pressure, Hypertension
Introduction
Lower levels of 25-hydroxyvitamin D (25[OH]D) are strongly and independently associated with an increased risk of developing hypertension.[1] Consistent with these findings, animal and human studies suggest that vitamin D deficiency may increase activity of the renin angiotensin system (RAS), both systemically and in the kidney. In mice, knock out of either the vitamin D receptor or the 1 α-hydroxylase gene (which converts 25[OH]D to 1,25[OH]D) upregulates RAS activity and induces hypertension[2,3], and treatment of such mice with 1,25-dihydroxyvitamin D suppresses RAS activity [3]. Human data support these findings. Individuals with low 25(OH)D levels have increased systemic and kidney-specific renin angiotensin system (RAS) activation, while hypertensive individuals with polymorphisms in the vitamin D receptor also have increased RAS activation [4–7]. In addition, a small open label and uncontrolled study of obese subjects with vitamin D deficiency found that treatment with ergocalciferol reduced kidney-specific RAS activity and blood pressure[8]. Thus, RAS activation may be a mechanism linking vitamin D with incident hypertension. However there have been no randomized controlled trials that have examined this question.
We therefore performed a randomized, double-blind, placebo-controlled trial to evaluate the effects of ergocalciferol (50,000 U/week) for 8 weeks on kidney-specific and systemic RAS activation in a population of overweight or obese individuals with vitamin D deficiency. Overweight and obese individuals were selected as they represent a clinically important population in which to study the effect of vitamin D repletion on blood pressure. These individuals constitute approximately two thirds of the adult US population, have increased RAS activity, are at increased risk for the development of hypertension, and are more likely to have vitamin D insufficiency relative to lean individuals[9,10]. Significantly, the relationship between high BMI and elevated RAS activity appears to be attenuated among individuals who are vitamin D replete[5].
Methods
Study Population
Participants were recruited from the greater Boston area from 2011 through 2014 using several mechanisms. First, we contacted participants through a Partners Healthcare database that included individuals who had previously expressed an interest in participating in clinical trials. Second, we recruited through local media outlets, such as billboards placed in nearby colleges and universities, local newspapers, and through online advertisement (such as Craiglist). Inclusion criteria were overweight or obese individuals (defined by a body mass index [BMI] ≥25 kg/m2), with vitamin D deficiency (defined as serum 25(OH)D ≤20ng/ml) and no known history of hypertension. Subjects needed to have a screening systolic blood pressure <140 mmHg, diastolic blood pressure <90 mmHg, and could not be taking blood pressure lowering medications. Potentially eligible subjects were excluded if they were pregnant or had a history of diabetes, coronary artery disease, chronic kidney disease (defined as an estimated glomerular filtration rate [eGFR] using the Modification of Diet in Renal Disease [MDRD] estimating equation of <60 mL/min/1.73m2)[11], hypercalcemia, history of kidney stones, or a known active malignancy (except non-melanoma skin cancer). All subjects provided written informed consent. The study was approved by the Institutional Review Board at Brigham and Women’s Hospital.
Study Design
The study flow of this randomized, double-blind, placebo-controlled trial is shown in Figure 1. After an initial phone screen, 489 individuals were asked to come in for a screening visit, in which a study physician explained the study and performed a detailed history and physical exam. Information about past medical history including medication use was obtained, as were measurements of blood pressure, serum 25(OH)D, serum creatinine, liver function tests, and electrolytes. Ninety three individuals who meet eligibility criteria and provided informed consent underwent concealed 1:1 randomization, stratified by race and sex, using a computer based algorithm to be treated with ergocalciferol 50,000 IU or matching placebo, once weekly for 8 weeks. The unblinded research pharmacist, who supervised the randomization and provided subjects with study medication, formulated all pills (active treatment and placebo) to be physically indistinguishable (ie, sight, smell, and weight). All other individuals involved in the study were blinded, including the investigators, subjects, outcome assessors, and data analyzers.
Figure 1.
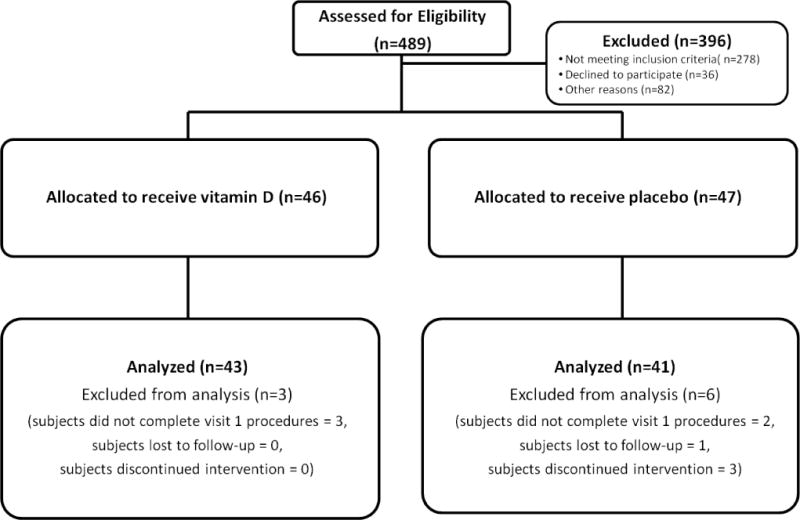
Study design showing numbers screened (N=489), found eligible and randomized (N=93) and analyzed.
Study Measurements
Following the screening visit, willing and eligible subjects were scheduled for three more visits, including a baseline visit (after which study medication was provided), a check-up visit at 4 weeks, and a final visit at 8 weeks (after which study medication was discontinued). At the baseline and 8-week visits, subjects were admitted to the hospital and underwent measurement of kidney-specific and systemic RAS activity; after discharge, 24-hour ambulatory blood pressure was assessed.
Kidney-specific RAS
Participants underwent salt loading three days prior to RAS measurement at baseline and at 8 weeks of treatment. Participants were instructed to consume 3 packets of chicken or vegetarian bouillon daily (150 mmol of sodium per day) starting two days prior to and on the day of admission to the Center for Clinical Investigation (CCI) at Brigham and Women’s Hospital (BWH). Successful salt loading was confirmed with an overnight 12-hour urine collection performed in the CCI (urine sodium content ≥75 mmol/12 hours). Following an overnight 8 hour fast, participants remained supine for the duration of the study. Two intravenous catheters were inserted, one for infusion and one for blood collection. An 8 mg/kg loading dose of para-aminohippurate (PAH) was administered 60 minutes before the administration of 25mg of oral captopril. This loading dose was followed by a continuous infusion of PAH at 12mg/min to achieve plasma PAH concentration in the middle of the range at which tubular secretion dominates excretion. At this concentration of PAH, clearance is independent of plasma PAH levels, and effective renal plasma flow (RPF) is defined as the rate of PAH clearance calculated from steady state plasma PAH concentrations, as described previously. Effective RPF is normalized to body surface area of 1.73m2. Three pre-captopril measurements and three post-captopril measurements of RPF were made. The change in RPF in response to captopril is a measure of the vasodilator effect from inhibiting AngII mediated vascular tone and therefore the degree of kidney-specific RAS activity. Although necessarily indirect, the bio-assay of renal vasodilator responsiveness to inhibition of the RAS has been demonstrated to provide reproducible measures of renal specific RAS activity in multiple studies, employing various agents in many patient subsets [12,13].
Systemic RAS
During the first morning of each inpatient CCI visit, subjects remained supine for >60 minutes prior to blood collection for measurement of plasma renin activity (PRA) and serum AngII levels. PRA was measured by radioimmunoassay (Diasorin, Stillwater, MN). AngII was measured using a double-antibody radioimmunoassay (ALPCO, Salem, NH). Both PRA and AngII assays were performed by the Brigham Research Assay Core (BRAC) with intra-assay CVs of 4.6 to 10% and inter-assay CVs of 5.6 to 7.6% for PRA, and intra-assay CVs of 7.1 to 11.3% and inter-assay CV of 5.8 to 18.1% for AngII.
ABP Measurement
Upon discharge from the CCI (following the baseline and 8 week in-patient visits), all subjects were fitted with an ambulatory blood pressure monitor (Space-Labs 90207 Ambulatory BP Device, Redmond, WA) that was to be worn for 24 hours. The monitors were pre-set to measure systolic and diastolic blood pressure every 30 minutes from 06:00 to 20:00 and every 60 minutes from 22:00 to 06:00. Each participant recorded in a sleep diary the time that they went to bed in the evening and the time that they rose in the morning. A 24 hour ambulatory measurement was considered complete if ≥70% of the programmed blood pressure measurements were successfully recorded, as has previously been described [14]. We assessed the change in mean 24 hour systolic blood pressure, taken as the 24 hour systolic blood pressure at 8 weeks minus the corresponding blood pressure from baseline. In each case, mean 24 hour systolic blood pressure was the average of all systolic blood pressure readings recorded during the 24 hour period following discharge from the CCI. Mean diastolic blood pressure, mean asleep systolic blood pressure, and percent nocturnal dipping were secondary outcomes. Mean awake and asleep systolic blood pressures were defined as the average of systolic blood pressures during the periods of wakefulness and sleeping per participant’s sleep diary. Nocturnal dipping was defined as the difference in mean awake systolic blood pressure and mean asleep systolic blood pressure divided by mean awake blood pressure.
Other measurements
Demographic, clinical and laboratory data were obtained at the outpatient screening visit. Serum 25(OH)D was measured at screening visit, baseline inpatient visit, 4 week check-up visit, and at the 8 week follow-up inpatient visit. All serum 25(OH)D measurements were performed using the LIAISON® direct competitive chemlluminescent immunoassay (DiaSorin, Stillwater, MN) with an intra-assay CV of 5.4% and an inter-assay CV of 10.6%.
Statistical Analysis
The distribution of baseline characteristics in each treatment group was assessed to describe the population recruited and to confirm effective randomization of subjects; T-tests were used to assess differences in continuous characteristics by treatment group, and Chi-square tests were used for categorical variables. The effect of treatment assignment on serum 25(OH)D levels at baseline, 4 weeks and 8 weeks of treatment was analyzed using T-tests. We compared mean kidney-specific RAS (RPF response to captopril), systemic RAS (levels of PRA and AngII), and 24-hour ambulatory blood pressure at baseline and at 8 weeks within each treatment arm using paired T-tests. We tested for the effect of treatment assignment (ergocalciferol versus placebo) on changes in these endpoints using repeated measures analysis.
In sensitivity analyses, we compared the baseline characteristics of subjects who completed the study with those who failed to complete the 8 week study. In addition, we evaluated the association of the 8 week change in serum 25(OH)D level with corresponding change in RAS and ambulatory blood pressure measures from baseline to 8 weeks. Statistical significance was set for P>0.05. All analyses were performed using the SAS statistical package (version 9.4; SAS Institute Inc, Cary, NC).
Sample size calculations were performed assuming the standard deviations for SBP and DBP were 8 mmHg, and 6 mmHg. With 45 participants allocated to each treatment, and assuming 10% drop out (final N=40 per group), we expected to have 80% statistical power to detect a treatment difference of 5.0mmHg in SBP and 3.8 mmHg in DBP at a two-sided 0.05 significance level using repeated measures analysis.
Results
A total of 489 individuals were screened. Of these, 93 eligible to participants were randomized (46 to ergocalciferol and 47 to placebo; Figure 1). From those randomized, 84 subjects completed the study and were analyzed (43 assigned ergocalciferol and 41 assigned placebo). Five subjects discontinued the study prior to receiving study medication (3 and 1, respectively, in the ergocalciferol and placebo groups who did not complete the baseline visit, and 1 in the placebo group who developed hypertension prior to the baseline visit). Four subjects in the placebo group discontinued the study prior to the 8-week visit, including 1 who was lost to follow-up and 3 who dropped out.
The randomized population had a mean age of 37 (±12) years and a mean body mass index (BMI) of 33.9±5.6 kg/m2. The population was 67% female and 33% black. At baseline, the mean 25(OH)D level was 14.5±5.5 ng/ml, the mean blood pressure was 118/77±10/8 mmHg, and the mean estimated glomerular filtration rate (eGFR) was 108±20 ml/min/1.73m2. There was no significant difference in any of the baseline characteristics between the ergocalciferol and placebo groups, including baseline blood pressure and serum 25(OH)D levels (Table 1).
Table 1.
Baseline characteristics of all randomized participants
| Vitamin D Arm (N=46) |
Placebo (N=47) |
P Value* | |
|---|---|---|---|
| Mean (SD) | |||
| Age (years) | 39.3 (12.3) | 34.7 (11.3) | 0.07 |
| Body mass index (kg/m2) | 33.5 (5.2) | 34.3 (6.6) | 0.48 |
| Serum 25(OH)D# (ng/ml) | 14.7 (5.4) | 14.3 (5.6) | 0.71 |
| Serum calcium (mg/dl) | 9.5 (0.3) | 9.5 (0.4) | 0.98 |
| Serum phosphate (mg/dl) | 3.4 (0.5) | 3.5 (0.5) | 0.79 |
| Serum parathyroid hormone (pg/ml) | 45.5 (19.6) | 43.7 (17.9) | 0.65 |
| Serum creatinine (mg/dl) | 0.8 (0.2) | 0.8 (0.2) | 0.42 |
| eGFR% (ml/min/1.73m2) | 104 (18) | 112 (20) | 0.08 |
| Systolic blood pressure (mmHg) | 118 (10) | 118 (10) | 0.98 |
| Diastolic blood pressure (mmHg) | 77 (7) | 77 (8) | 0.68 |
| N (%) | |||
| Black (%) | 15 (33) | 16 (34) | 0.56 |
| Men (%) | 15 (33) | 16 (34) | 0.88 |
| Median (IQR) | |||
| Urine sodium (mmol/day) | 293 (217 – 397) | 312 (232 – 400) | 0.68 |
| Urine microalbumin (mg/day) | 8.0 (4.4 – 16.4) | 6.8 (3.6 – 13.8) | 0.42 |
| Urine creatinine (mg/day) | 1479 (1302 – 1995) | 1626 (1317 - 1921) | 0.60 |
T-test used for normally distributed continuous variables and Wilcoxon used for non-normally distributed variables and Chi-squared test for categorical variables.
25(OH)D - 25-hydroxyvitmain D
eGFR – estimated glomerular filtration rate.
Serum levels of 25(OH)D substantially improved with ergocalciferol. Baseline 25(OH)D increased from a mean of 14.7±5.4 ng/ml to 27.8±8.1 ng/ml at 4 weeks and to 30.3±7.5 ng/ml at 8 weeks. In contrast, mean 25(OH)D in the placebo group was 14.3±5.6 at baseline, 16.8±15.5 at 4 weeks, and 17.4±18.8 at 8 weeks. A statistically significant separation in 25(OH)D levels between the treatment groups was apparent by 4 weeks and persisted at 8 weeks (p <0.0001, Figure 2). Correction of vitamin D deficiency (to a level above 20 ng/ml) was achieved in 95% of subjects assigned ergocalciferol and 22% of subjects assigned placebo (p-value <0.0001). Of the 84 subjects who completed the study for measurement of kidney-specific RAS (43 in the ergocalciferol group and 41 in the placebo group), ambulatory blood pressure monitoring was analyzed in 56 (67%) who had more than 70% of the programmed blood pressure measurements successfully recorded at both the baseline and 8-week visits (29 and 27 in the ergocalciferol and placebo groups, respectively).
Figure 2.
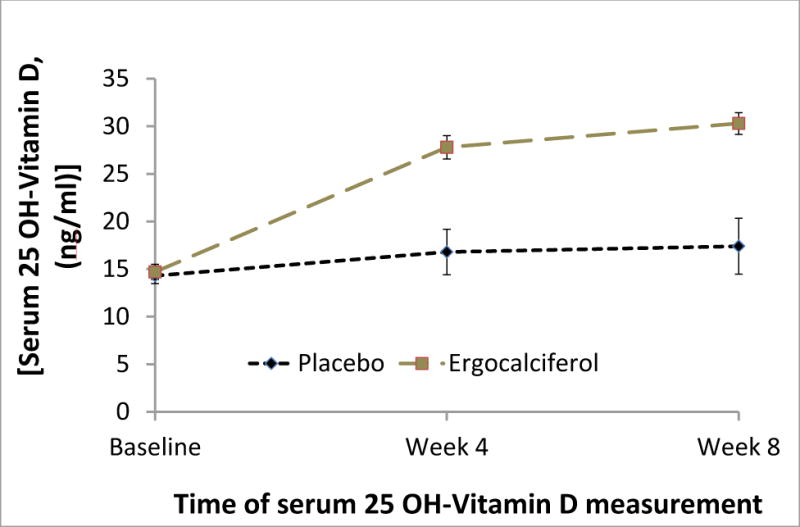
The effect of treatment assignment on mean serum 25(OH)D ± standard error at baseline, week 4 (check-up) and at week 8 in the two treatment groups. Comparisons of mean serum 25(OH)D levels between treatment groups were significant at weeks 4 and 8 (p-value < 0.0001) but not at baseline (p-value 0.5).
There were no clinically significant or statistically significant differences in the primary outcome, change in kidney-specific RAS activity, between baseline and 8 weeks follow-up in either treatment group. The response of RPF to captopril was measured as 33.9±56.1 ml/min/1.73m2 at baseline and 35.7±47.7 ml/min/1.73m2 at 8 weeks in the ergocalciferol group (p-value=0.83); in the placebo group it was 37.3±46.9 ml/min/1.73m2 at baseline and 35.9±26.2 ml/min/1.73m2 at 8 weeks (p-value=0.78). There was no difference between the 8-week change in RPF response with ergocalciferol and the corresponding 8-week change with placebo (p-value=0.72, Table 2). Systemic RAS activity measured by plasma renin activity (PRA) did not change significantly following 8 weeks of treatment with either ergocalciferol (from 0.34±0.37 ng/ml/hour to 0.36±0.44 ng/ml/hour; p-value=0.72) or placebo (0.42±0.44 ng/ml/hour to 0.44±0.80 ng/ml/hour; p-value=0.85). Similarly, serum angiotensin II (AngII) levels did not change significantly after 8 weeks of treatment with either ergocalciferol or placebo.
Table 2.
Effects of Vitamin D repletion on renal and systemic renin angiotensin system activity
| Parameter | Vitamin D N=43 |
Placebo N=41 |
Treatment effect | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 8−Week | Pˆ | Baseline | 8−Week | Pˆ | P& | |
| Mean (SD) | Mean (SD) | ||||||
| Pre-captopril RPF!, (mL/min/1.73m2) | 519.9 (100.5) | 529.4 (99.8) | 0.28 | 537.6 (98.1) | 529.5 (98.1) | 0.26 | 0.36 |
| Post-captopril RPF!, (mL/min/1.73m2) | 553.8 (113.1) | 565.1 (115.2) | 0.28 | 574.9 (99.5) | 563.2 (97.1) | 0.32 | 0.84 |
| Change in RPF!, (mL/min/1.73m2) | 33.9 (56.1) | 35.7 (47.7) | 0.83 | 37.3 (46.9) | 35.9 (26.2) | 0.78 | 0.62 |
| PRA#, (ng/mL/hr) | 0.34 (0.37) | 0.36 (0.44) | 0.72 | 0.42 (0.44) | 0.44 (0.80) | 0.85 | 0.77 |
| ATII%, (pg/mL) | 19.6 (5.9) | 19.5 (4.4) | 0.92 | 19.9 (4.5) | 19.7 (7.1) | 0.89 | 0.57 |
RPF – renal plasma flow
PRA – plasma renin activity
ATII – Angiotensin II
P-value for paired t-test comparing RAS activity at baseline and at 8-weeks in each treatment assignment.
P-value for repeated measures analysis for the effect of treatment assignment on RAS activity
There was no significant change in mean 24-hour systolic blood pressure or other ambulatory measurements following treatment with either ergocalciferol or placebo (Table 3). In sensitivity analyses, individual’s changes in RPF or blood pressure measurements between baseline and 8 weeks were not correlated with changes in serum 25(OH)D during the same period (Table 4).
Table 3.
Effects of Vitamin D repletion on ambulatory blood pressure
| Parameter | Vitamin D N= 29 |
Placebo N= 27 |
Treatment effect | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 8−Week | Pˆ | Baseline | 8−Week | Pˆ | P& | |
| Mean (SD) | Mean (SD) | ||||||
| Overall systolic blood pressure | 120.4 (10.2) | 121.6 (8.3) | 0.29 | 123.7 (7.6) | 124.7 (9.6) | 0.38 | 0.92 |
| Overall diastolic blood pressure | 71.6 (6.4) | 72.0 (5.6) | 0.54 | 73.7 (5.8) | 74.7 (7.2) | 0.32 | 0.64 |
| Awake systolic blood pressure | 122.4 (10.9) | 124.2 (9.1) | 0.16 | 126.4 (7.4) | 127.6 (9.2) | 0.36 | 0.80 |
| Asleep systolic blood pressure | 113.9 (11.0) | 114.7 (8.1) | 0.45 | 116.5 (8.7) | 117.4 (11.4) | 0.55 | 0.97 |
| Dipping | 7.3 (6.3) | 7.5 (5.7) | 0.86 | 7.8 (5.1) | 8.1 (6.0) | 0.87 | 0.98 |
P-value for paired t-test comparing ABP at baseline and at 8-weeks in each treatment assignment.
P-value for repeated measures analysis of the effect of treatment assignment on ABP.
Table 4.
Correlation between the change in serum 25(OH)D and the change in renin-angiotensin system activity and blood pressure measurements following treatment with either ergocalciferol or placebo for 8 weeks.
| Parameter correlated with change in serum 25 OH-Vitamin D | ρ! | p-value | |
|---|---|---|---|
| RAS | |||
| ∆ Pre-captopril RPF | 0.11 | 0.30 | |
| ∆ Post-captopril RPF | 0.03 | 0.80 | |
| ∆ Change in RPF | −0.06 | 0.56 | |
| ∆ PRA | −0.07 | 0.51 | |
| ∆ AT II | 0.06 | 0.59 | |
| ABP | |||
| ∆ 24hr Mean SBP | −0.001 | 0.99 | |
| ∆ 24hr mean DBP | −0.08 | 0.54 | |
| ∆ Asleep SBP | 0.08 | 0.58 | |
| ∆ Awake SBP | −0.09 | 0.51 | |
| ∆ Dipping percentage | −0.16 | 0.24 | |
Pearson correlation coefficient.
Adverse events did not differ significantly between treatment arms (Table 5). The most common adverse event was abdominal discomfort, which occurred in 5 and 4 subjects in the ergocalciferol and placebo arms, respectively.
Table 5.
Adverse events among those that received study medications
| Ergocalciferol (N=43) | Placebo (N=45) | |
|---|---|---|
| Serious Adverse Events | ||
| All | 0 | 0 |
| Rash | 0 | 0 |
| Minor Adverse Events | ||
| All | 6 | 6 |
| Abdominal discomfort | 5 | 4 |
| Dry Mouth | 0 | 1 |
| Elevated serum phosphorous | 0 | 1 |
| Elevated potassium | 1 | 0 |
Discussion
In this randomized, placebo-controlled trial, we found that correcting vitamin D deficiency in obese individuals with vitamin D deficiency without hypertension neither reduced kidney-specific or systemic RAS activity nor lowered blood pressure. These findings suggest that low vitamin D levels are not a modifiable risk factor for increased RAS activity or development of hypertension.
Considerable epidemiological data have demonstrated a robust association of low serum levels of vitamin D with risk of hypertension[1]. Consistent with these findings, animal studies found that vitamin D receptor knock out mice had increased renin activity, increased circulating AngII levels, and developed hypertension that could be reversed with RAS blockade[2,15,16]. In separate studies, mice unable to activate 25(OH)D due to 1-alpha-hydroxylase deficiency exhibited a similar phenotype as animals lacking the vitamin D receptor[3]. In this model, supplementation with 1,25(OH)2D reversed the phenotype, supporting the role of vitamin D in RAS activation and the development of hypertension. Detailed animal experiments have shown that vitamin D increases the intracellular calcium concentrations leading to decreased renin secretion from the juxtaglomerular apparatus[17].
Human cross-sectional studies support the association of low serum vitamin D levels with upregulation of RAS activity. The largest of these (N=3296) found that PRA levels were significantly higher in those with vitamin D deficiency, defined by 25(OH)D levels < 20 μg/L, as compared with those whose levels were >30 μg/L (plasma renin concentration 12.0 versus 10.8 pg/ml, p-value 0.004), as were plasma angiotensin II concentrations (21.0 ng/ml versus 18.0 ng/ml, p-value <0.001) [18]. In a smaller study, where renal specific RAS activity was measured as the RPF response in AngII infusion, individuals with lower serum 25(OH)D levels had higher renal specific RAS activity with lower serum 25(OH)D levels[4]. However, only one study has examined the effect of correcting vitamin D deficiency on RAS activation. In that open-label study of 14 obese subjects with hypertension and serum 25(OH)D levels <25 ng/ml, treatment with ergocalciferol (15,000 IU daily for 4 weeks) reduced kidney-specific RAS activation, measured as the renal-vascular sensitivity to AngII. Baseline RPF increased by 5% (p-value <0.01) following an increase in mean serum 25(OH)D levels from 18.2 ±1.8 ng/ml to 51.9 ± 3.1 ng/ml. There was also an increased response in RPF to AngII infusion following treatment with ergocalciferol (p-value < 0.05). These changes in RPF suggested there is decreased constitutive kidney-specific RAS activity with increased 25(OH)D levels, and that low vitamin D is a modifiable risk factor for increased RAS activity [8].
In contrast to this prior open-label uncontrolled study, the current study, which is a larger, double-blinded placebo-control trial, revealed no change in kidney-specific or systemic RAS activity with successful correction of vitamin D deficiency. Compared with the open-label study, we recruited individuals who had similar baseline 25(OH)D levels and BMI, but who were younger, had lower baseline blood pressure and higher baseline RPF. These differences in baseline characteristics (blood pressure and RPF) may indicate that our population had lower baseline RAS activation, potentially explaining differences between the studies. The current trial also used a lower dose of ergocalciferol to replete vitamin D than the open label study (50,000 IU per week as compared with 15,000 IU per day), and consequently produced a less dramatic increase in 25(OH)D levels (which increased from 18 to 52 ng/mL in the open label study), despite treating for a longer period of time. However, unless increasing serum 25(OH)D above a threshold level (between 30 and 52 ng/mL) is required to have an effect on RAS activity, then our trial of 84 participants, three times larger than the open label trial, would be expected to detect an effect of vitamin D supplementation on RAS activity. Perhaps more likely is that the smaller and uncontrolled intervention produced a spurious finding that was not be reproduced by the current randomized, controlled trial.
In addition to the lack of effect on the RAS, we also did not find an effect of vitamin D supplementation on blood pressure. Genetic analyses, specifically Mendelian randomization studies, found that individuals with genetically lower serum 25(OH)D levels have an increased incidence of hypertension, implying that vitamin D deficiency may play a role in the development of hypertension[19]. However, most but not all interventional studies show no blood pressure benefit from repleting with vitamin D. To date, there have been over 50 randomized, placebo-controlled trials of vitamin D supplementation that reported blood pressure as an outcome[20–31]. These trials have included individuals with and without baseline hypertension, with varying baseline 25(OH)D levels, and have used different formulations of vitamin D, including active vitamin D, vitamin D2, and vitamin D3, to correct 25(OH)D levels. A recent meta-analysis synthesized the results of 46 trials (4541 participants) and found no overall effect of vitamin D supplementation on either systolic or diastolic blood pressure, a finding consistent across the range of baseline blood pressure and serum 25(OH)D levels[20]. The results of our study, in which ambulatory blood pressure was measured at baseline and at 8 weeks are consistent with this recent meta-analysis and suggests that vitamin D supplementation does not improve any ambulatory blood pressure parameter, including nocturnal blood pressure and nocturnal dipping.
The discrepancy between Mendelian randomization studies and clinical trials may result from vitamin D having a relatively small effect on blood pressure. As an example, the largest genetic analysis included 142,255 individuals, and found that a 10% increase in genetically determined 25(OH)D concentration was associated with a 0.37 mmHg lower systolic pressure (95% CI, 0.003 to 0.73 mmHg lower) [19]. Thus, for a randomized clinical trial to have adequate statistical power to detect such a small benefit on blood pressure, tens of thousands of individuals would need to be recruited and followed for many years; such a study is unlikely to be performed. The largest trial is still ongoing (VITAL Hypertension Trial [ClinicalTrials.gov Identifier NCT01653678]), in which 25,874 men and women have been recruited to take 2000 IU of cholecalciferol or placebo per day for up to 5 years. However, VITAL is not powered to detect such small effects on blood pressure which, even if real, would be of marginal clinical importance.
Our study has several limitations that deserve mention. First, we recruited individuals with serum 25(OH)D levels less than 20 ng/ml, and it may be that a more vitamin D deficient population would have had a greater response to correction of 25(OH)D levels. However, several of the observational studies that described an association between low 25(OH)D levels and increased incidence of hypertension included individuals with 25(OH)D levels similar to those in this study. Second, we chose to replete low serum 25(OH)D levels using ergocalciferol, and it is possible that active vitamin D analogues would have produced to a measurable response. However, using active vitamin D analogues would have been an unconventional approach to treating low serum 25(OH)D levels in individuals with normal renal function, and would likely have lead to more adverse events specifically hypercalcemia. In addition, the previous unblinded study found significant effects of conventional vitamin D supplements (rather than active vitamin D) on the endpoints that we measured. Third, it is possible that low serum 25(OH)D levels may not necessarily identify individuals with low levels of bioactive vitamin D, due to inter-individual differences in vitamin D-binding protein[32]. Some of the individuals recruited may have had low levels of vitamin D-binding protein with low total but normal (or even high) bioactive 25(OH)D concentrations, such that supplementation would be expected to have no effect[32]. Finally the 8 week duration of treatment may have been inadequate to affect RAS activity or blood pressure. Although we were able to replete serum 25(OH)D levels to normal levels in the treatment group by 8 weeks, it is possible that the full effects of repletion on the RAS and blood pressure require a longer period of correction. However, this possibility is unlikely given the short duration of the one prior study that found a benefit on RAS activity and of several prior trials that found benefits on blood pressure.
We conclude that in contrast to animal experiments, observational studies, and open-label uncontrolled interventions, this randomized, double-blind, placebo-controlled trial found no effect of vitamin D supplementation on RAS activity or blood pressure in vitamin D deficient individuals.
Supplementary Material
Condensed abstract.
Lower circulating 25-hydroxyvitamin D (25[OH]D) is associated with increased (renin-angiotensin-system) RAS activity and BP in humans, with rodent models suggesting causation. In a randomized control trial investigating the effects of ergocalciferol supplementation on the RAS, 84 vitamin D deficient, overweight individuals, without hypertension, were randomized to 8 weeks treatment with ergocalciferol or placebo. 25[OH]D levels increased from 14.7 to 30.3 ng/mL in the ergocalciferol group. Kidney-specific and systemic RAS activity and 24-hour ambulatory BP were not significantly changed with ergocalciferol treatment. These findings are not consistent with the hypothesis that vitamin D is a modifiable target for reducing blood pressure.
Acknowledgments
This study was funded by a grant from the NIH/NHLBI (1R01HL105440-01).
Sources of support: This study was funded by a grant from the NIH/NHLBI (1R01HL105440-01).
Footnotes
Conflict of Interest: Gary C. Curhan. is a consultant for Allena Pharmaceuticals and Astra Zeneca and receives royalties from Uptodate. Ciaran J. McMullan, Leigh Borgi, Naomi Fisher, and John P. Forman have no competing financial interests.
References
- 1.Kunutsor SK, Apekey TA, Steur M. Vitamin D and risk of future hypertension: meta-analysis of 283,537 participants. European journal of epidemiology. 2013;28(3):205–221. doi: 10.1007/s10654-013-9790-2. [DOI] [PubMed] [Google Scholar]
- 2.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. The Journal of clinical investigation. 2002;110(2):229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney international. 2008;74(2):170–179. doi: 10.1038/ki.2008.101. [DOI] [PubMed] [Google Scholar]
- 4.Forman JP, Williams JS, Fisher ND. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension. 2010;55(5):1283–1288. doi: 10.1161/HYPERTENSIONAHA.109.148619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaidya A, Forman JP, Williams JS. Vitamin D and the vascular sensitivity to angiotensin II in obese Caucasians with hypertension. Journal of human hypertension. 2011;25(11):672–678. doi: 10.1038/jhh.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaidya A, Forman JP, Hopkins PN, Seely EW, Williams JS. 25-Hydroxyvitamin D is associated with plasma renin activity and the pressor response to dietary sodium intake in Caucasians. Journal of the renin-angiotensin-aldosterone system: JRAAS. 2011;12(3):311–319. doi: 10.1177/1470320310391922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaidya A, Sun B, Forman JP, Hopkins PN, Brown NJ, Kolatkar NS, et al. The Fok1 vitamin D receptor gene polymorphism is associated with plasma renin activity in Caucasians. Clinical endocrinology. 2011;74(6):783–790. doi: 10.1111/j.1365-2265.2011.03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaidya A, Sun B, Larson C, Forman JP, Williams JS. Vitamin D3 therapy corrects the tissue sensitivity to angiotensin ii akin to the action of a converting enzyme inhibitor in obese hypertensives: an interventional study. The Journal of clinical endocrinology and metabolism. 2012;97(7):2456–2465. doi: 10.1210/jc.2012-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988-1994 compared with 2000-2004. The American journal of clinical nutrition. 2008;88(6):1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelber RP, Gaziano JM, Manson JE, Buring JE, Sesso HD. A prospective study of body mass index and the risk of developing hypertension in men. American journal of hypertension. 2007;20(4):370–377. doi: 10.1016/j.amjhyper.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney international. 2005;67(6):2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 12.Fisher ND, Jan Danser AH, Nussberger J, Dole WP, Hollenberg NK. Renal and hormonal responses to direct renin inhibition with aliskiren in healthy humans. Circulation. 2008;117(25):3199–3205. doi: 10.1161/CIRCULATIONAHA.108.767202. [DOI] [PubMed] [Google Scholar]
- 13.Hollenberg NK, Fisher ND. Renal circulation and blockade of the renin-angiotensin system. Is angiotensin-converting enzyme inhibition the last word? Hypertension. 1995;26(4):602–609. doi: 10.1161/01.hyp.26.4.602. [DOI] [PubMed] [Google Scholar]
- 14.Parati G, Stergiou G, O’Brien E, Asmar R, Beilin L, Bilo G, et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. Journal of hypertension. 2014;32(7):1359–1366. doi: 10.1097/HJH.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 15.Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocrine reviews. 2008;29(6):726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. American journal of physiology Endocrinology and metabolism. 2005;288(1):E125–132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 17.Beierwaltes WH. The role of calcium in the regulation of renin secretion. American journal of physiology Renal physiology. 2010;298(1):F1–F11. doi: 10.1152/ajprenal.00143.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomaschitz A, Pilz S, Ritz E, Grammer T, Drechsler C, Boehm BO, et al. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clinica chimica acta; international journal of clinical chemistry. 2010;411(17-18):1354–1360. doi: 10.1016/j.cca.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 19.Vimaleswaran KS, Cavadino A, Berry DJ, Jorde R, Dieffenbach AK, Lu C, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. The lancet Diabetes & endocrinology. 2014;2(9):719–729. doi: 10.1016/S2213-8587(14)70113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beveridge LA, Struthers AD, Khan F, Jorde R, Scragg R, Macdonald HM, et al. Effect of Vitamin D Supplementation on Blood Pressure: A Systematic Review and Meta-analysis Incorporating Individual Patient Data. JAMA internal medicine. 2015;175(5):745–754. doi: 10.1001/jamainternmed.2015.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dreyer G, Tucker AT, Harwood SM, Pearse RM, Raftery MJ, Yaqoob MM. Ergocalciferol and microcirculatory function in chronic kidney disease and concomitant vitamin d deficiency: an exploratory, double blind, randomised controlled trial. PloS one. 2014;9(7):e99461. doi: 10.1371/journal.pone.0099461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kampmann U, Mosekilde L, Juhl C, Moller N, Christensen B, Rejnmark L, et al. Effects of 12 weeks high dose vitamin D3 treatment on insulin sensitivity, beta cell function, and metabolic markers in patients with type 2 diabetes and vitamin D insufficiency - a double-blind, randomized, placebo-controlled trial. Metabolism: clinical and experimental. 2014;63(9):1115–1124. doi: 10.1016/j.metabol.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Frandsen TB, Pareek M, Hansen JP, Nielsen CT. Vitamin D supplementation for treatment of seasonal affective symptoms in healthcare professionals: a double-blind randomised placebo-controlled trial. BMC research notes. 2014;7:528. doi: 10.1186/1756-0500-7-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zoccali C, Curatola G, Panuccio V, Tripepi R, Pizzini P, Versace M, et al. Paricalcitol and endothelial function in chronic kidney disease trial. Hypertension. 2014;64(5):1005–1011. doi: 10.1161/HYPERTENSIONAHA.114.03748. [DOI] [PubMed] [Google Scholar]
- 25.Gagnon C, Daly RM, Carpentier A, Lu ZX, Shore-Lorenti C, Sikaris K, et al. Effects of combined calcium and vitamin D supplementation on insulin secretion, insulin sensitivity and beta-cell function in multi-ethnic vitamin D-deficient adults at risk for type 2 diabetes: a pilot randomized, placebo-controlled trial. PloS one. 2014;9(10):e109607. doi: 10.1371/journal.pone.0109607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramly M, Ming MF, Chinna K, Suboh S, Pendek R. Effect of vitamin D supplementation on cardiometabolic risks and health-related quality of life among urban premenopausal women in a tropical country–a randomized controlled trial. PloS one. 2014;9(10):e110476. doi: 10.1371/journal.pone.0110476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mozaffari-Khosravi H, Loloei S, Mirjalili MR, Barzegar K. The effect of vitamin D supplementation on blood pressure in patients with elevated blood pressure and vitamin D deficiency: a randomized, double-blind, placebo-controlled trial. Blood pressure monitoring. 2015;20(2):83–91. doi: 10.1097/MBP.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 28.Arora P, Song Y, Dusek J, Plotnikoff G, Sabatine MS, Cheng S, et al. Vitamin D therapy in individuals with prehypertension or hypertension: the DAYLIGHT trial. Circulation. 2015;131(3):254–262. doi: 10.1161/CIRCULATIONAHA.114.011732. [DOI] [PubMed] [Google Scholar]
- 29.Sadiya A, Ahmed SM, Carlsson M, Tesfa Y, George M, Ali SH, et al. Vitamin D supplementation in obese type 2 diabetes subjects in Ajman, UAE: a randomized controlled double-blinded clinical trial. European journal of clinical nutrition. 2015;69(6):707–711. doi: 10.1038/ejcn.2014.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilz S, Gaksch M, Kienreich K, Grubler M, Verheyen N, Fahrleitner-Pammer A, et al. Effects of vitamin D on blood pressure and cardiovascular risk factors: a randomized controlled trial. Hypertension. 2015;65(6):1195–1201. doi: 10.1161/HYPERTENSIONAHA.115.05319. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell DM, Leder BZ, Cagliero E, Mendoza N, Henao MP, Hayden DL, et al. Insulin secretion and sensitivity in healthy adults with low vitamin D are not affected by high-dose ergocalciferol administration: a randomized controlled trial. The American journal of clinical nutrition. 2015;102(2):385–392. doi: 10.3945/ajcn.115.111682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. The New England journal of medicine. 2013;369(21):1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


