Abstract
Monovalent Hepatitis B vaccine (HepB) is heat stable, making it suitable for storage outside cold chain (OCC) at 37 °C for 1 month. We conducted an OCC project in the Solomon Islands to determine the feasibility of and barriers to national implementation and to evaluate impact on coverage. Healthcare workers at 13 facilities maintained monovalent HepB birth dose (HepB-BD) OCC for up to 28 days over 7 months. Vaccination data were recorded for children born during the project and those born during 7 months before the project. Timely HepB-BD coverage among facility and home births increased from 30% to 68% and from 4% to 24%, respectively. Temperature excursions above 37 °C were rare, but vaccine wastage was high and shortages common. Storing HepB OCC can increase HepB-BD coverage in countries with insufficient cold chain capacity or numerous home births. High vaccine wastage and unreliable vaccine supply must be addressed for successful implementation.
Keywords: Hepatitis B vaccine, Outside cold chain, Hepatitis B birth dose
1. Introduction
Chronic hepatitis B virus (HBV) infection can result in liver cirrhosis and cancer [1]. Infection during early childhood is strongly associated with progression to chronic infection [2]. The prevalence of chronic HBV infection in the Solomon Islands is high, estimated at 21% among all ages in 2007, and 13% among 0–9 year olds that attended the national reference hospital in 1994 [3,4]. The World Health Organization (WHO) recommends children receive a birth dose of hepatitis B vaccine (HepB), as soon as possible after birth followed by at least two additional doses to prevent infection [5]. In the Solomon Islands, HepB and HepB birth dose (HepB-BD) were introduced into the routine immunization program in 1990 and 2001, respectively. In 2015, coverage estimates were at 98% for three doses of HepB and 65% for HepB-BD [6].
Monovalent HepB is relatively heat stable making it suitable for storage outside cold chain (OCC). Storage of HepB OCC could help to increase HepB-BD coverage with no increase in reported adverse effects where access to health care is geographically challenging, home births are common, or health facilities lack adequate cold chain capacity [7,8,10–15]. Evidence shows that HepB can be stored OCC at 37 °C for 1 month or 45 °C for 1 week, and that persons administered vaccine stored at 37 °C for 1 month have similar mean geometric titers and seroconversion rates as those who receive vaccine stored at 2–8 °C for 1 month [7,8,10–12]. Storage of HepB OCC to improve timely administration of vaccine is now supported by the WHO Strategic Advisory Group of Experts on immunization [16]. Given this evidence, we conducted a pilot project in the Solomon Islands to determine the feasibility of and barriers to national implementation of a HepB-BD OCC program and to evaluate impact on coverage.
2. Material and methods
We selected 14 health facilities (HFs) from Guadalcanal, Makira, and Western provinces which lacked cold chain for vaccine storage, had >10 births per year, and were within a day of travel from the provincial capital. Each facility stored single dose, monovalent HepB (LG Lifescience) OCC for up to 28 days during a 7 month period (August 2015–February 2016). Facility health care workers (HCWs) were primarily responsible for monthly vaccine collection from the provincial pharmacy, although provincial EPI coordinators could also deliver vaccine during their monthly supervisory visits.
HepB-BD and BCG administration dates, dates of birth, location and status of birth were collected from immunization and birth registries for children born during the project and, for comparison, for children born during the 7 months before the project (January 2015–July 2015). BCG administration dates functioned as a quality control for HepB-BD administration with the caveat that BCG is frequently given after the perinatal period while HepB-BD needs to be given within 24 h of birth. Any adverse events following vaccination were recorded. Vaccine storage box temperature was recorded every 35 min by a LogTag™ thermometer for the 7-month project. Vaccine was discarded if the vaccine vial monitor (VVM) reached its temperature discard threshold or if the vaccine had reached either the a priori determined discard time point of 28 days or its expiration date, with the number of discarded vials recorded. After the project, HCWs discussed the successes and challenges of the project in a focus group. Participation was voluntary, and HCWs consent to participate was obtained.
Only births at a HF selected for the project or home births within the HF’s catchment area were included in the analysis. Day of HepB-BD and BCG administration post-birth was calculated for each child. Vaccines with illogical administration dates were excluded as was any child that died within 24 h of birth. HepB-BD was defined as a HepB-BD administered within <42 days of life. This follows WHO recommendations that the birth dose be provided as soon as possible after birth or during the first contact with health facilities at any time up to the time of the first primary dose, usually at 42 days old [16]. Timely HepB-BD was defined as a HepB-BD administered within 24 h of birth (0 or 1 days post-birth); late HepB-BD was defined as a HepB-BD administered 2 to <42 days after birth. BCG was categorized as either a BCG dose administered within 24 h of birth (0 or 1 days post-birth) or a BCG dose administered after 2 days up until 7 months. Vaccine coverage for HepB-BD, timely HepB-BD, and BCG was calculated for each HF and by birth location. Coverage among children born during the pre-project and project periods at each HF was compared using Wilcoxon paired rank test in SAS 9.4 (Cary, NC).
This evaluation was determined not to be human subject research by the Centers for Disease Control and Prevention human subjects review board and approved by the National Health Research and Ethics Committee of the Solomon Islands Ministry of Health and Medical Services.
3. Results
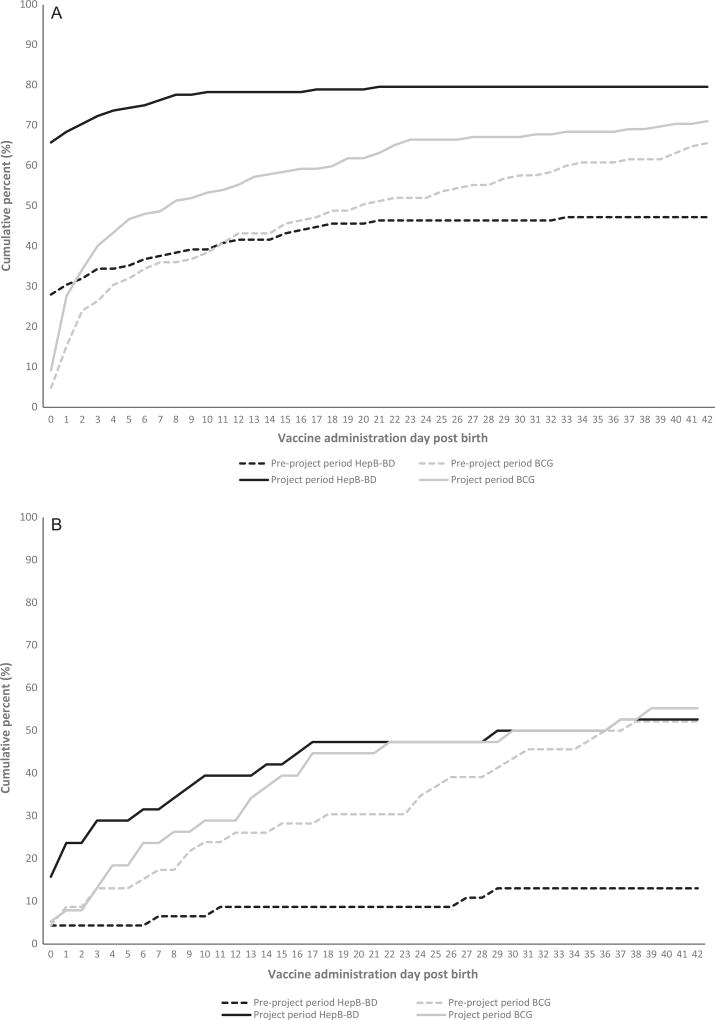
Of the 14 selected HFs, one was excluded as it had no assigned HCW; six had refrigerators installed after selection, but maintained HepB-BD OCC for the project. During January 2015–February 2016 there were 364 births, of which 278 (76%) were HF births. At two HFs, home births accounted for >50% of births. Among HF births, one newborn that died within 24 h was excluded; timely HepB-BD coverage increased from 30% (n = 38/125) during the preproject period to 68% (n = 104/152) during the project period (p = 0.0005; Table 1; Fig. 1A). Meanwhile, BCG 24-h coverage increased from 15% (n = 19/125) to 28% (n = 42/152) (p = 0.078). By 42 days post-birth, HepB-BD coverage had reached 80% (n = 121/152) during the project period with 85% (n = 104/122) of all vaccinated infants having received timely HepB-BD (Fig. 1A). HFs without cold chain (n = 7) increased timely HepB-BD coverage from 0% (n = 0/41) to 51% (n = 36/70; p = 0.016), and 24-h BCG coverage from 0% (n = 0/41) to 3% (n = 2/70; p = 0.5). Among home births, two newborns that died within 24 h were excluded; timely HepB-BD coverage increased from 4% (n = 2/46) during the preproject period to 24% (n = 9/38) during the project period (p = 0.063), while BCG coverage remained the same (p = 0.375; Table 1). By 42 days post-birth, HepB-BD coverage had reached 53% (n = 20/38) during the project period with 41% (n = 9/22) of all vaccinated infants having received timely HepB-BD (Fig. 1B). Two potential adverse events following HepB-BD administration were identified: thigh abscess in two children who were administered the vaccine two days apart at the same HF. Based on investigations, these might have been related to a short-term infection control issue at the facility.
Table 1.
Hepatitis B vaccine birth dose (HepB-BD) and BCG vaccine coverage by project period and timing of vaccine administration among health facility and home births—Guadalcanal, Makira, and Western provinces, Solomon Islands.
| Pre-project perioda
|
Project periodb
|
||||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Health facility births | No. of births | 125 | – | 152 | – |
| Received HepB-BDc | 59 | 47 | 121 | 78 | |
| Timing of HepB-BD | |||||
| Timely (≤24 h) | 38 | 30 | 104 | 68 | |
| Late (2–41 days) | 21 | 17 | 17 | 11 | |
| Received BCG | 109 | 87 | 119 | 78 | |
| Timing of BCG | |||||
| ≤24 h | 19 | 15 | 42 | 28 | |
| 2 days–7 months | 89 | 71 | 77 | 51 | |
| Home births | No. of births | 46 | – | 38 | – |
| Received HepB-BD | 6 | 13 | 20 | 53 | |
| Timing of HepB-BD | |||||
| Timely (≤24 h) | 2 | 4 | 9 | 24 | |
| Late (2–41 days) | 4 | 9 | 11 | 29 | |
| Received BCG | 42 | 91 | 26 | 68 | |
| Timing of BCG | |||||
| ≤24 h | 3 | 7 | 3 | 8 | |
| 2 days-7 months | 39 | 85 | 23 | 61 | |
Pre-project period covered January 2015–July 2015, inclusive.
Project period covered August 2015–February 2016, inclusive.
Defined as vaccinated before age 42 days, 2 children were excluded from the project period of health facility births due to illogical HepB vaccination dates; n – number, % – percent, No. – number, HepB-BD – hepatitis B vaccine birth dose, BCG – bacille Calmette-Guerin vaccine. Home births included those attended or not by a skilled birthing attendant and any births enroute to the health facility.
Fig. 1.
Cumulative hepatitis B vaccine birth dose (HepB-BD) and BCG vaccine coverage by age and project period reported by selected health facilities — Guadalcanal, Makira, and Western provinces, Solomon Islands (A) Health facility births (B) Home births. Pre-project period covered January 2015–July 2015; project period covered August 2015–February 2016.
The median OCC vaccine storage temperature recorded was 27.8 °C (IQR 26.5–29.5 °C), with a maximum of 46.6 °C. Deviations above 37 °C were rare, only occurring at 30/92,492 (0.03%) of the recorded time points (range by HF 0–0.16% (12/7600)). Vaccine wastage was high, 79/230 (34%) doses were discarded as they had reached the discard point of 28 days and 9/230 (4%) because the VVM had reached the discard point (one HF was excluded as it did not report vaccine wastage). Vaccine shortages were common; HCWs reported being unable to collect vaccines due to inclement weather, competing health activities, being unwilling to leave the HF if they were the sole provider, and having to use their own time and money to collect the vaccine. EPI coordinators also reported that they did not deliver vaccine. Another issue was the long annual leave (≈2 months), which resulted in the HF closing or assigning a replacement HCW who was not trained in OCC storage. All HCWs felt storing the HepB-BD OCC facilitated vaccine availability. They suggested using ante-natal information to improve vaccine forecasting, and standardizing the order and delivery system, increasing the number of satellite vaccine delivery posts, and increasing the number of HCWs at HFs considered for implementing HepB-BD OCC to improve vaccine supply.
4. Discussion
This study showed that storing HepB-BD OCC increased coverage among HF and home births, HCWs stored and handled monovalent HepB vaccine OCC well, and vaccine storage temperatures above 37 °C were rare. The WHO Regional Office of the Western Pacific endorses the use of HepB-BD OCC, and multiple studies conducted in the region have shown improvements in coverage [9,10,12–15]. However, few discussed program implementation challenges. In the Solomon Islands, the biggest challenges identified were vaccine stock outs and the need for better vaccine forecasting to decrease wastage. Vaccine supply relied heavily upon individual HCW motivation, which would not be sustainable in a long-term, nationwide program. In Lao PDR, program sustainability challenges were identified three months after an OCC pilot study, with 45% of intervention clinics having a stock out of HepB compared to 0% during the study [15]. Since monovalent HepB for storage OCC would need to be delivered monthly, compared with the routine bi-monthly Expanded Program of Immunization deliveries in the Solomon Islands, involving the National Medical Store and provincial pharmacy officers in HepB-BD OCC training and development of standard operating procedures may facilitate supply issues. Similarly, the Lao PDR pilot study also observed temperature deviations and vaccine wastage comparable to that seen in the Solomon Islands, although 2-dose vials were used and higher wastage would be expected in that setting where the second dose should be discarded in line with the multi-dose vial policy [15,17].
Although informative and consistent, this data is not representative of the Solomon Islands because of the convenience sampling and the inability to account for children who were born at home and never presented for medical care (and thus were not registered in the HF). At some HFs, the number of births was particularly low and the project time-frame was restricted due to staff vacation. Furthermore, although immunization and birth registry data quality was generally good, it varied by HF.
5. Conclusions
The results of this project and the substantial body of literature suggest the Solomon Islands and countries facing similar challenges should consider introducing a HepB-BD OCC program, especially where low birth dose coverage is associated with no or unreliable access to cold chain. However, further training on vaccine forecasting would be essential before implementing a national program. Furthermore, the WHO Strategic Advisory Group of Experts on immunization now supports countries that choose to implement an OCC policy for monovalent HepB [16].
Acknowledgments
We would like to acknowledge each of the healthcare workers at the 13 health facilities included in this study for their engagement and support of this activity.
Funding
This work was supported by the United Nations fund for children (UNICEF).
Abbreviations
- HBV
hepatitis B virus
- WHO
World Health Organization
- HepB
hepatitis B vaccine
- HepB-BD
hepatitis B vaccine birth dose
- OCC
outside the cold chain
- HF
health facility
- HCWs
healthcare workers
- EPI
Expanded Programme on Immunization
- BCG
bacille Calmette-Guerin vaccine
- VVM
vaccine vial monitor
- Lao PDR
Lao People’s Democratic Republic
Footnotes
Conflicts of interest
None.
Disclaimer
The findings and conclusions in this paper are those of the authors and do not necessarily reflect the position of the Centers for Disease Control and Prevention.
References
- 1.Lok ASF, McMahon BJ. Chronic hepatitis B. Hepatology. 2001;34:1225–41. doi: 10.1053/jhep.2001.29401. [DOI] [PubMed] [Google Scholar]
- 2.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335–52. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Western Pacific Regional Plan for Hepatitis B Control Through Immunization. 2007 Available from: < http://www.wpro.who.int/immunization/documents/docs/POA_HepB.pdf>.
- 4.Furusyo N, Hayashi J, Kakuda K, et al. Markedly high seroprevalence of hepatitis B virus infection in comparison to hepatitis C virus and human T lymphotropic virus type-1 infections in selected Solomon Islands populations. Am J Trop Med Hyg. 1999;61:85–91. doi: 10.4269/ajtmh.1999.61.85. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Hepatitis B vaccines. Wkly Epidemiol Rec. 2009;84:405–20. [Google Scholar]
- 6.World Health Organization. [Accessed May 6, 2016];Solomon Islands: WHO and UNICEF estimates of immunization coverage: 2015 revision. Available from: < http://www.who.int/immunization/monitoring_surveillance/data/slb.pdf>.
- 7.World Health Organization. Temperature Sensitivity of Vaccines. 2006 Available from: < http://apps.who.int/iris/bitstream/10665/69387/1/WHO_IVB_06.10_eng.pdf>.
- 8.Van Damme P, Cramm M, Safary A, Vandepapeliere P, Meheus A. Heat stability of a recombinant DNA hepatitis B vaccine. Vaccine. 1992;10:366–7. doi: 10.1016/0264-410x(92)90064-q. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Preventing Mother to Child Transmission of Hepatitis B: Operational Field Guidelines for Delivery of Birth Dose of Hepatitis B Vaccine. 2006 Available from: < http://www.wpro.who.int/hepatitis/hepb_operationalfieldguidelines.pdf>.
- 10.Otto BF, Suarnawa IM, Stewart T, et al. At-birth immunisation against hepatitis B using a novel pre-filled immunisation device stored outside the cold chain. Vaccine. 1999;18:498–502. doi: 10.1016/s0264-410x(99)00242-x. [DOI] [PubMed] [Google Scholar]
- 11.Sutanto A, Suarnawa IM, Nelson CM, Stewart T, Soewarso TI. Home delivery of heat-stable vaccines in Indonesia: outreach immunization with a prefilled, single-use injection device. Bull World Health Organ. 1999;77:119–26. [PMC free article] [PubMed] [Google Scholar]
- 12.Hipgrave DB, Tran TN, Huong VM, et al. Immunogenicity of a locally produced hepatitis B vaccine with the birth dose stored outside the cold chain in rural Vietnam. Am J Trop Med Hyg. 2006;74:255–60. [PubMed] [Google Scholar]
- 13.Wang L, Li J, Chen H, et al. Hepatitis B vaccination of newborn infants in rural China: evaluation of a village-based, out-of-cold-chain delivery strategy. Bull World Health Organ. 2007;85:688–94. doi: 10.2471/BLT.06.037002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huong VM, Hipgrave D, Hills S, Nelson C, Hien DS, Cuong NV. Out-of-coldchain delivery of the hepatitis B birth dose in four districts of Vietnam. PATH (Program for Appropriate Technology in Health); 2006. Available from: < http://www.path.org/publications/files/TS_hepb_coldchain_vietnam.pdf>. [Google Scholar]
- 15.Kolwaite AR, Xeuatvongsa A, Ramirez-Gonzalez A, et al. Hepatitis B vaccine stored outside the cold chain setting: a pilot study in rural Lao PDR. Vaccine. 2016;34:3324–30. doi: 10.1016/j.vaccine.2016.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, October 2016 – conclusions and recommendations. Weekly Epi Record. 2016;91:561–84. [Google Scholar]
- 17.World Health Organization. WHO policy statement: Multi-dose vial policy (MDVP) Revision 2014. 2014 Available from: < http://apps.who.int/iris/bitstream/10665/135972/1/WHO_IVB_14.07_eng.pdf>.