Abstract
The in-depth characterization of sex differences relevant to human physiology requires the judicious use of a variety of animal models and human clinical data. Nonhuman primates (NHPs) represent an important experimental system that bridges rodent studies and clinical investigations. NHP studies have been especially useful in understanding the role of sex hormones in development and metabolism and also allow the elucidation of the effects of pertinent dietary influences on physiology pertinent to disease states such as obesity and diabetes. This chapter summarizes the current state of our understanding of androgen effects on male and female NHP metabolism relevant to hypogonadism in human males and polycystic ovary syndrome in human females, as well as the interaction between altered androgen levels and dietary restriction and excess, in particular the western-style diet that underlies significant human pathophysiology.
Introduction
There is a growing appreciation of the role of sex differences in biology that has been inadequately addressed in previous biomedical research as well as in clinical trials. This has led to a renewed emphasis on evaluation of the role of sex in important aspects of physiology and pathology, largely driven by the realization that sex differences may affect the efficacy or adverse effects of established and emerging therapies for human disease. The importance of sex differences was illustrated by the recent demonstration that the majority of mammalian phenotypic traits are influenced by sex [1]. A major aspect of sex differences is their effect on metabolism [2] [3] [4]which is particularly relevant in light of the worldwide increase in obesity and its complications such as cardiovascular disease [5]. While the most obvious (and most studied) factor in determining sex differences is the role of sex steroids such as estrogens and androgens, it is important to note that many examples of sex differences are more directly due to the presence and copy number of sex chromosomes that can be independent of sex steroid effects per se. These studies have employed powerful rodent models such as the “four core genotypes” and XY* systems, in which sex chromosome vs sex steroid effects can be distinguished [6]; [7]; [8];[3] [9]. Connections between sex chromosome complement and sex steroid effects can obviously occur as well, exemplified by the effect of sex on adrenal androgens [10].
A comprehensive and translatable understanding of the effects of sex on major physiological processes such as metabolism requires the study of appropriate experimental systems. Clinical studies provide directly relevant information, while rodent systems allow the use of elegant genetic models. Clinical studies have obvious logistical and ethical limitations, while rodent metabolic control mechanisms do not always extrapolate to humans [11]; [12] [3]. Nonhuman primates (NHP), particularly macaque species such as rhesus and cynomolgus, represent important pre-clinical models that combine greater similarity to human physiology than rodents with greater possibilities for experimental intervention than human studies. NHPs exhibit sex differences in basic biology as well as in complex physiology [13]; [14]; [15]; [16].
With respect to the control of metabolism in particular, there are two crucial tissues that illustrate the similarities between NHPs and humans and differences from rodents that make the NHPs especially important experimental systems. The first is the pancreatic islet that regulates glucose metabolism through insulin and glucagon. Basic islet architecture in primate (macaque and human) islets involves a lower proportion of insulin-producing β cells and extensive intermingling of cell types, which results in primarily heterotypic interactions [17]; [18]; [19];[20] [21]. In contrast, rodent islets are composed of a central core comprised of β cells surrounded by a thin layer of non-β (α, δ, and PP) cells. Thus, interactions between insulin- and glucagon-producing cells are much more pronounced in primate islets. Other structural features that distinguish primate from rodent islets include aspects of vascularization [22,23] and basement membrane composition [24]; [25]; [26], innervation [27], and gene expression patterns [28]; [29]; [30]. These differences underlie important differences in function such as proliferative capacity and hormone secretion [31]; [32]; [33].
Another important metabolic tissue is the set of adipose depots that play the major role in lipid metabolism as well as important roles in glucose metabolism. Humans and NHPs have a variety of specialized adipose depots [34]; [35]; [36], including several white adipose tissue (WAT) depots and brown adipose tissue. Rodents and primates, however, differ in the anatomy and exact types of WAT [37]; [38]. Additionally, rodent and primate adipose tissue differ in gene expression and regulation as well as lipolysis control [39]; [40]; [41]; [42]; [43].
These species differences in major organs that regulate metabolism and their conservation between NHPs and human, makes NHPs invaluable tools for the elucidation of important aspects of sex-specific control of metabolism. While the role of estrogens in metabolic control is crucial [44,45], the role of androgens is also important in the metabolic state of both males and females [46]. In this chapter, we review the effects of androgens on various aspects of metabolism in NHP models of altered androgen levels as well as their interactions with diet.
Effects of androgens on male NHP metabolism
Our previous NHP studies demonstrated that androgen deprivation under conditions of a low-fat chow diet did not result in the development of obesity or insulin resistance [47], suggesting that other diet-related factors and/or increased caloric intake may increase the vulnerability of hypogonadal males to metabolic disturbance. This study also showed that androgen deprivation for one year achieved via surgical orchiectomy induced abnormal cellular morphology in retroperitoneal WAT [47]. Morphological alterations induced by androgen deprivation included a multilocular phenotype (the presence of multiple lipid droplets) and an increase in a percentage of smaller adipocytes (Figure 1). Furthermore, adipocyte insulin signaling via the Akt pathway and adipogenic gene expression were decreased in androgen- deprived males. In contrast, orchidectomized males receiving a physiological dose of testosterone (T) during the last six months of the study displayed a normal unilocular WAT phenotype (a single central lipid droplet), an increased percentage of larger adipocytes, improved adipocyte insulin sensitivity, and increased expression of adipogenic genes.
Figure 1. Testosterone deficiency induces a multilocular phenotype in male WAT.
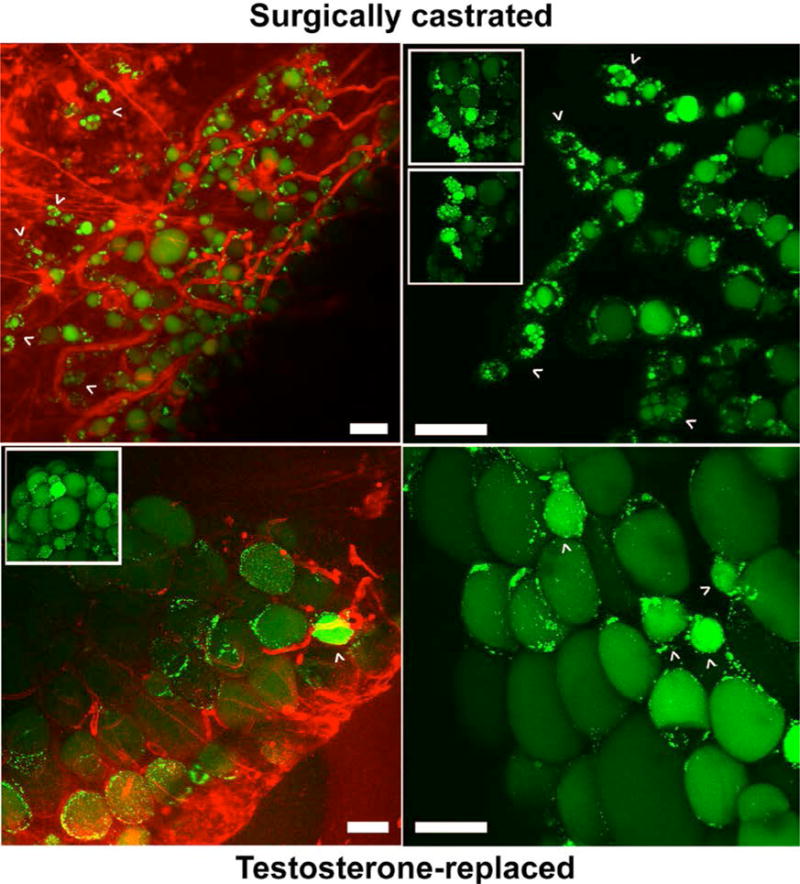
Insulin-stimulated WAT explants from castrated (CAS, A, upper panels, and B, both panels) and testosterone-replaced (TEST, A, lower panels) adult male macaques were labeled with green fluorescent fatty acid BODIPY-C12 (lipid droplets) and red fluorescent wheat germ agglutinin (blood vessels) and analyzed by confocal microscopy. Arrowheads indicate small multilocular adipocytes in CAS (upper panels) and small unilocular adipocytes in TEST (lower panels) WAT. Scale bar, 50 μm. Adapted from [47].
Effects of androgens on female NHP metabolism
Effects of prenatal hyperandrogenism
When early- to mid-gestation prenatal hyperandrogenism is derived from transplacental delivery of experimentally induced maternal hyperandrogenism [48]; [49], exposed female NHPs exhibit a variety of polycystic ovary syndrome (PCOS)-like neuroendocrine, ovarian, endocrine and metabolic traits, including type 2 diabetes mellitus (T2DM) ([50]). With specific regard to metabolic dysfunction, it must be noted that all these prenatal hyperandrogenic manipulations were performed on NHPs fed a non-obesogenic low-fat, high fiber diet.
Visceral adipocytes in female NHPs exposed to early-gestation hyperandrogenism demonstrate differential methylation in ~100-300 gene promoter sites when they reach infancy or adulthood [51]. Bioinformatic analysis of this epigenetic reprogramming identified altered TGF-β signaling as the most functionally defective pathway, implicating alterations in TGF-β receptor-mediated bone morphogenetic protein and antimullerian hormone regulation of adipogenesis [51]. One phenotypic consequence was the preferential accumulation of abdominal fat in visceral compared to subcutaneous (SC) depots as BMI increases, in contrast to preferential lipid accumulation in SC depots in controls [52]; [53]. Such differential adipose accumulation is consistent with impaired maturation of SC adipocytes, accompanied by accelerated commitment of adipocyte stem cells to preadipocytes in androgen-exposed female NHPs, as evidenced by disproportionally increased numbers of small SC adipocytes together with a reciprocal decrease in C/EBPα and an increase in Zfp423 gene expression, respectively [54]. C/EBPα is an androgen-regulated transcription factor enabling adipocyte maturation, while Zfp423 is an insulin-regulated transcription factor committing adipose stem cells to a pre-adipocyte phenotype [54]. Such a phenomenon may diminish SC abdominal storage of lipid and therefore explain why visceral fat, as an alternative fat depot, increases in amount with BMI in androgen-exposed, but not control, female NHPs [53]. Interestingly, an increase in the number of small SC adipocytes is linked with metabolic dysfunction in humans [55], as is an alternative increase in SC adipocyte size in some PCOS women [56], implying that a change in either direction away from the optimal size of SC adipocytes might have metabolic consequences.
Together, these NHP adipogenic findings suggest that when energy intake exceeds the capacity of normal SC adipose to safely store fat, excess free fatty acid (FFA) becomes deposited in abnormal locations, such as muscle, liver and pancreas, where FFAs induce oxidative/endoplasmic reticulum stress tightly linked with insulin resistance and inflammation [56]; [57]; [58]; [59]; [60]. This sequence of events is important because metabolic dysfunction in humans likely results from ectopic lipid accumulation in nonadipose cells [59]. Such a notion is consistent with abdominal fat or visceral fat correlating negatively with insulin sensitivity in androgen-exposed female NHPs, alone [53], and the progressive appearance of excess FFA, pancreatic beta cell decompensation, insulin resistance, and higher incidence of type 2 diabetes mellitus in these same female NHPs [61]; [62]; [63].
Interestingly, pancreatic dysfunction appears as early as the newborn infant in female NHPs exposed to hyperandrogenism during early gestation. Transient newborn hypoglycemia, excessive numbers of β cells and small pancreatic islets, together with inappropriate β cell compensation and increased body weight, suggest that transient gestational hyperglycemia unexpectedly induced by maternal hyperandrogenism may contribute to fetal reprogramming of metabolic function in androgen-exposed female NHPs [64]; [65].
Exposure of female NHPs to maternal androgen excess during late gestation, in contrast, while inducing greater total body adiposity in adulthood [52] does not induce metabolic dysfunction, including an absence of T2DM [62]; [53].
Effects of postnatal hyperandrogenism
The models of prenatal hyperandrogenemia described above have examined how early exposure to androgens can program changes in adult metabolism. These findings have clear implications for congenital adrenal hyperplasia, wherein elevated androgens produced by the fetal adrenal gland can result in masculinization of female offspring at birth and lifelong metabolic and reproductive complications [66]. This model also offers insight into PCOS, since many reproductive and metabolic phenotypes of PCOS are recreated in models of prenatal androgen exposure. However, whether gestational hyperandrogenemia happens in women who go on to develop PCOS is unclear. Clinical studies have identified elevated androgens around the time of puberty in a population of girls that appear at increased risk for the development of PCOS, indicating that this later postnatal developmental time window may also have profound effects to program adult metabolism [67,68]. Adolescence is also a critical time for the programming of obesity, lending further support to the hypothesis that androgen elevation during this time may have long-lasting effects on metabolism [69].
An NHP model of postnatal androgen treatment has been developed to investigate how androgen exposure during puberty may alter adult metabolism. Importantly, this model uses a 4-fold excess of T to imitate the similar increase in androgens observed in peripubertal girls at risk for the development of PCOS in adulthood [67], and treatments were initiated between 1-2.5 years of age. Initial pilot studies in prepubertal 1-yr-old animals did not reveal significant differences in weight or insulin resistance between control and androgen (T)-treated animals (T group) consuming a chow diet [70]. However, challenge with a western-style diet (WSD) later in development did reveal increased weight gain in androgen-treated females, indicating a worsened adaptive response to this caloric challenge. Hyperandrogenemia also reduced basal lipolytic activity and the expression of hormone-sensitive lipase in visceral omental (OM)-WAT [71]. Surprisingly, these effects were only observed during the luteal phase, when the levels of estrogens and progesterone are higher compared to menses. This same study revealed increased fatty acid uptake and insulin signaling in OM-WAT ex vivo. The effects of androgens on fatty acid uptake and lipolysis were observed at menses, under conditions of low estrogen and progesterone levels, but not during the luteal phase.
More recent studies have examined the effects of androgen treatment beginning at the initiation of puberty (roughly 2.5 years of age) and demonstrated that this treatment also appeared to cause negative metabolic outcomes. In particular, increased weight and fat mass gain as well as increased abdominal circumference was observed in androgen-treated females [72]. However, this study included androgen-treated females on a control diet and a WSD, and many effects that were statistically associated with androgen exposure appeared to be driven largely by the combination of WSD and androgens (see following section). Hyperandrogenemia was also associated with an altered pattern of physical activity, with androgen-treated females showing a delay in the usual puberty-associated decline in activity, followed by a more rapid decline later in the study, corresponding to the period when weight gain was observed. Similar to the findings in hyperandrogenized prepubertal animals described above, peripubertal treatment with androgens resulted in impaired lipolysis ex vivo [73]. Basal lipolytic activity was significantly reduced in OM and SC-WAT, while the β-adrenergic lipolytic response was significantly decreased only in SC-WAT (Figure 2). These findings of postnatal androgen effects on whole-body metabolism and adipocyte expansion are largely consistent with findings in rodents [74–76].
Figure 2. The suppression of lipolysis by androgens in female WAT.
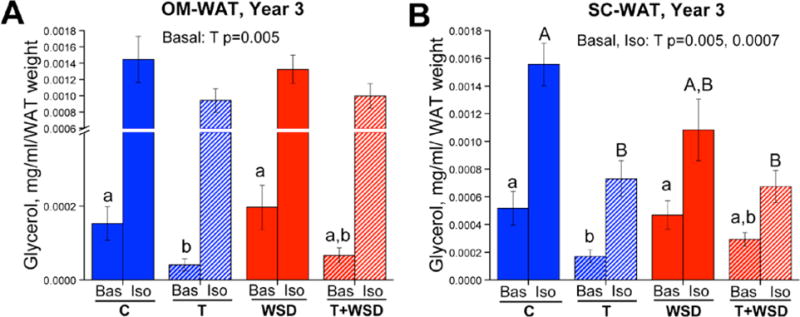
Female rhesus macaques were randomly assigned at 2.5 years of age (near menarche) to receive either cholesterol (C; n = 20) or testosterone (T; n = 20)-containing silastic implants to elevate T levels 5-fold above baseline. Half of each of these groups was then fed either a low-fat monkey chow diet or a WSD, resulting in four treatment groups (C, control diet; T alone; WSD alone; T + WSD; n = 10/group) that were maintained until the current analyses were performed at 5.5 years of age (3 years of treatment, young adults). OM (panel A) and SC-WAT (panel B) biopsies were collected and analyzed longitudinally for changes in basal (Bas) and isoproterenol (Iso)-stimulated lipolysis. In year 3 of treatment, basal lipolysis was blunted in the T and T + WSD groups in both WAT depots, while isoproterenol-stimulated lipolysis was significantly blunted in the T and T + WSD groups only in SC-WAT. Adapted from [49].
In addition to the long-term effects of androgen on whole-body metabolism in females, androgens exert acute effects on adipocyte physiology. In contrast to the effects of long-term postnatal androgen exposure, acute ex vivo treatment of female retroperitoneal WAT explants with the nonaromatizable androgen dihydrotestosterone increased basal but reduced insulin-stimulated fatty acid uptake [47]. Additionally, androgen-stimulated basal fatty acid uptake was greater in WAT of ovariectomized females compared to WAT of intact females and ovariectomized females replaced with estrogen and progesterone in vivo [47].
Interactions between androgens and diet
Androgen deprivation in NHP males
Our earlier studies in males [47] did not address the effect of androgen deprivation on SC-WAT and OM-WAT and was limited to a non-obesogenic low-fat control chow diet. In a follow-up study, we compared the effect of androgen deprivation in intact vs orchidectomized middle-aged male rhesus macaques, using three types of diet [77]. Both groups of animals were maintained for two months on a chow diet and then shifted to a WSD. Following six months on a WSD, the individual caloric intake was reduced by 30% (on chow diet). This experimental design allowed us to collect and longitudinally study WAT biopsies derived from the same anatomical sites. Androgen deprivation did not have a significant effect on the WSD-induced increase in the average size of OM and SC adipocytes, while both groups developed insulin resistance. However, orchidectomized animals exhibited less reduction in the size of OM and SC adipocytes after caloric restriction, which was associated with persistent insulin resistance.
Hyperandrogenemia in NHP females
We have also examined the interaction of peripubertal hyperandrogenemia and WSD on female metabolism. Strikingly, animals receiving androgens and WSD (T+WSD group) beginning at puberty did worse metabolically than animals receiving either treatment alone ([72]). This was observed for measures of body weight, fat gained over the experiment, and measures of insulin resistance. Adipocytes also appeared to be affected by the combined treatment, with larger visceral adipocytes and increased fatty acid uptake in visceral WAT in the T+WSD group compared to all other groups. Female rhesus macaques exposed to hyperandrogenemia and WSD exhibited an increase in the average size of visceral adipocytes that correlated with greater insulin resistance compared to animals treated with T or WSD alone [72]; [73] (Figure 3). As noted above, certain metabolic effects were often statistically identified as being driven by either diet or androgen in isolation; however, the group receiving both androgens and WSD was often significantly different from all other groups, indicating a more extreme metabolic phenotype when both conditions were present. In addition, the T+WSD group showed metabolic heterogeneity, with some animals maintaining metabolic parameters similar to controls and some developing a worsened metabolic phenotype (increased weight, increased fasting insulin, etc.). This variability resembles the clinical picture of PCOS, where hyperandrogenemia is associated with obesity and insulin resistance in the majority of, but not all, patients [78,79].
Figure 3. The effect of hyperandrogenemia on female adipocytes.
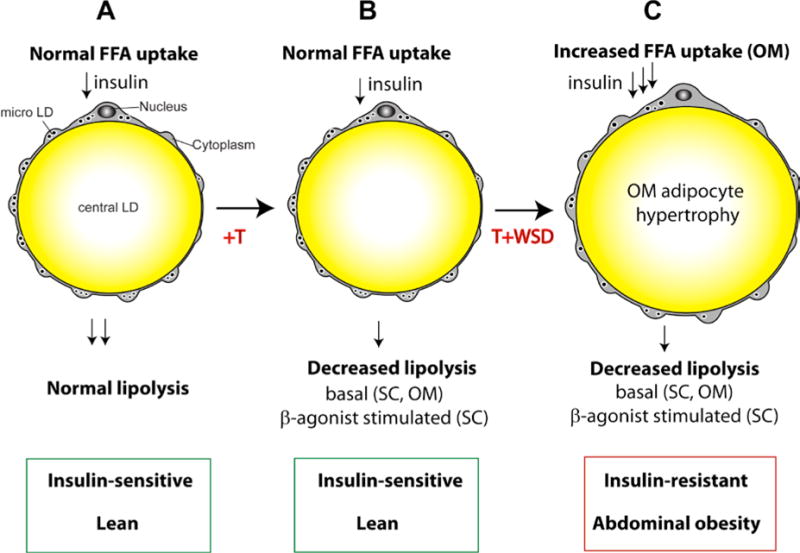
A, Insulin-stimulated free fatty acid (FFA) uptake in adipocytes is coupled to unidirectional FFA esterification, followed by the packaging of triglyceride into the central lipid droplet (LD). Micro LDs are strategically located at the interface of the cytoplasm, endoplasmic reticulum, and the central LD, being responsible for and/or associated with insulin-stimulated triglyceride synthesis and packaging in unilocular adipocytes [80]. The opposite process of triglyceride degradation, termed lipolysis, is potentiated by β-adrenergic stimuli provided by local sympathetic innervation. B, hyperandrogenemia (T excess) inhibits basal lipolysis both in subcutaneous (SC) and visceral omental (OM) WAT depots, while significantly suppressing β-agonist-stimulated lipolysis in SC-WAT. In animals fed a control low-fat chow diet, the T-induced lipolytic defect does not evoke adipocyte hypertrophy and insulin resistance. C, T-induced suppression of lipolysis persists in animals fed a WSD with the same depot specificity. Additionally, insulin-stimulated FFA uptake in OM adipocytes is significantly elevated in response to a combined exposure to hyperandrogenemia and WSD. In combination, elevated FFA uptake and suppressed lipolysis are associated with OM adipocyte hypertrophy, abdominal obesity, and systemic insulin resistance.
Conclusions
NHP males
These NHP studies suggest that T, at least in the presence of low-fat diet, is essential for the maintenance of normal WAT morphology (Figure 1), adipogenic gene expression, and adipocyte insulin sensitivity (Figure 4). Furthermore, T is involved in adipocyte hypotrophy following the transition from WSD to caloric restriction (Figure 4). Although the mechanism of male obesity associated with low T and therapeutic androgen deprivation is currently unknown, it is possible that it is related to the development of sarcopenia (muscle loss) observed in T-deficient males (Figure 3). Because skeletal muscle is responsible for the majority of glucose disposal and fatty acid β-oxidation, reduced muscle mass may result in insufficient substrate utilization and increased fat storage in WAT. Furthermore, androgen deprivation and muscle loss in males may also evoke secondary effects through reduced overall physical activity.
Figure 4. The effect of T deficiency on male adipocytes and skeletal muscle.
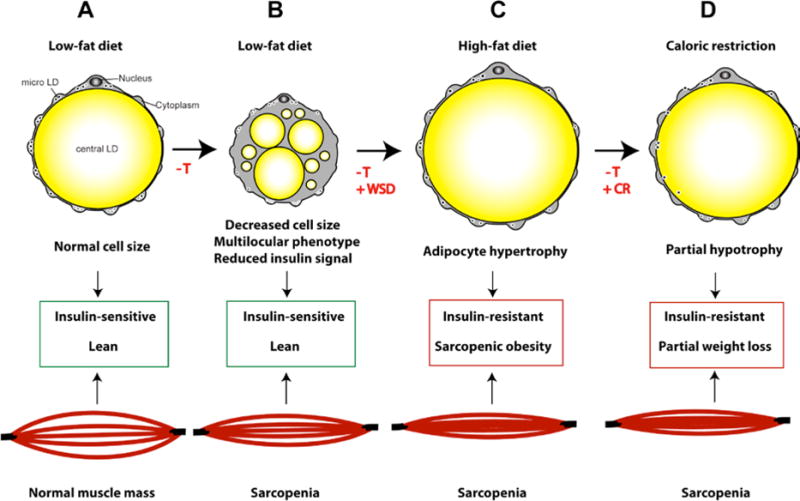
A, The cytoarchitecture of a unilocular adipocyte (described in Figure 3A). B, in control diet-fed animals, T deficiency induces a multilocular phenotype, the appearance of a population of smaller adipocytes, impairs insulin signaling in WAT, and triggers rapid muscle loss (sarcopenia). C, WSD stimulates visceral (OM) and SC-WAT hypertrophy, resulting in sarcopenic obesity and insulin resistance. D, the reversal of WSD with caloric restriction causes partial normalization of adipocyte size and reduces fat mass, but does not eliminate systemic insulin resistance and the progression of sarcopenia.
NHP females
Our research has indicated that both prenatal and postnatal androgen exposure in females can cause metabolic dysfunction. Early-to-mid, but not late, gestational exposure to androgens produces postnatal defects in insulin secretion and action, as well as increased body weight accumulation, in female offspring as early as infancy, well preceding pathological defects in adulthood leading to increased incidence of T2DM. Adipogenic constraint may amplify accelerated weight (and likely lipid) accumulation into lipotoxicity and its adult sequelae. Postnatal exposure to androgens before puberty is associated with increased insulin resistance, similar to the human condition of PCOS. Our studies indicate that peripubertal hyperandrogenemia inhibits lipolytic responsiveness (Figures 2 and 3) and accelerates fatty acid uptake in female WAT (Figure 3), while estrogen and/or progesterone can protect female WAT from androgen-induced lipid overload. These studies suggest that an increase in visceral WAT mass is associated with ectopic lipid deposition in visceral organs and skeletal muscle, being principally responsible for the development of peripheral insulin resistance. In contrast, SC-WAT can play a protective role against systemic lipotoxicity and insulin resistance. Our studies show that hyperandrogenemia alters the functional properties of visceral adipocytes, which leads to visceral obesity and metabolic disturbances.
The various NHP studies described above support the notion that androgens play important roles in organ-specific (adipose) as well as systemic aspects of metabolism, including a requirement for androgens in male adipose function and metabolic control and the adverse effects of androgen deficiency, and the similar effects of androgen excess in females. The specific molecular mechanisms that result in sex-specific effects on metabolism of the same ligand acting through the same receptor remain to be elucidated. Also requiring more understanding are the interactions between androgens, developmental windows, and diet that are just beginning to be appreciated in conditions such as PCOS, and that are at play in males as well.
Contributor Information
Cadence True, Division of Cardiometabolic Health, Oregon National Primate Research Center, Oregon Health & Science University, Portland, OR.
David H. Abbott, Department of Obstetrics and Gynecology and the Wisconsin National Primate Research Center, University of Wisconsin, Madison, WI
Charles T. Roberts, Jr., Division of Cardiometabolic Health, Oregon National Primate Research Center, Oregon Health & Science University, Portland, OR
Oleg Varlamov, Division of Cardiometabolic Health, Oregon National Primate Research Center, Oregon Health & Science University, Portland, OR.
References
- 1.Karp NA, Mason J, Beaudet AL, Benjamini Y, Bower L, Braun RE, Brown SDM, Chesler EJ, Dickinson ME, Flenniken AM, Fuchs H, Angelis MH, Gao X, Guo S, Greenaway S, Heller R, Herault Y, Justice MJ, Kurbatova N, Lelliott CJ, Lloyd KCK, Mallon AM, Mank JE, Masuya H, McKerlie C, Meehan TF, Mott RF, Murray SA, Parkinson H, Ramirez-Solis R, Santos L, Seavitt JR, Smedley D, Sorg T, Speak AO, Steel KP, Svenson KL, Wakana S, West D, Wells S, Westerberg H, Yaacoby S, White JK. Prevalence of sexual dimorphism in mammalian phenotypic traits. Nature communications. 2017;8:15475. doi: 10.1038/ncomms15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varlamov O, Bethea CL, Roberts CT., Jr Sex-specific differences in lipid and glucose metabolism. Frontiers in endocrinology. 2014;5:241. doi: 10.3389/fendo.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mauvais-Jarvis F, Arnold AP, Reue K. A Guide for the Design of Pre-clinical Studies on Sex Differences in Metabolism. Cell metabolism. 2017;25(6):1216–1230. doi: 10.1016/j.cmet.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biology of sex differences. 2015;6:14. doi: 10.1186/s13293-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spence JD, Pilote L. Importance of sex and gender in atherosclerosis and cardiovascular disease. Atherosclerosis. 2015;241(1):208–210. doi: 10.1016/j.atherosclerosis.2015.04.806. [DOI] [PubMed] [Google Scholar]
- 6.De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22(20):9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Frontiers in neuroendocrinology. 2009;30(1):1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP, Reue K. The number of x chromosomes causes sex differences in adiposity in mice. PLoS genetics. 2012;8(5):e1002709. doi: 10.1371/journal.pgen.1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold AP. Conceptual frameworks and mouse models for studying sex differences in physiology and disease: why compensation changes the game. Experimental neurology. 2014;259:2–9. doi: 10.1016/j.expneurol.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rehman KS, Carr BR. Sex differences in adrenal androgens. Seminars in reproductive medicine. 2004;22(4):349–360. doi: 10.1055/s-2004-861551. [DOI] [PubMed] [Google Scholar]
- 11.Kowalski GM, Bruce CR. The regulation of glucose metabolism: implications and considerations for the assessment of glucose homeostasis in rodents. American journal of physiology Endocrinology and metabolism. 2014;307(10):E859–871. doi: 10.1152/ajpendo.00165.2014. [DOI] [PubMed] [Google Scholar]
- 12.Bunner AE, Chandrasekera PC, Barnard ND. Knockout mouse models of insulin signaling: Relevance past and future. World journal of diabetes. 2014;5(2):146–159. doi: 10.4239/wjd.v5.i2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Resko JA, Roselli CE. Prenatal hormones organize sex differences of the neuroendocrine reproductive system: observations on guinea pigs and nonhuman primates. Cellular and molecular neurobiology. 1997;17(6):627–648. doi: 10.1023/A:1022534019718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Key C, Ross C. Sex differences in energy expenditure in non-human primates. Proceedings Biological sciences. 1999;266(1437):2479–2485. doi: 10.1098/rspb.1999.0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reinius B, Saetre P, Leonard JA, Blekhman R, Merino-Martinez R, Gilad Y, Jazin E. An evolutionarily conserved sexual signature in the primate brain. PLoS genetics. 2008;4(6):e1000100. doi: 10.1371/journal.pgen.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson EA, Nicolini Y, Shetler M, Suomi SJ, Ferrari PF, Paukner A. Experience-independent sex differences in newborn macaques: Females are more social than males. Scientific reports. 2016;6:19669. doi: 10.1038/srep19669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, Powers AC. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2005;53(9):1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- 18.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(7):2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosco D, Armanet M, Morel P, Niclauss N, Sgroi A, Muller YD, Giovannoni L, Parnaud G, Berney T. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes. 2010;59(5):1202–1210. doi: 10.2337/db09-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolensek J, Rupnik MS, Stozer A. Structural similarities and differences between the human and the mouse pancreas. Islets. 2015;7(1):e1024405. doi: 10.1080/19382014.2015.1024405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arrojo e Drigo R, Ali Y, Diez J, Srinivasan DK, Berggren PO, Boehm BO. New insights into the architecture of the islet of Langerhans: a focused cross-species assessment. Diabetologia. 2015;58(10):2218–2228. doi: 10.1007/s00125-015-3699-0. [DOI] [PubMed] [Google Scholar]
- 22.Brissova M, Shostak A, Fligner CL, Revetta FL, Washington MK, Powers AC, Hull RL. Human Islets Have Fewer Blood Vessels than Mouse Islets and the Density of Islet Vascular Structures Is Increased in Type 2 Diabetes. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2015;63(8):637–645. doi: 10.1369/0022155415573324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohrs CM, Chen C, Jahn SR, Stertmann J, Chmelova H, Weitz J, Bahr A, Klymiuk N, Steffen A, Ludwig B, Kamvissi V, Wolf E, Bornstein SR, Solimena M, Speier S. Vessel network architecture of adult human islets promotes distinct cell-cell interactions in situ and is altered after transplantation. Endocrinology. 2017 doi: 10.1210/en.2016-1184. [DOI] [PubMed] [Google Scholar]
- 24.Otonkoski T, Banerjee M, Korsgren O, Thornell LE, Virtanen I. Unique basement membrane structure of human pancreatic islets: implications for beta-cell growth and differentiation. Diabetes, obesity & metabolism. 2008;10(Suppl 4):119–127. doi: 10.1111/j.1463-1326.2008.00955.x. [DOI] [PubMed] [Google Scholar]
- 25.Virtanen I, Banerjee M, Palgi J, Korsgren O, Lukinius A, Thornell LE, Kikkawa Y, Sekiguchi K, Hukkanen M, Konttinen YT, Otonkoski T. Blood vessels of human islets of Langerhans are surrounded by a double basement membrane. Diabetologia. 2008;51(7):1181–1191. doi: 10.1007/s00125-008-0997-9. [DOI] [PubMed] [Google Scholar]
- 26.Kharouta M, Miller K, Kim A, Wojcik P, Kilimnik G, Dey A, Steiner DF, Hara M. No mantle formation in rodent islets – the prototype of islet revisited. Diabetes research and clinical practice. 2009;85(3):252–257. doi: 10.1016/j.diabres.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren PO, Caicedo A. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell metabolism. 2011;14(1):45–54. doi: 10.1016/j.cmet.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacDonald MJ, Longacre MJ, Stoker SW, Kendrick M, Thonpho A, Brown LJ, Hasan NM, Jitrapakdee S, Fukao T, Hanson MS, Fernandez LA, Odorico J. Differences between human and rodent pancreatic islets: low pyruvate carboxylase, atp citrate lyase, and pyruvate carboxylation and high glucose-stimulated acetoacetate in human pancreatic islets. The Journal of biological chemistry. 2011;286(21):18383–18396. doi: 10.1074/jbc.M111.241182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai C, Brissova M, Hang Y, Thompson C, Poffenberger G, Shostak A, Chen Z, Stein R, Powers AC. Islet-enriched gene expression and glucose-induced insulin secretion in human and mouse islets. Diabetologia. 2012;55(3):707–718. doi: 10.1007/s00125-011-2369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amisten S, Atanes P, Hawkes R, Ruz-Maldonado I, Liu B, Parandeh F, Zhao M, Huang GC, Salehi A, Persaud SJ. A comparative analysis of human and mouse islet G-protein coupled receptor expression. Scientific reports. 2017;7:46600. doi: 10.1038/srep46600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butler AE, Cao-Minh L, Galasso R, Rizza RA, Corradin A, Cobelli C, Butler PC. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia. 2010;53(10):2167–2176. doi: 10.1007/s00125-010-1809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genevay M, Pontes H, Meda P. Beta cell adaptation in pregnancy: a major difference between humans and rodents? Diabetologia. 2010;53(10):2089–2092. doi: 10.1007/s00125-010-1848-z. [DOI] [PubMed] [Google Scholar]
- 33.Conrad E, Dai C, Spaeth J, Guo M, Cyphert HA, Scoville D, Carroll J, Yu WM, Goodrich LV, Harlan DM, Grove KL, Roberts CT, Jr, Powers AC, Gu G, Stein R. The MAFB transcription factor impacts islet alpha-cell function in rodents and represents a unique signature of primate islet beta-cells. American journal of physiology Endocrinology and metabolism. 2016;310(1):E91–E102. doi: 10.1152/ajpendo.00285.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hudson JC, Baum ST, Frye DM, Roecker EB, Kemnitz JW. Age and sex differences in body size and composition during rhesus monkey adulthood. Aging (Milano) 1996;8(3):197–204. doi: 10.1007/BF03339677. [DOI] [PubMed] [Google Scholar]
- 35.Colman RJ, Ramsey JJ, Roecker EB, Havighurst T, Hudson JC, Kemnitz JW. Body fat distribution with long-term dietary restriction in adult male rhesus macaques. The journals of gerontology Series A, Biological sciences and medical sciences. 1999;54(7):B283–290. doi: 10.1093/gerona/54.7.b283. [DOI] [PubMed] [Google Scholar]
- 36.Varlamov O, Somwar R, Cornea A, Kievit P, Grove KL, Roberts CT., Jr Single-cell analysis of insulin-regulated fatty acid uptake in adipocytes. American journal of physiology Endocrinology and metabolism. 2010;299(3):E486–496. doi: 10.1152/ajpendo.00330.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.K I. Nutrition and Health. Vol. 2. Humana Press; 2014. Adipose Tissue and Adipokines in Health and Disease. [DOI] [Google Scholar]
- 38.Chusyd DE, Wang D, Huffman DM, Nagy TR. Relationships between Rodent White Adipose Fat Pads and Human White Adipose Fat Depots. Frontiers in nutrition. 2016;3:10. doi: 10.3389/fnut.2016.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mynatt RL, Stephens JM. Agouti regulates adipocyte transcription factors. American journal of physiology Cell physiology. 2001;280(4):C954–961. doi: 10.1152/ajpcell.2001.280.4.C954. [DOI] [PubMed] [Google Scholar]
- 40.Lindroos J, Husa J, Mitterer G, Haschemi A, Rauscher S, Haas R, Groger M, Loewe R, Kohrgruber N, Schrogendorfer KF, Prager G, Beck H, Pospisilik JA, Zeyda M, Stulnig TM, Patsch W, Wagner O, Esterbauer H, Bilban M. Human but not mouse adipogenesis is critically dependent on LMO3. Cell metabolism. 2013;18(1):62–74. doi: 10.1016/j.cmet.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guller I, McNaughton S, Crowley T, Gilsanz V, Kajimura S, Watt M, Russell AP. Comparative analysis of microRNA expression in mouse and human brown adipose tissue. BMC genomics. 2015;16:820. doi: 10.1186/s12864-015-2045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zuriaga MA, Fuster JJ, Gokce N, Walsh K. Humans and Mice Display Opposing Patterns of “Browning” Gene Expression in Visceral and Subcutaneous White Adipose Tissue Depots. Frontiers in cardiovascular medicine. 2017;4:27. doi: 10.3389/fcvm.2017.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sengenes C, Zakaroff-Girard A, Moulin A, Berlan M, Bouloumie A, Lafontan M, Galitzky J. Natriuretic peptide-dependent lipolysis in fat cells is a primate specificity. American journal of physiology Regulatory, integrative and comparative physiology. 2002;283(1):R257–265. doi: 10.1152/ajpregu.00453.2001. [DOI] [PubMed] [Google Scholar]
- 44.Newell-Fugate AE. The role of sex steroids in white adipose tissue adipocyte function. Reproduction. 2017;153(4):R133–R149. doi: 10.1530/REP-16-0417. [DOI] [PubMed] [Google Scholar]
- 45.Clegg D, Hevener AL, Moreau KL, Morselli E, Criollo A, Van Pelt RE, Vieira-Potter VJ. Sex Hormones and Cardiometabolic Health: Role of Estrogen and Estrogen Receptors. Endocrinology. 2017 doi: 10.1210/en.2016-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schiffer L, Kempegowda P, Arlt W, O’Reilly MW. MECHANISMS IN ENDOCRINOLOGY: The sexually dimorphic role of androgens in human metabolic disease. European journal of endocrinology. 2017;177(3):R125–R143. doi: 10.1530/EJE-17-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varlamov O, White AE, Carroll JM, Bethea CL, Reddy A, Slayden O, O’Rourke RW, Roberts CT., Jr Androgen effects on adipose tissue architecture and function in nonhuman primates. Endocrinology. 2012;153(7):3100–3110. doi: 10.1210/en.2011-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Resko JA, Buhl AE, Phoenix CH. Treatment of pregnant rhesus macaques with testosterone propionate: observations on its fate in the fetus. Biology of reproduction. 1987;37(5):1185–1191. doi: 10.1095/biolreprod37.5.1185. [DOI] [PubMed] [Google Scholar]
- 49.Abbott DH, Barnett DK, Levine JE, Padmanabhan V, Dumesic DA, Jacoris S, Tarantal AF. Endocrine antecedents of polycystic ovary syndrome in fetal and infant prenatally androgenized female rhesus monkeys. Biology of reproduction. 2008;79(1):154–163. doi: 10.1095/biolreprod.108.067702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abbott DH, Levine JE, Dumesic DA. Translational Insight Into Polycystic Ovary Syndrome (PCOS) From Female Monkeys with PCOS-like Traits. Current pharmaceutical design. 2016;22(36):5625–5633. doi: 10.2174/1381612822666160715133437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu N, Kwon S, Abbott DH, Geller DH, Dumesic DA, Azziz R, Guo X, Goodarzi MO. Epigenetic mechanism underlying the development of polycystic ovary syndrome (PCOS)-like phenotypes in prenatally androgenized rhesus monkeys. PloS one. 2011;6(11):e27286. doi: 10.1371/journal.pone.0027286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eisner JR, Dumesic DA, Kemnitz JW, Colman RJ, Abbott DH. Increased adiposity in female rhesus monkeys exposed to androgen excess during early gestation. Obesity research. 2003;11(2):279–286. doi: 10.1038/oby.2003.42. [DOI] [PubMed] [Google Scholar]
- 53.Bruns CM, Baum ST, Colman RJ, Dumesic DA, Eisner JR, Jensen MD, Whigham LD, Abbott DH. Prenatal androgen excess negatively impacts body fat distribution in a nonhuman primate model of polycystic ovary syndrome. Int J Obes (Lond) 2007;31(10):1579–1585. doi: 10.1038/sj.ijo.0803638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keller E, Chazenbalk GD, Aguilera P, Madrigal V, Grogan T, Elashoff D, Dumesic DA, Abbott DH. Impaired preadipocyte differentiation into adipocytes in subcutaneous abdominal adipose of PCOS-like female rhesus monkeys. Endocrinology. 2014;155(7):2696–2703. doi: 10.1210/en.2014-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLaughlin T, Lamendola C, Coghlan N, Liu TC, Lerner K, Sherman A, Cushman SW. Subcutaneous adipose cell size and distribution: relationship to insulin resistance and body fat. Obesity. 2014;22(3):673–680. doi: 10.1002/oby.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manneras-Holm L, Leonhardt H, Kullberg J, Jennische E, Oden A, Holm G, Hellstrom M, Lonn L, Olivecrona G, Stener-Victorin E, Lonn M. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. The Journal of clinical endocrinology and metabolism. 2011;96(2):E304–311. doi: 10.1210/jc.2010-1290. [DOI] [PubMed] [Google Scholar]
- 57.de Zegher F, Lopez-Bermejo A, Ibanez L. Adipose tissue expandability and the early origins of PCOS. Trends in endocrinology and metabolism: TEM. 2009;20(9):418–423. doi: 10.1016/j.tem.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome–an allostatic perspective. Biochimica et biophysica acta. 2010;1801(3):338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 59.Sorensen TI, Virtue S, Vidal-Puig A. Obesity as a clinical and public health problem: is there a need for a new definition based on lipotoxicity effects? Biochimica et biophysica acta. 2010;1801(3):400–404. doi: 10.1016/j.bbalip.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 60.Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Current diabetes reports. 2005;5(1):70–75. doi: 10.1007/s11892-005-0071-7. [DOI] [PubMed] [Google Scholar]
- 61.Eisner JR, Dumesic DA, Kemnitz JW, Abbott DH. Timing of prenatal androgen excess determines differential impairment in insulin secretion and action in adult female rhesus monkeys. The Journal of clinical endocrinology and metabolism. 2000;85(3):1206–1210. doi: 10.1210/jcem.85.3.6453. [DOI] [PubMed] [Google Scholar]
- 62.Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Human reproduction update. 2005;11(4):357–374. doi: 10.1093/humupd/dmi013. [DOI] [PubMed] [Google Scholar]
- 63.Zhou R, Bruns CM, Bird IM, Kemnitz JW, Goodfriend TL, Dumesic DA, Abbott DH. Pioglitazone improves insulin action and normalizes menstrual cycles in a majority of prenatally androgenized female rhesus monkeys. Reproductive toxicology. 2007;23(3):438–448. doi: 10.1016/j.reprotox.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abbott DH, Bruns CR, Barnett DK, Dunaif A, Goodfriend TL, Dumesic DA, Tarantal AF. Experimentally induced gestational androgen excess disrupts glucoregulation in rhesus monkey dams and their female offspring. American journal of physiology Endocrinology and metabolism. 2010;299(5):E741–751. doi: 10.1152/ajpendo.00058.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nicol LE, O’Brien TD, Dumesic DA, Grogan T, Tarantal AF, Abbott DH. Abnormal infant islet morphology precedes insulin resistance in PCOS-like monkeys. PloS one. 2014;9(9):e106527. doi: 10.1371/journal.pone.0106527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005;365(9477):2125–2136. doi: 10.1016/S0140-6736(05)66736-0. [DOI] [PubMed] [Google Scholar]
- 67.McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, Helm KD, Yoo R, Chang RJ, Foster CM, Caprio S, Marshall JC. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. The Journal of clinical endocrinology and metabolism. 2007;92(2):430–436. doi: 10.1210/jc.2006-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Apter D, Butzow T, Laughlin GA, Yen SS. Accelerated 24-hour luteinizing hormone pulsatile activity in adolescent girls with ovarian hyperandrogenism: relevance to the developmental phase of polycystic ovarian syndrome. The Journal of clinical endocrinology and metabolism. 1994;79(1):119–125. doi: 10.1210/jcem.79.1.8027216. [DOI] [PubMed] [Google Scholar]
- 69.Dietz WH. Critical periods in childhood for the development of obesity. The American journal of clinical nutrition. 1994;59(5):955–959. doi: 10.1093/ajcn/59.5.955. [DOI] [PubMed] [Google Scholar]
- 70.McGee WK, Bishop CV, Pohl CR, Chang RJ, Marshall JC, Pau FK, Stouffer RL, Cameron JL. Effects of hyperandrogenemia and increased adiposity on reproductive and metabolic parameters in young adult female monkeys. American journal of physiology Endocrinology and metabolism. 2014;306(11):E1292–1304. doi: 10.1152/ajpendo.00310.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Varlamov O, Chu MP, McGee WK, Cameron JL, O’Rourke RW, Meyer KA, Bishop CV, Stouffer RL, Roberts CT., Jr Ovarian cycle-specific regulation of adipose tissue lipid storage by testosterone in female nonhuman primates. Endocrinology. 2013;154(11):4126–4135. doi: 10.1210/en.2013-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.True CA, Takahashi DL, Burns SE, Mishler EC, Bond KR, Wilcox MC, Calhoun AR, Bader LA, Dean TA, Ryan ND, Slayden OD, Cameron JL, Stouffer RL. Chronic combined hyperandrogenemia and western-style diet in young female rhesus macaques causes greater metabolic impairments compared to either treatment alone. Human Reproduction. 2017:1–12. doi: 10.1093/humrep/dex246. doi: https://doi.org/10.1093/humrep/dex246. [DOI] [PMC free article] [PubMed]
- 73.Varlamov O, Bishop C, Handu M, Takahashi D, Srinivasan S, White A, Roberts CTJ. Combined androgen excess and Western-style diet accelerates adipose tissue dysfunction in young adult, female nonhuman primates. Human Reproduction. 2017:1–11. doi: 10.1093/humrep/dex244. doi: https://doi.org/10.1093/humrep/dex244. [DOI] [PMC free article] [PubMed]
- 74.Alexanderson C, Eriksson E, Stener-Victorin E, Lystig T, Gabrielsson B, Lonn M, Holmang A. Postnatal testosterone exposure results in insulin resistance, enlarged mesenteric adipocytes, and an atherogenic lipid profile in adult female rats: comparisons with estradiol and dihydrotestosterone. Endocrinology. 2007;148(11):5369–5376. doi: 10.1210/en.2007-0305. [DOI] [PubMed] [Google Scholar]
- 75.Nilsson C, Niklasson M, Eriksson E, Bjorntorp P, Holmang A. Imprinting of female offspring with testosterone results in insulin resistance and changes in body fat distribution at adult age in rats. The Journal of clinical investigation. 1998;101(1):74–78. doi: 10.1172/JCI1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kauffman AS, Thackray VG, Ryan GE, Tolson KP, Glidewell-Kenney CA, Semaan SJ, Poling MC, Iwata N, Breen KM, Duleba AJ, Stener-Victorin E, Shimasaki S, Webster NJ, Mellon PL. A Novel Letrozole Model Recapitulates Both the Reproductive and Metabolic Phenotypes of Polycystic Ovary Syndrome in Female Mice. Biology of reproduction. 2015;93(3):69. doi: 10.1095/biolreprod.115.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cameron JL, Jain R, Rais M, White AE, Beer TM, Kievit P, Winters-Stone K, Messaoudi I, Varlamov O. Perpetuating effects of androgen deficiency on insulin resistance. Int J Obes (Lond) 2016;40(12):1856–1863. doi: 10.1038/ijo.2016.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. The American journal of medicine. 2001;111(8):607–613. doi: 10.1016/s0002-9343(01)00948-2. [DOI] [PubMed] [Google Scholar]
- 79.Ovalle F, Azziz R. Insulin resistance, polycystic ovary syndrome, and type 2 diabetes mellitus. Fertility and sterility. 2002;77(6):1095–1105. doi: 10.1016/s0015-0282(02)03111-4. [DOI] [PubMed] [Google Scholar]
- 80.Chu M, Sampath H, Cahana DY, Kahl CA, Somwar R, Cornea A, Roberts CT, Jr, Varlamov O. Spatiotemporal dynamics of triglyceride storage in unilocular adipocytes. Molecular biology of the cell. 2014;25(25):4096–4105. doi: 10.1091/mbc.E14-06-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]


