Abstract
Vestitol and neovestitol are bioactive isoflavonoids isolated from Brazilian red propolis, an unique Apis melifera type of propolis botanically originated from Dalbergia ecastophyllum. While these molecules have relevant biological effects, including anticancer and immunomodulatory activities, their mechanism(s) of action and the affected pathways remain largely unknown. Here, we carried out a pharmacogenomic analysis to investigate the effects of vestitol and neovestitol on the whole-genome expression in human tumor cells, particularly cancer-related target proteins. HeLa cells were exposed to the compounds at IC20 and genomic information of treated cells was analyzed using the Illumina transcriptome system and GeneGo MetaCore software. Our results showed that vestitol (IC20 = 214.7 μM) reduced the expression of genes enrolled with the alpha tubulin (fold −3.7), tubulin in microtubules (fold −3.7) and histone h3 (fold = −3.03) and that treatment with neovestitol (IC20 = 102.91 μM) downregulated prostaglandin E synthase gene (fold = −3.12), which are considered ideal targets for anticancer therapy. These data open avenues for the study of vestitol and neovestitol as potential promising candidates for anticancer therapy. Toxicological, non-clinical and clinical validation of the findings presented herein is needed.
Keywords: Vestitol, neovestitol, tubulin microtubules, histone H3, prostaglandin E2, HeLa cells
Introduction
Propolis is a vegetal resinous substance collected by bees whose chemical composition depends on the overall biodiversity surrounding the beehives. In contrast with other countries, which have a limited diversity of propolis types, Brazil has been reported to harbor at least thirteen distinct types of propolis based on their physicochemical characteristics (Park et al., 2002; Alencar et al., 2007). This can be largely attributed to the great biodiversity of the preserved native areas in the country.
Brazilian red propolis (BRP) is an isoflavonoid-rich propolis botanically originated from Dalbergia ecastophyllum in the mangrove biome (Silva et al., 2008). In a recent review by our group we discuss the several biological activities of this unique type of propolis, particularly antimicrobial, antifungal, anti-inflammatory, and antiproliferative properties (Freires et al., 2016). While antimicrobial and immunomodulatory mechanisms of BRP isolated compounds have been established in the literature, its antiproliferative mechanisms on tumor cells (Alencar et al., 2007; Awale et al., 2008; de Mendonça et al., 2015) remain to be fully understood. Here, we report for the first time the effects of vestitol and neovestitol, two isoflavonoids isolated from BRP, on the whole-genome expression in human tumor cells. Important cancer-related proteins were significantly downregulated upon treatment with the compounds and could be considered critical targets for anticancer therapy.
Material and methods
Isolation of vestitol and neovestitol from BRP
BRP samples were collected in Maceio (9°40′ S, 35°41′ W), Alagoas State, in Northeastern Brazil. The extraction and isolation procedures were carried out as detailed elsewhere (Oldoni et al., 2011), yielding vestitol (Figure 1A) and neovestitol (Figure 2A) compounds (≥ 98 % purity each, measured on high performance liquid chromatography).
Figure 1. Pharmacogenomic analyses.
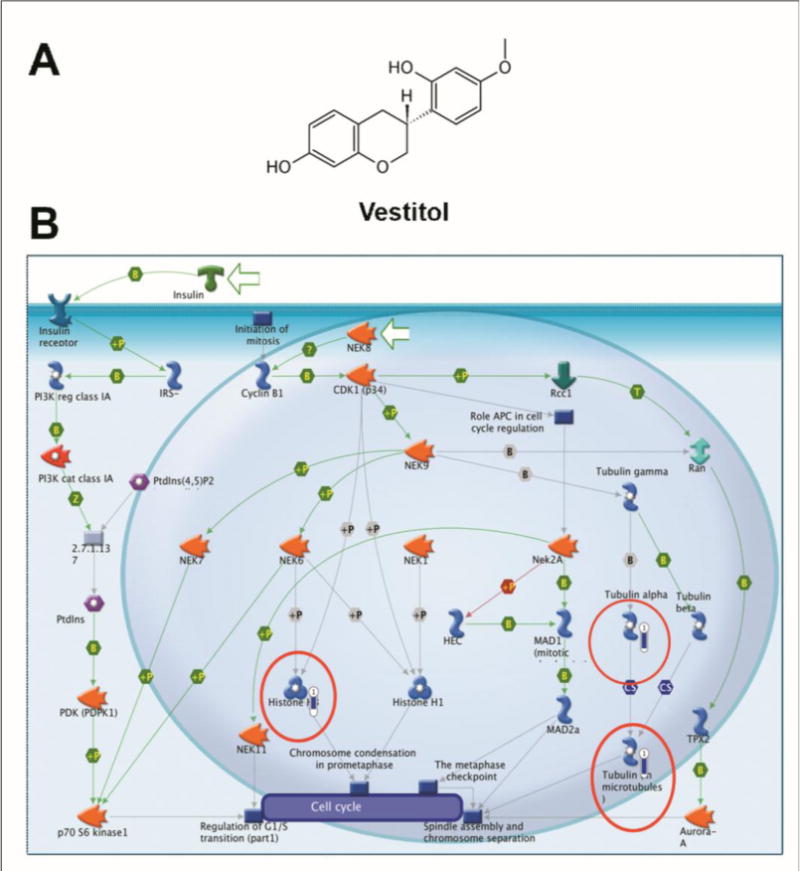
(A) Chemical structure of vestitol. (B) Canonic map representing the effect of vestitol treatment (IC20) in HeLa cells, causing down-regulation of genes enrolled in the alpha tubulin (fold −3.7), tubulin in microtubules (fold −3.7) and histone H3 (fold = −3.03). The thermometer next to each component indicates the magnitude of the modulation of gene expression (structures circulated in red; MetaCore, Thomson Reuters).
Figure 2. Pharmacogenomic analyses.
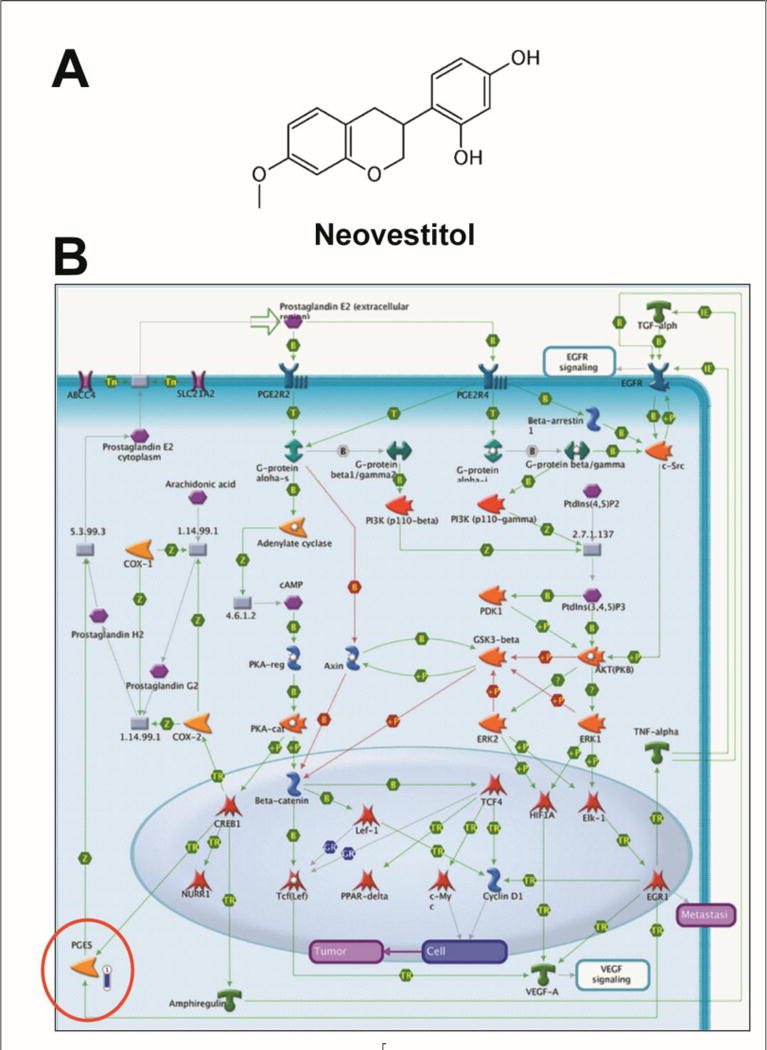
(A) Chemical structure of neovestitol. (B) Canonic map representing the effect of neovestitol treatment (IC20) in HeLa cells, causing down-regulation of genes enrolled with the PGE2 pathways in cancer (fold = −3.12). The thermometer next to the component indicates the magnitude of the modulation of gene expression (structure circulated in red; MetaCore, Thomson Reuters).
Cytotoxicity Assay
The cytotoxicity effects of vestitol and neovestitol on HeLa cells were assessed using the MTT assay. Briefly, HeLa cells (ATCC CCL-2) were seeded onto a 96-well plate (1×105 cells/well) and incubated at 37°C in 5 % CO2 (DMEM medium). After 24 hours, the compounds were added at 0.37, 3.7, 37 and 370 μM concentrations. The negative control group was incubated with vehicle (0.1% DMSO). Twenty-four hours later, 3 mg/mL MTT solution (in DMEM) was added to each well and the plate was further incubated for 3 h at 37 °C in 5 % CO2. Subsequently, the supernatant of each well was removed and ethanol was added to dissolve any deposited formazan. The optical density (OD) of each well was measured at 570 nm with a microplate reader (ASYS, UVM340, Biochrom, UK). Based on these measurements, the concentration that led to 20 % growth inhibition (IC20) compared to negative control was calculated and used for the pharmacogenomic analysis.
To compare the toxic effects of vestitol and neovestitol on cancer and non-cancer cell lines, we carried out a cytotoxicity assay (MTT method as described previously) using Human Gingival Fibroblasts (HGF-1 - ATCC CRL-2014) treated with vestitol (214.7 μM) and neovestitol (102.9 μM). These concentrations were previously determined in a cytotoxicity assay with HeLa cells and correspond to the respective IC20 of vestitol and neovestitol on HeLa cells.
Pharmacogenomic analysis
HeLa cells were treated with vestitol and neovestitol (IC20) under the conditions previously described. Negative control group was treated with vehicle (0.1% DMSO). After 24 h, total RNA was extracted from the cells and cDNA was synthetized. The total cDNA was then transferred to the Human HT-12 BeadChip V4 (Illumina Inc., San Diego, CA, USA) and bioinformatics data analyses were performed using the GeneGo MetaCore software (Thomson Reuters, New York, NY, USA). This technique analyzes 34,602 genes through 47,231 probes (representing the human transcriptome), generating canonic maps that indicate the genes affected by the treatment and is a very useful tool for evaluating multiple targets affected by natural products.
Results and Discussion
BRP and its active compounds vestitol and neovestitol have been reported to have antiproliferative properties against many cancer cells types (Alencar et al., 2007; Awale et al., 2008; de Mendonça et al., 2015; Shults et al., 2017), but the mechanisms underlying this effect are not fully elucidated. This short communication is the first report to show that vestitol (IC20 on HeLa cells = 214.7 μM) is capable to down-regulate the alpha tubulin (fold −3.7), tubulin in microtubules (fold −3.7) and histone H3 (fold = −3.03) genes (Figure 1B) and that neovestitol (IC20 on HeLa cells = 102.9 μM) down-regulate the prostaglandin E synthase gene (PGES; fold = −3.12; Figure 2B). These structures are important targets for cancer treatment, as briefly discussed below.
Alpha-tubulin and tubulin in microtubules have many vital cellular roles, acting as cytoskeleton components guaranteeing the cell shape, intracellular transporters, and, mainly, moving descendant chromosomes in cell division during mitosis (Perez, 2009). The importance of microtubules in mitosis makes this structure an ideal target for cancer treatment, since molecules able to affect this structure may effectively block the cancer cell cycle progression (Perez, 2009). The most important example of this statement is taxol (Paclitaxel, Bristol-Myers Squibb), a natural compound isolated from Taxus brevifolia, an important chemotherapeutic agent for the treatment of several types of cancer, by acting on microtubules (Wani et al., 1971). Taxol and other taxanes interact directly with the β-subunit of microtubules, resulting in the stabilization of these structures and leading the cancer cell to apoptosis (Perez, 2009). Therefore, tubulin is likely to be a potential target of vestitol and such mechanism may mediate the antiproliferative activity of this isoflavon.
The histone H3 is located in the nucleosome and is a fundamental unit of the chromatin. This structure maintains the DNA condensation into the cell during mitosis and is essential for the correct separation of sister chromatids and the formation of two daughter cells (Wei et al., 1999). We believe that the downregulation of histone H3 by vestitol may help explain how this molecule leads cancer cells to death.
Gene down-regulation promoted by vestitol may take place due to several reasons, some of which may include epigenetic phenomena, such as the histone acetylation, phosphorylation or methylation reactions. These events affect the interaction between histone and DNA and thereby favor or reduce the access of effector molecules to the DNA, leading to increased or decreased gene expression or even gene silencing (Biel et al., 2005; Cao and Zhang, 2004).
Prostaglandin E synthase (PGES) is also an important target for cancer treatment. This enzyme is responsible for the formation of prostaglandin E2 (PGE2), one of the most common prostanoid. After synthesis, PGE2 acts in peripheral inflamed tissues and in the spinal cord, being considered as a crucial mediator of inflammatory pain sensitization and other pathologies, including cancer (Kochel et al., 2017). Several authors have reported the participation of PGE2 as a pro-tumorigenic factor for many types of cancer, including pulmonary, colonic, mammary, intestinal, breast, and skin tumors (Kochel et al., 2017).
PGES gene expression is modulated by the NF-κB transcription factor, an important signaling pathway on inflammation and cancer diseases since its activation leads to the PGES gene expression (Korbecki et al., 2013). Previously, our group found that treatment with neovestitol decreases the NF-κB transcription factor activation (Bueno-Silva et al., 2017). The present results help to explain the mechanism of action of neovestitol in malignant cells, where this molecule reduces NF-κB activation, leading to a decrease in the PGES gene expression and ultimately reducing cancer cell activity and promoting cell death.
The cytotoxic effects of BRP crude extract are supported by other authors in several types of cancer cells such as cervical (IC50 of 7.45 μg/ml) (Alencar et al., 2007); glioblastoma (IC50 of 34.27 μg/ml); ovary (IC50 of 28.76 μg/ml); colon (IC50 of 25.26 μg/ml) (de Mendonça et al., 2015); and pancreas (IC50 of 10 μg/mL) (Awale et al., 2008). Vestitol was also tested, being effective at 50 μM against human pancreatic cancer cells by eliminating these cell tolerance against nutrient starvation (Awale et al., 2008); and showed selective cytotoxicity on leukaemia cells (IC50 from 2 to 7 μM) (Shults et al., 2017). In both of these cases, vestitol was better than the conventional anticancer drugs 5-fluorouracil and taxol (Awale et al., 2008); and was twice more active than doxorubicin (Shults et al., 2017).
In order to verify the cytotoxicity of vestitol and neovestitol against non-malignant cells, we performed a viability assay using HGF-1 cell line. Preliminary results show that vestitol was found to be more toxic on HGF-1 cells when compared to HeLa cells. At 214.7 μM, vestitol decreased HGF-1 cell viability by 51.2% and HeLa cell viability by 20%. However, treatment with neovestitol at 102.9 μM did not affect HGF-1 viability, with no differences as compared to the control, but it did reduce HeLa cell viability by 80% (data not shown).
Further research is warranted to test the toxicity of these molecules on other cell lines and in in vivo models. It is worth noting that the in vitro selectiveness against malignant cells is not an essential criterion for cancer treatment research, given that some drugs currently used in clinical practice for cancer treatment present non-selective toxic effects against malignant and non-malignant cells (Haglund et al., 2012).
Altogether, these findings suggest that the BRP isoflavonoids vestitol and neovestitol may be responsible for the BRP cytotoxic effects via distinct mechanisms.
Conclusion
Our data indicate that vestitol and neovestitol isolated from BRP are promising molecules for cancer treatment by downregulating expression of important cancer-related genes involved in alpha-tubulin, tubulin in microtubule, histone H3 and prostaglandin E synthesis. These findings demonstrate that these isoflavonoids could be considered potential promising candidates for future research in oncological therapy.
Acknowledgments
The authors are grateful to Mr. Alessandro Esteves (in memoriam) for providing the red propolis samples.
Funding
This research was supported by the São Paulo Research Foundation (FAPESP, grants no. 2012/22378-2 and 2011/23635-6). Research reported in this publication was also supported by the National Center for Complementary and Alternative Medicine of the National Institutes of Health under award number R00AT006507. The content is solely the responsibility of the authors, and does not represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
All authors declare that they have no conflicts of interest.
References
- Alencar SM, Oldoni TL, Castro ML, Cabral IS, Costa-Neto CM, Cury JA, et al. Chemical composition and biological activity of a new type of Brazilian propolis: red propolis. J Ethnopharmacol. 2007;113(2):278–283. doi: 10.1016/j.jep.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Awale S, Li F, Onozuka H, Esumi H, Tezuka Y, Kadota S. Constituents of Brazilian red propolis and their preferential cytotoxic activity against human pancreatic PANC-1 cancer cell line in nutrient-deprived condition. Bioorg Med Chem. 2008;16(1):181–189. doi: 10.1016/j.bmc.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Biel M, Wascholowski V, Giannis A. Epigenetics–an epicenter of gene regulation: histones and histone-modifying enzymes. Angew Chem Int Ed Engl. 2005;44(21):3186–3216. doi: 10.1002/anie.200461346. [DOI] [PubMed] [Google Scholar]
- Cao R, Zhang Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev. 2004;14(2):155–164. doi: 10.1016/j.gde.2004.02.001. [DOI] [PubMed] [Google Scholar]
- de Mendonça IC, Porto IC, do Nascimento TG, de Souza NS, Oliveira JM, Arruda RE, et al. Brazilian red propolis: phytochemical screening, antioxidant activity and effect against cancer cells. BMC Complement Altern Med. 2015;15:357. doi: 10.1186/s12906-015-0888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freires IA, de Alencar SM, Rosalen PL. A pharmacological perspective on the use of Brazilian Red Propolis and its isolated compounds against human diseases. Eur J Med Chem. 2016;110:267–279. doi: 10.1016/j.ejmech.2016.01.033. [DOI] [PubMed] [Google Scholar]
- Haglund C, Aleskog A, Nygren P, Gullbo J, Höglund M, Wickström M, et al. In vitro evaluation of clinical activity and toxicity of anticancer drugs using tumor cells from patients and cells representing normal tissues. Cancer Chemother Pharmacol. 2012;69(3):697–707. doi: 10.1007/s00280-011-1746-1. [DOI] [PubMed] [Google Scholar]
- Kochel TJ, Reader JC, Ma X, Kundu N, Fulton AM. Multiple drug resistance-associated protein (MRP4) exports prostaglandin E2 (PGE2) and contributes to metastasis in basal/triple negative breast cancer. Oncotarget. 2017;8(4):6540–6554. doi: 10.18632/oncotarget.14145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbecki J, Baranowska-Bosiacka I, Gutowska I, Chlubek D. The effect of reactive oxygen species on the synthesis of prostanoids from arachidonic acid. J Physiol Pharmacol. 2013;64(4):409–421. [PubMed] [Google Scholar]
- Oldoni TL, Cabral ISL, d´Arce MAB, Rosalen PL, Ikegaki M, Nascimento AM, Alencar SM. Isolation and analysis of bioactive isoflavonoids and chalcone from a new type of Brazilian propolis. Separation and modification technology. 2011;77(2):208–213. [Google Scholar]
- Park YK, Alencar SM, Aguiar CL. Botanical origin and chemical composition of Brazilian propolis. J Agric Food Chem. 2002;50(9):2502–2506. doi: 10.1021/jf011432b. [DOI] [PubMed] [Google Scholar]
- Perez EA. Microtubule inhibitors: Differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol Cancer Ther. 2009;8(8):2086–2095. doi: 10.1158/1535-7163.MCT-09-0366. [DOI] [PubMed] [Google Scholar]
- Shults EE, Shakirov MM, Pokrovsky MA, Petrova TN, Pokrovsky AG, Gorovoy PG. Phenolic compounds from Glycyrrhiza pallidiflora Maxim. and their cytotoxic activity. Nat Prod Res. 2017;31(4):445–452. doi: 10.1080/14786419.2016.1188094. [DOI] [PubMed] [Google Scholar]
- Silva BB, Rosalen PL, Cury JA, Ikegaki M, Souza VC, Esteves A, et al. Chemical composition and botanical origin of red propolis, a new type of brazilian propolis. Evid Based Complement Alternat Med. 2008;5(3):313–316. doi: 10.1093/ecam/nem059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93(9):2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- Wei Y, Yu L, Bowen J, Gorovsky MA, Allis CD. Phosphorylation of histone H3 is required for proper chromosome condensation and segregation. Cell. 1999;97(1):99–109. doi: 10.1016/s0092-8674(00)80718-7. [DOI] [PubMed] [Google Scholar]


