Abstract
Persistent responding to food cues may underlie the difficulty to resist palatable foods and to maintain healthy eating habits. Renewal of responding after extinction is a model of persistent food seeking that can be used to study the underlying neural mechanisms. In context-mediated renewal, a return to the context in which the initial cue-food learning occurred induces robust responding to the cues that were extinguished elsewhere. Previous work found sex differences in context-mediated renewal and in the recruitment of the ventromedial prefrontal cortex (vmPFC) during that behavior. Males exhibited renewal of responding to food cues and had higher Fos induction in the prelimbic area (PL) of the vmPFC, while females failed to exhibit renewal of responding and had lower Fos induction in the PL. The main aim of the current study was to determine key components of the PL circuitry mediating renewal. The focus was on inputs from three areas important in appetitive associative learning and contextual processing: the amygdala, ventral hippocampal formation, and the paraventricular nucleus of the thalamus. The goal was to determine whether neurons from these areas that send direct projections to the PL (identified with a retrograde tracer) are selectively activated (Fos induction) during renewal and whether they are differently recruited in males and females. The Fos induction patterns demonstrated that the PL-projecting neurons in each of these areas were recruited in a sex-specific way that corresponded to the behavioral differences between males and females. These pathways were selectively activated in the male experimental group—the only group that showed renewal behavior. The findings suggest the pathways from the ventral hippocampal formation, paraventricular nucleus of the thalamus, and basolateral amygdala to the PL mediate renewal in males. The lack of recruitment in females suggests that under activation of these pathways may underlie their lack of renewal.
Keywords: Ventromedial prefrontal cortex, hippocampal formation, paraventricular nucleus of the thalamus, sex differences, renewal, appetitive conditioning
1. Introduction
Cues associated with food can stimulate food seeking and consumption independently of hunger (for review see Petrovich, 2013), and these behaviors can reappear after extinction because the original learned associations continue to exist (Bouton, 2004). This persistent responding to food cues may help explain the difficulty associated with adopting healthy eating habits (Boutelle & Bouton, 2015). Renewal of responding to food cues after extinction is a model of persistent food seeking that can be used to study the underlying neural mechanisms (Calu, Chen, Kawa, Nair, & Shaham, 2014). In context-mediated renewal, a return to the context in which the initial cue-food learning occurred induces robust responding to the cues that were extinguished elsewhere (Bouton & King, 1983). The neural substrates underlying context-mediated renewal of responding to food cues are still being elucidated. The ventromedial prefrontal cortex (vmPFC) is crucial in renewal of appetitive and fear responding (Eddy, Todd, Bouton, & Green, 2016; Knapska & Maren, 2009; Orsini, Kim, Knapska, & Maren, 2011; Rhodes & Killcross, 2007; Villaruel et al., 2017; Wang, Jin, & Maren, 2016; Willcocks & McNally, 2013). Recently, we found sex differences in context-mediated renewal (Anderson & Petrovich, 2016) and differential vmPFC recruitment in males and females during this behavior (Anderson & Petrovich, 2016). Males exhibited renewal of responding to food cues and had higher Fos induction in the prelimbic area (PL) of the vmPFC. In contrast, females did not exhibit renewal of responding and had lower Fos induction in the PL compared to females in the control group. A follow up study demonstrated that the vmPFC is causal during renewal of responding in a sex-specific way (Anderson & Petrovich, 2017); chemogenetic silencing the vmPFC in males during renewal disrupted responding, while stimulating the vmPFC in females induced responding.
In the current study, the main goal was to determine key components of the PL circuitry mediating renewal. We combined retrograde tracing and Fos induction detection to identify PL-input neurons that are activated during the test for context-mediated renewal in male and female rats. The PL has distinct connections with three areas implicated in appetitive learning: the amygdala, the ventral hippocampal formation, and the paraventricular nucleus of the thalamus (PVT) (Fanselow & Dong, 2010; Hoover & Vertes, 2007; Li & Kirouac, 2012; Moga et al., 1990; Petrovich, Canteras, & Swanson, 2001; Pitkanen, Pikkarainen, Nurminen, & Ylinen, 2000; Reppucci & Petrovich, 2015; Sesack, Deutch, Roth, & Bunney, 1989; Swanson & Petrovich, 1998). The hippocampal formation is critical for contextual processing, including context-dependent renewal (Fanselow, 2000; Holland & Bouton, 1999; Marinelli, Funk, Juzytsch, Li, & Le, 2007; Orsini et al., 2011), the PVT is important for context-induced renewal and is involved in the regulation of food consumption (Anderson & Petrovich, 2016; Bhatnagar & Dallman, 1999; Cole, Mayer, & Petrovich, 2015; Hamlin, Clemens, Choi, & McNally, 2009), and the amygdala is important for appetitive associative learning (Cole, Hobin, & Petrovich, 2015; Crombag & Shaham, 2002; Holland & Petrovich, 2005). Notably, these regions were recruited (Fos induction) during renewal in a sex-specific way that suggested their inputs to the PL may be causal in this behavior (Anderson & Petrovich, 2016). Thus, here we examined whether neurons in the hippocampal formation, PVT, and the amygdala that send direct projections to the PL are selectively activated during renewal and whether they are differentially recruited in males and females.
In these experiments we tested females in different phases of the estrous cycle to determine if different estradiol levels at test mediate differences in renewal responding. Previously, estradiol replacement to ovariectomized rats induced renewal responding, while ovariectomized females without estradiol replacement did not exhibit this behavior (Anderson & Petrovich, 2015).
Determining which PL-projecting neurons are specifically recruited during renewal behavior and how they differ in male and female rats is needed for our better understanding of the fundamental neural circuit mechanisms of reward driven behaviors and potential sites of sex differences. This knowledge about the neural mechanisms of Pavlovian renewal is also important for our understanding of the resilience of food cue to influence our consumption and diet choices.
2. Methods
2.1 Subjects
16 adult male and 32 female Long-Evans rats (250-275g at arrival; Charles River Laboratories) were individually housed and maintained on a 12 h light/dark cycle (lights on at 7:00). Males and females were housed in separate colony rooms. Subjects were acclimated to the colony room for two days prior to brain surgery to receive a retrograde tracer. Animals were given a week to recover post-surgery during which they were weighed and handled daily and were given ad libitum access to standard laboratory chow (18% Protein Rodent Diet #2018, Harlan Teklad Global Diets, Madison, WI) and water, except as otherwise noted. Vaginal smears of the female rats were obtained daily between 15:00-16:00 to ensure normal estrous cycling starting four days prior to behavioral testing. All housing and testing procedures were in compliance with the National Institute of Health Guidelines for Care and Use of Laboratory Animals and were approved by the Boston College Institutional Animal Care and Use Committee.
2.2 Surgical Procedure
Animals were briefly anesthetized with isoflurane (5%; Baxter Healthcare Corporation, Deerfield, IL, USA), and then deeply anesthetized with a mixture (1 ml/kg body weight) of ketamine (50 mg/mL; Fort Dodge Animal Health, Fort Dodge, Iowa) and xylazine (10 mg/mL; LLOYD Laboratories, Shenandoah, IA, USA; delivered via intramuscular injection). While under anesthesia, animals received unilateral stereotaxically placed infusions into the prelimbic area of the ventromedial prefrontal cortex (PL) of Alexa Fluor-594 conjugated cholera toxin B (CTb; Life Technologies, Carlsbad, CA; Figure 1a) delivered at a rate of 0.1μl/min for 4 minutes (0.4 μl totally volume; 5 μg/μl; relative to bregma anterior-posterior [AP]:+2.8mm, mediolateral [ML]: +/− 0.7mm, dorsoventral [DV]: −4.7mm). The injector remained in the site for 10 minutes post-infusion to allow for the diffusion of CTb. A 10 μl Hamilton syringe with 32 gauge cannula driven by a motorized stereotaxic injector (Stoelting, Wood Dale, IL) was used to deliver microinjections. Stereotaxic surgeries were performed according to the procedures for aseptic technique in survival surgery and postoperative care approved by Boston College IACUC. Behavioral experiments started one week after surgery to allow for recovery and sufficient transport of the tracer.
Figure 1.
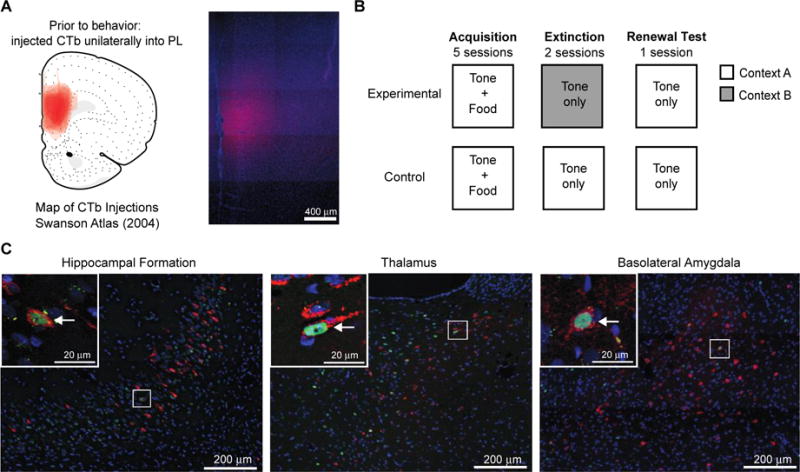
Experimental Design. (A) Map of all CTb injections in the PL of male and female rats (left) and a representative image (right). (B) Between-subjects context-dependent renewal protocol. (C) Representative images of CTb and Fos labeling in the hippocampal formation (CA1v), paraventricular nucleus of the thalamus and the basolateral amygdala. Insets in upper left of each image show magnified area in corresponding rectangles. Fos-positive (green), CTb-positive (red), double-labeled (CTb+Fos) neurons (arrow).
2.3 Apparatus
The behavioral training was conducted in identical behavioral chambers (30 × 28 × 30 cm; Coulbourn Instruments, Allentown, PA) located in a room different from the colony housing rooms. The chambers had aluminum top and sides, clear Plexiglas rear wall and front hinged door and a floor of stainless steel rods 5 mm thick spaced 15 mm apart. Chambers contained a recessed food cup (3.2 × 4.2 cm) and a 4 W house light. Each chamber was located in a sound- and light-attenuating cubicle (79 × 53 × 53 cm), which was equipped with a ventilation fan (55 dB) and video camera attached to a recording system (Coulbourn Instruments, Allentown, PA). The conditioned stimulus (CS) was a 10 seconds long tone (75dB, 2kHz). The unconditioned stimulus (US) consisted of two food pellets (45 mg pellets, formula 5TUL; Test Diets, Richmond, IN, USA) delivered to the food cup. Chambers were modified in visual, tactile, and olfactory features, to create two distinct environments. For Context 1, a black Plexiglas panel was placed on top of the grid floor (so that rats could not see or feel the grids), and the doors to the cubicles were closed. For Context 2, a black Plexiglas panel was inserted diagonally across the side of the chamber creating a wall, and the doors to the cubicle were left open, and 1% acetic acid in water solution (Fisher Scientific, Fair Lawn, NJ) was sprayed onto the tray below the grid floor.
2.4 Behavioral training procedure
All behavioral training and testing occurred between 9:00 and 14:00. A week before start of training, all rats were food deprived and their daily food allotment was restricted to gradually reach 85% of their body weight; they were maintained at this weight for the duration of the experiment. All rats received 1 g of the food pellets (US) in the home cage the day before the training started to familiarize them with the pellets. The training consisted of three phases: conditioning (acquisition), extinction, and renewal test (Figure 1b). The training protocol followed an “ABA” design where conditioning acquisition and extinction occurred in different contexts and renewal occurred in the same context as acquisition. During the acquisition phase, rats were trained for five days, with one 34-minute training session per day. During each session they received eight presentations of the tone (CS), each immediately followed with delivery of food pellets (US) into the food cup. The acquisition training occurred in Context A (Context 1 for half of the rats, and Context 2 for the other half). During the extinction phase, rats received two 34-minute sessions (one session per day), each with eight presentations of the CS alone, with no USs. Rats in the experimental condition received extinction training in a context different than the training context (ABA), while rats in the control condition remained in the same context across all training phases (AAA). The test for renewal was one 34-minute session with eight CS presentations and no USs, conducted in the acquisition context. The intertrial interval was 110-326 seconds and the length varied randomly across trials and training sessions. All sessions were recorded and stored on DVDs for behavioral analysis. After the second extinction session, females rats in the experimental and control groups were divided into two groups based on estrous cycle phase so that during the renewal test half of the females were in a high estradiol phase (Proestrus; High E) while the other half of the females were in a low estradiol phase (Metestrus or early Diestrus; Low E). This resulted in four female groups: High E Control, High E Experimental, Low E Control, Low E Experimental.
2.5 Behavioral Measures
Trained observers, ‘blind’ to experimental condition or sex of the rats, analyzed animals’ behavior from the video recordings. The primary measure of conditioning (conditioned response, CR) was the expression of ‘food cup behavior’ during the CS. The food cup behavior was defined by distinct nose pokes into the recessed food cup, or by rats standing in front of and directly facing the food cup. Behavior was scored every 1.25 seconds during each 10 second preCS and CS periods. At each observation only one behavior was recorded (food cup or other). The number of CRs was summed and converted to a percentage of the total time during each period an animal expressed food cup behavior.
2.6 Histological procedures
Ninety minutes after the end of renewal tests, rats were anaesthetized with tribromoethanol (375 mg/kg body weigh, i. p.) and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde in 0.1 M borate buffer. The brains were stored for 20-24hrs at 4°C in a paraformaldehyde and 12% sucrose mixture and then rapidly frozen in hexanes cooled with dry ice and stored at −80°C. To determine the location and spread of the CTb injections, brain tissue containing the prefrontal cortex was cut into 30μm coronal sections using a microtome and collected into four adjacent series. One series was mounted unstained for identification of CTb location in the PL. Another series was mounted and stained with thionin for identification of anatomical borders as defined in Swanson (2004) (Swanson, 2004). Of those brains that had well-defined and localized injections (described below), the remaining part of the brains were cut into 30μm coronal sections and collected into four adjacent series. One series was used to identify Fos expression with an anti-Fos antibody and fluorescent-conjugated secondary antibody (AlexaFluor-488), following a standard immunohistochemistry protocol, described below (Figure 1c). Another series was mounted and stained with thionin for identification of anatomical borders.
2.7 Immunohistochemistry
Immediately following slicing, sections were incubated for 72 h at 4°C in a blocking solution [KPBS containing 0.3 % Triton X-100, 2 % normal donkey serum (017-000-001; Jackson ImmunoResearch, West Grove, PA, USA)], with the primary antibody: anti-c-Fos antibody raised in rabbit (1:10,000; Millipore, Billerica, MA). After rinses in KPBS, tissue was incubated for 1 h in the dark in the blocking solution containing the secondary antibody: anti-rabbit Alexa 488 (1:200; A21206; Invitrogen, Carlsbad, CA, USA). Following rinses in KPBS, in semidarkness tissue was mounted onto slides (SuperFrost Plus), dried, coverslipped with Vectashield HardSet Mounting Medium with DAPI (H-1500; Vector Labs, Burlingame, CA, USA), and stored at 4°C until analysis.
2.8 Image acquisition and analysis
The acquisition of images was conducted with a Zeiss Axio Image Z2 fluorescence microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) and attached Hamamatsu ORCA-R2 camera (Bridgewater, NJ, USA). To determine the location and extent of CTb injection sites, images of the areas with tracer deposits and the adjacent thionin-stained tissue were acquired at 10×. Neuroanatomical borders were drawn onto the thionin-stained image and then transposed to the adjacent fluorescently image using ImageJ software (NIH). The injection sites were then drawn on computerized versions of the standard rat brain atlas templates (Swanson, 2004) using illustration software (Adobe Illustrator CS5.5). All CTb injections were analyzed and well-defined and localized injections were identified based on the following criteria: at least 50% of the target region contained CTb and with less than 25% of the injection spread outside the target region (Figure 2).
Figure 2.
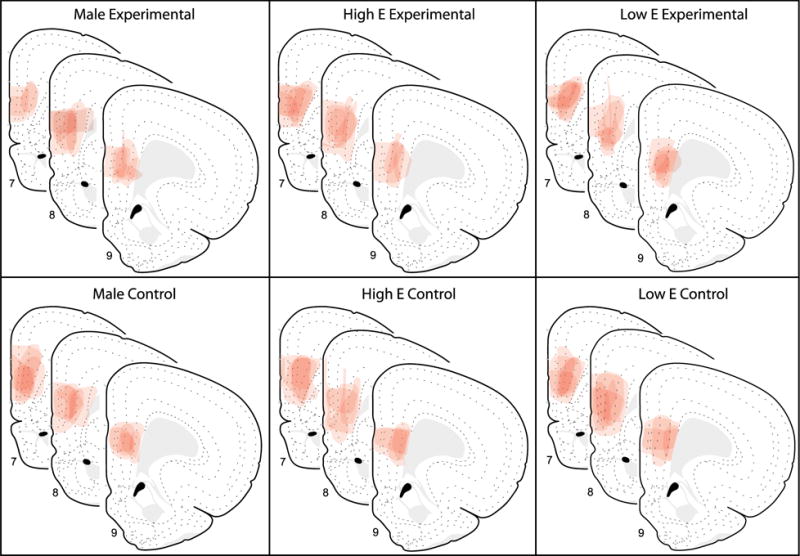
Illustration of CTb placements in the PL across groups. The extent of CTb injections in the PL for each group is shown on modified Swanson atlas templates (Swanson (2004); atlas levels 7, 8, 9; +3.6, +3.2 and +2.8 mm from bregma, respectively).
Retrograde labeling and Fos were analyzed within the ventral hippocampus, paraventricular nucleus of the thalamus, and the basolateral area of the amygdala (Figure 1c). Within the hippocampal formation, the ventral field CA1, Ammon’s horn (Levels 34-39 as defined in the Swanson atlas (2004), −4.45mm to −6.60mm from Bregma; all subsequent levels refer to Swanson atlas, all subsequent measurements refer to mm from Bregma) and ventral subiculum (Levels 34-39, −4.45mm to −6.60mm) were analyzed. Within the paraventricular nucleus of the thalamus, the anterior (PVTa; Levels 24-27, −1.33mm to −2.00mm) and posterior (PVTp; Levels 28-32, −2.45mm to −3.90mm) parts were analyzed separately. Within the basolateral area of the amygdala, the anterior part of the basolateral nucleus (BLAa; Levels 25-29, −1.53mm to −2.85 mm), the posterior part of the basolateral nucleus (BLAp; Levels 28-34, −2.45mm to −4.45mm), the posterior part of the basomedial nucleus (BMAp; Levels 28-32, −2.45mm to −4.20mm), and the lateral nucleus (LA; Levels 25-32, −1.53mm to −3.90mm) were analyzed. The analysis followed parcellation and nomenclature as defined in the Swanson atlas (2004). Consecutive images were taken at 20X through the regions of interest (one image per atlas level). Images were pseudocolored with red for CTb, green for Fos, and blue for DAPI (nuclear counterstain). Single CTb, single Fos and double-labeled (CTb and Fos) neurons were determined based on characteristic nuclear (Fos) or cytoplasmic (CTb) labeling. Single (CTb or Fos) and double-labeled (CTb and Fos) neurons were counted. Double-labeled neurons were expressed as a percentage of total CTb neurons.
2.9 Statistical Analysis
Behavioral (i.e. food cup behavior) and neuronal data analyses were conducted with ANOVAs and post hoc t-tests, as appropriate. For the ANOVAs, the factors of Group (Experimental, Control) and Sex (Male, Female) were used, unless otherwise stated. In all cases, p < 0.05 was considered significant. SPSS software was used for all statistical analyses. All data are presented as means ± SEM. For the behavioral statistical analysis, total CRs during the preCS and CS periods, as well as Elevation (CS-preCS responding), were analyzed. 2 male rats were excluded due to poor health. The final groups, after exclusions due to unacceptable tracer injection sites, were n=5 per group: Male Control, Male Experimental, High E Control, High E Experimental, Low E Control, Low E Experimental.
3. Results
3.1 Behavior
3.1.1 Acquisition
Behavioral data are presented as Elevation scores (CS-preCS responding) to show CS-specific responding above baseline (Figure 3). During acquisition, all rats showed an increase in food cup responding (CR) across the training sessions during the tone (CS) presentations when Elevation scores were compared (Repeated measures ANOVA Greenhouse-Geisser correction, F(3.506,91.167)=56.319, p=0.000). There were no Sex or Group differences (p>0.05, both). This increase was also significant for total CRs (without subtracting preCS responding) during the CSs (Repeated measures ANOVA Greenhouse-Geisser correction, F(3.284,85.381)=63.728, p=0.000), and there were no Sex or Group differences (p>0.05, both). There was a significant effect in responding during preCS across training sessions due to variability across sessions (Repeated measures ANOVA Greenhouse-Geisser correction, F(3.719, 107.858)=3.492, p=0.012); however, preCS responding remained low throughout training (average percentage of food cup behavior across session (mean ± SEM), Male Experimental: 20 ± 5, Male Control: 25 ± 5, Female Experimental: 23 ± 3, Female Control: 23 ± 4) and the first session was not significantly different from the last acquisition session for any groups (Paired t-test, ps>0.05). During the last acquisition session (Acquisition 5), all rats showed high CRs during CSs compared to their low responding during preCS (t(29)= −17.418, p=0.000). An ANOVA (Sex and Group) confirmed that there were no significant differences across groups in responding during CS or preCS (p>0.05, both).
Figure 3.
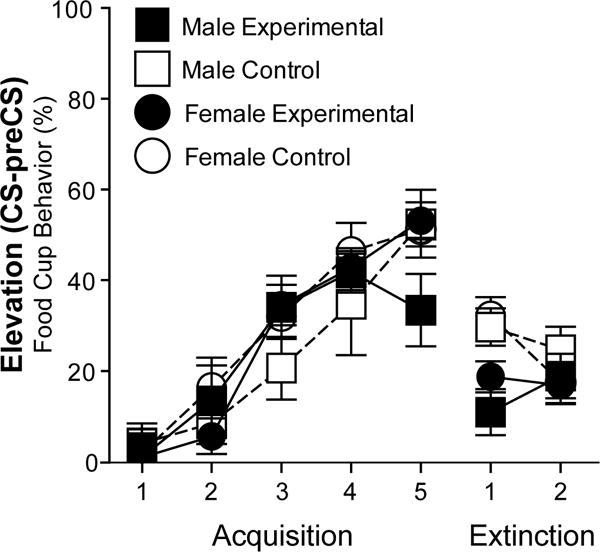
Conditioned responses during acquisition and extinction. Elevation scores represent percentage of time rats expressed food cup behavior (mean ± SEM) during CS above the baseline (preCS) during each Acquisition and Extinction training session.
3.1.2 Extinction
All rats showed low responding during extinction (Figure 3). There was a statistically significant decrease in responding during CSs (total CRs and Elevation) across the last acquisition session, the first extinction session, and the second extinction session for all groups (Repeated measures ANOVA Greenhouse-Geisser correction for Elevation: F(1.831,45.781)=56.854, p=0.000; for total CRs: F(1.946,48.638)=118.662, p=0.000). When responding across the two extinction sessions was compared, there was a significant effect of session for the total CRs during CSs (Repeated measures ANOVA Greenhouse-Geisser correction, F(1,25)=14.266, p=0.001) but not for Elevation (p>0.05). For total CRs, there was also an effect of Group (F(1,25)=6.849, p=0.015), while there were no effects of Sex or Group for Elevation (ps>0.05). The group effect was driven by higher responding in the control groups (tested in the acquisition context) during the first extinction session (One-way ANOVA on Extinction Session 1 total CRs, F(1,26)=12.379, p=0.002). This group effect was transient and by the second extinction session there were no differences in responding across groups (One-way ANOVA on Extinction Session 2 total CRs, ps>0.05). All groups showed similar, low responding in preCS periods during each extinction sessions (p>0.05, percentage of time rats expressed food cup behavior during preCS average across extinction sessions (mean ± SEM): Male Experimental: 9 ± 3, Male Control: 14 ± 6, Female Experimental: 10 ± 2, Female Control: 16 ± 4).
3.1.3 Renewal
During the test for renewal, only male groups showed differential responding (Figure 4; Table 1). There was no effect of Estradiol condition on responding of females (Figure 4; ANOVA (Estradiol Condition by Group); p>0.05)), and therefore High and Low Estradiol groups were analyzed together. An ANOVA (Sex and Group) revealed a significant effect of Sex by Group interaction on CRs during CSs (F(1,26)=10.570, p=0.003; Table 1) but no effect of Sex or Group (p>0.05, both). Post hoc tests confirmed that the males in the experimental group had significantly more CRs compared to the males in the control condition (t(6.984)= −4.501, p=0.003) while females were not significantly different (p>0.05). Additionally, there was a significant effect of Sex by Group interaction on responses during preCSs (F(1,26)=4.822, p=0.037) but no significant differences between groups (p>0.05), most likely due to opposite patterns in males and females (Table 1, higher preCS responding in males in the experimental group and females in the control group compared to males in the control group and females in the experimental group). To account for the difference in preCS responding, we examined Elevation Scores (CS-preCS responding) by group (Figure 4). Males in the experimental group had significantly higher elevation scores compared to the males in the control group (t(7.160)= −2.963, p=0.020). Females in the experimental group and control groups did not show differential responding (p>0.05). Interestingly, both female groups showed high responding, similar to the experimental male group (p>0.05).
Figure 4.
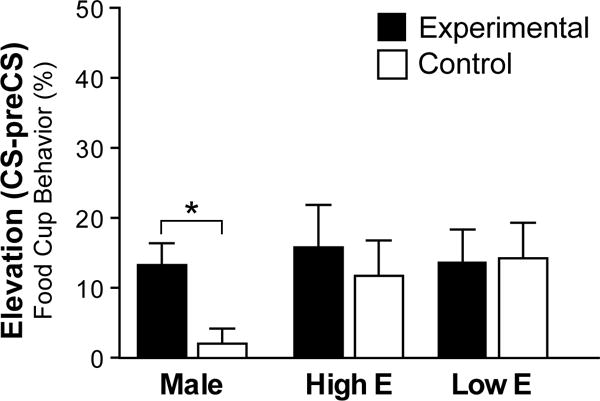
Conditioned responses during the test for renewal. Elevation scores represent percentage of time rats expressed food cup behavior during CS minus preCS during the test for renewal. Bars are mean ± SEM. * indicates p < 0.01.
Table 1.
Conditioned responses during the test for renewal. Percentage of time (mean ± SEM) rats expressed food cup behavior during preCS and CS.
| Male | Female | |||
|---|---|---|---|---|
| Exp | Control | Exp | Control | |
| preCS | 17.2 ± 5 | 7.8 ± 5 | 9.8 ± 2 | 17.5 ± 4 |
| CS | 30.3 ± 3* | 9.7 ± 4 | 24.4 ± 3 | 30.3 ± 5 |
indicates difference from same-sex control.
p < 0.05.
3.2 Neuronal Analysis
3.2.1 Retrograde Tracer Injection Sites
The location and spread of CTb injection sites were analyzed throughout the rostro-caudal extent of the PL based on the Swanson brain atlas (Swanson, 2004). Acceptable injections (see methods for the criteria) were confined predominately within the PL (n=30) and were centered within the mid rostro-caudal extent of the PL (Figure 2; Swanson atlas levels 7, 8 and 9; +3.6, +3.2 and +2.8 from bregma, respectively).
3.2.2 Fos Induction
Retrograde labeling and Fos were analyzed within the ventral hippocampal formation (SUBv, CA1v), paraventricular nucleus of the thalamus (PVTa, PVTp), and the basolateral area of the amygdala (BLAa, BLAp, BMAp, LA). Single (CTb or Fos) and double-labeled (CTb and Fos) neurons were counted. Double-labeled neurons were also expressed as a percentage of total CTb neurons in each animal.
Because there were no behavioral differences between High and Low Estradiol groups for all statistical analyses these two groups were collapsed together by group.
3.2.3 Hippocampal formation
3.2.3.1 SUBv
In the SUBv, there was overall more total Fos induction in males compared to females and more Fos induction the experimental groups compared to control groups (Figure 5). This was supported by an ANOVA, which found Sex (F(1,26)=52.523, p=0.000) and Group (F(1,26)=5.517, p=0.027) but no Sex by Group interaction effects (p>0.05). Post hoc comparisons confirmed females in the experimental group had significantly higher Fos induction compared to females in the control group (t(15.850)= −2.216, p=0.042), while higher Fos in male experimental group compared to male controls was not significant (p>0.05). Males in the experimental group were significantly different from females in the experimental group however (t(4.073)=3.160, p=0.033), as well as from females in the control group (t(4.160)=3.517, p=0.023).
Figure 5.
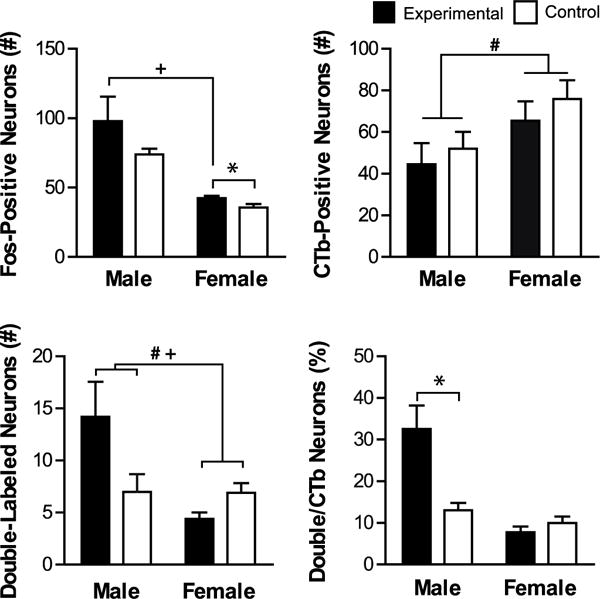
Fos induction in SUBv-PL projecting neurons. Shown are mean values (± SEM) for total number of Fos-positive, CTb-positive, and double-labeled (CTb + Fos) neurons, as well as percentage of CTb neurons that were double-labeled. # denotes significant main effect of Sex and + denotes significant main effect of Sex by Group. * denotes significant difference between experimental and same-sex control. p < 0.05.
There were more CTb neurons in the female groups compared to the male groups (Figure 5). This was confirmed with a significant effect of Sex on the number of CTb-positive neurons (F(1,26)=4.607, p=0.041), and there was no effect of Group or Sex by Group interaction (p>0.05).
There were overall more double-labeled (Fos+CTb) neurons in males compared to females and the highest number was in the experimental males (Figure 5). There was a significant effect of Sex and Sex by Group on the number of double-labeled neurons (Sex: F(1,26)=10.722, p=0.003, Sex by Group: F(1,26)=0.293, p=0.004) but no effect of Group (p>0.05). Post hoc t-tests found that the number of double-labeled neurons in the males in the control and experimental groups were not significantly different (p>0.05). However, the number of Fos+CTb labeled neurons was significantly higher in the males in the experimental group compared to females in the experimental group (t(4.274)=2.875, p=0.042) but not compared to females in the control group (p>0.05).
The proportion of total CTb neurons that were double-labeled (Fos+CTb) was the highest in the males in the experimental group compared to all other groups (Figure 5). There were significant effects of Sex, Group, and Sex by Group on the percentage of double-labeled neurons (Sex: F(1,26)=31.414, p=0.00, Group: F(1,26)=12.256, p=0.002; Sex by Group: F(1,26)=19.212, p=0.000; Figure 5). These effects were driven by a statistically higher percentage of CTb+Fos neurons in males in the experimental group compared to males in the control group (t(4.762)= −3.323, p=0.023). Female groups did not differ in the percentage of CTb+Fos neurons (p>0.05).
Since there were sex differences in the number of CTb-positive neurons in the SUBv, we additionally examined males and females separately using individual t-tests. Consistent with the original analysis, males in the experimental group had a significantly higher percentage of double-labeled cells (t(8)= −3.323, p=0.010) and females in the experimental group had significantly more total Fos induction (t(18)= −2.216, p=0.040) and fewer double-labeled neurons (t(18)=2.209, p=0.040).
3.2.3.2 CA1v
In the CA1v, there was more overall Fos induction in the male groups compared to the female groups (Figure 6). This was confirmed with a significant effect of Sex on the number of Fos-positive neurons (F(1,26)=32.160, p=0.000), but no effect of Group or Sex by Group (p>0.05).
Figure 6.
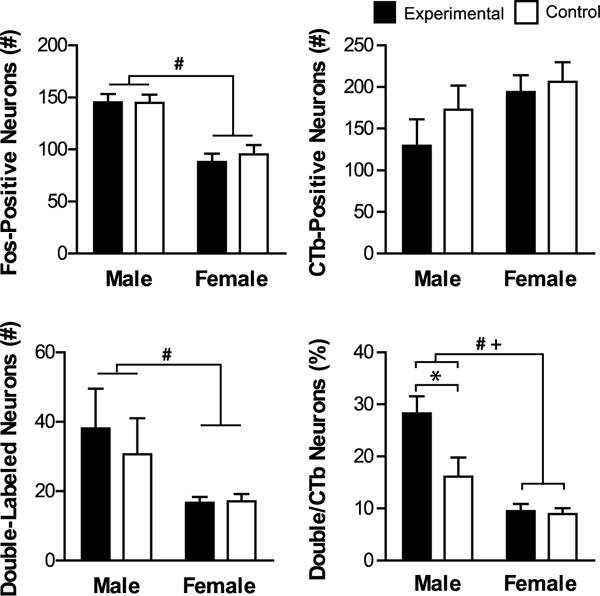
Fos induction in CA1v-PL projecting neurons. Shown are mean values (± SEM) for total number of Fos-positive, CTb-positive, and double-labeled (CTb + Fos) neurons, as well as percentage of CTb neurons that were double-labeled. # denotes significant main effect of Sex and + denotes significant main effect of Sex by Group. * denotes significant difference between experimental and same-sex control. p < 0.05.
There were no differences between groups in the number of CTb-positive neurons (p>0.05).
There were more double-labeled neurons in the male groups compared to the female groups (Figure 6). This was confirmed with a significant effect of Sex on the number of double-labeled neurons (F(1,26)=9.662, p=0.005), and there was no effect of Group or Sex by Group interaction (p>0.05).
The proportion of CTb neurons that were double-labeled was the highest in the males in the experimental group compared to all other groups (Figure 6). There were significant effects of Sex, Group and Sex by Group on the percentage of double-labeled neurons (Sex: F(1,26)=38.765, p=0.00, Group: F(1,26)=9.261, p=0.005; Sex by Group: F(1,26)=7.762, p=0.010). These effects were driven by a statistically higher percentage in males in the experimental group compared to males in the control group (t(7.902)= −2.483, p=0.038). Males in the experimental group were also significantly different from females in the experimental (t(5.429)=5.318, p=0.002) and control (t(4.934)=5.613, p=0.003) groups. Female groups did not differ in the percentage of CTb+Fos neurons (p>0.05).
3.2.4 Paraventricular Nucleus of the Thalamus
3.2.4.1 PVTa
Within the PVTa, Fos induction was highest in males in the experimental group (Figure 7). This was confirmed with significant effects of Sex and Sex by Group (Sex: F(1,26)=7.142, p=0.013, Sex by Group: F(1,26)=5.522, p=0.027), but no effect of Group (p>0.05). Males in the experimental group had higher Fos induction than males in the control group, but this trend did not reach significance (t(5.335)= −2.377, p=0.060). Males in the experimental group had significantly higher Fos induction from females in the experimental (t(8.875)=3.118, p=0.013) and control (t(6.711)=2.456, p=0.045) groups.
Figure 7.
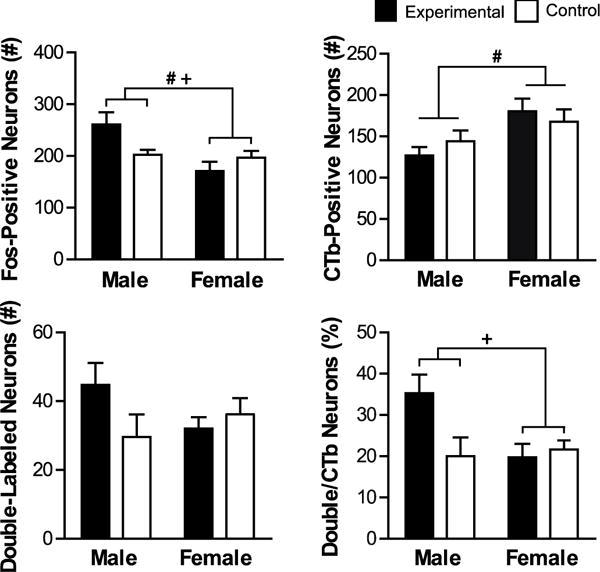
Fos induction in PVTa-PL projecting neurons. Shown are mean values (± SEM) for total number of Fos-positive, CTb-positive, and double-labeled (CTb + Fos) neurons, as well as percentage of CTb neurons that were double-labeled. # denotes significant main effect of Sex and + denotes significant main effect of Sex by Group. p <0.05.
There were more CTb neurons in the female groups compared to the male groups (Figure 7). There was a significant effect of Sex on the number of CTb-positive neurons (F(1,26)=5.651, p=0.025) but no effect of Group or Sex by Group (p>0.05). The effect of Sex was driven by higher number of CTb-positive neurons in females compared to males (t(27.379)= −2.898, p=0.007).
There were no differences between groups in the number of double-labeled neurons (p>0.05).
The percentage of CTb-positive neurons that were double-labeled with Fos was highest in the male experimental group (Figure 7). There was a significant effect of Sex by Group (F(1,26)=4.924, p=0.035), and there was no effect of Group or Sex (p>0.05).
Post hoc t-test comparison found that difference between male experimental and control groups did not reach significance (t(7.882)= −2.080, p=0.072), however males in the experimental group were significantly different from females in the experimental group (t(8.052)=2.761, p=0.024).
Since there were sex differences in the number of CTb-positive neurons in the PVTa, similar to SUBv, we additionally examined males and females separately using individual t-tests. Consistent with the original analysis, males in the experimental group had a significantly higher total Fos induction compared to males in the control group (t(8)= −2.377, p=0.045). There were no differences between females in the experimental and control groups (p>0.05).
3.2.4.2 PVTp
Within the PVTp, total Fos induction was higher in males compared to females (Figure 8). There was a main effect of Sex on total number of Fos neurons (F(1,26)=9.816, p= 0.004), and no effect of Group or Sex by Group interaction (p>0.05), which was driven by higher Fos induction in males compared to females (t(17.685)=3.152, p= 0.006).
Figure 8.
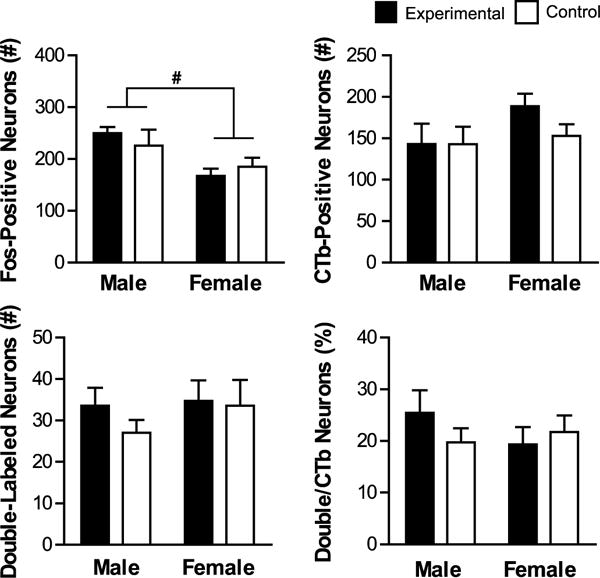
Fos induction in PVTp-PL projecting neurons. Shown are mean values (± SEM) for total number of Fos-positive, CTb-positive, and double-labeled (CTb + Fos) neurons, as well as percentage of CTb neurons that were double-labeled. # denotes significant main effect of Sex. p < 0.05.
There were no other differences between groups in the PVTp for number of CTb-positive neurons, double-labeled neurons or percentage of double- labeled neurons (p>0.05).
3.2.5 Basolateral Area of the Amygdala
3.2.5.1 BLAa
Within the BLAa, Fos induction was highest in males in the experimental group (Figure 9). This was confirmed with an ANOVA with a main effect of Sex and Sex by Group (Sex: F(1,26)=7.446, p=0.011, Sex by Group: F(1,26)=5.554, p=0.026), and there was no effect of Group (p>0.05). Post hoc t-test found that the difference in Fos induction between males in the control experimental groups did not reach significance (p>0.05). Males in the experimental group were significantly different from females in the experimental group (t(5.563)=2.506, p=0.049) but not from females in the control group (t(4.777)=2.356, p=0.067)
Figure 9.
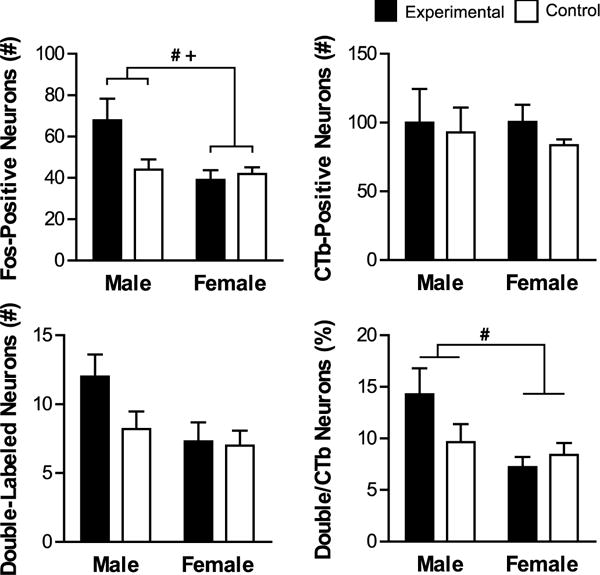
Fos induction in BLAa-PL projecting neurons. Shown are mean values (± SEM) for total number of Fos-positive, CTb-positive, and double-labeled (CTb + Fos) neurons, as well as percentage of CTb neurons that were double-labeled. # denotes significant main effect of Sex and + denotes significant main effect of Sex by Group. p < 0.05.
There were no significant differences between groups in the number of CTb-positive neurons or double-labeled neurons (p>0.05, all).
The males in the experimental group had the highest percentage of CTb neurons that were double labeled (Figure 9). There was an effect of Sex (F(1,26)=7.575, p=0.011) but no effects of Group or Sex by Group interaction (p>0.05) on the percentage of double-labeled neurons as a portion of the total number of CTb-positive neurons. This was driven by a significantly higher percentage in males in the experimental group compared to females in the experimental group (t(5.309)=2.608, p=0.045).
3.2.5.2 BLAp
Within the BLAp, there were no differences between groups for Fos induction, CTb-positive neurons, double-labeled neurons, or percentage of double labeled neurons (p>0.05 for all; Table 2).
Table 2.
Counts for all brain regions analyzed. Total number of Fos-positive, CTb-positive, double-labeled (CTb + Fos) neurons, and percentage of CTb neurons that were double-labeled. Results are displayed as a mean ± SEM.
| Brain Region | Male | Female | |||
|---|---|---|---|---|---|
|
|
|||||
| Experimental | Control | Experimental | Control | ||
| SUBv | Fos+ | 97 ± 17 | 74 ± 4 | 42 ± 2* | 36 ± 2 |
| CTb# | 45 ± 6 | 52 ± 8 | 65 ± 9 | 75 ± 9 | |
| Double# + | 14 ± 3 | 7 ± 2 | 4 ± 1 | 7 ± 1 | |
| Percentage# + | 33 ± 6* | 13 ± 2 | 8 ± 1 | 10 ± 2 | |
|
| |||||
| CA1v | Fos# | 145 ± 8 | 145 ± 8 | 88 ± 8 | 95 ± 9 |
| CTb | 129 ± 32 | 173 ± 29 | 193 ± 20 | 206 ± 23 | |
| Double# | 38 ± 11 | 31 ± 10 | 17 ± 2 | 17 ± 2 | |
| Percentage# + | 28 ± 3* | 16 ± 4 | 10 ± 1 | 9 ± 1 | |
|
| |||||
| PVTa | Fos# + | 261 ± 23 | 203 ± 9 | 171 ± 18 | 197 ± 13 |
| CTb# | 127 ± 10 | 144 ± 13 | 181 ± 15 | 168 ± 15 | |
| Double | 45 ± 6 | 30 ± 7 | 32 ± 3 | 36 ± 5 | |
| Percentage+ | 35 ± 5 | 20 ± 4 | 20 ± 3 | 22 ± 2 | |
|
| |||||
| PVTp | Fos# | 250 ± 11 | 226 ± 31 | 168 ± 14 | 185 ± 17 |
| CTb | 143 ± 24 | 143 ± 21 | 189 ± 15 | 153 ± 13 | |
| Double | 34 ± 4 | 27 ± 3 | 35 ± 5 | 34 ± 6 | |
| Percentage | 25 ± 4 | 20 ± 3 | 19 ± 3 | 21 ± 3 | |
|
| |||||
| BLAa | Fos# + | 68 ± 10 | 44 ± 5 | 39 ± 5 | 42 ± 3 |
| CTb | 99 ± 24 | 93 ± 18 | 101 ± 12 | 83 ± 4 | |
| Double | 12 ± 2 | 8 ± 1 | 7 ± 1 | 7 ± 1 | |
| Percentage# | 14 ± 3 | 10 ± 2 | 7 ± 1 | 8 ± 1 | |
|
| |||||
| BLAp | Fos | 50 ± 7 | 39 ± 3 | 35 ± 4 | 38 ± 4 |
| CTb | 105 ± 24 | 157 ± 30 | 160 ± 18 | 179 ± 20 | |
| Double | 6 ± 1 | 7 ± 1 | 8 ± 1 | 9 ± 1 | |
| Percentage | 6 ± 1 | 6 ± 2 | 5 ± 1 | 5 ± 1 | |
|
| |||||
| BMAp | Fos# | 41 ± 8 | 36 ± 6 | 24 ± 2 | 30 ± 3 |
| CTb | 50 ± 16 | 66 ± 13 | 80 ± 7 | 80 ± 10 | |
| Double | 5 ± 1 | 4 ± 1 | 4 ± 1 | 5 ± 1 | |
| Percentage+ | 10 ± 2 | 6 ± 2 | 6 ± 1 | 7 ± 1 | |
|
| |||||
| LA | Fos# | 51 ± 7 | 41 ± 5 | 27 ± 2 | 34 ± 5 |
| CTb | 43 ± 11 | 50 ± 13 | 68 ± 10 | 66 ± 14 | |
| Double | 4 ± 2 | 3 ± 1 | 4 ± 1 | 5 ± 1 | |
| Percentage | 8 ± 3 | 7 ± 2 | 5 ± 1 | 6 ± 1 | |
Abbreviations: BLAa- anterior part of the basolateral nucleus of the amygdala, BLAp- posterior part of the basolateral nucleus of the amygdala, BMAp- posterior part of the basomedial nucleus of the amygdala, CA1v- ventral field CA1, Ammon’s horn, LA- lateral nucleus of the amygdala, PVTa- anterior part of the paraventricular nucleus of the thalamus, PVTp- posterior part of the paraventricular nucleus of the thalamus, SUBv- ventral subiculum.
denotes significant main effect of Sex and
denotes significant main effect of Sex by Group.
denotes significant difference compared to same-sex control.
p < 0.05.
3.2.5.3 BMAp
Within the BMAp, there was more Fos induction in the male groups compared to the female groups (Table 2). There was an effect of Sex (F(1,26)=6.414, p= 0.018) but not of Group or Sex by Group (p>0.05) on total amount of Fos induction. However, post hoc t-tests revealed no significant differences between groups (p>0.05 for all).
There were no significant differences between groups in the number of CTb-positive neurons or double-labeled neurons (p>0.05, all).
The percentage of CTb neurons that were double-labeled was highest in males in the experimental group (Table 2). There was a significant effect of Sex by Group (F(1,26)=4.979, p=0.034) but not of Sex or of Group (p>0.05). However, post hoc t-tests revealed no differences between groups.
3.2.5.4 LA
Within the LA, there was more Fos induction in the male groups compared to the female groups (Table 2). This was confirmed with an effect of Sex on total number of Fos neurons (F(1,26)=9.458, p= 0.005). There were no effects of Group or Sex by Group (p>0.05).
There were no other differences between groups for number of CTb neurons, number of double-labeled neurons, or percentage of CTb neurons (p>0.05 for all; Table 2).
4. Discussion
Here, we identified recruitment of key pathways to the PL during context-dependent renewal of conditioned responding to food cues in male and female rats. Specifically we examined PL-projecting neurons within areas important in associative learning and contextual processing: the hippocampal formation, thalamus and basolateral area of the amygdala. To accomplish this, we used a retrograde tracer (CTb) to identify PL-projecting neurons in each area and Fos induction to examine their recruitment during context-mediated renewal. We found projections to the PL from the ventral hippocampal formation (SUBv and CA1v), thalamus (PVTa) and basolateral area of the amygdala (BLAa) were recruited during renewal in a sex-specific way.
Within the ventral hippocampal formation, we found selective recruitment of PL-projecting neurons in both the SUBv and CA1v during renewal responding. In the SUBv, male rats in the experimental group that showed renewal had significantly higher Fos induction in the SUBv-PL pathway (CTb-positive) neurons. Females in both conditions had similar behavior and similar SUBv-PL pathway recruitment. In addition to higher specific Fos induction in the PL-projecting neurons in the experimental group, males also had significantly higher total Fos induction than females. This is in contrast to prior findings that both males and females in the experimental groups had higher Fos induction than their sex-matched controls in the SUBv (Anderson & Petrovich, 2016). A methodological difference in sampling is potentially the reason why these results differ. Anderson & Petrovich (2016) examined the entire dorso-ventral and medio-lateral extent within the sampling area, while in the current study the total Fos was counted within a more constrained area where PL projecting neurons were concentrated.
In the CA1v, as in the SUBv, males in the experimental group had the highest percentage of PL-projecting neurons that were recruited during renewal compared to all other groups. In addition, males had higher overall Fos induction and higher numbers of PL-projecting neurons with Fos compared to females. Taken together, these results suggest that the pathways from the ventral hippocampal formation (SUBv and CA1v) to PL are important mediators of responding during renewal. These pathways were selectively activated in males that showed renewal but under activated in both groups of females, which did not show differential responding. These activation patterns supports the hypothesis that the ventral hippocampal pathways to PL mediate contextual information during renewal of responding in a sex-specific way.
The current findings are in agreement with previous evidence the ventral hippocampal formation is important for context-dependent aversive and appetitive renewal (Anderson & Petrovich, 2016; Herry et al., 2008; Hobin, Ji, & Maren, 2006; Jin & Maren, 2015). Additionally, the hippocampal formation is a known site of sex differences in associative conditioning (Chang et al., 2009; Gresack, Schafe, Orr, & Frick, 2009; Maren, De Oca, & Fanselow, 1994; Matsuda et al., 2015). The hippocampal formation function is influenced by estradiol levels, which could change its responsiveness to associative cues and hinder associative learning (Gupta, Sen, Diepenhorst, Rudick, & Maren, 2001; Khayum, de Vries, Glaudemans, Dierckx, & Doorduin, 2014; Shors, Chua, & Falduto, 2001; Shors, Falduto, & Leuner, 2004). This is in part why we examined females during different stages in the estrous cycle on test day. However there were no behavioral differences between females tested under high and low estradiol levels, which indicates previously observed estradiol effects (with chronic estradiol replacement; Anderson & Petrovich, 2016) are not mediated acutely during test.
In the PVTa, similar to the SUBv, males in the experimental group—the only group that showed renewal— had the highest percentage of PL-projecting neurons that were recruited during renewal. These results suggest that the PVTa-PL pathway, like SUBv-PL pathway, may have been under activated or inhibited in the females during test. These findings build upon prior evidence the PVT is recruited and necessary during appetitive renewal. The PVT and its inputs are important for context-dependent and cue-induced reinstatement of alcohol seeking (Hamlin et al., 2009; Marchant, Furlong, & McNally, 2010; Wedzony et al., 2003).
It should be noted that in the SUBv and PVTa, there were sex differences in the number of CTb neurons. In both regions, there were more CTb labeled neurons in females compared to males. This could be due to differences in the sizes of injections or different connectional patterns in males and females. As shown in Figure 2, the injection sites were evenly matched in size and distribution across sex and experimental conditions, which suggests an intriguing possibility that in females more neurons in SUBv and PVTa send inputs to PL than in males. However, these findings should be interpreted with caution, as more work is needed, including quantitative analyses, in order to determined if these differences are due to structural sex differences. Here, to account for individual differences in the number of CTb labeled neurons we expressed double-labeled (CTb+Fos) neurons as a percent of total number of CTb labeled cells.
The Fos induction patterns in the BLAa were similar to those in the PVTa and SUBv. The males in the experimental group had a significantly higher percentage of CTb+Fos positive neurons compared to females in the experimental group, suggesting that BLAa-PL pathways are recruited in a sex-specific manner. The male experimental group also had higher total Fos induction compared to females in the experimental group. Interestingly, prior work (Anderson & Petrovich, 2016) did not find Fos induction differences in the BLAa between conditions or sexes. This is likely due to methodological difference in sampling areas between the studies, as discussed above; the entire dorso-ventral and medio-lateral BLAa extent was analyzed in Anderson & Petrovich (2016), while in the current study the total Fos was counted within a more constrained area of BLAa where PL projecting neurons were concentrated (Reppucci & Petrovich, 2015). The current findings are in general agreement that the basolateral area of the amygdala is important in context-dependent renewal of drug or fear cues (Crombag, Bossert, Koya, & Shaham, 2008; Orsini et al., 2011) and more broadly in appetitive conditioning tasks (Cole, Hobin, et al., 2015; Cole, Powell, & Petrovich, 2013; Crombag et al., 2008; Holland & Petrovich, 2005; Petrovich, 2013; Wassum & Izquierdo, 2015).
Notably, there was less activation of PL-pathways from the SUBv/CA1v, PVTa, and BLAa in the experimental females compared to experimental males, suggesting that either a lack of recruitment or an inhibition of these pathways may have been the reason of lack of renewal behavior in female rats. Interestingly, there was less total Fos induction in females, both in the areas where there were differences in PL-projecting neurons (SUBv, CA1v, PVTa, BLAa) and in other areas where there were no differences in the PL-pathways recruitment (PVTp, BMAp and LA). Taken together, these patterns suggest overall less activation in females compared to males.
Previously, we found the behavior of females in context-mediated renewal of responding was inconsistent and successful renewal depended on estradiol. Ovariectomized females with a steady dosage of estradiol delivered via silastic capsule exhibited renewal responding but intact and ovariectomized females without estradiol replacement did not (Anderson & Petrovich, 2015). In the current study, we tested females for renewal at high and low estradiol stages in the estrous cycle in order to determine if acute estradiol was mediating responding at test. We found no behavioral differences between the two groups, and neither group showed renewal compared to their matched controls, but both groups had high responding that was similar to males in the experimental group. This suggests that estradiol may not be critical for renewal acutely at test, instead it may be important during extinction learning, especially considering that estradiol has an established impact on fear extinction learning. Fear extinction during proestrus (high estradiol and progesterone) is enhanced, compared to metestrus (low estradiol and progesterone) phase, and an estrogen receptor-beta agonist facilitated extinction recall in intact female rats (Lebron-Milad & Milad, 2012; Milad, Igoe, Lebron-Milad, & Novales, 2009). Estradiol and progesterone administered together before or immediately after extinction training increased the rate of extinction learning (Milad et al., 2009). It is likely that estradiol has similar modulatory effects in appetitive extinction, which would explain why estradiol had an impact when given continuously throughout training and testing (Anderson & Petrovich, 2015) but not when tested acutely the day of test in the current study.
Based on this, it is very likely the lack of differences in responding between female groups during renewal test was, at least in part, due to their estradiol levels during extinction. Given that we found no differences between males and females during extinction learning, the postulated effects of estradiol could have occurred during extinction consolidation which would later impact extinction recall at test. In support of this hypothesis both groups of females had high responding during the test for renewal, similar to experimental males. This similar responding is particularly interesting when considering the patterns of Fos induction: only males in the experimental group had distinctly higher Fos induction. This suggests that Fos induction in the male experimental group likely reflects behavioral guidance during renewal, rather than activity.
In the current study, the responding during extinction training sessions did not gradually decrease, rather it was low across both sessions and was initially lower in the experimental groups. This is consistent we our prior observation that rats tested in a novel context during extinction had lower responding during the initial trials compared to rats tested in the acquisition context. Importantly, the behavior was similar across sexes and there were no sex or group differences during the last extinction session.
Here, we focused on the PL, however the ILA is likely also important during renewal of food cue responding, but potentially mediating a distinct function. The ILA was recruited during renewal of responding, similar to the PL (Anderson & Petrovich, 2016). This is in agreement with similar function of PL and ILA in other appetitive tasks (Moorman, James, McGlinchey, & Aston-Jones, 2015) and with recent work that showed similar patterns of activation in PL and ILA inputs from ventral hippocampal formation neurons during fear renewal (Wang et al., 2016). However, there is evidence for distinct functions of the PL and ILA during extinction and renewal of fear (Knapska & Maren, 2009; Mendoza, Sanio, & Chaudhri, 2015; Willcocks & McNally, 2013). Furthermore, optogenetic stimulation of ILA neurons reduced context-induced renewal of responding to sucrose cues (Villaruel et al., 2017). Further work is necessary to characterize how pathways to the ILA are recruited and to determine if there is a functional dissociation between the PL and ILA circuitries during renewal responding.
5. Conclusions
The current study identified sex-specific recruitment of PL inputs during context-dependent renewal of appetitive behavior. The patterns of Fos induction in the neurons in the SUbv/CA1v, PVTa, and BLAa corresponded to the behavioral differences between males and females, and suggest under activation in females. These findings enhance our understanding of the neural circuit mechanisms underlying sex differences in appetitive associative learning and memory. More broadly, there are implications for future translational work investigating maladaptive eating habits in males and females.
Highlights.
Investigated key components of prelimbic circuitry during renewal in both sexes
Replicated males show renewal of responding to a food cue, females do not
Examined Fos induction in amygdala, hippocampus and thalamus PL-projecting neurons
Found sex-specific recruitment of PL-projecting neurons during renewal
Pathways were selectively activated in males, but not recruited in females
Acknowledgments
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award R01DK085721 to GDP. We thank Heather Mayer, Amanda Jenkins, Hannah Yoon and Connor Milone for technical assistance. We thank Bret Judson and the Boston College Imaging Core for infrastructure and support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson LC, Petrovich GD. Renewal of conditioned responding to food cues in rats: Sex differences and relevance of estradiol. Physiol Behav. 2015;151:338–344. doi: 10.1016/j.physbeh.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LC, Petrovich GD. Sex specific recruitment of a medial prefrontal cortex-hippocampal-thalamic system during context-dependent renewal of responding to food cues in rats. Neurobiol Learn Mem. 2016;139:11–21. doi: 10.1016/j.nlm.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LC, Petrovich GD. Ventromedial prefrontal cortex mediates sex-specific persistent cognitive drive for food. Sci Rep. 2017 doi: 10.1038/s41598-018-20553-4. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman MF. The paraventricular nucleus of the thalamus alters rhythms in core temperature and energy balance in a state-dependent manner. Brain Res. 1999;851(1-2):66–75. doi: 10.1016/s0006-8993(99)02108-3. [DOI] [PubMed] [Google Scholar]
- Boutelle KN, Bouton ME. Implications of learning theory for developing programs to decrease overeating. Appetite. 2015 doi: 10.1016/j.appet.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. 2004/10/07 ed. [DOI] [PubMed] [Google Scholar]
- Bouton ME, King DA. Contextual control of the extinction of conditioned fear: Tests for the associative value of the context. Journal of Experimental Psychology: Animal Behavior Processes. 1983;9(3):248–265. [PubMed] [Google Scholar]
- Calu DJ, Chen YW, Kawa AB, Nair SG, Shaham Y. The use of the reinstatement model to study relapse to palatable food seeking during dieting. Neuropharmacology. 2014;76(Pt B):395–406. doi: 10.1016/j.neuropharm.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YJ, Yang CH, Liang YC, Yeh CM, Huang CC, Hsu KS. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor beta. Hippocampus. 2009;19(11):1142–1150. doi: 10.1002/hipo.20581. [DOI] [PubMed] [Google Scholar]
- Cole S, Hobin MP, Petrovich GD. Appetitive associative learning recruits a distinct network with cortical, striatal, and hypothalamic regions. Neuroscience. 2015;286:187–202. doi: 10.1016/j.neuroscience.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Mayer HS, Petrovich GD. Orexin/Hypocretin-1 receptor antagonism selectively reduces cue-induced feeding in sated rats and recruits medial prefrontal cortex and thalamus. Sci Rep. 2015;5:16143. doi: 10.1038/srep16143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Powell DJ, Petrovich GD. Differential recruitment of distinct amygdalar nuclei across appetitive associative learning. Learn Mem. 2013;20(6):295–299. doi: 10.1101/lm.031070.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116(1):169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- Eddy MC, Todd TP, Bouton ME, Green JT. Medial prefrontal cortex involvement in the expression of extinction and ABA renewal of instrumental behavior for a food reinforcer. Neurobiol Learn Mem. 2016;128:33–39. doi: 10.1016/j.nlm.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110(1-2):73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Schafe GE, Orr PT, Frick KM. Sex differences in contextual fear conditioning are associated with differential ventral hippocampal extracellular signal-regulated kinase activation. Neuroscience. 2009;159(2):451–467. doi: 10.1016/j.neuroscience.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats(1) Brain Res. 2001;888(2):356–365. doi: 10.1016/S0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, Choi EA, McNally GP. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur J Neurosci. 2009;29(4):802–812. doi: 10.1111/j.1460-9568.2009.06623.x. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454(7204):600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16(2):174–182. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- Holland PC, Bouton ME. Hippocampus and context in classical conditioning. Curr Opin Neurobiol. 1999;9(2):195–202. doi: 10.1016/S0959-4388(99)80027-0. [DOI] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD. A neural systems analysis of the potentiation of feeding by conditioned stimuli. Physiol Behav. 2005;86(5):747–761. doi: 10.1016/j.physbeh.2005.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212(2):149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Jin J, Maren S. Fear renewal preferentially activates ventral hippocampal neurons projecting to both amygdala and prefrontal cortex in rats. Sci Rep. 2015;5:8388. doi: 10.1038/srep08388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayum MA, de Vries EF, Glaudemans AW, Dierckx RA, Doorduin J. In vivo imaging of brain estrogen receptors in rats: a 16alpha-18F-fluoro-17beta-estradiol PET study. J Nucl Med. 2014;55(3):481–487. doi: 10.2967/jnumed.113.128751. [DOI] [PubMed] [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem. 2009;16(8):486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebron-Milad K, Milad MR. Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biol Mood Anxiety Disord. 2012;2(1):3. doi: 10.1186/2045-5380-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain Struct Funct. 2012;217(2):257–273. doi: 10.1007/s00429-011-0360-7. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Furlong TM, McNally GP. Medial dorsal hypothalamus mediates the inhibition of reward seeking after extinction. J Neurosci. 2010;30(42):14102–14115. doi: 10.1523/jneurosci.4079-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, De Oca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res. 1994;661(1-2):25–34. doi: 10.1016/0006-8993(94)91176-2. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Li Z, Le AD. Effects of opioid receptor blockade on the renewal of alcohol seeking induced by context: relationship to c-fos mRNA expression. Eur J Neurosci. 2007;26(10):2815–2823. doi: 10.1111/j.1460-9568.2007.05898.x. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Matsuzawa D, Ishii D, Tomizawa H, Sutoh C, Shimizu E. Sex differences in fear extinction and involvements of extracellular signal-regulated kinase (ERK) Neurobiol Learn Mem. 2015;123:117–124. doi: 10.1016/j.nlm.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Sanio C, Chaudhri N. Inactivating the infralimbic but not prelimbic medial prefrontal cortex facilitates the extinction of appetitive Pavlovian conditioning in Long-Evans rats. Neurobiol Learn Mem. 2015;118:198–208. doi: 10.1016/j.nlm.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164(3):887–895. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moga MM, Herbert H, Hurley KM, Yasui Y, Gray TS, Saper CB. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J Comp Neurol. 1990;295(4):624–661. doi: 10.1002/cne.902950408. [DOI] [PubMed] [Google Scholar]
- Moorman DE, James MH, McGlinchey EM, Aston-Jones G. Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res. 2015;1628(Pt A):130–146. doi: 10.1016/j.brainres.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci. 2011;31(47):17269–17277. doi: 10.1523/JNEUROSCI.4095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD. Forebrain networks and the control of feeding by environmental learned cues. Physiol Behav. 2013;121:10–18. doi: 10.1016/j.physbeh.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Rev. 2001;38(1-2):247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Reppucci CJ, Petrovich GD. Organization of connections between the amygdala, medial prefrontal cortex, and lateral hypothalamus: a single and double retrograde tracing study in rats. Brain Struct Funct. 2015 doi: 10.1007/s00429-015-1081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SE, Killcross AS. Lesions of rat infralimbic cortex enhance renewal of extinguished appetitive Pavlovian responding. Eur J Neurosci. 2007;25(8):2498–2503. doi: 10.1111/j.1460-9568.2007.05486.x. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290(2):213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21(16):6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Falduto J, Leuner B. The opposite effects of stress on dendritic spines in male vs. female rats are NMDA receptor-dependent. Eur J Neurosci. 2004;19(1):145–150. doi: 10.1046/j.1460-9568.2003.03065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain A laboratory guide with printed and electronic templates for data, models and schematics. Elsevier; Amsterdam: 2004. [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21(8):323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Villaruel FR, Lacroix F, Sanio C, Sparks DW, Chapman CA, Chaudhri N. Optogenetic Activation of the Infralimbic Cortex Suppresses the Return of Appetitive Pavlovian-Conditioned Responding Following Extinction. Cereb Cortex. 2017:1–12. doi: 10.1093/cercor/bhx275. [DOI] [PubMed] [Google Scholar]
- Wang Q, Jin J, Maren S. Renewal of extinguished fear activates ventral hippocampal neurons projecting to the prelimbic and infralimbic cortices in rats. Neurobiol Learn Mem. 2016 doi: 10.1016/j.nlm.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Izquierdo A. The basolateral amygdala in reward learning and addiction. Neurosci Biobehav Rev. 2015;57:271–283. doi: 10.1016/j.neubiorev.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedzony K, Koros E, Czyrak A, Chocyk A, Czepiel K, Fijal K, Bienkowski P. Different pattern of brain c-Fos expression following re-exposure to ethanol or sucrose self-administration environment. Naunyn Schmiedebergs Arch Pharmacol. 2003;368(5):331–341. doi: 10.1007/s00210-003-0811-7. [DOI] [PubMed] [Google Scholar]
- Willcocks AL, McNally GP. The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. Eur J Neurosci. 2013;37(2):259–268. doi: 10.1111/ejn.12031. [DOI] [PubMed] [Google Scholar]


