Abstract
Children with autism spectrum disorder (ASD) exhibit difficulties processing and encoding sensory information in daily life. Cognitive response to environmental change in control individuals is naturally dynamic, meaning it habituates or reduces over time as one becomes accustomed to the deviance. The origin of atypical response to deviance in ASD may relate to differences in this dynamic habituation. The current study of 133 children and young adults with and without ASD examined classic electrophysiological responses (MMN and P3a), as well as temporal patterns of habituation (i.e., N1 and P3a change over time) in response to a passive auditory oddball task. Individuals with ASD showed an overall heightened sensitivity to change as exhibited by greater P3a amplitude to novel sounds. Moreover, youth with ASD showed dynamic ERP differences, including slower attenuation of the N1 response to infrequent tones and the P3a response to novel sounds. Dynamic ERP responses were related to parent ratings of auditory sensory-seeking behaviors, but not general cognition. As the first large-scale study to characterize temporal dynamics of auditory ERPs in ASD, our results provide compelling evidence that heightened response to auditory deviance in ASD is largely driven by early sensitivity and prolonged processing of auditory deviance.
Keywords: autism spectrum disorder (ASD), dynamic habituation, auditory processing, event-related potentials (ERP), sensory abnormalities
1. Introduction
Detection of change in the environment is a fundamental element of human perception. Pre-attentive neural encoding of change mediates the automatic evaluation of incoming information and reflexively triggers attention to important information in the environment (Schröger, 1997). As such, disruption to mechanisms responsible for low-level processing of change may negatively affect attention orienting and environmental adaptation. Evidence suggests that these key elements of sensation and perception are disrupted among individuals with autism spectrum disorder (ASD), a neurodevelopmental disorder characterized by social communication impairments and restricted and repetitive behaviors and interests, and may contribute to common sensory-related features of the disorder (e.g., hypersensitivity, hyposensitivity, sensory-seeking behaviors) (Lane, Young, Baker, & Angley, 2010). Although auditory hypersensitivity is one of the most commonly reported sensory processing impairments in ASD (Gomes, Rotta, Pedroso, Sleifer, & Danesi, 2004; Tomchek & Dunn, 2007), there is evidence that it co-occurs with increased sensory seeking (Liss, Saulnier, Fein, & Kinsbourne, 2006).
Atypical electrophysiological responses to stimulus change at early stages of sensory information processing have been reported in ASD and associated with behavioral symptoms, such as sensory and attention problems (Cui, Wang, Liu, & Zhang, 2016; Keehn, Mueller, & Townsend, 2013; Orekhova & Stroganova, 2014). The auditory oddball task (Polich, 2007) is commonly used to assess initial detection and discrimination of an infrequent (deviant) auditory tone relative to a frequent tone by measuring an electrophysiological difference component known as the mismatch negativity (MMN)(R. Näätänen, Paavilainen, Rinne, & Alho, 2007). The MMN is measured and computed as the difference between electrophysiological responses to frequent and deviant stimuli across frontocentral electrodes approximately 100 – 200 ms post-stimulus onset, overlapping and in part driven by the N1 subcomponent (Jääskeläinen et al., 2004). If a stimulus is sufficiently deviant, such as an unexpected novel stimulus, the MMN is followed by a P3, a frontocentral positive-going ERP component occurring approximately 250 – 350 ms post stimulus that represents early attention orienting (Friedman, Cycowicz, & Gaeta, 2001; Polich, 2007).
Over the past three decades, studies of children and adults with ASD have reported atypical MMN/N1 and P3/P3a/P3b responses to the auditory oddball task (Cléry et al., 2013; Čeponienė et al., 2003; Ferri, Elia, Agarwal, Lanuzza, Musumeci, & Pennisi, 2003a; Gomot et al., 2011; Gomot, Giard, Adrien, Barthélémy, & Bruneau, 2002; Jansson-Verkasalo et al., 2003; Kemner, van der Gaag, Verbaten, & van Engeland, 1999; Kemner, Verbaten, Cuperus, Camfferman, & van Engeland, 1995; Korpilahti et al., 2007; Kujala et al., 2007; Kujala, Lepistö, Nieminen-von Wendt, Näätänen, & Näätänen, 2005; Lepistö, Nieminen-von Wendt, Wendt, Näätänen, & Kujala, 2007; Lepistö et al., 2006; Yu et al., 2015). However, discrepant findings in the literature regarding the timing and magnitude of these responses in ASD have led to different conclusions about how stages of neural processing diverge from typical development and how dysfunctional auditory deviance detection relates to behavioral impairments. All three potential patterns of results have been identified: (1) ASD group differences suggesting a reduced or hyposensitive ASD response (i.e., smaller amplitudes, slower latencies) for the MMN (M. A. Dunn, Gomes, & Gravel, 2008; Jansson-Verkasalo et al., 2003; Kujala et al., 2005; 2007; Lepistö et al., 2006; Seri, Cerquiglini, Pisani, & Curatolo, 1999) and P3/P3a/P3b (Courchesne, Kilman, Galambos, & Lincoln, 1984; Čeponienė et al., 2003; Ferri, Elia, Agarwal, Lanuzza, Musumeci, & Pennisi, 2003b; Kemner et al., 1995; Lepistö et al., 2006; 2007; 2005); (2) ASD group differences suggesting enhanced or hypersensitive response (i.e., larger amplitudes, faster latencies) for the MMN (Ferri, Elia, Agarwal, Lanuzza, Musumeci, & Pennisi, 2003b; Korpilahti et al., 2007; Lepistö et al., 2006) and P3/P3a/P3b (Ferri, Elia, Agarwal, Lanuzza, Musumeci, & Pennisi, 2003b; Gomot et al., 2002; 2011; Kujala et al., 2007; Lepistö et al., 2007); and (3) no group difference between ASD and controls, suggesting intact auditory processing (Dunn et al., 2008; Kemner et al., 1995). These distinct patterns of results in ASD may reflect methodological decisions, including stimulus types (i.e., kind of deviance) and task demands (i.e., passive or active task), but may also be driven by individual differences (e.g., cognitive abilities, development/maturation).
Critically, an additional gap in the existing research on auditory deviance detection in ASD is the contribution of dynamic processing across time, specifically habituation or sensitization of neural responses over the course of the experiment. Discrepancies in the literature may be explained by atypicalities in this dynamic pattern, with group differences potentially amplified, attenuated, or washed out entirely depending on the length of the experimental procedure, the type of deviant stimuli, and the number of ERP trials. Thus, the current study aims to expand on existing research by characterizing the temporal dynamics of electrophysiological response to two types of auditory deviance in ASD. The detection of environmental change requires complex integration of stimulus perception and encoding within memory and cognitive systems (Johnson, Spencer, Luck, & Schöner, 2009) and this process evolves over the course of an experiment (Rockstroh et al., 2011). Evidence of slower neural activation in ASD (Kleinhans, Johnson, & Richards, 2009; Kleinhans, Richards, Greenson, Dawson, & Aylward, 2015) suggests important differences may be tethered to dynamic patterns rather than an overall capacity for deviance detection. For instance, infants at risk for developing ASD exhibited a lack of habituation (i.e., they did not show decrement of the signal) during an auditory oddball task (Guiraud et al., 2011), consistent with accounts of atypical sensory processing. Work with school-age children with ASD similarly demonstrated a lack of habituation (Hudac et al., 2015) or atypically delayed responses due to sensitization (i.e., increase of signal) (Hudac et al., 2017) in social perception tasks.
In this study, we aimed to clarify the dynamic nature of auditory deviance detection in a large, carefully characterized sample of youth with ASD (n = 102) and without (n = 31) using a passive, auditory oddball task during EEG acquisition. We targeted the temporally dynamic aspect of discrimination between repeatedly presented “frequent” tones and (a) rare ”infrequent” tones (frequency deviance) or (b) unique, non-repeated “novel” sounds (novelty deviance) by measuring change in ERP response to these deviant sounds over time. To this end, we examined polynomial (i.e., linear, quadratic) effects of time on the N1 (frequency deviance) and P3a (novelty deviance) over the course of the experiment, as these ERP components are primary contributors to the averaged MMN and P3a signals. We anticipated atypical dynamic patterns in ASD would indicate a lack of habituation (Guiraud et al., 2011; Hudac et al., 2015). Lastly, considering known associations between behaviors and sensory sensitivities (Boyd et al., 2010; Boyd, McBee, Holtzclaw, Baranek, & Bodfish, 2009), we examined how individual ERP patterns were associated with sensory-related behaviors in ASD.
2. Material and methods
2.1. Participants
142 children and young adults age 4–23 years with ASD (N = 108; 22 female) and typically developing control children (N = 34; 11 female) were enrolled in this study and completed the EEG session. The final sample of 133 individuals comprises individuals who provided clean EEG data and characterization details are provided in Table 1. Parents gave written consent and participants provided written consent or written assent as chronological- and mental-age appropriate. The local ethical review board approved all research procedures.
Table 1. Participant characterization.
Participants included typically developing children (Control) and individuals with autism spectrum disorders (ASD). Abbreviations: SD, standard deviation; VIQ, verbal intelligence quotient; NVIQ, nonverbal intelligence quotient; ADOS-2, Autism Diagnostic Observation Schedule 2nd Edition; SA CSS, social affective calibrated severity score; RRB CSS, restricted and repetitive behavior calibrated severity score.
| Control | ASD | Differences | |||
|---|---|---|---|---|---|
|
| |||||
| Mean (SD) | Range | Mean (SD) | Range | p value | |
| N | 31 | 102 | |||
| Female : Male | 10:21 | 20:82 | .22 | ||
| Age (Years) | 13.27 (2.34) | 9–17 | 12.29 (3.56) | 4–23 | .15 |
| VIQ | 115.06 (13.91) | 94–139 | 81.3 (30.58) | 15–148 | <.0001 |
| NVIQ | 115.71 (16.39) | 90–159 | 82.26 (30.08) | 19–154 | <.0001 |
| Data retained (%) | 82.9 (11) | 47–97 | 68.81 (19.9) | 17–99 | <.0001 |
| Deviant conditions (%) | 82.28 (11.64) | 42–96 | 68.8 (20.38) | 15–99 | <.0001 |
| ADOS-2 Overall | 14.78 (5.08) | 6–26 | |||
| SA CSS | 7.72 (2.05) | 3–17 | |||
| RRB CSS | 7.38 (2.43) | 0–10 | |||
| Sensory Problems | |||||
| Hyper-reactive | 2.81 (1.01) | ||||
| Distractibility | 2.57 (0.93) | ||||
| Hypo-reactive | 3 (0.92) | ||||
| Sensory Seeking | 2.3 (1.28) | ||||
All participants received a standard battery of cognitive and behavioral assessments including the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) or the Differential Ability Scales-Second edition (Elliott, 2007), dependent on participant’s age. ASD diagnoses based on DSM-5 criteria were confirmed by expert clinician judgment following gold-standard assessment that included the Autism Diagnostic Interview-Revised (ADI-R) (Lord, Rutter, & Goode, 1989), the Autism Diagnostic Observation Schedule-2 (ADOS-2) (Lord, Rutter, & Le Couteur, 1994), and medical history interview. A majority of the subjects completed the task (94.4% in the ASD group; 97% in the control group). Six individuals with ASD were excluded from the current analyses for excessive movement during EEG (n = 2), inability to tolerate EEG net (n = 1), or lack of clinical consensus for an ASD diagnosis (n = 2). The control group consisted of individuals with neurotypical development, defined as individuals without any parent reported psychiatric or neurodevelopmental diagnoses and a lack of features of autism or subclinical concerns on the Social Communication Questionnaire (Rutter, Bailey, & Lord, 2003) (SCQ; all scores < 8) or clinical concerns on the Child Behavior Check List (Achenbach & Edelbrock, 1983) (CBCL; all total T-scores < 65). Following data processing, three control children were excluded for poor signal quality due to environmental noise (n = 1), excessive blinks (n = 1), or excessive movement (n = 1). Table 1 includes artifact-free trial values per group; there were significantly fewer artifact free trials from the ASD group, F(30, 101) = 3.27, p= .0004. The groups were matched on age and gender proportion (p > .05); however, statistical analyses (detailed in Section 2.2.4) included an evaluation of the relationship to temporal dynamics and key individual differences, including age, nonverbal cognition, and amount of data retained,
2.2. EEG task procedures and processing
2.2.1. Auditory oddball EEG task
Our experimental design was identical to prior work (Salmond, Vargha-Khadem, Gadian, De Haan, & Baldeweg, 2007). Participants watched a silent video of a trip to the zoo while passively attending to randomly presented frequent tones (70%, n=104 trials), infrequent tones (15%, n=78), and novel sounds (15%, n=78). Frequent and infrequent tones were sinusoid stimuli of either 1000 or 750 Hz, counterbalanced between subjects. Novel stimuli (e.g., chimes, cricket chirps, drums, whooshing noise) were identical to Salmond and colleagues (2007). Participants were given a short break halfway through the experiment (10–60 seconds). Sounds were presented for 210 ms with a random interval between 550–565 ms between trials and stimuli. A total of 520 stimuli were presented; however, analyses were restricted to the stimuli that followed a frequent tone in order to equalize the neurophysiological response from the adjacent stimuli (Woldorff, 1993). Thus, analyses targeted a total of 104 frequent tone trials and 78 trials each for the deviant conditions. Participants were seated 75 cm from a video monitor (SmartEye DR 120, 24″ widescreen display) and were instructed to sit still and attend to the videos. Sounds were presented at 65 dB at the child’s midline from Nata 3D speakers. Stimuli were displayed using E-Prime 2.0 software (Psychology Software Tools, Inc. Pittsburgh, PA) and the video subtended a visual angle of 14.6° by 28.9°.
2.2.2. EEG recording and preprocessing
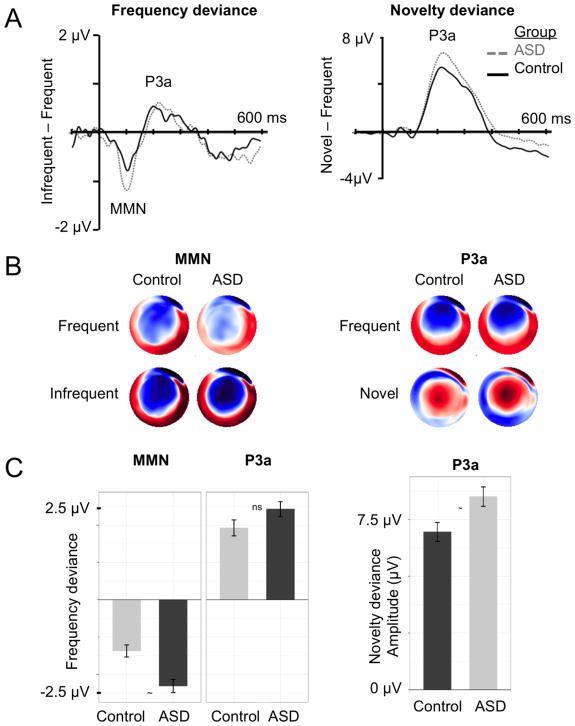
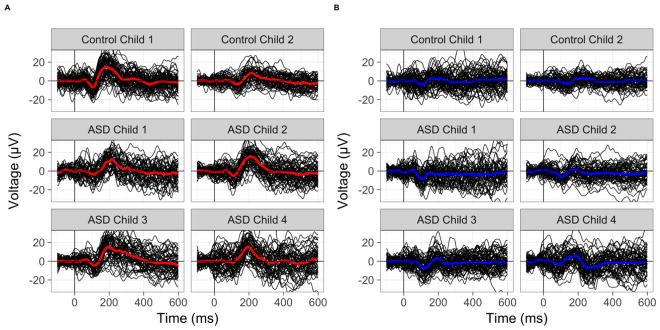
Continuous EEG was recorded from a high-density 128-channel geodesic net using Net Station 4.5.6 software integrated with a 400-series high-impedance amplifier (Electric Geodesics Inc, Eugene, OR). Electrode impedances were below 50 kohms to maximize signal-to-noise ratio, within the standard range for high-impedance amplifiers. During acquisition, EEG signals were referenced to the vertex electrode, analog filtered (0.1 Hz high-pass, 100 Hz elliptical low-pass), amplified, and digitized with a sampling rate of 1000 Hz; additionally, researchers observed and marked periods of off-task behavior or behaviors interfering with the EEG signal. The continuous EEG was filtered offline between 0.1–40 Hz. Segmented epochs extended from 100 ms pre-stimulus onset to 600 ms post-stimulus onset. Epochs marked during acquisition for improper attention or following automatic artifact detection were removed. Epochs were re-referenced to the average reference, baseline corrected, and lastly, averaged across epochs within individual. Temporal windows for ERP components were based upon previous literature (Näätänen, Tervaniemi, Sussman, Paavilainen, & Winkler, 2001; Opitz, Mecklinger, Cramon, & Kruggel, 1999; Orekhova & Stroganova, 2014) and visual inspection of the grand-averaged waveforms (see Figure 1A). Considering maturational changes across development, preliminary grand-averaged waveforms were generated across developmental age bins (see Figure SI1) to verify that the temporal windows were appropriate across age. Peak amplitude and latencies were extracted for the MMN/N1 between 60 and 150 ms and the P3a between 100 and 350 ms for the central medial electrodes (grand-average difference waveforms in Figure 1A). Examples of single-trial ERPs are provided in Figure 2.
Figure 1. Overall group differences in frequency deviance (left column) and novelty deviance (right column).
Panel A: Grand-averaged difference waveforms for individuals with (ASD; grey, dashed) and without ASD (Control; black, solid). Panel B: Topographic voltage distributions scaled between −2 μV (blue) and 2 μV (red). Panel C: Group averages with standard deviation noted by error bars. Asterisk highlights significant group difference (p <.05) in model controlling for age, nonverbal cognition, and amount of data retained.
Figure 2. Examples of single-trial waveforms.
Single-trial waveform values are presented as thin black lines. The thick colored line is the individual child’s grand-averaged waveform for Novel (red, Panel A) and Infrequent (blue, Panel B) conditions. Control children and ASD children 1 and 2 have nonverbal cognition scores > 80. ASD children 3 and 4 have nonverbal cognition scores of 54 and 26, respectively.
2.2.3. Auditory sensory behaviors
Sensory behavior abnormalities were assessed in individuals with ASD via parent report on the Sensory Profile caregiver questionnaire (Dunn, 1999) or by self-rating on the Adolescent/Adult Sensory Profile self-report (Brown & Dunn, 2002). Two individuals did not complete this battery and were excluded from these analyses. Items targeting behavioral response to auditory stimuli were rated on a 5-point scale. Self-report scores were transformed such that for both measures higher ratings reflecting more atypical behavior. Four continuous composites reflecting hyper-reactivity, hypo-reactivity, sensory seeking and distractibility were generated by averaging ratings across relevant items, based upon prior work evaluating auditory response behaviors in ASD (Tomchek & Dunn, 2007; Watling, Deitz, & White, 2001). See Table SI1 for domain items and internal consistency. Internal reliability was high within domain (Cronbach’s alpha range: .74 to .81) and domains were independent of one another (composite correlation range: r = .34 to .55).
2.2.4. Analytic plan
All analyses were conducted via SAS 9.3 (SAS Institute) using restricted maximum likelihood (REML) and Satterthwaite denominator degrees of freedom. A series of multilevel models were generated using PROC MIXED procedure to describe the variances and covariances separately for each ERP component (i.e. MMN, N1, P3a) and measurement (i.e., amplitude, latency). However, preliminary analyses indicated no significant group differences in latency; thus, results focused on amplitude measurements.
In order to determine whether the ERP signal was influence by age and by factors related to known group sample differences (e.g., cognitive abilities, amount of data retained), we tested a series of preliminary models to determine whether to include covariates for individual differences. Results indicated partial relevance of age and amount of data retained, specifically for frequency deviance (see Supplemental Materials for full details). Critically, no model indicated a significant effect of nonverbal cognition (p’s < .27) and was this factor was subsequently removed from analyses per best practices (Hoffman, 2007). Thus, in order to evaluate the effect of ASD over-and-above these individual difference factors, the final models were generated with fixed effects controlling for age (centered at 12 years) and percent of EEG data retained (centered at 50%).
3. Results
3.1. Group differences in deviance detection
First, we examined group differences in average discrimination between frequent and deviant sounds. MMN and P3a amplitude and latency difference components were computed for each individual within condition. The frequency deviance condition was defined as the response to infrequent tones minus the response to frequent tones and novelty deviance as the response to novel sounds minus the response to frequent tones. As shown in Figure 1, both groups exhibited classic MMN and P3a deviance responses across central medial electrodes (see Figure SI2; EGI Hydrocel net sensors 7, 31, 55, 80, and 106; EGI GSN net sensors 7, 32, 55, 81, 107 for n = 3; see Figures SI3 and SI4 for waveforms across the full topography). Difference component values for each group and all multilevel model group effects are reported in Table 2.
Table 2. Group characterization of difference waveform components, MMN and P3.
Means of peak amplitude (μV) and latency (ms) are presented for typically developing individuals (Control) and individuals with autism spectrum disorders (ASD). Group difference results are reported for a multilevel model with covariates (Group differences) for age and amount of EEG data. Abbreviations: M, mean; SD, standard deviation.
| Measurement | Component | Control | ASD | Group differences | ||
|---|---|---|---|---|---|---|
| M (SD) | M (SD) | F(1, 128) | p | Cohen’s d | ||
| Amplitude | Pitch MMN | −1.37 (0.9) | −2.31 (1.79) | 0.92 | 0.21 | 2.18 |
| Pitch P3a | 1.93 (1.19) | 2.43 (2.02) | 1.02 | 0.29 | −0.89 | |
| Novelty P3a | 6.95 (2.32) | 8.51 (4.32) | 4.64 | 0.06 | 1.40 | |
|
| ||||||
| Latency | Pitch MMN | 105.57 (19.92) | 103.25 (20.98) | 0.83 | 0.42 | 0.24 |
| Pitch P3a | 247.13 (46.04) | 243.52 (47.87) | 0.37 | 0.37 | 0.16 | |
| Novelty P3a | 236.3 (36.2) | 230.17 (35.44) | 0.44 | 0.28 | 0.35 | |
3.1.1. Overall frequency deviance (Pitch MMN & P3a)
Although individuals with ASD exhibited larger responses to frequency deviance, there were no significant group differences in frequency deviance (see Table 2). These findings suggest that TDs and individuals with ASD exhibit comparable ERP responses to auditory frequency deviance. However, older children exhibited reduced amplitudes for both MMN (F(1, 129) = 15.89, p = .0001) and P3a (F(1, 129) = 5.33, p = .023), consistent with prior work suggesting developmental or maturational shifts toward reduced amplitudes to auditory stimuli with age (Cheour, H T Leppänen, & Kraus, 2000; V. Mueller, Brehmer, Oertzen, Li, & Lindenberger, 2008; van Dinteren, Arns, Jongsma, Kessels, Roy P. C., 2014). Finally, participants contributing more data exhibited reduced MMN amplitudes (F(1, 129) = 16.21, p < .0001), suggesting potential attenuation of the MMN with greater signal-to-noise ratio (i.e., regression to the mean)(Barnett, van der Pols, & Dobson, 2005).
3.1.2. Overall novelty deviance (Novelty P3a)
Individuals with ASD exhibited marginally greater novelty deviance P3a amplitudes than the control group, F(1, 129) = 3.51, p = .063. Neither of the covariates were significant as fixed effects, F(1, 129)’s < 0.55, p’s > .46. These results indicate heightened P3a response to novelty deviance in ASD that is unrelated to age or cognitive abilities.
3.2. Patterns of dynamic change
The preceding analyses indicate comparable frequency deviance and heightened novelty deviance, on average, in ASD relative to controls. To address the dynamic manner in which information is processed, we examined temporal effects of ERP components that are primary contributors to the difference waveform (see SI5), namely the N1 (frequency deviance) and P3a (novelty deviance). Amplitudes of N1 and P3a components were extracted at the trial level (i.e. for each stimulus presentation rather than from averaged data). See Table 3 for group characterization of amplitude and group difference results.
Table 3. Group characterization of peak amplitude (μV) for waveform components, N1 and P3a.
Means of peak amplitude (μV) and latency (ms) are presented for typically developing individuals (Control) and individuals with autism spectrum disorders (ASD). Group difference results are reported for a multilevel model with covariates (Group differences) for age and amount of EEG data. Abbreviations: M, mean; SD, standard deviation.
| Component | Condition | Control | ASD | Group differences | |
|---|---|---|---|---|---|
| M (SD) | M (SD) | t- and p- value | Cohen’s d | ||
| N1 | Frequent | −5.09 (6.36) | −5.69 (7.42) | t(132)=0.91, p = 0.364 | 0.25 |
| Infrequent | −5.63 (6.26) | −6.49 (7.39) | t(141)=1.49, p = 0.138 | 0.33 | |
|
| |||||
| P3a | Frequent | 7.43 (6.58) | 8.92 (8.14) | t(131)=−1.26, p = 0.211 | 0.47 |
| Novelty | 11.87 (7.75) | 14.12 (9.62) | t(136)=−2.24, p = 0.026 | 0.61 | |
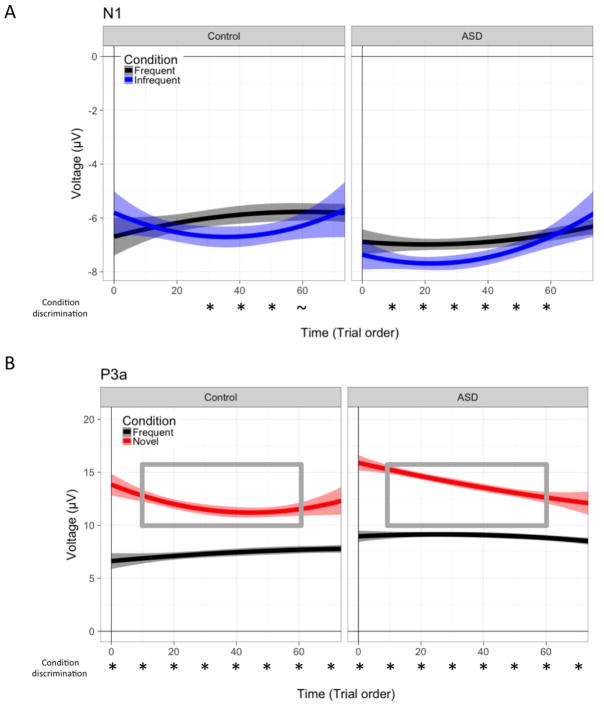
Intraclass correlations confirmed that a dominant portion (>87.9%; N1, ICC = .081; P3a, ICC = .121) of the variability was related to within-person fluctuations across trials, as compared to between-person differences on average. Polynomial effects of time (i.e., linear, quadratic, and cubic) were sequentially added to an unconditional model with an individual-level random intercept to determine the shape of change over time. Both the N1 and P3a components demonstrated significant linear and quadratic effects, F(1, 17000)’s > 4.38, p’s < .036, but non-significant cubic effects of time, F(1, 17000)’s < .85, p’s > .36. Thus, the best-fitting model included linear and quadratic terms, reflecting habituation slope and change in rate over time, respectively. After fitting the final model, frequency and novelty deviance effects were estimated within group via post-hoc pairwise comparisons at every tenth trial (e.g., at Trials 10, 20, etc.) using least square differences.
3.2.1. Frequency deviance: N1 temporal dynamics
The temporal dynamics of frequency deviance were determined by change in the N1 amplitude over time for frequent and infrequent tone conditions. As illustrated in Figure 3A and Table 4, both groups exhibited linear and quadratic time by condition interactions, (F[1, 17000] = 6.24, p = .013; F[1, 17000] = 5.80, p = .016). These interactions indicated that the N1 amplitude response to frequent tones did not change over time, either for linear or quadratic slope, p’s > .15, while N1 response to infrequent tones showed enhanced initial N1 response followed by attenuation towards the middle of the experiment. We conducted pairwise comparisons across condition every ten trials to better describe the timing of condition discrimination within each group. The ASD group showed frequency deviance from early in the experiment (trial 10) until trial 60 (p’s < .044). In contrast, the control group only exhibited discrimination from trial 30 through 50 (p’s < .019). These results suggest that the larger average MMN response in ASD is related to an earlier sensitivity to and prolonged processing of infrequent tones compared to frequent tones.
Figure 3. Dynamic patterns of group-averaged amplitude for N1 and P3a.
Group amplitude patterns are presented over time (i.e., experiment trial order) for N1 pitch deviance (top, Panel A) and P3a novel deviance (bottom, Panel B) for frequent tones (black), infrequent tones (blue), and novel sounds (red). Shading around the line represents a 95% confidence interval. Asterisks under the x-axis indicate within-group significant condition differences, p’s < .05. The grey inset box highlights the portion of the experiment in which novel amplitude is significantly greater for ASD relative to TYP, p’s < .05.
Table 4. Linear and quadratic patterns of dynamic change of peak amplitude.
Linear time reflects the change in slope (μV per ms). Quadratic time reflects the rate of slope change.
| Component Term | Condition | Control | ASD |
|---|---|---|---|
| N1 Linear | Frequent | 0.034 | −0.01 |
| Infrequent | −0.043 | −0.033 | |
|
| |||
| N1 Quadratic | Frequent | −0.0003 | 0.0001 |
| Infrequent | 0.0006 | 0.0006 | |
|
| |||
| P3a Linear | Frequent | 0.03 | 0.006 |
| Novelty | −0.121 | −0.084 | |
|
| |||
| P3a Quadratic | Frequent | −0.0002 | ~ 0 |
| Novelty | 0.0014 | 0.0008 | |
3.2.2. Novelty deviance: P3a temporal dynamics
Next, we examined novelty deviance as defined by P3a amplitude change over the course of the experiment, separately for frequent tones and novel sounds across both groups. The multilevel model indicated interactions between condition and linear and quadratic time, F(1, 1700)= 25.84, p < .0001 and F(1, 1700)= 14.75, p = .0001, respectively. Similar to the N1, there was no effect of time on the P3a amplitude for frequent tones (linear and quadratic term p’s > .16); however, in response to novel sounds the P3a showed negative linear change reflecting habituation (Figure 3B and Table 4). Moreover, there was a positive quadratic effect indicating that habituation to novel sounds decreased in rate toward the end of the experiment (main effect estimate = .0011, p =.0001). Pairwise comparisons indicated that the control group showed greater habituation and plateaued more quickly than the ASD group (as reflected by larger linear and quadratic effects of time; see Table 4). Group differences in novelty P3a amplitude were significant between Trials 10 and 60 (p’s < .042), suggesting overall group differences in P3a difference waveform are driven by the more rapid habituation of the control group.
3.3. Sensory behaviors in ASD
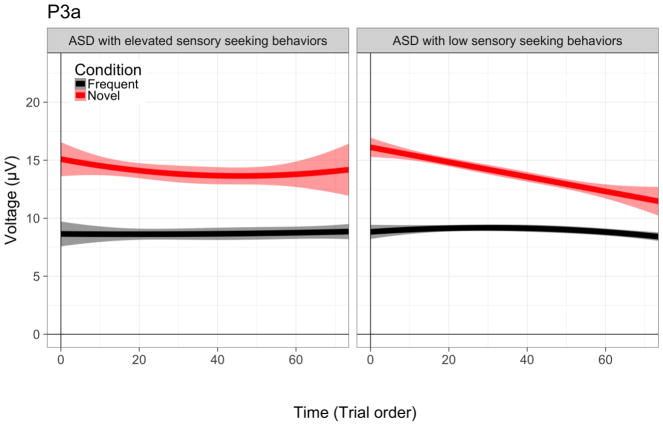
Next, we examined the association between temporal dynamics and severity of sensory abnormalities in four domains (hyper-reactivity, hypo-reactivity, distractibility and sensory-seeking). Sensory behaviors were not associated with frequency deviance including the difference waveform components (MMN/P3a), N1 linear time, or N1 quadratic slope. However, for the P3a, children with auditory sensory seeking problem behaviors exhibited atypical P3a amplitude changes over linear and quadratic time, F(95) = 5.82, p = 0.018 and F(95) = 6.02, p = 0.016, respectively. Specifically, sensory seeking children habituated more slowly than non-sensory seeking children (as noted by a smaller linear time effect) and did not exhibit a quadratic time effect. For illustration purposes only, we grouped children with elevated (n =23; SPCQ scores 3, 4, or 5) and with low (n = 77; SPCQ scores 1 or 2) sensory seeking problems to better depict our results (Figure 4).
Figure 4. Dynamic patterns of P3a amplitude for children and young adults with ASD with and without sensory seeking behaviors.
Amplitude patterns are presented over time (i.e., experiment trial order) P3a novel deviance for frequent tones (black) and novel sounds (red). Shading around the line represents a 95% confidence interval.
4. Discussion
We used electrophysiology to capture the temporal dynamics of electrophysiological response to auditory deviance in a large, well-characterized sample of youth with ASD. Our results showed that individuals with ASD exhibited enhanced neural responses to auditory deviance detection overall, suggesting a heightened sensitivity to change relative to controls. However, closer examination of dynamic change in this ERP component revealed the overall group difference was actually driven by early sensitivity to and prolonged processing of both infrequent and novel sounds among individuals with ASD. Of clinical significance, this pattern of response was further associated with increased incidence of sensory-seeking behaviors in individuals with ASD. Our data clarify the nature of auditory deviance processing in ASD and emphasize that factors influencing the dynamic neural response are critical to accurate evaluation of group differences as well as phenotypic heterogeneity within ASD.
The results of the current study imply that the type of auditory deviance is relevant to the pattern of neural habituation. Specifically, in ASD, we found an early sensitivity to frequency deviance (i.e. N1 to infrequent tones) but normal early sensitivity to novelty deviance (i.e. P3a to novel sounds) and slow habituation to both conditions. It is important to underscore that despite these distinct dynamic patterns, both findings indicated a common prolonged processing of auditory deviance. Thus, we propose a hierarchical, shared deviance detection system that is differentially sensitive to tone versus novel category stimuli. This is consistent with animal literature, in which the macaque brain is understood to engage an initial network of brain regions (including the auditory cortex, thalamus, and striatum) to encode deviant stimulus properties, followed by activation of higher-order global processing regions (Uhrig, Dehaene, & Jarraya, 2014). If this temporal pattern of activation reflects the monkey homolog to the human MMN/N1 and P3/P3a response (Gil-da-Costa, Stoner, Fung, & Albright, 2013; Paller, Zola-Morgan, Squire, & Hillyard, 1988), then by extension, our results may reflect an early enhanced perceptual and subcortical system in ASD (i.e., early N1 sensitivity) and a potentially more responsive involuntary attention system (i.e., slower P3a habituation). Additional support for this hypothesis includes evidence of increased activation within Heschl’s gyrus in response to frequency and duration (i.e., stimuli length) deviance (Samson et al., 2011), and increased prefrontal and inferior parietal activation (Gomot, Belmonte, Bullmore, Bernard, & Baron-Cohen, 2008) in response to novelty deviance.
Unlike prior work reporting superior frequency discrimination in ASD (Bonnel et al., 2010; Jones et al., 2009), the overall MMN response was comparable in ASD and controls. It has been proposed that the MMN is elicited within the cerebral cortex by a temporal generator associated with memory updating and a frontal generator related to attention allocation (Alho, 1995; Deouell, 2007; Giard, Perrin, Pernier, & Bouchet, 1990; Jemel, Achenbach, Müller, & Röpcke, 2002; R. Näätänen & Michie, 1979). In contrast, the novelty P3a response is thought to reflect attention orienting (Escera & Corral, 2007; Friedman et al., 2001), generated by cortical surfaces in cingulate, frontal, and right parietal areas (Tsolaki et al., 2017; Volpe et al., 2007). In light of our findings, heightened novelty P3a responses in ASD may reflect increased automatic responsivity of the attention system (i.e., allocation and orienting), aligned with work suggesting a role for the P3a in involuntary switching of attention (Čeponienė et al., 2003; K. C. Squires, Squires, & Hillyard, 1975). Alternatively, the prolonged P3a processing in ASD may reflect increased low-level processing, which would be consistent with proposals suggesting enhanced low-level perception explains abnormal sensory behaviors in ASD (Mottron & Burack, 2001; Mottron, Dawson, Soulières, Hubert, & Burack, 2006).
One of the main challenges of pinpointing basic cognitive mechanisms is determining whether patterns are consistent across the wide range of phenotypic heterogeneity in ASD. Our results indicated that children with sensory-seeking problem behaviors exhibited particularly slow habituation to novel sounds, aligned with other work linking sensory abnormalities with deviance responses (Gomot et al., 2011). Our unique strategy reveals that the origin of ASD differences in deviance detection are less global (i.e., top-down attentional control), instead, reflecting a deficiency in online, dynamic processing. In fact, children with atypical sensory behaviors in the absence of social communication deficits exhibit greater white matter pathology (i.e., reduced fractional anisotropy connectivity within the splenium of the corpus callosum compared to controls) than children with ASD (Chang et al., 2014). Considering the role of the posterior portion of the corpus callosum in somatosensory integration, our results for the subgroup of individuals with sensory-seeking behaviors and ASD may indeed reflect reduced network efficiency, or in other words, increased neural cost of information processing (Poldrack, 2015). Possible implications for this subgroup may be the inability to properly filter irrelevant perceptual information due to over-extended processing, critical for higher-order cognitive processes (Shiffrin & Schneider, 1977).
Importantly, our sample included children with extremely low cognitive abilities (i.e., 21 of 102 children with nonverbal IQ < 50), which is distinct from previous work and addresses a common limitation in the literature (Jack & A Pelphrey, 2017; Kasari, Brady, Lord, & Tager-Flusberg, 2013; Schauder & Bennetto, 2016; Tager-Flusberg & Kasari, 2013). Notably, 94.4% of subjects with ASD successfully provided enough data for analysis, underscoring the overall success of this paradigm and our electrophysiological acquisition procedures, even in the absence of cognitive reappraisal (Sculthorpe, Ouellet, & Campbell, 2009). Further, our results indicate the MMN and novelty P3a amplitudes were not related to nonverbal cognition, consistent with prior ERP and magnetoencephalography studies (Cantiani et al., 2016; Lepistö et al., 2006; Roberts et al., 2011; Yu et al., 2015). In other words, children with low cognitive abilities are just as likely to exhibit robust deviance detection (i.e., condition discrimination). This is in contrast to proposals that associate the MMN with cognitive decline in other clinical and aging populations (R. Näätänen & Kujala, 2011; R. Näätänen et al., 2011). It may be the case that differences between children with low and moderate cognitive abilities would be more evident with more complex speech sounds, rather than sinusoidal stimuli. For instance, considering recent work linking speech (consonant) differentiation to cognitive abilities (Key, Yoder, & Stone, 2016) and the present results, future work should explore whether the temporal dynamics associated with ASD speech perception are also related to intellectual disability.
There are several important limitations to acknowledge. First, although our experimental design was identical to Salmond and colleagues (2007), the number of stimuli used in this paradigm (520 trials) is low compared to other passive, non-speech auditory oddball ERP studies (>1000 trials). It is possible that the opportunity for afnd subsequent inclusion of too few trials improperly biased the ASD results towards a pattern of longer deviance processing. However, it is notable that several longer paradigms found an enhanced ASD response similar to our results (Ferri, Elia, Agarwal, Lanuzza, Musumeci, & Pennisi, 2003b; Gomot et al., 2011; Yu et al., 2015). In addition, our participants included children and youth from across a wide age range that encompasses varying stages of brain maturation. The temporal windows chosen for the MMN/N1 and P3a components were consistent across developmental age bins (see Figure SI1), yet future work should continue to evaluate individual differences in component latency.
5. Conclusions
In sum, this is the first large-scale study to characterize the temporal dynamics associated with deviance detection in individuals with ASD. We find that individuals with ASD exhibit a heightened response driven by early detection of frequency deviance (i.e., tone discrimination) and reduced habituation (i.e., prolonged processing of deviance) in response to novel sounds. Our results contribute an important link between the dynamic nature of neural responses and sensory abnormalities, specifically sensory-seeking behaviors. This work will further advance our understanding of the mixed findings and heterogeneity of deviance detection in ASD and generate a foundation for future work targeting the process by which individuals with ASD perceive changes in a perpetually dynamic world.
Supplementary Material
Highlights.
Children with ASD exhibit increased ERP responses to auditory changes.
Temporal dynamics indicate earlier and prolonged detection of auditory deviants.
Individual differences are related to sensory-seeking problems in ASD.
Acknowledgments
We would like to thank the children and families for their participation in this study. We would also like to thank the clinicians and coordinators of the Bernier Lab who helped collect clinical and behavioral data, including Ari Wallace, Jennifer Gerdts, Rachel Earl, Jessica Peterson, and Sandy Trinh. This work was supported by the National Institutes of Mental Health [MH100047 Bernier; MH10028 Webb/Bernier], by the National Institute of Child Health and Human Development to the University of Washington’s Center on Human Development and Disability [U54 HD083091], and by Autism Speaks [6061, Webb/Bernier].
Footnotes
Conflicts of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM, Edelbrock C. Manual for the child behavior checklist and revised child behavior pro le. Burlington: University of Vermont; 1983. [Google Scholar]
- Alho K. Cerebral generators of mismatch negativity (MMN) and its magnetic counterpart (MMNm) elicited by sound changes. Ear and Hearing. 1995;16(1):38–51. doi: 10.1097/00003446-199502000-00004. [DOI] [PubMed] [Google Scholar]
- Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. International Journal of Epidemiology. 2005;34(1):215–220. doi: 10.1093/ije/dyh299. http://doi.org/10.1093/ije/dyh299. [DOI] [PubMed] [Google Scholar]
- Bonnel A, McAdams S, Smith B, Berthiaume C, Bertone A, Ciocca V, et al. Enhanced pure-tone pitch discrimination among persons with autism but not Asperger syndrome. Neuropsychologia. 2010;48(9):2465–2475. doi: 10.1016/j.neuropsychologia.2010.04.020. http://doi.org/10.1016/j.neuropsychologia.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Boyd BA, Baranek GT, Sideris J, Poe MD, Watson LR, Patten E, Miller H. Sensory Features and Repetitive Behaviors in Children with Autism and Developmental Delays. Autism Research. 2010;3(2):78–87. doi: 10.1002/aur.124. http://doi.org/10.1002/aur.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd BA, McBee M, Holtzclaw T, Baranek GT, Bodfish JW. Relationships among repetitive behaviors, sensory features, and executive functions in high functioning autism. Research in Autism Spectrum Disorders. 2009;3(4):959–966. doi: 10.1016/j.rasd.2009.05.003. http://doi.org/10.1016/j.rasd.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C, Dunn W. Adolescent/Adult Sensory Pro le manual. NY: The Psychological Corporation; 2002. [Google Scholar]
- Cantiani C, Choudhury NA, Yu YH, Shafer VL, Schwartz RG, Benasich AA. From Sensory Perception to Lexical-Semantic Processing: An ERP Study in Non-Verbal Children with Autism. PLoS ONE. 2016;11(8):e0161637–31. doi: 10.1371/journal.pone.0161637. http://doi.org/10.1371/journal.pone.0161637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YS, Owen JP, Desai SS, Hill SS, Arnett AB, Harris J, et al. Autism and sensory processing disorders: shared white matter disruption in sensory pathways but divergent connectivity in social-emotional pathways. PLoS ONE. 2014;9(7):e103038. doi: 10.1371/journal.pone.0103038. http://doi.org/10.1371/journal.pone.0103038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheour M, Leppänen HTP, Kraus N. Mismatch negativity (MMN) as a tool for investigating auditory discrimination and sensory memory in infants and children. Clinical Neurophysiology. 2000;111(1):4–16. doi: 10.1016/s1388-2457(99)00191-1. http://doi.org/10.1016/S1388-2457(99)00191-1. [DOI] [PubMed] [Google Scholar]
- Cléry H, Bonnet-Brilhault F, Lenoir P, Barthélémy C, Bruneau N, Gomot M. Atypical visual change processing in children with autism: an electrophysiological study. Psychophysiology. 2013;50(3):240–252. doi: 10.1111/psyp.12006. http://doi.org/10.1111/psyp.12006. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Kilman BA, Galambos R, Lincoln AJ. Autism: Processing of novel auditory information assessed by event-related brain potentials. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section. 1984;59(3):238–248. doi: 10.1016/0168-5597(84)90063-7. http://doi.org/10.1016/0168-5597(84)90063-7. [DOI] [PubMed] [Google Scholar]
- Cui T, Wang PP, Liu S, Zhang X. P300 amplitude and latency in autism spectrum disorder: a meta-analysis. European Child & Adolescent Psychiatry. 2016:1–15. doi: 10.1007/s00787-016-0880-z. http://doi.org/10.1007/s00787-016-0880-z. [DOI] [PubMed]
- Čeponienė R, Lepistö T, Shestakova A, Vanhala R, Alku P, Näätänen R, Yaguchi K. Speech-sound-selective auditory impairment in children with autism: they can perceive but do not attend. Proceedings of the National Academy of Sciences. 2003;100(9):5567–5572. doi: 10.1073/pnas.0835631100. http://doi.org/10.1073/pnas.0835631100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deouell LY. The Frontal Generator of the Mismatch Negativity Revisited. Journal of Psychophysiology. 2007;21(3–4):188–203. http://doi.org/10.1027/0269-8803.21.34.188. [Google Scholar]
- Dunn MA, Gomes H, Gravel J. Mismatch negativity in children with autism and typical development. Journal of Autism and Developmental Disorders. 2008;38(1):52–71. doi: 10.1007/s10803-007-0359-3. http://doi.org/10.1007/s10803-007-0359-3. [DOI] [PubMed] [Google Scholar]
- Dunn W. Sensory profile: Users manual. San Antonio: 1999. [Google Scholar]
- Elliott CD. Differential Ability Scales-ll. San Antonio, TX: Pearson; 2007. [Google Scholar]
- Escera C, Corral MJ. Role of Mismatch Negativity and Novelty-P3 in Involuntary Auditory Attention. Journal of Psychophysiology. 2007;21(3–4):251–264. http://doi.org/10.1027/0269-8803.21.34.251. [Google Scholar]
- Ferri R, Elia M, Agarwal N, Lanuzza B, Musumeci SA, Pennisi G. The mismatch negativity and the P3a components of the auditory event-related potentials in autistic low-functioning subjects. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2003a;114(9):1671–1680. doi: 10.1016/s1388-2457(03)00153-6. [DOI] [PubMed] [Google Scholar]
- Ferri R, Elia M, Agarwal N, Lanuzza B, Musumeci SA, Pennisi G. The mismatch negativity and the P3a components of the auditory event-related potentials in autistic low-functioning subjects. Clinical Neurophysiology. 2003b;114(9):1671–1680. doi: 10.1016/s1388-2457(03)00153-6. http://doi.org/10.1016/S1388-2457(03)00153-6. [DOI] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neuroscience & Biobehavioral Reviews. 2001;25(4):355–373. doi: 10.1016/s0149-7634(01)00019-7. http://doi.org/10.1016/S0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Giard MH, Perrin F, Pernier J, Bouchet P. Brain generators implicated in the processing of auditory stimulus deviance: a topographic event-related potential study. Psychophysiology. 1990;27(6):627–640. doi: 10.1111/j.1469-8986.1990.tb03184.x. [DOI] [PubMed] [Google Scholar]
- Gil-da-Costa R, Stoner GR, Fung R, Albright TD. Nonhuman primate model of schizophrenia using a noninvasive EEG method. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(38):15425–15430. doi: 10.1073/pnas.1312264110. http://doi.org/10.2307/42713338?ref=search-gateway:adf120d23f387a2cb8b18beed5666ee6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes E, Rotta NT, Pedroso FS, Sleifer P, Danesi MC. Auditory hypersensitivity in children and teenagers with autistic spectrum disorder. Arquivos De Neuro-Psiquiatria. 2004;62(3B):797–801. doi: 10.1590/s0004-282x2004000500011. [DOI] [PubMed] [Google Scholar]
- Gomot M, Belmonte MK, Bullmore ET, Bernard FA, Baron-Cohen S. Brain hyper-reactivity to auditory novel targets in children with high-functioning autism. Brain. 2008;131(9):2479–2488. doi: 10.1093/brain/awn172. http://doi.org/10.1093/brain/awn172. [DOI] [PubMed] [Google Scholar]
- Gomot M, Blanc R, Cléry H, Roux S, Barthélémy C, Bruneau N. Candidate electrophysiological endophenotypes of hyper-reactivity to change in autism. Journal of Autism and Developmental Disorders. 2011;41(6):705–714. doi: 10.1007/s10803-010-1091-y. http://doi.org/10.1007/s10803-010-1091-y. [DOI] [PubMed] [Google Scholar]
- Gomot M, Giard MH, Adrien JL, Barthélémy C, Bruneau N. Hypersensitivity to acoustic change in children with autism: electrophysiological evidence of left frontal cortex dysfunctioning. Psychophysiology. 2002;39(5):577–584. doi: 10.1017.S0048577202394058. [DOI] [PubMed] [Google Scholar]
- Guiraud JA, Kushnerenko E, Tomalski P, Davies K, Ribeiro H, Johnson MH BASIS Team. Differential habituation to repeated sounds in infants at high risk for autism. Neuroreport. 2011;22(16):845–849. doi: 10.1097/WNR.0b013e32834c0bec. http://doi.org/10.1097/WNR.0b013e32834c0bec. [DOI] [PubMed] [Google Scholar]
- Hoffman L. Multilevel models for examining individual differences in within-person variation and covariation over time. Multivariate Behavioral Research. 2007;42(4):609–629. [Google Scholar]
- Hudac CM, Kresse A, Aaronson B, DesChamps TD, Webb SJ, Bernier RA. Modulation of mu attenuation to social stimuli in children and adults with 16p11.2 deletions and duplications. Journal of Neurodevelopmental Disorders. 2015;7(1):25–25. doi: 10.1186/s11689-015-9118-5. http://doi.org/10.1186/s11689-015-9118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudac CM, Stessman HAF, DesChamps TD, Kresse A, Faja S, Neuhaus E, et al. Exploring the heterogeneity of neural social indices for genetically distinct etiologies of autism. Journal of Neurodevelopmental Disorders. 2017;9(1):24. doi: 10.1186/s11689-017-9199-4. http://doi.org/10.1186/s11689-017-9199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack A, Pelphrey AK. Annual Research Review: Understudied populations within the autism spectrum - current trends and future directions in neuroimaging research. Journal of Child Psychology and Psychiatry. 2017;58(4):411–435. doi: 10.1111/jcpp.12687. http://doi.org/10.1111/jcpp.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson-Verkasalo E, Ceponiene R, Kielinen M, Suominen K, Jäntti V, Linna SL, et al. Deficient auditory processing in children with Asperger Syndrome, as indexed by event-related potentials. Neuroscience Letters. 2003;338(3):197–200. doi: 10.1016/s0304-3940(02)01405-2. [DOI] [PubMed] [Google Scholar]
- Jääskeläinen IP, Ahveninen J, Bonmassar G, Dale AM, Ilmoniemi RJ, Levänen S, et al. Human Posterior Auditory Cortex Gates Novel Sounds to Consciousness. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(17):6809–6814. doi: 10.1073/pnas.0303760101. http://doi.org/10.2307/3372165?ref=search-gateway:3fdd1f16fdb9f60ebbbd4b0fb537fe01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemel B, Achenbach C, Müller BW, Röpcke B. Mismatch negativity results from bilateral asymmetric dipole sources in the frontal and temporal lobes. Brain Topography. 2002 doi: 10.1023/a:1019944805499. [DOI] [PubMed] [Google Scholar]
- Johnson JS, Spencer JP, Luck SJ, Schöner G. A Dynamic Neural Field Model of Visual Working Memory and Change Detection. Psychological Science. 2009;20(5):568–577. doi: 10.1111/j.1467-9280.2009.02329.x. http://doi.org/10.1111/j.1467-9280.2009.02329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CRG, Happ F, Baird G, Simonoff E, Marsden AJS, Tregay J, et al. Auditory discrimination and auditory sensory behaviours in autism spectrum disorders. Neuropsychologia. 2009;47(13):2850–2858. doi: 10.1016/j.neuropsychologia.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Kasari C, Brady N, Lord C, Tager-Flusberg H. Assessing the Minimally Verbal School-Aged Child With Autism Spectrum Disorder. Autism Research. 2013;6(6):479–493. doi: 10.1002/aur.1334. http://doi.org/10.1002/aur.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Mueller RA, Townsend J. Atypical attentional networks and the emergence of autism. Neuroscience & Biobehavioral Reviews. 2013;37(2):164–183. doi: 10.1016/j.neubiorev.2012.11.014. http://doi.org/10.1016/j.neubiorev.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemner C, van der Gaag RJ, Verbaten M, van Engeland H. ERP differences among subtypes of pervasive developmental disorders. Biological Psychiatry. 1999;46(6):781–789. doi: 10.1016/s0006-3223(99)00003-7. [DOI] [PubMed] [Google Scholar]
- Kemner C, Verbaten MN, Cuperus JM, Camfferman G, van Engeland H. Auditory event-related brain potentials in autistic children and three different control groups. Biological Psychiatry. 1995;38(3):150–165. doi: 10.1016/0006-3223(94)00247-Z. http://doi.org/10.1016/0006-3223(94)00247-Z. [DOI] [PubMed] [Google Scholar]
- Key AP, Yoder PJ, Stone WL. Consonant differentiation mediates the discrepancy between non-verbal and verbal abilities in children with ASD. Journal of Intellectual Disability Research. 2016;60(5):478–490. doi: 10.1111/jir.12286. http://doi.org/10.1111/jir.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Johnson LC, Richards T. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. American Journal of …. 2009;166(4):467–475. doi: 10.1176/appi.ajp.2008.07101681. http://doi.org/10.1176/appi.ajp.2008.07101681. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Greenson J, Dawson G, Aylward E. Altered Dynamics of the fMRI Response to Faces in Individuals with Autism. Journal of Autism and Developmental Disorders. 2015;46(1):232–241. doi: 10.1007/s10803-015-2565-8. http://doi.org/10.1007/s10803-015-2565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpilahti P, Jansson-Verkasalo E, Mattila ML, Kuusikko S, Suominen K, Rytky S, et al. Processing of affective speech prosody is impaired in Asperger syndrome. Journal of Autism and Developmental Disorders. 2007;37(8):1539–1549. doi: 10.1007/s10803-006-0271-2. http://doi.org/10.1007/s10803-006-0271-2. [DOI] [PubMed] [Google Scholar]
- Kujala T, Aho E, Lepistö T, Jansson-Verkasalo E, Nieminen-von Wendt T, von Wendt L, Näätänen R. Atypical pattern of discriminating sound features in adults with Asperger syndrome as reflected by the mismatch negativity. Biological Psychology. 2007;75(1):109–114. doi: 10.1016/j.biopsycho.2006.12.007. http://doi.org/10.1016/j.biopsycho.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Kujala T, Lepistö T, Nieminen-von Wendt T, Näätänen P, Näätänen R. Neurophysiological evidence for cortical discrimination impairment of prosody in Asperger syndrome. Neuroscience Letters. 2005;383(3):260–265. doi: 10.1016/j.neulet.2005.04.048. http://doi.org/10.1016/j.neulet.2005.04.048. [DOI] [PubMed] [Google Scholar]
- Lane AE, Young RL, Baker AEZ, Angley MT. Sensory processing subtypes in autism: association with adaptive behavior. Journal of Autism and Developmental Disorders. 2010;40(1):112–122. doi: 10.1007/s10803-009-0840-2. http://doi.org/10.1007/s10803-009-0840-2. [DOI] [PubMed] [Google Scholar]
- Lepistö T, Kujala T, Vanhala R, Alku P, Huotilainen M, Näätänen R. The discrimination of and orienting to speech and non-speech sounds in children with autism. Brain Research. 2005;1066(1–2):147–157. doi: 10.1016/j.brainres.2005.10.052. http://doi.org/10.1016/j.brainres.2005.10.052. [DOI] [PubMed] [Google Scholar]
- Lepistö T, Nieminen-von Wendt T, von Wendt L, Näätänen R, Kujala T. Auditory cortical change detection in adults with Asperger syndrome. Neuroscience Letters. 2007;414(2):136–140. doi: 10.1016/j.neulet.2006.12.009. http://doi.org/10.1016/j.neulet.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Lepistö T, Silokallio S, Nieminen-von Wendt T, Alku P, Näätänen R, Kujala T. Auditory perception and attention as reflected by the brain event-related potentials in children with Asperger syndrome. Clinical Neurophysiology. 2006;117(10):2161–2171. doi: 10.1016/j.clinph.2006.06.709. http://doi.org/10.1016/j.clinph.2006.06.709. [DOI] [PubMed] [Google Scholar]
- Liss M, Saulnier C, Fein D, Kinsbourne M. Sensory and attention abnormalities in autistic spectrum disorders. Autism. 2006;10(2):155–172. doi: 10.1177/1362361306062021. http://doi.org/10.1177/1362361306062021. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S. Autism Diagnostic Observation Schedule: A standardized investigator-based instrument. Journal of Autism and Developmental Disorders. 1989 doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mottron L, Burack JA. Enhanced perceptual functioning in the development of autism. Lawrence Erlbaum Associates Publishers; 2001. [Google Scholar]
- Mottron L, Dawson M, Soulières I, Hubert B, Burack J. Enhanced Perceptual Functioning in Autism: An Update, and Eight Principles of Autistic Perception. Journal of Autism and Developmental Disorders. 2006;36(1):27–43. doi: 10.1007/s10803-005-0040-7. http://doi.org/10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Mueller V, Brehmer Y, Oertzen von T, Li S-C, Lindenberger U. Electrophysiological correlates of selective attention: A lifespan comparison. BMC Neuroscience. 2008;9(1) doi: 10.1186/1471-2202-9-18. http://doi.org/10.1186/1471-2202-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näätänen R, Kujala T. The Mismatch Negativity and Its Magnetic Equivalent: An Index of Language Impairment or More General Cognitive Decline in Autism? Biological Psychiatry. 2011;70(3):212–213. doi: 10.1016/j.biopsych.2011.05.024. http://doi.org/10.1016/j.biopsych.2011.05.024. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Michie PT. Early selective-attention effects on the evoked potential: a critical review and reinterpretation. Biological Psychology. 1979 doi: 10.1016/0301-0511(79)90053-x. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Kujala T, Kreegipuu K, Carlson S, Escera C, Baldeweg T, Ponton C. The mismatch negativity: an index of cognitive decline in neuropsychiatric and neurological diseases and in ageing. Brain. 2011;134(Pt 12):3435–3453. doi: 10.1093/brain/awr064. http://doi.org/10.1093/brain/awr064. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Paavilainen P, Rinne T, Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2007;118(12):2544–2590. doi: 10.1016/j.clinph.2007.04.026. http://doi.org/10.1016/j.clinph.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Tervaniemi M, Sussman E, Paavilainen P, Winkler I. “Primitive intelligence” in the auditory cortex. Trends in Neurosciences. 2001;24(5):283–288. doi: 10.1016/s0166-2236(00)01790-2. http://doi.org/10.1016/S0166-2236(00)01790-2. [DOI] [PubMed] [Google Scholar]
- Opitz B, Mecklinger A, Cramon DY, Kruggel F. Combining electrophysiological and hemodynamic measures of the auditory oddball. Psychophysiology. 1999;36(1):142–147. doi: 10.1017/s0048577299980848. http://doi.org/10.1017/S0048577299980848. [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA. Arousal and attention re-orienting in autism spectrum disorders: evidence from auditory event-related potentials. Frontiers in Human Neuroscience. 2014;8:34. doi: 10.3389/fnhum.2014.00034. http://doi.org/10.3389/fnhum.2014.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller KA, Zola-Morgan S, Squire LR, Hillyard SA. P3-like brain waves in normal monkeys and in monkeys with medial temporal lesions. Behavioral Neuroscience. 1988;102(5):714–725. doi: 10.1037//0735-7044.102.5.714. http://doi.org/10.1037/0735-7044.102.5.714. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Is “efficiency” a useful concept in cognitive neuroscience? Developmental Cognitive Neuroscience. 2015;11:12–17. doi: 10.1016/j.dcn.2014.06.001. http://doi.org/10.1016/j.dcn.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. http://doi.org/10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TPL, Cannon KM, Tavabi K, Blaskey L, Khan SY, Monroe JF, et al. Auditory Magnetic Mismatch Field Latency: A Biomarker for Language Impairment in Autism. Biological Psychiatry. 2011;70(3):263–269. doi: 10.1016/j.biopsych.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockstroh B, Müller MM, Heinz A, Wagner M, Berg P, Elbert T. Modulation of Auditory Responses During Oddball Tasks. 2011 doi: 10.1016/0301-0511(95)05175-9. http://doi.org/10.2307/40599285?ref=search-gateway:2cf15d0e581a9a27fcdb9d7849e6e901. [DOI] [PubMed]
- Rutter M, Bailey A, Lord C. The Social Communication Questionnaire. 2003 http://doi.org/10.1186/1742-2094-11-110.
- Salmond CH, Vargha-Khadem F, Gadian DG, De Haan M, Baldeweg T. Heterogeneity in the patterns of neural abnormality in autistic spectrum disorders: evidence from ERP and MRI. Cortex. 2007;43(6):686–699. doi: 10.1016/s0010-9452(08)70498-2. [DOI] [PubMed] [Google Scholar]
- Samson F, Hyde KL, Bertone A, Soulières I, Mendrek A, Ahad P, et al. Atypical processing of auditory temporal complexity in autistics. Neuropsychologia. 2011;49(3):546–555. doi: 10.1016/j.neuropsychologia.2010.12.033. http://doi.org/10.1016/j.neuropsychologia.2010.12.033. [DOI] [PubMed] [Google Scholar]
- Schauder KB, Bennetto L. Toward an Interdisciplinary Understanding of Sensory Dysfunction in Autism Spectrum Disorder: An Integration of the Neural and Symptom Literatures. Frontiers in Neuroscience. 2016 Jun;10 doi: 10.3389/fnins.2016.00268. http://doi.org/10.3389/fnins.2016.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröger E. On the detection of auditory deviations: a pre-attentive activation model. Psychophysiology. 1997;34(3):245–257. doi: 10.1111/j.1469-8986.1997.tb02395.x. [DOI] [PubMed] [Google Scholar]
- Sculthorpe LD, Ouellet DR, Campbell KB. MMN elicitation during natural sleep to violations of an auditory pattern. Brain Research. 2009;1290:52–62. doi: 10.1016/j.brainres.2009.06.013. http://doi.org/10.1016/j.brainres.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Seri S, Cerquiglini A, Pisani F, Curatolo P. Autism in tuberous sclerosis: evoked potential evidence for a deficit in auditory sensory processing. Clinical Neurophysiology. 1999;110(10):1825–1830. doi: 10.1016/s1388-2457(99)00137-6. [DOI] [PubMed] [Google Scholar]
- Shiffrin RM, Schneider W. Controlled and automatic human information processing: II. Perceptual learning, automatic attending and a general theory. Psychological Review. 1977;84(2):127–190. http://doi.org/10.1037/0033-295X.84.2.127. [Google Scholar]
- Squires KC, Squires NK, Hillyard SA. Decision-related cortical potentials during an auditory signal detection task with cued observation intervals. Journal of Experimental Psychology: Human Perception and Performance. 1975;1(3):268–279. doi: 10.1037//0096-1523.1.3.268. http://doi.org/10.1037/0096-1523.1.3.268. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Kasari C. Minimally Verbal School-Aged Children with Autism Spectrum Disorder: The Neglected End of the Spectrum. Autism Research. 2013;6(6):468–478. doi: 10.1002/aur.1329. http://doi.org/10.1002/aur.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchek SD, Dunn W. Sensory processing in children with and without autism: a comparative study using the short sensory profile. Am J Occup Ther. 2007;61(2):190–200. doi: 10.5014/ajot.61.2.190. [DOI] [PubMed] [Google Scholar]
- Tsolaki AC, Kosmidou V, Kompatsiaris IY, Papadaniil C, Hadjileontiadis L, Adam A, Tsolaki M. Brain source localization of MMN and P300 ERPs in mild cognitive impairment and Alzheimer’s disease: a high-density EEG approach. Neurobiology of Aging. 2017;55:190–201. doi: 10.1016/j.neurobiolaging.2017.03.025. http://doi.org/10.1016/j.neurobiolaging.2017.03.025. [DOI] [PubMed] [Google Scholar]
- Uhrig L, Dehaene S, Jarraya B. A Hierarchy of Responses to Auditory Regularities in the Macaque Brain. Journal of Neuroscience. 2014;34(4):1127–1132. doi: 10.1523/JNEUROSCI.3165-13.2014. http://doi.org/10.1523/JNEUROSCI.3165-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dinteren R, Arns M, Jongsma MLA, Kessels Roy PC. P300 Development across the Lifespan: A Systematic Review and Meta-Analysis. PLoS ONE. 2014;9(2) doi: 10.1371/journal.pone.0087347. http://doi.org/10.1371/journal.pone.0087347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe U, Mucci A, Bucci P, Merlotti E, Galderisi S, Maj M. The cortical generators of P3a and P3b: A LORETA study. Brain Research Bulletin. 2007;73(4–6):220–230. doi: 10.1016/j.brainresbull.2007.03.003. http://doi.org/10.1016/j.brainresbull.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Watling RL, Deitz J, White O. Comparison of Sensory Profile scores of young children with and without autism spectrum disorders. Am J Occup Ther. 2001;55(4):416–423. doi: 10.5014/ajot.55.4.416. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Woldorff MG. Distortion of ERP averages due to overlap from temporally adjacent ERPs: analysis and correction. Psychophysiology. 1993;30(1):98–119. doi: 10.1111/j.1469-8986.1993.tb03209.x. http://doi.org/10.1111/j.1469-8986.1993.tb03209.x. [DOI] [PubMed] [Google Scholar]
- Yu L, Fan Y, Deng Z, Huang D, Wang S, Zhang Y. Pitch Processing in Tonal-Language-Speaking Children with Autism: An Event-Related Potential Study. Journal of Autism and Developmental Disorders. 2015;45(11):3656–3667. doi: 10.1007/s10803-015-2510-x. http://doi.org/10.1007/s10803-015-2510-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.