Abstract
Follicular CD8+ T cells (fCD8 cells) reside within B cell follicles, believed to be immune privileged sites of HIV/SIV infection. We have observed comparable levels of fCD8 cells between chronically SIV infected rhesus macaques with low viral loads (LVL) and high viral loads (HVL), raising the question concerning their contribution to viremia control. Here we aimed to clarify the role of SIV-specific fCD8 cells in lymph nodes (LNs) over the course of SIV infection in rhesus macaques. We observed that fCD8, T follicular helper (Tfh), and T follicular regulatory (Tfreg) cells were all elevated in chronic SIV infection. fCD8 cells of LVL animals tended to express more Gag-specific granzyme B and exhibited significantly greater killing than HVL animals, and their cell frequencies negatively correlated with viremia, suggesting a role in viremia control. Env- and Gag-specific IL-21+Tfh of LVL but not HVL macaques negatively correlated with viral load, suggesting better provision of T cell help to fCD8 cells. Tfreg positively correlated with fCD8 cells in LVL animals and negatively correlated with viremia, suggesting a potential benefit of Tfregs via suppression of chronic inflammation. In contrast, in HVL macaques, Tfreg and fCD8 cell frequencies tended to negatively correlate, and a positive correlation was seen between Tfreg cell number and viremia, suggesting possible dysfunction and suppression of an effective fCD8 cell immune response. Our data suggest that control of virus-infected cells in B cell follicles not only depends on fCD8 cell cytotoxicity but also on complex fCD8 cell associations with Tfh and Tfreg.
Introduction
Among the earliest manifestations of the epidemic disease that came to be known as the acquired immune deficiency syndrome (AIDS) was persistent, generalized lymphadenopathy, first seen in homosexual males (1, 2). Lymph node follicles were subsequently identified as important sites of replication and trapping of the etiologic agent of the disease, HIV (3, 4), as well as of SIV in the rhesus macaque model (5). Subsequently, CD4+ T helper cells in lymph node follicles, now known as T follicular helper (Tfh) cells (6, 7) were identified as key targets of both HIV (8 –10) and SIV infection (11, 12) in secondary lymphoid tissue.
During HIV infection, Tfh cells in B cell follicles produce HIV and are responsible for persistent virus transcription in long-term aviremic individuals treated with anti-retroviral therapy (ART) (13). Significantly higher concentrations of SIV-producing cells have been reported to occur in B cell follicles compared to extrafollicular regions of the spleen, lymph node (LN), and gut-associated lymphoid tissues of SIV-infected macaques during chronic asymptomatic infection (14). Furthermore, residual SIV infection has been localized in B cell follicles of rhesus macaques undergoing fully suppressive ART (15). Such observations have suggested that germinal center (GC) Tfh cells comprise an immune privileged site for HIV/SIV replication (14, 16, 17), which may not be readily accessible to ART or to antiviral CD8+ T cells which lack expression of the follicular homing molecule, CXCR5. Thus, the production of HIV/SIV in GC Tfh cells represents a major obstacle to obtaining a functional cure for HIV/SIV infection.
In HIV infection, CD8+ T cells, especially Gag-specific CTL (18, 19), play a role in control of viral load. Early studies showed that depletion of CD8+ T cells in SIV-infected animals impaired viremia control (20, 21). Furthermore, cytotoxic CD8+ T cells were detected in lymph node GC of HIV-infected individuals (22, 23), as well as in lymph nodes of SIV infected non-human primates (24, 25). However, lymph nodes, among other tissues, have come to be considered sanctuaries where reservoirs of Tfh cells infected with HIV or SIV can persist (15, 26). The observation that tetramer-positive CD8+ T cells, although present in extrafollicular areas of LNs of HIV-infected subjects were mostly absent in follicles, provided a rationale for the persistence of HIV/SIV in lymphoid Tfh cells (16). The growing focus of the field on obtaining an HIV cure, requiring elimination of viral reservoirs, has stimulated new studies on quantitation and functional capability of CD8+ T cells in lymphoid follicles.
In healthy humans, a subset of CD8+ T cells was reported to use CXCR5 to enter B cell follicles (27). CXCR5+CD8+ T cells, termed follicular cytotoxic T cells (Tfc cells), were subsequently identified in the LCMV mouse model and shown to enter B cell follicles and eradicate LCMV-infected Tfh cells and B cells infected with the herpesvirus, MuHV-4 (28). CXCR5+CD8+ T cells were also reported to express lower levels of inhibitory receptors than the CXCR5− subset and exhibited more potent cytotoxicity against chronic LCMV infection (29). With regard to HIV/SIV infection, CD8+ T cells with Tfc characteristics, including potential cytotoxic function, have been identified in lymphoid follicles. CXCR5+CD8+ T cells in individuals infected with HIV were shown to correlate inversely with viral load (29). Granzyme B+ CM-9-tetramer+ (SIV Gag-specific) CD8+ T cells in lymphoid follicles of mucosal tissue were enriched in chronically SIV-infected macaques that controlled infection compared to non-controllers, and correlated inversely with lymphoid Tfh cells (30). Similarly, CXCR5+ CD8+ T cells (termed fCD8 cells) were increased in LN follicles and GC of HIV-infected individuals, and exhibited expression of both granzyme B and perforin. In the presence of anti-HIV/anti-CD3 bispecific antibody these cells exhibited better killing in vitro compared to non-fCD8 cells (31). Overall, these reports substantiate that fCD8 are located in B cell follicles of secondary lymphoid tissue, the site of active HIV/SIV replication, and that they might be key contributors to viremia control.
Here we aimed to clarify the role of viral-specific fCD8 cells over the course of SIV infection in rhesus macaques. While previous studies have suggested that fCD8 cells might contribute to viremia control in chronic viral infections, they are observed in chronically SIV-infected macaques exhibiting high viral loads (HVL). This raises the question of whether fCD8 cells in HVL animals are functional. Further, the interactions of other cell populations in B cell follicles and their effect on fCD8 cells have not been fully explored. Here we show that in low viral load (LVL) animals, fCD8 cells are associated with viremia control while associations of Tfh with fCD8 cells suggest provision of help through secretion of IL-21. In contrast, in HVL animals Tfh apparently fail to provide adequate help, T follicular regulatory cells (Tfreg) are associated with diminished fCD8 cells and increased viremia, and fCD8 overall are not correlated with a protective outcome.
Materials and Methods
Study animals and sample collection
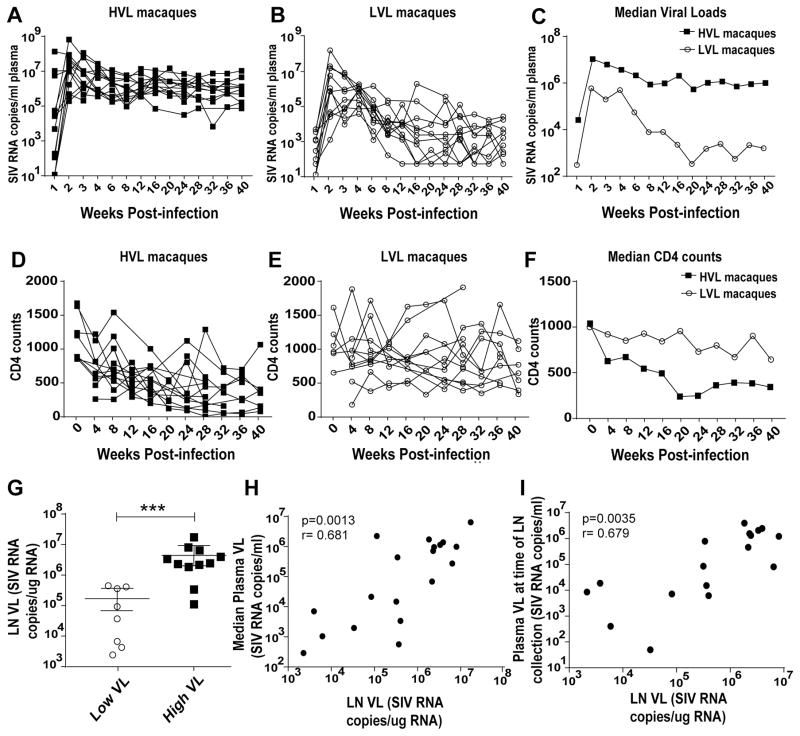
Rhesus macaques (27 females, 24 males) were maintained at Advanced Bioscience Laboratories, Inc. (Rockville, MD) and at the NCI animal facility (Bethesda, MD) under the guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care and according to the recommendations of the Guide for the Care and Use of Laboratory Animals. Protocols and procedures were approved by the Institutional Animal Care and Use Committee of the respective facility. Inguinal LN biopsy specimens were collected from: 1) 12 naïve macaques (includes pre-immunization/infection samples from 4 of the acute group and 1 each from the LVL and HVL groups with no apparent clustering in the paired values); 2) 15 acutely infected macaques (samples obtained within 9 to 16 days of infection with SIVmac251), 12 samples were used for flow cytometry and 5 were used in killing and ELISPOT assays; 3) 24 chronically infected macaques (samples obtained 40–50 weeks post-SIVmac251 infection). These were previously vaccinated with replicating adenovirus-SIV recombinants and boosted with SIV gp120 or gp140 as described (32), or with either ALVAC-SIV recombinant plus SIV gp120 protein or SIV DNA plasmid plus gp120 protein (Musich et al., manuscript in preparation). They were divided into LVL (11 macaques) and HVL (13 macaques) groups. One macaque each in the LVL and HVL groups had received empty Ad vector and adjuvant only. Samples from 11 of each group were used for flow cytometry, and 5 from each group were used in killing and ELISPOT assays. Median plasma viral loads over weeks 12 until necropsy for LVL and HVL animals were <3 X 104 and >7 X 104 SIV RNA copies per mL of plasma, respectively. Geometric means of the median values for the HVL and LVL macaques were 1.20 X 106 and 1.86 X 103, respectively. CD4 counts were not performed as routinely as the viral loads, however, median CD4 counts from the week 12 setpoint onward ranged from 641 to 923 for LVL macaques and from 248 to 552 for the HVL macaques. The means of the median CD4 counts for this time period were 805 and 384 for the LVL and HVL macaques, respectively. For LN viral loads, LN cells were lysed using Qiazol Lysis reagent (Qiagen) and stored at −70ºC until RNA extraction and viral load determination by droplet digital PCR as previously described (33). Figure 1 illustrates the viral loads (Fig. 1A–C), CD4 counts (Fig. 1D–F), and LN viral loads (Fig. 1G) of macaques in the LVL and HVL groups. LN viral loads were directly correlated with both the median plasma viral loads during chronic infection (Fig. 1H) as well as with plasma viral loads at the time of LN collection (Fig. 1I). This is consistent with a previous report that significantly correlated plasma viral loads with SIV+ follicular cells assessed by in situ hybridization (14). None of the macaques was diagnosed with clinical AIDS.
Figure 1.
Virological and immunological status of the study animals. (A–B) Viral load of HVL and LVL animals, respectively, over the course of infection. (C) Median viral load of the HVL and LVL groups post-infection. (D–E) CD4 counts of HVL and LVL animals, respectively, post infection. (F) Median CD4 counts of the HVL and LVL groups post-infection. (G) LN viral loads of the macaques. LN cells from 8 LVL and 11 HVL macaques were available for analysis. (H) Correlation between LN viral loads and median plasma viral load. (I) Correlation between LN viral loads and plasma viral loads at the time of LN collection. Viral loads at time of LN collection for 2 HVL macaques were not determined. Data of panel G were analyzed by the Mann-Whitney test. Data of panels H–I were analyzed by the Spearman correlation test. * * * P < 0.001.
LN biopsies were minced and passed through a 40-um cell strainer after lysis of red blood cells. Cells were washed and resuspended in R10 (RPMI 1640 containing 10% FBS, 2 mM L-glutamine, 1% nonessential amino acids, 1% sodium pyruvate, and antibiotics). Cells were viably frozen in FBS + 10% DMSO, and thawed for all assays. Additional LN biopsies from 3 naïve, 5 acute, 4 LVL and 5 HVL animals were collected in OCT (Sakura Finetek USA INC) and frozen in liquid nitrogen for immunohistochemical analysis.
Flow cytometric detection of fCD8, Tfh and Tfreg cells
LN cells were stained with aqua Live/Dead viability dye (Invitrogen, Carlsbad, CA) at room temperature (RT) for 15 min in PBS, washed with FACS wash, and surface stained with the following anti-human fluorochrome-conjugated mAbs cross-reactive with rhesus macaque antigens: AlexaFluor700 anti–CD3 (SP34-2), BV650 anti-CD8 (RPA-T8), BV711 anti-CD4 (L200), PE-Cy™7 anti-CD28 (CD28.2), all from BD Biosciences, San Jose, CA; PE-Cy5 anti-CD95 (DX2), APC-eFluor780 anti-CCR7 (3D12) from eBioscience, San Diego, CA; BV786 anti-CD25 (BC96), BV605 anti-PD-1 (EH12.2H7) from Biolegend, San Diego, CA; PE anti-CXCR5 (710D82.1) from the NIH Nonhuman Primate Reagent Resource, Boston, MA. For detection of Tfreg cells, after surface staining cells were permeabilized with Foxp3/Transcription Factor Staining buffer (eBioscience) and stained intracellularly with PerCP-Cy5.5 anti-Foxp3 (236A/E7) (BD Biosciences). At least 500,000 singlet events were acquired on a SORP LSR II (BD Biosciences) and analyzed using FlowJo Software (FlowJo, Ashland, OR). For all samples, gating was established using a combination of isotype and fluorescence-minus-one (FMO) controls.
Cytokine staining of SIV-specific Tfh and fCD8 cells
SIV-specific production of granzyme B, perforin and IL-21 by LN-resident fCD8 and Tfh cells was assayed by stimulating LN cells in the presence of 2 ug/ml anti-CD49d and PE-Cy™7 anti-CD28 (CD28.2) (BD Biosciences), BD GolgiPlug, BD GolgiStop, and APC-eFluor780 anti-CCR7 (3D12) at the manufacturer’s recommended concentrations. One million cells were stimulated with pooled peptides spanning the SIVmac239 Gag (125 peptides) or Env (218 peptides) protein. Peptides were 15-mers overlapping by 11; each peptide at 1 μg/mL (NIH AIDS Research and Reference Reagent Program). Following incubation for 6 h at 37 °C in 5% CO2, cells were stained with Live/Dead aqua dye followed by surface staining and permeabilization with Foxp3/Transcription Factor Staining buffer. Intracellular staining was performed with Alexa 647 anti–IL-21 (3A3-N2.1) (BD Biosciences); FITC anti-Perforin (Pf-344) (Mabtech Inc, Cincinnati, OH); and PE-Txred anti- GrazB (GB11) (Invitrogen Life Technologies, Carlsbad, CA). Acquisition and analysis was as described above. SIVmac239 Gag or Env-specific granzyme B, perforin and IL-21 responses were calculated by subtracting the % unstimulated response from % peptide stimulated response. Specific mean fluorescence intensity (MFI) was calculated as the peptide stimulated response minus the unstimulated response.
Cell Sorting
LN cells from acutely and chronically infected animals were stained with BV711 anti-CD4 (L200), FITC anti-CD8 (RPA-T8) (BD Biosciences) and PE anti-CXCR5 (710D82.1) (NIH Nonhuman Primate Reagent Resource). Aqua Live/Dead viability dye was used to exclude dead cells. After staining, cells were washed, passed through a 40-um cell strainer, and sorted on an Astrios EQ flow cytometer. Three groups of live cells were sorted (CD8+CXCR5−, CD8+CXCR5+ and CD4+) with purity of >85%.
ELISPOT Assay
The ELISPOT assay was conducted using monkey IFN-γ ELISPOT PLUS (HRP) kits. The 96 well polyvinylidene plates were pre-coated with anti-IFN-γ monoclonal antibody (mAb MT126L). After washing and blocking the plates, CD8+CXCR5− or CD8+CXCR5+ sorted LN cells were serially diluted and plated in duplicate at 1 X 105, 0.5 X 105, and 0.25 X 105 cells/well. Cells were stimulated by adding either SIVmac239 Env or Gag pooled peptides (1 ug/ml each peptide) and incubating for 20h at 37°C. Subsequently, plates were washed with phosphate-buffered saline (PBS) before the addition of biotinylated anti-IFN-γ MAb (7-B6-1) at 1 μg/ml. Following incubation at RT for 120 min, plates were washed with PBS and incubated with the manufacturer’s recommended dilution of streptavidin-conjugated HRP for 60 min at RT. Following washes with PBS, spots resulting from individual IFN-γ-producing cells were visualized after a 15-min reaction with TMB ready to use substrate solution, counted with an Eliphoto-Counter (CTL analyzer) and recorded per 106 CD8+ T cells. As negative controls, cells were plated without peptide. SIV-specific spots were calculated by subtracting spots resulting from unstimulated cells.
Killing Assay
Cytotoxic activity of CD8+ T cells was assayed using a modified flow cytometry-based killing assay (34). Sorted autologous CD4+ T cells, used as targets, were fluorescently labeled according to the manufacturer’s recommendation with the CellTrace™ Violet dye kit (Thermo Fisher Scientific/LifeTechnologies, Grand Island, NY). The labeled CD4+ cells were washed and pulsed with SIVmac239 Gag pooled peptides (1 ug/ml each peptide) for an hour. A portion of the cells was not peptide pulsed and served as control. Target cells (with/without peptide pulsing) were washed twice and 10,000/well were plated in R-10 in U-bottom 96-well plates. Effector CD8+CXCR5− or CD8+CXCR5+ sorted LN cells were placed in different wells at 25:1 effector-to-target (E:T) ratios to a final volume of 200 μl. Plates were incubated at 37°C for 5 h. After incubation, cells were labeled by adding 0.25 μl of yellow LIVE/DEAD viability dye (Invitrogen) in 100 μl PBS per well. Plates were washed twice with PBS and cells were re-suspended in 200 μl 2% PBS–paraformaldehyde solution, transferred to acquisition tubes, and read on a LSRII. Killing was measured by incorporation of the yellow Live/Dead viability dye in CellTrace™ Violet+ target cells. Specific killing was defined as % killing of peptide pulsed targets – % killing of targets without peptide pulsing.
Immunohistochemical detection of GCs and GC-resident fCD8 and Tfh cells
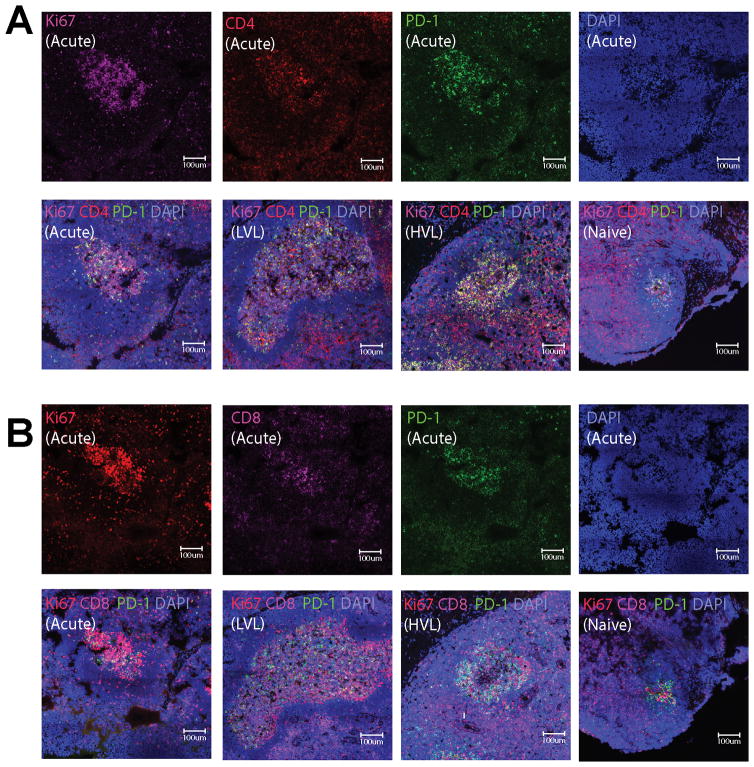
Adjacent LN sections were stained for Tfh and fCD8 cells, respectively. Briefly, 6-μm sections were cut, adhered to salinized slides, and pretreated in 1 mM EDTA (pH 8.0) in a Presto pressure cooker (National Presto Industries, Eau Claire, WI) at 121°C for 35sec to unmask antigens. Sections were blocked with normal horse serum, and incubated overnight at 4°C with the following primary antibodies: rabbit monoclonal anti-human CD4 (Clone: EPR6855, Cat#: ab133616, 1:200, Abcam) or mouse monoclonal anti-human CD8 (Clone: RPA-T8, Cat#: 557084, 1:200, BD Biosciences), goat anti-human PD-1 (AF1086, 1:40, R&D Systems), and mouse monoclonal anti-human Ki67 (Clone: MM1, Cat#: VP-K452, 1:200, Vector Laboratories) for CD4 and PD-1 staining combination or rabbit monoclonal anti-human Ki67 (Clone: EPR3610, Cat#: ab92742, 1:200, Abcam) for CD8 and PD-1 staining combination. After washing in PBS, slides were incubated at RT for 2 h with the following secondary antibodies for CD4 staining: AlexaFluor 594 donkey anti-rabbit IgG, AlexaFluor 488 donkey anti-goat IgG and AlexaFluor 647 donkey anti-mouse IgG and counterstained with DAPI (4′,6-diamidino-2-phenylindole). For CD8 staining secondary antibodies included AlexaFluor 647 donkey anti-mouse IgG, AlexaFluor 488 donkey anti-goat IgG and AlexaFluor 594 donkey anti-rabbit IgG with DAPI counterstaining. The secondary antibodies (diluted 1:200) were from Life Technologies. After washing in PBS, slides were cover slipped and examined. Images were collected on a LSM780 confocal microscope with Zen software (Carl Zeiss, Inc, Thornwood, NY) using Plan-Apochromat 40X, 1.40 NA Zeiss objective and GaASP detector. Panels of single focal plane images were acquired with pixel size of 415 nm or 519 nm at 12-bit image depth with averaging (setting 2) and pixel dwell time of 1.58 us in the following channels: Magenta (Ex 633 nm; Em 650–758), Red (Ex 561 nm; Em 570–694 nm), Green (Ex 488 nm; Em 490–553 nm), and Blue (Ex 405 nm; Em 410–470 nm). For publication, images were scaled to 8-bit RGB identically, with a linear LUT and exported in TIFF format. Figures were made from TIFF images in Adobe Illustrator without any change in resolution. Scalebars are provided for each image.
Quantification of GC-resident fCD8 and Tfh cells in fixed LNs
Ki67 staining defined GCs, as both light and dark zones have a high cell proliferation rate (35). The total area of individual GCs was determined by abundance of Ki67 and PD-1. The quantification of fCD8 and CD4+ Tfh in GCs was performed as described (36). Cells expressing CD4 or CD8 were extracted from the entire confocal images by first using the interactive segmentation software ilastik (v. 1.1.5) (37) to separate cells from the background based on features defined by color/intensity, edges, and texture in all image channels. Although the ilastik segmentation efficiently delineated cells from the image background, groups of cells were not adequately separated from each other. Therefore, the segmentation mask, along with the raw confocal images was imported into custom software written in MATLAB (R2015a, Mathworks, Natick, MA), with use of the image processing toolbox. The custom software performed morphological erosion on the segmentation mask to isolate individual cells, which were then each given a unique identification number, and then returned to their original size by use of morphological dilation. The boundary of the GC was then outlined manually based on the Ki67 and PD-1 expression, and the number of cells in each GC was counted. To aid comparisons between different conditions, the normalized cell count in each image was obtained by dividing the number of cells in the GC by the area (in mm^2) of the GC.
Statistical analysis
The Mann-Whitney test was used for comparisons between different groups of animals and the Wilcoxon signed rank test for paired differences within the same group of animals. Correlations were assessed using the Spearman rank correlation test. Exact permutation p values were calculated. When small numbers of observed values yielded inadequate power for nonparametric tests, outcomes combined across groups were modeled using analysis of variance (ANOVA) after appropriate transformations to meet distributional assumptions. In Figures 3A–B, numbers of cells in multiple GCs per animal were analyzed by repeated measures ANOVA, and p values for the multiple pairwise comparisons of the groups were corrected using Tukey’s method. In all other figures, p values reported are not corrected for multiple comparisons. Analyses were performed using GraphPad Prism (GraphPad Software Inc.) and SAS/STAT software version 12.1 (SAS Institute, Cary, NC).
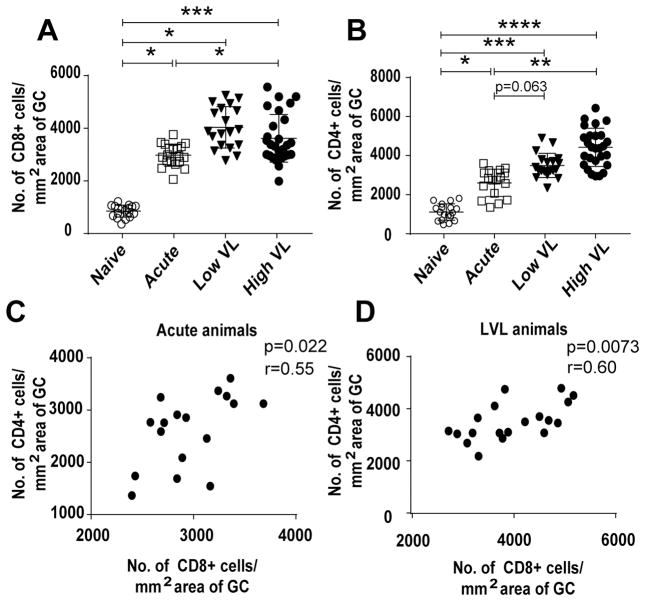
Figure 3.
Quantitation of CD4+ and CD8+ cells in GCs of rhesus macaques. (A) Frequency of CD8+ cells in GCs of naïve and acutely and chronically SIV infected macaques. (B) CD4+ cell frequency in GCs of all groups of macaques. (C–D) Correlation between the number of CD8+ and CD4+ cells in acutely infected and LVL animals, respectively. Data analyzed by repeated measures ANOVA (A, B) and the Spearman correlation test (C–D). In (A) and (B), p values for multiple pairwise comparisons of the groups were corrected using Tukey’s method. Horizontal and vertical bars denote mean and standard deviation. * P < 0.05; * * P < 0.01; * * * P < 0.001, * * * * P < 0.0001.
Results
CD8+ and CD4+ cells accumulate in LN follicular regions over the course of SIV infection
The number of CD8+ and CD4+ cells in LN GCs was determined immunohistochemically. Representative staining of LN from acutely and chronically infected (LVL and HVL) and naïve macaques is shown (Figs. 2A, B). Eighteen GCs (7, 2, and 9 respectively) of 3 naïve, 19 GCs (7, 8, 2, 1, 1 respectively) of 5 acute, 19 GCs of 4 LVL (5, 6, 6, 2 respectively) and 28 GCs (4, 5, 6, 6, 7 respectively) of 5 HVL animals were analyzed. Some of the animals had less defined GCs in the tissue sections examined. We chose more defined areas for analysis which resulted in lower GC counts in 1 of the naïve and 4 of the 14 infected animals. A higher number of CD8+ cells accumulated in GCs of the acutely infected compared to naïve animals, with even higher accumulation in the chronically infected animals. However, no difference in the number of CD8+ cells between LVL and HVL animals was observed (Fig. 3A). Compared to LN of naïve macaques, an increased number of CD4+ cells were seen in acutely and chronically infected animals (Fig. 3B). A positive correlation was observed between CD8+ and CD4+ cells/mm2 of tissue in the acute and LVL animals (Figs. 3C, D), suggesting that the interaction between CD8+ and CD4+ starts early after exposure and continues during chronic infection in LVL but not HVL animals. Thus, CD4+ cells might contribute to the proliferation of CD8+ cells in the acute and LVL animals.
Figure 2.
Localization of CD4 and CD8 cells in GCs of rhesus macaques. (A) Representative imaging of GCs for CD4+ cells. Immunofluorescence staining for Ki67 (purple), CD4 (red), PD-1 (green) and nuclei (blue) of a LN section from an acutely infected macaque (top panels). Representative staining of GCs from SIV-infected macaques (acutely-infected and LVL and HVL chronically-infected macaques) and a naïve macaque as indicated (bottom panels). (B) Representative imaging of GCs for CD8+ cells. Immunofluorescence staining for Ki67 (red), CD8 (purple), PD-1 (green) and nuclei (blue) of a LN section from an acutely-infected macaque (top panels). Representative staining of GCs from SIV-infected macaques (acutely-infected and LVL and HVL chronically-infected macaques) and a naïve macaque as indicated (bottom panels).
SIV specific fCD8 cells accumulate in B cell follicles of SIV-infected LNs
LNs from all macaque groups (see methods) were further analyzed by flow cytometry to detect SIV-specific fCD8 cells over the course of infection. The relative expression of CCR7 and CXCR5 on CD8+ T cells determines their localization within LNs (38, 39). CD8+ T cells expressing high CXCR5 and low CCR7, defined as fCD8 cells, are localized in B cell follicles and were gated as shown (Fig. 4A). Increased frequencies of fCD8 cells were observed in LNs of acute- and chronically-infected macaques, but no difference in the level of fCD8 cells was seen between LVL and HVL groups (Fig. 4B). As CD3 cells were not stained in the immunohistochemical analysis we examined flow cytometry data from the same lymph nodes to determine the fraction of cells that were CD3 positive as well as both CD4 and CD8 positive. The percentage of CD3+ T cells in the follicular CD4+ population ranged from 78.8 to 97.5%; the percentage in the follicular CD8+ population ranged from 70.9 to 98.9%. The percentage of CD4+CD8+ T cells among the total follicular CD4+ and CD8+ cells ranged from 0.3 to 4.5%. Overall, the percentage of fCD8 cells was highest in the chronic phase of infection regardless of viral load as also observed for CD8+ cells in GCs immunohistochemically (Fig.3A).
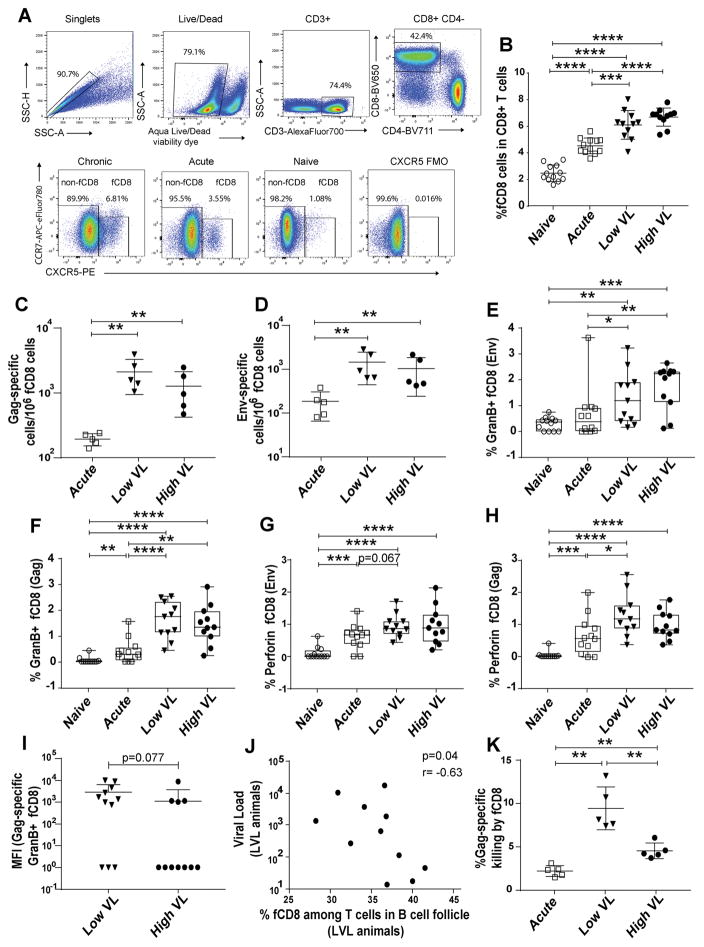
Figure 4.
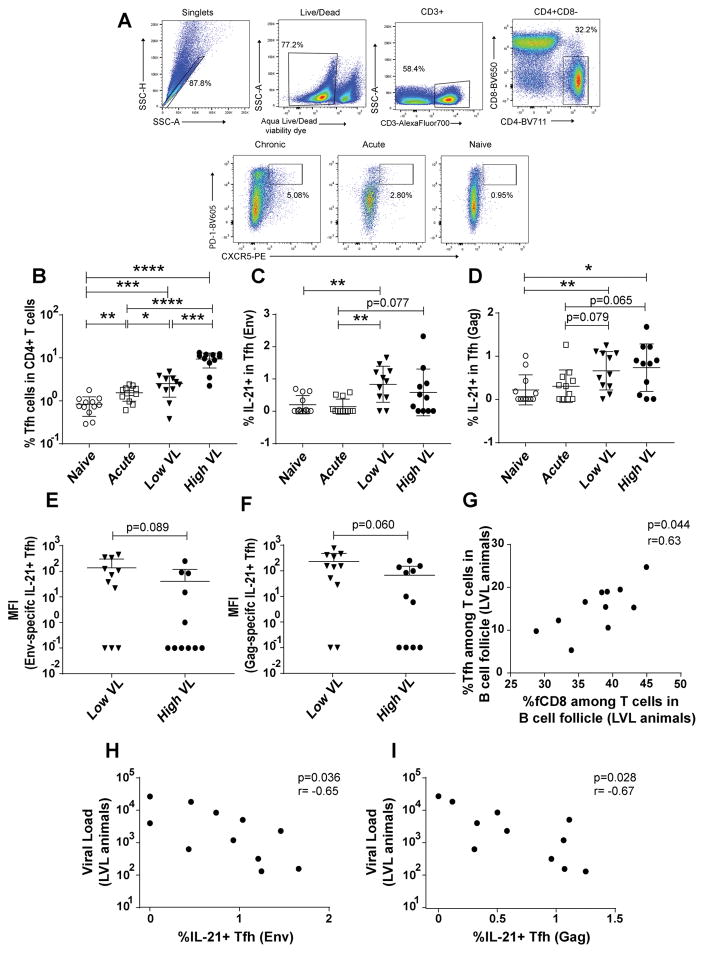
fCD8 cells during SIV infection: frequency, functionality and association with viremia control. (A) Representative gating of fCD8 cells. A representative CXCR5 FMO control is shown. (B) Frequency of fCD8 cells in LN of naïve and acutely and chronically SIV infected macaques. (C–D) Frequency of SIV Gag- and Env-specific fCD8 cells determined by ELISPOT in acutely and chronically infected macaques. (E–H) Frequency of Env-and Gag-specific Granzyme B+ and perforin+ fCD8 cells in all macaque groups following stimulation with Env and Gag peptides. (I) SIV Gag-specific Granzyme B expression on fCD8 cells of LVL and HVL macaques, shown by MFI. Frequencies and MFI were calculated as the peptide-stimulated response minus the unstimulated response. MFI calculated as 0 were assigned a value of 1 for plotting. (J) Correlation between median chronic viral load and percentage of fCD8 cells among T cells (CD3+CXCR5+) in B cell follicles of LVL macaques. (K) Gag-specific killing by fCD8 cells of SIV-infected macaques. Data of panels B–I and K analyzed by the Mann-Whitney test; data of panel J were analyzed by the Spearman correlation test. Horizontal and vertical bars denote mean and standard deviation. * P < 0.05; * * P < 0.01; * * * P < 0.001, * * * * P < 0.0001.
To further investigate the expansion of fCD8 cells over the course of SIV infection, the frequency of SIV Env and Gag specific fCD8 cells was determined by ELISPOT assay in acutely and chronically infected macaques. Cells of both specificities were observed in infected animals, with greater levels seen in chronically infected macaques. However, no significant difference was obtained between LVL and HVL groups (Figs. 4C, D).
fCD8 cells in LVL animals are associated with viremia control
Granzyme B and perforin producing fCD8 cells were next assessed following stimulation with SIV Env and Gag peptides. Upon 6 hours stimulation with peptide, the expression of CXCR5 on CD8 T cells remained unchanged (data not shown). Higher frequencies of granzyme B+ (Figs. 4E, F) and perforin+ (Figs. 4G, H) cells were observed in chronically-infected animals compared to naïve and acutely-infected animals. While the frequency of granzyme B+ cells was comparable between LVL and HVL animals, fCD8 cells of LVL compared to HVL animals tended to express more Granzyme B in response to Gag peptide stimulation as shown by higher MFI, suggesting a greater cytotoxic potential (Fig. 4I).
To assess the potential impact of greater Gag-specific granzyme B fCD8 cell responses in LVL macaques on viremia control, correlation analyses were performed between fCD8 cells and median plasma viremia during chronic infection (weeks 12 to time of necropsy) of HVL and LVL macaques and viremia at the time of necropsy of acutely infected macaques. A significant negative correlation was observed between fCD8 cells in B cell follicles and median viral load in LVL animals (Fig. 4J), but not in the other groups of animals (data not shown) suggesting that fCD8 cells might contribute to viremia control in LVL animals.
Killing capacity was next investigated as a mechanism by which fCD8 cells might contribute to viremia control. CD8+CXCR5+ and autologous CD4+ cells were sorted from LNs of acute, LVL and HVL animals. CD4+ cells pulsed with SIVmac239 Gag peptides were used as targets and CD8+CXCR5+ fCD8 cells as effectors. fCD8 cells from LVL animals killed target cells more efficiently compared to those from HVL and acutely infected animals (Fig. 4K), further supporting the notion that fCD8 cells contributed to control of viral load in the LVL animals.
Tfh are associated with fCD8 activity in SIV infected animals
CD8+ T cells control virus replication with the help of CD4+ T cells which provide IL-21, essential for maintaining CD8+ T cell functionality (40, 41). The control might be compromised in the absence of CD4+ T cell help (42). An increase in Tfh cells in LNs occurs following both HIV and SIV infection (11, 43), however, IL-21 production by the cells has been reported to be significantly reduced (44). Thus, we investigated LN Tfh-fCD8 cell interactions. Tfh cells were gated as shown (Fig. 5A) and analyzed in the four macaque groups. Overall Tfh percentages were elevated in acutely and chronically SIV infected animals compared to naïve macaques; highest frequencies were observed in HVL animals (Fig. 5B). Comparable data were obtained on GC CD4+ cells immunohistochemically (Fig. 3B). Percentages of Env- (Fig. 5C) and Gag-specific IL-21+ Tfh cells (Fig. 5D) were highest in chronically infected macaques with no difference between HVL and LVL groups. IL-21 expression levels in response to both Env and Gag peptide stimulation, reported as MFI, although not significant tended to be higher in the LVL macaques compared to the HVL macaques (Figs. 5E, F). Notably in LVL macaques, but not in the other infected animals, percentages of Tfh and fCD8 cells were significantly correlated (Fig. 5G). Percentages of both Env- and Gag-specific IL-21+ Tfh in LVL group but not the other groups, also correlated inversely with viral load (Figs. 5H, I). These results are consistent with IL-21+Tfh cells providing help to fCD8 cells in maintaining control of viral load.
Figure 5.
Association of Tfh and fCD8 cells in B cell follicles. (A) Representative gating of Tfh cells. (B) Frequency of Tfh cells among CD4+ cells in LN of the four macaque groups (C–D) Percentage of IL-21+ Tfh cells in response to Gag (C) and Env (D) peptide stimulation. (E–F) IL-21 expression on Env- (E) and Gag- (F) specific Tfh cells determined by MFI in HVL and LVL animals. Frequencies and MFI were calculated as the peptide-stimulated response minus the unstimulated response. MFI calculated as 0 were assigned a value 0.1 for plotting. (G) Correlation between Tfh and fCD8 cell frequencies among T cells (CD3+CXCR5+) in B cell follicles of LVL macaques. (H–I) Correlation between Env- (H) and Gag-specific (I) IL-21+ Tfh cells and median chronic viral load of LVL animals. The data of (B–F) were analyzed by Mann-Whitney test and (G–I) were analyzed by Spearman correlation test. Horizontal and vertical bars denote mean and standard deviation. * P < 0.05; * * P < 0.01; * * * P < 0.001, * * * * P < 0.0001.
Tfreg-fCD8 cell associations suggest suppression of CD8 cell activity in HVL SIV infected animals
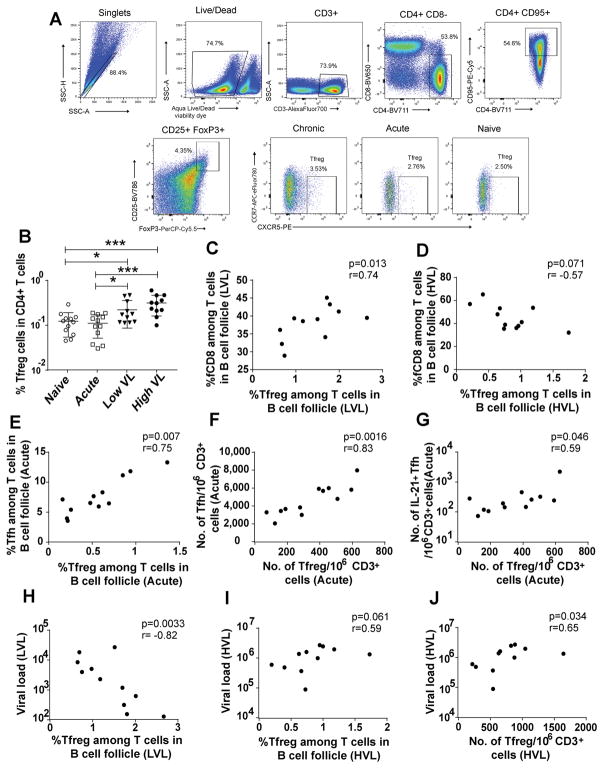
Suppressive T regulatory (Treg) cells expand in chronic infections, including HIV infection (45). They are potentially beneficial, limiting non-specific immune activation, or detrimental, suppressing effective antigen-specific immune responses (46). Simonetta and Bourgeois suggested that a balance between positive and negative Treg effects might determine disease outcome (47). In HIV patients reduced immune activation and LVL have been associated with increased levels of Treg (48, 49). However, in both HIV and SIV infection, Treg accumulation in lymphoid tissues has been associated with high viremia (50). Decreased HIV-specific T cell responses in chronic infection, which contributes to viral persistence, has been associated with mucosal Treg (51). Thus, here we examined the effect of Tfreg on fCD8 cells and viral loads over the course of SIV infection. Tfreg cells were gated as shown (Fig. 6A), and comparable frequencies of Tfreg were observed among naïve and acutely-infected animals, with significantly elevated levels seen in chronically-infected macaques, although no large difference was observed between the LVL and HVL groups (Fig. 6B).
Figure 6.
Association of Tfreg with fCD8 and Tfh cells in B cell follicles. (A) Representative gating of Tfreg cells. (B) Frequency of Tfreg cells among CD4+ cells in LN of four animal groups. (C–D) Correlation between Tfreg and fCD8 cell frequencies of LVL (C) or HVL (D) animals. (E) Correlation between Tfreg and Tfh frequencies in acutely infected macaques. (F–G) Correlation between Tfreg number and Tfh number (F) or IL-21+ Tfh number (G) in acutely infected macaques. (H–I) Correlation between the frequencies of Tfreg in LVL (H) or HVL (I) macaques and median chronic viral loads. (J) Correlation between Tfreg number and median chronic viral load in HVL macaques. The percentages in panels C–E and H–I were taken among T cells (CD3+CXCR5+) in B cell follicles of the macaques. Numbers of Tfreg, Tfh, and IL-21+Tfh cells in panels F, G, and J were normalized to one million CD3+ cells. The data of (B) analyzed by Mann-Whitney test; (C–J) were analyzed by Spearman correlation test. Horizontal and vertical bars denote mean and standard deviation. * P < 0.05; * * P < 0.01; * * * P < 0.001, * * * * P < 0.0001.
To investigate the impact of Tfreg cells on disease progression, we examined relationships between frequencies of follicular T cell subpopulations determined by flow cytometry. In acutely infected macaques no significant correlation was observed between Tfreg and fCD8 cells. However, during chronic infection, Tfreg in the LVL animals exhibited a significant positive correlation with fCD8 cells (Fig. 6C), whereas, a negative trend was observed in HVL animals (Fig. 6D). This suggests that while Tfreg have a beneficial effect in the LVL macaques, perhaps helping to control immune activation, they may exert suppressive effects on fCD8 in HVL animals. In contrast to the lack of relationship between Tfreg and fCD8 cell frequencies in the acute animals, a positive correlation was observed between Tfreg and Tfh in the acutely infected animals (Fig. 6E) which was lost in both groups of chronically infected macaques. Because CD4 T cells are lost during HIV/SIV infection, the absolute number of Tfreg cells is also important in predicting HIV infection outcomes (47). In acutely infected animals, a positive correlation was observed between the number of Tfreg cells and the number of Tfh cells (Fig. 6F) as well as with the number of IL-21+ Tfh cells (Fig. 6G). These correlations were lost in the chronic phase of infection, suggesting that Tfreg play a positive role on Tfh in the early phase of infection.
We also investigated relationships between Tfreg and viremia levels. A strong negative correlation between Tfreg and chronic median viral load was observed in LVL animals (Fig. 6H). In view of the positive correlation between fCD8 cells and Tfreg in LVL macaques (Fig. 6C), this result is consistent with Tfreg contributing to viremia suppression. However, the percentage of Tfreg in HVL animals showed a positive trend with the median viral load (Fig. 6I) and the number of Tfreg was also positively correlated with viremia (Fig. 6J). The trend of negative correlation between fCD8 and Tfreg frequencies (Fig. 6D), together with the trend of positive correlation of Tfreg frequency with viral load and the significant association of Tfreg number with viral load in HVL macaques (Figs. 6I, J), suggest that Tfreg contribute to the high viremia in these animals.
Higher frequency and function of SIV-specific fCD8 cells compared to non-fCD8 cells
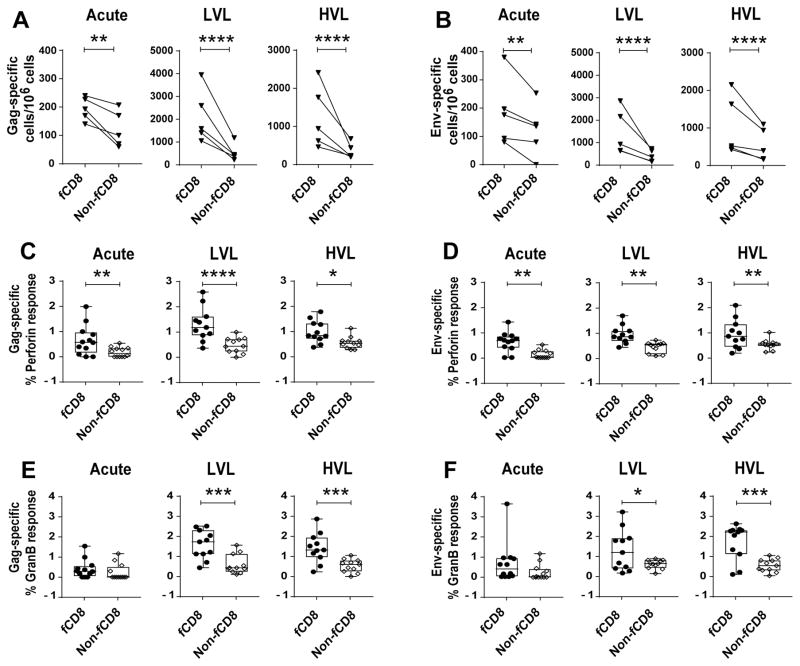
As antiviral CD8+ T cells are important in the control of HIV/SIV replication and Tfh cells in B-cell follicles are key sites of productive HIV/SIV infection (8–12, 52), we compared fCD8 within the B cell follicle with non-fCD8 (CD8+CXCR5−) T cells with regard to their impact on viral replication in vivo and viral load control. Higher frequencies of SIV Gag- and Env-specific fCD8 compared to non-fCD8 T cells were observed in SIV-infected macaques by ELISPOT assay (Fig. 7A, B). To investigate functionality, we examined frequencies of granzyme B- and perforin-positive cells in each compartment by flow cytometry. A previous study reported equivocal results in chronically SIV-infected macaques. Higher percentages of granzyme B-positive cells were found flow cytometrically in CXCR5+ CTL including CCR7− cells, indicating the fCD8 population, whereas perforin-positive cells detected by in situ tetramer staining were more prevalent in the non-fCD8 population (14). Here, flowcytometric analysis revealed higher percentages of perforin+ SIV Gag- and Env-specific fCD8 compared to non-fCD8 in LN of all SIV-infected macaques (Fig. 7C, D) together with greater frequencies of Granzyme B+ SIV Gag- and Env-specific fCD8 in LVL and HVL animals (Fig. 7E, F). The data suggest that SIV specific CD8+ T cells accumulate over the course of infection in B cell follicles where they are available to combat virus infected cells.
Figure 7.
Comparison of SIV-specific frequency and cytokine producing cells in fCD8 and non-fCD8 cells. (A–B) Frequency of SIV-Gag and Env-specific fCD8 and non-fCD8 cells in LN of SIV-infected macaques. (C–F) Percentage of SIV-specific perforin+ (C, D) and Granzyme B+ (E, F) fCD8 and non-fCD8 cells in response to Gag (C, E) or Env (D, F) peptide stimulation. The data of (A–F) were analyzed with ANOVA. Horizontal and vertical bars denote mean and standard deviation. * P < 0.05; * * P < 0.01; * * * P < 0.001, * * * * P < 0.0001.
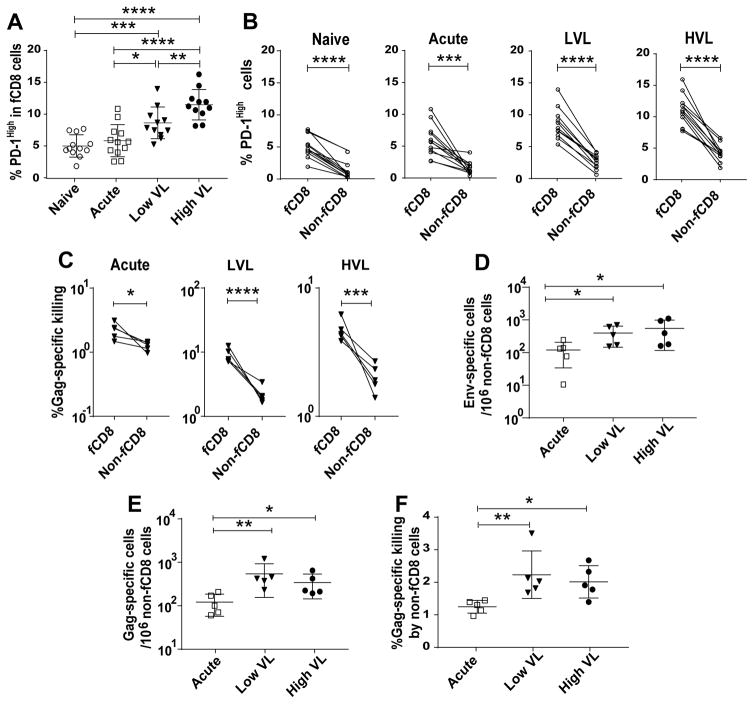
A caveat in this regard involves the PD-1/PD-L1 pathway which contributes to the exhaustion of HIV-specific CD8+ T cells. In both HIV- (53) and SIV-specific CD8+ T cells (54) PD-1 is upregulated and positively correlated with high plasma viremia and negatively correlated with CD4+ T cell counts. Blocking of the PD-1/PD-L1 interaction in the SIV-macaque model has reversed exhaustion of antigen-specific CD8+ T cells (54). Moreover, inhibitory PD-1 has been associated with lower functionality of CD8+ T cells (55) and with decreased HIV-specific CD8+ T-cell proliferation (56). While PD-1+ T cells have been associated with poor functionality, these “exhausted” CD8+ T cells have also been shown to play a vital role in controlling viral replication (57). Here we assessed PD-1 expression on fCD8 cells at different stages of SIV infection. Elevated frequencies of PD-1+ fCD8 cells were seen in chronically infected animals, highest in HVL animals (Fig. 8A). This result suggested that fCD8 cells of HVL macaques might have lower functionality compared to fCD8 cells of the other macaque groups. In fact, fCD8 cells of LVL macaques exhibited greater SIV-specific killing compared to both acutely-infected and HVL macaques (Fig. 4K).
Figure 8.
Comparison of phenotypic and functional properties of fCD8 and non-fCD8 cells. (A) Percentage of PD-1High fCD8 cells among four groups of macaques. (B) PD-1High fCD8 and non-fCD8 cells in naive and SIV-infected animals. (C) Specific killing by fCD8 and non-fCD8 cells of SIV-infected macaques. (D–E) Frequencies of SIV Env- and Gag-specific non-fCD8 cells in acutely and chronically SIV infected macaques, determined by ELISPOT. (F) Gag-specific killing by non-fCD8 cells among acutely and chronically-infected macaques. The data of (A, D–F) analyzed by Mann-Whitney test and (B–C) analyzed by ANOVA. Horizontal and vertical bars denote mean and standard deviation. * P < 0.05; * * P < 0.01; * * * P < 0.001, * * * * P < 0.0001.
We further compared frequencies of PD-1+ fCD8 and non-fCD8 cells. In all macaque groups, greater percentages of PD-1 positive cells were observed in the fCD8 populations (Fig. 8B). Killing assays performed using fCD8 or non-fCD8 cells as effectors with Gag peptide pulsed autologous CD4+ T targets revealed a higher percentage of killing by the fCD8 cells compared to the non-fCD8 cells, regardless of the viral load or duration of infection (Fig. 8C), and despite displaying high PD-1 frequencies, as PD-1 expression has been reported unreliable as a marker for immune exhaustion in chronic SIV infection (58). Higher frequencies of SIV Env- and Gag- specific non-fCD8 cells were observed in chronically infected animals compared to acutely infected ones (Fig. 8D, E), with no difference between LVL and HVL macaques and no difference in their killing capacity (Fig. 8F). Unlike fCD8 cells of LVL macaques, the frequency of non-fCD8 cells in all the LVL animals did not correlate negatively with viral load (data not shown). Therefore, killing attributed to non-fCD8 cells might not be a major contributing factor in viremia control of the LVL animals studied. Recent data following in vivo CD8+ T cell depletion of SIV-infected macaques, showed that while SIV-positive cells increased in both follicular and extrafollicular areas, the increase was approximately two-fold greater in the extrafollicular region (59). The authors concluded that both follicular and extrafollicular CD8+ T cells can suppress viral replication in vivo. Whether this difference from our findings results from the fact that our animals were vaccinated whereas the CD8 depleted macaques were not, or because the latter macaques had only been infected for 59 days while our macaques were studied after 40 to 50 weeks of infection will require further investigation.
Discussion
Our investigation of three LN follicular T cell populations of rhesus macaques revealed correlative interactions and associations with maintenance of low viremia during SIV chronic infection. As recently reported for HIV infected patients (31) and SIV infected macaques (30), fCD8 cells were elevated here in chronically infected macaques. While similar fCD8 frequencies between LVL and HVL macaques and similar frequencies of SIV Gag- and Env-specific cells and granzyme B- and perforin-positive cells were seen, granzyme B expression tended to be greater in fCD8 of LVL macaques. These cells also exhibited significantly greater killing compared to HVL macaques (Fig. 4K). This greater functionality suggests a mechanism by which LVL macaques maintain viremia control, supported by the negative correlation of their fCD8 frequency and viral load (Fig. 4J). Connick et al. in an earlier study reported using a mixed model analysis that SIV-specific CTL in both follicular and extrafollicular compartments of combined lymph node and spleen were inversely correlated with chronic tissue viral load of all chronically SIV-infected macaques (14). The study is not strictly comparable, however to the one here. The animal populations were somewhat different, as the earlier study used macaques infected a median of 22 weeks while our macaque samples were obtained 40 to 50 weeks post-infection. More important differences include the fact that we correlated total fCD8 cells with plasma viral loads, while Connick et al. correlated dominant SIV-specific CTL in follicular and extrafollicular compartments with SIV RNA-positive cells, perhaps contributing to the different results.
Tfh cells in macaque LNs exhibited elevated frequencies associated with increasing viremia, as reported earlier (10, 12). Notably, however, Env-specific IL-21+ Tfh were significantly higher in LVL macaques and tended to be higher in HVL animals (Fig. 5C) while Gag-specific IL-21+ Tfh tended to be higher in both LVL and HVL animals compared to the acute group. Tfh and fCD8 cell frequencies were directly correlated in LVL (Fig. 5G) but not HVL macaques. Importantly, both Env- and Gag-specific IL-21+ Tfh cells of LVL animals were significantly negatively correlated with viral loads (Figs. 5H, I). These results are consistent with adequate help being provided by Tfh cells to fCD8 cells in LVL but not HVL animals.
We confirmed an increased abundance of Tfreg cells over the course of infection (50), although no difference was observed between LVL and HVL animals. Tfreg cells have shown negative effects on Tfh cells in HIV and SIV infection, including inhibition of proliferation, ICOS expression, and IL-4 and IL-21 production (60). While a positive correlation between Tfreg and Tfh in acutely infected animals was seen (Fig. 6E), it was lost in chronic infection. A significant positive correlation between Tfreg and fCD8 cells in the LVL animals (Fig. 6C), as well as a negative correlation between Tfreg and viral load (Fig. 6H), suggested that Tfreg positively impact fCD8 cells which in turn control viremia. Importantly, in chronic viral infections, Tregs have been shown to suppress CD8+ T cell activity in a contact-dependent manner (61). Moreover, in situ staining of lymph node tissue sections for SIV-specific CD8+ T cells and FoxP3-positive Tregs revealed significant numbers of these cells in direct contact (59), further suggesting cell interactions. Strikingly, however, a significant negative correlation was seen between Tfreg and fCD8 cell frequencies in HVL macaques (Fig. 6D). In addition, a strong positive trend was seen between Tfreg cell frequency in HVL animals and their viral loads (Fig. 6I), along with a significant positive correlation between Tfreg cell number and viral load (Fig. 6J). These data suggest suppression of fCD8 cells by Tfreg might contribute to the loss of viremia control in the HVL animals.
While here we focused on CD4+ Tfreg cells, CD8+ Tfregs have been shown to make up most of the CD8 T cells within follicles of both humans and macaques (62) and to impair both Tfh and GC B cell responses. As they have been characterized as having limited cytotoxic potential, they could be critical in maintaining persistent HIV and SIV infection of lymphoid follicles.
Overall these results illustrate the complexity of cellular activities within LN follicles, and indicate assessment of a single T cell population and associated molecular mechanisms will be insufficient to fully understand pathogen control. Detection of fCD8 cells in B cell follicles of secondary lymphoid organs in humans, as well as in murine and macaque models (14, 16, 17, 27–31) suggested that fCD8 cells enter B cell follicles and contribute to eradication of infected cells. While HIV specific killing in vitro was reported in the presence of bispecific antibody (31), this is the first report showing SIV peptide-specific killing of target cells by fCD8 cells isolated from LN. While fCD8 cells of LVL animals had higher killing capacity and were negatively correlated with viremia, fCD8 cells were also detected in HVL animals. Their higher expression of PD-1 is not necessarily firm evidence of immune exhaustion (58) and other mechanisms may contribute to their impaired viremia control. While Tfh cell frequency was highest in HVL animals, these cells tended to have lower frequencies and/or expression of IL-21 suggesting dysfunction and diminished support of fCD8 cells. In fact, unlike LVL animals, the percentage of IL-21+ Tfh in HVL animals did not correlate with reduced viral load or fCD8 cell frequency. In addition, a negative impact of Tfreg on fCD8, suggested by the negative correlation in HVL macaques (Fig. 6D), is consistent with lower viral control by fCD8 cells in these animals.
The frequencies of SIV Env- and Gag-specific fCD8 compared to non-fCD8 cells were higher in LN of infected animals (Figs. 7A, B). Furthermore, the frequencies of SIV-specific Granzyme B+ and perforin+ cells were higher in fCD8 compared to non-fCD8 populations (Figs. 7C, D). These data explain the higher SIV-specific killing by fCD8 cells compared to non-fCD8 cells, and are consistent with results obtained in the LCMV mouse model, where fCD8 cells exhibited more potent cytotoxicity than the CXCR5− subset (29). SIV-specific killing in non-fCD8 cell populations was higher in the chronic phase compared to the acute phase, but no difference in killing was observed between LVL and HVL animals. Moreover, the frequency of SIV-specific non-fCD8 cells did not correlate with viral loads, suggesting that the activity of fCD8 cells might be more important for viremia control.
Overall, our results suggest that elimination of virus-infected cells in B cell follicles does not only depend on the cytotoxicity of fCD8 cells but also interaction of fCD8 cells with other T follicular cells including Tfh and Tfreg. We should note that all but one in each group of the chronically infected HVL and LVL macaques in our study had been previously vaccinated, so our results cannot be generalized to what might occur in natural infection. Whether the vaccination regimen had long-term effects on follicular T cell populations in the macaques 40 to 50 weeks after infection remains to be determined. A recent abstract reported that a DNA/MVA SHIV vaccine regimen elicited SHIV-specific CXCR5+ CD8+ T cells in the blood of rhesus macaques and were enhanced when the regimen was adjuvanted with CD40L (63). The frequency of these cells was inversely correlated with peak viral load following SHIV infection. Future studies will be critical in determining if immunization can induce potent fCD8 cells leading to protection or enhanced control of viremia. In addition to prophylactic vaccines, further understanding of how to direct the differentiation and function of fCD8 and other follicular T cell populations might lead to the design of new strategies for eliminating HIV/SIV reservoirs.
Acknowledgments
This study was supported by the Intramural Research Program of the NIH, National Cancer Institute.
We gratefully acknowledge the staffs at the NCI animal facility and Advanced BioScience Laboratories, Inc., and Tanya Hoang and Sophia Brown (Vaccine Branch, NCI) for laboratory and flow cytometry support. PE anti-CXCR5 was obtained through the NIH Nonhuman Primate Reagent Resource. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: SIVmac239 Env and SIVmac239 Gag peptides (complete sets).
References
- 1.CDC. Persistent generalized lymphadenopathy among homosexual males. MMWR. 1982;31:249–251. [PubMed] [Google Scholar]
- 2.Ioachim HL, Lerner CW, Tapper ML. Lymphadenopathies in homosexual men. Relationships with the acquired immune deficiency syndrome. JAMA. 1983;250:1306–1309. doi: 10.1001/jama.250.10.1306. [DOI] [PubMed] [Google Scholar]
- 3.Tenner-Racz K, Racz P, Bofill M, Schulz-Meyer A, Dietrich M, Kern P, Weber J, Pinching AJ, Veronese-Dimarzo F, Popovic M, Klatzmann D, Gluckman JC, Janossy G. HTLV-III/LAV viral antigens in lymph nodes of homosexual men with persistent generalized lymphadenopathy and AIDS. Am J Pathol. 1986;123:9–15. [PMC free article] [PubMed] [Google Scholar]
- 4.Biberfeld P, Chayt KJ, Marselle LM, Biberfeld G, Gallo RC, Harper ME. HTLV-III expression in infected lymph nodes and relevance to pathogenesis of lymphadenopathy. Am J Pathol. 1986;125:436–442. [PMC free article] [PubMed] [Google Scholar]
- 5.Ringler DJ, Wyand MS, Walsh DG, MacKey JJ, Chalifoux LV, Popovic M, Minassian AA, Sehgal PK, Daniel MD, Desrosiers RC, King NW. Cellular localization of simian immunodeficiency virus in lymphoid tissues. I. Immunohistochemistry and electron microscopy. Am J Pathol. 1989;134:373–383. [PMC free article] [PubMed] [Google Scholar]
- 6.Breitfeld D, Lohl, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1551. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McIlroy D, Autran B, Cheynier R, Wain-Hobson S, Clauvel JP, Oksenhendler E, Debre P, Hosmalin A. Infection frequency of dendritic cells and CD4+ T lymphocytes in spleens of human immunodeficiency virus-positive patients. J Virol. 1995;69:4737–4745. doi: 10.1128/jvi.69.8.4737-4745.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haase AT. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu Rev Immunol. 1999;17:625–656. doi: 10.1146/annurev.immunol.17.1.625. [DOI] [PubMed] [Google Scholar]
- 10.Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, Ambrozak DR, Sandler NG, Timmer KJ, Sun X, Pan L, Poholek A, Rao SS, Brenchley JM, Alam SM, Tomaras GD, Roederer M, Douek DC, Seder RA, Germain RN, Haddad EK, Koup RA. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest. 2012;122:3281–3294. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu H, Wang X, Malam N, Aye PP, Alvarez X, Lackner AA, Veazey RS. Persistent Simian Immunodeficiency Virus Infection Drives Differentiation, Aberrant Accumulation, and Latent Infection of Germinal Center Follicular T Helper Cells. J Virol. 2015;90:1578–1587. doi: 10.1128/JVI.02471-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banga R, Procopio FA, Noto A, Pollakis G, Cavassini M, Ohmiti K, Corpataux JM, de Leval L, Pantaleo G, Perreau M. PD-1(+) and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat Med. 2016;22:754–761. doi: 10.1038/nm.4113. [DOI] [PubMed] [Google Scholar]
- 14.Connick E, Folkvord JM, Lind KT, Rakasz EG, Miles B, Wilson NA, Santiago ML, Schmitt K, Stephens EB, Kim HO, Wagstaff R, Li S, Abdelaal HM, Kemp N, Watkins DI, MaWhinney S, Skinner PJ. Compartmentalization of simian immunodeficiency virus replication within secondary lymphoid tissues of rhesus macaques is linked to disease stage and inversely related to localization of virus-specific CTL. J Immunol. 2014;193:5613–5625. doi: 10.4049/jimmunol.1401161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, Hagen SI, Shoemaker R, Deleage C, Lucero C, Morcock D, Swanson T, Legasse AW, Axthelm MK, Hesselgesser J, Geleziunas R, Hirsch VM, Edlefsen PT, Piatak M, Jr, Estes JD, Lifson JD, Picker LJ. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med. 2015;21:132–139. doi: 10.1038/nm.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connick E, Mattila T, Folkvord JM, Schlichtemeier R, Meditz AL, Ray MG, McCarter MD, Mawhinney S, Hage A, White C, Skinner PJ. CTL fail to accumulate at sites of HIV-1 replication in lymphoid tissue. J Immunol. 2007;178:6975–6983. doi: 10.4049/jimmunol.178.11.6975. [DOI] [PubMed] [Google Scholar]
- 17.Folkvord JM, Armon C, Connick E. Lymphoid follicles are sites of heightened human immunodeficiency virus type 1 (HIV-1) replication and reduced antiretroviral effector mechanisms. AIDS Res Hum Retroviruses. 2005;21:363–370. doi: 10.1089/aid.2005.21.363. [DOI] [PubMed] [Google Scholar]
- 18.Edwards BH, Bansal A, Sabbaj S, Bakari J, Mulligan MJ, Goepfert PA. Magnitude of functional CD8+ T-cell responses to the gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J Virol. 2002;76:2298–2305. doi: 10.1128/jvi.76.5.2298-2305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, de Pierres C, Mncube Z, Mkhwanazi N, Bishop K, van der Stok M, Nair K, Khan N, Crawford H, Payne R, Leslie A, Prado J, Prendergast A, Frater J, McCarthy N, Brander C, Learn GH, Nickle D, Rousseau C, Coovadia H, Mullins JI, Heckerman D, Walker BD, Goulder P. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 20.Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, Irwin CE, Safrit JT, Mittler J, Weinberger L, Kostrikis LG, Zhang L, Perelson AS, Ho DD. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 22.Devergne O, Peuchmaur M, Crevon M-C, Trapani JA, Maillot P, Galanaud M-C, Emilie D. Activation of cytotoxic cells in hyperplastic lymph nodes from HIV-infected patients. AIDS. 1991;5:1071–1079. doi: 10.1097/00002030-199109000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Tenner-Racz, Racz KP, Thome C, Meyer CG, Anderson PJ, Schlossman SF, Letvin NL. Cytotoxic effector cell granules recognized by the monoclonal antibody TIA-1 are present in CD8+ lymphocytes in lymph nodes of human immunodeficiency virus-1-infected patients. Am J Pathol. 1993;142:1750–1758. [PMC free article] [PubMed] [Google Scholar]
- 24.Reimann KA, Snyder GB, Chalifoux LV, Waite BCD, Miller MD, Yamamoto O, Spertini H, Letvin NL. An activated CD8+ lymphocyte appears in lymph nodes of rhesus monkeys early after infection with simian immunodeficiency virus. J Clin Invest. 1991;88:1113–1120. doi: 10.1172/JCI115410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg YJ, Kosco MH, Lewis MG, Leon EC, Greenhouse JJ, Bieg KE, Eddy GA, Zack PM. Changes in follicular dendritic cell and CD8+ cell function in macaque lymph nodes following infection with SIV251. Adv Exp Med Biol. 1993;329:417–23. doi: 10.1007/978-1-4615-2930-9_70. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz C, Bouchat S, Margan C, Gautier V, Van Lint C, Rohr O, Le Douce V. On the way to find a cure: purging latent HIV-1 reservoirs. Biochem Pharmacol. 2017 doi: 10.1016/j.bcp.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Quigley MF, Gonzalez VD, Granath A, Andersson J, Sandberg JK. CXCR5+ CCR7− CD8 T cells are early effector memory cells that infiltrate tonsil B cell follicles. 2007;37:3352–3362. doi: 10.1002/eji.200636746. [DOI] [PubMed] [Google Scholar]
- 28.Leong YA, Chen Y, Ong HS, Wu D, Man K, Deleage C, Minnich M, Meckiff BJ, Wei Y, Hou Z, Zotos D, Fenix KA, Atnerkar A, Preston S, Chipman JG, Beilman GJ, Allison CC, Sun L, Wang P, Xu J, Toe JG, Lu HK, Tao Y, Palendira U, Dent AL, Landay AL, Pellegrini M, Comerford I, McColl SR, Schacker TW, Long HM, Estes JD, Busslinger M, Belz GT, Lewin SR, Kallies A, Yu D. CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol. 2016;17:1187–1196. doi: 10.1038/ni.3543. [DOI] [PubMed] [Google Scholar]
- 29.He R, Hou S, Liu C, Zhang A, Bai Q, Han M, Yang Y, Wei GG, Shen T, Yang X, Xu L, Chen X, Hao Y, Wang P, Zhu C, Ou J, Liang H, Ni T, Zhang X, Zhou X, Deng K, Chen Y, Luo Y, Xu J, Qi H, Wu Y, Ye L. Follicular CXCR5− expressing CD8(+) T cells curtail chronic viral infection. Nature. 2016;537:412–428. doi: 10.1038/nature19317. [DOI] [PubMed] [Google Scholar]
- 30.Mylvaganam GH, Rios D, Abdelaal HM, Iyer S, Tharp G, Mavinger M, Hicks S, Chahroudi A, Ahmed R, Bosinger SE, Williams R, Skinner PJ, Velu V, Amara RR. Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection. Proc Natl Acad Sci U S A. 2017;114:1976–1981. doi: 10.1073/pnas.1621418114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrovas C, Ferrando-Martinez S, Gerner MY, Casazza JP, Pegu A, Deleage C, Cooper A, Hataye J, Andrews S, Ambrozak D, Del Rio Estrada PM, Boritz E, Paris R, Moysi E, Boswell KL, Ruiz-Mateos E, Vagios I, Leal M, Ablanedo-Terrazas Y, Rivero A, Gonzalez-Hernandez LA, McDermott AB, Moir S, Reyes-Teran G, Docobo F, Pantaleo G, Douek DC, Betts MR, Estes JD, Germain RN, Mascola JR, Koup RA. Follicular CD8 T cells accumulate in HIV infection and can kill infected cells in vitro via bispecific antibodies. Sci Transl Med. 2017:9. doi: 10.1126/scitranslmed.aag2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuero I, Mohanram V, Musich T, Miller L, Vargas-Inchaustegui DA, Demberg T, Venzon D, Kalisz I, Kalyanaraman VS, Pal R, Ferrari MG, LaBranche C, Montefiori DC, Rao M, Vaccari M, Franchini G, Barnett SW, Robert-Guroff M. Mucosal B Cells Are Associated with Delayed SIV Acquisition in Vaccinated Female but Not Male Rhesus Macaques Following SIVmac251 Rectal Challenge. PLoS Pathog. 2015;11:e1005101. doi: 10.1371/journal.ppat.1005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vargas-Inchaustegui DA, Helmold Hait S, Chung HK, Narola J, Hoang T, Robert-Guroff M. Phenotypic and functional characterization of circulatory, splenic, and hepatic NK cells in simian immunodeficiency virus-controlling macaques. J Immunol. 2017;199:3202–3211. doi: 10.4049/jimmunol.1700586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vargas-Inchaustegui DA, Ying O, Demberg T, Robert-Guroff M. Evaluation of Functional NK Cell Responses in Vaccinated and SIV-Infected Rhesus Macaques. Front Immunol. 2016;7:340. doi: 10.3389/fimmu.2016.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vargas-Inchaustegui DA, Demers A, Shaw JM, Kang G, Ball D, Tuero I, Musich T, Mohanram V, Demberg T, Karpova TS, Li Q, Robert-Guroff M. Vaccine Induction of Lymph Node-Resident Simian Immunodeficiency Virus Env-Specific T Follicular Helper Cells in Rhesus Macaques. J Immunol. 2016;196:1700–1710. doi: 10.4049/jimmunol.1502137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sommer C, Straehle C, Koethe U, Hamprecht FA. ilastik: Interactive Learning and Segmentation Toolkit, abstr 8th IEEE International Symposium on Biomedical Imaging (ISBI 2011); 2011/3/30; IEEE; 2011. [Google Scholar]
- 38.Hardtke S, Ohl L, Forster R. Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node folicles and is essential for efficient B-cell help. Blood. 2005;106:1924–1931. doi: 10.1182/blood-2004-11-4494. [DOI] [PubMed] [Google Scholar]
- 39.Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007;179:5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- 40.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 43.Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, Flanders MD, Cutler S, Yudanin N, Muller MI, Davis I, Farber D, Hartjen P, Haag F, Alter G, Schulze zur Wiesch J, Streeck H. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest. 2012;122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, Connick E, Meditz A, Freeman GJ, Abesada-Terk G, Jr, Jacobson JM, Brooks AD, Crotty S, Estes JD, Pantaleo G, Lederman MM, Haddad EK. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med. 2013;19:494–499. doi: 10.1038/nm.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol. 2004;78:2454–2459. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chevalier MF, Weiss L. The split personality of regulatory T cells in HIV infection. Blood. 2013;121:29–37. doi: 10.1182/blood-2012-07-409755. [DOI] [PubMed] [Google Scholar]
- 47.Simonetta F, Bourgeois C. CD4+FOXP3+ Regulatory T-Cell Subsets in Human Immunodeficiency Virus Infection. Front Immunol. 2013;4:215. doi: 10.3389/fimmu.2013.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eggena MP, Barugahare B, Jones N, Okello M, Mutalya S, Kityo C, Mugyenyi P, Cao H. Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol. 2005;174:4407–4414. doi: 10.4049/jimmunol.174.7.4407. [DOI] [PubMed] [Google Scholar]
- 49.Prendergast A, Prado JG, Kang YH, Chen F, Riddell LA, Luzzi G, Goulder P, Klenerman P. HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. AIDS. 2010;24:491–502. doi: 10.1097/QAD.0b013e3283344895. [DOI] [PubMed] [Google Scholar]
- 50.Nilsson J, Boasso A, Velilla PA, Zhang R, Vaccari M, Franchini G, Shearer GM, Andersson J, Chougnet C. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108:3808–3817. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaw JM, Hunt PW, Critchfield JW, McConnell DH, Garcia JC, Pollard RB, Somsouk M, Deeks SG, Shacklett BL. Increased frequency of regulatory T cells accompanies increased immune activation in rectal mucosae of HIV-positive noncontrollers. J Virol. 2011;85:11422–11434. doi: 10.1128/JVI.05608-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mylvaganam GH, Velu V, Hong JJ, Sadagopal S, Kwa S, Basu R, Lawson B, Villinger F, Amara RR. Diminished viral control during simian immunodeficiency virus infection is associated with aberrant PD-1hi CD4 T cell enrichment in the lymphoid follicles of the rectal mucosa. J Immunol. 2014;193:4527–4536. doi: 10.4049/jimmunol.1401222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 54.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, Ahmed R, Amara RR. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–9258. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 57.Speiser DE, Utzschneider DT, Oberle SG, Munz C, Romero P, Zehn D. T cell differentiation in chronic infection and cancer: functional adaptation or exhaustion? Nat Rev Immunol. 2014;14:768–774. doi: 10.1038/nri3740. [DOI] [PubMed] [Google Scholar]
- 58.Hong JJ, Amancha PK, Rogers K, Ansari AA, Villinger F. Re-evaluation of PD-1 expression by T cells as a marker for immune exhaustion during SIV infection. PLoS One. 2013;8:e60186. doi: 10.1371/journal.pone.0060186. [DOI] [PMC free article] [PubMed] [Google Scholar]; Li, et al. J Virol. 90:11168. doi: 10.1128/JVI.01332-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li S, Folkvord JM, Rakasz EG, Abdelaal HM, Wagstaff KR, Kovacs J, Kim HO, Sawahata R, MaWhinner S, Masopust D, Connick E, Skinner PJ. Simian immunodeficiency virus-producing cells in follicles are partially suppressed by CD8+ cells in vivo. J Virol. 2016;90:11168–11180. doi: 10.1128/JVI.01332-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miles B, Miller SM, Folkvord JM, Kimball A, Chamanian M, Meditz AL, Arends T, McCarter MHD, Levy DN, Rakasz EG, Skinner PJ, Connick E. Follicular regulatory T cells impair follicular T helper cells in HIV and SIV infection. Nat Commun. 2015;6:8608. doi: 10.1038/ncomms9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park HJ, Park JS, Jeong YH, Son J, Ban YH, Lee BH, Chen L, Chang J, Chung DH, Choi I, Ha SJ. PD-1 upregulated on regulatory T cells during chronic virus infection enhances the suppression of CD8+ T cell immune response via the interaction with PD-L1 expressed on CD8+ T cells. J Immunol. 2015;194:5801–5811. doi: 10.4049/jimmunol.1401936. [DOI] [PubMed] [Google Scholar]
- 62.Miles B, Miller SM, Folkvord JM, Levy DN, Rakasz EG, Skinner PJ, Connick E. Follicular Regulatory CD8 T cells impair the germinal center response in SIV and ex vivo HIV infection. PLoS Pathog. 2016;12:e1005924. doi: 10.1371/journal.ppat.1005924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Velu V, Styles T, Reddy PBJ, Hicks S, Gangadhara S, Amara RR. Induction of CXCR5+ follicular CD8 T cells by CD40L adjuvanted DNA/MVA vaccination is associated with enhanced control of pathogenic SHIV infection. J Immunol. 2017;198(1 Suppl):73, 14. [Google Scholar]