Abstract
Screening for asymptomatic peripheral artery disease (aPAD) with the ankle-brachial index (ABI) test is hypothesized to reduce disease progression and cardiovascular (CV) events by identifying individuals who may benefit from early initiation of medical therapy. Using a Markov model, we evaluated the cost-effectiveness of initiating medical therapy (e.g. statin & ACE-inhibitor) after a positive ABI screen in 65-years old patients. We modeled progression to symptomatic PAD (sPAD) and CV events with and without ABI screening evaluating differences in costs and quality adjusted life years (QALYs). The cost of the ABI test, physician visit, new medication, CV events, and interventions for sPAD were incorporated in the model. We performed sensitivity analysis on model variables with uncertainty. Our model found an incremental cost of $338 and incremental QALY of 0.00380 with one-time ABI screening resulting in an incremental cost-effectiveness ratio (ICER) of $88,758/QALY over a 35-year period. The variables with the largest effects in the ICER were aPAD disease prevalence, cost of monthly medication after a positive screen and 2-year medication adherence rates. Screening high-risk populations, such as tobacco users where the prevalence of PAD may be 2.5× higher, decreases the ICER to $24,092/QALY. Our analysis indicates the cost effectiveness of one-time screening for aPAD depends on prevalence, medication costs, and adherence to therapies for CV disease risk reduction. Screening in higher-risk populations under favorable assumptions about medication adherence results in the most favorable cost effectiveness, but limitations in the primary data preclude definitive assessment of cost effectiveness.
I. Introduction
Approximately half of the patients with peripheral artery disease (PAD) do not report symptoms.1-2 Population screening to identify asymptomatic PAD (aPAD) with the ankle-brachial-index (ABI) test has been hypothesized to reduce cardiovascular (CV) related events and delay the progression of aPAD to symptomatic PAD (sPAD) with the initiation of medical therapy and lifestyle changes.3-6 The ABI tests can diagnose PAD (index < 0.9) with high sensitivity (75%) and specificity (90%) in patients with symptomatic disease.7-8 The ABI test is relatively inexpensive and poses minimal risk to the patient.9
Professional societies have varying recommendations regarding appropriate use of PAD screening with the ABI test (See Table 1) as evidence supporting population based screening for aPAD with the ABI test is limited.10-15 A previous cost-effective analysis advocated screening for aPAD as it may be cost effective by initiating anti-platelet therapy as a result of a positive screen16; however, no randomized control trials have been conducted to show that aPAD screening alone will reduce CV related events and mortality.17 In 2006, the United States Preventive Services Task Force (USPSTF) recommended against ABI screening as the potential for unnecessary angiograms would outweigh the benefits.18 Screening for aPAD was re-evaluated in 2013 by the USPSTF, considering risk factor modification with a positive ABI test, and the overall recommendation changed from “against” to indeterminate.10 This study aims to evaluate the cost-effectiveness of implementing ABI screening in asymptomatic adults who may benefit from initiation of medical therapy and lifestyle changes.
Table 1.
ABI Screening Recommendations from Societal Guidelines
| Society | Recommendation | Year | Ref |
|---|---|---|---|
| US Preventive Services Task Force | Insufficient evidence that screening for PAD leads to clinically important benefits. Risk reduction interventions are recommended for high-risk individuals with known CVD or diabetes. | 2013 | 10 |
| American College of Cardiology / American Heart Association | In patients at increased risk of PAD but without history or physical examination findings suggestive of PAD, measurement of the resting ABI is reasonable. (Class IIa) | 2016/17 | 11 |
| American College of Preventive Medicine | No routine screening is recommended; clinicians should be alert to symptoms of PAD in patients with risk factors (age≥50, smoking history, diabetes) | 2011 | 12 |
| American Diabetes Association | Screen patients with diabetes (symptomatic or asymptomatic and > 50 years old or have at least one other risk factor (smoking, hypertension, hyperlipidemia, diabetes > 10 years) | 2015 | 13 |
| European Society of Cardiology | Consider screening in patients with coronary artery disease | 2011 | 14 |
| Society of Vascular Surgery | Screening reasonable if used to improve risk stratification, preventive care, and medical management in asymptomatic patients at increased risk (adults > 70, smokers, diabetes, abdominal pulse examination or cardiovascular disease) | 2015 | 15 |
II. Methods
This cost-effectiveness analysis uses a decision tree and Markov model to predict the costs and health outcomes of aPAD screening with the ABI test. The model tracks costs in 2017 U.S. dollars from the healthcare sector perspective, and evaluates health outcomes in quality-adjusted life years (QALYs).19 All costs are discounted by 3% per year. Modeling was performed with TreeAge software (Williamstown, MA). Published literature, including meta-analyses when available, were used for input values into our model. If specific values were not available for aPAD alone, values based on sPAD were used from the literature. Variables with uncertainty underwent sensitivity analysis to include the range of uncertainty.
Markov model
The overall model compares patients screened with the ABI test to patients without screening to determine differential costs and QALYs. The Markov model incorporates the natural history of PAD from the healthy state to aPAD followed by sPAD, surgical intervention, amputation, and death (See Figure 1). Patients with aPAD, sPAD, and amputation can progress to CV related events modeled as acute myocardial infarction (MI) and acute stroke. All patients progress to death according to age-adjusted20 and disease-related rates (see Table 2). The benefits of aPAD screening are accounted for a decrease in progression to sPAD from aPAD and lower rates of acute stroke and MI due to medication initiation and lifestyle changes after a positive ABI test.
Figure 1.
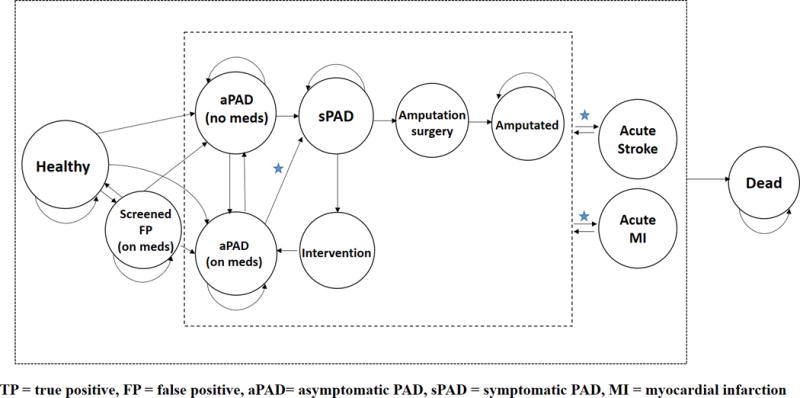
Markov model states and transitions. The cost-effectiveness of the ABI test is performed by comparing ABI screening to no ABI screening. This captures the differences in cost and QALY in patients with aPAD not on medication “aPAD (no meds)” to patients with aPAD and assigned medication “aPAD (on meds)” after a positive ABI screening. The benefits of ABI screening are noted by stars, where there is a slower progression to sPAD and lower incident rate of acute stroke and MI due to medication initiation and lifestyle changes after a positive ABI test. See Methods section for further details.
Table 2.
Markov Model - Baseline Values
| Utilitiy score (age dependent)21 | Value | Source |
|---|---|---|
| Healthy state | 1.0 | – |
| Screened FP state | 1.0 | – |
| Screened TP state (aPAD on meds) | 0.9 | † |
| aPAD state no meds | 0.85 | † |
| sPAD state | 0.79 | † |
| Intervention state | 0.7 | † |
| Amputated state | 0.68 | 22 |
| Dead state | 0.0 | – |
| Acute MI – first month | 0.60 | 23 |
| Acute MI – one year | 0.73 | 25 |
| Acute Stroke – first month | 0.64 | 24 |
| Acute stroke – one year | 0.70 | 24 |
| 2017 Costs (Adjusted for 3% discount/ year) | Cost | Source |
|---|---|---|
| Cost in healthy state | $0.0 | – |
| Cost of doctor visit* | $200 ($50-250) | 26 |
| Monthly cost of medication* | $11.12 ($2-80) | 27 |
| Monthly cost in screened FP state | $11.12 | 27 |
| Monthly cost in aPAD state | $459.70 | 28 |
| Monthly cost in screened TP state (aPAD + meds) | $470.82 | 27, 28 |
| Monthly cost in sPAD state | $1004.80 | 28 |
| Yearly cost in sPAD surgery state | $25,670.57 | 36 |
| Yearly cost in amputation surgery state | $80,162.93 | 37 |
| Cost in dead state | $0.0 | – |
| Cost of screening (ABI test)* | $100 ($20-300) | 9 |
| Cost of Acute MI – first month | $25,485.59 | 42 |
| Cost of Acute MI – one year | $23,540.29 | 42 |
| Cost of Acute Stroke – first month | $18,984.58 | 44 |
| Cost of Acute Stroke – one year | $21,409.06 | 45 |
| Transition Probabilities | Definition/ Values | Source |
|---|---|---|
| Proportion aPAD population already on medication* | 96% (5-99%) | 29-32 |
| Probability of quitting medication* | 40% in first 2 years, then stable thereafter (28-88%) | 33 |
| Progression probability from aPAD to sPAD | 7% in 5 years | 34 |
| sPAD to amputation surgery | 5% in 5 years | 34 |
| sPAD to endovascular surgery | 21% in 5 years | 34 |
| Relative decrease in probability of progressing from aPAD to sPAD if on medication/ lifestyle changes* | 25% (10-90%) | 35 |
| Healthy to death | Based on age | 20 |
| Amputated to death | 71% mortality in the first 3 years, then sPAD death rate thereafter. | 38 |
| Endovascular surgery to death | 0.5% | 39 |
| Amputation surgery to death | 13.5% | 40 |
| Acute MI to Death – first month | 15.3% | 42 |
| Acute MI to Death – one year | 20.2% | 42 |
| Acute Stroke to Death – first month | 14.1% | 43 |
| Acute Stroke to Death – one year | 31.1% | 43 |
| aPAD/ sPAD to MI | 6.8% - 1 year | 41 |
| aPAD/ sPAD to Stroke | 6% - 1 year | 41 |
| Amputation to MI | 5% - 1 year | 41 |
| Amputation to Stroke | 4.3% - 1 year | 41 |
| Relative reduction of MI with medication* | 0.5 (0.15-85) | 46-50 |
| Relative decrease of stroke with medication* | 0.5 (0.15-0.85) | 46-50 |
| Other Variables | Definition/ Values | Source |
|---|---|---|
| ABI test Sensitivity* | 75% (60-100%) | 7,8 |
| ABI Test Specificity* | 90% (85- 100%) | 7,8 |
| Start age* | 65 (45-65) | – |
| Start prevalence of PAD* | 9% (5-40%) | 1 |
FP – false positive, TP – True positive, aPAD – asymptomatic PAD, sPAD symptomatic PAD, MI – myocardial infarction
Subject to sensitivity analysis: base case (range)
extrapolated from healthy state to amputation state
–assumption from base-case model
The healthy and death states have a QALY of 1.0 0, respectively and are adjusted according to the patient’s age-adjusted QALY: at age 35, 45, 55, 65, 75 the QALY is 0.8, 0.8, 0.78, 0.78, 0.76, respectively.21 The QALY assigned to aPAD, sPAD, or amputated state is extrapolated from the amputated state of 0.68.22 Patients who experience an acute MI or stroke also have reduced QALYs at one-month: 0.60 and 0.64 respectively.23,24 After 1-year the QALY for acute MI improves to 0.73 and 0.70 for stroke.24,25 Each cycle in the model is one month and the effects of aPAD screening are analyzed up to 35 years.
Patient population
We set our base-case model to screen patients in the U.S. population at 65 years of age. The model considers age ranges above and below 65 years old incorporating the age-related mortality and QALYs.20-21 Prevalence of PAD at specific ages are included in the model: at age 45, 55, 65, 75, 85 the prevalence is 2%, 4%, 9%, 18%, 30%, respectively.1 High risk populations, e.g. tobacco users, are considered in the model by increasing the initial prevalence rate of the population screened.
PAD Screening
The model assumes ABI screening in a primary care setting, where a positive test triggers medicine and lifestyle intervention. We set the base-case cost of the ABI test in the outpatient setting at $100 and include a sensitivity analysis for this cost.9 The costs of accompanying physician visits are also included in the model set at a base case of $200.26 We set the base-case sensitivity and specificity of the ABI test in detecting aPAD at 75% and 90%, respectively, and perform sensitivity analyses of these values as the sensitivity and specificity of the ABI test is not well established for aPAD.7, 8
Treatment Strategies after a Positive Screening Result
A positive ABI test assigns statin and ACE-inhibitor medication as well as lifestyle modification counseling with the goal of improving both CV and PAD-related outcomes. Patients with a positive ABI test are noted as “aPAD (on meds)” in Figure 1. The monthly costs of starting an ACE-inhibitor and statin are $11.12, based on Veterans Affair pharmaceutical prices and dispensing fees.27 The cost of medications undergo sensitivity analysis given the variation in drug costs. The monthly cost of aPAD care without medications is $459.70 and $470.82 after medication costs are added with a positive ABI test.28 Patients in the aPAD group may already be on a statin therapy and/or ACE-inhibitor therapy due to other comorbidities and we set the baseline of patients already on medication at 96%.29-32 This proportion of the population already eligible for similar medical therapy is considered in a one-way sensitivity analysis given eligible patients may not be taking medication due to patient non-compliance or varying prescribing patterns. Long-term medication non-compliance after a positive ABI test is also incorporated into the model based on a 2-year non-compliance rate of 40%, which also undergoes sensitivity analysis.33 Patients who are non-compliant with medical therapy transition back to the “aPAD (no meds)” state (See Figure 1). Patients can also be in a false positive state “Screened FP (on meds)” with a misdiagnosis from the initial ABI test.
Transitions
The starting healthy state, which can progress to aPAD according to age-based prevalence. Patients with aPAD progress to sPAD over time (7% over 5 years) based on the natural history of disease.34 This progression rate is reduced with medication initiation and lifestyle modification, set at 25% reduction over 5 years.35 Patients with sPAD can undergo surgical intervention to return to the aPAD state on medications, with an associated yearly cost of $25,670.57.36 The sPAD state can also progress to the amputated state, which carries higher mortality and costs ($80,162.93/year).37,38 Peri-operative mortality is considered when patients undergo a surgical intervention for sPAD and amputation.39, 40
The transition from aPAD, sPAD, and amputated states to CV events (i.e. acute MI and stroke) are based on yearly incident rates.41 Patients who progress to acute MI and stroke have associated 30-day and 1-year mortality.42,43 If patients do not progress to death after an MI or stroke, they then return to their original state (e.g. aPAD, sPAD, or amputation), and can have a recurrence of an acute MI or stroke according to yearly incident rates. The associated first month and yearly cost of MI and stroke are noted in Table 2.42,44,45 The reduction of CV events and subsequent mortality is reduced by 50% with initiation of medication and lifestyle changes, and is subject to sensitivity analysis.46-50 All healthy and diseased states in the model can progress to death. For further details regarding transitions see Table 2 and Supplemental Table 1.
Sensitivity Analysis
Model variables with uncertainty underwent sensitivity analysis as noted in Table 2. To determine variables that influence the ICER the most, we conducted one-way sensitivity analyses for each variable of interest given uncertainties in the literature.
III. Results
The base case model for screening the general population costs $338 more compared to no screening. The increased cost is due to the ABI test, physician visit, and cost of medication for a positive ABI test. There is a mild offset in the overall costs due to slower progression of disease for screened patients, who require less surgical intervention and amputation. The screened patients also have an increase of QALYs of 0.00380, or 1.39 days, compared to no screening. The overall ICER is $88,758/QALY demonstrating that aPAD screening increases the overall QALY with an associated increase in costs, see Figure 2. Removing the benefits of medication effects on MI and stroke increases the ICER to $309,065 (248% increase) and removing the benefits of medication on PAD progression increases the ICER to $93,309 (5.1% increase).
Figure 2.
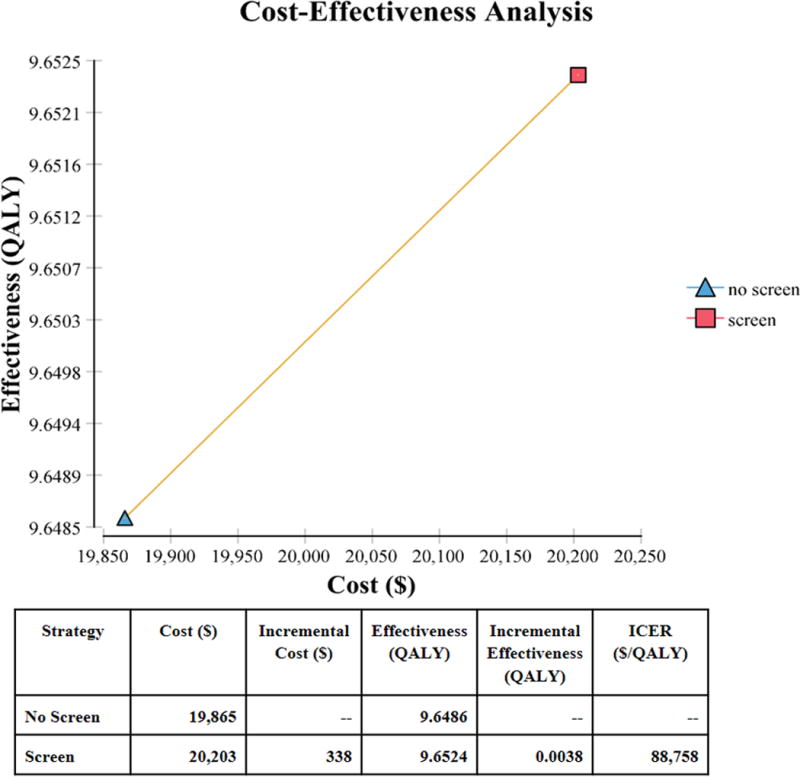
Cost-effectiveness frontier comparing screening and no screening with the ABI test by costs and QALY (Quality Adjusted Life Years). ICER – Incremental Cost-Effectiveness Ratio
Our sensitivity analyses of variables with uncertainty are shown in Figure 3. The top three variables affecting the ICER are starting prevalence of the population being screened, monthly cost medication after a positive ABI screen, and medication adherence rates. One way sensitivities for these variables are shown in Figure 4. The variables that decrease the ICER below $50,000/ QALY is the starting prevalence, medication adherence, and percentage of patients already eligible for medical therapy. Screening high-risk populations, such as tobacco users, where the prevalence of PAD is predicted to be 23%, decreases the ICER to $24,092/QALY, a 73% decrease from the base case (Figure 4a), with favorable assumptions about adherence to risk-reducing medications.
Figure 3.
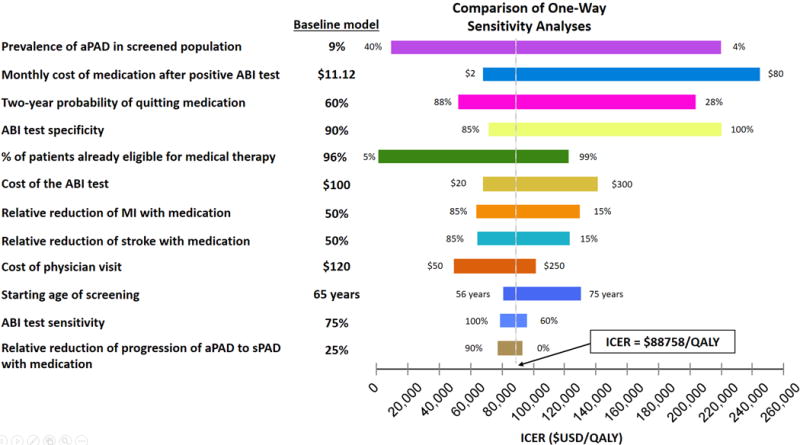
One-way sensitivity analysis for input variables with uncertainties. The variables are arranged from top to bottom starting with the largest range of ICER depending on the uncertainty range. The baseline value for each variable is noted by the vertical line indicating the ICER of $88,758/QALY. aPAD – asymptomatic PAD, sPAD – symptomatic PAD, MI – myocardial infarction.
Figure 4.
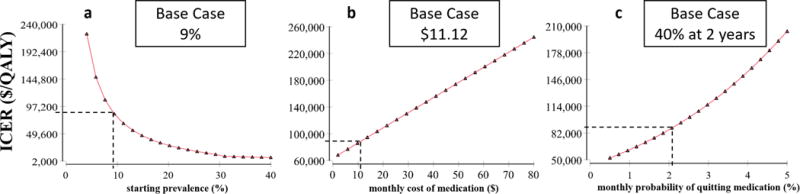
Sensitivity analysis for the top three variables affecting the ICER of aPAD screening with the ABI test: a. starting prevalence, b. monthly cost of medications, c. monthly probability of quitting medication.
When the screened population already on medical therapy is reduced to 70% from the base model of 96% the ICER decreases to $20,687/QALY (77% decrease, see Supplemental Figure 1b). The optimal age for initial aPAD screening was found between 55 and 65 years-old (See Supplemental Figure 1f). The initial decrease in ICER from 45 to 55 years of age is attributed to an increasing prevalence of disease, while the increasing ICER after 65 years of age is due to the increasing all-cause mortality with age. Other variables affecting the ICER are shown with accompanying one-way sensitivity analyses in Supplemental Figure 1.
IV. Discussion
Our base case analysis found benefit from a one-time ABI screening to detect aPAD and initiate early medical therapy, reducing CV events and mitigate progression of aPAD to sPAD. With favorable assumptions about the effect of screening on the use of CV risk reducing medications, the ICER was $88,758 per QALY with ABI screening compared to no screening over a 35 year period, which is between the previously reported societal willingness to pay threshold of $50,000 and $100,000.51 Our results are similar to previous studies showing benefit from initiation of medication therapy in patients with aPAD.16,48 However, our model found a slightly higher ICER compared to these studies due to a variety of factors discussed below. As we also discuss below, empirical evidence regarding the effect of ABI screening on risk-reducing medication remains sparse, suggesting caution in interpreting our findings.
Effects of prevalence, high risk populations, and age
The cost-effectiveness for aPAD screening improves when there is a higher prevalence of disease in the screened population (See Figure 4a). Screening high-risk populations, such as tobacco users where the prevalence of PAD may be 2.5× higher than the general population, decreases the ICER to $24,092/QALY (See Table 1). Currently, the ABI test is not reimbursed in patients without symptoms. This favorable ICER shows ABI testing will be most cost effective in high risk populations. Regarding age, our model found the highest return for screening when the patient is 55-66 years old (See Supplemental Figure 1f). Although an older population has a higher prevalence of PAD, higher all-cause mortality diminishes the benefits of population screening at older ages.
Medication effects after a positive ABI test
We note that similar to AAA screening, which may lead to a reduction in both disease and all-cause mortality,52,53 the focus of aPAD screening should lead to risk modifications to decrease CV related events and slow progression to sPAD. The medication cost after a positive ABI screen is relatively inexpensive and is noted at $11.12 per month in our base-case scenario, as statin and ACE-inhibitor prices have decreased with generic production.27 At this low cost, we observe a benefit in ABI screening; however, small increases in medication costs can make ABI screening less cost-effective (See Figure 4b). We also note that prescribers may vary in choosing the cheapest medication, thus reducing the cost-effectiveness of aPAD screening.54
Medication non-adherence for chronic disease can cost the health care system $100 billion annually and it is unknown how a patient would increase adherence to medication and lifestyle recommendations with a positive ABI.55 Previous reports have shown that statin adherence is only 40% at two years even with financial incentives to patients and providers to reach LDL goals.56 We speculate that medication adherence rates improves with screening as a diagnosis of PAD provides evidence of atherosclerosis disease. Patients with coronary artery disease (CAD) have higher compliance rates for statin medication when there is symptomatic disease compared to asymptomatic disease.33 We also note that diagnosing PAD may help with risk stratification in CAD patients and support the use of more aggressive lipid goals similar to proposed adjunct testing with carotid ultrasound and coronary calcium scoring.57,58
A critique of PAD screening is that many patients who are eligible for screening may already be on statins, ACE-inhibitors, and anti-platelets for general management of their CV risk factors per current primary care guidelines and thus not benefit from screening. We considered 96% of patients already eligible for therapy in our model as this reflected previous population studies.29-32 Although 4-5% of patients eligible for new medication may seem small this can account for 4.9 million US adults with PAD without established CVD.32 Furthermore, we note that medication adherence is imperfect as seen in real world data regarding patients who are eligible for cardio-protective medicine.59 Therefore the ICER may be lower if the screened population’s eligibility for therapy is below our baseline model of 96% (See Supplemental Figure 1b).
The long-term effects of medication on aPAD patients encompass one of the larger uncertainties in the predicted ICER, including the hazard ratio for CV related events in patients with aPAD. Randomized control trials have not shown a benefit in antiplatelet regimen in primary prevention of CV-related events,60 but statins and ACE-inhibitors provide improvements to CV related mortality in aPAD patients.49,50 Our baseline model incorporates a 50% reduction in overall CV related events as this includes synergistic medication effects and possible lifestyle changes with a positive screen. Previous studies have also demonstrated favorable outcomes with lifestyle changes such as diet modification and exercise after CAD diagnosis.61
Limitations of the ABI test
The ABI test carries a wide range of uncertainty and limitations. Cost-effectiveness of screening is limited by the specificity and sensitivity of the ABI test (Supplemental Figure 1a., 1h.). More expensive diagnostic tests such as duplex ultrasound, CT angiography, and MRI angiography provide higher diagnostic capabilities assessing vascular flow and morphology but are associated with higher costs, risk and time. Although we do not consider these diagnostic imaging modalities in our model, as these tests are usually reserved for patients with symptoms or non-healing wounds, we note that increasing the cost of the ABI test from $100 to $300 in our model increases the ICER to $141,309 (see Supplemental Figure 1c). Therefore costly follow-up tests beyond the ABI test (e.g. CT scan which may costs thousands of dollars) need to be avoided in routine practice to make aPAD screening cost-effective.
Although the ABI test has high accuracy with an ROC area of 0.87 in symptomatic patients, there is far less data in asymptomatic patients and there are patients for whom screening may more commonly lead to inaccurate results, such as diabetic patients and those with pretibial calcification.7 A previous study found that toe-brachial indices and skin perfusion may be more cost-effective in diagnosing PAD in diabetic patients with foot wounds.62 Currently, new and easy-to-use technology is being evaluated to asses flow to the lower extremities that has the potential to more accurately detect PAD with high ease of use.63 We emphasize that introduction of new technology must be evaluated for its sensitivity, specificity, and cost to determine the cost-effectiveness in future analyses. We also note that a lower specificity leads to a lower ICER for aPAD screening (Supplemental Figure 1h). This is explained by an advantage in false positive patients benefiting from early statin therapy64-65 as well as disease prevalence increasing with age.
Limitations and barriers to implementing ABI screening
It is unclear what the direct and indirect costs would be from implementing aPAD screening in a primary care clinic. Given the increasing logistical demands placed on primary care physicians, it is of utmost importance that further evaluation of PAD screening evaluates the feasibility and cost of implementing and interpreting the ABI test. For example, examining how will patient counseling and medication adherence will be affected with a positive ABI screen.66 Furthermore, other studies are needed to determine when it is appropriate for primary care providers to refer to a specialist for a positive ABI test in the asymptomatic patient to balance “unnecessary” revascularization procedures or supervised exercise therapy instead.18,67
We made simplifying assumptions in our analysis. First, we used sPAD literature values when aPAD values were unavailable, such as the true benefits of medication initiation in patients with aPAD and prevalence of asymptomatic disease. To address this limitation we performed sensitivity analyses, acknowledging that the aPAD population is difficult to identify without prior screening and observational studies of aPAD are limited. Second, we did not differentiate between an open or endovascular approach to treat of sPAD. As the level of disease (e.g. aorto-iliac vs. femoral and tibal disease) and patient co-morbidities vary we considered the average cost of intervention over the course of 1 year after an intervention is pursued. We also interpolated QALYs given the lack of evidence and variability of disease states and patient demographics.68 Furthermore, it is possible that aPAD patients may have worse functional status than patients with sPAD, highlighting the variation in patient complexity and severity of asymptomatic disease.69 Combining the paucity of studies and uncertainty in the variables outlined above, further investigation is required prior to implementing large scale aPAD screening.
Conclusions
Our analysis demonstrates that cost effectiveness of screening for aPAD with the ABI test depends on the prevalence of disease in the initial population being screened, monthly medication costs, and adherence to medical therapies for CV risk reduction. The lack of high-quality data leads to an indeterminate conclusion on whether aPAD screening with the ABI test is truly below the generally accepted thresholds for cost effectiveness. Further studies are warranted to not only understand the cost-effectiveness of aPAD screening, but also to improve overall PAD and CV related health outcomes.
Supplementary Material
Supplemental Figure 1. Remaining one-way sensitivity analysis for variables with uncertainty affecting the ICER (see Figure 3). Note for Figure 1g. the U-shape above between 55 and 65 years old is due to interpolation of the prevalence curve.
Supplemental Table 1. Additional details of transitions in Markov Model.
Acknowledgments
This work was supported by a National Institutes of Health, National Center for Advancing Translational Science, Clinical and Translational Science Award (TL1 TR000084) and NIH / NHLBI (5T3HL098049). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
COI disclosure:
Douglas K. Owens is a member of the United States Preventive Service Task Force (USPSTF). This article does not necessarily represent the views and policies of the USPSTF.
References
- 1.Criqui MH, Aboyans V. Epidemiology of Peripheral Artery Disease. Circulation Research. 2015;116:1509–1526. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 2.McDermott M, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 3.Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, et al. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: a systematic review and meta-analysis. Hypertension. 2012 Aug;60(2):556–62. doi: 10.1161/HYPERTENSIONAHA.112.194779. [DOI] [PubMed] [Google Scholar]
- 4.Smith SC, Benjamin EJ, Bonow RO, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011 Nov;124(22):2458–73. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 5.Ramos R, García-Gil M, Comas-Cufí M, et al. Statins for Prevention of Cardiovascular Events in a Low-Risk Population With Low Ankle Brachial Index. J Am Coll Cardiol. 2016 Feb 16;67(6):630–40. doi: 10.1016/j.jacc.2015.11.052. [DOI] [PubMed] [Google Scholar]
- 6.Hankey GJ, Norman PE, Eikelboom JW. Medical treatment of peripheral arterial disease. JAMA. 2006 Feb 1;295(5):547–53. doi: 10.1001/jama.295.5.547. [DOI] [PubMed] [Google Scholar]
- 7.Alahdab F, Wang AT, Elraiyah TA, et al. A systematic review for the screening for peripheral arterial disease in asymptomatic patients. J Vasc Surg. 2015 Mar;61(3 Suppl):42S–53S. doi: 10.1016/j.jvs.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Xu D, Zou L, Xing Y, et al. Diagnostic value of ankle-brachial index in peripheral arterial disease: a meta-analysis. Can J Cardiol. 2013;29:492–8. doi: 10.1016/j.cjca.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 9.2016 Medicare Parkt B Fee Schedule (CPT code 93922) http://www.wallachsurgical.com/site/assets/files/2834/updated_2016_summit_doppler_2016_pfs_and_icd-10_final.pdf (Accessed 12 October 2016)
- 10.Moyer VA, U.S. Preventive Services Task Force Screening for peripheral artery disease and cardiovascular disease risk assessment with the ankle-brachial index in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013 Sep 3;159(5):342–8. doi: 10.7326/0003-4819-159-5-201309030-00008. [DOI] [PubMed] [Google Scholar]
- 11.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017 Mar 21;69(11):1465–1508. doi: 10.1016/j.jacc.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Lim LS, Haq N, Mahmood S, et al. ACPM Prevention Practice Committee; American College of Preventive Medicine Atherosclerotic cardiovascular disease screening in adults: American College Of Preventive Medicine position statement on preventive practice. Am J Prev Med. 2011 Mar;40(3):381.e1–10. doi: 10.1016/j.amepre.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Peripheral arterial disease in people with diabetes. Clin Diabetes. 2004;22(4):181–189. [Google Scholar]
- 14.Tendera M, Aboyans V, Bartelink ML, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries * The task force on the diagnosis and treatment of peripheral artery diseases of the European society of cardiology (ESC) Eur Heart J. 2011;32(22):2851–2906. doi: 10.1093/eurheartj/ehr211. [DOI] [PubMed] [Google Scholar]
- 15.Society for Vascular Surgery Lower Extremity Guidelines Writing Group. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. J Vasc Surg. 2015 Mar;61(3 Suppl):2S–41S. doi: 10.1016/j.jvs.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Vaidya A, Joore MA, Ten Cate-Hoek AJ, et al. Screen or not to screen for peripheral arterial disease: guidance from a decision model. BMC Public Health. 2014 Jan 29;14:89. doi: 10.1186/1471-2458-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crawfird F, Welch K, Andras A, et al. Ankle brachial index for the diagnosis of lower limb peripheral arterial disease. Cochrane Database Syst Rev. 2016 Sep 14;9:CD010680. doi: 10.1002/14651858.CD010680.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Preventive Services Task Force. Screening for Peripheral Arterial Disease: Recommendation Statement. AHRQ Publication no. 05-0583-A-EF. Agency for Healthcare Research and Quality. 2005 [Google Scholar]
- 19.Torrance GW, Feeny D. Utilities and quality-adjusted life years. Int J Technol Assess Health Care. 1989;5(4):559–75. doi: 10.1017/s0266462300008461. [DOI] [PubMed] [Google Scholar]
- 20.2014 Actuarial Life Table. https://www.ssa.gov/oact/STATS/table4c6.html (accessed 30 October 2016)
- 21.Fryback DG, Dunham NC, Palta M, et al. US norms for six generic health-related quality-of-life indexes from the National Health Measurement study. Med Care. 2007 Dec;45(12):1162–70. doi: 10.1097/MLR.0b013e31814848f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redekop WK, Stolk EA, Kok E, et al. Diabetic foot ulcers and amputations: estimates of health utility for use in cost-effectiveness analyses of new treatments. Diabetes Metab. 2004 Dec;30(6):549–56. doi: 10.1016/s1262-3636(07)70154-4. [DOI] [PubMed] [Google Scholar]
- 23.Rancic NK, Petrovic BD, Apostolovic SR. Health-related quality of life in patients after the acute myocardial infarction. Cent Eur J Med. 2013 Apr;8(2):266–72. [Google Scholar]
- 24.Luengo-Fernandez R, Gray AM, Bull L, et al. Quality of life after TIA and stroke: ten-year results of the Oxford Vascular Study. Neurology. 2013 Oct 29;81(18):1588–95. doi: 10.1212/WNL.0b013e3182a9f45f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pilote L, Lauzon C, Huynh T, et al. Quality of life after acute myocardial infarction among patients treated at sites with and without on-site availability of angiography. Arch Intern Med. 2002 Mar 11;162(5):553–9. doi: 10.1001/archinte.162.5.553. [DOI] [PubMed] [Google Scholar]
- 26.Kaiser Permanente 2016 Sample Fee List. http://info.kaiserpermanente.org/info_assets/estimating_your_cost/PDFs/scal_fees.pdf (accessed 30 October 2016)
- 27.2016 National Acquisition Center. http://www.va.gov/nac/Pharma/List (Accessed 30 October 2016)
- 28.Mahoney EM, Wang K, Keo HH, et al. Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Circ Cardiovasc Qual Outcomes. 2010 Nov;3(6):642–51. doi: 10.1161/CIRCOUTCOMES.109.930735. [DOI] [PubMed] [Google Scholar]
- 29.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–43. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 30.Kundhal KK, Chin SL, Harrison L, et al. Patterns of medical therapy in patients with peripheral artery disease in a tertiary care centre in Canada. Can J Cardiol. 2007 Apr;23(5):357–361. doi: 10.1016/s0828-282x(07)70768-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joosten MM, Pai JK, Bertoia ML, et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012 Oct 24;308(16):1660–7. doi: 10.1001/jama.2012.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pande RL, Perlstein TS, Beckman JA, et al. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation. 2011 Jul 5;124(1):17–23. doi: 10.1161/CIRCULATIONAHA.110.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002 Jul 24-31;288(4):462–7. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 34.Sigvant B, Lundin F, Wahlberg E. The Risk of Disease Progression in Peripheral Arterial Disease is Higher than Expected: A Meta-Analysis of Mortality and Disease Progression in Peripheral Arterial Disease. Eur J Vasc Endovasc Surg. 2016 Mar;51(3):395–403. doi: 10.1016/j.ejvs.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 35.Pedersen TR, Kjekshus J, Pyorala K, et al. Effect of simvastatin on ischemic signs and symptoms in the Scandinavian Simvastatin Survival Study. Am J Cardiol. 1998;81(3):333–335. doi: 10.1016/s0002-9149(97)00904-1. [DOI] [PubMed] [Google Scholar]
- 36.Jaff MR, Cahill KE, Yu AP, et al. Clinical outcomes and medical care costs among medicare beneficiaries receiving therapy for peripheral arterial disease. Ann Vasc Surg. 2010 Jul;24(5):577–87. doi: 10.1016/j.avsg.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 37.MacKenzie EJ, Jones AS, Bosse MJ, et al. Health-care costs associated with amputation or reconstruction of a limb-threatening injury. J Bone Joint Surg Am. 2007 Aug;89(8):1685–92. doi: 10.2106/JBJS.F.01350. [DOI] [PubMed] [Google Scholar]
- 38.Kristensen MT, Holm G, Kirketerp-Møller K, et al. Very low survival rates after non-traumatic lower limb amputation in a consecutive series: what to do? Interact Cardiovasc Thorac Surg. 2012 May;14(5):543–547. doi: 10.1093/icvts/ivr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeRubertis BG, Faries PL, McKinsey JF, et al. Shifting paradigms in the treatment of lower extremity vascular disease: a report of 1000 percutaneous interventions. Annual Surgery. 2007 Sep;246(3):415–22. doi: 10.1097/SLA.0b013e31814699a2. discussion 422-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aparna S, Manesh RP, et al. Lower extremity amputation in peripheral artery disease: improving patient outcomes. Vasc Health Risk Manag. 2014;10:417–424. doi: 10.2147/VHRM.S50588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones WS, Patel MR, Dai D, Vemulapalli S, Subherwal S, Stafford J, Peterson ED. High mortality risks after major lower extremity amputation in Medicare patients with peripheral artery disease. Am Heart J. 2013 May;165(5):809–15. 815.e1. doi: 10.1016/j.ahj.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Likosky DS, Zhou W, Malenka DJ, et al. Growth in medicare expenditures for patients with acute myocardial infarction: a comparison of 1998 through 1999-2008. JAMA Intern Med. 2013 Dec 9-23;173(22):2055–61. doi: 10.1001/jamainternmed.2013.10789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fonarow GC, Smith EE, Reeves MJ, et al. Hospital-level variation in mortality and rehospitalizatino for medicare beneficiaries with acute ischemic stroke. Stroke. 2011 Jan;42(1):159–66. doi: 10.1161/STROKEAHA.110.601831. [DOI] [PubMed] [Google Scholar]
- 44.Rymer MM, Armstrong EP, Meredith NR, et al. Analysis of the costs and payments of a coordinated stroke center and regional stroke network. Stroke. 2013 Aug;44(8):2254–9. doi: 10.1161/STROKEAHA.113.001370. [DOI] [PubMed] [Google Scholar]
- 45.Godwin KM, Wasserman J, Ostwald SK. Cost associated with stroke: outpatient rehabilitative services and medication. Top Stroke Rehabil. 2011 Oct;18(Suppl 1):676–84. doi: 10.1310/tsr18s01-676. [DOI] [PubMed] [Google Scholar]
- 46.Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for Prevention of Cardiovascular Disease in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2016 Nov 15;316(19):2008–2024. doi: 10.1001/jama.2015.15629. [DOI] [PubMed] [Google Scholar]
- 47.Heart Protection Study Collaborative Group. Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg. 2007;45:645–54. doi: 10.1016/j.jvs.2006.12.054. discussion: 653-4. [DOI] [PubMed] [Google Scholar]
- 48.Signvant B, Henriksson M, Lundin F, Wahlberg E. Asymptomatic peripheral arterial disease: is pharmacological prevention of cardiovascular risk cost-effective? Eur J Cardiovasc Prev Rehabil. 2011 Apr;18(2):254–61. doi: 10.1177/1741826710389368. [DOI] [PubMed] [Google Scholar]
- 49.Vidula H, Tian L, Liu K, Criqui M, Ferrucci L, Guralnik J, et al. Comparison of effects of statin use on mortality in patients with peripheral arterial disease with versus without elevated C-reactive protein and D-dimer levels. Am J Cardiol. 2010;105:1348–52. doi: 10.1016/j.amjcard.2009.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ostergren J, Sleight P, Dagenais G, Danisa K, Bosch J, Qilong Y, et al. Impact of ramipril in patients with evidence of clinical or subclinical peripheral arterial disease. Eur Heart J. 2004;25:17–24. doi: 10.1016/j.ehj.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 51.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness – the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014 Aug 28;371(9):796–7. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 52.Lindholt JS, Norman P. Screening for abdominal aortic aneurysm reduces overall mortality in men. A meta-analysis of the mid- and long-term effects of screening for abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2008 Aug;36(2):167–71. doi: 10.1016/j.ejvs.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 53.Lindholt JS, Søgaard R. Population screening and intervention for vascular disease in Danish men (VIVA): a randomised controlled trial. Lancet. 2017 Aug 25; doi: 10.1016/S0140-6736(17)32250-X. [DOI] [PubMed] [Google Scholar]
- 54.Ryskina KL, Pesko MF, Gossey JT, et al. Brand Name Statin Prescribing in a Resident Ambulatory Practice: Implications for Teaching Cost-Conscious Medicine. J Grad Med Educ. 2014 Sep;6(3):484–8. doi: 10.4300/JGME-D-13-00412.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 56.Asch DA, Troxel AB, Stewart WF, et al. Effect of Financial Incentives to Physicians, Patients, or Both on Lipid Levels: A Randomized Clinical Trial. Jama. 314(18):1926–35. doi: 10.1001/jama.2015.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fokes FG, Murray GD, Butcher I, et al. Ankle Brachial Index Collaboration Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008 Jul 9;300(2):197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Kempen BJ, Ferket BS, Steyerberg EW, et al. Comparing the cost-effectiveness of four novel risk markers for screening asymptomatic individuals to prevent cardiovascular disease (CVD) in the US population. Int J Cardiol. 2016 Jan 15;203:422–31. doi: 10.1016/j.ijcard.2015.10.171. [DOI] [PubMed] [Google Scholar]
- 59.Kumar A, Fonarow GC, Eagle KA, et al. Regional and practice variation in adherence to guideline recommendations for secondary and primary prevention among outpatients with atherothrombosis or risk factors in the United States: a report from the REACH Registry. Crit Pathw Cardiol. 2009 Sep;8(3):104–11. doi: 10.1097/HPC.0b013e3181b8395d. [DOI] [PubMed] [Google Scholar]
- 60.Fowkes FG, Price JF, Stewart MC, et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010 Mar 3;303(9):841–8. doi: 10.1001/jama.2010.221. [DOI] [PubMed] [Google Scholar]
- 61.Cole JA, Smith SM, Hart N, et al. Systematic Review of the Effect of Diet and Exercise Lifestyle Interventions in the Secondary Prevention of Coronary Heart Disease. Cardiol Res Pract. 2011;2011:232351. doi: 10.4061/2011/232351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barshes NR, Flores E, Belkin M, et al. The accuracy and cost-effectiveness of strategies used to identify peripheral artery disease among patients with diabetic foot ulcers. J Vasc Surg. 2016 Dec;64(6):1682–1690.e3. doi: 10.1016/j.jvs.2016.04.056. [DOI] [PubMed] [Google Scholar]
- 63.King DH, Paulson WD, Al-Qaisi M, et al. Volume blood flow, static pressure ratio and venous conductance in native arterio-venous fistulae: three surveillance methods compared. J Vasc Access. 2015 May-Jun;16(3):211–7. doi: 10.5301/jva.5000324. [DOI] [PubMed] [Google Scholar]
- 64.Otto CM. Statins for primary prevention of cardiovascular disease. BMJ. 2016 Nov 24;355:i6334. doi: 10.1136/bmj.i6334. [DOI] [PubMed] [Google Scholar]
- 65.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol—lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005 Oct 8;366(9493):1267–78. doi: 10.1016/S0140-6736(05)67394-1. Epub 2005 Sep 27. [DOI] [PubMed] [Google Scholar]
- 66.Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011 Apr;86(4):304–314. doi: 10.4065/mcp.2010.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mohler ER, III, Treat-Jacobson D, Reilly MP, et al. Utility and barriers to performance of the ankle-brachial index in primary care practice. Vasc Med. 2004;9(4):253–260. doi: 10.1191/1358863x04vm559oa. [DOI] [PubMed] [Google Scholar]
- 68.Moriarty JP, Murad MH, Shah ND, et al. Society for Vascular Surgery Committee on Comparative Effectiveness A systematic review of lower extremity arterial revascularization economic analyses. J Vasc Surg. 2011 Oct;54(4):1131–1144.e1. doi: 10.1016/j.jvs.2011.04.058. [DOI] [PubMed] [Google Scholar]
- 69.McDermott M, Guralnik J, Ferrucci L, et al. Asymptomatic peripheral arterial disease is associated with more adverse lower extremity characteristics than intermittent claudication. Circulation. 2008;117:2484–91. doi: 10.1161/CIRCULATIONAHA.107.736108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Remaining one-way sensitivity analysis for variables with uncertainty affecting the ICER (see Figure 3). Note for Figure 1g. the U-shape above between 55 and 65 years old is due to interpolation of the prevalence curve.
Supplemental Table 1. Additional details of transitions in Markov Model.


