Abstract
Nonalcoholic Fatty Liver Disease (NAFLD) is the hepatic manifestation of metabolic syndrome, and its rising prevalence parallels the rise in obesity and diabetes. Historically thought to result from overnutrition and sedentary lifestyle, recent evidence suggests that diets high in sugar (from sucrose and/or high fructose corn syrup (HFCS)) not only increases the risk for NAFLD, but also, nonalcoholic steatohepatitis (NASH). Here we review the experimental and clinical evidence that fructose precipitates fat accumulation in the liver, due to both increased lipogenesis and impaired fat oxidation. Recent evidence suggests that the predisposition to fatty liver is linked with metabolism of fructose by fructokinase C, resulting in ATP consumption, nucleotide turnover and uric acid generation that mediate fat accumulation. Alterations in gut permeability, microbiome, and associated endotoxemia contributes to the risk of NAFLD and NASH. Early clinical studies suggest that reducing sugary beverages and total fructose intake, especially from added sugars, may have a significant benefit on reducing hepatic fat accumulation. We suggest larger, more definitive trials to determine if lowering sugar/HFCS intake, and/or blocking uric acid generation, may help reduce NAFLD and its downstream complications of cirrhosis and chronic liver disease.
Keywords: hepatic steatosis, hepatic inflammation, insulin resistance, sugar consumption, uric acid
“Apicius made the discovery, that we may employ the same artificial method of increasing the size of the liver of the sow, as of that of the goose; it consists in cramming them with dried figs, and when they are fat enough, they are drenched with wine mixed with honey, and immediately killed.”
Pliny the Elder (The Natural History 1ST Century AD, eds John Bostock, Thomas Henry Riley)
Fructose is a simple sugar that is present in fruit and honey, but is also a major component in the two most commonly used sweeteners, sucrose (table sugar, a disaccharide of fructose and glucose), and high fructose corn syrup (HFCS, a mixture of fructose and glucose monosaccharides). Intake of fructose has increased markedly over the last several hundred years in parallel with the rise in intake of sucrose and HFCS, and currently the intake of added sugars approaches 15 percent of overall energy intake in the average western diet, with higher intakes among younger individuals (adolescents and adults in their twenties) and among ethnic minorities (African American, Hispanic, Native American, and Pacific Islanders) (1–4).
The association of fructose with fatty liver dates back to Pliny the Elder who noted that the famous Roman chef, Marcus Apicius, would make fatty liver (foie gras) by feeding geese dates (a rich source of fructose). Later the German chemist, Justus von Liebig, made the observation that simple carbohydrates stimulated fat accumulation in the liver. Indeed, by the 1960s numerous scientists reported that fructose was distinct from glucose in its unique ability to increase both plasma triglycerides and liver fat (5–7). Metabolic studies in which fructose was labeled further showed a 2 to 3-fold greater labeling of plasma and liver triglycerides than that observed with glucose (8). However, overall the amount of fructose being converted to triglycerides was relatively small (1 to 3% of the fructose), and did not account for the lipogenic response observed (9, 10). This led some scientists to question the importance of fructose as a means for stimulating lipid synthesis and accumulation.
However, the importance of fructose reemerged with a report in this journal linking intake of sugary sweetened beverages, and in particular fructose, with non-alcoholic fatty liver disease (NAFLD) (11), an association that has been confirmed in numerous other studies and is now a major area of research (12–15). Here we provide an update on the association and potential mechanisms by which fructose causes fatty liver. One of the key findings is that it is not the fructose molecule itself that is primarily responsible for making triglycerides, but rather fat accumulates in the liver by the general activation of lipogenesis while at the same time blocking fatty acid oxidation (16, 17). Indeed, the weight of studies strongly suggest that sucrose and HFCS are likely major risk factors for NAFLD.
The Discovery of NAFLD and Its Association with Metabolic Syndrome
An association of diabetes with liver disease and gout has been known for over 120 years (18) and is strongly associated with insulin resistance. Type 2 diabetes mellitus is the strongest predictor for NAFLD-related hepatic fibrosis and cirrhosis (19). However, the recognition that people with obesity and prediabetes could develop NAFLD emerged only in the last several decades (20–22). Many patients with NAFLD show characteristics observed in subjects with metabolic syndrome, including elevated plasma triglycerides, low HDL cholesterol, impaired fasting glucose levels, an increased waist circumference, and elevated blood pressure (23). Indeed, NAFLD can be viewed as another clinical manifestation of metabolic syndrome, similar to that of hyperuricemia, systemic inflammation (elevated C reactive protein), and microalbuminuria.
NAFLD was not recognized as a clinical entity until the 1980s (20–22), but has been increasing in prevalence, and may progress to nonalcoholic steatohepatitis (NASH) or cirrhosis and eventual liver transplantation (24, 25). NAFLD is also the most common chronic liver disease in children and adolescents especially in obese patients and has even been detected in infants of mothers with gestational diabetes, making this disorder relevant across a wide spectrum of ages (26–28). Thus, identifying the etiologies of NAFLD represents a major goal.
Soft Drinks and Added Sugar are Associated with Fatty Liver
While fructose is present in honey and fruits, the major source of fructose is from sucrose and HFCS, especially in sugary sweetened beverages. While sucrose containing drinks have equal amounts of glucose and fructose, HFCS-containing beverages have varying ratios, usually varying from a 55/45 or 65/35 fructose:glucose ratio (29).
Experimental Studies
Dietary fructose, sucrose, or HFCS have been shown to have a special tendency to induce fatty liver in experimental animals (6, 17, 30–34), as well as inflammation (35). To develop the fatty liver, it usually takes atleast 8–24 weeks on high fructose diet with more progressive disease with longer exposure (36). Often the administration of fructose also induces other features of metabolic syndrome as well, including elevated blood pressure, elevated serum triglycerides, and insulin resistance (37). In part the fatty liver may be due to increased energy intake, as high fructose intake induces leptin resistance in rats (38, 39). However, if diet is controlled so that the control group ingests the same amount of total energy, the fructose-fed rats will still develop features of metabolic syndrome, although weight gain will not be different between groups (37, 40). Indeed, one can even induce fatty liver with a calorically restricted diet if the diet is high (40%) in sugar (41). Others have also reported that high fructose diet can induce fatty liver in the absence of weight gain (35).
Fructose has also been administered to primates. In one study in cynomolgus monkeys (M. fascicularis), the administration of fructose was shown to result in both an increase in liver fat and hepatic fibrosis after seven years, with the degree of fibrosis correlating with time of fructose exposure (42). Fructose-induced metabolic syndrome can also be induced in rhesus monkeys (43).
Based on comparative studies in which isocaloric diets were administered using sucrose (glucose-fructose disaccharide) or a 50:50 mixture of glucose and fructose monosaccharides, the monosaccharide mixture appears to induce more fatty liver, although the differences are slight (34). This may relate to differences in absorption or other pharmacokinetics.
Endogenously generated fructose may also have a role in fatty liver and NAFLD (44). For example, the administration of high concentrations of glucose in drinking water will lead to obesity, insulin resistance and fatty liver in mice over time (44). Our group reported that the high portal vein levels of glucose can induce the expression of aldose reductase in the liver, which can convert the glucose to sorbitol, which is then further metabolized to fructose by sorbitol dehydrogenase (the polyol pathway). Indeed, glucose fed mice show increased fructose levels in their liver, and when fructose metabolism is blocked (by giving glucose to fructokinase knockout mice) the animals are almost completely protected from fatty liver and insulin resistance, and are partially protected from obesity (44).
The NAFLD so commonly observed in diabetes may also represent the effects of endogenous fructose accumulation. Indeed, either knocking down aldose reductase mRNA in the liver, or treatment with aldose reductase inhibitors, can attenuate hepatic steatosis in the type 2 diabetic (db db) mouse (45).
Clinical Studies
Sugary sweetened beverage drink intake is also strongly associated with NAFLD in humans. Ouyang et al. (11) compared NAFLD subjects without cirrhosis to controls that were matched for age, sex and BMI. Subjects with NAFLD had a 2 to 3-fold higher intake of fructose from sugary sweetened beverages than controls, and this was associated with an increased expression of fructokinase in the liver (11). Subsequently the association of fructose from soft drinks has been associated with NAFLD in children, adolescents and adults, where it correlates in a dose-dependent manner with the severity of hepatic fibrosis (11, 13, 46–52). Fructose intake has also been shown to predict the development of NAFLD (53).
Clinical studies also suggest a role for fructose in NAFLD. For example, the administration of sugary beverages for 6 months to humans resulted in increases in liver fat confirmed by magnetic resonance spectroscopy (54). Conversely, the restriction of fructose for 9 days in children with a high baseline fructose intake resulted in both a reduction in liver fat and de novo lipogenesis compared to controls fed an isocaloric diet (55). In a subset of the same study, there was also an improvement in other features of the metabolic syndrome, including diastolic blood pressure, serum triglycerides and insulin resistance (56).
While the experimental and clinical studies suggest an association of fructose intake with NAFLD, there is one epidemiological study from Finland that found an inverse relationship between fructose intake and NAFLD, but in this population less than 10 percent consumed soft drinks, and fruit intake was much more prevalent (57). While fruits contain fructose, they are less likely to induce metabolic syndrome due to the lower fructose content per fruit (compared to a soft drink) and also because they contain constituents (flavonols, epicatechin, ascorbate, and other antioxidants) that may combat the effects of fructose (58).
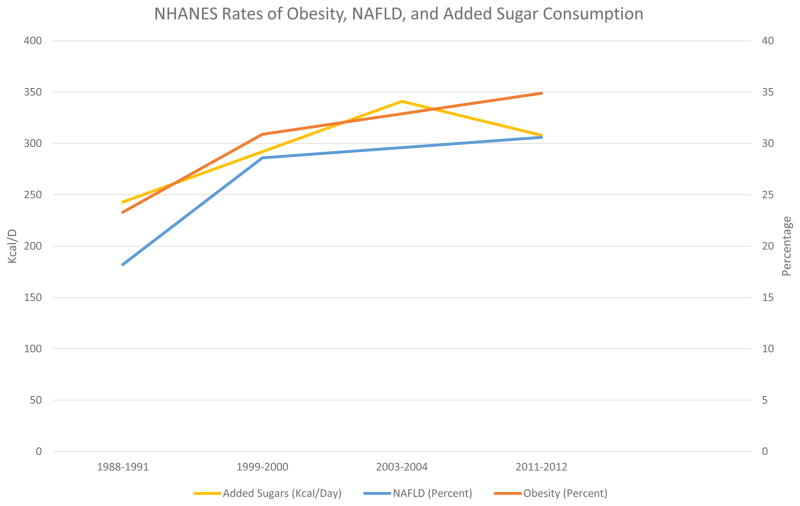
In addition to the aforementioned clinical studies, Figure 1 shows the rise in documented NAFLD prevalence in the National Health and Nutrition Examination Survey (NHANES) database, using a validated, noninvasive measurement (59), the United States Fatty Liver Index (US FLI), in relationship to the rise in obesity and also the rise in added sugar consumption (refined beet, and sugar cane sucrose and HFCS) intake in the periods from 1988–1991, 1999–2000, 2003–2004 and 2011–2012 (60–62). As can be seen, there is a definite association between fructose intake from added sugars and the rise in obesity and NAFLD.
Figure 1.
Association of Added Sugar Consumption with rates of NAFLD, Obesity in NHANES data.
Fructose Effect on Lipogenesis and Fat Oxidation
Fructose intake has been shown to stimulate de novo lipogenesis in animals, as well as to block hepatic β-fatty acid oxidation (16, 17, 63). Similarly, studies in humans have also shown that fructose stimulates de novo lipogenesis and blocks fatty acid oxidation in the liver (13, 63–66). Acutely (hours) fructose stimulates thermogenesis and metabolic rate (67, 68), but chronically fructose (days to weeks) has been shown to reduce resting energy expenditure (66). The mechanisms for these effects are discussed below, but it is readily evident how these processes could lead to fat accumulation in the liver and elsewhere.
Differences in Fructose and Glucose Metabolism
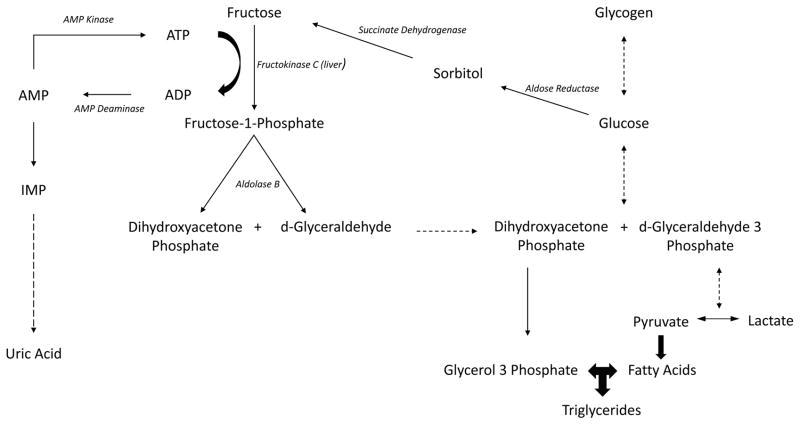
In order to understand how fructose intake might predispose to the development of fatty liver, one must know how fructose metabolism is distinct from glucose metabolism. Glucose is metabolized primarily by glucokinase or hexokinase, whereas fructose is principally metabolized by fructokinase. Fructokinase utilizes ATP to phosphorylate fructose to fructose-1-phosphate, followed by the metabolism by aldolase B to generate D-glyceraldehyde and dihydroxyacetone phosphate. From this stage on, fructose metabolism is similar to glucose metabolism, and results in the generation of glucose, glycogen, and triglycerides (69). Thus, the unique aspect of fructose metabolism lies in its first two enzymatic steps (Figure 2).
Figure 2.
Interaction of Fructose, Glucose and Polyol Pathway with uric acid and triglycerides
The principal isoform of fructokinase in the liver is fructokinase C, which phosphorylates fructose rapidly and without any negative feedback control, resulting in a drop of ATP and intracellular phosphate (70–73). The fall in intracellular phosphate activates the enzyme, adenosine monophosphate (AMP) deaminase, that converts AMP to inosine monophosphate (IMP), resulting in purine nucleotide turnover that culminates in the formation of uric acid (74). Fructose also stimulates the synthesis of uric acid from amino acid precursors (75, 76). The ability of fructose to induce ATP depletion was shown in humans with both intravenous (49, 70, 77, 78) and orally (79) administered fructose. Likewise, an acute rise in uric acid also occurs following fructose ingestion (80–82).
Thus, the unique aspect of fructose compared to glucose is that when fructose is metabolized there is a transient decrease in intracellular phosphate and ATP levels associated with nucleotide turnover and uric acid generation. This fall in ATP level induces a series of reactions, including a transient block in protein synthesis, an induction in oxidative stress, and mitochondrial dysfunction that turn out to have a key role in fructose-mediated effects (17, 70, 83).
Fructokinase, the Principal Enzyme Driving Fructose-Induced Fatty Liver
As mentioned, the hepatic metabolism of fructose by fructokinase C results in the breakdown of AMP to IMP and the generation of uric acid (Figure 2), which is primarily due to a fall in intracellular phosphate that occurs following the rapid phosphorylation of fructose by fructokinase C in the liver (70, 71). In contrast, fructokinase A is a second isoform of fructokinase and is more ubiquitously expressed, but differs from fructokinase C in that it phosphorylates fructose less efficiently and does not cause significant ATP depletion (84, 85). We have observed that mice lacking both fructokinase C and A are protected from fructose-induced fatty liver, while fructokinase A-knockout mice develop worse fatty liver compared to wild type animals despite ingesting similar amounts of fructose (85). These animals also show evidence for greater metabolism of fructose through the fructokinase C pathway and have higher intrahepatic uric acid levels (85). In addition, another study by Softic, et al. (85) found fructose, but not glucose drove lipogenic enzymes, and insulin resistance through fructokinase, and that fructokinase levels are elevated in fructose fed mice as well as obese humans with NASH (86). Thus, these studies suggest that there is a unique property of the fructokinase C pathway that leads to hepatic steatosis, and raises the possibility that it may relate to the transient ATP depletion, intracellular phosphate depletion or uric acid generation (16).
Gut Permeability and the Microbiome
One potential mechanism by which fructokinase may drive fatty liver is via actions on the gut. While fructokinase C is the main enzyme in the liver that metabolizes fructose, it is also highly expressed in the small intestine. We have found that the metabolism of fructose in the intestine results in disruption of the tight junctions and that this is not observed in fructokinase knockout mice (87). This likely is responsible for the increased gut permeability that has been observed with fructose ingestion (88, 89). Indeed, studies by Bergheim’s group have shown that the increase in gut permeability results in endotoxin getting into the portal vein, which is an important trigger for fatty liver formation (90). Indeed, the administration of antibiotics to reduce the endotoxemia can improve the fatty liver (88, 89). Endotoxemia has also been shown to be elevated in children with NAFLD (91). Thus, the microbiome may have a role in fructose-induced fatty liver through an interaction with fructose metabolism in the intestinal wall. Finally, fructose has been found to alter the gut microbiome, which also favors NAFLD development along with increased gut permeability through loss of tight junctions leading to more progressive disease (92–94).
Role of the Immune System
As noted, endotoxemia has been identified as a mechanism by which fructose may augment NAFLD (88). Endotoxemia acts in part by activating the innate immune system, and inflammation is known to have a role in NAFLD, especially for the transition from steatosis to steatohepatitis and cirrhosis (95). In this regard, a role for T cells and NK cells (but not B lymphocytes) in fructose-induced NAFLD has been shown experimentally through the use of mice genetically modified that lack T cell or NK cell function (96).
Uric acid and its Role in Fructose-Mediated NAFLD
Fructose generates Uric acid
As discussed earlier, fructose is the only common carbohydrate that generates uric acid during its metabolism (Figure 2), and levels rise in the circulation within minutes, and can be noted postprandially in subjects eating fructose-rich meals (80, 82, 97). Fructose also increases uric acid levels in the liver (17). Fructose also stimulates the synthesis of uric acid from amino acid precursors (75, 76), and diets high in fructose are associated with increases in fasting serum uric acid levels (98). Some, but not all epidemiological studies, have also linked high fructose intake with increases in fasting serum uric acid (99, 100).
Experimental studies
One of the striking findings was the observation that fructose-induced metabolic syndrome could be partially inhibited by treatment with allopurinol, a xanthine oxidase inhibitor that blocks uric acid generation (37). Subsequent studies showed that xanthine oxidase inhibitors such as allopurinol or febuxostat could reduce fatty liver from fructose (33) as well as in a genetic model of NAFLD (101), diabetes-induced fatty liver (102), high fat diet associated NAFLD (103), and alcohol-induced fatty liver (104). This effect appears to be mediated by improvement of uric acid and/or effects of blocking xanthine oxidase-induced oxidative stress. Furthermore, acutely raising uric acid by the administration of a uricase inhibitor resulted in an acute increase in liver triglycerides and hepatic expression of fatty acid synthase (FAS) (105), and incubation of liver cells (HepG2 cells) with uric acid also resulted in an increase in intracellular triglycerides (17, 83).
Additional studies identified potential mechanisms by which fructose-induced rise in uric acid can stimulate hepatic lipogenesis. First, fructose was found to induce mitochondrial oxidative stress that was mediated by uric acid-induced activation of NADPH oxidase with its translocation to mitochondria (17). The mitochondria contains a large number of enzymes, but two in particular are known to be sensitive to oxidative stress, that being aconitase-2 (in the Kreb cycle) and enoyl CoA hydratase (involved in β-fatty acid oxidation) (106, 107). Fructose and uric acid have been shown to reduce aconitase-2 activity, leading to an accumulation of citrate that moves into the cytoplasm and activates lipogenesis by stimulating ATP citrate lyase (17). Choi et al. (82) further showed that the initial oxidative stress in the mitochondria is due to NADPH oxidase, but later there is stimulation of mitochondrial oxidative stress via the electron transport chain, and that these lead to endoplasmic reticulum (ER) stress, the activation of SREBP-1c, and further stimulation of lipogenesis via activation of acetyl CoA carboxylase-1 and FAS (83). Others have also shown fructose-induced induction of the transcription factor, SREBP-1c (83, 108–110) as well as the carbohydrate Responsive-Element Binding Protein (ChREBP) (33, 111). The stimulation of ChREBP results in the stimulation of glucose-6-phosphatase that may mediate some of the gluconeogenic effects of fructose (112).
We also documented that fructose-induced uric acid can impair fatty acid oxidation. While mitochondrial oxidative stress may be partially responsible for lowering enoyl CoA hydratase-1 activity, we also found that the activity of this enzyme is regulated by AMP-activated protein kinase (AMPK) and AMP Deaminase-2 (AMPD) (16). As mentioned, the rapid metabolism of fructose leads to intracellular phosphate, GTP and ATP depletion, with the stimulation of both AMPK and AMPD activity. However, the stimulation of AMPD tends to dominate, possibly by removing AMP substrate, but also by generating uric acid which feeds back to inhibit AMPK (16, 113). The combined effects of inhibition of AMPK, coupled with AMPD overactivity, results in an inhibition of enoyl A CoA hydratase and the accumulation of lipid (16), as well as the stimulation of gluconeogenesis (113).
This process of reducing AMPK and stimulating AMPD can also result in a reduction in hepatic intracellular ATP levels. Baseline resting ATP levels are low in diabetic subjects with NAFLD and fall further following fructose challenge (77). Subjects with NAFLD who have a higher serum uric acid level show a greater fall in ATP levels following the same fructose challenge (49). Thus, one potential consequence of uric acid is that it may have a role not only in stimulating lipogenesis and gluconeogenesis, but also in blocking fatty acid oxidation, and this may lead to a relatively low hepatic ATP state.
Thus these studies document that the lipogenic response to fructose is not from metabolism of the fructose molecule itself, but rather from the general stimulation of lipogenesis with a block in fatty acid oxidation. Thus, studies using labeled acetate document lipogenesis better (65) than by following labeled fructose (9, 10).
Soluble uric acid has also been shown to have other proinflammatory effects that could play a role in NAFLD, including the activation of the transcription factor NFκB, stimulation of chemokines such as monocyte chemoattractant protein-1, and the stimulation of NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasomes (114, 115).
Clinical Studies
Epidemiological studies have also linked hyperuricemia with NAFLD in both adults (11, 116–122) and children (117–119) in both cross-sectional and longitudinal studies (123, 124) (Table 1). A liver biopsy study also found that in subjects with NAFLD, the higher the serum uric acid the greater the NAFLD score, lobular inflammation and steatosis grade (122). In addition, a meta-analysis found a dose-dependent rise in incidence of NAFLD by 3% for every 1 mg/dL increase in serum uric acid, even after accounting for factors of the metabolic syndrome and other lifestyle factors (125). Finally, a larger meta-analysis of 55,573 patients found a OR of 1.92 (1.59–2.31) for NAFLD occurrence when comparing the highest to lowest serum uric acid (126). While most subjects with NAFLD are obese, NAFLD can also occur in subjects with normal and low BMI, and elevated uric acid also is common in these subjects (17, 127).
Table 1.
Studies with reported Uric Acid in NAFLD patients compared to non-NAFLD patients.
| Study | Uric Acid (mg/dL) and NAFLD | P Values |
|---|---|---|
| Ouyang X, et al. (2008), Journal of Hepatology | NAFLD 6.8 ± 1.6 vs non- NAFLD 4.8 ± 0.9 | P=0.03 |
| Sirota JC, et al. (2013), Metabolism | Non-NAFLD 5.09 ± 0.02 vs Mild NAFLD 5.19 ± 0.06 vs Moderate NAFLD 5.89 ± 0.06 vs Severe NAFLD 6.35 ± 0.10 | P<0.0001 |
| Liu J, et al. (2016), Hepatol Res | Obese Hyperuricemia (>7.0mg/dL males and >6.0mg/dL females) OR 1.692(1.371–2.087) Non-obese hyperuricemia 2.559(1.870–3.503) | Not calculated |
| Liang J, et al. (2015), Eur Rev Med Pharmacol Sci | OR for NAFLD+: Uric Acid <3.7 OR 1, 3.7–<4.49 OR 1.53(1.17–1.99), 4.49–<5.24 OR 2.22(1.71–2.89), 5.24–<6.11 OR 2.64(2.02–3.44), ≥6.11 OR 3.71(2.83–4.88) | P<0.001 |
| Ryu S, et al. (2011), Metabolism | Hyperuricemia (>7.0mg/dL males only) OR 1.21(1.07–1.38) for NAFLD= | P=0.004 |
| Sartorio A, et al. (2007), European Journal of Clinical Nutrition | NAFLD 6.6 ± 1.7 vs non-NAFLD 5.9 ± 1.6 | P<0.0001 |
| Sullivan JS, et al. (2015), Pediatr Obes | NAFLD 7.5 ± 1.4 vs obese non-NAFLD 6.1 ± 1.6* vs lean non-NAFLD 4.5 ± 1.6# | *P=0.04 #P=0.0007 |
| Li Y, Xu C, Yu C, Xu L, & Miao M (2009), Journal of Hepatology | NAFLD 6.2 ± 1.5 vs non-NAFLD 5.4 ± 1.4 | P<0.001 |
Model adjusted for sex, age, BMI, Systolic Blood Pressure, Diastolic Blood Pressure, total cholesterol, HDL, LDL, log of triglycerides, Log AST, Log ALT.
Model adjusted for age, BMI, smoking, alcohol intake, exercise, total cholesterol, HDL, triglycerides, glucose, systolic blood pressure, insulin, hsCRP, and presence of metabolic syndrome
Hyperuricemia is also associated with NASH, the intermediate stage and progressive form of NAFLD. In a study of adolescents, hyperuricemia independently predicted [OR 2.5 (1.87–2.83)] the presence of NASH after adjusting for age, sex and other components of the metabolic syndrome (51). Hyperuricemia in males has also been found to associate more strongly with NASH than simple steatosis and was significantly associated with hepatocyte ballooning, BMI, and younger age in multivariate analysis (128).
One small randomized controlled study evaluated patients with ultrasound-diagnosed NAFLD to determine whether allopurinol treatment (n=17) was superior to placebo (N=14). They reported significant reductions in cytokeratin 18 (a marker for hepatic apoptosis and NASH (129)) (P=0.006), lower ALT and AST levels (P<0.001 and P=0.013), and improved total cholesterol and triglycerides levels (P=0.01 and P=0.038) at 3 months (130).
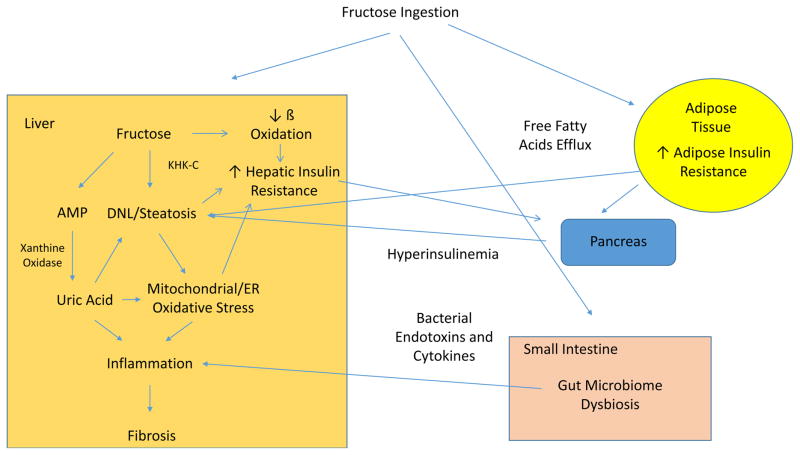
A diagram showing how fructose with its metabolite uric acid may play a role in NAFLD is shown in Figure 3. This does not include a larger role uric acid likely plays in the metabolic syndrome including effects on adipose tissue and islet cells (131).
Figure 3.
Fructose Mechanism mediating development and progression of NAFLD
Modulating Factors
Factors that may Exacerbate Fructose-induced NAFLD
High fat diets may also contribute to fatty liver. Indeed, when fructose is combined with high fat diet, much more severe fatty liver occurs in mice (132). One potential mechanism is that high fat diets also induce mitochondrial oxidative stress (133), similar to fructose. Nevertheless, mice lacking fructokinase show marked protection from fatty liver and insulin resistance, documenting the key role for fructose in western diet-induced NAFLD (132).
Alcohol ingestion also is well known to induce fatty liver and chronic liver disease that can be histologically similar to NAFLD. Alcohol combined with fructose was associated with worsening metabolic features (hyperlipidemia), although interestingly there did not appear to be a synergistic effect on inducing liver injury (134).
As mentioned, high glycemic diets can induce endogenous fructose production (44). However, we have found that high salt diets can also induce hepatic aldose reductase expression (due to effects on osmolarity) leading to endogenous fructose production and NAFLD in mice, and mice lacking fructokinase are protected (Lanaspa MA, manuscript under review). High salt diets are independently associated with metabolic syndrome/diabetes (135, 136) and NAFLD (137). Thus, it seems likely that both high glycemic diets and/or high salt diets might exacerbate fructose-induced NAFLD.
In addition, genetic factors also likely play a role in fructose-induced NAFLD. For instance, Hispanic children who were homozygous for the patatin-like phospholipase domain-containing protein 3 (PNPLA3) gene variant rs738409 were found to have a positive correlation between liver fat content and carbohydrate (r=0.38, p=0.02) and total sugar (r=0.33, p=0.04) intake (138). A similar correlation was made in Italian adolescents with the variant and liver fat content and SSB intake (139). Also of note in 18 adult NAFLD patients (matched for liver fat content), a 6 day low calorie, low carbohydrate diet revealed reductions in liver fat content, but was 2.5 fold greater in those who were PNPLA3 GG homozygotes (n=8) vs CC homozygotes (n=10) (140). Less is known about interaction with other genes such as transmembrane 6 superfamily member 2 (TM6SF2) which regulates VLDL secretion and Glucokinase Regulatory Gene (GCKR) that regulates glycolytic pathway. Overall, further investigations are warranted in this area, but initial data suggest a role of genetic polymorphisms interacting with fructose in the pathogenesis of NAFLD.
Protective Factors for Fructose-Induced NAFLD
Omega 3 fatty acids, such as found in fish oils and in Mediterranean diets, may also protect against NAFLD (141). For example, mice on a western diet (high fat, high fructose) were partially protected from developing NAFLD if they were supplemented with combined omega-3 fatty acids with flavanols (142). Likewise, fish oil treatment was found to improve hypertriglyceridemia and insulin resistance in fructose-fed macaques, although liver fat was not assessed in that study (143). A short term fructose feeding study in humans also suggested fish oil might blunt the development of hypertriglyceridemia and de novo lipogenesis (64). Further studies investigating this pathway are needed.
Likewise, there is evidence that many substances found in natural fruits, such as the flavanols, epicatechin, vitamin C and other antioxidants may also protect against fructose-induced metabolic syndrome (58, 144, 145). This may explain why intake of natural fruits are not associated with NAFLD. Fruit juices, which are associated with metabolic syndrome, contain higher amounts of fructose and are often ingested rapidly, leading to higher fructose concentrations that would cause greater ATP consumption and depletion.
Other Amplifying Mechanisms
High Sugar Exposure May Enhance the Metabolic Effects of Fructose
Repeated exposure to sugar is known to upregulate the transport of fructose through the Glut5 transporter (41, 146) and also increase fructokinase levels in the liver (41). Uptake of fructose is also enhanced by glucose (147). Interestingly, this may increase the risk for fatty liver. A study by Sullivan et al investigated the absorption of fructose in lean children, obese children, and obese children with biopsy proven NAFLD. While fructose malabsorption was common in lean children, it was less in obese children and children with NAFLD absorbed almost all of the oral fructose challenge (119). In addition, blood levels of fructose were lower in the obese children with NAFLD, suggesting they also metabolized the fructose more rapidly. Furthermore, Jin et al reported that children with NAFLD show a greater rise in serum triglycerides in response to fructose compared to lean controls (148).
Uric acid as an Amplification Mechanism
An elevated serum uric acid may also function to amplify fructose effects by creating a positive feedback system. For example, uric acid may feedback to increase endogenous fructose production by stimulating AR (149, 150) and may also stimulate fructose metabolism by increasing expression and activity of fructokinase (33). In contrast, high concentrations of uric acid can block xanthine oxidase (151, 152). Thus, high concentrations of uric acid may act to stimulate upstream metabolism of fructose and which will further promote purine metabolism end-products including uric acid.
Limitations
While the evidence for fructose as a risk factor for NAFLD seems very likely, large clinical trials are still lacking. Nevertheless, there are some groups that have argued that fructose intake may not increase the risk for NAFLD, especially in short term (4 weeks or less) trials that compare fructose to isocaloric diets (153) as well as in studies in which fructose-associated hypercaloric diets (154). However, the development of fatty liver with fructose takes months in animals (85, 132) and most of the trials were probably too short to note this effect. Table 2 lists some of these trials, with the longest ones done by Stanhope, et al. and Maersk, et al. at 10 weeks and 6 months respectively showing differences in either visceral fat, or liver and visceral fat being higher in fructose diet compared to glucose or other isocaloric beverage despite similar changes in weight. Other studies noted are shorter duration, although the majority supports fructose worsening lipid profiles or insulin sensitivity compared to glucose that are likely mechanisms in the development of NAFLD. Of note, studies funded by the food industry, and/or in which the authors are funded by the food industry, often fail to show a relationship between sugar intake and metabolic disease (155, 156).
Table 2.
Studies comparing fructose compared to glucose or other isocaloric intake on NAFLD, Insulin Resistance and Lipids
| Study | Corhort | Intervention | NAFLD Measurement | Liver Enzymes | Insulin Resistance | Lipids |
|---|---|---|---|---|---|---|
| Maersk M, et al., 2012(54) | 47 overweight nondiabetic patients (30 female) | 1L/d SSB (10), isocaloric milk (12), diet cola (12), and water (13) for 6 months | 132–143% increase in liver fat over other beverages | NA | NA | NA |
| Johnson RD, Et al., 2013(161) | 32 centrally overweight males age 18–50. | Overfeeding fructose (25%) (n=15) vs glucose (25%) (n=17) 2 weeks | No difference | No Difference | No difference HOMA-IR | No difference in triglycerides |
| Aeberli I, et al., 2013.(162) | 9 healthy normal weight males age 21–25. | 3 weeks crossover of medium fructose (40 g/d) (MF), and high fructose (HF), high glucose (HG), and high sucrose (HS) beverage at (80 g/d) | NA | NA | Decreased hepatic insulin sensitivity in HF compared to HG | Increased total cholesterol and LDL in MF, HF, and HS, but not HG. Free fatty acids only elevated in MF. |
| LeCoultre V, et al. 2013 (163) | 55 normal weight males (mean age 22.5) | 6–7 days on weight maintenance diet followed by 6–7 day 1.5g/kg/d (n=7), 3g/kg/d (n=17), or 4g/kg/d fructose, 3g/kg/d glucose (n=11) or 30% saturated fats overfeed. | Higher intrahepatic fat in 3g/kg/d and 4g/kg/d fructose, 3g/kg/day glucose, and saturated fat compared to baseline, with tendency for higher levels in fructose diets | NA | 4g/kg/d Fructose and 3g/kg/d of Glucose increased hepatic glucose production. 4g/kg/d and 3g/kg/d Fructose decreased hepatic insulin sensitivity | NA |
| Silbernagel G, et al., 2011. (164) | 20 healthy normal weight (mean age 30.5) (12 males, 8 females | 4 week over feeding with 150g fructose or glucose | No difference | No difference | No difference | Increased triglycerides in fructose compared to glucose |
| Stanhope K, et al. 2009. (65) | 32 patients (age 42–71) Overweight or obese (25–35 kg/m2) (16 female) | 10 week trial with 25% glucose vs 25% fructose added to diet; 2 weeks inpatient energy balanced diet, followed by 8 week ad libitum diet | Not assessed though visceral adipose tissue, a marker for liver fat, was significantly higher in fructose, but not glucose diet | NA | Fasting insulin, glucose, and decreased insulin sensitivity in fructose diet, but not glucose diet | Increased fasting triglycerides in glucose, not fructose, but higher post prandial triglycerides as well as fasting apoB, LDL, and oxidized LDL in fructose diet only |
| Schwarz JM, et al., 2015 (165) | 8 healthy males (age 18–65) with BMI <30kg/m2 | Cross over 9 day study on isocaloric weight maintaining diet of either 25% fructose or fructose portion substituted with complex carbohydrates | Significant increase in liver fat by 137% in fructose diet compared to complex carbohydrate diet | NA | Higher endogenous glucose production during hyperinsulinemia in high fructose vs complex carbohydrate diet | Increased de novo lipogenesis in high fructose as compared to complex carbohydrate diet |
Experimentally, the data that uric acid may have a role in NAFLD is countered by a report in which exogenously administered uric acid reversed hepatic steatosis since it can function as an antioxidant (157). However, there may be differences from exogenous uric acid from intracellular uric acid in terms of its effects on oxidative stress (17, 158). Finally, there is still much to learn about fructose metabolism that is not well understood. For example, fructose is now known to stimulate FGF21, which may counter some of the negative effects of fructose on craving, metabolic syndrome and liver disease (159, 160). Identifying how this factor modulates fructose responses is clearly of major interest.
Conclusions
In summary, there has been a marked rise in sugar and HFCS intake that has paralleled the rise of NAFLD. Experimentally the fructose component of sugar and HFCS appears to have a major role in inducing fatty liver by both stimulating de novo lipogenesis and blocking β-fatty acid oxidation. Evidence suggests these effects are due to the unique metabolism of fructose by fructokinase that leads to a fall in ATP with nucleotide turnover and uric acid generation. The prooxidative and proinflammatory effects of uric acid lead to increases in gut permeability and endotoxemia that exacerbates the lipogenic process in the liver, and coupled with mitochondrial dysfunction results in NAFLD. Clinically the intake of sugary sweetened beverages is strongly linked with NAFLD. Reducing sugar or HFCS intake may have a major benefit on NAFLD. Clinical studies to investigate the potential benefit of lowering uric acid should also be performed. While there are many causes of NAFLD, the intake of fructose containing sugars is likely to have a major role.
Lay Summary.
In this paper we discuss the role of fructose, a monosaccharide sugar, in the development of NonAlcoholic Fatty Liver Disease (NAFLD). We examine evidence both in animal models, and clinical data to support the notion that fructose is a kye player in the recent NAFLD epidemic.
Acknowledgments
Grant Funding: AMD and MFA receive funding support from NIH/NIDDK (PI: Diehl; DK 061703-09 and MPI: Abdelmalek: R01DK093568). KDH is supported by a grant of National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2015R1A2A1A15053374, NRF-2017R1A2B2005849).
Abbreviations
- NAFLD
Nonalcoholic Fatty Liver Disease
- NASH
Nonalcoholic steatohepatitis
- HFCS
high fructose corn syrup
- NHANES
National Health and Nutrition Examination Survey
- US FLI
United States Fatty Liver Index
- FAS
fatty acid synthase
- AMPD
AMP-activated protein kinase, AMP Deaminase 2
- NLRP3
NOD-like receptor family pyrin domain containing 3
- AR
aldose reductase
- SDH
sorbitol dehydrogenase
- PNPLA3
patatin-like phospholipase domain-containing protein 3
- TM6SF2
transmembrane 6 superfamily member 2
- GCKR
Glucokinase Regulatory Gene
Footnotes
Authors’ Contributions: TJ and RJJ provided the main work of reviewing literature, formatting, the paper, and creation of table/figures along with editing the paper. MFA, SS, KJN, MG, AMD, CR, DHK, TN, LGS, MK, YS, HRR, and LGS-L all provided editorial feedback on the paper for additions and grammatical corrections.
Authors’ Disclosures: RJJ and ML discloses they are inventors on patents related to blocking fructose metabolism as a means to reduce sugar craving and metabolic syndrome. RJJ, LGL, DRT, and ML also have equity in Colorado Research Partners LLC, which is a startup company interested in developing novel fructokinase inhibitors. Dr Johnson has also received honoraria from Danone, Astra Zeneca and is on the Scientific Board of Kibow, Inc. RJJ, ML and TJ have submitted a patent for V1b antagonists for the treatment of IR and fatty liver disease. LGS-L receives research support from Danone Research and Kibow Biotech, Inc. MK, MG, CR, YS, KJN, DHK SS, KJN, MFA, AMD, HRR do not have any relevant disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saab KR, Kendrick J, Yracheta JM, Lanaspa MA, Pollard M, Johnson RJ. New Insights on the Risk for Cardiovascular Disease in African Americans: The Role of Added Sugars. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2014040393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yracheta JM, Alfonso J, Lanaspa MA, Roncal-Jimenez C, Johnson SB, Sanchez-Lozada LG, et al. Hispanic Americans living in the United States and their risk for obesity, diabetes and kidney disease: Genetic and environmental considerations. Postgrad Med. 2015;127(5):503–10. doi: 10.1080/00325481.2015.1021234. [DOI] [PubMed] [Google Scholar]
- 3.Yracheta JM, Lanaspa MA, Le MT, Abdelmalak MF, Alfonso J, Sanchez-Lozada LG, et al. Diabetes and Kidney Disease in American Indians: Potential Role of Sugar-Sweetened Beverages. Mayo Clin Proc. 2015;90(6):813–23. doi: 10.1016/j.mayocp.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Johnson RJ, Lanaspa MA, Sanchez-Lozada LG, Rivard CJ, Bjornstad PS, Merriman T, et al. Fat storage syndrome in Pacific peoples: a combination of environment and genetics? Pac Health Dialog. 2014;20(1):11–6. [PubMed] [Google Scholar]
- 5.Macdonald I. Influence of fructose and glucose on serum lipid levels in men and pre- and postmenopausal women. Am J Clin Nutr. 1966;18(5):369–72. doi: 10.1093/ajcn/18.5.369. [DOI] [PubMed] [Google Scholar]
- 6.al-Nagdy S, Miller DS, Yudkin J. Changes in body composition and metabolism induced by sucrose in the rat. Nutr Metab. 1970;12(4):193–219. doi: 10.1159/000175293. [DOI] [PubMed] [Google Scholar]
- 7.Laube H, Klor HU, Fussganger R, Pfeiffer EF. The effect of starch, sucrose, glucose and fructose on lipid metabolism in rats. Nutr Metab. 1973;15(4):273–80. doi: 10.1159/000175450. [DOI] [PubMed] [Google Scholar]
- 8.Maruhama Y, Macdonald I. Incorporation of orally administered glucose-U-14C and fructose-U-14C into the triglyceride of liver, plasma, and adipose tissue of rats. Metabolism. 1973;22(9):1205–15. doi: 10.1016/0026-0495(73)90208-4. [DOI] [PubMed] [Google Scholar]
- 9.Nikkila EA. Control of plasma and liver triglyceride kinetics by carbohydrate metabolism and insulin. Adv Lipid Res. 1969;7:63–134. doi: 10.1016/b978-0-12-024907-7.50009-9. [DOI] [PubMed] [Google Scholar]
- 10.Sun SZ, Empie MW. Fructose metabolism in humans - what isotopic tracer studies tell us. Nutr Metab (Lond) 2012;9(1):89. doi: 10.1186/1743-7075-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, et al. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48(6):993–9. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vos MB, Lavine JE. Dietary fructose in nonalcoholic fatty liver disease. Hepatology. 2013;57(6):2525–31. doi: 10.1002/hep.26299. [DOI] [PubMed] [Google Scholar]
- 13.Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010;7(5):251–64. doi: 10.1038/nrgastro.2010.41. [DOI] [PubMed] [Google Scholar]
- 14.Stanhope KL, Schwarz JM, Havel PJ. Adverse metabolic effects of dietary fructose: results from the recent epidemiological, clinical, and mechanistic studies. Curr Opin Lipidol. 2013;24(3):198–206. doi: 10.1097/MOL.0b013e3283613bca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le KA, Tappy L. Metabolic effects of fructose. Curr Opin Clin Nutr Metab Care. 2006;9(4):469–75. doi: 10.1097/01.mco.0000232910.61612.4d. [DOI] [PubMed] [Google Scholar]
- 16.Lanaspa MA, Cicerchi C, Garcia G, Li N, Roncal-Jimenez CA, Rivard CJ, et al. Counteracting roles of AMP deaminase and AMP kinase in the development of fatty liver. PLoS One. 2012;7(11):e48801. doi: 10.1371/journal.pone.0048801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanaspa MA, Sanchez-Lozada LG, Choi YJ, Cicerchi C, Kanbay M, Roncal-Jimenez CA, et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem. 2012;287(48):40732–44. doi: 10.1074/jbc.M112.399899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harley G. Remarks on Diabetes and Gout in their Relationship to Liver Disease, with Hints Regarding their Scientific Treatment. Br Med J. 1893;1(1691):1097–101. doi: 10.1136/bmj.1.1691.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frankel JJ, Asbury CE, Jr, Baker LA. Hepatic insufficiency and cirrhosis in diabetes mellitus. AMA Arch Intern Med. 1950;86(3):376–90. doi: 10.1001/archinte.1950.00230150059004. [DOI] [PubMed] [Google Scholar]
- 20.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55(7):434–8. [PubMed] [Google Scholar]
- 21.Spech HJ, Liehr H, Mitschke H. Nonalcoholic fatty liver hepatitis and fatty cirrhosis mimicking alcoholic liver diseases. Z Gastroenterol. 1983;21(11):651–9. [PubMed] [Google Scholar]
- 22.Diehl AM, Goodman Z, Ishak KG. Alcohollike liver disease in nonalcoholics. A clinical and histologic comparison with alcohol-induced liver injury. Gastroenterology. 1988;95(4):1056–62. [PubMed] [Google Scholar]
- 23.Paschos P, Paletas K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia. 2009;13(1):9–19. [PMC free article] [PubMed] [Google Scholar]
- 24.Calzadilla Bertot L, Adams LA. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int J Mol Sci. 2016;17(5) doi: 10.3390/ijms17050774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ray K. NAFLD-the next global epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):621. doi: 10.1038/nrgastro.2013.197. [DOI] [PubMed] [Google Scholar]
- 26.Mosca A, Della Corte C, Sartorelli MR, Ferretti F, Nicita F, Vania A, et al. Beverage consumption and paediatric NAFLD. Eat Weight Disord. 2016;21(4):581–8. doi: 10.1007/s40519-016-0315-3. [DOI] [PubMed] [Google Scholar]
- 27.Love-Osborne KA, Nadeau KJ, Sheeder J, Fenton LZ, Zeitler P. Presence of the metabolic syndrome in obese adolescents predicts impaired glucose tolerance and nonalcoholic fatty liver disease. J Adolesc Health. 2008;42(6):543–8. doi: 10.1016/j.jadohealth.2007.11.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brumbaugh DE, Tearse P, Cree-Green M, Fenton LZ, Brown M, Scherzinger A, et al. Intrahepatic fat is increased in the neonatal offspring of obese women with gestational diabetes. J Pediatr. 2013;162(5):930–6. e1. doi: 10.1016/j.jpeds.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ventura EE, Davis JN, Goran MI. Sugar content of popular sweetened beverages based on objective laboratory analysis: focus on fructose content. Obesity (Silver Spring) 2011;19(4):868–74. doi: 10.1038/oby.2010.255. [DOI] [PubMed] [Google Scholar]
- 30.Bruckdorfer KR, Khan IH, Yudkin J. Dietary carbohydrate and fatty acid synthetase activity in rat liver and adipose tissue. Biochem J. 1971;123(2):7P. doi: 10.1042/bj1230007p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ackerman Z, Oron-Herman M, Grozovski M, Rosenthal T, Pappo O, Link G, et al. Fructose-induced fatty liver disease: hepatic effects of blood pressure and plasma triglyceride reduction. Hypertension. 2005;45(5):1012–8. doi: 10.1161/01.HYP.0000164570.20420.67. [DOI] [PubMed] [Google Scholar]
- 32.Collison KS, Maqbool ZM, Inglis AL, Makhoul NJ, Saleh SM, Bakheet RH, et al. Effect of dietary monosodium glutamate on HFCS-induced hepatic steatosis: expression profiles in the liver and visceral fat. Obesity (Silver Spring) 2010;18(6):1122–34. doi: 10.1038/oby.2009.502. [DOI] [PubMed] [Google Scholar]
- 33.Lanaspa MA, Sanchez-Lozada LG, Cicerchi C, Li N, Roncal-Jimenez CA, Ishimoto T, et al. Uric Acid stimulates fructokinase and accelerates fructose metabolism in the development of Fatty liver. PLoS ONE. 2012;7(10):e47948. doi: 10.1371/journal.pone.0047948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanchez-Lozada LG, Mu W, Roncal C, Sautin YY, Abdelmalek M, Reungjui S, et al. Comparison of free fructose and glucose to sucrose in the ability to cause fatty liver. Eur J Nutr. 2010;49(1):1–9. doi: 10.1007/s00394-009-0042-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schultz A, Neil D, Aguila MB, Mandarim-de-Lacerda CA. Hepatic adverse effects of fructose consumption independent of overweight/obesity. Int J Mol Sci. 2013;14(11):21873–86. doi: 10.3390/ijms141121873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asgharpour A, Cazanave SC, Pacana T, Seneshaw M, Vincent R, Banini BA, et al. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J Hepatol. 2016;65(3):579–88. doi: 10.1016/j.jhep.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290(3):F625–31. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 38.Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am J Physiol Regul Integr Comp Physiol. 2008;295(5):R1370–5. doi: 10.1152/ajpregu.00195.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shapiro A, Tumer N, Gao Y, Cheng KY, Scarpace PJ. Prevention and reversal of diet-induced leptin resistance with a sugar-free diet despite high fat content. Br J Nutr. 2011;106(3):390–7. doi: 10.1017/S000711451100033X. [DOI] [PubMed] [Google Scholar]
- 40.Reungjui S, Roncal CA, Mu W, Srinivas TR, Sirivongs D, Johnson RJ, et al. Thiazide diuretics exacerbate fructose-induced metabolic syndrome. J Am Soc Nephrol. 2007;18(10):2724–31. doi: 10.1681/ASN.2007040416. [DOI] [PubMed] [Google Scholar]
- 41.Roncal-Jimenez CA, Lanaspa MA, Rivard CJ, Nakagawa T, Sanchez-Lozada LG, Jalal D, et al. Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake. Metabolism. 2011;60(9):1259–70. doi: 10.1016/j.metabol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cydylo MA, Davis AT, Kavanagh K. Fatty liver promotes fibrosis in monkeys consuming high fructose. Obesity (Silver Spring) 2017;25(2):290–3. doi: 10.1002/oby.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bremer AA, Stanhope KL, Graham JL, Cummings BP, Wang W, Saville BR, et al. Fructose-fed rhesus monkeys: a nonhuman primate model of insulin resistance, metabolic syndrome, and type 2 diabetes. Clin Transl Sci. 2011;4(4):243–52. doi: 10.1111/j.1752-8062.2011.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lanaspa MA, Ishimoto T, Li N, Cicerchi C, Orlicky DJ, Ruzicky P, et al. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat Commun. 2013;4:2434. doi: 10.1038/ncomms3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu L, Lin J, Xu F, Gao Y, Zhang C, Liu Y, et al. Inhibition of aldose reductase activates hepatic peroxisome proliferator-activated receptor-alpha and ameliorates hepatosteatosis in diabetic db/db mice. Exp Diabetes Res. 2012;2012:789730. doi: 10.1155/2012/789730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, et al. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51(6):1961–71. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collison KS, Saleh SM, Bakheet RH, Al-Rabiah RK, Inglis AL, Makhoul NJ, et al. Diabetes of the Liver: The Link Between Nonalcoholic Fatty Liver Disease and HFCS-55. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.58. [DOI] [PubMed] [Google Scholar]
- 48.Dekker MJ, Su Q, Baker C, Rutledge AC, Adeli K. Fructose: a highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am J Physiol Endocrinol Metab. 2010;299(5):E685–94. doi: 10.1152/ajpendo.00283.2010. [DOI] [PubMed] [Google Scholar]
- 49.Abdelmalek MF, Lazo M, Horska A, Bonekamp S, Lipkin EW, Balasubramanyam A, et al. Higher dietary fructose is associated with impaired hepatic adenosine triphosphate homeostasis in obese individuals with type 2 diabetes. Hepatology. 2012;56(3):952–60. doi: 10.1002/hep.25741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nomura K, Yamanouchi T. The role of fructose-enriched diets in mechanisms of nonalcoholic fatty liver disease. J Nutr Biochem. 2012;23(3):203–8. doi: 10.1016/j.jnutbio.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Mosca A, Nobili V, De Vito R, Crudele A, Scorletti E, Villani A, et al. Serum uric acid concentrations and fructose consumption are independently associated with NASH in children and adolescents. Journal of hepatology. 2017;66(5):1031–6. doi: 10.1016/j.jhep.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 52.Hamza RT, Ahmed AY, Rezk DG, Hamed AI. Dietary fructose intake in obese children and adolescents: relation to procollagen type III N-terminal peptide (P3NP) and non-alcoholic fatty liver disease. J Pediatr Endocrinol Metab. 2016;29(12):1345–52. doi: 10.1515/jpem-2016-0015. [DOI] [PubMed] [Google Scholar]
- 53.O’Sullivan TA, Oddy WH, Bremner AP, Sherriff JL, Ayonrinde OT, Olynyk JK, et al. Lower fructose intake may help protect against development of nonalcoholic fatty liver in adolescents with obesity. J Pediatr Gastroenterol Nutr. 2014;58(5):624–31. doi: 10.1097/MPG.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 54.Maersk M, Belza A, Stodkilde-Jorgensen H, Ringgaard S, Chabanova E, Thomsen H, et al. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr. 2012;95(2):283–9. doi: 10.3945/ajcn.111.022533. [DOI] [PubMed] [Google Scholar]
- 55.Schwarz JM, Noworolski SM, Erkin-Cakmak A, Korn NJ, Wen MJ, Tai VW, et al. Effects of Dietary Fructose Restriction on Liver Fat, De Novo Lipogenesis, and Insulin Kinetics in Children With Obesity. Gastroenterology. 2017;153(3):743–52. doi: 10.1053/j.gastro.2017.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lustig RH, Mulligan K, Noworolski SM, Tai VW, Wen MJ, Erkin-Cakmak A, et al. Isocaloric fructose restriction and metabolic improvement in children with obesity and metabolic syndrome. Obesity (Silver Spring) 2015 doi: 10.1002/oby.21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanerva N, Sandboge S, Kaartinen NE, Mannisto S, Eriksson JG. Higher fructose intake is inversely associated with risk of nonalcoholic fatty liver disease in older Finnish adults. Am J Clin Nutr. 2014;100(4):1133–8. doi: 10.3945/ajcn.114.086074. [DOI] [PubMed] [Google Scholar]
- 58.Vasdev S, Gill V, Parai S, Longerich L, Gadag V. Dietary vitamin E and C supplementation prevents fructose induced hypertension in rats. Mol Cell Biochem. 2002;241(1–2):107–14. doi: 10.1023/a:1020835229591. [DOI] [PubMed] [Google Scholar]
- 59.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2015;41(1):65–76. doi: 10.1111/apt.13012. [DOI] [PubMed] [Google Scholar]
- 61.An R. Prevalence and Trends of Adult Obesity in the US, 1999–2012. ISRN Obes. 2014;2014:185132. doi: 10.1155/2014/185132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord. 1998;22(1):39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 63.Softic S, Cohen DE, Kahn CR. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig Dis Sci. 2016;61(5):1282–93. doi: 10.1007/s10620-016-4054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Faeh D, Minehira K, Schwarz JM, Periasamy R, Park S, Tappy L. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes. 2005;54(7):1907–13. doi: 10.2337/diabetes.54.7.1907. [DOI] [PubMed] [Google Scholar]
- 65.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119(5):1322–34. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, et al. Consumption of fructose-sweetened beverages for 10 weeks reduces net fat oxidation and energy expenditure in overweight/obese men and women. Eur J Clin Nutr. 2012;66(2):201–8. doi: 10.1038/ejcn.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwarz JM, Acheson KJ, Tappy L, Piolino V, Muller MJ, Felber JP, et al. Thermogenesis and fructose metabolism in humans. Am J Physiol. 1992;262(5 Pt 1):E591–8. doi: 10.1152/ajpendo.1992.262.5.E591. [DOI] [PubMed] [Google Scholar]
- 68.Mizobe T, Nakajima Y, Ueno H, Sessler DI. Fructose administration increases intraoperative core temperature by augmenting both metabolic rate and the vasoconstriction threshold. Anesthesiology. 2006;104(6):1124–30. doi: 10.1097/00000542-200606000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steinmann B, Gitzelmann R, Van den Berghe G. Disorders of Fructose Metabolism. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The Metabolic and Molecular Basis of Inherited Disease. New York: McGraw-Hill; 2001. pp. 1489–520. [Google Scholar]
- 70.Maenpaa PH, Raivio KO, Kekomaki MP. Liver adenine nucleotides: fructose-induced depletion and its effect on protein synthesis. Science. 1968;161(847):1253–4. doi: 10.1126/science.161.3847.1253. [DOI] [PubMed] [Google Scholar]
- 71.Smith CM, Rovamo LM, Raivio KO. Fructose-induced adenine nucleotide catabolism in isolated rat hepatocytes. Can J Biochem. 1977;55(12):1237–40. doi: 10.1139/o77-185. [DOI] [PubMed] [Google Scholar]
- 72.van den Berghe G, Bronfman M, Vanneste R, Hers HG. The mechanism of adenosine triphosphate depletion in the liver after a load of fructose. A kinetic study of liver adenylate deaminase. Biochem J. 1977;162(3):601–9. doi: 10.1042/bj1620601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kurtz TW, Kabra PM, Booth BE, Al-Bander HA, Portale AA, Serena BG, et al. Liquid-chromatographic measurements of inosine, hypoxanthine, and xanthine in studies of fructose-induced degradation of adenine nucleotides in humans and rats. Clin Chem. 1986;32(5):782–6. [PubMed] [Google Scholar]
- 74.Van den Berghe G. Fructose: metabolism and short-term effects on carbohydrate and purine metabolic pathways. Prog Biochem Pharmacol. 1986;21:1–32. [PubMed] [Google Scholar]
- 75.Emmerson BT. Effect of oral fructose on urate production. Ann Rheum Dis. 1974;33(3):276–80. doi: 10.1136/ard.33.3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raivio KO, Becker A, Meyer LJ, Greene ML, Nuki G, Seegmiller JE. Stimulation of human purine synthesis de novo by fructose infusion. Metabolism. 1975;24(7):861–9. doi: 10.1016/0026-0495(75)90133-x. [DOI] [PubMed] [Google Scholar]
- 77.Cortez-Pinto H, Chatham J, Chacko VP, Arnold C, Rashid A, Diehl AM. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. Jama. 1999;282(17):1659–64. doi: 10.1001/jama.282.17.1659. [DOI] [PubMed] [Google Scholar]
- 78.Nair S, VPC, Arnold C, Diehl AM. Hepatic ATP reserve and efficiency of replenishing: comparison between obese and nonobese normal individuals. Am J Gastroenterol. 2003;98(2):466–70. doi: 10.1111/j.1572-0241.2003.07221.x. [DOI] [PubMed] [Google Scholar]
- 79.Bawden SJ, Stephenson MC, Ciampi E, Hunter K, Marciani L, Macdonald IA, et al. Investigating the effects of an oral fructose challenge on hepatic ATP reserves in healthy volunteers: A P MRS study. Clin Nutr. 2015 doi: 10.1016/j.clnu.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 80.Perheentupa J, Raivio K. Fructose-induced hyperuricaemia. Lancet. 1967;2(7515):528–31. doi: 10.1016/s0140-6736(67)90494-1. [DOI] [PubMed] [Google Scholar]
- 81.Stirpe F, Della Corte E, Bonetti E, Abbondanza A, Abbati A, De Stefano F. Fructose-induced hyperuricaemia. Lancet. 1970;2(7686):1310–1. doi: 10.1016/s0140-6736(70)92269-5. [DOI] [PubMed] [Google Scholar]
- 82.Le MT, Frye RF, Rivard CJ, Cheng J, McFann KK, Segal MS, et al. Effects of high-fructose corn syrup and sucrose on the pharmacokinetics of fructose and acute metabolic and hemodynamic responses in healthy subjects. Metabolism. 2012;61(5):641–51. doi: 10.1016/j.metabol.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choi YJ, Shin HS, Choi HS, Park JW, Jo I, Oh ES, et al. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Lab Invest. 2014;94(10):1114–25. doi: 10.1038/labinvest.2014.98. [DOI] [PubMed] [Google Scholar]
- 84.Diggle CP, Shires M, McRae C, Crellin D, Fisher J, Carr IM, et al. Both isoforms of ketohexokinase are dispensable for normal growth and development. Physiological genomics. 2010;42a(4):235–43. doi: 10.1152/physiolgenomics.00128.2010. [DOI] [PubMed] [Google Scholar]
- 85.Ishimoto T, Lanaspa MA, Le MT, Garcia GE, Diggle CP, Maclean PS, et al. Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc Natl Acad Sci U S A. 2012;109(11):4320–5. doi: 10.1073/pnas.1119908109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Softic S, Gupta MK, Wang GX, Fujisaka S, O’Neill BT, Rao TN, et al. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J Clin Invest. 2017 doi: 10.1172/JCI94585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnson RJ, Rivard C, Lanaspa MA, Otabachian-Smith S, Ishimoto T, Cicerchi C, et al. Fructokinase, Fructans, Intestinal Permeability, and Metabolic Syndrome: An Equine Connection? J Equine Vet Science. 2013;33(2):120–6. doi: 10.1016/j.jevs.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bergheim I, Weber S, Vos M, Kramer S, Volynets V, Kaserouni S, et al. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol. 2008;48(6):983–92. doi: 10.1016/j.jhep.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 89.Spruss A, Bergheim I. Dietary fructose and intestinal barrier: potential risk factor in the pathogenesis of nonalcoholic fatty liver disease. J Nutr Biochem. 2009;20(9):657–62. doi: 10.1016/j.jnutbio.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 90.Thuy S, Ladurner R, Volynets V, Wagner S, Strahl S, Konigsrainer A, et al. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr. 2008;138(8):1452–5. doi: 10.1093/jn/138.8.1452. [DOI] [PubMed] [Google Scholar]
- 91.Jin R, Willment A, Patel SS, Sun X, Song M, Mannery YO, et al. Fructose induced endotoxemia in pediatric nonalcoholic Fatty liver disease. Int J Hepatol. 2014;2014:560620. doi: 10.1155/2014/560620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jegatheesan P, Beutheu S, Freese K, Waligora-Dupriet AJ, Nubret E, Butel MJ, et al. Preventive effects of citrulline on Western diet-induced non-alcoholic fatty liver disease in rats. Br J Nutr. 2016;116(2):191–203. doi: 10.1017/S0007114516001793. [DOI] [PubMed] [Google Scholar]
- 93.Jegatheesan P, Beutheu S, Ventura G, Sarfati G, Nubret E, Kapel N, et al. Effect of specific amino acids on hepatic lipid metabolism in fructose-induced non-alcoholic fatty liver disease. Clin Nutr. 2016;35(1):175–82. doi: 10.1016/j.clnu.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 94.Ritze Y, Bardos G, Claus A, Ehrmann V, Bergheim I, Schwiertz A, et al. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice. PLoS One. 2014;9(1):e80169. doi: 10.1371/journal.pone.0080169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate Immunity and Inflammation in NAFLD/NASH. Dig Dis Sci. 2016;61(5):1294–303. doi: 10.1007/s10620-016-4049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bhattacharjee J, Kumar JM, Arindkar S, Das B, Pramod U, Juyal RC, et al. Role of immunodeficient animal models in the development of fructose induced NAFLD. J Nutr Biochem. 2014;25(2):219–26. doi: 10.1016/j.jnutbio.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 97.Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, et al. Consumption of fructose- but not glucose-sweetened beverages for 10 weeks increases circulating concentrations of uric acid, retinol binding protein-4, and gamma-glutamyl transferase activity in overweight/obese humans. Nutr Metab (Lond) 2012;9(1):68. doi: 10.1186/1743-7075-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reiser S, Powell AS, Scholfield DJ, Panda P, Ellwood KC, Canary JJ. Blood lipids, lipoproteins, apoproteins, and uric acid in men fed diets containing fructose or high-amylose cornstarch. Am J Clin Nutr. 1989;49(5):832–9. doi: 10.1093/ajcn/49.5.832. [DOI] [PubMed] [Google Scholar]
- 99.Choi JW, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: The third national health and nutrition examination survey. Arthritis Rheum. 2007;59(1):109–16. doi: 10.1002/art.23245. [DOI] [PubMed] [Google Scholar]
- 100.Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. Bmj. 2008;336(7639):309–12. doi: 10.1136/bmj.39449.819271.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baldwin W, McRae S, Marek G, Wymer D, Pannu V, Baylis C, et al. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes. 2011;60(4):1258–69. doi: 10.2337/db10-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang W, Wang C, Ding XQ, Pan Y, Gu TT, Wang MX, et al. Quercetin and allopurinol reduce liver thioredoxin-interacting protein to alleviate inflammation and lipid accumulation in diabetic rats. Br J Pharmacol. 2013;169(6):1352–71. doi: 10.1111/bph.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nakatsu Y, Seno Y, Kushiyama A, Sakoda H, Fujishiro M, Katasako A, et al. The xanthine oxidase inhibitor febuxostat suppresses development of nonalcoholic steatohepatitis in a rodent model. Am J Physiol Gastrointest Liver Physiol. 2015;309(1):G42–51. doi: 10.1152/ajpgi.00443.2014. [DOI] [PubMed] [Google Scholar]
- 104.Kono H, Rusyn I, Bradford BU, Connor HD, Mason RP, Thurman RG. Allopurinol prevents early alcohol-induced liver injury in rats. J Pharmacol Exp Ther. 2000;293(1):296–303. [PubMed] [Google Scholar]
- 105.Tapia E, Cristobal M, Garcia-Arroyo FE, Soto V, Monroy-Sanchez F, Pacheco U, et al. Synergistic effect of uricase blockade plus physiological amounts of fructose-glucose on glomerular hypertension and oxidative stress in rats. Am J Physiol Renal Physiol. 2013;304(6):F727–36. doi: 10.1152/ajprenal.00485.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Addabbo F, Montagnani M, Goligorsky MS. Mitochondria and reactive oxygen species. Hypertension. 2009;53(6):885–92. doi: 10.1161/HYPERTENSIONAHA.109.130054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Addabbo F, Ratliff B, Park HC, Kuo MC, Ungvari Z, Csiszar A, et al. The Krebs cycle and mitochondrial mass are early victims of endothelial dysfunction: proteomic approach. Am J Pathol. 2009;174(1):34–43. doi: 10.2353/ajpath.2009.080650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Herman MA, Samuel VT. The Sweet Path to Metabolic Demise: Fructose and Lipid Synthesis. Trends in endocrinology and metabolism: TEM. 2016;27(10):719–30. doi: 10.1016/j.tem.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miyazaki M, Dobrzyn A, Man WC, Chu K, Sampath H, Kim HJ, et al. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J Biol Chem. 2004;279(24):25164–71. doi: 10.1074/jbc.M402781200. [DOI] [PubMed] [Google Scholar]
- 110.Nagai Y, Yonemitsu S, Erion DM, Iwasaki T, Stark R, Weismann D, et al. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab. 2009;9(3):252–64. doi: 10.1016/j.cmet.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koo HY, Miyashita M, Cho BH, Nakamura MT. Replacing dietary glucose with fructose increases ChREBP activity and SREBP-1 protein in rat liver nucleus. Biochem Biophys Res Commun. 2009;390(2):285–9. doi: 10.1016/j.bbrc.2009.09.109. [DOI] [PubMed] [Google Scholar]
- 112.Kim MS, Krawczyk SA, Doridot L, Fowler AJ, Wang JX, Trauger SA, et al. ChREBP regulates fructose-induced glucose production independently of insulin signaling. J Clin Invest. 2016;126(11):4372–86. doi: 10.1172/JCI81993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cicerchi C, Li N, Kratzer J, Garcia G, Roncal-Jimenez CA, Tanabe K, et al. Uric acid-dependent inhibition of AMP kinase induces hepatic glucose production in diabetes and starvation: evolutionary implications of the uricase loss in hominids. FASEB J. 2014;28(8):3339–50. doi: 10.1096/fj.13-243634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wan X, Xu C, Lin Y, Lu C, Li D, Sang J, et al. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. J Hepatol. 2016;64(4):925–32. doi: 10.1016/j.jhep.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 115.Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41(6):1287–93. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- 116.Sirota JC, McFann K, Targher G, Johnson RJ, Chonchol M, Jalal DI. Elevated serum uric acid levels are associated with non-alcoholic fatty liver disease independently of metabolic syndrome features in the United States: Liver ultrasound data from the National Health and Nutrition Examination Survey. Metabolism. 2013;62(3):392–9. doi: 10.1016/j.metabol.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sartorio A, Del Col A, Agosti F, Mazzilli G, Bellentani S, Tiribelli C, et al. Predictors of non-alcoholic fatty liver disease in obese children. Eur J Clin Nutr. 2007;61(7):877–83. doi: 10.1038/sj.ejcn.1602588. [DOI] [PubMed] [Google Scholar]
- 118.Vos MB, Colvin R, Belt P, Molleston JP, Murray KF, Rosenthal P, et al. Correlation of vitamin E, uric acid, and diet composition with histologic features of pediatric NAFLD. J Pediatr Gastroenterol Nutr. 2012;54(1):90–6. doi: 10.1097/MPG.0b013e318229da1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sullivan JS, Le MT, Pan Z, Rivard C, Love-Osborne K, Robbins K, et al. Oral fructose absorption in obese children with non-alcoholic fatty liver disease. Pediatr Obes. 2015;10(3):188–95. doi: 10.1111/ijpo.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li Y, Xu C, Yu C, Xu L, Miao M. Association of serum uric acid level with non-alcoholic fatty liver disease: a cross-sectional study. J Hepatol. 2009;50(5):1029–34. doi: 10.1016/j.jhep.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 121.Kuo CF, Yu KH, Luo SF, Chiu CT, Ko YS, Hwang JS, et al. Gout and risk of non-alcoholic fatty liver disease. Scand J Rheumatol. 2010;39(6):466–71. doi: 10.3109/03009741003742797. [DOI] [PubMed] [Google Scholar]
- 122.Petta S, Camma C, Cabibi D, Di Marco V, Craxi A. Hyperuricemia is associated with histological liver damage in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2011;34(7):757–66. doi: 10.1111/j.1365-2036.2011.04788.x. [DOI] [PubMed] [Google Scholar]
- 123.Lee JW, Cho YK, Ryan M, Kim H, Lee SW, Chang E, et al. Serum uric Acid as a predictor for the development of nonalcoholic Fatty liver disease in apparently healthy subjects: a 5-year retrospective cohort study. Gut Liver. 2010;4(3):378–83. doi: 10.5009/gnl.2010.4.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu C, Yu C, Xu L, Miao M, Li Y. High serum uric acid increases the risk for nonalcoholic Fatty liver disease: a prospective observational study. PLoS ONE. 2010;5(7):e11578. doi: 10.1371/journal.pone.0011578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu Z, Que S, Zhou L, Zheng S. Dose-response Relationship of Serum Uric Acid with Metabolic Syndrome and Non-alcoholic Fatty Liver Disease Incidence: A Meta-analysis of Prospective Studies. Sci Rep. 2015;5:14325. doi: 10.1038/srep14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou Y, Wei F, Fan Y. High serum uric acid and risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Clin Biochem. 2016;49(7–8):636–42. doi: 10.1016/j.clinbiochem.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 127.Kim HJ, Lee KE, Kim DJ, Kim SK, Ahn CW, Lim SK, et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med. 2004;164(19):2169–75. doi: 10.1001/archinte.164.19.2169. [DOI] [PubMed] [Google Scholar]
- 128.Sertoglu E, Ercin CN, Celebi G, Gurel H, Kayadibi H, Genc H, et al. The relationship of serum uric acid with non-alcoholic fatty liver disease. Clin Biochem. 2014;47(6):383–8. doi: 10.1016/j.clinbiochem.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 129.Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50(4):1072–8. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sylvia S, Sedhom OAB, Salama Salwa H, Mokhles Mohamad A. Assessment of the therapeutic effect of allopurinol in patients with non-alcoholic fatty liver disease associated with hyperuricemia by cytokeratin 18. 9th Euro Global Gastroenterology Conference; Valenica Spain. 2016. [Google Scholar]
- 131.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, Shafiu M, Sundaram S, Le M, et al. Sugar, uric Acid, and the etiology of diabetes and obesity. Diabetes. 2013;62(10):3307–15. doi: 10.2337/db12-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ishimoto T, Lanaspa MA, Rivard CJ, Roncal-Jimenez CA, Orlicky DJ, Cicerchi C, et al. High-fat and high-sucrose (western) diet induces steatohepatitis that is dependent on fructokinase. Hepatology. 2013;58(5):1632–43. doi: 10.1002/hep.26594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119(3):573–81. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Alwahsh SM, Xu M, Schultze FC, Wilting J, Mihm S, Raddatz D, et al. Combination of alcohol and fructose exacerbates metabolic imbalance in terms of hepatic damage, dyslipidemia, and insulin resistance in rats. PLoS One. 2014;9(8):e104220. doi: 10.1371/journal.pone.0104220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hu G, Jousilahti P, Peltonen M, Lindstrom J, Tuomilehto J. Urinary sodium and potassium excretion and the risk of type 2 diabetes: a prospective study in Finland. Diabetologia. 2005;48(8):1477–83. doi: 10.1007/s00125-005-1824-1. [DOI] [PubMed] [Google Scholar]
- 136.Ma Y, He FJ, MacGregor GA. High salt intake: independent risk factor for obesity? Hypertension. 2015;66(4):843–9. doi: 10.1161/HYPERTENSIONAHA.115.05948. [DOI] [PubMed] [Google Scholar]
- 137.Huh JH, Lee KJ, Lim JS, Lee MY, Park HJ, Kim MY, et al. High Dietary Sodium Intake Assessed by Estimated 24-h Urinary Sodium Excretion Is Associated with NAFLD and Hepatic Fibrosis. PLoS One. 2015;10(11):e0143222. doi: 10.1371/journal.pone.0143222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Davis JN, Le KA, Walker RW, Vikman S, Spruijt-Metz D, Weigensberg MJ, et al. Increased hepatic fat in overweight Hispanic youth influenced by interaction between genetic variation in PNPLA3 and high dietary carbohydrate and sugar consumption. Am J Clin Nutr. 2010;92(6):1522–7. doi: 10.3945/ajcn.2010.30185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nobili V, Liccardo D, Bedogni G, Salvatori G, Gnani D, Bersani I, et al. Influence of dietary pattern, physical activity, and I148M PNPLA3 on steatosis severity in at-risk adolescents. Genes Nutr. 2014;9(3):392. doi: 10.1007/s12263-014-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sevastianova K, Kotronen A, Gastaldelli A, Perttila J, Hakkarainen A, Lundbom J, et al. Genetic variation in PNPLA3 (adiponutrin) confers sensitivity to weight loss-induced decrease in liver fat in humans. Am J Clin Nutr. 2011;94(1):104–11. doi: 10.3945/ajcn.111.012369. [DOI] [PubMed] [Google Scholar]
- 141.Simopoulos AP. Dietary omega-3 fatty acid deficiency and high fructose intake in the development of metabolic syndrome, brain metabolic abnormalities, and non-alcoholic fatty liver disease. Nutrients. 2013;5(8):2901–23. doi: 10.3390/nu5082901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vauzour D, Rodriguez-Ramiro I, Rushbrook S, Ipharraguerre IR, Bevan D, Davies S, et al. n-3 Fatty acids combined with flavan-3-ols prevent steatosis and liver injury in a murine model of NAFLD. Biochim Biophys Acta. 2017;1864(1):69–78. doi: 10.1016/j.bbadis.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 143.Bremer AA, Stanhope KL, Graham JL, Cummings BP, Ampah SB, Saville BR, et al. Fish oil supplementation ameliorates fructose-induced hypertriglyceridemia and insulin resistance in adult male rhesus macaques. J Nutr. 2014;144(1):5–11. doi: 10.3945/jn.113.178061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hu QH, Zhang X, Pan Y, Li YC, Kong LD. Allopurinol, quercetin and rutin ameliorate renal NLRP3 inflammasome activation and lipid accumulation in fructose-fed rats. Biochem Pharmacol. 2012;84(1):113–25. doi: 10.1016/j.bcp.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 145.Gutierrez-Salmean G, Meaney E, Lanaspa MA, Cicerchi C, Johnson RJ, Dugar S, et al. A randomized, placebo-controlled, double-blind study on the effects of (−)-epicatechin on the triglyceride/HDLc ratio and cardiometabolic profile of subjects with hypertriglyceridemia: Unique in vitro effects. Int J Cardiol. 2016;223:500–6. doi: 10.1016/j.ijcard.2016.08.158. [DOI] [PubMed] [Google Scholar]
- 146.Korieh A, Crouzoulon G. Dietary regulation of fructose metabolism in the intestine and in the liver of the rat. Duration of the effects of a high fructose diet after the return to the standard diet. Arch Int Physiol Biochim Biophys. 1991;99(6):455–60. [PubMed] [Google Scholar]
- 147.Rumessen JJ, Gudmand-Hoyer E. Absorption capacity of fructose in healthy adults. Comparison with sucrose and its constituent monosaccharides. Gut. 1986;27(10):1161–8. doi: 10.1136/gut.27.10.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jin R, Le NA, Liu S, Farkas Epperson M, Ziegler TR, Welsh JA, et al. Children with NAFLD are more sensitive to the adverse metabolic effects of fructose beverages than children without NAFLD. J Clin Endocrinol Metab. 2012;97(7):E1088–98. doi: 10.1210/jc.2012-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang Y, Hong Q, Huang Z, Xue P, Lv Y, Fu B, et al. ALDR enhanced endothelial injury in hyperuricemia screened using SILAC. Cell Physiol Biochem. 2014;33(2):479–90. doi: 10.1159/000358628. [DOI] [PubMed] [Google Scholar]
- 150.Huang Z, Hong Q, Zhang X, Xiao W, Wang L, Cui S, et al. Aldose reductase mediates endothelial cell dysfunction induced by high uric acid concentrations. Cell Commun Signal. 2017;15(1):3. doi: 10.1186/s12964-016-0158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Radi R, Tan S, Prodanov E, Evans RA, Parks DA. Inhibition of xanthine oxidase by uric acid and its influence on superoxide radical production. Biochim Biophys Acta. 1992;1122(2):178–82. doi: 10.1016/0167-4838(92)90321-4. [DOI] [PubMed] [Google Scholar]
- 152.Tan S, Radi R, Gaudier F, Evans RA, Rivera A, Kirk KA, et al. Physiologic levels of uric acid inhibit xanthine oxidase in human plasma. Pediatr Res. 1993;34(3):303–7. doi: 10.1203/00006450-199309000-00013. [DOI] [PubMed] [Google Scholar]
- 153.Chiu S, Sievenpiper JL, de Souza RJ, Cozma AI, Mirrahimi A, Carleton AJ, et al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr. 2014;68(4):416–23. doi: 10.1038/ejcn.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chung M, Ma J, Patel K, Berger S, Lau J, Lichtenstein AH. Fructose, high-fructose corn syrup, sucrose, and nonalcoholic fatty liver disease or indexes of liver health: a systematic review and meta-analysis. Am J Clin Nutr. 2014;100(3):833–49. doi: 10.3945/ajcn.114.086314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schillinger D, Tran J, Mangurian C, Kearns C. Do Sugar-Sweetened Beverages Cause Obesity and Diabetes? Industry and the Manufacture of Scientific Controversy. Ann Intern Med. 2016;165(12):895–7. doi: 10.7326/L16-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bes-Rastrollo M, Schulze MB, Ruiz-Canela M, Martinez-Gonzalez MA. Financial conflicts of interest and reporting bias regarding the association between sugar-sweetened beverages and weight gain: a systematic review of systematic reviews. PLoS medicine. 2013;10(12):e1001578. doi: 10.1371/journal.pmed.1001578. dicsussion e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Garcia-Ruiz I, Rodriguez-Juan C, Diaz-Sanjuan T, del Hoyo P, Colina F, Munoz-Yague T, et al. Uric acid and anti-TNF antibody improve mitochondrial dysfunction in ob/ob mice. Hepatology. 2006;44(3):581–91. doi: 10.1002/hep.21313. [DOI] [PubMed] [Google Scholar]
- 158.Yu MA, Sanchez-Lozada LG, Johnson RJ, Kang DH. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens. 2010;28(6):1234–42. [PubMed] [Google Scholar]
- 159.Dushay JR, Toschi E, Mitten EK, Fisher FM, Herman MA, Maratos-Flier E. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Mol Metab. 2015;4(1):51–7. doi: 10.1016/j.molmet.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fisher FM, Kim M, Doridot L, Cunniff JC, Parker TS, Levine DM, et al. A critical role for ChREBP-mediated FGF21 secretion in hepatic fructose metabolism. Mol Metab. 2017;6(1):14–21. doi: 10.1016/j.molmet.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Johnston RD, Stephenson MC, Crossland H, Cordon SM, Palcidi E, Cox EF, et al. No difference between high-fructose and high-glucose diets on liver triacylglycerol or biochemistry in healthy overweight men. Gastroenterology. 2013;145(5):1016–25. e2. doi: 10.1053/j.gastro.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 162.Aeberli I, Hochuli M, Gerber PA, Sze L, Murer SB, Tappy L, et al. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: a randomized controlled trial. Diabetes Care. 2013;36(1):150–6. doi: 10.2337/dc12-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lecoultre V, Egli L, Carrel G, Theytaz F, Kreis R, Schneiter P, et al. Effects of fructose and glucose overfeeding on hepatic insulin sensitivity and intrahepatic lipids in healthy humans. Obesity (Silver Spring) 2013;21(4):782–5. doi: 10.1002/oby.20377. [DOI] [PubMed] [Google Scholar]
- 164.Silbernagel G, Machann J, Unmuth S, Schick F, Stefan N, Haring HU, et al. Effects of 4-week very-high-fructose/glucose diets on insulin sensitivity, visceral fat and intrahepatic lipids: an exploratory trial. The British journal of nutrition. 2011;106(1):79–86. doi: 10.1017/S000711451000574X. [DOI] [PubMed] [Google Scholar]
- 165.Schwarz JM, Noworolski SM, Wen MJ, Dyachenko A, Prior JL, Weinberg ME, et al. Effect of a High-Fructose Weight-Maintaining Diet on Lipogenesis and Liver Fat. J Clin Endocrinol Metab. 2015;100(6):2434–42. doi: 10.1210/jc.2014-3678. [DOI] [PMC free article] [PubMed] [Google Scholar]